Abstract
To improve the combustion performance of boron powder, a method was developed for synthesizing boron–magnesium–titanium (B-Mg-Ti) ternary composite powders with controlled metal content. Boron–magnesium (B-Mg) base materials were first prepared via electrical explosion, followed by the incorporation of titanium powder at varying mass fractions (1 wt.%, 3 wt.%, 5 wt.%, and 7 wt.%) through mechanical ball milling. Field emission scanning electron microscopy (FE-SEM) revealed that the addition of titanium promoted a more uniform dispersion of magnesium within the boron agglomerates. Moreover, nanoscale titanium particles were observed to be embedded on the particle surfaces, confirming successful microscale composite formation. Particle size distribution was measured using a Malvern 3000 laser particle size analyzer, and results showed that the particle size of the ternary composites decreased gradually with increasing titanium content. Specific surface area was determined via the Brunauer–Emmett–Teller (BET) method, with all samples exhibiting values greater than 15 m2/g, indicating good surface reactivity. Furthermore, the rheological behavior of the B-Mg-Ti composite powders, when combined with terminal hydroxyl polybutadiene (HTPB)—a typical binder in solid propellants—was evaluated. Viscosity measurements were conducted using a rotational rheometer at constant temperatures of 20 °C and 70 °C. The results demonstrated a marked decrease in viscosity with increasing titanium content, suggesting that titanium incorporation enhances the flowability of the composite powders. This study systematically evaluated the influence of titanium content on the structural and physicochemical properties of B-Mg-Ti composite powders, thereby providing a valuable experimental foundation for the optimized design of boron-based combustion systems and the enhancement of their processing and application performance.
1. Introduction
To fully harness the combustion potential of boron in energetic formulations [], extensive research has been devoted to the development of boron-based composites with various additives [,,,,,,]. Among these, boron–magnesium (B-Mg) composites have garnered significant attention due to the well-established pro-ignition effect of magnesium. It has been demonstrated that magnesium, when added at approximately 8 wt.%, can reduce the ignition delay time of boron from ~1800 ms to less than 100 ms, while also marginally enhancing the total heat release during combustion [,,,]. However, the introduction of magnesium into propellants presents trade-offs: small amounts result in limited improvement, whereas excessive magnesium can diminish the overall energy output and reduce the specific impulse of the propellant system []. Recently, titanium has emerged as a promising alternative or supplementary additive to modify the combustion behavior of boron. Although titanium’s combustion enthalpy (19.7 kJ/g) is lower than that of both boron and magnesium, it combusts efficiently and is widely used in inorganic thermites and ignition compositions. Importantly, titanium can also function as a combustion promoter by disrupting the formation of dense oxide shells, which are known to inhibit boron ignition. This makes titanium a valuable trade-off despite its lower intrinsic energy [,]. Given the limited performance improvement achievable through single-metal additives, this study explores the development of a ternary boron–magnesium–titanium (B-Mg-Ti) composite. The objective is to combine the advantages of magnesium and titanium to produce a composite with enhanced combustion-related properties. In this formulation, the total metal content is fixed at 8 wt.%, and magnesium is partially replaced by titanium in varying proportions (0 wt.%, 1 wt.%, 3 wt.%, 5 wt.%, and 7 wt.%). The resulting composite powders are characterized in terms of particle size distribution, compositional uniformity, specific surface area, and rheological behavior to assess their suitability for subsequent combustion performance evaluations.
Traditional approaches for synthesizing boron–metal composites—such as vapor phase deposition [], pyrolytic coating [], high-energy ball milling [,,], and pulsed electric explosion []—face limitations in processing boron due to its high viscosity and poor plasticity. These challenges are exacerbated when attempting to fabricate ternary composites with homogeneous distribution and controlled composition. To address these issues, this study adopts a two-step method: a boron–magnesium composite base powder is first prepared via pulsed electric explosion with a fixed magnesium content. The base powder is then passivated and subjected to wet ball milling with titanium powder in an anhydrous ethanol environment to form the ternary composite. Our findings show that the electric explosion B-Mg base powder exhibits enhanced micro-area compositing with titanium particles after ball milling, confirming the efficacy of this combined technique in producing a uniform ternary powder. Additionally, the influence of titanium content on key physicochemical properties—including particle size, specific surface area, and viscosity (with hydroxyl-terminated polybutadiene, HTPB, as the binder)—was systematically investigated. The goal is to identify the formulation with the most favorable properties for future combustion performance testing.
2. Experiment
2.1. B-Mg Composite Powder Preparation
Commercial boron powder with a purity of 95 wt.% was selected as both the reference sample and the primary raw material for the preparation of the composite materials. The main impurities in the boron powder are oxygen and magnesium, which exist primarily in compound form, with an oxygen content of approximately 2.8 wt.% and a magnesium content of 1.9 wt.%. The second raw material was magnesium wire with a purity of 99 wt.% and a diameter of 0.25 mm. Both the boron powder and magnesium wire were supplied by BGRIMM Advanced Materials Science & Technology Co., Ltd. located in Beijing, China. The third raw material was titanium powder with a particle size of less than 45 μm and a purity of ≥99.5 wt.%. It was supplied by Sichuan Xin Shu Tai New Materials Co., Ltd. located in Chengdu, China. To synthesize the ternary B-Mg-Ti composite, the B-Mg base powder was first prepared using a pulsed electric explosion method []. A schematic diagram of the electric explosion apparatus is presented in Figure 1. As an illustrative example for producing a powder with 8 wt.% magnesium content, the following procedure was followed.
