Impaired Spermatogenesis in Infertile Patients with Orchitis and Experimental Autoimmune Orchitis in Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Infertile Patients
2.2. Histopathological Classification of Testicular Biopsies
2.3. Animals
2.4. Induction of Experimental Autoimmune Orchitis (EAO)
2.5. CD45 Immunohistochemistry
2.6. Ki67 Immunohistochemistry
2.7. AMH Immunohistochemistry
2.8. Periodic Acid-Schiff (PAS) Staining
2.9. Seminiferous Tubule Diameter and Wall Thickness Determination
2.10. Quantification of Sertoli Cells and Spermatogonia in Human Biopsies
2.11. Determination of Sertoli Cell Number in Rat with Orchitis
2.12. Determination of Sperm Parameter in Rat Epididymis
2.13. TUNEL Technique
2.14. Statistical Analysis
3. Results
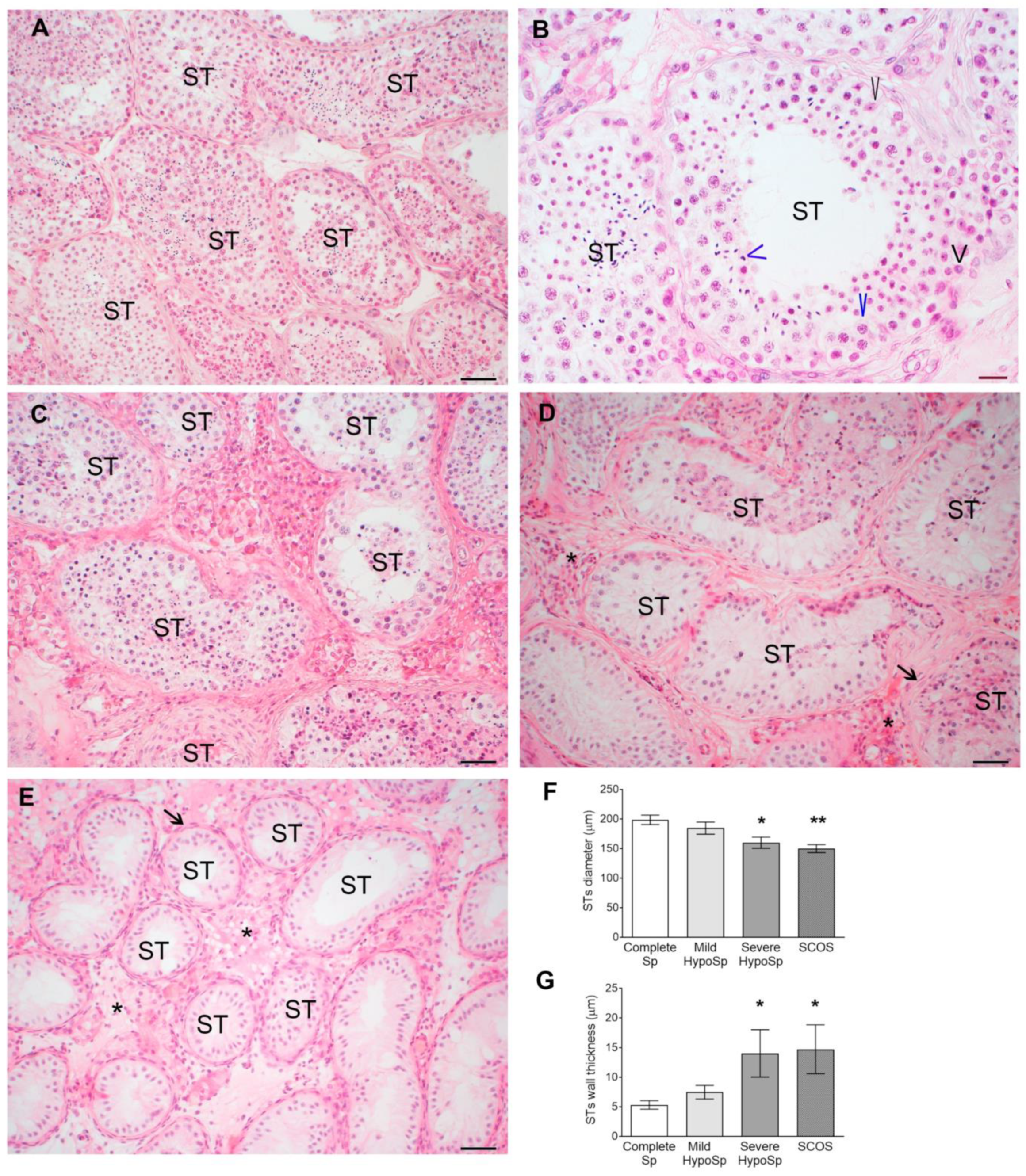
3.1. Histopathology of Testicular Biopsies from Infertile Patients and Hormonal Profile
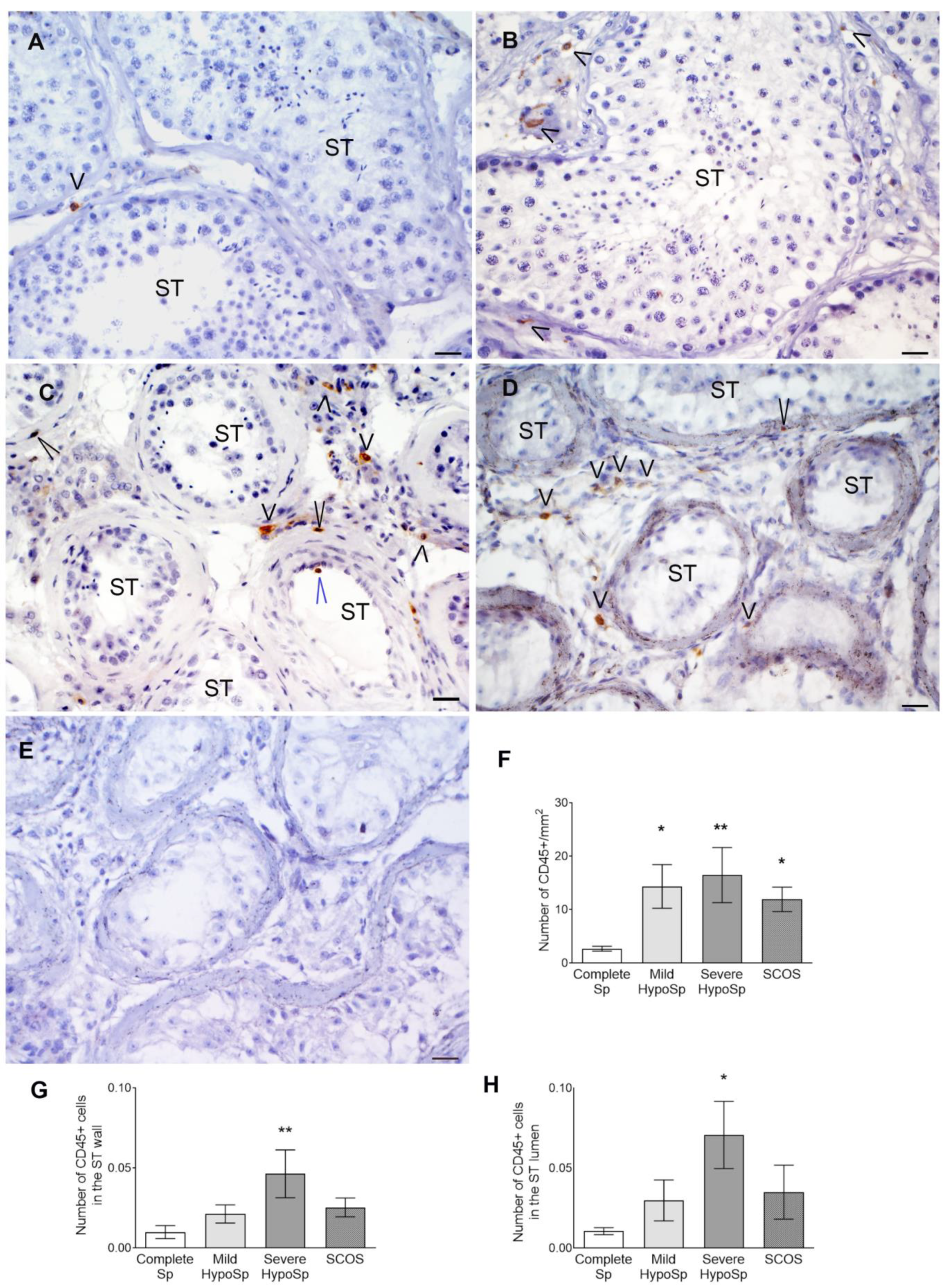
3.2. CD45+ Cells Increase in Testicular Biopsies
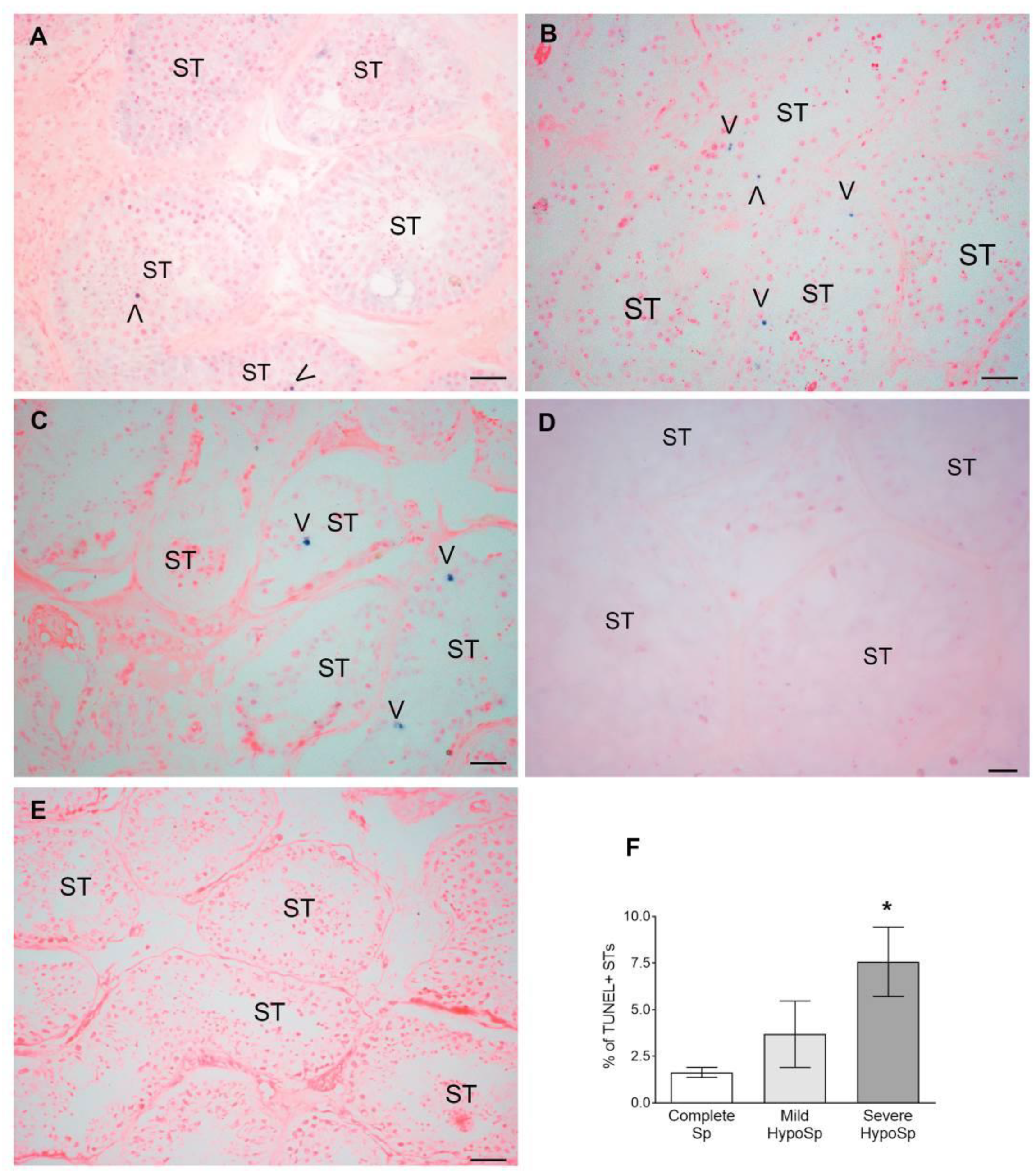
3.3. Meiotic Germ Cells Are Reduced and Die by Apoptosis in Human Orchitis
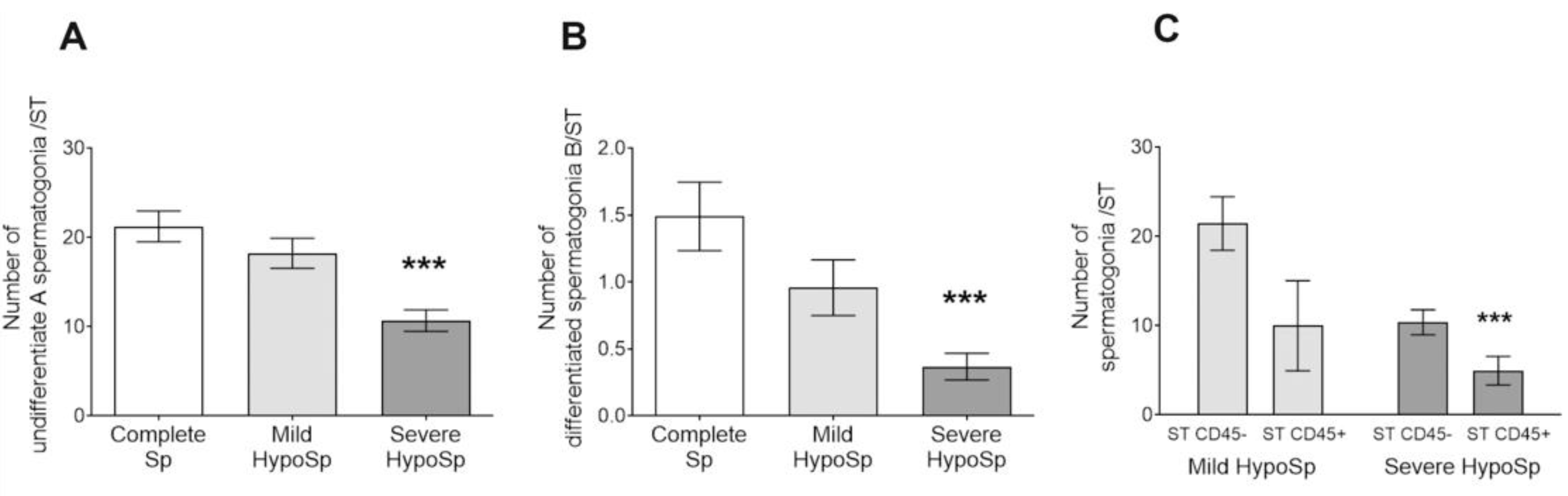
3.4. The Number of Undifferentiated Type A and Differentiated Type B Spermatogonia Decreased in Human Orchitis
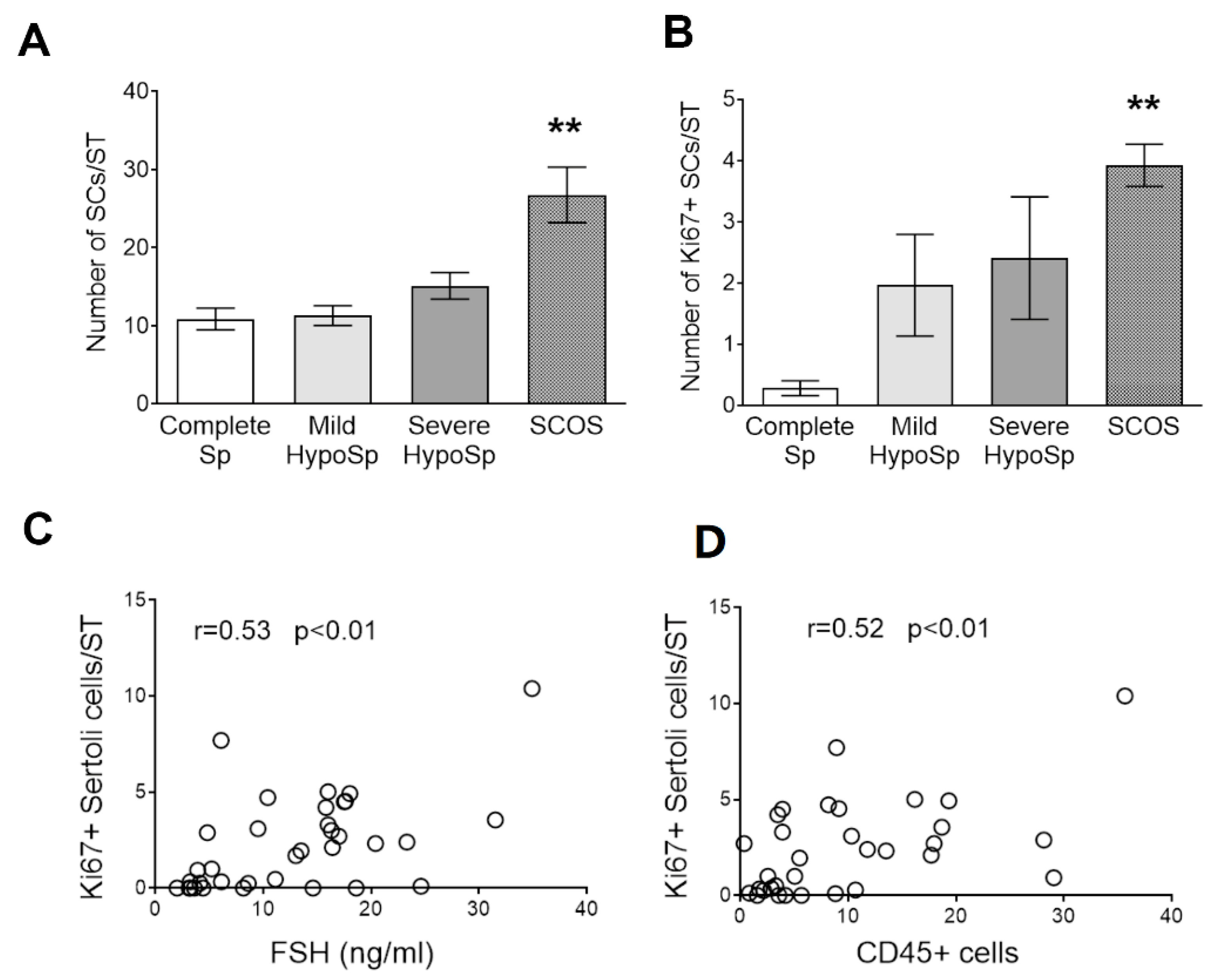
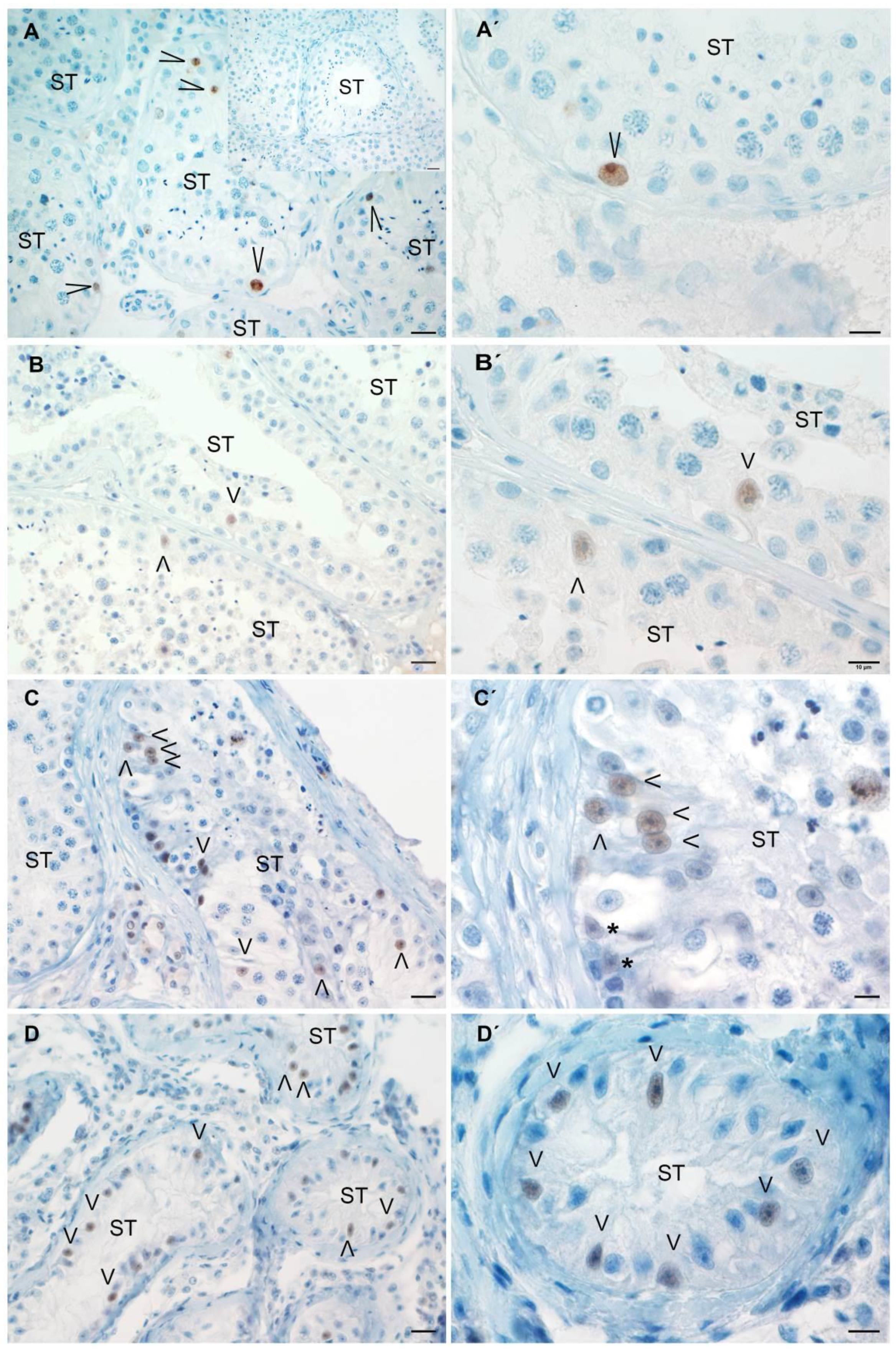
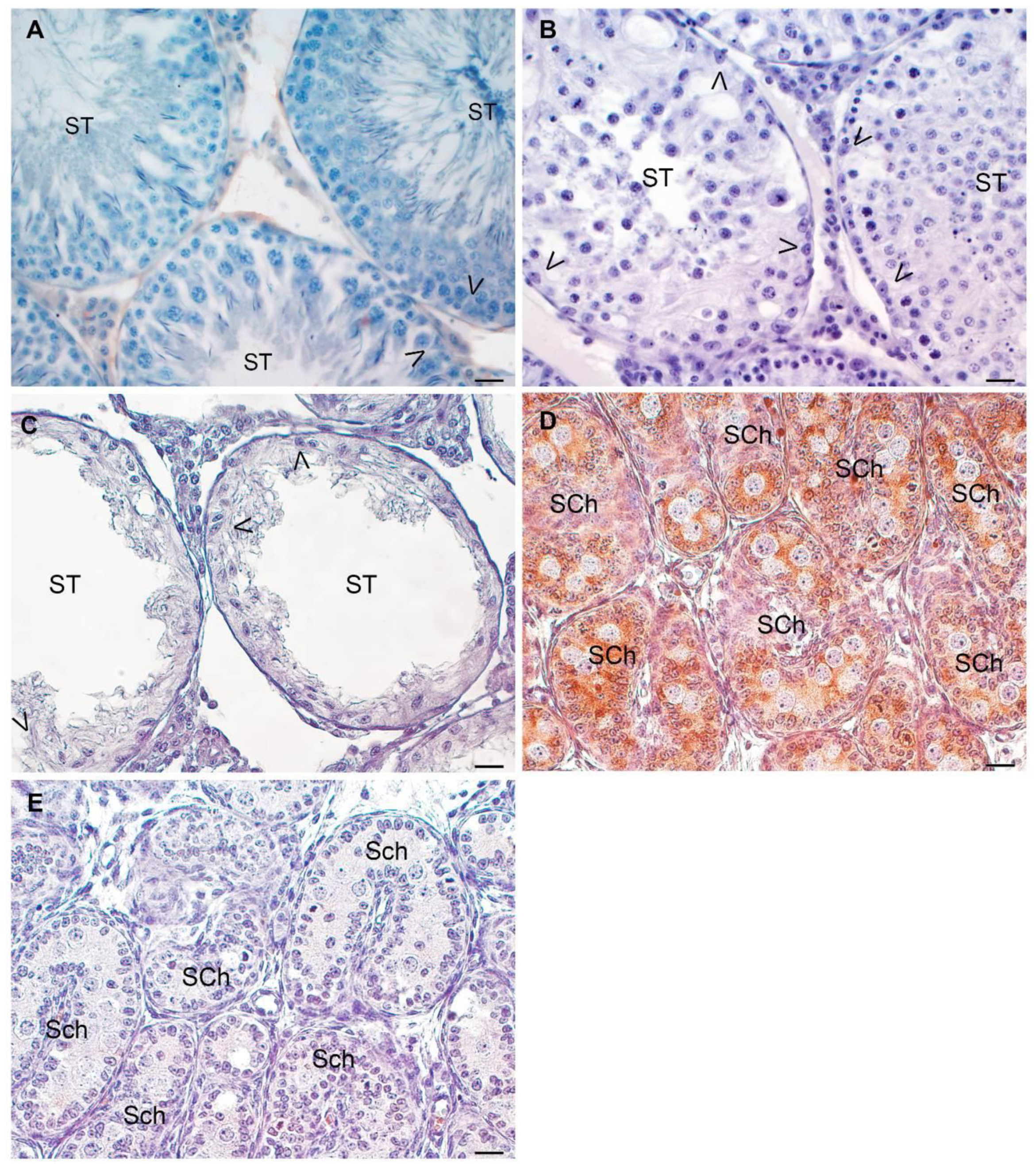
3.5. Analysis of Sertoli Cell Number and Ki67 Expression in Testicular Biopsies
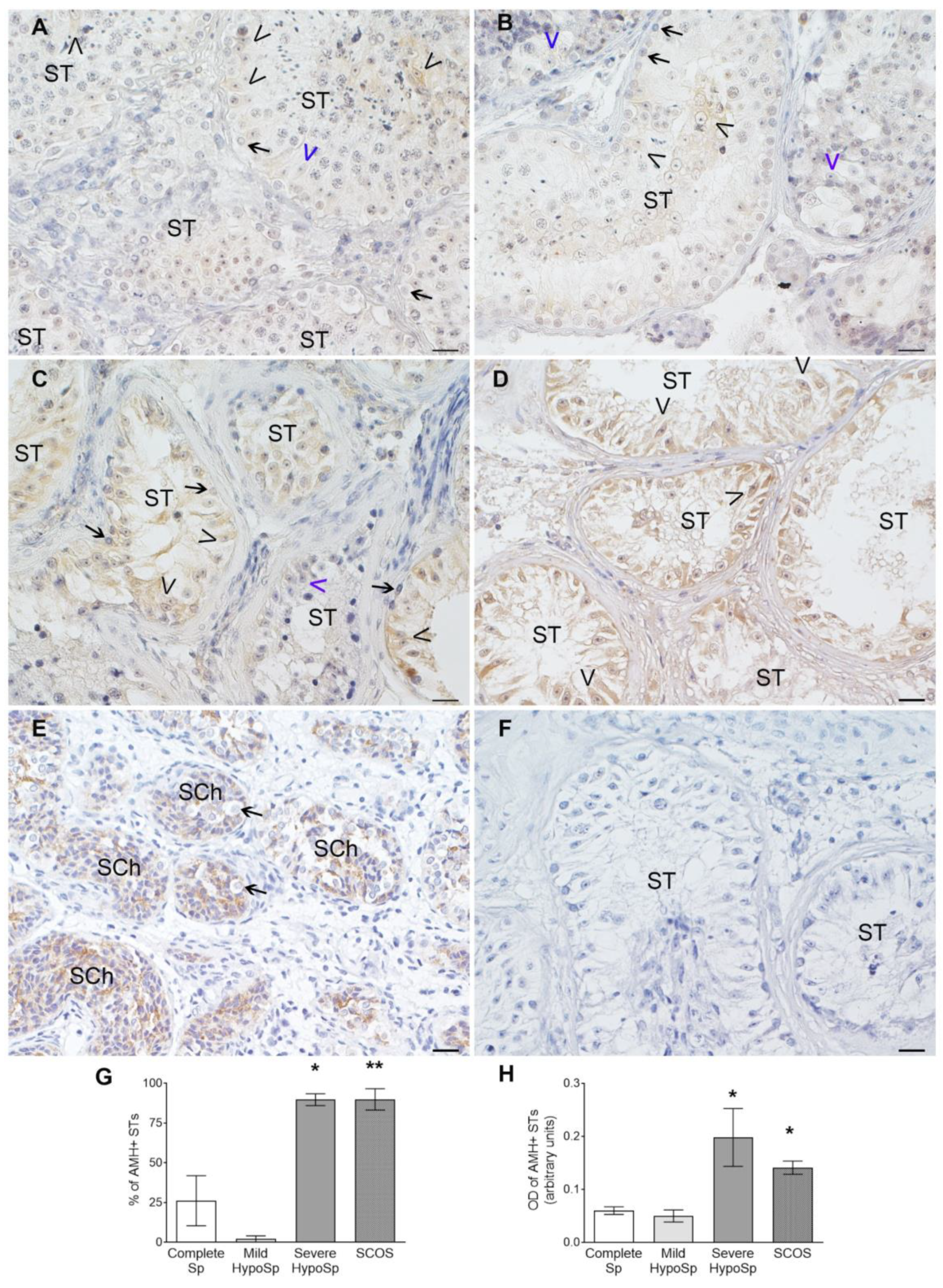
3.6. Analysis of AMH Expression in Testicular Biopsies with Orchitis
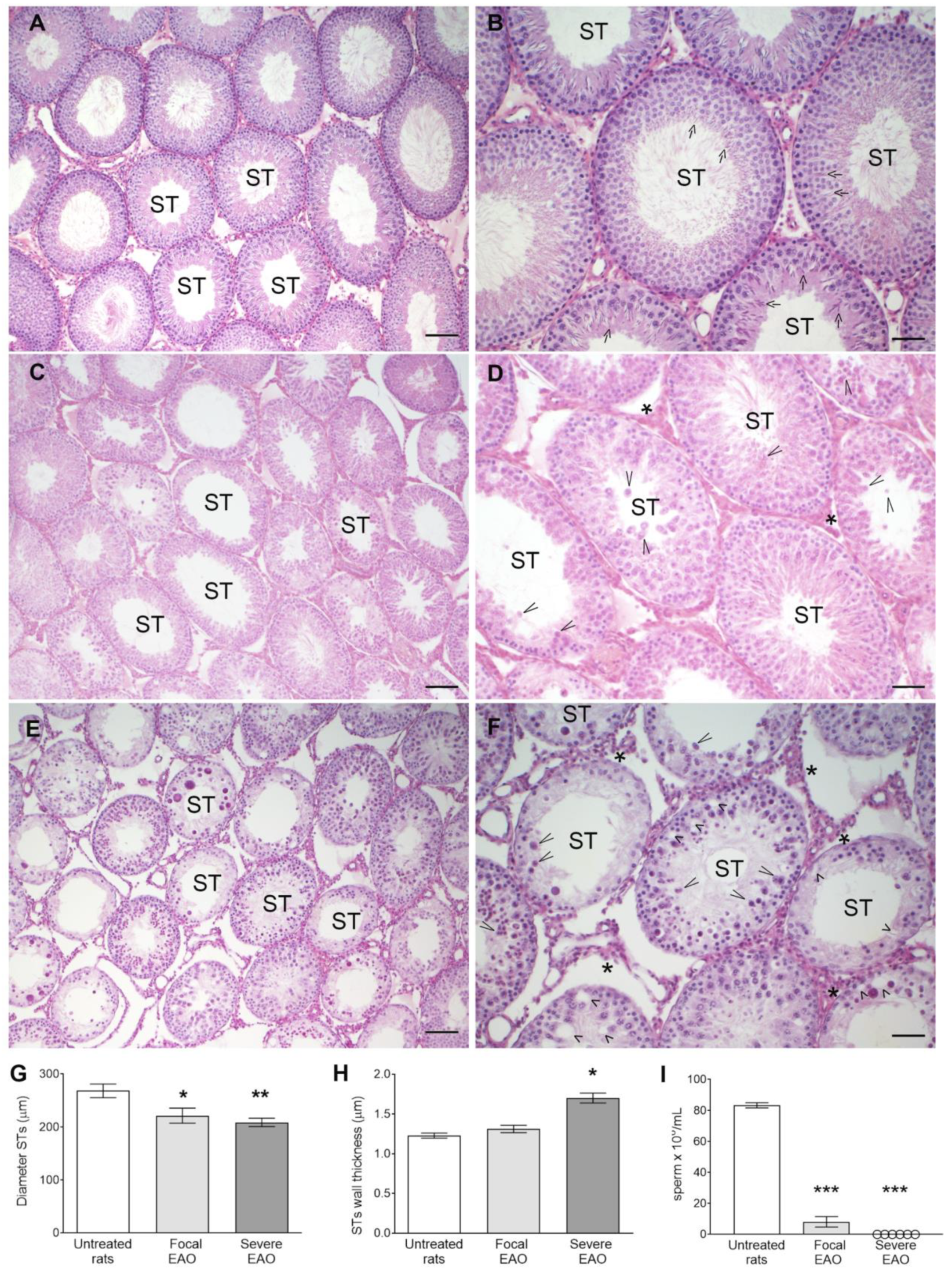
3.7. EAO Histopathology and Hormonal Profile
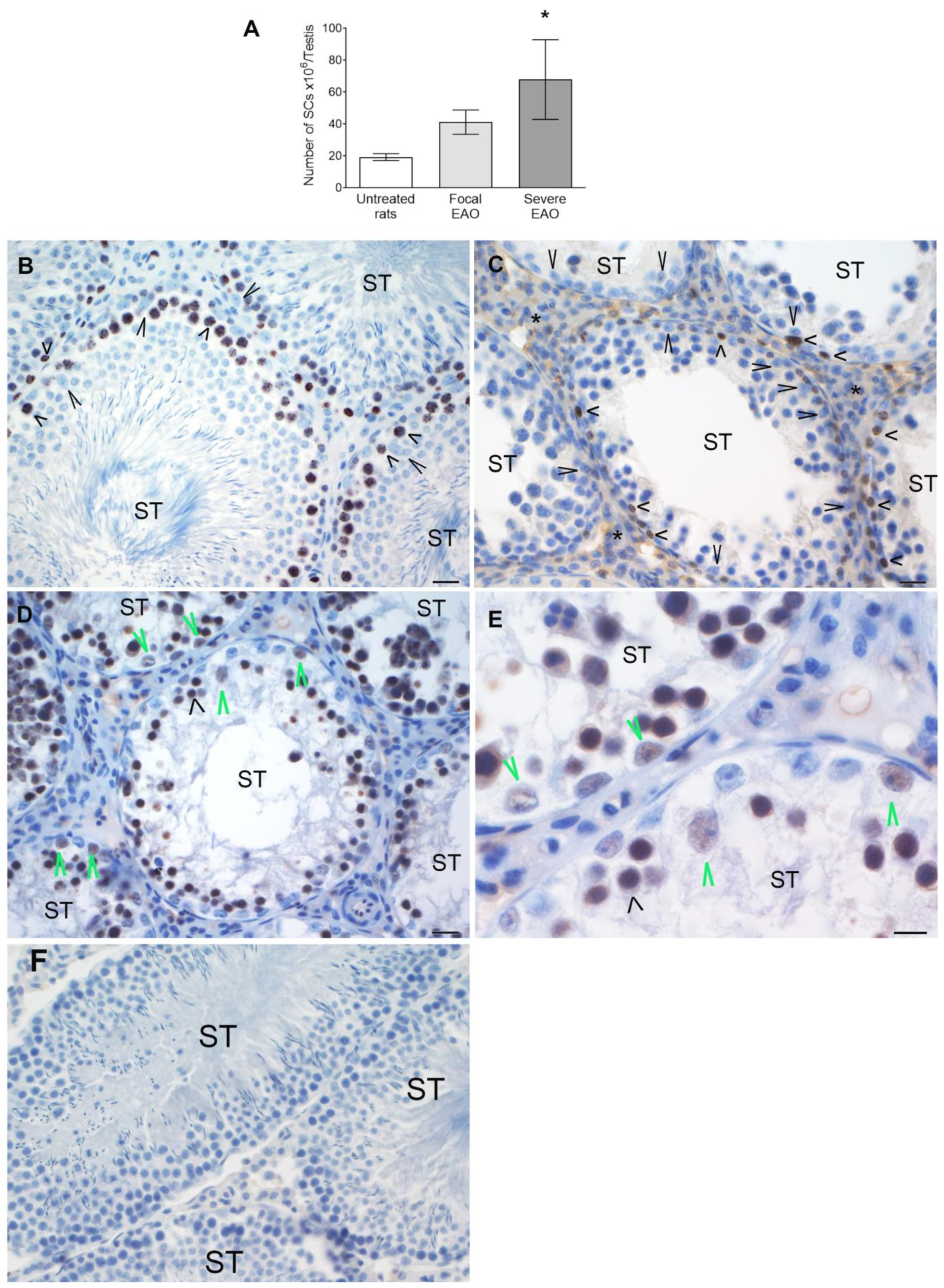
3.8. Study of Sertoli Cells in Rats with EAO
3.9. Human Orchitis and Experimental Autoimmune Orchitis (EAO)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weidner, W.; Anderson, R.U. Evaluation of Acute and Chronic Bacterial Prostatitis and Diagnostic Management of Chronic Prostatitis/Chronic Pelvic Pain Syndrome with Special Reference to Infection/Inflammation. Int. J. Antimicrob. Agents 2008, 31, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Pilatz, A.; Kilb, J.; Kaplan, H.; Fietz, D.; Hossain, H.; Schüttler, C.G.; Diemer, T.; Bergmann, M.; Domann, E.; Weidner, W.; et al. High Prevalence of Urogenital Infection/Inflammation in Patients with Azoospermia Does Not Impede Surgical Sperm Retrieval. Andrologia 2019, 51, e13401. [Google Scholar] [CrossRef] [PubMed]
- Fijak, M.; Schneider, E.; Klug, J.; Bhushan, S.; Hackstein, H.; Schuler, G.; Wygrecka, M.; Gromoll, J.; Meinhardt, A. Testosterone Replacement Effectively Inhibits the Development of Experimental Autoimmune Orchitis in Rats: Evidence for a Direct Role of Testosterone on Regulatory T Cell Expansion. J. Immunol. 2011, 186, 5162–5172. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Terayama, H.; Hirai, S.; Qu, N.; Lustig, L.; Itoh, M. Experimental Autoimmune Orchitis as a Model of Immunological Male Infertility. Med. Mol. Morphol. 2012, 45, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Fijak, M.; Pilatz, A.; Hedger, M.P.; Nicolas, N.; Bhushan, S.; Michel, V.; Tung, K.S.K.; Schuppe, H.-C.; Meinhardt, A. Infectious, Inflammatory and ‘Autoimmune’ Male Factor Infertility: How Do Rodent Models Inform Clinical Practice? Hum. Reprod. Update 2018, 24, 416–441. [Google Scholar] [CrossRef] [PubMed]
- Lustig, L.; Guazzone, V.A.; Theas, M.S.; Pleuger, C.; Jacobo, P.; Pérez, C.V.; Meinhardt, A.; Fijak, M. Pathomechanisms of Autoimmune Based Testicular Inflammation. Front. Immunol. 2020, 11, 583135. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, M.E.; Méndez, C.S.; Glienke, L.; Sobarzo, C.M.; Ferraris, M.J.; Pisera, D.A.; Lustig, L.; Jacobo, P.V.; Theas, M.S. Unraveling the Effect of the Inflammatory Microenvironment in Spermatogenesis Progression. Cell Tissue Res. 2023, 392, 581–604. [Google Scholar] [CrossRef] [PubMed]
- Suescun, M.O.; Calandra, R.S.; Lustig, L. Alterations of Testicular Function after Induced Autoimmune Orchitis in Rats. J. Androl. 1994, 15, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Jarazo Dietrich, S.; Fass, M.I.; Jacobo, P.V.; Sobarzo, C.M.A.; Lustig, L.; Theas, M.S. Inhibition of NOS-NO System Prevents Autoimmune Orchitis Development in Rats: Relevance of NO Released by Testicular Macrophages in Germ Cell Apoptosis and Testosterone Secretion. PLoS ONE 2015, 10, e0128709. [Google Scholar] [CrossRef]
