Abstract
Essential oils (EOs) extracted from aromatic or medicinal plants are biodegradable, safe, and regarded as alternatives to chemical pesticides to reduce fungal species attacking different crops. In this study, thirty EOs at 0.5 mg/mL were evaluated for in vitro growth inhibition of the main postharvest fungi, which are Alternaria alternata, Botrytis cinerea, and Penicillium italicum. Cinnamomum verrum EO completely inhibited the mycelial growth of A. alternata and B. cinerea, and Syzygium aromaticum EO completely inhibited the mycelia of A. alternata. B. cinerea mycelial growth was completely inhibited by Gautheria fragrantissima, Cymbopogon nardus, Pelargonium asperum, and Cupressus sempervirens EOs. G. fragrantissima EO inhibited the mycelia growth of P. italicum by 98%. Overall, B. cinerea displayed the highest sensitivity to EOs than P. italicum and A. alternata. G. fragrantissima, C. sempervirens, C. nardus, P. asperum, Mentha piperita, Foeniculum vulgare, C. verrum, and S. aromaticum EOs showed the highest inhibition for these three pathogens. Minimum inhibitory concentrations were lower for C. verrum and S. aromaticum EOs, ranging between 0.31 and 0.45 mg/mL and 0.37 to 0.57 mg/mL, respectively, against the three pathogens. The tested EOs inhibited the in vitro growth of three of the main postharvest fungal pathogens. Further studies are needed to confirm these activities in vivo.
1. Introduction
Food loss and waste are issues of importance to global food security, and according to the Food and Agriculture Organization of the United Nations, 45% of all fruits and vegetables are lost or wasted every year [1]. This waste occurs along the entire food chain (from field to consumer) and needs to be analyzed and monitored due to its impact on the development of the food sector. Contamination of fruit and vegetables by pathogenic microorganisms is a major factor in reducing yields and market quality. The use of fungicides is a common practice as a postharvest treatment to control fruit decay. In recent years, it has been necessary to achieve the United Nations’ Sustainable Development Goals (SDGs) and the Farm to Fork Strategy of the European Green. In addition, fresh fruit loss, waste prevention, and management are included among the 17 SDGs (targets 12.2 and 12.3) within Agenda 2030 for Sustainable Development. Also, the Commission under the Farm to Fork Strategy of the European Green Deal aims to reduce the use of dangerous compounds in agriculture and achieve at least 25% of the EU’s agricultural land under organic farming by 2030. Nowadays, environment-friendly alternatives, such as essential oils, are being developed and tested for antifungal activities against postharvest pathogens. Essential oils (EOs) are a rich source of broad-spectrum antifungal plant-derived metabolites that inhibit both fungal growth and their production of toxic metabolites [2,3,4].
EOs are extracted from different plant species to evaluate the bioactive compounds able to extend the shelf life of packed foods. Some of these EOs that displayed effectiveness in reducing fungal decay are tea seed [5], camellia [6], oregano [7], cinnamon [8], lemongrass [9], sunflower seed [10], Citrus sinensis [11], Ziziphora persica [12], clove [13], and fennel [14]. As a result, the production and consumption of essential oils have expanded over the world in recent years [15]. The total EO content of plants is generally low and rarely exceeds 1%, with the exception of some cases, for example, clove (Syzygium aromaticum) and nutmeg (Myristica fragrans), which could reach 10% [16].
There are limitations to incorporating these EOs, such as their low solubility in water, high volatility, and possible toxicity at high concentrations [17]. Despite these drawbacks, they present numerous positive aspects in food packaging, such as the promotion of food shelf life, inhibition of microbiological deterioration by fungi and bacteria, and delay in fruit ripening [17]. Recently, there has been a great interest in using EOs as possible natural substitutes for conventional synthetic fungicides [18,19]. Plants rich in terpenes, alkaloids, or phenolic compounds with low or no residual effect could act as bioinsecticides, biofungicides, and bioherbicides to replace the classical chemicals and reduce the impact on the environment [20,21].
EO components could act as antifungal agents due to their accumulation in the lipophilic hydrocarbon molecules of the cell lipid bilayer; such action also allows the easier transfer of other EO constituents to the inner part of the cell. Water solubility and lipophilic properties of the EOs may explain their difference in activities [22].
In this study, thirty EOs were analyzed in vitro regarding their efficacy toward three fungal species responsible for different rots on harvested fruits and during cold storage. These fungal species were classified according to their general reaction against thirty EOs extracted from varied plant species before determining the MIC required.
2. Results
2.1. Response of Alternaria alternata towards 30 EOs
Mycelial growth of A. alternata in PDA was determined 21 days post incubation at 10 °C in the dark. The activity of EOs towards A. alternata varied considerably, from zero to high efficacy, as expressed in Figure 1, showing a total lack of efficiency from −11% (H19, Z. officinale) to a total efficacy of 100% (H13, S. aromaticum and H28, C. verrum). Negative values for such an EO indicate that it is without any antifungal activity; on the contrary, it stimulates fungal growth better than the control, thus playing the role of a nutrient favorable to fungal growth. Intermediate reactions were expressed by different EOs. Mycelial growth inhibition by respective EOs can be visualized in Figure 2.
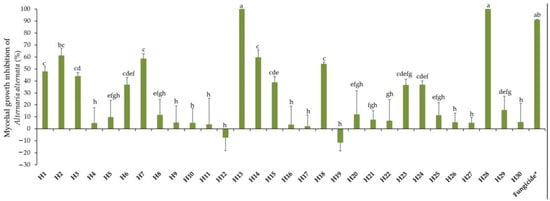
Figure 1.
Mycelial growth inhibition of A. alternata by thirty essential oils used at 0.5 mg/mL. H1, C. martinii; H2, F. vulgare; H3, L. nobilis; H4, M. alternifolia; H5, S. sclarea; H6, C. odoranta; H7, P. cablin; H8, M. quinquenervia; H9, B. carterii; H10, L. angustifolia; H11, C. sinensis; H12, C. paradisii; H13, S. aromaticum; H14, M. piperita; H15, G. fragrantissima; H16, A. dracunculus; H17, C. limon; H18, D. carota; H19, Z. officinale; H20, P. crispum; H21, C. reticulata; H22, C. aurantium bergamia; H23, C. nardus; H24, P. asperum; H25, J. communis; H26, C. nobile; H27, C. atlantica; H28, C. verrum; H29, C. sempervirens; H30, O. basilicum, after 21 days of incubation at 10 °C in the dark. * 25 g/L difenoconazole + 25 g/L fludioxonil. Data are expressed as means ± Standard Error (SE, n = 3). Data with different letters are significantly different (p ≤ 0.05, Fisher’s LSD tests).
