When Nature Requires a Resource to Be Used—The Case of Callinectes sapidus: Distribution, Aggregation Patterns, and Spatial Structure in Northwest Europe, the Mediterranean Sea, and Adjacent Waters
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Callinectes sapidus Invasion History
2.2. Distribution, Aggregation Pattern, and Spatial Structure Analyses
2.3. Callinectes sapidus Detection Methods
3. Results
3.1. Invasion History and Spatial–Temporal Patterns of Callinectes sapidus Distribution
3.2. Aggregation Patterns and Spatial Structure
- NEW—at a local spatial scale, the O–GOG* analysis showed two areas with statistically significant cold spots (99%) along the Atlantic coast of France and the Netherlands (corresponding to older records of Callinectes sapidus). The other records had non-significant index values. No hot spots and spatial outliers were detected. Medium density values were recorded in the NWE area.
- MAW—the O–GOG* analysis showed a very statistically significant cold spot (99%) in the Levantine Basin corresponding to the initial direction of spread. Hot spots (99%) were detected in the western Mediterranean Basin and eastern Atlantic Ocean. Maximum density values were recorded in the same area. Hot spots of up to 95% were also detected along the Tyrrhenian Coast of Italy and around Sicily. The occurrences in the Adriatic and Ionian Sea had non-significant index values. High spatial outliers were detected in the Levant and north Aegean Sea, and low outliers were detected in the central Mediterranean Sea.
3.3. Key Characteristics of Distribution
- NWE—from 1900, i.e., the year of the first record on the Atlantic coasts of France, to 1973, the central tendencies, measured as median and mean centres, were found in the Netherlands and Belgium, respectively. The directional dispersion of distribution and trends was concentrated along the coast, from the southwest (France) to the northeast (Denmark), with a very elongated and narrow ellipse. In the second period, i.e., 1975–1995, there is an evident contraction of the ellipse, with a strong reduction in y dispersion (from 631 to 231 km) and median and mean centres approaching each other. In the 1996–2018 period, the central tendencies diverge somewhat, and the distribution is dispersed again along the x-axis with the maximum XStdDist recorded, changing its shape but not its direction.
- MAW—from 1940, i.e., the year of the first record on the Egyptian coast, to 2000, the central tendencies, measured as median and mean centres, were found in the Aegean Sea, close to each other. The directional dispersion of distribution and trends was from southeast, in the Levantine Basin, to northwest, in the central Mediterranean Sea. In the 2001–2011 period, the central tendencies were in the southern Adriatic (Albanian coasts). The distribution’s directional dispersion changed in shape and direction, showing a considerable westward dispersion: the XStdDist ranged from 1459 km to 437 and the YStdDist from 494 km to 1552 (Table 2). This period showed the highest dispersion of Callinectes sapidus over time. This expansion is also confirmed by the distribution key characteristics in the third period (2012–2023). The ellipse is elongated from Greece to Spain, and the direction extends towards the Strait of Gibraltar. Central tendencies are shifted further west, on the western coast of Sardinia.
3.4. Callinectes sapidus Detection Methods
4. Discussion
Callinectes sapidus in the Mediterranean Sea and Adjacent Waters: A Resource to Be Used
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- IUCN. Guidelines for the prevention of biodiversity loss caused by alien invasive species. In Secondary Guidelines for the Prevention of Biodiversity Loss Caused by Alien Invasive Species; IUCN: Gland, Switzerland, 2000. [Google Scholar]
- IPBES Intergovernmental Science–Policy Platform on Biodiversity and Ecosystem Services. In Summary for Policymakers of the Thematic Assessment Report on Invasive Alien Species and Their Control of the Intergovernmental Science–Policy Platform on Biodiversity and Ecosystem Services; Roy, H.E., Pauchard, A., Stoett, P., Renard Truong, T., Bacher, S., Galil, B.S., Hulme, P.E., Ikeda, T., Sankaran, K.V., McGeoch, M.A., et al., Eds.; IPBES: Bonn, Germany, 2023. [Google Scholar] [CrossRef]
- Williams, A.B. The swimming crabs of the genus Callinectes (Decapoda: Portunidae). Fish. Bull. 1974, 72, 685–798. [Google Scholar]
- Streftaris, N.; Zenetos, A. Alien marine species in the Mediterranean—The 100 ‘worst invasives’ and their impact. Mediterr. Mar. Sci. 2006, 7, 87–118. [Google Scholar] [CrossRef]
- Clavero, M.; Franch, N.; Bernardo–Madrid, R.; López, V.; Abelló, P.; Queral, J.M.; Mancinelli, G. Severe, rapid and widespread impacts of an Atlantic blue crab invasion. Mar. Poll. Bull. 2022, 176, 113479. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. The Atlantic blue crab Callinectes sapidus in southern European coastal waters: Distribution, impact and prospective invasion management strategies. Mar. Poll. Bull. 2017, 119, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Kevrekidis, K.; Kevrekidis, T.; Mogias, A.; Boubonari, T.; Kantaridou, F.; Kaisari, N.; Malea, P.; Dounas, C.; Thessalou–Legaki, M. Fisheries Biology and Basic Life–Cycle Characteristics of the Invasive Blue Crab Callinectes sapidus Rathbun in the Estuarine Area of the Evros River (Northeast Aegean Sea, Eastern Mediterranean). J. Mar. Sci. Eng. 2023, 11, 462. [Google Scholar] [CrossRef]
- Nehring, S. Invasion history and success of the American blue crab Callinectes sapidus Rathbun, 1896 in European and adjacent waters. In the Wrong Place–Alien Marine Crustaceans: Distribution, Biology and Impacts Invading Nature; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer Series in Invasion Ecology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 607–624. [Google Scholar]
- Van Engel, W.A. The blue crab and its fishery in Chesapeake Bay. Part I. Reproduction, early development, growth and migration. Commer. Fish. Rev. 1958, 20, 6–17. [Google Scholar]
- Williams, A.B. Marine decapod crustaceans of the Carolinas. Fish. Bull. 1965, 65, 1–298. [Google Scholar]
- Hines, A.H.; Johnson, E.G.; Young, A.C.; Aguilar, R.; Kramer, M.A.; Goodison, M.; Zmora, O.; Zohar, Y. Release strategies for estuarine species with complex migratory life cycles: Stock enhancement of Chesapeake blue crabs (Callinectes sapidus). Rev. Fish. Sci. 2008, 16, 175–185. [Google Scholar] [CrossRef]
- Mancinelli, G.; Carrozzo, L.; Costantini, M.L.; Rossi, L.; Marini, G.; Pinna, M. Occurrence of the Atlantic blue crab Callinectes sapidus Rathbun, 1896 in two Mediterranean coastal habitats: Temporary visitor or permanent resident? Estuar. Coast. Shelf Sci. 2013, 135, 46–56. [Google Scholar] [CrossRef]
- Cilenti, L.; Pazienza, G.; Scirocco, T.; Fabbrocini, A.; D’Adamo, R. First record of ovigerous Callinectes sapidus (Rathbun, 1896) in the Gargano Lagoons (south–west Adriatic Sea). BioInvasions Rec. 2015, 4, 281–287. [Google Scholar] [CrossRef]
- Hines, A.H. Ecology of juvenile and adult blue crabs. Chapter 14. In The Blue Crab: Callinectes Sapidus; Kennedy, V.S., Cronin, L.E., Eds.; Maryland Sea Grant Program: College Park, MD, USA, 2007; pp. 565–654. [Google Scholar]
- Taylor, D.L.; Fehon, M.M. Blue crab (Callinectes sapidus) population structure in southern New England tidal rivers: Patterns of shallow water, unvegetated habitat use and quality. Estuaries Coasts 2021, 44, 1320–1343. [Google Scholar] [CrossRef] [PubMed]
- Hines, A.H.; Haddon, A.M.; Wiechert, L.A. Guild structure and foraging impact of blue crabs and epibenthic fish in a subestuary of Chesapeake Bay. Mar. Ecol. Prog. Ser. 1990, 67, 105–126. [Google Scholar] [CrossRef]
- Belgrad, B.A.; Griffen, B.D. The influence of diet composition on fitness of the blue crab, Callinectes sapidus. PLoS ONE 2016, 11, e0145481. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.L.; Fehon, M.M.; Cribari, K.J.; Scro, A.K. Blue crab Callinectes sapidus dietary habits and predation on juvenile winter flounder Pseudopleuronectes americanus in southern New England tidal rivers. Mar. Ecol. Progr. Ser. 2022, 681, 145–167. [Google Scholar] [CrossRef]
- Rady, A.; Sallam, W.S.; Abdou, N.E.I.; El-Sayed, A.A.M. Food and feeding habits of the blue crab, Callinectes sapidus (Crustacea: Decapoda: Portunidae) with special reference to the gastric mill structure. Egypt. J. Aquat. Biol. Fish. 2018, 22, 417–431. [Google Scholar]
- Mancinelli, G.; Guerra, M.T.; Alujević, K.; Raho, D.; Zotti, M.; Vizzini, S. Trophic flexibility of the Atlantic blue crab Callinectes sapidus in invaded coastal systems of the Apulia region (SE Italy): A stable isotope analysis. Estuar. Coast. Shelf Sci. 2017, 198, 421–431. [Google Scholar] [CrossRef]
- Oussellam, M.; Selfati, M.; El Ouamari, N.; Bazairi, H. Using the new SEICAT methodology to study the socio–economic impacts of the American blue crab Callinectes sapidus from Marchica lagoon, Morocco. AACL Bioflux 2021, 14, 3231–3241. Available online: http://bioflux.com.ro/docs/2021.3231-3241.pdf (accessed on 14 March 2024).
- Falautano, M.; Perzia, P.; Castriota, L. First record of the Lessepsian fish Parexocoetus mento in Italian waters and GIS–based spatial and temporal distribution in Mediterranean Sea. J. Mar. Biol. Assoc. U. K. 2020, 100, 1163–1169. [Google Scholar] [CrossRef]
- Castriota, L.; Falautano, M.; Maggio, T.; Perzia, P. The Blue Swimming Crab Portunus segnis in the Mediterranean Sea: Invasion Paths, Impacts and Management Measures. Biology 2022, 11, 1473. [Google Scholar] [CrossRef]
- Perzia, P.; Spinelli, A.; Interdonato, F.; Castriota, L. Ecological indicators from spatial statistics to describe the Atlantic fangtooth moray distribution in Mediterranean Sea. Trans. GIS 2022, 26, 2802–2817. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, 160. [Google Scholar] [CrossRef]
- Lipej, L.; Mavric, B.; Paliska, D. New northernmost record of the blunthead pufferfish, Sphoeroides pachygaster (osteichthyes: Tetraodontidae) in the Mediterranean Sea/Nuova segnalazione a nord del pesce palla liscio, Sphoeroides pachygaster (Osteichthyes: Tetraodontidae), nel mare Mediterraneo. Ann. Ser. Hist. Nat. 2013, 23, 103–114. [Google Scholar]
- ESRI. ArcGIS Desktop Help: Release 10.3; Environmental Systems Research Institute: Redlands, CA, USA, 2011. [Google Scholar]
- Mitchell, A. The ESRI Guide to GIS Analysis, Volume 2; ESRI Press: Redlands, CA, USA, 2005; ISBN 1–58948–116–X. [Google Scholar]
- Scott, L.M.; Janikas, M.V. Spatial statistics in ArcGIS. In Handbook of Applied Spatial Analysis: Software Tools, Methods and Applications; Fischer, M.M., Getis, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 27–41. [Google Scholar]
- Lucchetti, A.; Petetta, A.; Bdioui, M.; Gökçe, G.; Saber, M.; Sacchi, J.; Özbilgin, H.; Carlson, A.; Carpentieri, P. Catalogue of Fishing Gear in the Mediterranean and Black Sea Region; FAO Fisheries and Aquaculture Technical Paper No. 695; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Hill, J.; Fowler, D.L.; Avyle, M.V. Species Profiles: Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (Mid-Atlantic)—Blue Crab; U.S. Army Corps of Engineers: Vicksburg, MI, USA, 1989. [Google Scholar]
- Marchessaux, G.; Bosch–Belmar, M.; Cilenti, L.; Lago, N.; Mangano, M.C.; Marsiglia, N.; Sarà, G. The invasive blue crab Callinectes sapidus thermal response: Predicting metabolic suitability maps under future warming Mediterranean scenarios. Front. Mar. Sci. 2022, 9, 1055404. [Google Scholar] [CrossRef]
- Giordani Soika, A. II Neptunus pelagicus (L.) nell’alto Adriatico. Natura 1951, 42, 18–20. [Google Scholar]
- Serbetis, C. Un nouveau crustacé comestible en Mer Egée Callinectes sapidus Rath. (Decapode Brach.). Proc. Gen. Fish. Counc. Medit. 1959, 5, 505–507. [Google Scholar]
- Banoub, M.W. Survey of the blue crab Callinectes sapidus (Rath.) in lake Edku in 1960. Notes Mem. Alex. Inst. Hydrobiol. 1963, 69, 1–18. [Google Scholar]
- Tortonese, E. La comparsa di Callinectes sapidus Rathb. (Decapoda Brachyura) nel Mar Ligure. Doriana 1965, 4, 1–3. [Google Scholar]
- Zibrowius, H. Assessing scale and impact of ship–transported alien fauna in the Mediterranean. In Alien Marine Organisms Introduced by Ships in the Mediterranean and Black Seas; CIESM: Bd de Suisse, Monaco, 2002; 136p. [Google Scholar]
- Milori, E.; Zhori, A.; Agolli, I.; Beqiraj, S. Distribution of the invasive blue crab Callinectes sapidus Rathbun, 1896 along the Albanian Coast. In Proceedings of 4th ESENIAS Workshop: International Workshop on IAS in Agricultural and Non–Agricultural Areas in ESENIAS Region, Çanakkale, Turkey, 16–17 December 2013; pp. 96–100. [Google Scholar]
- Ceyhunlu, A.I.; Ceribasi, G.; Ahmed, N.; Al–Najjar, H. Climate change analysis by using sen’s innovative and trend analysis methods for western black sea coastal region of turkey. J. Coast. Conserv. 2021, 25, 50. [Google Scholar] [CrossRef]
- Azzurro, E.; Bolognini, L.; Dragičević, B.; Drakulović, D.; Dulčić, J.; Fanelli, E.; Grati, F.; Kolitari, J.; Lipej, L.; Magaletti, E.; et al. Detecting the occurrence of indigenous and non–indigenous megafauna through fishermen knowledge: A complementary tool to coastal and port surveys. Mar. Poll. Bull. 2019, 147, 229–236. [Google Scholar] [CrossRef]
- Fuentes, M.A.; Torrent, L.; Barrera, S.; Boix, D. Rapid invasion of the American blue crab Callinectes sapidus Rathbun, 1896 in the North–East of the Iberian Peninsula. BioInvasions Rec. 2019, 8, 113–118. [Google Scholar] [CrossRef]
- Ragkousis, M.; Zenetos, A.; Ben Souissi, J.; Hoffman, R.; Ghanem, R.; Taşkın, E.; Muresan, M.; Karpova, E.; Slynko, E.; Katsanevakis, S.; et al. Unpublished Mediterranean and Black Sea records of marine alien, cryptogenic, and neonative species. BioInvasions Rec. 2023, 12, 339–369. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Poursanidis, D.; Hoffman, R.; Rizgalla, J.; Rothman, B. –S.S.; Levitt–Barmats, Y.; Hadjioannou, L.; Trkov, D.; Garmendia, J.M.; Joxe, M.; et al. Unpublished Mediterranean records of marine alien and cryptogenic species. BioInvasions Rec. 2020, 9, 165–182. [Google Scholar] [CrossRef]
- Izquierdo–Gómez, D. Synergistic Use of Facebook, Online Questionnaires and Local Ecological Knowledge to Detect and Reconstruct the Bioinvasion of the Iberian Peninsula by Callinectes sapidus Rathbun, 1896. Biol. Invasions 2022, 24, 1059–1082. [Google Scholar] [CrossRef]
- Maggio, T.; Perzia, P.; Falautano, M.; Visconti, G.; Castriota, L. From LEK to LAB: The case of the blue crab Portunus segnis in the Pelagie Islands Marine Protected Area, central Mediterranean Sea. Ocean. Coast. Manag. 2022, 219, 106043. [Google Scholar] [CrossRef]
- Politico. Available online: https://www.politico.eu/article/italy-blue-crab-invasion-eat-them (accessed on 3 April 2024).