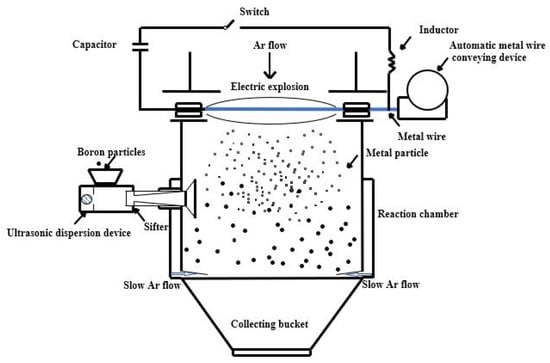
Figure 1.
Schematic diagram of the electric explosion apparatus used for the preparation of B-Mg base materials.
The equipment was first evacuated to below −0.095 MPa using a vacuum pump, after which the chamber was backfilled with argon to a pressure of 0.015 MPa to establish an inert gas environment. The magnesium wire was mounted in an automatic wire feeder, which advanced the wire at a constant speed of 50 mm/s. Simultaneously, 11.5 g of vacuum-dried boron powder was loaded into the inlet. The powder was dispersed into the reaction chamber as individual particles with the aid of an ultrasonic dispersion device. To prevent particle agglomeration and address single-sided deposition issues common in vapor-phase methods, a vent at the base of the chamber allowed a slow, upward argon gas flow. This generated a weak circulatory motion, enabling the boron particles to remain suspended and uniformly distributed within the chamber. Once the chamber was stabilized, a high-voltage DC power supply was activated and maintained at 15 kV to trigger the electric explosion. Each 100 mm section of magnesium wire was completely exploded within 5 s, yielding approximately 0.01 g of fine magnesium powder per explosion. The process was programmed to repeat 100 times, resulting in approximately 1 g of magnesium powder dispersed within the boron matrix. After the explosions were completed, the system was powered down and left undisturbed for 15 min to allow the magnesium to fully adsorb onto the boron surface. Subsequently, the argon inflow was stopped, and the internal pressure of the chamber was increased to 0.02 MPa, facilitating powder sedimentation into a collection hopper. The collected composite was then transferred to an argon-filled glovebox, where it was gently passivated by introducing a small amount of oxygen. Approximately 10 g of passivated B–Mg base powder was obtained from each batch. For the base powders with different magnesium contents (1 wt.%, 3 wt.%, 5 wt.%, 7 wt.%, and 8 wt.%), the samples were designated as CBM1, CBM3, CBM5, CBM7, and CBM8, respectively. The same processing method was applied to all samples, with only the wire feed amount and the number of explosions adjusted to achieve the target compositions.
2.2. B-Mg-Ti Composite Powder Preparation
To prepare the ternary B-Mg-Ti composite powder, titanium powder was doped into the B-Mg base powder using a mechanical alloying approach. Mechanical alloying induces repeated cold welding, plastic deformation, and work hardening at the atomic scale, ultimately promoting atomic diffusion and alloy formation []. This technique is well-suited for synthesizing the ternary composites in this study. A LM-1 series vertical stirring ball mill (LM-1 series. Wuxi Longda drying powder equipment Co., Ltd. located in Wuxi, China) was employed for the mechanical alloying process. Titanium powder was added to the B-Mg base powder in mass ratios corresponding to target titanium contents of 0 wt.%, 1 wt.%, 3 wt.%, 5 wt.%, and 7 wt.%. The sample IDs and respective titanium powder masses are detailed in Table 1. Notably, sample boron was retained as a control group without any ball milling, while BM8 was obtained by ball milling the CBM8 base powder to ensure consistency in the processing conditions of the subsequent samples. To serve as a process control agent, 300 mL of anhydrous ethanol was added to each batch. In each experiment, the designated mass of B-Mg base powder and titanium powder were placed in an 800 mL milling jar, along with 1000 g of zirconia milling balls (3 mm diameter). Ball milling was conducted at a rotation speed of 400 RPM for 20 h. Upon completion, the milled samples were retrieved via vacuum filtration and subsequently transferred to a vacuum oven for drying at ambient temperature for 12 h to ensure complete evaporation of the ethanol solvent.

Table 1.
Number table of prepared samples.
3. Results and Discussion
3.1. Powder Size Distribution
The laser particle size distributions of the B-Mg base powder, titanium powder, boron powder, and the ball-milled composite powder were measured using a Malvern Mastersizer 3000 laser particle size analyzer(Malvern Panalytical Ltd., Malvern, UK). The results are shown in Figure 2.