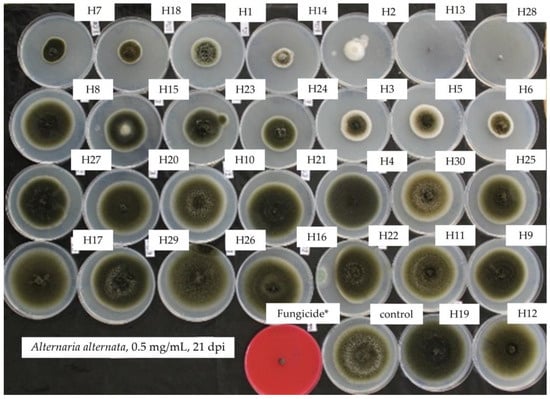
Figure 2.
Mycelial growth inhibition of A. alternata in PDA amended with the essential oils at 0.5 mg/mL showing total inhibition of mycelia (upper) and progressively normal growth (bottom) (red plate amended with difenoconazole + fludioxonil showing total inhibition). H1, C. martinii; H2, F. vulgare; H3, L. nobilis; H4, M. alternifolia; H5, S. sclarea; H6, C. odoranta; H7, P. cablin; H8, M. quinquenervia; H9, B. carterii; H10, L. angustifolia; H11, C. sinensis; H12, C. paradisii; H13, S. aromaticum; H14, M. piperita; H15, G. fragrantissima; H16, A. dracunculus; H17, C. limon; H18, D. carota; H19, Z. officinale; H20, P. crispum; H21, C. reticulata; H22, C. aurantium bergamia; H23, C. nardus; H24, P. asperum; H25, J. communis; H26, C. nobile; H27, C. atlantica; H28, C. verrum; H29, C. sempervirens; H30, O. basilicum, after 21 days of incubation at 10 °C in the dark. * 25 g/L difenoconazole + 25 g/L fludioxonil.
2.2. Response of Botrytis cinerea towards 30 EOs
Mycelial growth of B. cinerea in PDA was rated after 8 days of incubation at 10 °C in the dark. The activity of the EOs towards B. cinerea varied greatly from highly to weakly effective, as represented in Figure 3, showing a progressive efficacy from 22% (H5) to 100% (H28, H23, H29, H24, and H15). Nine (H2, H14, H15, H16, H23, H24, H28, and H29) EOs were effective in controlling this fungus, and there was no significant difference between them and the fungicide. Intermediate reactions were expressed by different EOs. Mycelial growth inhibition by respective EOs from total inhibition to normal growth filling the whole Petri dish can be visualized in Figure 4.
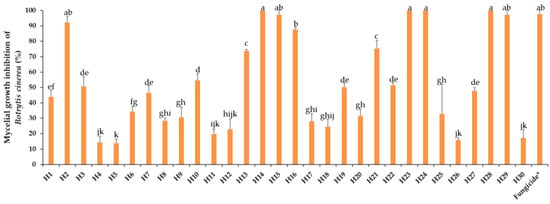
Figure 3.
Mycelial growth inhibition of B. cinerea by the thirty essential oils tested at 0.5 mg/mL. H1, C. martinii; H2, F. vulgare; H3, L. nobilis; H4, M. alternifolia; H5, S. sclarea; H6, C. odoranta; H7, P. cablin; H8, M. quinquenervia; H9, B. carterii; H10, L. angustifolia; H11, C. sinensis; H12, C. paradisii; H13, S. aromaticum; H14, M. piperita; H15, G. fragrantissima; H16, A. dracunculus; H17, C. limon; H18, D. carota; H19, Z. officinale; H20, P. crispum; H21, C. reticulata; H22, C. aurantium bergamia; H23, C. nardus; H24, P. asperum; H25, J. communis; H26, C. nobile; H27, C. atlantica; H28, C. verrum; H29, C. sempervirens; H30, O. basilicum, after 8 days of incubation at 10 °C in the dark. * 25 g/L difenoconazole + 25 g/L fludioxonil. Data are expressed as means ± SE (n = 3). Data with different letters are significantly different (p ≤ 0.05, Fisher’s LSD tests).
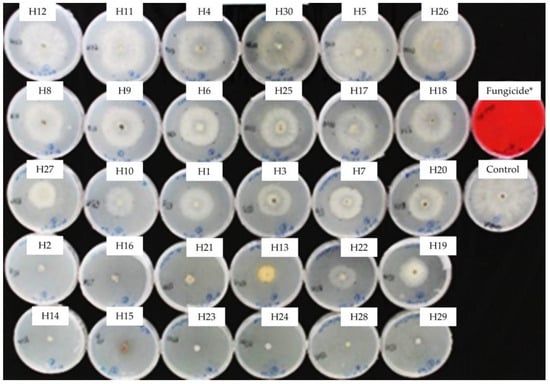
Figure 4.
Mycelial growth inhibition of B. cinerea in PDA amended with the essential oils used at 0.5 mg/mL. H1, C. martinii; H2, F. vulgare; H3, L. nobilis; H4, M. alternifolia; H5, S. sclarea; H6, C. odoranta; H7, P. cablin; H8, M. quinquenervia; H9, B. carterii; H10, L. angustifolia; H11, C. sinensis; H12, C. paradisii; H13, S. aromaticum; H14, M. piperita; H15, G. fragrantissima; H16, A. dracunculus; H17, C. limon; H18, D. carota; H19, Z. officinale; H20, P. crispum; H21, C. reticulata; H22, C. aurantium bergamia; H23, C. nardus; H24, P. asperum; H25, J. communis; H26, C. nobile; H27, C. atlantica; H28, C. verrum; H29, C. sempervirens; H30, O. basilicum, after 8 days of incubation at 10 °C in the dark. * 25 g/L difenoconazole + 25 g/L fludioxonil.