- Invasivesnet on, X. Available online: https://twitter.com/Invasivesnet/status/1413430446755655681 (accessed on 3 April 2024).
- Archivio Bollettino. Available online: https://www.archiviobollettino.unict.it/gallery/il-granchio-reale-blu-la-specie-invasiva-buona-da-mangiare (accessed on 3 April 2024).
- Seaman, A.N.; Franzidis, A.; Nelson, M. Considering invasive alien species as food source: Current motivations and future implications for controlling through consumption. Geogr. Rev. 2024, 1–18. [Google Scholar] [CrossRef]
- Vecchioni, L.; Russotto, S.; Arculeo, M.; Marrone, F. On the occurrence of the invasive Atlantic blue crab Callinectes sapidus Rathbun 1896 (Decapoda: Brachyura: Portunidae) in Sicilian inland waters. Nat. Hist. Sci. 2022, 9, 43–46. [Google Scholar] [CrossRef]
- Adams, D.H.; Engel, M.E. Mercury, lead, and cadmium in blue crabs, Callinectes sapidus, from the Atlantic coast of Florida, USA: A multipredator approach. Ecotoxicol. Environ. Saf. 2014, 102, 196–201. [Google Scholar] [CrossRef]
- Reichmuth, J.M.; Weis, P.; Weis, J.S. Bioaccumulation and depuration of metals in blue crabs, Callinectes sapidus (Rathbun) from a contaminated and clean estuary. Environ. Pollut. 2010, 158, 361–368. [Google Scholar] [CrossRef]
- Tekin, S.; Pazi, I. POP levels in blue crab (Callinectes sapidus) and edible fish from the eastern Mediterranean coast. Environ. Sci. Pollut. Res. 2017, 24, 509–518. [Google Scholar] [CrossRef]
- Renzi, M.; Cilenti, L.; Scirocco, T.; Grazioli, E.; Anselmi, S.; Broccoli, A.; Pauna, V.; Provenza, F.; Specchiulli, A. Litter in alien species of possible commercial interest: The blue crab (Callinectes sapidus Rathbun, 1896) as case study. Mar. Poll. Bull. 2020, 157, 111300. [Google Scholar] [CrossRef]
- Fakhri, Y.; Hoseinvandtabar, S.; Heidarinejad, Z.; Borzoei, M.; Bagheri, M.; Dehbandi, R.; Khaneghah, A.M. The concentration of potentially hazardous elements (PHEs) in the muscle of blue crabs (Callinectes sapidus) and associated health risk. Chemosphere 2021, 279, 130431. [Google Scholar] [CrossRef]
- Aliko, V.; Beqiraj, E.G.; Qirjo, M.; Cani, M.; Rama, A.; Bego, K.; Reka, A.; Faggio, C. Plastic invasion tolling: First evaluation of microplastics in water and two crab species from the nature reserve lagoonary complex of Kune-Vain, Albania. Sci. Total Environ. 2022, 849, 157799. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. On the Atlantic blue crab (Callinectes sapidus Rathbun 1896) in southern European coastal waters: Time to turn a threat into a resource? Fish. Res. 2017, 194, 1–8. [Google Scholar] [CrossRef]
- Official Journal of the Italian Republic, General Series. 2024, 165. Available online: https://www.gazzettaufficiale.it/eli/gu/2024/01/26/21/sg/pdf (accessed on 14 March 2024).
- 2023, Official Journal of the Italian Republic, General Series. 2023, 284. Available online: https://www.gazzettaufficiale.it/eli/id/2023/12/05/23A06621/sg (accessed on 14 March 2024).
- Hamdi, M.; Hajji, S.; Affes, S.; Taktak, W.; Maâlej, H.; Nasri, M.; Nasri, R. Development of a controlled bioconversion process for the recovery of chitosan from blue crab (Portunus segnis) exoskeleton. Food Hydrocoll. 2018, 77, 534–548. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and chitosans: Characteristics, eco–friendly processes, and applications in cosmetic science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef]
- Stagg, C.; Whilden, M. The history of Chesapeake Bay’s blue crab (Callinectes sapidus): Fisheries and management. Investig. Mar. 1997, 25, 93–104. [Google Scholar] [CrossRef]
- Simantiris, N.; Violaris, I.G.; Avlonitis, M. Computing Invasive Species Population Based on a Generalized Random Walk Process: Application to Blue Crab (Callinectes sapidus). J. Mar. Sci. Eng. 2023, 11, 1282. [Google Scholar] [CrossRef]
- Bouvier, E.L. Sur un Callinectes sapidus M. Rathbun trouvé à Rochefort. Bull. Mus. Natl. Hist. Nat. 1901, 7, 16–17. [Google Scholar]
- Holthuis, L.B.; Gottlieb, E. The occurrence of the American blue crab, Callinectes sapidus Rathbun, in Israel waters. Bull. Res. Counc. Israel 1955, 5, 154–156. [Google Scholar]
- Holthuis, L.B.; Gottlieb, E. An annotated list of the decapod Crustacea of the Mediterranean coast of Israel, with an appendix listing the Decapoda of the eastern Mediterranean. Bull. Res. Counc. Israel 1958, 7b, 1–126. [Google Scholar]
- Holthuis, L.B. Report on a collection of Crustacea Decapoda and Stomatopoda from Turkey and the Balkans. Zool. Verh. 1961, 47, 1–67. [Google Scholar]
- Amanieu, M.; Le Dantec, J. Sur la présence accidentelle de Callinectes sapidus M. Rathbun à l’embouchure de la Gironde. Rev. Trav. Inst. Pêches Marit. 1961, 25, 339–343. [Google Scholar]
- Zelenka, G. Notes ornithologiques sur la Grèce du Nord (Aout 1962). Nos Oiseaux 1964, 27, 189–203. [Google Scholar]
- George, C.J.; Athanasiou, V. The occurrence of American blue crab, Callinectes sapidus Rathbun, in the coastal waters of Lebanon. Doriana 1965, 4, 1–3. [Google Scholar]
- Kinzelbach, R. Die Blaue Schwimmkrabbe (Callinectes sapidus) ein Neuburger im Mittelmeer. Nat. Mus. 1965, 95, 293–296. [Google Scholar]
- Gorgy, S. Les pêcheries et le milieu marin dans le secteur Méditerranéen de la République Arabe Unie. Rev. Trav. Inst. Pêches Marit. 1966, 30, 25–92. [Google Scholar]
- Bulgurkov, K. Occurrence of Callinectes sapidus Rathbun (Crustacea–Decapoda) in Black Sea. Proc. Res. Inst. Fish. Oceanogr. Varna 1968, 9, 97–99. [Google Scholar]
- Holthuis, L. Enkele interessante Nederlandse Crustacea. Bijdragen tot de faunistiek van Nederland, 1. Zool. Bijdr. Leiden 1969, 11, 34–48. [Google Scholar]
- Demetropoulos, A.; Neocleous, D. The Fishes and Crustaceans of Cyprus. Fish. Bull. Min. Agric. Nat. Res. Cyprus 1969, 1, 3–21. [Google Scholar]
- Ramadan, S.E.; Dowidar, N.M. Brachyura (Decapoda, Crustacea) from the Mediterranean waters of Egypt. Thalass. Jugosl. 1972, 8, 127–139. [Google Scholar]
- Froglia, C. Segnalazione di alcuni crostacei nuovi o rari per l’Adriatico. Quad. Lab. Tecnol. Pesca 1972, 1, 43–52. [Google Scholar]
- Georgiadis, C.; Georgiadis, G. Zur kenntnis der Crustacea Decapoda del Golfes von Thessaloniki. Crustaceana 1974, 26, 239–248. [Google Scholar] [CrossRef]
- Cavaliere, A.; Berdar, A. Presenza di Callinectes sapidus Rathbun (Decapoda Brachyura) nello Stretto di Messina. Boll. Pesca Piscic. Idrobiol. 1975, 30, 315–322. [Google Scholar]
- Shaverdashvili, R.S.; Ninua, N.S. New find of crab Callinectes sapidus Rathbun, 1896 in the Black Sea. Nauchnyye Dokl. Vyss. Shkoly 1975, 9, 19–20. [Google Scholar]
- Maury, A. A propos du “Crabe bleu”. Bull. Tr. Soc. Géol. Normandie Des Amis Du Muséum Du Havre 1975, 62, 25. [Google Scholar]
- Gaudêncio, M.J.; Guerra, M.T. Note on the blue crab Callinectes sapidus Rathbun 1896 (Crustacea Decapoda Brachyura) capture in the Tagus estuary. Bol. Inst. Nac. Investig. Pescas 1979, 2, 67–73. [Google Scholar]
- Shiber, J.C. Brachyurans from Lebanese waters. Bull. Mar. Sci. 1981, 31, 864–875. [Google Scholar]
- Kocatas, A.; Katagan, T. Crustacean fauna of Turkish coastal lagoons. Rapp. Proc. Verb. Réun. 1983, 28, 231–233. [Google Scholar]
- Monin, V.L. New find of the blue crab Callinectes sapidus (Decapoda, Brachyura) in the Black Sea. Zool. Zhurnal 1984, 63, 1100–1101. [Google Scholar]
- Lewinsohn, C.; Holthuis, L.B. The Crustacea Decapoda of Cyprus. Zool. Verh. 1986, 230, 3–64. [Google Scholar]
- Vincent, T. Les captures de Callinectes sapidus (Rathbun, 1896) en Baie de Seine, entre 1975 et 1984. Bull. Tr. Soc. Géol. Normandie Des Amis Du Muséum Du Havre 1986, 73, 13–15. [Google Scholar]
- Abdel–Razek, F.A. Crab fishery of the Egyptian waters with notes on the bionomics of Portunus pelagicus (L.). Acta Adriat. 1987, 28, 143–154. [Google Scholar]
- Snovsky, Z.; Galil, B. The occurence of the American Blue Crab, Callinectes sapidus Rathbun, in the Sea of Galilee. Isr. J. Acquacult. 1990, 42, 62–63. [Google Scholar]
- Mizzan, L. Presence of swimming crabs of the genus Callinectes (Stimpson) (Decapoda, Portunidae) in the Venice Lagoon (North Adriatic Sea, Italy). Boll. Mus. Civ. St. Nat. Venezia 1993, 42, 31–43. [Google Scholar]
- Baker, M.; Noureddin, S.; Hamoud, N.; Mayhoub, H.; Youssef, A.K. Effect of hydrochemical characteristics of coastal waters of Lattakia City on zoo– and phyto–plankton communities. Tishreen Univ. J. Stud. Sci. Res. 1994, 2, 71–125. [Google Scholar]
- Gomoiu, M.T.; Skolka, M. Changements récents dans la biodiversité de la Mer Noire dus aux immigrants. In Danube Delta–Black Sea System under Global Changes Impact Geo–Eco–Marina; Romanian Centre of Marine Geology and Geoecology: București, Romania, 1996; pp. 34–47. [Google Scholar]
- Enzenross, R.; Enzenross, L.; Bingel, F. Occurrence of Blue crab, Callinectes sapidus (Rathbun, 1896) (Crustacea, Brachyura) on the Turkish Mediterranean and the adjacent Aegean Coast and its size distribution in the Bay of Iskenderun. Turkish J. Zool. 1997, 21, 113–122. [Google Scholar] [CrossRef]
- Gökoğlu, M.; Oray, I.K. Antalya Körfezi’nde Mavi Yengeç Avcılığı Üzerine Bir Araştırma. II. In Su Ürünleri Avlama ve İşleme Teknolojisi Workshop, İstanbul, Türkiye, 6–7 Mart 1997; İstanbul Üniversitesi Su Ürünleri Fakültesi Avlama ve İşleme Teknolojisi Bölümü: İstanbul, Türkiye, 1997; Volume 97, pp. 6–7. [Google Scholar]
- Gokoglu, M.; Aydın, H.; Çiloglu, E. Antalya Körfezi’ndeki Ekonomik Öneme Sahip Yengeçlerin Avcılıgĭ Üzerine Bir Araştırma. Doğu Anadolu Bölgesi III. In Proceedings of the Su Urünleri Sempozyumu, Erzurum, Türkiye, 10–12 June 1998; pp. 637–643. [Google Scholar]
- Zaitsev, Y. Samoe Sinee v Mire (Most Blue in the World); Izd. OON: New York, NY, USA, 1998; pp. 1–142. [Google Scholar]
- Gomoiu, M.T.; Skolka, M. Creoterea biodiversitãþii prin imigrare—Noi specii în fauna României. Analele Univ. Ovidius Constanþa Ser. Biol. Ecol. 1998, 2, 181–202. [Google Scholar]
- Pessani, D.; Salton, L. Planktonic larval stages of Brachyura in the Gulf of Tigullio (Ligurian Sea, Italy). Invertebr. Reprod. Dev. 1998, 33, 201–208. [Google Scholar] [CrossRef]
- Türeli, C. İskenderun Körfezi’ndeki Mavi Yengeç (Callinectes sapidus RATHBUN, 1896)’in Biyolojik Özellikleri. Doktora Tezi, Çukurova Üniversitesi Fen Bilimleri Enstitüsü Su Üruünleri Anabilim Dalı, Adana, Turkey, 1999. [Google Scholar]
- Vincent, T. Callinectes sapidus (Decapoda, Brachyura, Portunidae). Essai de synthèse sur 23 ans d’observations en Baie de Seine (Normandie, France). Bull. tr. Soc. Géol. Normandie Des Amis Du Muséum Du Havre 1999, 86, 13–17. [Google Scholar]
- Egemen, Ö.; Önen, M.; Büyükişik, B.; Hoşsucu, B.; Sunlu, U.; Gökpinar, Ş.; Cırık, S. Güllük Lagünü (Ege Denizi, Türkiye) Ekosistemi. Turkish J. Zool. 1999, 23, 927–947. [Google Scholar]
- Türelı, C.; Çelık, M.; Erdem, Ü. Comparison of Meat Composition and Yield of Blue Crab (Callinectes sapidus RATHBUN, 1896) and Sand Crab (Portunus pelagicus LINNE, 1758) Caught in Iskenderun Bay, North–East Mediterranean. Turk. J. Vet. Anim. Sci. 2000, 24, 195–203. [Google Scholar]
- Petrescu, I.; Papadopol, N.; Nicolaev, S. O nouă specie pentru fauna de decapode din apele marine româneşti, Callinectes sapidus Rathbun 1896. Analele Univ. Ovidius Din Constanta Ser. Biol. Ecol. 2000, 6, 222–228. [Google Scholar]
- Zaitsev, Y.; Öztürk, B. Exotic Species in the Aegean, Marmara, Black, Azov and Caspian Seas; Turkish Marine Research Foundation: Istanbul, Turkey, 2001; 267p. [Google Scholar]
- Gennaio, R. I Bacini di Ugento: Aspetti Botanici, Faunistici e Paesaggistici; Martano Editrice: Lecce, Italy, 2001; 133p. [Google Scholar]
- ICES. Report of the Working Group on Introductions and Transfers of Marine Organisms (WGITMO), 21–23 March 2001, Barcelona, Spain; ICES CM 2001/ACME:08; ICES: Copenhagen, Denmark, 2001. [Google Scholar]
- Türeli, C.; Erdem, Ü.; Çelik, M. Seasonal Variation and Meat Composition of Blue Crab (Callinectes sapidus, RATHBUN, 1896) Caught in Iskenderun Bay, North–East Mediterranean. Turk. J. Vet. Anim. Sci. 2002, 26, 1435–1439. [Google Scholar]
- Başusta, N.; Kumlu, M.; Gökçe, M.A.; Göçer, M. Yumurtalık Koyu’nda dip trolü ile yakalanan türlerin mevsimsel değişimi ve verimlilik indeksi. E.U. Su Urünleri Dergisi 2002 E.U. J. Fish. Aquat. Sci. 2002, 19, 29–34. [Google Scholar]
- WWF/ADENA. 2002. Doñana y Cambio Climático. Available online: https://wwfes.awsassets.panda.org/downloads/donana_y_cambio_climatico_1.pdf?55601/Donana-y-el-Cambio-Climatico-Informe-2002 (accessed on 16 January 2013).