- Rey, R.; al-Attar, L.; Louis, F.; Jaubert, F.; Barbet, P.; Nihoul-Fékété, C.; Chaussain, J.L.; Josso, N. Testicular Dysgenesis Does Not Affect Expression of Anti-Müllerian Hormone by Sertoli Cells in Premeiotic Seminiferous Tubules. Am. J. Pathol. 1996, 148, 1689–1698. [Google Scholar] [PubMed]
- Rowley, M.J.; Heller, C.G. Quantitation of the Cells of the Seminiferous Epithelium of the Human Testis Employing the Sertoli Cell as a Constant. Z. Zellforsch. 1971, 115, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Nebel, B.R.; Murphy, C.J. Damage and Recovery of Mouse Testis after 1000 r Acute Localized X-Irradiation, with Reference to Restitution Cells, Sertoli Cell Increase, and Type A Spermatogonial Recovery. Radiat. Res. 1960, 12, 626. [Google Scholar] [CrossRef]
- McLachlan, R.I.; Rajpert-De Meyts, E.; Hoei-Hansen, C.E.; De Kretser, D.M.; Skakkebaek, N.E. Histological Evaluation of the Human Testis—Approaches to Optimizing the Clinical Value of the Assessment: Mini Review. Hum. Reprod. 2007, 22, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Christensen, A.K. Morphometric Analysis of Leydig Cells in the Normal Rat Testis. J. Cell Biol. 1980, 84, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Suescun, M.O.; Calandra, R.S.; Lustig§, L. Increased Testosterone Production in Vitro by Leydig Cells from Rats with Severe Autoimmune Orchitis. Int. J. Androl. 1998, 20, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-L.; Huang, S.-T.; Huang, H.-C.; Chen, Y.; Hsu, Y.-C. The Reliability of Ultrasonographic Measurements for Testicular Volume Assessment: Comparison of Three Common Formulas with True Testicular Volume. Asian J. Androl. 2009, 11, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, V.G.; Munuce, M.J.; Cohen, D.J.; Marín-Briggiler, C.I.; Busso, D.; Visconti, P.E.; Cuasnicú, P.S. Bicarbonate Is Required for Migration of Sperm Epididymal Protein DE (CRISP-1) to the Equatorial Segment and Expression of Rat Sperm Fusion Ability. Biol. Reprod. 