2.3. Response of Penicillium italicum towards 30 EOs
Mycelial growth of P. italicum in PDA was assessed 23 days post incubation at 10 °C in the dark. The activity of the EOs towards P. italicum varied greatly from lack of efficiency to highly effective, as expressed in Figure 5, showing a total lack of efficiency from −1.9% (H4) to 98% (H15). No significant differences were registered between the fungicide and the EOs H1, H2, H13, H15, H23, H28, and H29. Intermediate reactions were expressed by different EOs. Mycelial growth inhibition by respective EOs can be visualized in Figure 6.
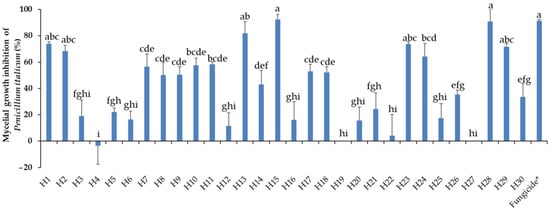
Figure 5.
Mycelial growth inhibition of P. italicum by the thirty essential oils used at 0.5 mg/mL. H1, C. martinii; H2, F. vulgare; H3, L. nobilis; H4, M. alternifolia; H5, S. sclarea; H6, C. odoranta; H7, P. cablin; H8, M. quinquenervia; H9, B. carterii; H10, L. angustifolia; H11, C. sinensis; H12, C. paradisii; H13, S. aromaticum; H14, M. piperita; H15, G. fragrantissima; H16, A. dracunculus; H17, C. limon; H18, D. carota; H19, Z. officinale; H20, P. crispum; H21, C. reticulata; H22, C. aurantium bergamia; H23, C. nardus; H24, P. asperum; H25, J. communis; H26, C. nobile; H27, C. atlantica; H28, C. verrum; H29, C. sempervirens; H30, O. basilicum, after 23 days of incubation at 10 °C in the dark. * 25 g/L difenoconazole + 25 g/L fludioxonil. Data are expressed as means ± SE (n = 3). Data with different letters are significantly different (p ≤ 0.05, Fisher’s LSD tests).
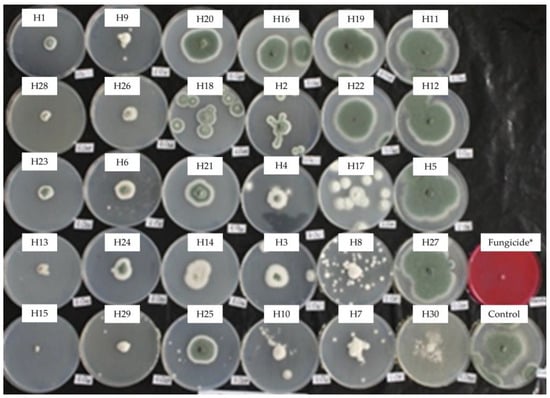
Figure 6.
Mycelial growth inhibition of P. italicumi in PDA amended with the essential oils at 0.5 mg/mL. H1, C. martinii; H2, F. vulgare; H3, L. nobilis; H4, M. alternifolia; H5, S. sclarea; H6, C. odoranta; H7, P. cablin; H8, M. quinquenervia; H9, B. carterii; H10, L. angustifolia; H11, C. sinensis; H12, C. paradisii; H13, S. aromaticum; H14, M. piperita; H15, G. fragrantissima; H16, A. dracunculus; H17, C. limon; H18, D. carota; H19, Z. officinale; H20, P. crispum; H21, C. reticulata; H22, C. aurantium bergamia; H23, C. nardus; H24, P. asperum; H25, J. communis; H26, C. nobile; H27, C. atlantica; H28, C. verrum; H29, C. sempervirens; H30, O. basilicum, after 23 days of incubation at 10 °C in the dark. * 25 g/L difenoconazole + 25 g/L fludioxonil.
2.4. Comparison between the Essential Oil Inhibitory Activity and between Fungi Tolerance
The antifungal activity heat map of 30 EOs toward B. cinerea, P. italicum, and A. alternata is summarized in Figure 7. The heat map showed valuable information concerning the interaction between fungal species and the EOs. There are two clusters for the fungal species, one for B. cinerea and another for A. alternanta and P. italicum, having a similar mycelial growth inhibition pattern. The EOs of G. fragrantissima (H15), C. sempervirens (H29), C. nardus (H23), P. asperum (H24), M. piperita (H14), F. vulgare (H2), C. verrum (H28), and S. aromaticum (H13) were grouped in a separate cluster exerting a higher inhibition to B. cinerea compared to A. alternata and P. italicum that were less sensitive to the same EOs. This cluster also includes the fungicide. On the other hand, the EOs of C. paradisii (H12), M. alternifolia (H4), S. sclarea (H5), C. nobile (H26), O. basilicum (H30), J. communis (H25), P. crispum (H20), C. odoranta (H6), and L. nobilis (H3) were distinct in another cluster characterized by a level of inhibition of the three fungi lower than 50%.
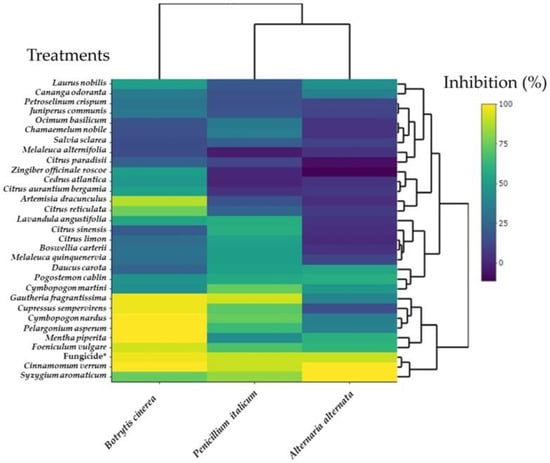
Figure 7.
Heat map of the mycelial growth inhibition (%) of B. cinerea, P. italicum, and A. alternata by thirty essential oils at 8–23 days post-inoculation. Positive control is fungicide *: 25 g/L difenoconazole + 25 g/L fludioxonil.