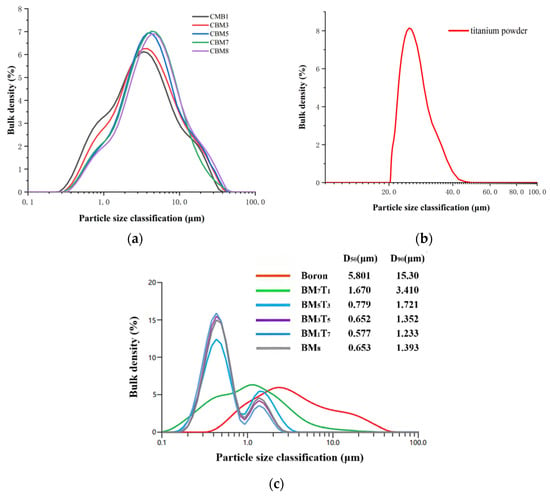
Figure 2.
Laser particle size distribution of (a) B–Mg base powde; (b) titanium powder; (c) boron powder and ball-milled composite powder.
As shown in Figure 2, the particle size of the composite powder after ball milling is significantly reduced compared to that of the B-Mg base powder and titanium powder. Moreover, the particle size of the ternary composite powders gradually decreases with increasing titanium content, indicating that the addition of titanium enhances the shear force transmission between the grinding media and the B-Mg base powder during the ball milling process. This enhanced mechanical interaction promotes the fragmentation of boron particles and facilitates more effective micro-scale compositing between boron and metal. It is worth noting that in Figure 2c, both the raw boron sample and the BM7T1 sample exhibit bimodal particle size distributions. However, the secondary peak is relatively small, and the overall distribution is broader. The peak position of BM7T1 shifts further to the left compared to the raw boron sample, and its curve exhibits a steeper decline in the tail, corresponding to smaller D50 and D90 values, which confirms the formation of finer composite powder after processing. In contrast, the other samples display more pronounced bimodal distribution characteristics. With increasing titanium content, the height difference between the two peaks gradually increases, suggesting a trend toward greater non-uniformity in particle size distribution. This type of bimodal structure typically includes a primary peak near D50 and a secondary peak near D90, indicating the coexistence of fine and coarse particles.
3.2. Phase Analysis
The samples were scanned using a D8 ADVANCE X-ray Diffraction (XRD) instrument (Bruker AXS GmbH, Karlsruhe, Germany). For example, the results for sample BM3T5 are shown in Figure 3.
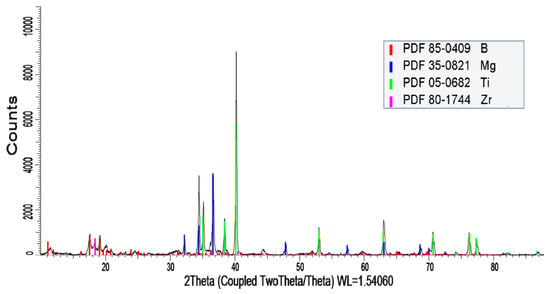
Figure 3.
XRD pattern of the BM3T5.
In Figure 3, the prominent diffraction peaks are identified as B (red), Mg (blue), and Ti (green). In addition, a few weak peaks corresponding to Zr (introduced from zirconium milling media) are observed. No diffraction peaks associated with other compounds were detected. This indicates that the B-Mg-Ti composite powder was physically combined through mechanical ball milling without the formation of new compounds, thereby effectively avoiding the reduction in combustion reactivity and calorific value typically associated with compound formation in energetic materials.
3.3. Microscopic Characterization
For ease of description, the composite powders are denoted using the subscript notation BMxTy, where x and y represent the weight percentages of magnesium and titanium, respectively. For example, a sample containing 1 wt.% magnesium and 7 wt.% titanium is denoted as BM1T7. The morphology and elemental distribution of the prepared composites were examined using a Hitachi SU5000 Field Emission Scanning Electron Microscope (FESEM) (Hitachi High-Technologies Corporation, Tokyo, Japan). Compositional analysis was conducted using XFlash 6130 Energy Dispersive Spectroscopy (EDS) (Bruker AXS GmbH, Karlsruhe, Germany) in conjunction with surface mapping to evaluate the homogeneity of metal distribution on the boron base. Representative micrographs of the starting boron powder and a composite sample are shown in Figure 4a, taken using secondary electrons, which reveals the general morphology of the particles, while Figure 4b, captured using backscattered electrons, highlights the heavier metal elements as brighter regions, enabling clear distinction between metal particles and boron aggregates. In Figure 4c, EDS elemental mapping confirms that both magnesium and titanium are dispersed across the boron particle surfaces. The co-localization of boron with elevated metal concentrations suggests that the metallic constituents are effectively deposited onto the boron aggregates during the preparation process.