- Bashtannyy, R.; Webster, L.; Raaymakers, S. First Black Sea Conference on Ballast Water Control and Management, Odessa, Ukraine, 10–12 October 2001: Conference Report; GloBallast Monograph Series No. 3. IMO: London, UK, 2002; 112p. [Google Scholar]
- Galil, B.; Froglia, C.; Noel, P.Y. Crustacean Decapods and Stomatopods. In CIESM Atlas of Exotic Species in the Mediterranean; Briand, F., Ed.; CIESM Publishers: Bd de Suisse, Monaco, 2002; Volume 2, pp. 1–192. [Google Scholar]
- Atar, H.H.; Seçer, S. Width/Length–Weight relationships of the blue crab (Callinectes sapidus Rathbun 1896) population living in Beymelek lagoon lake. Turk. J. Vet. Anim. Sci. 2003, 27, 443–447. [Google Scholar]
- Gökoğlu, N.; Yerlikaya, P. Determination of proximate composition and mineral contents of blue crab (Callinectes sapidus) and swim crab (Portunus pelagicus) caught off the Gulf of Antalya. Food Chem. 2003, 80, 495–498. [Google Scholar] [CrossRef]
- Revkov, N.K. Taxonomical composition of the bottom fauna at the Black Sea Crimean coast. In Modern Condition of the Biodiversity of the Coastal Zone of Crimea (Black Sea Region); Eremeev, V.N., Gaevskaya, A.V., Eds.; Ekosi–Gidrophizika: Sevastopol, Ukraine, 2003; pp. 209–218. [Google Scholar]
- Çelik, M.; Türeli, C.; Çelik, M.; Yanar, Y.; Erdem, Ü.; Küçükgülmez, A. Fatty acid composition of the blue crab (Callinectes sapidus Rathbun, 1896) in the northeastern Mediterranean. Food Chem. 2004, 88, 271–273. [Google Scholar] [CrossRef]
- Özcan, T.; Katagan, T.; Kocatas, A. Brachyuran crabs from Iskenderun Bay (southeastern Turkey). Crustaceana 2005, 78, 237–243. [Google Scholar] [CrossRef]
- Çekiç, M.; Dal, T.; Başusta, N.; Gökçe, M.A. Comparison of two different types of basket trap on fish catches in Iskenderun Bay. Turk. J. Vet. Anim. Sci. 2005, 29, 743–749. [Google Scholar]
- Bisconti, M.; Silvi, E. Prima segnalazione di Callinectes sapidus Rathbun, 1896 (Crustacea, Decapoda, Brachyura) nella provincia di Livorno. Quad. Mus. Stor. Nat. Livorno 2005, 18, 1–6. [Google Scholar]
- Türkmen, A.; Türkmen, M.; Tepe, Y.; Mazlum, Y.; Oymael, S. Metal concentrations in blue crab (Callinectes sapidus) and mullet (Mugil cephalus) in Iskenderun Bay, Northern East Mediterranean, Turkey. Bull. Environ. Contam. Toxicol. 2006, 77, 186–193. [Google Scholar] [CrossRef]
- Küçükgülmez, A.; Çelik, M.; Yanar, Y.; Ersoy, B.; Çikrikçi, M. Proximate composition and mineral contents of the blue crab (Callinectes sapidus) breast meat, claw meat and hepatopancreas. Int. J. Food Sci. Technol. 2006, 41, 1023–1026. [Google Scholar] [CrossRef]
- Gökçe, G.; Erguden, D.; Sangun, L.; Cekic, M.; Alagoz, S. Width/length–weight and relationships of the blue crab (Callinectes sapidus Rathbun, 1986) population living in Camlik Lagoon Lake (Yumurtalik). Pak. J. Biol. Sci. 2006, 9, 1460–1464. [Google Scholar] [CrossRef]
- ICES. Report of the Working Group on Introductions and Transfers of Marine Organisms (WGITMO), 16–17 March 2006, Oostende, Belgium, 2006; ICES CM 2006/ACME:05; ICES: Copenhagen, Denmark, 2006. [Google Scholar]
- Gennaio, R.; Scordella, G.; Pastore, M. Occurrence of Blue Crab Callinectes sapidus (Rathbun, 1896, Crustacea, Brachyura), in the Ugento Ponds Area (Lecce, Italy). Thalass. Salentina 2006, 29, 29–39. [Google Scholar]
- Cabal, J.; Pis Millán, J.A.; Arronte, J.C. A new record of Callinectes sapidus Rathbun, 1896 (Crustacea: Decapoda: Brachyura) from the Cantabrian Sea, Bay of Biscay, Spain. Aquat. Invasions 2006, 1, 186–187. [Google Scholar] [CrossRef]
- Micu, S.; Micu, D. Proposed IUCN regional status of all Crustacea: Decapoda from the Romanian Black Sea. Ann. Sci. Univ. AlICuza Iasi Sect Biol Anim. 2006, 52, 7–38. [Google Scholar]
- ICES. Report of the Working Group on Introductions and Transfers of Marine Organisms (WGITMO), 21–23 March 2007, Dubrovnik, Croatia, 2007; ICES CM 2007/ACME:05; ICES: Copenhagen, Denmark, 2007; 160p. [Google Scholar]
- Shiganova, T. Introduced Species. In The Black Sea Environment. The Handbook of Environmental Chemistry; Kostianoy, A.G., Kosarev, A.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 5Q. [Google Scholar] [CrossRef]
- Scaravelli, D.; Mordenti, O. Segnalazioni faunistiche n. 83–87. 83—Callinectes sapidus Rathbun, 1896 (Crustacea Brachyura Portunidae). Quad. Studi Nat. Romagna 2007, 24, 155–160. [Google Scholar]
- Hasan, H.; Zeini, A.; Noel, P.Y. The Marine Decapod Crustacea of the Area of Lattakia, Syria. Crustaceana 2008, 81, 513–536. [Google Scholar] [CrossRef]
- Küçükgülmez, A.; Çelik, M. Amino acid composition of blue crab (Callinectes sapidus) from the North Eastern Mediterranean Sea. J. Appl. Biol. Sci. 2008, 2, 39–42. [Google Scholar]
- Tuncer, S.; Bilgin, S. First record of Callinectes sapidus Rathbun, 1896 (Crustacea: Decapoda: Brachyura) in the Dardanelles, Canakkale, Turkey. Aquat. Invasions 2008, 3, 469. [Google Scholar] [CrossRef]
- Florio, M.; Breber, P.; Scirocco, T.; Specchiulli, A.; Cilenti, L.; Lumare, L. Exotic species in Lesina and Varano lakes, Gargano National Park (Italy). Transit. Waters Bull. 2008, 2, 69–79. [Google Scholar] [CrossRef]
- Onofri, V.; Dulčić, J.; Conides, A.; Matić–Skoko, S.; Glamuzina, B. The occurrence of the blue crab, Callinectes sapidus Rathbun, 1896 (Decapoda, Brachyura, Portunidae) in the eastern Adriatic (Croatian coast). Crustaceana 2008, 81, 403–409. [Google Scholar] [CrossRef]
- Gülșahin, A.; Erdem, M. Length–weight relationships in blue crab, Callinectes sapidus (Rathbun, 1896) in Köycegiz Dalyan Lagoon Area–Turkey. J. Fish. Sci. 2009, 3, 24–31. [Google Scholar]
- Diripasko, O.A.; Izergin, L.V.; Koshkalda, A.I. First finds of the blue crab Callinectes sapidus (Portunidae, Decapoda) in the Sea of Azov. Vestn. Zool. 2009, 43, 529–532. [Google Scholar]
- Kevrekidis, K. Callinectes sapidus (Decapoda, Brachyura): An allochthonous species in Thermaikos Gulf. Fish. News 2010, 340, 44–49. [Google Scholar]
- Beqiraj, S.; Kashta, L. The establishment of blue crab Callinectes sapidus Rathbun, 1896 in the Lagoon of Patok, Albania (south–east Adriatic Sea). Aquat. Invasions 2010, 5, 219–221. [Google Scholar] [CrossRef]
- Dulcic, J.; Dragicevic, B.; Lipej, L. New record of the blue crab, Callinectes sapidus Rathbun, 1896 (Decapoda: Brachyura) in the Adriatic Sea. Ann. Ser. Hist. Nat. 2010, 20, 23–28. [Google Scholar]
- Skolka, M.; Preda, C. Alien invasive species at the Romanian Black Sea Coast—Present and perspectives. Trav. Mus. Natl. Hist. Nat. Grigore Antipa 2010, 53, 443–467. [Google Scholar] [CrossRef]
- Khvorov, S.A. Decapods (Decapoda). In Vselentsy v Bioraznoobrazii i Produktivnosti Azovskogo i Chernogo Morei (Invaders in Biodiversity and Productivity of the Sea of Azov and Black Sea); Matishov, G.G., Boltachev, A.R., Eds.; YuNTs RAN: Rostov on Don, Russia, 2010; pp. 70–75. [Google Scholar]
- Nehring, S.; van der Meer, U. First record of a fertilized female blue crab, Callinectes sapidus Rathbun, 1896 (Crustacea: Decapoda: Brachyura), from the German Wadden Sea and subsequent secondary prevention measures. Aquat. Invasions 2010, 5, 215–218. [Google Scholar] [CrossRef]
- Mehanna, S.F.; El–Aiatt, A.A.A. Fisheries characteristics and population dynamics of the blue swimmer crab Portunus pelagicus (Linnaeus, 1766) from Bardawil lagoon. Egypt. J. Aquat. Biol. Fish 2011, 15, 393–406. [Google Scholar]
- Mutlu, C.; Türkmen, M.; Türkmen, A.; Tepe, Y. Comparison of metal concentrations in tissues of blue crab, Callinectes sapidus from Mediterranean lagoons. Bull. Environ. Contam. Toxicol. 2011, 87, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Ayas, D.; Özogul, Y. The chemical composition of carapace meat of sexually mature blue crab (Callinectes sapidus, Rathbun 1896) in the Mersin Bay. J. Fish. Sci. 2011, 5, 262. [Google Scholar] [CrossRef]
- Tureli Bilen, C.; Korkcu, P.; Ibrikci, T. Application of artificial neural networks (ANNs) for weight predictions of blue crabs (Callinectes sapidus Rathbun, 1896) using predictor variables. Mediterr. Mar. Sci. 2011, 12, 439–446. [Google Scholar] [CrossRef]
- Eleftheriou, M.; Anagnostopoulou–Visilia, E.; Anastasopoulou, E.; Ates, A.S.; Cavas, L.; Cem, C.; Ulha, M.; Cevik, F.; Delos, A.L.; Derici, O.B.; et al. New Mediterranean Biodiversity Records (December 2011). Mediterr. Mar. Sci. 2011, 12, 491–508. [Google Scholar] [CrossRef]
- Dulcic, J.; Tutman, P.; Matic–Skoko, S.; Glamuzina, B. Six years from first record to population establishment: The case of the blue crab, Callinectes sapidus Rathbun, 1896 (Brachyura, Portunidae) in the Neretva River delta (south–eastern Adriatic Sea, Croatia). Crustaceana 2011, 84, 1211–1220. [Google Scholar] [CrossRef]
- Özcan, T. The swimming crab Portunus segnis (Forskål, 1775): Host for the barnacle Chelonibia platula (Ranzani, 1818) from the Turkish coast. J. Black Sea Mediterr. Environ. 2012, 18, 271–278. [Google Scholar]
- Thessalou–Legaki, M.; Aydogan, O.; Bekas, P.; Bilge, G.; Boyaci, Y.O.; Brunelli, E.; Circosta, V.; Crocetta, F.; Durucan, F.; Erdem, M.; et al. New Mediterranean biodiversity records (December 2012). Mediterr. Mar. Sci. 2012, 13, 312–327. [Google Scholar] [CrossRef]
- Kevrekidis, K.; Avramoglou, K.; Efstathiadis, J.; Chintiroglou, C. Population aspects of the allochtonous species Callinectes sapidus (Decapoda: Brachyura) in Methoni Bay (Thermaikos Gulf): Preliminary results. In Proceedings of the 10th Panhellenic Symposium of Oceanography and Fisheries; Hellenic Center for Marine Research: Gournes Gouvon, Greece, 2012. [Google Scholar]
- Mačić, V.; Kljajić, Z. A review of the introduced species in Montenegrian coastal sea. In 41st Annual Conference of the Serbian Water Pollution Control Society “WATER 2012”, Divčibare, Serbia, 5–7 June 2012; Serbian Water Pollution Control Society: Belgrade, Serbia, 2012; pp. 255–260. [Google Scholar]
- Giansante, C. Segnalazioni faunistiche n. 119–124. Quad. Studi E Not. Stor. Nat. Della Romagna 2012, 36, 207–208, ISSN 1123-6787. [Google Scholar]
- Castriota, L.; Andaloro, F.; Costantini, R.; De Ascentiis, A. First record of the Atlantic crab Callinectes sapidus Rathbun, 1896 (Crustacea: Brachyura: Portunidae) in Abruzzi waters, central Adriatic Sea. Acta Adriat. 2012, 53, 467–471. [Google Scholar]
- Pashkov, A.N.; Reshetnikov, S.I.; Bondarev, K.B. The capture of the blue crab (Callinectes sapidus, Decapoda, Crustacea) in the Russian sector of the Black Sea. Russ. J. Biol. Invasions 2012, 3, 22–28. [Google Scholar] [CrossRef]
- Sumer, C.; Teksam, I.; Karatas, H.; Beyhan, T.; Aydin, C.M. Growth and Reproduction Biology of the Blue Crab, Callinectes sapidus Rathbun, 1896, in the Beymelek Lagoon (Southwestern Coast of Turkey). Turk. J. Fish. Aquat. Sci. 2013, 13, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Zenetos, A.; Koutsogiannopoulos, D.; Ovalis, P.; Poursanidis, D. The role played by citizen scientists in monitoring marine alien species in Greece. Cah. Biol. Mar. 2013, 54, 419–426. [Google Scholar]
- Brusco, A.; Marchianò, R.; Puntillo, D.; Tripepi, S.; Sperone, E.; Cozza, R. Flora & Fauna Acquatiche della Riserva Naturale Regionale della Foce del Crati; Edizione Amici della Terra Italia/Ente Gestore Riserve Tarsia–Crati: Tarsia, Italy, 2013; 112p. [Google Scholar]
- Bilecenoglu, M.; Alfaya, J.E.F.; Azzurro, E.; Baldacconi, R.; Boyacı, Y.Ö.; Circosta, V.; Compagno, L.J.V.; Coppola, F.; Deidun, A.; Durgham, H.; et al. New Mediterranean marine biodiversity records (December, 2013). Mediterr. Mar. Sci. 2013, 14, 463–480. [Google Scholar] [CrossRef]
- Mancinelli, G.; Carrozzo, L.; Marini, G.; Costantini, M.L.; Pagliara, M.; Pinna, M. The co–occurrence of Callinectes sapidus Rathbun, 1896 (Brachyura: Portunidae) and the parasitic dinoflagellate Hematodinium sp. (Dinoflagellata: Syndinidae) in two transitional water ecosystems of the Apulia coastline (South–Italy). Transitional Waters Bull. 2013, 7, 32–42. [Google Scholar] [CrossRef]
- Kevrekidis, K.; Antoniadou, C.; Avramoglou, K.; Efstathiadis, J.; Chintiroglou, C. Population structure of the blue crab Callinectes sapidus in Thermaikos Gulf (Methoni Bay). In Proceedings of the 15th Pan–Hellenic Congress of Ichthyologists, Thessaloniki, Greece, 10–13 October 2013; pp. 113–116. [Google Scholar]
- Castejón, D.; Guerao, G. A new record of the American blue crab, Callinectes sapidus Rathbun, 1896 (Decapoda: Brachyura: Portunidae), from the Mediterranean coast of the Iberian Peninsula. BioInvasions Rec. 2013, 2, 141–143. [Google Scholar] [CrossRef]
- Tureli–Bilen, C.; Yesilyurt, I.N. Growth of blue crab, Callinectes sapidus, in the Yumurtalik Cove, Turkey: A molt process approach. Cent. Eur. J. Biol. 2014, 9, 49–57. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Acar, Ü.; Ammar, I.; Balci, B.; Bekas, P.; Belmonte, M.; Chintiroglou, C.; Consoli, P.; Dimiza, M.; Fryganiotis, K.; et al. New Mediterranean Biodiversity Records (October, 2014). Mediterr. Mar. Sci. 2014, 15, 675–695. [Google Scholar] [CrossRef]
- Ribeiro, F.; Veríssimo, A. A new record of Callinectes sapidus in a western European estuary (Portuguese coast). Mar. Biodivers. Rec. 2014, 7, e36. [Google Scholar] [CrossRef]
- Sierra, J.; Mínguez, M. Los Pescadores Capturan en la Albufera un Ejemplar del Invasor Cangrejo Azul. Levante, 2 de Octubre de 2014. Available online: https://www.levante–emv.com/valencia/2014/10/02/pescadores–capturan–albufera–ejemplar–invasor–12730112.html (accessed on 31 January 2024).