2.5. Minimum Inhibitory Concentration
Different EO concentrations (0, 0.05, 0.1, 0.2, 0.3, and 0.4 mg/mL) were tested on six EOs selected based on two arbitrary criteria: (i) average mycelial growth inhibition of at least 78% towards the 30 EOs at 0.5 mg/mL, and (ii) inhibition of mycelial growth of at least 98% towards at least one out of the three fungal species. According to these criteria, the selected six EOs were Foeniculum vulgare (H2), Syzygium aromaticum (H13), Gautheria fragrantissima (H15), Cymbopogon nardus (H23), Pelargonium asperum (H24), and Cinnamomum verrum (H28) that were used to determine by linear regression the lowest concentration (MIC analysis) that exerted a total mycelial growth inhibition of each fungal species.
Linear regression was determined by relating mycelial growth inhibitions compared to the control to the corresponding concentration (0.05, 0.1, 0.2, 0.3, and 0.4 mg/mL) for every EO toward A. alternata (Table 1), B. cinerea (Table 2), and P. italicum (Table 3). Linear regression was performed for each pathogen. For P. italicum, linear regression was done only with the most effective EOs, which were Cinnamomum verrum (H28) and Syzygium aromaticum (H13).

Table 1.
Mycelial growth inhibition of A. alternata by the six essential oils from Foeniculum vulgare (H2), Syzygium aromaticum (H13), Gautheria fragrantissima (H15), Cymbopogon nardus (H23), Pelargonium asperum (H24), and Cinnamomum verrum (H28) after 21 days of incubation at 10 °C in the dark.

Table 2.
Mycelial growth inhibition of Botrytis cinerea by the six essential oils from Foeniculum vulgare (H2), Syzygium aromaticum (H13), Gautheria fragrantissima (H15), Cymbopogon nardus (H23), Pelargonium asperum (H24), and Cinnamomum verrum (H28) after 21 days of incubation at 10 °C in the dark.

Table 3.
Mycelial growth inhibition of Penicillium italicum by the two essential oils of Syzygium aromaticum (H13) and Cinnamomum verrum (H28) after 23 days of incubation at 10 °C in the dark.
Table 4 summarizes the equations of linear regression of every EO by fungal species. The coefficient of determination R2 of each equation was, in most cases, high, ranging between 0.96 and 0.62, except in three cases that were 0.27, 0.43, and 0.48. It is possible to determine the MIC using these equations by assigning the value 100 to y (y = 100) in order to find the value of x, which is the MIC. The results of the analysis are shown in Figure 8.

Table 4.
The equation of linear regression and the corresponding determination coefficient R2 determined for every fungal species and essential oil.
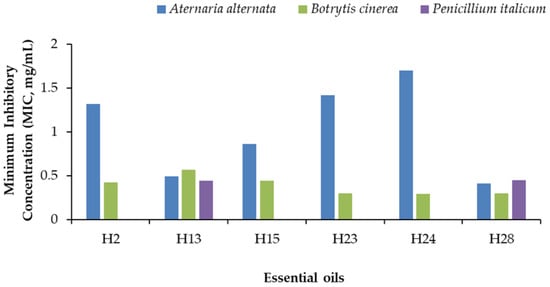
Figure 8.
Minimum Inhibitory Concentration (MIC) of six essential oils against A. alternata, B. cinerea, and P. italicum. H2, F. vulgare; H13, S. aromaticum; H15, G. fragrantissima; H23, C. nardus; H24, P. asperum; H28, C. verrum.
The EOs of H28 and H13 showed a broad spectrum of antifungal action since they completely inhibited mycelial growth of A. alternata, B. cinerea, and P. italicum at low concentrations (MIC) ranging between 0.31 and 0.45 mg/mL for H28 and between 0.37 and 0.57 mg/mL for H13. The low MIC values of these six EOs were registered against B. cinerea, ranging between 0.29 and 0.44 mg/mL. In comparison, the highest MIC values were towards A. alternata (0.85–1.71 mg/mL).
3. Discussion
In vitro analysis is the first step in a program aimed at studying a wide range of biological resources in order to identify the most bioactive products and to determine their working concentration range. This is particularly important for EOs since low concentrations can be ineffective, and high concentrations may result in phytotoxic or leave residues on treated plants [23]. Mani-Lopez et al. [24] pointed out that the antifungal activity of EOs must be demonstrated before undertaking analyses on target sites or mechanisms of action. This is true because, as far as we know, the activity of EOs varied greatly from highly effective to ineffective and from a general to a specific efficacy according to the target pathogen.
The use of 0.5 mg/mL was an appropriate dose to perform the right classification of the 30 EOs; such results were subsequently specified by MIC analyses. Our results were consistent concerning the effectiveness of cinnamon bark EO, which was a powerful product in reducing fungal growth at low doses (0.31–0.45 mg/mL) irrespective of the fungal species. Similarly, clove buds EO has also proven to be effective (0.37–0.57 mg/mL), whatever the fungal species, with an advantage of 0.06–0.11 mg/mL in favor of cinnamon bark EO. These two EOs are of particular interest for further studies on their use as a biological disinfectant, e.g., as a biological ingredient in a formulation suitable for post-harvest fruit coating.
In vitro studies by Sukatta et al. [25] using inverted Petri plates showed that the antifungal activity of clove and cinnamon volatile oils against six fungi caused the postharvest decay of grapes. An inverted Petri plate means the mycelium of the fungus is placed on the center of the Petri dish containing a nutrient medium such as PDA, and the oil pipetted on a filter paper is kept on the coverlid of the same Petri dish. They reported the MICs for the six fungi ranged from 200 to 800 mg mL−1 using clove bud EO and 50 to 800 mg mL−1 using cinnamon bark EO. This is in accordance with our results that cinnamon oil is slightly more effective against fungi than clove oil. Parker et al. [26] tested the antifungal activities of 21 EOs against human fungal pathogens such as Candida auris. They found the most effective EOs able to inhibit and kill the fungi were those of lemongrass, clove bud, and cinnamon bark when in direct contact and at concentrations safe for topical use.