2004, 70, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Theas, S.; Rival, C.; Lustig, L. Germ Cell Apoptosis in Autoimmune Orchitis: Involvement of the Fas-FasL System. Am. J. Reprod. Immunol. 2003, 50, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Di Persio, S.; Saracino, R.; Fera, S.; Muciaccia, B.; Esposito, V.; Boitani, C.; Berloco, B.P.; Nudo, F.; Spadetta, G.; Stefanini, M.; et al. Spermatogonial Kinetics in Humans. Development 2017, 144, 3430–3439. [Google Scholar] [CrossRef] [PubMed]
- Cavicchia, J.C.; Sacerdote, F.L.; Ortiz, L. The Human Blood-Testis Barrier in Impaired Spermatogenesis. Ultrastruct. Pathol. 1996, 20, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Meroni, S.B.; Galardo, M.N.; Rindone, G.; Gorga, A.; Riera, M.F.; Cigorraga, S.B. Molecular Mechanisms and Signaling Pathways Involved in Sertoli Cell Proliferation. Front. Endocrinol. 2019, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Theas, M.S. Germ Cell Apoptosis and Survival in Testicular Inflammation. Andrologia 2018, 50, e13083. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, S.; Jiang, S.; Huang, Z.; Chen, X.; Guo, H.; Li, M.; Zheng, S. Evaluation of Immune Status in Testis and Macrophage Polarization Associated with Testicular Damage in Patients with Nonobstructive Azoospermia. Am. J. Rep. Immunol. 2021, 86, e13481. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.-G.; Yu, C.-F.; Novak, N.; Bieber, T.; Zhu, C.-H.; Schuppe, H.-C.; Haidl, G.; Allam, J.-P. Immunodeviation towards a Th17 Immune Response Associated with Testicular Damage in Azoospermic Men: Testicular Th17 Immune Response in Azoospermic Men. Int. J. Androl. 2011, 34, e536–e545. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Calandra, R.S.; Lustig, L.; Meineke, V.; Köhn, F.M.; Vogt, H.-J.; Mayerhofer, A. Number, Distribution Pattern, and Identification of Macrophages in the Testes of Infertile Men. Fertil. Steril. 2002, 78, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, A.; Walenta, L.; Mayer, C.; Eubler, K.; Welter, H. Human Testicular Peritubular Cells, Mast Cells and Testicular Inflammation. Andrologia 2018, 50, e13055. [Google Scholar] [CrossRef] [PubMed]