- Sierra, J. Nuevas Capturas Confirman la Invasión del Cangrejo azul. Levante, 28 de Octubre de 2014. 2014. Available online: https://www.levante–emv.com/valencia/2014/10/27/nuevas–capturas–confirman–invasion–cangrejo–12711862.html (accessed on 31 January 2024).
- Gönülal, O.; Güreşen, S.O. A list of macrofauna on the continental shelf of Gökçeada Island (northern Aegean Sea) with a new record (Gryphus vitreus Born, 1778) (Brachiopoda, Rhynchonellata) for the Turkish seas. J. Black Sea/Medit. Environ. 2014, 20, 228–252. [Google Scholar]
- Carrozzo, L.; Potenza, L.; Carlino, P.; Costantini, M.L.; Rossi, L.; Mancinelli, G. Seasonal abundance and trophic position of the Atlantic blue crab Callinectes sapidus Rathbun 1896 in a Mediterranean coastal habitat. Rend. Lincei Sci. Fis. Nat. 2014, 25, 201–208. [Google Scholar] [CrossRef]
- Stasolla, G.; Innocenti, G. New records of the invasive crabs Callinectes sapidus Rathbun, 1896 and Percnon gibbesi (H. Milne Edwards, 1853) along the Italian coasts. Bioinvasions Rec. 2014, 3, 39–43. [Google Scholar] [CrossRef]
- Kapiris, K.; Apostolidis, C.; Baldacconi, R.; Başusta, N.; Bilecenoglu, M.; Bitar, G.; Bobori, D.C.; Boyaci, Y.Ö.; Dimitriadis, C.; Djurović, M.; et al. New Mediterranean marine biodiversity records (April, 2014). Mediterr. Mar. Sci. 2014, 15, 198–212. [Google Scholar] [CrossRef]
- Yağlıoğlu, D.; Turan, C.; Öğreden, T. First record of blue crab Callinectes sapidus (Rathbun, 1896) (Crustacea, Brachyura, Portunidae) from the Turkish Black Sea coast. J. Black Sea Med. Env. 2014, 20, 13–17. [Google Scholar]
- Özdemir, S.; Gökçe, G.; Çekiç, M. Determination of size selectivity of traps for blue crab (Callinectes sapidus Rathbun, 1896) in the Mediterranean Sea. Tarım Bilimleri Dergisi 2015, 21, 256–261. [Google Scholar] [CrossRef]
- Genc, T.O.; Yilmaz, F. Bioaccumulation indexes of metals in blue crab inhabiting specially protected area Koycegiz Lagoon (Turkey). Indian J. Anim. Sci. 2015, 85, 94–99. [Google Scholar] [CrossRef]
- Ak, O.; Haşimoğlu, A.; Bayram, K. Southeastward expansion of the blue crab Callinectes sapidus (Rathbun, 1896) in the Black Sea. Cah. Biol. Mar. 2015, 56, 397–399. [Google Scholar]
- Pujol, J.A.; Banos, J.M.; Banos, A.; Sanchez, M.; Gonzalez–Wanguemert, M. 2015 AHSA 2015. Available online: http://ahsa.org.es/desembocadura–del–rio–segura/el–cangrejo–azul–americano–callinectes–sapidus–localizado–en–la–desembocadura–del–rio–segura (accessed on 31 January 2024).
- Manfrin, C.; Chung, J.; Turolla, E.; Giulianini, P. First occurrence of Callinectes sapidus (Rathbun, 1896) within the Sacca di Goro (Italy) and surroundings. Check List 2015, 11, 1–4. [Google Scholar] [CrossRef]
- Abdel Razek, F.A.; Ismaiel, M.; Ameran, M.A.A. Occurrence of the blue crab Callinectes sapidus, Rathbun, 1896, and its fisheries biology in Bardawil Lagoon, Sinai Peninsula, Egypt. Egypt. J. Aquat. Res. 2016, 42, 223–229. [Google Scholar] [CrossRef]
- Dailianis, T.; Akyol, O.; Babali, N.; Bariche, M.; Crocetta, F.; Gerovasileiou, V.; Ghanem, R.; Gökoğlu, M.; Hasiotis, T.; Izquierdo-Muñoz, A.; et al. New Mediterranean Biodiversity Records (July 2016). Mediterr. Mar. Sci. 2016, 17, 608–626. [Google Scholar] [CrossRef]
- Türeli, C.; Miller, T.J.; Gündogdu, S.; Yesilyurt, I.N. Growth and mortality of blue crab (Callinectes sapidus) in the north–eastern Mediterranean Sea. J. Fish. Sci. 2016, 10, 56. [Google Scholar]
- Perdikaris, C.; Konstantinidis, E.; Gouva, E.; Ergolavou, A.; Klaoudatos, D.; Nathanailides, C.; Paschos, I. Occurrence of the invasive crab species Callinectes sapidus Rathbun, 1896 in NW Greece. Walailak J. Sci. Tech. 2016, 13, 503–510. [Google Scholar]
- Karachle, P.K.; Angelidis, A.; Apostolopoulos, G.; Ayas, D.; Ballesteros, M.; Bonnici, C.; Brodersen, M.M.; Castriota, L.; Chalari, N.; Cottalorda, J.M.; et al. New Mediterranean Biodiversity Records (March 2016). Mediterr. Mar. Sci. 2016, 17, 230–252. [Google Scholar] [CrossRef]
- Generalitat Valenciana. Situación Actual del Cangrejo Azul en la Comunitat Valenciana. Evolución de la Especie en el Período 2014–2016; Technical Report; Direcció General de Medi Natural i Avaluació Ambiental, Conselleria d’Agricultura, Medi Ambient, Canvi Climàtic i Desenvolupament Rural, Generalitat Valenciana: Valencia, Spain, 2016. [Google Scholar]
- Gonzalez–Wanguemert, M.; Pujol, J.A. First record of the Atlantic blue crab Callinectes sapidus (Crustacea: Brachyura: Portunidae) in the Segura river mouth (Spain, southwestern Mediterranean Sea). Turkish J. Zool. 2016, 40, 615–619. [Google Scholar] [CrossRef]
- Kapiris, K.; Tsionki, I.; Kavadas, S. The crustacean fauna composition of the Sperchios Estuary (Maliakos Gulf). In Proceedings of the 8th Congress of the Hellenic Ecological Society, Thessaloniki, Greece, 20–23 October 2016; p. 268. [Google Scholar]
- Daban, I.B.; Cengiz, Ö.; Tuncer, S. Further range expansion of the blue crab Callinectes sapidus (Rathbun, 1896) (Crustacea: Decapoda: Brachyura) in Turkish waters, Northern Aegean Sea: Insight into distribution depth. Cah. Biol. Mar. 2016, 57, 175–178. [Google Scholar]
- Zotti, M.; Del Coco, L.; De Pascali, S.A.; Migoni, D.; Vizzini, S.; Mancinelli, G.; Fanizzi, F.P. Comparative analysis of the proximate and elemental composition of the blue crab Callinectes sapidus, the warty crab Eriphia verrucosa, and the edible crab Cancer pagurus. Heliyon 2016, 2, e00075. [Google Scholar] [CrossRef]
- Cecere, E.; Petrocelli, A.; Belmonte, M.; Portacci, G.; Rubino, F. Activities and vectors responsible for the biological pollution in the Taranto Seas (Mediterranean Sea, southern Italy): A review. Environ. Sci. Pollut. Res. 2016, 23, 12797–12810. [Google Scholar] [CrossRef]
- Bañón, R.; Cuesta, J.A.; Almón, B.; Pérez–Dieste, J.; Trigo, J.E.; Ríos, M.B. First records of two decapod crustaceans, the caramote prawn Penaeus kerathurus and the blue crab Callinectes sapidus from Galician waters (NE Atlantic). Cah. Biol. Mar. 2016, 57, 323–328. [Google Scholar]
- Dizdarević, S.; Gajić, A.; Kahrić, A.; Tomanić, J. First finding of the blue crab, Callinectes sapidus Rathbun, 1896 (Malacostraca: Portunidae), in Bosnia and Herzegovina. Uzizaž 2016, 12, 5–9. [Google Scholar]
- Mancinelli, G.; Glamuzina, B.; Petrić, M.; Carrozzo, L.; Glamuzina, L.; Zotti, M.; Raho, D.; Vizzini, S. The trophic position of the Atlantic blue crab Callinectes sapidus Rathbun 1896 in the food web of Parila Lagoon (South Eastern Adriatic, Croatia): A first assessment using stable isotopes. Mediterr. Mar. Sci. 2016, 17, 634–643. [Google Scholar] [CrossRef]
- Papadopol, N.C.; Curlişcă, A. The North American blue crab, Callinectes sapidus Rathbun, 1896 (Portunidae/Decapoda) has a tendency to become a common species in Romanian waters. In Sustainable Use, Protection of Animal World and Forest Management in the Context of Climate Change, IX-th International Conference of Zoologists, 12–13 October 2016; Academy of Sciences of Moldova: Chisinau, Moldavia, 2016; pp. 217–218. [Google Scholar]
- Manfrin, C.; Comisso, G.; Dell’Asta, A.; Bettoso, N.; Sook Chung, J. The return of the Blue Crab, Callinectes sapidus Rathbun, 1896, after 70 years from its first appearance in the Gulf of Trieste, northern Adriatic Sea, Italy (Decapoda: Portunidae). Check List 2016, 12, 2006. [Google Scholar] [CrossRef]
- Župan, I.; Karaga, A.; Šarić, T.; Kanski, D. Blue crab Callinectes sapidus Rathbun, 1896 continues invasion: First case of entering into freshwater ecosystem in the Mediterranean (Nature Park Vransko Lake, Adriatic Sea). Cah. Biol. Mar. 2016, 57, 81–84. [Google Scholar]
- Ben Souissi, J.; Abidi, A.; Ounifi–Ben Amor, K.; Chaffai, A.; Rifi, M. Nouvelle invasion du golfe de Gabès par un crabe bleu d’origine Atlantique: Première occurrence de Callinectes sapidus Rathbun, 1896 en Tunisie (Méditerranée Centrale). XVIIèmes Journées Tunis. Des Sci. Mer Îles Kerkennah 2017, 18–21. [Google Scholar]
- Aldık, R.; Cengizler, I. The investigation of bacteria, parasite and fungi in blue crabs (Callinectes sapidus, Rathbun 1896) caught from Akyatan lagoon in east Mediterranean Sea. J. Adv. Vetbio Sci. Tech. 2017, 2, 11–17. [Google Scholar]
- Çoğun, H.Y.; First, Ö.; Aytekin, T.; Firidin, G.; Varkal, H.; Temiz, Ö.; Kargin, F. Heavy metals in the blue crab (Callinectes sapidus) in Mersin Bay, Turkey. Bull. Environ. Contam. Toxicol. 2017, 98, 824–829. [Google Scholar] [CrossRef]
- Lipej, L.; Acevedo, I.; Akel, E.H.K.; Anastasopoulou, A.; Angelidis, A.; Azzurro, E.; Castriota, L.; Çelik, M.; Cilenti, L.; Crocetta, F.; et al. New Mediterranean Biodiversity Records (March 2017). Mediterr. Mar. Sci. 2017, 18, 179–201. [Google Scholar] [CrossRef]
- Katselis, G.N.; Koutsikopoulos, C. The establishment of blue crab Callinectes sapidus Rathbun, 1896 in the Lagoon Pogonitsa (Amvrakikos Gulf, Western Greece). In Trends in Fisheries and Aquatic Animal Health; Berilis, P., Ed.; Bentham Science: Sharjah, United Arab Emirates, 2017; pp. 299–306. [Google Scholar] [CrossRef]
- Aydin, M. First record of Blue Crab Callinectes sapidus (Rathbun 1896) from the Middle Black Sea Coast. Turkish J. Marit. Marine Sci. 2017, 3, 121–124. [Google Scholar]
- Marković, O.; Durović, M. Occurrence of the invasive crustacean species along the Montenegrin coast (South Adriatic). In Proceedings of the ISEM7, Sutomore, Montenegro, 4–7 October 2017; pp. 65–68. [Google Scholar]
- Milori, E.; Qorri, L.; Ibrahimi, E.; Beqiraj, S. Data on the distribution, population structure and establishment of the invasive blue crab Callinectes sapidus Rathbun, 1896 (Decapoda, Brachyura, Portunidae) in the Lagoon of Viluni (South–East Adriatic Sea, Albania). Albanian J. Agric. Sci. Spec. Ed. 2017, 485–492. [Google Scholar]
- Marković, O.; Đurović, M.; Rađenović, T. New locality in the south Adriatic Sea (Montenegrin coast) for the alien crab Callinectes sapidus Rathbun, 1896. In Proceedings of the “11th Colloquium Crustacea Decapoda Mediterranean”, The Crustacean Society Mid-Year Meeting, Barcelona, Spain, 19–22 June 2017. [Google Scholar]
- Noel, P. Le crabe bleu américain Callinectes sapidus (Rathbun, 1896). In Muséum National d’Histoire Naturelle Ed., 11 Octobre 2017. Inventaire National du Patrimoine Naturel; 2017; pp. 1–30. Available online: https://inpn.mnhn.fr/docs-web/docs/download/206717 (accessed on 19 October 2023).