In terms of chemical composition and according to the supplier of these EOs, E-cinnamaldehyde and cinnamyl acetate for cinnamon bark, eugenol and eugenyl acetate for clove buds are the major components of the respective EOs. This suggests that these oil constituents (E-cinnamaldehyde and cinnamyl acetate for cinnamon bark, eugenol and eugenyl acetate for clove buds) are owed to the fair effectiveness against the fungi tested in this research. The antifungal activities of cinnamon leaf EO were studied in vitro against wood-decay fungi in Taiwan. This study showed that this EO and its main component, cinnamaldehyde, exhibited strong antifungal activity at 50–100 ppm [27]. Our results and those of published works [28,29,30,31] agree that EOs from cinnamon bark or from clove bud possess a broad-spectrum antifungal activity.
Although from different plant species and harvested from different countries, the EOs of the aerial part of Ceylon lemongrass (H23) and that of geranium leaves (H24) unexpectedly showed similar patterns among the four fungal species. Thus, mycelial growth inhibition at 500 ppm was (99, 100, 81, 39%) and (100, 100, 71, 39%), respectively, for the four fungal species treated either with Ceylon lemongrass or with geranium leaves EOs (Table 2). Close chemical components of their respective EOs (citronellol and geraniol as major constituents) could explain this similarity of effectiveness characterized by a high activity against B. cinerea, a moderate activity towards P. italicum, but a low activity against A. alternata.
Methyl salicylate, a volatile form of salicylic acid responsible for increased membrane permeability, is the result of salicylic acid’s methylation. It can play an important role in systemic acquired resistance signaling and defense against pests, microbial pathogens, and antagonists [32,33]. Fragrant wintergreen leaves EO (H15), whose methyl salicylate is the major component, exhibited in our experiments high activity against the fungal species except for A. alternata, to which it was relatively tolerant at the concentration used. In another study, the fruit essential oil of Alchornea cordifolia, rich in methyl salicylate and citronellol, showed antifungal activity against Aspergillus niger and antibacterial activity against Staphylococcus aureus [34]. In addition to its antifungal activity, methyl salicylate was reported to increase the health-promoting properties of cherry fruit consumption with a positive effect on delaying the fruit postharvest senescence process [35]. Therefore, the EO of fragrant wintergreen leaves can play a double role in postharvest foodstuffs as an antifungal and an anti-senescence.
The essential oil of grapefruit peel (H12), whose main compound is limonene, showed the lowest efficacy against the four fungal species, in particular against A. alternata (−7%) and P. italicum (16%). In addition, the essential oils containing limonene, linalool, linalyl acetate, terpineol, or terpinene were the least effective, especially against A. alternata (1% to 14%) and to a lesser extent against P. italicum (−2% to 59%) or B. cinerea (22% to 83%). The EOs rich in limonene are from tea tree leaves (H4), the flowering top of clary sage (H5), sweet orange zest (H11), lemon zest (H17), fruit of black pepper (H20), mandarin zest (H21), and bergamot zest (H22). Thus, it seems that limonene has limited antifungal activity contrary to what has been observed with E-cinnamaldehyde or eugenol. However, Hao et al. [36] suggested that D-limonene has the potential to prevent the foodborne opportunistic yeast Candida tropicalis contamination in the food industry based on the observed disruption and alteration of cell wall integrity. Nevertheless, Kishore et al. [28] tested the effectiveness of five EO compounds (citral, eugenol, geraniol, limonene, and linalool) together with clove and cinnamon EOs for growth inhibition of 14 fungi using paper disc agar diffusion. They pointed out that limonene and linalool showed the least antifungal activity, which led to the removal of them from further tests. Such a result is in accordance with our finding that limonene and EOs rich in limonene (mainly those from citrus) are not advisable to consider as appropriate antifungal control agents because of their narrow spectrum antifungal activity.
Furthermore, our results allowed the issuing of a global classification concerning the degree of sensitivity of the fungal species by taking into account their reaction toward the totality of the EOs. B. cinerea was the most sensitive fungal species among the tested and, therefore, easier to control, especially when it is on apparent tissues such as those of post-harvest fruits. This pathogen is a serious problem for stored foodstuffs, even in cold storage. Several essential oils have shown high efficacy against it, which indicates the relative ease with which this pathogen could be controlled. Cinnamomum zeylanicum EO in the vapor phase was tested in vitro against B. cinerea, Monilinia fructicola, Monilinia fructigena, and Monilinia laxa. Based on mycelial growth inhibition, the volatiles of C. zeylanicum EO consistently showed higher inhibition against B. cinerea, suggesting a specific mode of action [37]. Vapor phase and direct contact via growth media of EOs could act differently against postharvest fungi [37,38], but this hypothesis needs confirmation. On the other hand, A. alternata and, to a lesser extent, P. italicum appear to have a greater ability to tolerate the various antifungal treatments. This property should be taken into account when a control strategy is planned.
Because of the tolerance observed in Alternaria spp. populations to different fungicides (QoIs, benzimidazole, fludioxonil, cyprodinil, boscalid, and pyraclostrobin) [39,40,41], it was suggested the need to study Alternaria isolates not previously exposed to fungicides to have a better estimate of the shifts in sensitivity [42]. Here, we have used different sources of EOs not previously applied as fungicides on A. alternata, and yet this tolerance was well observed. This tolerance could be due to an innate mechanism in this species (rigid cell wall, for example) allowing protection against adverse conditions. Furthermore, unlike some other fungal species, Alternaria spp. appear to be able to grow on host plants despite an environment characterized by water or thermal stress. Thus, Fagodiya et al. [42] emphasized that Alternaria blight severity in soybean fields increased with increasing temperature (19.8–32.5 °C) and sunshine hours, decreasing rainfall and relative humidity. Any agronomic factor that increases stress on the crop can act as a precursor for Alternaria infection, as well as the severity of the outbreak. Thus, there is evidence that Aternaria spp. possesses factors that allow it to withstand adverse conditions, including antifungal treatments, even before induction of mutation in response to any stress.
Penicillium spp. has also been relatively tolerant to many EOs. It appears that it uses a different mechanism than Alternaria, allowing it a moderate ability to tolerate an adverse environment such as antifungal compounds. Oiki et al. [43] tested Penicillium strains with no history of fungicide exposure, collected from various environmental and clinical sources, and despite this, they found fungicide-resistant strains, questioning where and how these Penicillium strains became resistant. The same situation arises in our analyses, suggesting that innate genetic variability in fungal species has occurred naturally without being related to a response to synthetic fungicide pressure.