- Hentrich, A.; Wolter, M.; Szardening-Kirchner, C.; Lüers, G.H.; Bergmann, M.; Kliesch, S.; Konrad, L. Reduced Numbers of Sertoli, Germ, and Spermatogonial Stem Cells in Impaired Spermatogenesis. Mod. Pathol. 2011, 24, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.W.; Lamb, D.J.; Wheeler, T.M.; Lipshultz, L.I.; Kim, E.D. In Situ End-Labeling of Human Testicular Tissue Demonstrates Increased Apoptosis in Conditions of Abnormal Spermatogenesis. Fertil. Steril. 1997, 68, 1065–1069. [Google Scholar] [CrossRef]
- Steger, K. The Proliferation of Spermatogonia in Normal and Pathological Human Seminiferous Epithelium: An Immunohistochemical Study Using Monoclonal Antibodies against Ki-67 Protein and Proliferating Cell Nuclear Antigen. Mol. Hum. Reprod. 1998, 4, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Edelsztein, N.Y.; Valeri, C.; Lovaisa, M.M.; Schteingart, H.F.; Rey, R.A. AMH Regulation by Steroids in the Mammalian Testis: Underlying Mechanisms and Clinical Implications. Front. Endocrinol. 2022, 13, 906381. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.-C.; Chen, Y.-T.; Chang, C.; Chang, Y.-C.; Lin, H.-J.; Huang, K.-E.; Kang, H.-Y. Up-Regulation of SOX9 in Sertoli Cells from Testiculopathic Patients Accounts for Increasing Anti-Mullerian Hormone Expression via Impaired Androgen Receptor Signaling. PLoS ONE 2013, 8, e76303. [Google Scholar] [CrossRef] [PubMed]
- Buzzard, J.J.; Wreford, N.G.; Morrison, J.R. Thyroid Hormone, Retinoic Acid, and Testosterone Suppress Proliferation and Induce Markers of Differentiation in Cultured Rat Sertoli Cells. Endocrinology 2003, 144, 3722–3731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yao, C.; Xing, X.; Jing, T.; Li, P.; Zhu, Z.; Yang, C.; Zhai, J.; Tian, R.; Chen, H.; et al. Single-Cell Analysis of Developing and Azoospermia Human Testicles Reveals Central Role of Sertoli Cells. Nat. Commun. 2020, 11, 5683. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.F.A.; França, L.R.; Hess, R.A.; Costa, G.M.J. Sertoli Cells Are Capable of Proliferation into Adulthood in the Transition Region between the Seminiferous Tubules and the Rete Testis in Wistar Rats. Cell Cycle 2016, 15, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.F.A.; Hess, R.A.; Batlouni, S.R.; Wnuk, N.T.; Tavares, A.O.; Abarikwu, S.O.; Costa, G.M.J.; França, L.R. Insights into Differentiation and Function of the Transition Region between the Seminiferous Tubule and Rete Testis. Differentiation 2021, 120, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Aiyama, Y.; Tsunekawa, N.; Kishi, K.; Kawasumi, M.; Suzuki, H.; Kanai-Azuma, M.; Kurohmaru, M.; Kanai, Y. A Niche for GFRα1-Positive Spermatogonia in the Terminal Segments of the Seminiferous Tubules in Hamster Testes. Stem Cells 2015, 33, 2811–2824. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Fröysa, B.; Söder, O. Endotoxin and Proinflammatory Cytokines Modulate Sertoli Cell Proliferation in Vitro. J. Reprod. Immunol. 2004, 61, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Boitani, C.; Fröysa, B.; Söder, O. Interleukin-1 Is a Potent Growth Factor for Immature Rat Sertoli Cells. Mol. Cell. Endocrinol. 2002, 186, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Lardone, M.C.; Piottante, A.; Valdevenito, R.; Ebensperger, M.; Castro, A. Histological and Hormonal Testicular Function in Oligo/Azoospermic Infertile Men. Andrologia 2013, 45, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Billur, D.; Kizil, S.; Ozkavukcu, S.; Topal Celikkan, F.; Aydos, K.; Erdemli, E. Evaluation of Blood–Testis Barrier Integrity in Terms of Adhesion Molecules in Nonobstructive Azoospermia. Andrologia 2020, 52, e13636. [Google Scholar] [CrossRef] [PubMed]
- Tung, K.S.K.; Harakal, J.; Qiao, H.; Rival, C.; Li, J.C.H.; Paul, A.G.A.; Wheeler, K.; Pramoonjago, P.; Grafer, C.M.; Sun, W.; et al. Egress of Sperm Autoantigen from Seminiferous Tubules Maintains Systemic Tolerance. J. Clin. Investig. 2017, 127, 1046–1060. [Google Scholar] [CrossRef]