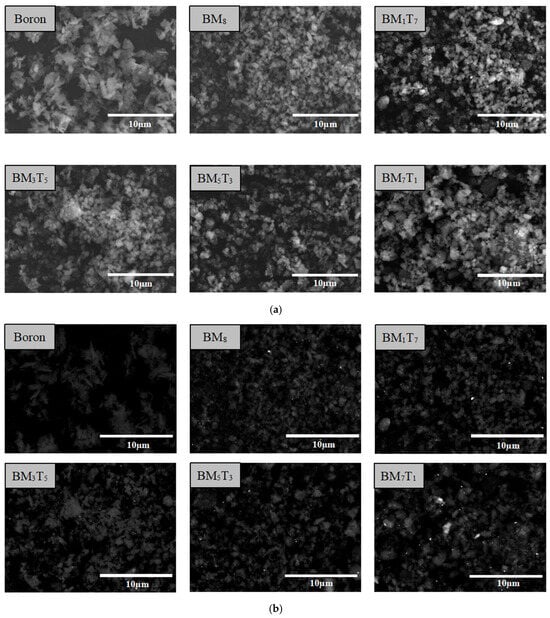
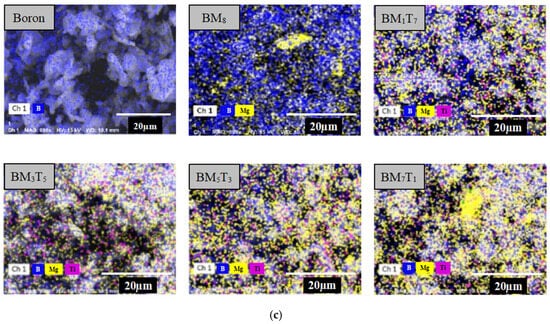
Figure 4.
SEM images (a,b) and EDS surface scanning analysis images (c) of the boron powder and composite samples.
In particular, samples BM8, BM1T7, BM5T3, and BM7T1 were further analyzed using INCA Energy Dispersive Spectroscopy (EDS) (Oxford Instruments, Oxford, UK) with ZAF (atomic number-absorption-fluorescence) correction to improve quantitative accuracy. The corresponding X-ray fluorescence spectra were plotted to verify the elemental composition and further confirm the effective integration of magnesium and titanium within the boron-based composite matrix.
As shown in Figure 5, taking BM5T3 as an example, in the B element map, the color at the edges of some large red agglomerates (indicated by arrows) transitions from red to yellow, green, and blue, indicating a noticeable decrease in radiation intensity at the boundaries. The Mg element map shows a similar pattern, where the edges of certain bright red regions are surrounded by green/blue areas (indicated by arrows), suggesting a lower Mg density at the periphery. This implies that the B-Mg matrix has achieved a high degree of integration after electrical explosion and subsequent ball milling. The apparent diffusion of Mg into the boron agglomerates further supports the good composite formation. In contrast, titanium exhibits a strong spectral absorption effect, with only a slight decrease in intensity at the edges, indicating that despite its uniform surface distribution, Ti forms relatively few micro-regions of composite with B and Mg. Notably, the edge attenuation observed in the B-Mg maps becomes more pronounced with increasing Mg content, which is most evident in the BM5T3 sample. This may be attributed to a more complete coating of boron by magnesium following the electrical explosion process. During ball milling, titanium likely facilitates this process by transmitting mechanical forces and enhancing the shearing action, thereby promoting the dispersion of magnesium within the boron matrix. To verify this hypothesis, the BM5T3 sample was subjected to carbon embedding (due to its magnetic nature) for further EDS analysis and JEM2100 high-resolution transmission electron microscopy (HRTEM) (JEOL Ltd., Tokyo, Japan) observation. As shown in Figure 6, composite powder particles containing relatively large titanium inclusions were selected to highlight the differences in microstructure.
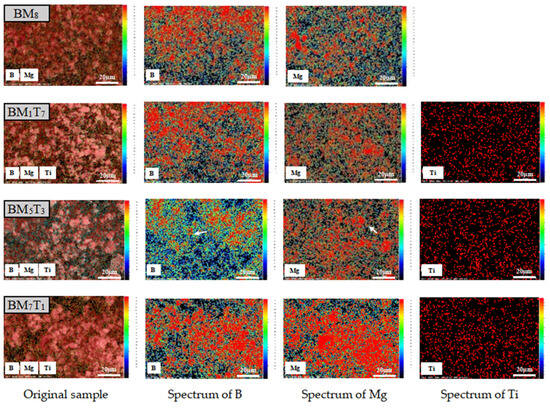
Figure 5.
Fluorescence spectrogram of BM8, BM1T7, BM5T3, and BM7T1 samples after ZAF correction.
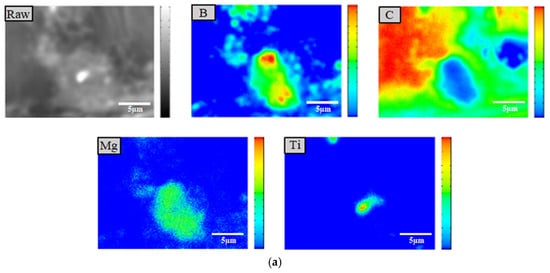
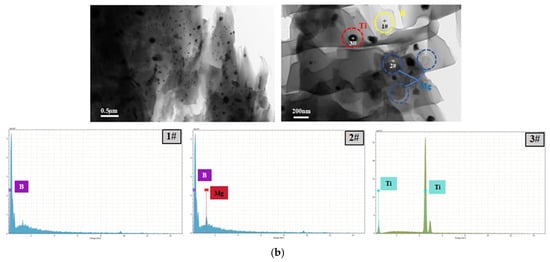
Figure 6.