- Suaria, G.; Pierucci, A.; Zanello, P.; Fanelli, E.; Chiesa, S.; Azzurro, E. Percnon gibbesi (H. Milne Edwards, 1853) and Callinectes sapidus (Rathbun, 1896) in the Ligurian Sea: Two additional invasive species detections made in collaboration with local fishermen. BioInvasions Rec. 2017, 6, 147–151. [Google Scholar] [CrossRef]
- Rady, A.; Sallam, W.S.; Abdou, N.E.I.; El Sayed, A.A.M. Biological Aspects on the Blue Crab, Callinectes sapidus (Rathbun, 1896) Inhabiting the Bardawil Lagoon, Northern Sinai, Egypt. Egypt Acad. J. Biol. Sci. B. Zool. 2018, 10, 61–77. [Google Scholar]
- Chartosia, N.; Anastasiadis, D.; Bazairi, H.; Crocetta, F.; Deidun, A.; Despalatović, M.; Di Martino, V.; Dimitriou, N.; Dragičević, B.; Dulčić, J.; et al. New Mediterranean Biodiversity Records (July 2018). Mediterr. Mar. Sci. 2018, 19, 398–415. [Google Scholar] [CrossRef]
- Hasan, H. The Current State of Exotic Crustacean Decapoda Fauna in Syrian Marine Waters (Update and Review). Tishreen Univ. J. Res. Sci. Stud. Biol. Sci. Ser. 2018, 40, 131–146. [Google Scholar]
- Türeli, C.; Yeşilyurt, İ.N.; Nevşat, İ.E. Female reproductive pattern of Callinectes sapidus Rathbun, 1896 (Brachyura: Portunidae) in Iskenderun Bay, Eastern Mediterranean. Zool. Middle East 2018, 64, 55–63. [Google Scholar] [CrossRef]
- Box, A.; Colomar, V.; Sureda, A.; Tejada, S.; Nuñez–Reyes, V.; Cohen–Sanchez, A.; Avila, T.; Forteza, V.; Castello, M.; Valverde, N.; et al. Primera cita de l’espècie Callinectes sapidus a les Illes Pitiuses. In VII Jornades de Medi Ambient de les Illes Balears; Societat d’Historia Natural de les Balears: Palma de Mallorca, Spain, 2018; pp. 299–300. [Google Scholar]
- Gil, A. Análisis de la Dieta de Callinectes sapidus (Rathbun, 1896) en Ambientes Recientemente Invadidos del Golfo de Valencia. Trabajo Final de Máster, Universitat Politècnica de València, Gandia, Spain, 2018; pp. 1–30. [Google Scholar]
- Garcia, L.; Pinya, S.; Colomar, V.; París, T.; Puig, M.; Rebassa, M.; Mayol, J. The first recorded occurrences of the invasive crab Callinectes sapidus Rathbun, 1896 (Crustacea: Decapoda: Portunidae) in coastal lagoons of the Balearic Islands (Spain). BioInvasions Rec. 2018, 7, 191–196. [Google Scholar] [CrossRef]
- Pagliara, P.; Mancinelli, G. Parasites affect hemocyte functionality in the hemolymph of the invasive Atlantic blue crab Callinectes sapidus from a coastal habitat of the Salento Peninsula (SE Italy). Mediterr. Mar. Sci. 2018, 19, 193–200. [Google Scholar] [CrossRef]
- Kevrekidis, K. Fishery characteristics and landings of the blue crab Callinectes sapidus in Thermaikos Gulf, northern Aegean Sea. In Proceedings of the 12th Panhellenic Symposium of Oceanography and Fisheries, Corfu, Greece, 30 May–3 June 2018; p. 208. [Google Scholar]
- Kevrekidis, K.; Antoniadou, C. Abundance and population structure of the blue crab Callinectes sapidus (Decapoda, Portunidae) in Thermaikos Gulf (Methoni Bay), northern Aegean Sea. Crustaceana 2018, 91, 641–657. [Google Scholar] [CrossRef]
- Garrido, M.; Noël, P. L’interminable Expansion de Callinectes sapidus Dans les Lagunes Corses. 6 Février 2018. Pôle Relais Lagunes 2018. Available online: https://pole-lagunes.org/linterminable-expansion-de-callinectes-sapidus-dansles-lagunes-corses/ (accessed on 14 April 2020).
- Mehanna, S.F.; Desouky, M.G.; Farouk, A.E. Population dynamics and fisheries characteristics of the blue crab Callinectes sapidus (Rathbun, 1896) as an invasive species in Bardawil Lagoon, Egypt. Egypt J. Aquat. Biol. Fish. 2019, 23, 599–611. [Google Scholar] [CrossRef]
- Oussellam, M.; El Ouamari, N.; Bazairi, H. First record of the American blue crab Callinectes sapidus from the Marchica Lagoon, Mediterranean coast of Morocco. In Proceedings of the 1st Mediterranean Symposium on the Non–Indigenous Species, Antalya, Turkey, 17–18 January 2019; pp. 103–104. [Google Scholar]
- Ayas, D.; Shaiek, M.; Ciftci, N.; Bakan, M. Some brachyuran crab records from coastal waters of the Mersin Bay, Northeastern Mediterranean coast of Turkey. NESciences 2019, 4, 174–181. [Google Scholar] [CrossRef]
- İlkyaz, A.T.; Tosunoğlu, Z.; Ünlüler, A.; Çetin Ünlüler., S. Köyceğiz Dalyani (Muğla) Mavi Yengecinin (Callinectes sapidus Rathbun, 1896) Boy, Büyüme Ve Üreme Özellikleri; Haziran: İzmir, Turkey, 2019; pp. 1–47. [Google Scholar]
- Benabdi, M.; Belmahi, A.E.; Grimes, S. First record of the Atlantic blue crab Callinectes sapidus Rathbun, 1896 (Decapoda: Brachyura: Portunidae) in Algerian coastal waters (southwestern Mediterranean). BioInvasions Rec. 2019, 8, 119–122. [Google Scholar] [CrossRef]
- Vasconcelos, P.; Carvalho, A.N.; Piló, D.; Pereira, F.; Encarnação, J.; Gaspar, M.B.; Teodósio, M.A. Recent and Consecutive Records of the Atlantic Blue Crab (Callinectes sapidus Rathbun, 1896): Rapid Westward Expansion and Confirmed Establishment along the Southern Coast of Portugal. Thalassas 2019, 35, 485–494. [Google Scholar] [CrossRef]
- Morais, P.; Gaspar, M.; Garel, E.; Baptista, V.; Cruz, J.; Cerveira, I.; Leitão, F.; Teodósio, M.A. The Atlantic blue crab Callinectes sapidus Rathbun, 1896 expands its non–native distribution into the ria Formosa lagoon and the Guadiana estuary (SW–Iberian Peninsula, Europe). BioInvasions Rec. 2019, 8, 123–133. [Google Scholar] [CrossRef]
- Guijarro–Garcia, E.; Vivas, M.; Garcia, E.; Barcala, E.; Trives, M.; Munoz–Vera, A. Atlantic blue crab (Callinectes sapidus Rathbun, 1896) in a protected coastal lagoon in SE Spain. In Proceedings of the XX Iberian Symposium on Marine Biology Studies (SIEBM XX), Braga, Portugal, 9–12 September 2019. [Google Scholar] [CrossRef]
- Giacobbe, S.; Lo Piccolo, M.; Scaduto, G. Forty–seven years later: The blue crab Callinectes sapidus Rathbun, 1896 (Crustacea Decapoda Portunidae) reappears in the Strait of Messina (Sicily, Italy). Biodivers. J. 2019, 10, 365–368. [Google Scholar] [CrossRef]
- Piras, P.; Esposito, G.; Meloni, D. On the occurrence of the blue crab Callinectes sapidus (Rathbun, 1896) in Sardinian coastal habitats (Italy): A present threat or a future resource for the regional fishery sector? BioInvasions Rec. 2019, 8, 134–141. [Google Scholar] [CrossRef]
- Ventura, M.P.; Salgado, S.Q.; de Arenas, J.H.N.; Cano, J.V.; Mata, P.R.; Soriano, J.L. Predation of the blue crab Callinectes sapidus Rathbun, 1896 on freshwater bivalves (Unionidae & Corbiculidae) in eastern Iberian Peninsula. Folia Conchyliol. 2019, 47, 3–9. [Google Scholar]
- Kampouris, T.E.; Porter, J.S.; Sanderson, W.G. Callinectes sapidus Rathbun, 1896 (Brachyura: Portunidae): An assessment on its diet and foraging behaviour, Thermaikos Gulf, NW Aegean Sea, Greece: Evidence for ecological and economic impacts. Crustac. Res. 2019, 48, 23–37. [Google Scholar] [CrossRef]
- Kevrekidis, K. Relative growth of the blue crab Callinectes sapidus in Thermaikos Gulf (Methoni Bay), northern Aegean Sea. Cah. Biol. Mar. 2019, 60, 395–397. [Google Scholar] [CrossRef]
- Labrune, C.; Amilhat, E.; Amouroux, J. –M.; Jabouin, C.; Gigou, A.; Noel, P. The arrival of the American blue crab, Callinectes sapidus Rathbun, 1896 (Decapoda: Brachyura: Portunidae), in the Gulf of Lions (Mediterranean Sea). BioInvasions Rec. 2019, 8, 876–881. [Google Scholar] [CrossRef]
- Munari, C.; Mistri, M. A new record of Callinectes sapidus Rathburn, 1896 along the Emilia–Romagna coast. Biol. Mar. Mediterr. 2019, 26, 318–319. [Google Scholar]
- Pezy, J.P.; Raoux, A.; Baffreau, A.; Dauvin, J.C. A well established population of the Atlantic blue crab Callinectes sapidus (Rathbun, 1896) in the English Channel. Cah. Biol. Mar. 2019, 60, 205–209. [Google Scholar] [CrossRef]
- İlkyaz, A.T.; Tosunoğlu, Z. A blue crab (Callinectes sapidus Rathbun, 1896) individual with partial albino: A case report. Ege J. Fish. Aquat. Sci. 2019, 36, 85–86. [Google Scholar] [CrossRef]
- Taybi, A.F.; Mabrouki, Y. The American blue crab Callinectes sapidus Rathbun, 1896 (Crustacea: Decapoda: Portunidae) is rapidly expanding through the Mediterranean coast of Morocco. Thalassas 2020, 36, 267–271. [Google Scholar] [CrossRef]
- González–Ortegón, E.; Jenkins, S.; Galil, B.S.; Drake, P.; Cuesta, J.A. Accelerated Invasion of Decapod Crustaceans in the Southernmost Point of the Atlantic Coast of Europe: A non–Natives’ Hot Spot? Biol. Invasions 2020, 22, 3487–3492. [Google Scholar] [CrossRef]
- Mili, S.; Ennouri, R.; Ghanem, R.; Rifi, M.; Jaziri, S.; Ben Souissi, J. Additonal and unusual records of bleu crabs Portunus segnis and Callinectes sapidus from the northeastern Tunisian waters (Central Mediterranean Sea). J. N. Sci. 2020, 14, 303–311. [Google Scholar]
- Cerri, J.; Chiesa, S.; Bolognini, L.; Mancinelli, G.; Grati, F.; Dragičević, B.; Dulčic, J.; Azzurro, E. Using online questionnaires to assess marine bio–invasions: A demonstration with recreational fishers and the Atlantic blue crab Callinectes sapidus (Rathbun, 1986) along three Mediterranean countries. Mar. Poll. Bull. 2020, 156, 111209. [Google Scholar] [CrossRef] [PubMed]
- Chic, O.; Garrabou, J. Global Marine Biodiversity Data from Seawatchers Marine Citizen Science Platform between 1980 and 2020. Blue Crab. 2020. Available online: https://www.gbif.org/ (accessed on 11 April 2022).