4. Materials and Methods
4.1. Origin of the Essential Oils
Thirty EOs were offered for sale by a local company named STDCE (Société Tunisienne de Distribution et de Commerce Electronique, sarl) located at Megrine in the vicinity of Tunis, Tunisia. These EOs are packed in a dark glass bottle of 10 mL, which is marked with the logo ‘Aroma Vegetal’. Some oils were chosen without any prior information on their efficacy, while others were selected based on our recent results [2,44]. The chosen EOs are listed in Table 5, together with information given by the supplier about plant species, vegetal part of oil extraction, major chemical components, and the provenience of plant products.

Table 5.
Details of the thirty essential oils used in this study, including the main volatile constituents defined according to the supplier.
4.2. Fungal Species
The fungal species A. alternata, B. cinerea, and P. italicum used in the present study were isolated from infected fruits (strawberry, peach, apricot, plum, nectarine, and orange) collected in cold storage. Pure cultures were transplanted into Petri dishes (diameter, 90 mm) with potato dextrose agar (PDA), then morphologically identified in the laboratory and conserved in PDA at 10 °C until use. These fungal species are considered the most damaging agents causing fruit decay during storage in Tunisia.
4.3. In Vitro Antifungal Activities on Mycelial Growth
The antifungal activities of EOs (Table 1) were assessed according to their contact phase effects on the mycelial growth of A. alternata, B. cinerea, and P. italicum. The EOs were dissolved in sterilized distilled water with 0.1% (v/v) Tween 20 (Sigma Aldrich, Steinheim, Germany) to obtain homogeneous emulsions, according to Moumni et al. [2,44]. The negative control was PDA containing 0.1% (v/v) Tween 20. The positive control was based on the fungicides as 25 g/L difenoconazole plus 25 g/L fludioxonil (Celest Extra 50 FS (CE); Syngenta, Cambridge, UK). The PDA was mixed and poured immediately into Petri dishes (diameter, 90 mm; 20 mL/plate), and after medium solidification, each plate was inoculated under aseptic conditions with a 6 mm plug, taken from the edge of actively growing cultures from each of the fungal species. The inoculated plates were sealed with Parafilm and incubated at 10 ± 2 °C in the dark for 7 to 23 days, depending on the speed of mycelial growth in the negative control at 10 °C of the fungal species. The orthogonal diameters of the colonies were measured when the negative control plates were completely covered by the mycelia.
A first experiment aimed to reveal the most effective EOs against each of the three fungal species was performed at a concentration of 0.5 mg/mL using the 30 EOs and the fungicide. After analysis, the retained EOs were used in a second experience by applying the EOs concentrations 0 (control, PDA), 0.05, 0.1, 0.2, 0.3, and 0.4 mg/mL in order to determine, by linear regression, the minimum concentration allowing 100% mycelial growth inhibition (MIC, minimum inhibitory concentration). The method used to dissolve the oils is the same as described above.
The experiments were carried out as three replicates per fungal species and treatment. Mycelial growth inhibition was calculated based on the Equation (1) [45]:
where dc and dt represent the mean diameter of the mycelial growth of the control and treated fungal species, respectively. The MIC for each selected EO and fungal species was determined based on the linear regression between EO concentrations (x) and respective mycelial growth inhibition (y = ax + b), considering (y) is equal to zero and the (x) is to be found out by this equation, which is the value of the MIC (mg/mL).
Mycelial growth inhibition (%) = [1 − dt/dc] × 100
4.4. Statistical Analysis
For the first set of experiments using 30 EOs, each experimental unit was represented by one EO (concentration 0.5 mg/mL) and one fungal species repeated 3 times, knowing that the protocol was completely randomized and was composed of 30 EOs and 3 fungal species. For the second set of experiments concerning the MIC, also completely randomized, each experimental unit was constituted by one EO, one fungal species, and one EO concentration. The protocol included 6 EOs at 6 concentrations each and 3 fungal species. Excel (version 2016) was used to determine the average, standard errors, and linear regression. All of the trials were repeated at least twice, and data are means ± standard error (SE) presented as histograms or linear regression equations. Analysis of variance was calculated using SPSS (version 20; IBM, Armonk, NY, USA). The data for inhibition of mycelial growth underwent analysis of variance (ANOVA). Means were compared using Fisher’s tests for protected least significant difference (LSD) at p < 0.05. Package ‘heatmaply’ was used to prepare the heat map figure with RStudio version 2023.03.0+386.
5. Conclusions
The present study has demonstrated the in vitro activities of thirty EOs and their effectiveness against the three important fungal pathogens: A. alternata, B. cinerea, and P. italicum. C. verrum and S. aromaticum EOs at 0.5 mg/mL presented the highest inhibitory activity for the three pathogens. These two EOs are promising biological candidates to be included in our next program as essential preservative ingredients in the coating formulation for postharvest fruits during storage to maintain the quality and extend shelf life.
Author Contributions
M.B.A., conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, funding acquisition; M.M., conceptualization, software, validation, writing—review and editing, visualization; G.R., validation, writing—review and editing, visualization, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
Ministry of Higher Education and Scientific Research of Tunisia funded this research in the framework of PRIMA, project acronym StopMedWaste projects ‘Innovative Sustainable technologies TO extend the shelf-life of Perishable MEDiterranean fresh fruit, vegetables and aromatic plants and to reduce WASTE’, Project Number 1556. EUPHRESCO Basic substances as an environmentally friendly alternative to synthetic pesticides for plant protection (BasicS)” project (Objective 2020-C-353).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- FAO Fruit and Vegetables–Your Dietary Essentials. Available online: https://www.fao.org/documents/card/en/c/cb2395en (accessed on 16 September 2023).