EPMA (a), HRTEM and EDS images (b) of the BM5T3 sample, showing elemental distribution, interfacial characteristics, and localized elemental composition.
Elemental mapping of carbon and boron obtained from EPMA 1720H Electron Probe Microanalysis (EPMA) (Shimadzu Corporation, Kyoto, Japan) clearly indicates that the low-contrast gray clusters in the image correspond to boron agglomerates. The high-contrast regions represent titanium particles, as confirmed by targeted elemental scans. The results also reveal that titanium is dispersed around some of the larger Ti particles. In the HRTEM images, the contrast among the three elements is more distinct: titanium appears brightest, followed by the B-Mg regions, while boron itself shows relatively low brightness. This contrast is consistent with the EDS results, as shown in Figure 6. Moreover, the HRTEM images reveal that smaller titanium particles are embedded within the boron agglomerates, whereas magnesium is completely dispersed throughout the boron matrix, exhibiting a flocculent morphology. This behavior may be attributed to the lower hardness of magnesium and the higher hardness of titanium. During the ball milling process, titanium plays a mechanically assisting role, effectively promoting the fragmentation of magnesium and facilitating its dispersion and embedding into the boron matrix, thereby forming a more compact and uniform composite structure.
3.4. Specific Surface Area
The B-Mg-Ti composite powder developed in this study is primarily intended for use as a high-energy fuel. Increasing the specific surface area of the composite powder is beneficial for enhancing the material’s reactivity, which in turn promotes energy release during combustion. Therefore, it is essential to investigate the changes in specific surface area between the composite powder and the untreated boron powder. According to the single-point Brunauer–Emmett–Teller (BET) adsorption theory, the specific surface area of a material can be calculated using the following formula:
where
Vm is the saturated adsorption of nitrogen on the sample surface in mL;
N is Avogadro’s constant (6.024 × 1023);
σ is the cross-sectional area of nitrogen molecules 0.162 nm2;
W is the sample mass;
The value of Vm can be obtained using the BET equation.
where
P is the partial pressure of nitrogen and P0 is the saturation vapor pressure at the adsorption temperature. The relative pressure () was selected in the range of 0.05–0.25, as values below 0.05 make it difficult to establish multilayer adsorption equilibrium, while values above 0.25 tend to promote capillary condensation. In the BET adsorption plot, is used as the x-axis and as the y-axis. The resulting BET adsorption plot of the sample is shown in Figure 7.
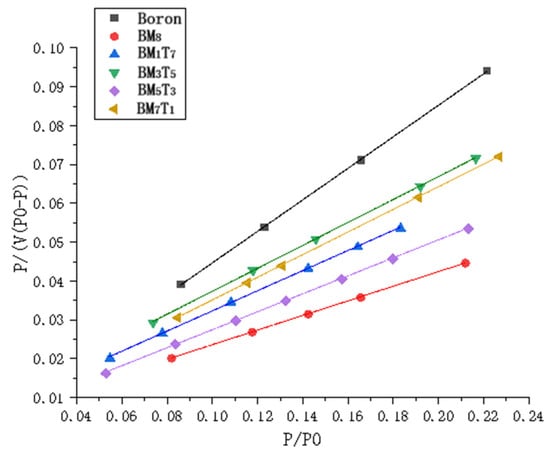
Figure 7.
Linear fitting of the boron powder and composite samples using the BET adsorption isotherm method for specific surface area determination.
The specific surface area of each sample was determined by calculating the slope and intercept of the linear region of the BET adsorption isotherm. These values were subsequently used to derive the BET constant C and the monolayer adsorption capacity Vm. Based on these parameters, the specific surface areas were calculated using the Multi-BET model, as shown in Figure 8. It should be noted that the error bars represent the standard deviation from three independent measurements, reflecting the reproducibility of the data. The results in Figure 8 clearly demonstrate that the composite powders exhibit significantly higher specific surface areas than the untreated boron powder, all exceeding 15 m2/g. However, no consistent or regular trend was observed between the variation in titanium content among the samples and their specific surface areas. Generally, finer powders exhibit larger specific surface areas. In this study, as the Ti content increases, the measured particle size of the composite powders follows the following order: BM7T1 > BM5T3 > BM3T5 > BM1T7. However, the actual specific surface area results show that BM3T5 has the highest value, while BM5T3 has the lowest. This discrepancy is mainly attributed to the drying process applied after wet ball milling. During drying, secondary agglomeration occurs in the powders. Although manual grinding was employed to break up these agglomerates, the effectiveness of this process in fully dispersing the particles is somewhat random. Consequently, samples with different Ti contents exhibit varying degrees of agglomeration, resulting in irregular specific surface area measurements. In contrast, the laser particle size distribution measurements display a consistent trend because the testing involves ultrasonic dispersion, ensuring a more uniform sample state during analysis. Therefore, the particle size results show a regular variation trend. In addition, the average pore width of all compositions remained within a narrow range of 2.6–2.8 nm, indicating that the mechanical ball milling process had a greater effect on specific surface area than on pore structure.