- Falsone, F.; Scannella, D.; Geraci, M.L.; Vitale, S.; Sardo, G.; Fiorentino, F. Further records of Callinectes sapidus (Rathbun, 1896) (Decapoda, Brachyura, Portunidae) in the Strait of Sicily. Mar. Biodivers. Rec. 2020, 13, 8. [Google Scholar] [CrossRef]
- Sercia, G.; Innocenti, G. First record of the crab Callinectes sapidus Rathbun, 1896 (Crustacea Decapoda Brachyura Portunidae) off Favignana (Sicily, Italy). Biodivers. J. 2020, 11, 871–874. [Google Scholar] [CrossRef]
- Pipitone, C.; Zenone, A.; Badalamenti, F.; D’Anna, G. First record of the blue crab Callinectes sapidus (Crustacea, Decapoda, Portunidae), a non-indigenous species in the central/southern Tyrrhenian Sea. Acta Adriat. 2020, 61, 101–106. [Google Scholar] [CrossRef]
- Box, A.; Colomar, V.; Sureda, A.; Tejada, S.; Nuñez–Reyes, V.; Cohen–Sanchez, A.; Avila, T.; Forteza, V.; Castello, M.; Valverde, N.; et al. Next step of the colonization of the Balearic Islands (Spain) by invasive Atlantic blue crab, Callinectes sapidus Rathbun, 1896 (Crustacea: Decapoda: Portunidae). BioInvasions Rec. 2020, 9, 259–265. [Google Scholar] [CrossRef]
- Culurgioni, J.; Diciotti, R.; Satta, C.T.; Camedda, A.; de Lucia, G.A.; Pulina, S.; Lugliè, A.; Brundu, R.; Fois, N. Distribution of the alien species Callinectes sapidus (Rathbun, 1896) in Sardinian waters (western Mediterranean). BioInvasions Rec. 2020, 9, 65–73. [Google Scholar] [CrossRef]
- Feidantsis, K.; Michaelidis, B.; Raitsos, D.Ε.; Vafidis, D. Seasonal cellular stress responses of commercially important invertebrates at different habitats of the North Aegean Sea. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 250, 110778. [Google Scholar] [CrossRef]
- Öztürk, R.Ç.; Terzi, Y.; Feyzioğlu, A.M.; Şahin, A.; Aydın, M. Genetic characterization of the invasive blue crab, Callinectes sapidus (Rathbun, 1896), in the Black Sea. Reg. Stud. Mar. Sci. 2020, 39, 101412. [Google Scholar] [CrossRef]
- Ceylan, Y. The blue crab (Callinectes sapidus, Rathbun, 1896) is spreading in the southern coast of the Black Sea. Mar. Sci. Technol. Bull. 2020, 9, 168–172. [Google Scholar] [CrossRef]
- Kamberi, E.; Beqiri, K.; Kolitari, J.; Buda, E.; Sadiku, E. The Occurrence of Blue Crab (Callinectes sapidus, Rathbun 1896) in the Vaini Lagoon. Albanian J. Agric. Sci. 2020, 19, 60–63. [Google Scholar]
- Glamuzina, L.; Conides, A.; Mancinelli, G.; Glamuzina, B. A Comparison of Traditional and Locally Novel Fishing Gear for the Exploitation of the Invasive Atlantic Blue Crab in the Eastern Adriatic Sea. J. Mar. Sci. Eng. 2021, 9, 1019. [Google Scholar] [CrossRef]
- Czerniejewski, P.; Kasowska, N.; Linowska, A.; Rybczyk, A. A new record of the invasive blue crab (Callinectes sapidus Rathbun, 1896) and his parasite from the Baltic basin. Oceanologia 2020, 62, 111–115. [Google Scholar] [CrossRef]
- Corsini–Foka, M.; Abdulghani, A.; Al Mabruk, S.A.A.; Abdulrraziq, A.A.; Ibrahim, S.M.; Scannella, D.; Zava, B.; Deidun, A.; Gianguzza, P. Invasive portunid crabs in Libyan waters: First record of the Atlantic blue crab Callinectes sapidus Rathbun, 1896 and range expansion of the swimming blue crab Portunus segnis (Forskål, 1775). BioInvasions Rec. 2021, 10, 885–893. [Google Scholar] [CrossRef]
- Castriota, L.; Falautano, M. Reviewing the invasion history of the blue crab Callinectes sapidus (Portunidae) in Sicily (Central Mediterranean): An underestimated alien species. Ann. Ser. Hist. Nat. 2021, 31, 1–8. [Google Scholar] [CrossRef]
- Hamida, C.; Kara, M.H. First documented record of the Atlantic blue crab Callinectes sapidus Rathbun, 1896 from the southwestern Mediterranean coasts. Crustaceana 2021, 94, 283–292. [Google Scholar] [CrossRef]
- Kara, M.H.; Chaoui, L. Strong invasion of Mellah lagoon (South–Western Mediterranean) by the American blue crab Callinectes sapidus Rathbun, 1896. Mar. Poll. Bull. 2021, 164, 112089. [Google Scholar] [CrossRef]
- Shaiek, M.; El Zrelli, R.; Crocetta, F.; Mansour, L.; Rabaoui, L. On the occurrence of three exotic decapods, Callinectes sapidus (Portunidae), Portunus segnis (Portunidae), and Trachysalambria palaestinensis (Penaeidae), in northern Tunisia, with updates on the distribution of the two invasive portunids in the Mediterranean Sea. BioInvasions Rec. 2021, 10, 158–169. [Google Scholar] [CrossRef]
- Png–Gonzalez, L.; Papiol, V.; Balbín, R.; Cartes, J.E.; Carbonell, A. Larvae of the blue crab Callinectes sapidus Rathbun, 1896 (Decapoda: Brachyura: Portunidae) in the Balearic Archipelago (NW Mediterranean Sea). Mar. Biodivers. Rec. 2021, 14, 21. [Google Scholar] [CrossRef]
- Brusco, A.; De Bonis, S.; Giorgio, A.; Marchianò, R. Presenza del granchio blu (Callinectes sapidus Rathbun, 1896) nella Riserva naturale regionale Foce del fiume Crati. Biol. Ambient. 2021, 35, 3–10. [Google Scholar] [CrossRef]
- Aslan, H.; Polito, M.J. Trophic ecology of the Atlantic blue crab Callinectes sapidus as an invasive non–native species in the Aegean Sea. Biol. Invasions 2021, 23, 2289–2304. [Google Scholar] [CrossRef]
- Milori, E.; Ruci, S.; Ibrahimi, E.; Beqiraj, S. State of Blue Crab Callinectes sapidus in the Lagoon of Orikum in Albania. J. Earth Environ. Sci. Res. 2021, 3, 157. [Google Scholar] [CrossRef]
- Tiralongo, F.; Villani, G.; Arciprete, R.; Mancini, E. Filling the gap on Italian records of an invasive species: First records of the Blue Crab, Callinectes sapidus Rathbun, 1896 (Decapoda: Brachyura: Portunidae), in Latium and Campania (Tyrrhenian Sea). Acta Adriat. 2021, 61, 99–104. [Google Scholar] [CrossRef]
- Efthymiadis, G.; Anastasiadou, C.; Gubili, C.; Sapounidis, A.; Liasko, R.; Exadactylos, A.; Koutrakis, M. Morphological differentiation and fecundity in Callinectes sapidus Rathbun, 1896 from North Aegean Sea. In Proceedings of the 10th Congress of the Hellenic Ecological Society (HELECOS 2021), Ioannina, Greece, 14–17 October 2021; p. 160. [Google Scholar]
- Pérez–Sorribes, L.; Gil–Climent, B. A new case of amphibian consumption by Atlantic blue crab (Callinectes sapidus) in the Iberian Mediterranean coast. Bol. Asoc. Herpetol. Esp. 2021, 32, 109–111. [Google Scholar]
- Milori, E.; Ruci, S.; Beqiraj, S. Population trend of the invasive blue crab Callinectes sapidus Rathbun,1896 in Patoku lagoon. Paripex Indian J. Res. 2021, 10, 135–140. [Google Scholar] [CrossRef]
- Tomanic, J. BLUE CRAB (Callinectes sapidus) along the Montenegrin Coast; Interreg—IPA CBC: Rome, Italy, 2021. [Google Scholar]
- Stefanov, T. Recent expansion of the alien invasive blue crab Callinectes sapidus (Rathbun, 1896) (Decapoda, Crustacea) along the Bulgarian coast of the Black Sea. Hist. Nat. Bulg. 2021, 42, 49–53. [Google Scholar] [CrossRef]
- Lipej, L.; Rogelja, M. Status of the invasive blue crab Callinectes sapidus Rathbun, 1896 (Brachyura: Portunidae) in Slovenia. Acta Biol. Slov. 2021, 64, 24–33. [Google Scholar] [CrossRef]
- Deidun, A.; Insacco, G.; Galdies, J.; Balistreri, P.; Zava, B. Tapping into hard–to–get information: The contribution of citizen science campaigns for updating knowledge on range–expanding, introduced and rare native marine species in the Malta–Sicily Channel. BioInvasions Rec. 2021, 10, 257–269. [Google Scholar] [CrossRef]
- Gaglioti, M.; Mancini, E. The invasive Callinectes sapidus (Rathbun, 1896) and the Native Carcinus aestuarii (Nardo, 1847) along the Latium Coast—The Essential Role of Citizen Scientists for Timely Reporting. 2021. Available online: https://www.researchgate.net/publication/349569938_The_invasive_Callinectes_sapidus_Rathbun_1896_and_the_native_Carcinus_aestuarii_Nardo_1847_along_the_Latium_coast-_The_essential_role_of_citizen_scientists_for_timely_reporting (accessed on 20 October 2023).
- Gaglioti, M.; Fiasca, R.; Radlo, P. One More Hint from the Blue Colonizer–Callinectes sapidus Strikes again in the Latium Coast (Central Tyrrhenian Sea). 2021. Available online: https://www.researchgate.net/profile/Martina_Gaglioti/publication/349774073_One_more_hint_from_the_blue_colonizer–_Callinectes_sapidus_strikes_again_in_the_Latium_coast_Central_Tyrrhenian_Sea/links/60414f27a6fdcc9c7812216d/One–more–hint–from–the–blue–colonizer–Callinectes–sapidus–strikes–again–in–the–Latium–coast–Central–Tyrrhenian–Sea.pdf (accessed on 20 October 2023).
- Chaouti, A.; Belattmania, Z.; Nadri, A.; Serrão, E.; Encarnação, J.; Teodósio, A.; Reani, A.; Sabour, B. The Invasive Atlantic Blue Crab Callinectes sapidus Rathbun 1896 Expands its Distributional Range Southward to Atlantic African Shores: First Records Along the Atlantic Coast of Morocco. BioInvasions Rec. 2022, 11, 227–237. [Google Scholar] [CrossRef]
- Ragheb, E.; Kamal, R.M.; Hasan, M.W.A. Species diversity of gillnet catches along the Egyptian Mediterranean coast of Alexandria. Egypt. J. Aquat. Res. 2022, 48, 281–289. [Google Scholar] [CrossRef]
- Zakzok, S.M.; Tawfik, M.M.; Mohammad, S.H.; Alkaradawe, R.M. Biometric Study, Condition Factor and Biochemical Composition of the Blue Crab Callinectes sapidus Rathbun, 1896. J. Fish. Environ. 2022, 46, 100–115. [Google Scholar]
- Chairi, H.; González–Ortegón, E. Additional records of the blue crab Callinectes sapidus Rathbun, 1896 in the Moroccan Sea, Africa. BioInvasions Rec. 2022, 11, 776–784. [Google Scholar] [CrossRef]
- Ben Abdallah, O.; Ben Hadj Hamida, N.; Labni, M.A.; Missaoui, H. Status of the Atlantic blue crab Callinectes sapidus Rathbun, 1896 in Tunisian waters (Central Mediterranean). In Proceedings of the 2nd Mediterranean Symposium on the non–indigenous Species, Genova, Italy, 22–23 September 2022; pp. 18–22. [Google Scholar]
- González–Ortegón, E.; Berger, S.; Encarnação, J.; Chairi, H.; Morais, P.; Teodósio, M.A.; Oliva–Paterna, F.J.; Schubart, C.D.; Cuesta, J.A. Free Pass Through the Pillars of Hercules? Genetic and Historical Insights into the Recent Expansion of the Atlantic Blue Crab Callinectes sapidus to the West and the East of the Strait of Gibraltar. Front. Mar. Sci. 2022, 9, 918026. [Google Scholar] [CrossRef]
- Deidun, A.; Galdies, J.; Marrone, A.; Sciberras, A.; Zava, B.; Corsini–Foka, M.; Gianguzza, P. The first confirmed record of the Atlantic blue crab Callinectes sapidus Rathbun, 1896 (Decapoda, Brachyura) from Maltese waters. BioInvasions Rec. 2022, 11, 238–243. [Google Scholar] [CrossRef]
- Agilkaya, G.S.; Korkmaz, C.; Karakurt, S.; Karaytug, S. Influences of Sex and Seasons on Levels of Heavy Metals in Muscle Tissues of Callinectes sapidus Obtained from the Göksu Delta. Thalassas 2022, 38, 1081–1089. [Google Scholar] [CrossRef]
- Khamassi, F.; Rjiba Bahri, W.; Mnari Bhouri, A.; Chaffai, A.; Soufi Kechaou, E.; Ghanem, R.; Ben Souissi, J. Biochemical composition, nutritional value and socio–economic impacts of the invasive crab Callinectes sapidus Rathbun, 1896 in central Mediterranean Sea. Mediterr. Mar. Sci. 2022, 23, 650–663. [Google Scholar] [CrossRef]
- Rjiba Bahri, W.; Chaffai, A.; Ghanem, R.; Ben Souissi, J. Eat alien invasive blue crabs: Yes, but without running health risks! In Proceedings of the 2nd Mediterranean Symposium on the non–indigenous Species, Genova, Italy, 22–23 September 2022; pp. 62–67. [Google Scholar]
- de Vries, H.; Lemmens, M. Observation.org, Nature Data from Around the World. Blue Crab. Available online: https://observation.org/ (accessed on 12 June 2022).