- Moumni, M.; Romanazzi, G.; Najar, B.; Pistelli, L.; Ben Amara, H.; Mezrioui, K.; Karous, O.; Chaieb, I.; Allagui, M.B. Antifungal activity and chemical composition of seven essential oils to control the main seedborne fungi of cucurbits. Antibiotics 2021, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Khalek, H.H.; Hammad, A.A.; El-Kader, R.M.A.; Youssef, K.A.; Abdou, D.A. Combinational inhibitory action of essential oils and gamma irradiation for controlling Aspergillus flavus and Aspergillus parasiticus growth and their aflatoxins biosynthesis in vitro and in situ conditions. Food Sci. Technol. Int. 2022, 28, 703–715. [Google Scholar] [CrossRef]
- Zhou, X.; Zeng, M.; Huang, F.F.; Qin, G.; Song, Z.; Liu, F. The potential role of plant secondary metabolites on antifungal and immunomodulatory effect. Appl. Microbiol. Biotechnol. 2023, 107, 4471–4492. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.T.H.; Koga, A.; Nkede, F.N.; Tanaka, F.; Tanaka, F. Application of edible coatings composed of chitosan and tea seed oil for quality improvement of strawberries and visualization of internal structure changes using X-ray computed tomography. Prog. Org. Coat. 2023, 183, 107730. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Dong, L.; Wen, Q.; Huang, W.; Li, T.; YE, J.; Xu, L.A. Analysis on the character diversity of fruit and seed of Camellia chekiangoleosa. J. Nanjing Norm. Univ. (Nat. Sci. Ed.) 2021, 45, 51. [Google Scholar]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Basaglia, R.R.; Pizato, S.; Santiago, N.G.; de Almeida, M.M.M.; Pinedo, R.A.; Cortez-Vega, W.R. Effect of edible chitosan and cinnamon essential oil coatings on the shelf life of minimally processed pineapple (Smooth cayenne). Food Biosci. 2021, 41, 100966. [Google Scholar] [CrossRef]
- Wani, S.M.; Gull, A.; Ahad, T.; Malik, A.R.; Ganaie, T.A.; Masoodi, F.A.; Gani, A. Effect of gum Arabic, xanthan and carrageenan coatings containing antimicrobial agent on postharvest quality of strawberry: Assessing the physicochemical, enzyme activity and bioactive properties. Int. J. Biol. Macromol. 2021, 183, 2100–2108. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, E.S.; Han, J. Enhancement of the water-resistance properties of an edible film prepared from mung bean starch via the incorporation of sunflower seed oil. Sci. Rep. 2020, 10, 13622. [Google Scholar] [CrossRef]
- Das, S.; Vishakha, K.; Banerjee, S.; Mondal, S.; Ganguli, A. Sodium alginate-based edible coating containing nanoemulsion of Citrus sinensis essential oil eradicates planktonic and sessile cells of food-borne pathogens and increased quality attributes of tomatoes. Int. J. Biol. Macromol. 2020, 162, 1770–1779. [Google Scholar] [CrossRef]
- Hamedi, H.; Kargozari, M.; Shotorbani, P.M.; Mogadam, N.B.; Fahimdanesh, M. A novel bioactive edible coating based on sodium alginate and galbanum gum incorporated with essential oil of Ziziphora persica: The antioxidant and antimicrobial activity, and application in food model. Food Hydrocoll. 2017, 72, 35–46. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Liu, S.; Gao, J.; Cui, S.W.; Xia, W. Coating white shrimp (Litopenaeus vannamei) with edible fully deacetylated chitosan incorporated with clove essential oil and kojic acid improves preservation during cold storage. Int. J. Biol. Macromol. 2020, 162, 1276–1282. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Bhandari, B.; Bai, B. Nanoemulsion-based edible coatings loaded with fennel essential oil/cinnamaldehyde: Characterization, antimicrobial property and advantages in pork meat patties application. Food Control 2021, 127, 108151. [Google Scholar] [CrossRef]
- Sharma, A.; Gumber, K.; Gohain, A.; Bhatia, T.; Sohal, H.S.; Mutreja, V.; Bhardwaj, G. Chapter 3—Importance of essential oils and current trends in use of essential oils (aroma therapy, agrofood, and medicinal usage). In Essential Oils; Ahmad, G., Ansari, N.M.J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 53–83. ISBN 9780323917407. [Google Scholar] [CrossRef]
- Khanam, M.; Dar, A.H.; Beg, F.; Khan, S.A.; Nayik, G.A.; Karabagias, I.K. Nutmeg essential oil. In Essential Oils; Academic Press: Cambridge, MA, USA, 2023; pp. 391–399. [Google Scholar]
- Oliveira Filho, J.G.D.; Duarte, L.G.; Silva, Y.B.; Milan, E.P.; Santos, H.V.; Moura, T.C.; Bandini, V.P.; Vitolano, L.E.S.; Nobre, J.J.; Moreira, C.T.; et al. Novel approach for improving papaya fruit storage with carnauba wax nanoemulsion in combination with Syzigium aromaticum and Mentha spicata essential oils. Coatings 2023, 13, 847. [Google Scholar] [CrossRef]
- Magri, A.; Curci, M.; Battaglia, V.; Fiorentino, A.; Petriccione, M. Essential oils in postharvest treatment against microbial spoilage of the rosaceae family fruits. AppliedChem 2023, 3, 196–216. [Google Scholar] [CrossRef]
- Rahman, M.M.; Wills, R.B.; Bowyer, M.C.; Golding, J.B.; Kirkman, T.; Pristijono, P. Potential control of postharvest fungal decay of citrus fruits by crude or photochemically changed essential oils—A review. Food Rev. Int. 2023, 1–18. [Google Scholar] [CrossRef]
- Fragkouli, R.; Antonopoulou, M.; Asimakis, E.; Spyrou, A.; Kosma, C.; Zotos, A.; Tsiamis, G.; Patakas, A.; Triantafyllidis, V. Mediterranean plants as potential source of biopesticides: An overview of current research and future trends. Metabolites 2023, 13, 967. [Google Scholar] [CrossRef]
- Javaid, A.; Ali, A.; Khan, I.H.; Ferdosi, M.F. Leaves of Chenopodium album as source of natural fungicides against Sclertium rolfsii. Arab. J. Chem. 2023, 16, 104677. [Google Scholar] [CrossRef]