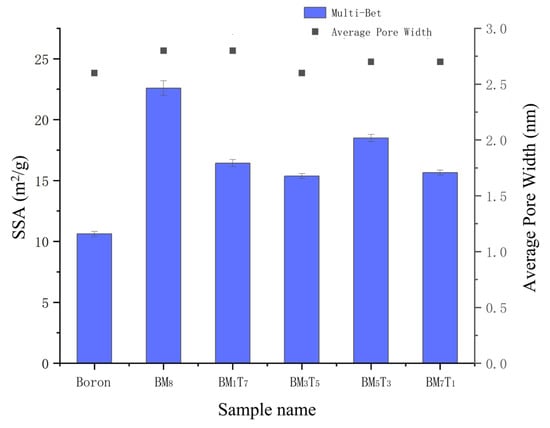
Figure 8.
SSA (Multi-Bet) and average pore width of the boron powder and composite samples.
3.5. Viscosity
Figure 9 presents the results of numerical fitting based on all experimental test data, illustrating the relationship between viscosity and the varying test parameters across all prepared samples.
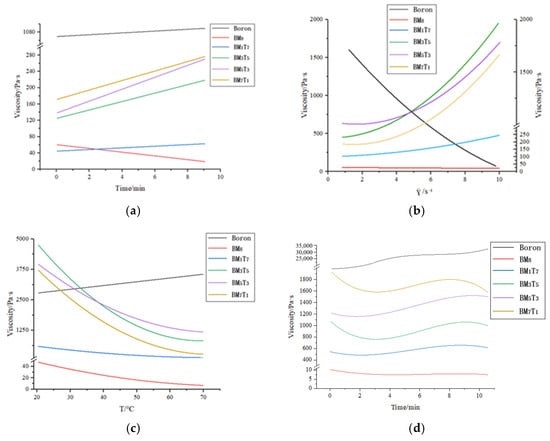
Figure 9.
Graph of experimental results for four stages of viscosity testing: (a) step1: Variation of sample viscosity with time at a constant temperature of 20 °C and a shear rate of 1.000 s−1; (b) step2: Viscosity variation of the sample as the shear rate increases from 1.000 s−1 to 10.000 s−1 at a constant temperature of 20 °C; (c) step3: Viscosity change of the sample at a constant shear rate of 1.000 s−1 as the temperature increases rapidly from 20 °C to 70 °C; (d) step4: Viscosity variation of the sample over 10 min at a constant temperature of 70 °C and a shear rate of 1.000 s−1.
The rheological behavior of the composite powders was evaluated through a four-stage viscosity test. In the first stage, conducted at 20 °C with a constant shear rate of 1.000 s−1 for 10 min, most composite samples exhibited a slow increase in viscosity, except BM8, which showed a gradual decline. The unprocessed boron powder displayed the highest initial viscosity, followed by composite powders in the order of BM7T1 > BM5T3 > BM3T5 > BM1T7. Notably, BM8 started with a higher viscosity than BM1T7 but reversed over time. These results suggest that higher titanium content corresponds to a general reduction in viscosity at this stage.
In the second stage, as the rotation speed increased from 1.000 s−1 to 10.000 s−1 at a constant 20 °C, the viscosity of the ternary composites rose exponentially, while the unprocessed boron powder showed a rapid decrease. BM8 exhibited minimal change. Initially, BM3T5 and BM5T3 surpassed BM1T7 in viscosity, with BM3T5 overtaking BM5T3 at a shear rate of 4.898 s−1, indicating a stronger response to shear. The viscosity of BM1T7 increased only slightly, remaining the lowest among the composites.
In the third stage, the platform rotation rate was maintained at 1.000 s−1 while the temperature was rapidly increased from 20 °C to 70 °C over a 15-min period. During this rapid heating process, the viscosity of the original boron sample exhibited an increasing trend. In contrast, all B-Mg-Ti ternary composite powders demonstrated a general decrease in viscosity. Among these, the BM3T5 sample initially exhibited the highest viscosity; however, in the 20 °C to 40 °C temperature range, its viscosity decreased sharply, falling below that of the BM5T3 sample but remaining higher than that of the BM7T1 sample. The BM1T7 sample consistently exhibited the lowest viscosity throughout this stage.