- Tahri, M.; Boutabia, L. First appearance of the American blue crab Callinectes sapidus Rathbun, 1896 (Crustacea: Decapoda: Brachyura) in fresh water of Western Mediterranean—Algeria. Afr. J. Ecol. 2022, 60, 1293–1296. [Google Scholar] [CrossRef]
- Bouhali, K.; Derbal, F.; Kara, M.H. First data on the biology and dynamics of the American blue crab Callinectes sapidus in Mellah Lagoon, Algeria. In Proceedings of the 2nd Mediterranean Symposium on the Non–Indigenous Species, Genova, Italy, 22–23 September 2022; pp. 83–84. [Google Scholar]
- Encarnação, J.; Krug, L.A.; Teodósio, M.A.; Morais, P. Coastal Counter–Currents Increase Propagule Pressure of an Aquatic Invasive Species to an Area Where Previous Introductions Failed. Estuaries Coasts 2022, 45, 2504–2518. [Google Scholar] [CrossRef]
- Di Muri, C.; Rosati, I.; Bardelli, R.; Cilenti, L.; Li Veli, D.; Falco, S.; Vizzini, S.; Katselis, G.N.; Kevrekidis, K.; Glamuzina, L.; et al. An individual-based dataset of carbon and nitrogen isotopic data of Callinectes sapidus in invaded Mediterranean waters. Biodiv. Data J. 2022, 10, e77516. [Google Scholar] [CrossRef] [PubMed]
- Navarro García, P. Asentamiento de Megalopas y Juveniles de Callinectes sapidus en Colectores Artificiales en las Golas de la Albufera de Valencia. Trabajo Fin de Máster, Universitat Politècnica de València, Valencia, Spain, 2022. [Google Scholar]
- Grech, D.; Pilloni, Z.; Burton, M.; Serra, E.; Brundu, G.; Baroli, M.; Porporato, E.M.D.; Massaro, G.; Ceccherelli, G.; Cerri, J.; et al. 2022 A local ecological knowledge approach for a collaborative NIS mapping in Sardinia (Italy). In Proceedings of the 2nd Mediterranean Symposium on the Non–Indigenous Species, Genova, Italy, 22–23 September 2022; pp. 42–47. [Google Scholar]
- Acar, S.; Gürkan, S.E.; Ateş, A.S.; Özdilek, Ş.Y. Presence of microplastics in stomach contents of blue crab Callinectes sapidus (Rathbun, 1896) in Canakkale Strait. In Proceedings of the AGRIBALKAN 2022, IV. Balkan Agricultural Congress, Edirne, Türkiye, 31 August–2 September 2022; pp. 368–373. [Google Scholar]
- Scalici, M.; Chiesa, S.; Mancinelli, G.; Rontani, P.M.; Voccia, A.; Nonnis Marzano, F. Euryhaline Aliens Invading Italian Inland Waters: The Case of the Atlantic Blue Crab Callinectes sapidus Rathbun, 1896. Appl. Sci. 2022, 12, 4666. [Google Scholar] [CrossRef]
- Veyssiere, D.; Garrido, M.; Massé, C.; Noël, P.; Romans, P. Etat des Connaissances sur le Crabe Bleu, Callinectes sapidus (Rathbun, 1896). Focus sur la Méditerranée et la Corse; Rapp. Office de l’Environnement de la Corse: Corse, France, 2022; 49p. [Google Scholar]
- Castellón Información. El cangrejo azul se expande sin control por la desembocadura del río Mijares. Castellón Información, 13 January 2017. Available online: https://www.castelloninformacion.com/cangrejo-azul-mijares/ (accessed on 31 January 2024).
- Marchessaux, G.; Gjoni, V.; Sarà, G. Environmental drivers of size–based population structure, sexual maturity and fecundity: A study of the invasive blue crab Callinectes sapidus (Rathbun, 1896) in the Mediterranean Sea. PLoS ONE 2023, 18, e0289611. [Google Scholar] [CrossRef] [PubMed]
- Oussellam, M.; Benhoussa, A.; Pariselle, A.; Rahmouni, I.; Salmi, M.; Agnèse, J. –F.; Selfati, M.; El Ouamari, N.; Bazairi, H. First and southern–most records of the American blue crab Callinectes sapidus Rathbun, 1896 (Decapoda, Portunidae) on the African Atlantic coast. BioInvasions Rec. 2023, 12, 403–416. [Google Scholar] [CrossRef]
- Bardelli, R.; Mancinelli, G.; Mazzola, A.; Vizzini, S. The Atlantic blue crab Callinectes sapidus spreading in the Tyrrhenian sea: Evidence of an established population in the Stagnone di Marsala (Sicily, southern Italy). Naše More 2023, 70, 177–183. [Google Scholar] [CrossRef]
- Tufan, B. Biochemical composition of different sex and body parts of blue crabs (Callinectes sapidus) caught from the middle Black Sea coast. Mar. Sci. Technol. Bull. 2023, 12, 104–110. [Google Scholar] [CrossRef]
- Nastase, A.; Honț, Ș.; Iani, M.; Paraschiv, M. First record of Callinectes sapidus (Blue Crab)(Rathbun, 1896)(Crustacea: Decapoda: Portunidae) in Romanian sea coasts of Danube Delta. Sci. Ann. Danube Delta Inst. 2023, 28, 169–174. [Google Scholar] [CrossRef]
- Glamuzina, L.; Pešić, A.; Marković, O.; Tomanić, J.; Pećarević, M.; Dobroslavić, T.; Brailo Šćepanović, M.; Conides, A.; Grđan, S. Population structure of the invasive Atlantic blue crab, Callinectes sapidus on the Eastern Adriatic coast (Croatia, Montenegro). Naše More 2023, 70, 153–159. [Google Scholar] [CrossRef]
- Ortega Jiménez, E.; Cuesta, J.A.; Laiz, I.; González–Ortegón, E. Feeding habits of the invasive Atlantic blue crab Callinectes sapidus (Decapoda, Brachyura, Portunidae) in a temperate European estuary. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Vella, A.; Giarrusso, E.; Monaco, C.; Mifsud, C.M.; Darmanin, S.A.; Raffa, A.; Tumino, C.; Peri, I.; Vella, N. New Records of Callinectes sapidus (Crustacea, Portunidae) from Malta and the San Leonardo River Estuary in Sicily (Central Mediterranean). Diversity 2023, 15, 679. [Google Scholar] [CrossRef]
- Battisti, C.; Chiesa, S.; Gallitelli, L.; Scalici, M. Further evidence of the occurrence of the Atlantic blue crab Callinectes sapidus (Rathbun 1896) (Crustacea: Decapoda: Portunidae) along the central Tyrrhenian coast. Natural History Sciences. Atti Soc. It. Sci. Nat. Mus. Civ. Stor. Nat. Milano 2023, 10, 63–68. [Google Scholar] [CrossRef]