- Jacobo, P. The Role of Regulatory T Cells in Autoimmune Orchitis. Andrologia 2018, 50, e13092. [Google Scholar] [CrossRef]
- Lee, N.P.Y.; Cheng, C.Y. Nitric Oxide/Nitric Oxide Synthase, Spermatogenesis, and Tight Junction Dynamics1. Biol. Reprod. 2004, 70, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Stammler, A.; Lüftner, B.U.; Kliesch, S.; Weidner, W.; Bergmann, M.; Middendorff, R.; Konrad, L. Highly Conserved Testicular Localization of Claudin-11 in Normal and Impaired Spermatogenesis. PLoS ONE 2016, 11, e0160349. [Google Scholar] [CrossRef] [PubMed]
- Washburn, R.L.; Hibler, T.; Thompson, L.A.; Kaur, G.; Dufour, J.M. Therapeutic Application of Sertoli Cells for Treatment of Various Diseases. Semin. Cell Dev. Biol. 2022, 121, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Jacobo, P.; Sobarzo Alvarez, C.M.; Pérez, C.V.; Amarilla, M.S.; Gliemke, L.; Bertinetti, B.; Frungieri, M.; Glisoni, R.J.; Theas, S. F127 (or p407) poloxamer as a promising gelling agent in Sertoli cell delivery for cell therapy of the testis. Rev. Med. 2019, 79 (Suppl. SIV), 280. [Google Scholar]
- Amiri, N.; Mohammadi, P.; Allahgholi, A.; Salek, F.; Amini, E. The Potential of Sertoli Cells (SCs) Derived Exosomes and Its Therapeutic Efficacy in Male Reproductive Disorders. Life Sci. 2023, 312, 121251. [Google Scholar] [CrossRef] [PubMed]
| Testosterone | LH | FSH | PRL | |
|---|---|---|---|---|
| (RV: 1.3–8.2 ng/mL) | (RV: 0.8–8.6 ng/mL) | (RV: 0.95–11.95 ng/mL) | (RV: 2.5–17.0 ng/mL) | |
| Complete Sp | 4.24 ± 0.71 (n = 8) | 4.1 ± 0.57 (n = 8) | 5.21 ± 1.06 (n = 8) | 8.9 ± 1.16 (n = 9) |
| Mild HypoSp | 4.77 ± 0.86 (n = 8) | 4.1 ± 0.57 (n = 9) | 9.55 ± 2.57 (n = 9) | 11.29 ± 2.44 (n = 9) |
| Severe HypoSp | 3.95 ± 0.46 (n = 8) | 9.5 ± 2.1 (n = 10) | 21.4 ± 4.1 (n = 10) * | 14.03 ± 2.67 (n = 10) |
| SCOS | 5.01 ± 0.68 (n = 8) | 6.28 ± 0.79 (n = 9) | 20.01 ± 1.96 (n = 10) * | 11.54 ± 2.31 (n = 6) |
| Human Mild HypoSp | Human Severe HypSp | SCOS | Rat Focal EAO | Rat Severe EAO | |
|---|---|---|---|---|---|
| Sperm count | azoospermia | azoospermia | azoospermia | decreased | azoospermia |