In the fourth stage, the samples were held at a constant temperature of 70 °C while the platform rotated at a fixed rate of 1.000 s−1 for 10 min. Under these conditions, the viscosity of the original boron sample exceeded 20,000 Pa·s, indicating significant thickening at an elevated temperature. In contrast, all B-Mg-Ti ternary composite powders exhibited markedly lower viscosities, all remaining below 2000 Pa·s, with the BM8 sample reaching as low as <10 Pa·s. The viscosity profiles of the composite powders remained relatively stable throughout this stage, suggesting the establishment of a dynamic equilibrium at 70 °C. The viscosity ranking of the samples from highest to lowest was BM7T1 > BM5T3 > BM3T5 > BM1T7, which mirrored the trend observed at 20 °C. These results further confirm that increasing titanium content effectively reduces the viscosity of the composite powders. Across all testing phases, the BM7T1 sample consistently maintained a viscosity below 1000 Pa·s, while the other samples exhibited varying degrees of fluctuation. Notably, the BM3T5 sample demonstrated the largest fluctuations, with its viscosity peaking near 5000 Pa·s during the early stages of the thermal ramp-up, followed by a sharp decline. This pattern was also observed during the speed-increase phase, where a rapid rise in viscosity was followed by an equally rapid drop during heating. Although the BM5T3 sample displayed a similar fluctuation trend, the rate and extent of change were less pronounced than those of BM3T5. The BM1T7 sample showed viscosity behavior consistent with that of BM7T1, both maintaining relatively low and stable values throughout the testing sequence.
The B-Mg-Ti composite powder prepared in this study is primarily intended for use as a fuel component in solid propellants. In particular, HTPB-based (hydroxyl-terminated polybutadiene) solid propellants commonly use HTPB as the binder. During propellant formulation, the mixture of boron powder and HTPB is achieved through stirring at a controlled temperature of 70 °C. However, the boron powder surface often contains boron oxide (B2O3) or boric acid, which can interact with the terminal hydroxyl groups (–OH) in HTPB through acid-base coordination or esterification reactions. These interactions can induce slight crosslinking or molecular entanglement, leading to a significant increase in system viscosity and consequently limiting the amount of boron powder that can be incorporated. To address this issue, magnesium and titanium are introduced to form a composite coating on the surface of boron particles, which effectively prevents direct contact between the oxidized boron surface (B2O3 or boric acid) and HTPB, thereby reducing the viscosity during mixing. Titanium is introduced via a ball milling process, which enhances the shear force exerted by the milling media on the base materials. This promotes the dispersion of magnesium within the boron aggregates, resulting in localized micro-scale composites of boron, magnesium, and titanium. The amount of titanium added influences the surface coating state of the powder, which in turn affects the rheological behavior of the composite. Notably, in practical applications at 70 °C, higher titanium content corresponds to lower viscosity, indicating that titanium plays a crucial role in viscosity control. Therefore, it is reasonable to conclude that an optimal ratio of magnesium and titanium in the B-Mg-Ti samples can be identified to maintain stable viscosity under the four-stage testing conditions.
4. Summary and Conclusions
Laser particle size distribution results indicate that the particle size of the ball-milled composite powders is significantly reduced compared to that of the B-Mg base powder and titanium powder. Moreover, the particle size of the ternary composite powders decreases progressively with increasing titanium content. This suggests that the addition of titanium during the ball milling process enhances the shear force transfer between the milling media and the B-Mg base powder. The intensified mechanical action facilitates the fragmentation of boron particles and promotes more effective micro-scale composite formation between boron and the metallic components.
Comprehensive microstructural analysis using SEM and EDS confirmed that the B-Mg-Ti ternary composite powders produced via the combined electric explosion and ball milling process exhibited uniform dispersion of magnesium and titanium within boron aggregates. High-resolution transmission electron microscopy (HRTEM) revealed that magnesium lost its crystalline structure and was diffusely distributed throughout the boron matrix. Electron probe microanalysis (EPMA) further demonstrated the presence of titanium as nanoscale particles embedded within the boron aggregates. These findings confirm that a well-integrated B-Mg-Ti composite structure was successfully formed.
Nitrogen adsorption analysis (BET) indicated an increase in specific surface area for all composite powders compared to the original boron powder, with all samples exhibiting values above 15 m2/g and average pore diameters between 2.4 and 2.6 nm. This increase appeared independent of titanium content. Rheological testing, conducted in four stages—namely, isothermal holding at 20 °C, shear rate increase, temperature ramping to 70 °C, and isothermal holding at 70 °C—showed that the viscosity of the ternary composite powders decreased with increasing titanium content. At 20 °C, viscosities remained below 1000 Pa·s, while at 70 °C, values remained below 2000 Pa·s. Notably, greater fluctuations in viscosity were observed during the dynamic shear and heating stages.
The incorporation of titanium was found to enhance the shear forces exerted by the grinding media during the ball milling process, thereby promoting the uniform dispersion of magnesium within the boron aggregates and facilitating the formation of localized B-Mg-Ti microstructures. These microstructural modifications led to improved rheological behavior, primarily through their influence on the average particle size and the surface metal cladding characteristics of the composite powders. Notably, the BM5T3 and BM3T5 samples, which featured balanced magnesium and titanium contents, demonstrated more stable viscosity profiles across all testing stages.