- Ammar, I. A recent study of biodiversity of Marine Zoobenthos in Al-Masab basin near Tartus, with Record of Non-Indigenous species for the first time in Syria. Damascus Univ. J. Basic Sci. 2023, 39, 67–87. [Google Scholar]
- Compa, M.; Perelló, E.; Box, A.; Colomar, V.; Pinya, S.; Sureda, A. Ingestion of microplastics and microfibers by the invasive blue crab Callinectes sapidus (Rathbun 1896) in the Balearic Islands, Spain. Environ. Sci. Pollut. Res. 2023, 30, 119329–119342. [Google Scholar] [CrossRef]
- Rifi, M.; Basti, L.; Rizzo, L.; Tanduo, V.; Radulovici, A.; Jaziri, S.; Uysal, İ.; Souissi, N.; Mekki, Z.; Crocetta, F. Tackling bioinvasions in commercially exploitable species through interdisciplinary approaches: A case study on blue crabs in Africa’s Mediterranean coast (Bizerte Lagoon, Tunisia). Estuar. Coast. Shelf Sci. 2023, 291, 108419. [Google Scholar] [CrossRef]
- Mali, S.; Shumka, S.; Pepa, B.; Osmani, M.; Hila, N. Further records of Callinectes sapidus (Rathbun, 1896) in central part of Adriatic coast in Albania. Thalass. Salentina 2023, 44, 41–48. [Google Scholar]
- ARPAV-ISPRA. Relazione Tecnica Congiunta ARPAV e ISPRA Relativa alla Presenza della Specie Callinectes Sapidus (Rathbun 1896) Nelle Lagune del Canarin e Scardovari Porto Tolle (RO); ARPAV-ISPRA Technical Report; ARPAV-ISPRA: Rome, Italy, 2023. [Google Scholar]
- González, J.A. Presencia Confirmada del Cangrejo Invaso (Brachyura, Portunidae) en Gran Canaria. Informe Científico Referencia De Experto 2023; 4 Fundación Canaria Parque Científico Tecnológico de la Universidad de Las Palmas de Gran Canaria: Las Palmas, Spain, 2023. [Google Scholar]
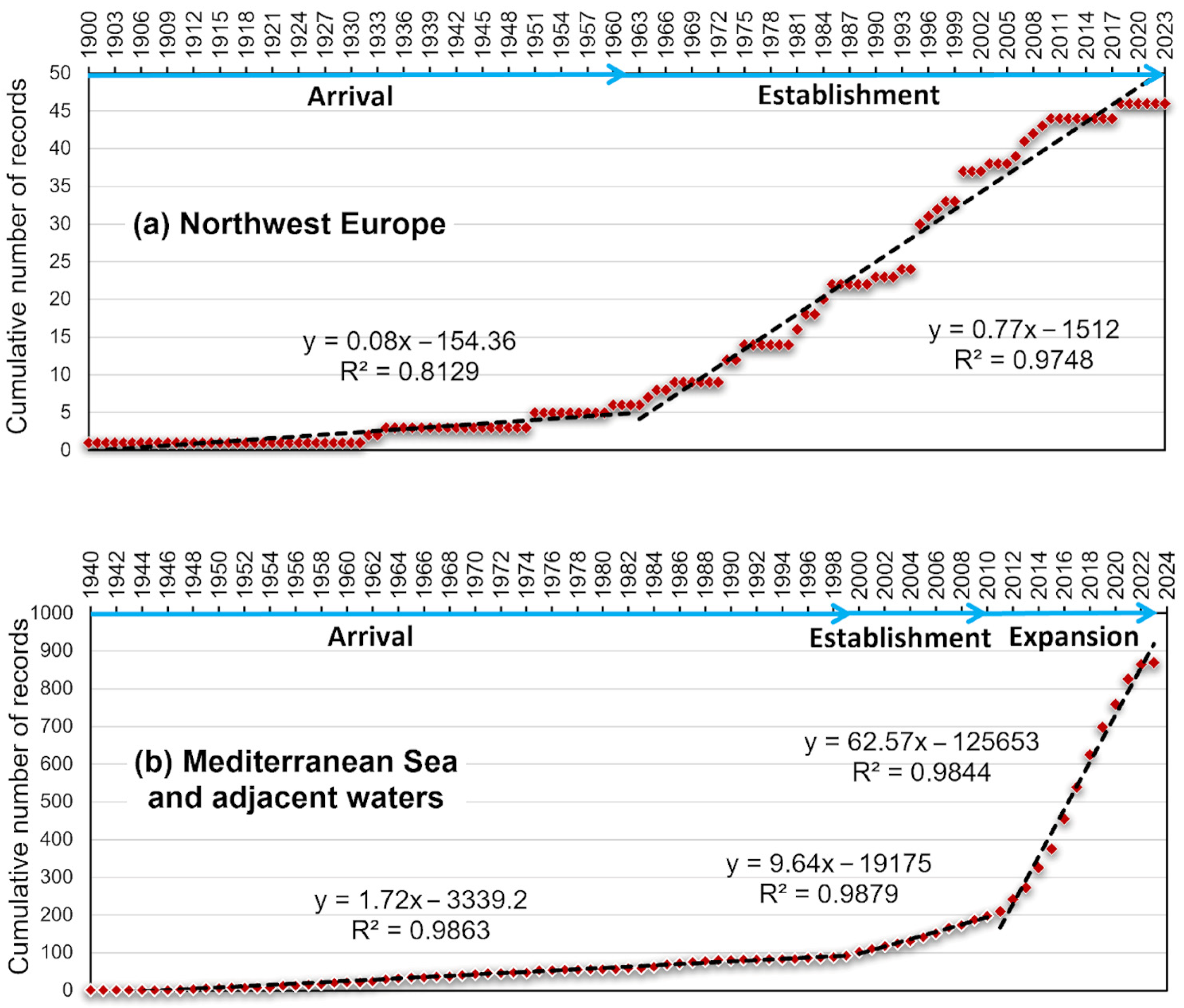
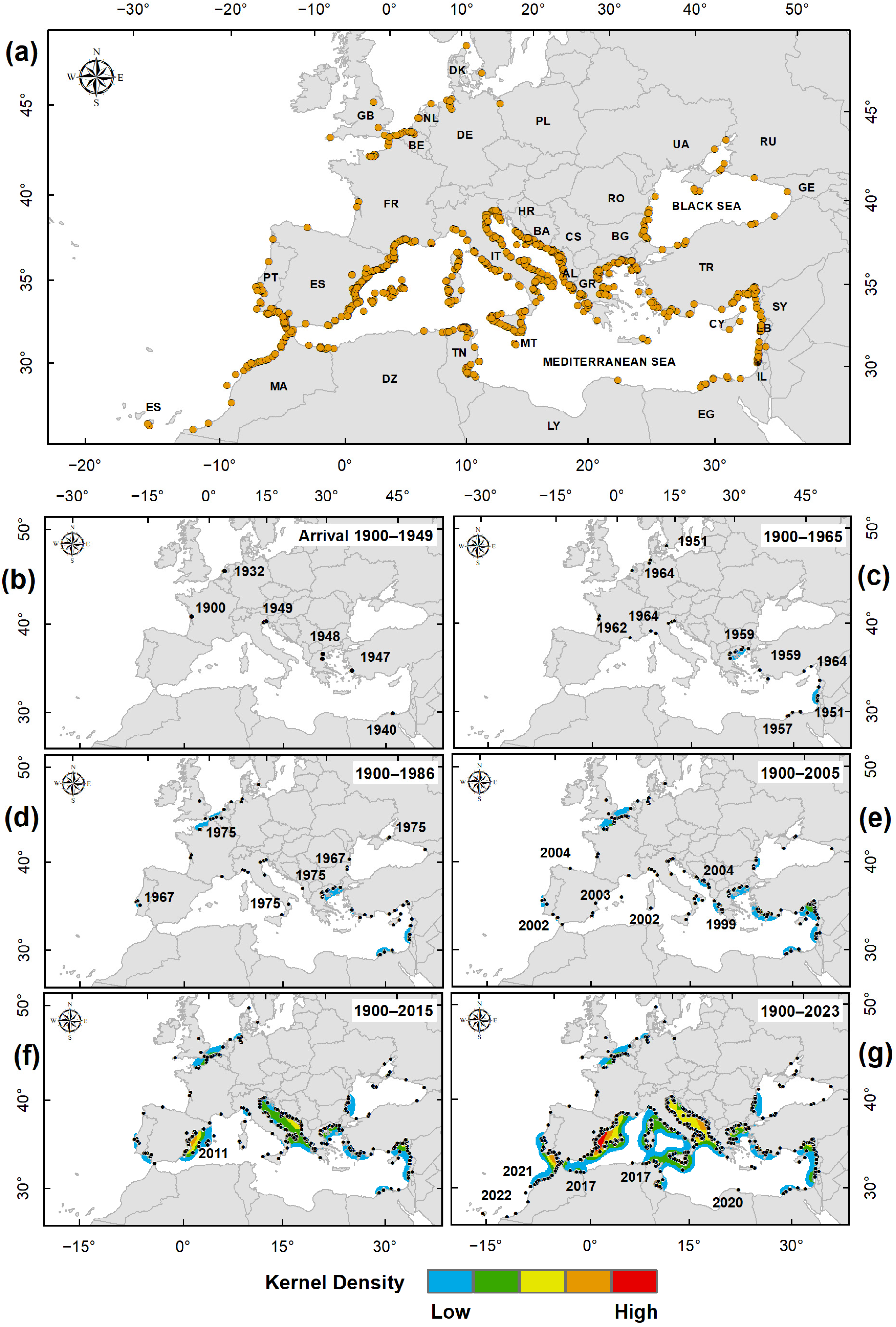
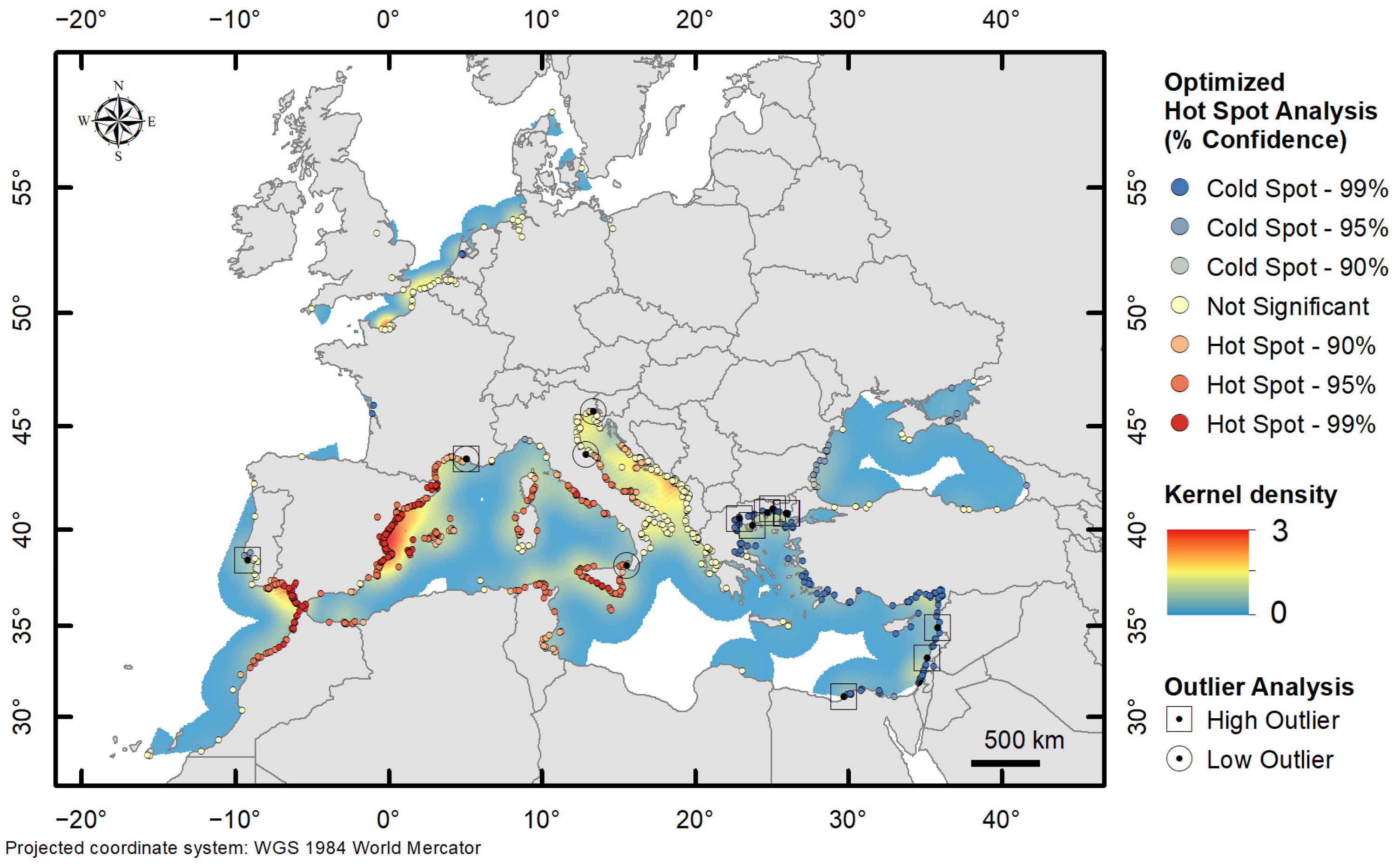

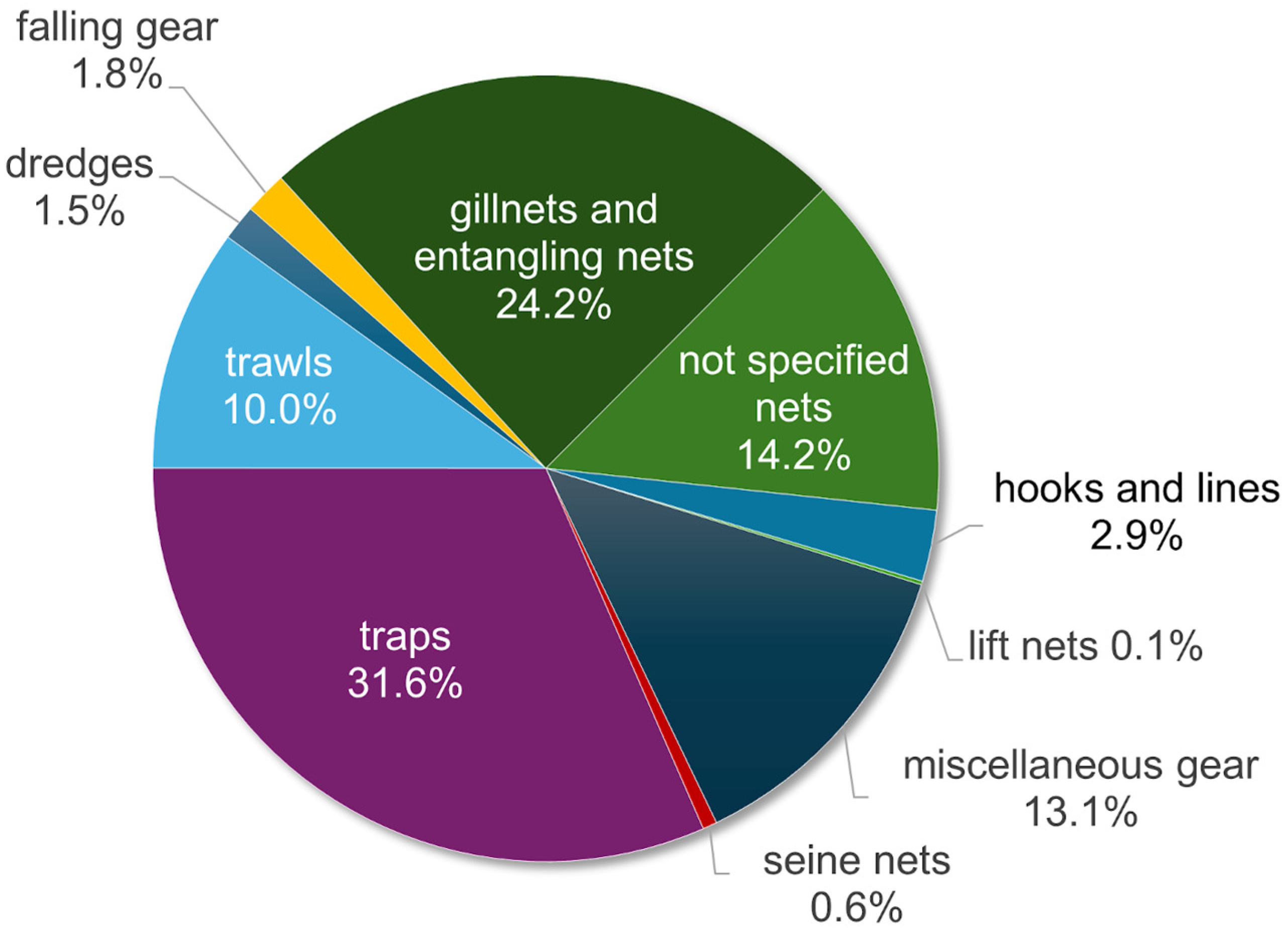
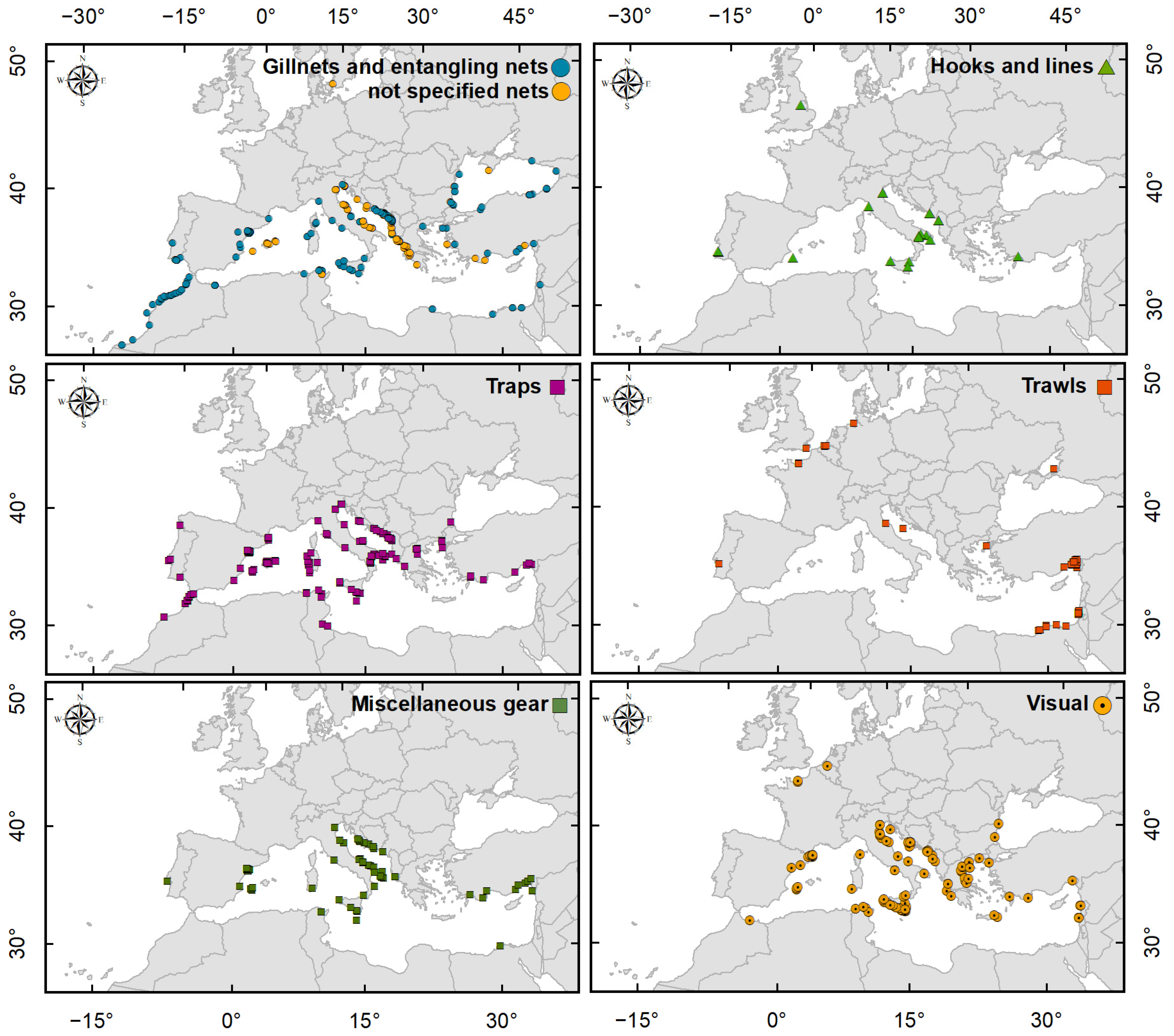
| Analysis/Indicator Name | Tools | Spatial Scale | Time Scale | Ecological Meaning |
|---|---|---|---|---|
| Temporal and spatial–temporal pattern | ||||
| Occurrence increase | Cumulative curve of occurrence | Global | All years | Occurrences increasing over time and space. Identification of invasion phases |
| Occurrence increase rate | Evaluation of the slopes of the cumulative curve by the Least Squares Method | Global | NWE: 1900–1962 1963–2018 MAW: 1940–1999 2000–2010 2011–2023 | The rate of specimens increasing over time |
| Density hotspots | Kernel density | Global | 1900–1949 1900–1965 1900–1986 1900–2005 1900–2015 1900–2023 | Expansion areas; nuclei of record aggregation; persistent occurrence areas; space–time occurrence density increase; highest-density areas |
| Aggregation patterns and spatial structure | ||||
| Global spatial autocorrelation | Global Moran’s I (GMI) NWE: cutoff distance = 350 km MAW: cutoff distance = 350 km | Global | All years | Distribution pattern: dispersion vs. random vs. clustering; change in spatial pattern over time |
| Statistically significant hot spots and cold spots | Optimised hot spot analysis (O–GOG*) NWE: distance band = 95 km MAW: distance band = 200 km | Local | All years | Initial and current direction of spread and identification of dispersion/settle areas |
| Spatial outliers | Anselin local Moran’s I (AMI) Cluster and outlier analysis NWE: distance band = 95 km MAW: distance band = 200 km | Local | All years | Recent records in proximity of group of older records and vice versa |
| Key characteristics of distribution | ||||
| Centre of gravity | Central tendency (mean centre—median centre) | Global | NWE: 1900–1973 1975–1995 1996–2018 MAW: 1940–2000 2001–2011 2012–2023 | Species concentration centre and its change over time |
| Directional dispersion | XStdDist and YStdDist (km); standard deviational ellipse (1 standard deviation) | Global | Species distribution in X and Y directions | |
| Directional trends | Rotation (°) Standard deviational ellipse (1 standard deviation) | Global | Directional trend in species dispersion | |
| Area | Years | Directional Dispersion XStdDist (km) | Directional Dispersion YStdDist (km) | Directional Trend Rotation (°) |
|---|---|---|---|---|
| NWE | 1900–1973 | 97 | 631 | 45 |
| 1975–1995 | 119 | 231 | 61 | |
| 1996–2018 | 175 | 558 | 59 | |
| MAW | 1940–2000 | 1459 | 494 | 99 |
| 2001–2011 | 438 | 1553 | 86 | |
| 2012–2023 | 425 | 1356 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castriota, L.; Falautano, M.; Perzia, P. When Nature Requires a Resource to Be Used—The Case of Callinectes sapidus: Distribution, Aggregation Patterns, and Spatial Structure in Northwest Europe, the Mediterranean Sea, and Adjacent Waters. Biology 2024, 13, 279. https://doi.org/10.3390/biology13040279
Castriota L, Falautano M, Perzia P. When Nature Requires a Resource to Be Used—The Case of Callinectes sapidus: Distribution, Aggregation Patterns, and Spatial Structure in Northwest Europe, the Mediterranean Sea, and Adjacent Waters. Biology. 2024; 13(4):279. https://doi.org/10.3390/biology13040279
Chicago/Turabian StyleCastriota, Luca, Manuela Falautano, and Patrizia Perzia. 2024. "When Nature Requires a Resource to Be Used—The Case of Callinectes sapidus: Distribution, Aggregation Patterns, and Spatial Structure in Northwest Europe, the Mediterranean Sea, and Adjacent Waters" Biology 13, no. 4: 279. https://doi.org/10.3390/biology13040279
APA StyleCastriota, L., Falautano, M., & Perzia, P. (2024). When Nature Requires a Resource to Be Used—The Case of Callinectes sapidus: Distribution, Aggregation Patterns, and Spatial Structure in Northwest Europe, the Mediterranean Sea, and Adjacent Waters. Biology, 13(4), 279. https://doi.org/10.3390/biology13040279