| Immune cell infiltrates | increased | increased | increased | increased [6] | increased [6] |
| STs diameter | unchanged | reduced | reduced | reduced | reduced |
| STs thickness | unchanged | increased | increased | unchanged | increased |
| Undifferentiated SPG | unchanged | reduced | nd | reduced [7] | reduced [7] |
| Differentiated SPG | unchanged | reduced | nd | reduced [7] | reduced [7] |
| Sertoli cell number | unchanged | unchanged | increased | unchanged | increased |
| Ki67+ Sertoli cells | unchanged | unchanged | increased | - | + |
| AMH+ Sertoli cells | - | increased | increased | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amarilla, M.S.; Glienke, L.; Munduruca Pires, T.; Sobarzo, C.M.; Oxilia, H.G.; Fulco, M.F.; Rodríguez Peña, M.; Maio, M.B.; Ferrer Viñals, D.; Lustig, L.; et al. Impaired Spermatogenesis in Infertile Patients with Orchitis and Experimental Autoimmune Orchitis in Rats. Biology 2024, 13, 278. https://doi.org/10.3390/biology13040278
Amarilla MS, Glienke L, Munduruca Pires T, Sobarzo CM, Oxilia HG, Fulco MF, Rodríguez Peña M, Maio MB, Ferrer Viñals D, Lustig L, et al. Impaired Spermatogenesis in Infertile Patients with Orchitis and Experimental Autoimmune Orchitis in Rats. Biology. 2024; 13(4):278. https://doi.org/10.3390/biology13040278
Chicago/Turabian StyleAmarilla, María Sofía, Leilane Glienke, Thaisy Munduruca Pires, Cristian Marcelo Sobarzo, Hernán Gustavo Oxilia, María Florencia Fulco, Marcelo Rodríguez Peña, María Belén Maio, Denisse Ferrer Viñals, Livia Lustig, and et al. 2024. "Impaired Spermatogenesis in Infertile Patients with Orchitis and Experimental Autoimmune Orchitis in Rats" Biology 13, no. 4: 278. https://doi.org/10.3390/biology13040278
APA StyleAmarilla, M. S., Glienke, L., Munduruca Pires, T., Sobarzo, C. M., Oxilia, H. G., Fulco, M. F., Rodríguez Peña, M., Maio, M. B., Ferrer Viñals, D., Lustig, L., Jacobo, P. V., & Theas, M. S. (2024). Impaired Spermatogenesis in Infertile Patients with Orchitis and Experimental Autoimmune Orchitis in Rats. Biology, 13(4), 278. https://doi.org/10.3390/biology13040278