Author Contributions
Methodology, Supervision, Investigation, Software and Writing—Review, Y.W.; Conceptualization, Visualization, Validation, Resources and Writing—Review, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by BGRIMM Technology Group scientific research Project (02-218).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The Author Yueguang Yu was employed by the company BGRIMM Technology Group. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Liu, S.; Han, L.; Liu, H.; Song, Y.; Liu, L.; Hu, S. Experimental and numerical study on ignition and combustion characteristics of boron-magnesium composite powders. Particuology 2024, 84, 12–29. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Li, H.-Y.; Li, H.; Zhao, X. Preparation and oxidative combustion property of boron-aluminum alloy. Chin. J. Nonferrous Met. 2021, 31, 890–898. [Google Scholar]
- Alexander, G.K.; Sorokin, I.V.; Selikhova, E.A.; Arkhipov, V.A. Effect of B, Fe, Ti, Cu nanopowders on the laser ignition of Al-based high-energy materials. Combust. Flame 2020, 222, 103–110. [Google Scholar]
- Cheng, L.; Yang, H.-T.; Yang, Y.; Yifan, L.; Yingyi, M.; Yanchun, L.; Dongming, S.; Houhe, C.; Artiaga, R. Preparation of B/Nitrocellulose/Fe particles and their effect on the performance of an ammonium perchlorate propellant. Combust. Flame 2020, 211, 456–464. [Google Scholar] [CrossRef]
- Yang, J.-L. Preparation and Properties of B/Al and B/Mg Composites; Nanjing University of Technology: Nanjing, China, 2015. [Google Scholar]
- Yu, R.-T.; MA, M.-M.; Zhang, R.; Qin, Z.; Liu, D. Ignition and combustion characteristics of single particles of boron-based composite metal fuels. Chin. J. Explos. Propellants 2022, 45, 862–869. [Google Scholar]
- Pang, W.-Q. New progressin the research of boron based composite energetic fuels. Chin. J. Explos. Propellants 2023, 46, 85–88. [Google Scholar]
- Glotov, O. Screening of metal fuels for use in composite propellants for ramjets. Progress. Arose Pace Sci. 2023, 143, 100954. [Google Scholar] [CrossRef]
- Obuchi, K.; Tanabe, M.; Kuwahara, T. Ignition characteristics of boron particles in the secondary combustor of ducted rockets, effects of magnalium particle addition. In Proceedings of the 46th AIAA Aerospace Sciences Meeting, Reno, NV, USA, 7–10 January 2008; pp. 7–10. [Google Scholar]
- Liu, J.-Z.; Xi, J.-F.; Wei, J.-Y.; Hu, Y.-R.; Zhang, Y.-W.; Wang, Y.; Zhou, J.-H. Effect of magnesium on the burning characteristics of boron particles. Acta Astronaut. 2014, 96, 89–96. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, W.; Zhou, X.; Deng, L. Study on Thermal Oxidation Characteristics of Magnesium Diboride. J. Inorg. Mater. 2019, 34, 873–878. [Google Scholar]
- Wu, X.-G.; Yan, Q.-L.; Guo, X.; He, G.; Pan, L. Combustion efficiency and pyrochemical properties of micron-sized metal particles as the components of modified double-base propellant. Acta Astronaut. 2010, 68, 296–304. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, D.-Y.; Zhu, H.; Zhang, W. Energy analysis of boron magnesium high energy oxygen rich propellant. Promot. Technol. 1998, 19, 78–81. [Google Scholar]
- Bondarchuk, S.; Matveev, A.; Promakhov, V.; Vorozhtsov, A.B.; Zhukov, A.S.; Zhukov, I.A. Synthesis and Properties of Energetics Metal Borides for Hybrid Solid-Propellant Rocket Engines; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 511–519. [Google Scholar]
- Matthew, T.F.; Brian, L.C.; Albert, E. Exploring the Effects of Reaction Conditions on Morphology and Stability of Sonochemically Generated Ti-Al-B Fuel Powders. Energy Fuels 2020, 34, 11373–11380. [Google Scholar]
- Zhang, G.; Fu, Z.; Wang, Y.; Wang, H.; Wang, W.; Zhang, J.; Lee, S.W.; Niihara, K. Boron-doped mullite derived from single-phase gels. Eur. Ceram. Soc. 2010, 30, 2435–2441. [Google Scholar] [CrossRef]
- Chintersingh, K.L.; Schoenitz, M.; Dreizin, E.L. Oxidation kinetics and combustion of boron particles with modified surface. Combust. Flame 2016, 173, 288–295. [Google Scholar] [CrossRef]
- Yang, H.-T. Preparation and Properties of Highly Active Boron Powder Composites; Materials Science and Engineering, Nanjing University of Technology: Nanjing, China, 2017. [Google Scholar]
- Yang, H.-T.; Xie, W.-X.; Zhao, Y.; Liu, Y.-F. Research and development of activated boron powder. Blasting Mater. 2018, 47, 1–7. [Google Scholar]
- Chintersingh, K.L.; Schoenitz, M.; Dreizir, E.L. Boron doped with iron Preparation and combustion in air. Combust. Flame 2019, 200, 286–295. [Google Scholar] [CrossRef]
- CN105458276A; A Method for Preparing Active Metal Composite Boron Powder. Bgrimm Advanced Materials Science & Technology Co., Ltd.: Beijing, China, 2016.
- Raman, R.K.S.; Gupta, R.; Koch, C.C. Grain growth behaviour and consolidation of ball-milled nanocrystalline Fe-10Cr alloy. Mater. Sci. Eng. A 2008, 494, 253–256. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).