- Assadpour, E.; Can Karaça, A.; Fasamanesh, M.; Mahdavi, S.A.; Shariat-Alavi, M.; Feng, J.; Kharazmi, M.S.; Rehman, A.; Jafari, S.M. Application of essential oils as natural biopesticides; recent advances. Crit. Rev. Food Sci. Nutr. 2023, 1–21. [Google Scholar] [CrossRef]
- Servili, A.; Feliziani, E.; Romanazzi, G. Exposure to volatiles of essential oils alone or under hypobaric treatment to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2017, 133, 36–40. [Google Scholar] [CrossRef]
- Mani-López, E.; Cortés-Zavaleta, O.; López-Malo, A. A review of the methods used to determine the target site or the mechanism of action of essential oils and their components against fungi. SN Appl. Sci. 2021, 3, 44. [Google Scholar] [CrossRef]
- Sukatta, U.; Haruthaithanasan, V.; Chantarapanont, W.; Dilokkunanant, U.; Suppakul, P. Antifungal activity of clove and cinnamon oil and their synergistic against postharvest decay fungi of grape in vitro. Kasetsart J. (Nat. Sci.) 2008, 42, 169–174. [Google Scholar]
- Parker, R.A.; Gabriel, K.T.; Graham, K.D.; Butts, B.K.; Cornelison, C.T. Antifungal activity of select essential oils against Candida auris and their interactions with antifungal drugs. Pathogens 2022, 11, 821. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, P.F.; Chang, S.T. Antifungal activities of essential oils and their constituents from indigenous cinnamon (Cinnamomum osmophloeum) leaves against wood decay fungi. Bioresour. Technol. 2005, 96, 813–818. [Google Scholar] [CrossRef]
- Kishore, G.K.; Pande, S.; Harish, S. Evaluation of essential oils and their components for broad-spectrum antifungal activity and control of late leaf spot and crown rot diseases in peanut. Plant Dis. 2007, 91, 375–379. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. The extracts of cinnamon and clove as potential biofungicides against strawberry grey mould. Plants 2020, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Shahina, Z.; Molaeitabari, A.; Sultana, T.; Dahms, T.E.S. Cinnamon leaf and clove essential oils are potent inhibitors of Candida albicans virulence traits. Microorganisms 2022, 10, 1989. [Google Scholar] [CrossRef]
- Al-Garadi, M.; Qaid, M.; Alqhtani, A.; Alhajj, M.; Al-abdullatif, A.; Al-Mufarrej, S. In vitro antimicrobial efficacy assessment of ethanolic and aqueous extracts of cinnamon (Cinnamomum verum) bark against selected microbes. Braz. J. Poult. Sci. 2023, 25, 1–15, eRBCA-2022-1682. [Google Scholar] [CrossRef]
- Singewar, K.; Fladung, M.; Robischon, M. Methyl salicylate as a signaling compound that contributes to forest ecosystem stability. Trees 2021, 35, 1755–1769. [Google Scholar] [CrossRef]
- Snoeren, T.A.L.; Mumm, R.; Poelman, E.H.; Yang, Y.; Pichersky, E.; Dicke, M. The herbivore-induced plant volatile methyl salicylate negatively affects attraction of the parasitoid Diadegma semiclausum. J. Chem. Ecol. 2010, 36, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Essien, E.E.; Newby, J.S.; Walker, T.M.; Setzer, W.N.; Ekundayo, O. Characterization and antimicrobial activity of volatile constituents from fresh fruits of Alchornea cordifolia and Canthium subcordatum. Medicines 2016, 3, 1. [Google Scholar] [CrossRef]
- Valverde, J.M.; Giménez, M.J.; Guillén, F.; Valero, D.; Martínez-Romero, D.; Serrano, M. Methyl salicylate treatments of sweet cherry trees increase antioxidant systems in fruit at harvest and during storage. Postharvest Biol. Technol. 2015, 109, 106–113. [Google Scholar] [CrossRef]
- Hao, Y.; Lin, Z.X.; Xiang, W.L.; Huang, M.; Tang, J.; Lu, Y.; Zhao, Q.H.; Zhang, Q.; Rao, Y.; Liu, L. Antifungal activity and mechanism of D-limonene against foodborne opportunistic pathogen Candida tropicalis. LWT-Food Sci. Technol. 2022, 159, 113144. [Google Scholar]
- Álvarez-García, S.; Moumni, M.; Romanazzi, G. Antifungal activity of volatile organic compounds from essential oils against the postharvest pathogens Botrytis cinerea, Monilinia fructicola, Monilinia fructigena, and Monilinia laxa. Front. Plant Sci. 2023, 14, 1274770. [Google Scholar] [CrossRef]
- Santoro, K.; Maghenzani, M.; Chiabrando, V.; Bosio, P.; Gullino, M.L.; Spadaro, D.; Giacalone, G. Thyme and savory essential oil vapor treatments control brown rot and improve the storage quality of peaches and nectarines, but could favor gray mold. Foods 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Iacomi-Vasilescu, B.; Avenot, H.; Bataille-Simoneau, N.; Laurent, E.; Guénard, M.; Simoneau, P. In vitro fungicide sensitivity of Alternaria species pathogenic to crucifers and identification of Alternaria brassicicola field isolates highly resistant to both dicarboximides and phenylpyrroles. Crop Prot. 2004, 23, 481–488. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Detection of isolates of Alternaria alternata with multiple-resistance to fludioxonil, cyprodinil, boscalid and pyraclostrobin in California pistachio orchards. Crop Prot. 2015, 78, 214–221. [Google Scholar] [CrossRef]
- Rosenzweig, N.; Hanson, L.E.; Mambetova, S.; Jiang, Q.W.; Guza, C.; Stewart, J.; Somohano, P. Fungicide sensitivity monitoring of Alternaria spp. causing leaf spot of sugarbeet (Beta vulgaris) in the Upper Great Lakes. Plant Dis. 2019, 103, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Fagodiya, R.K.; Trivedi, A.; Fagodia, B.L. Impact of weather parameters on Alternaria leaf spot of soybean incited by Alternaria alternata. Sci. Rep. 2022, 12, 6131. [Google Scholar] [CrossRef] [PubMed]
- Oiki, S.; Yaguchi, T.; Urayama, S.I.; Hagiwara, D. Wide distribution of resistance to the fungicides fludioxonil and iprodione in Penicillium species. PLoS ONE 2022, 17, e0262521. [Google Scholar] [CrossRef]
- Moumni, M.; Allagui, M.B.; Mezrioui, K.; Ben Amara, H.; Romanazzi, G. Evaluation of seven essential oils as seed treatments against seedborne fungal pathogens of Cucurbita maxima. Molecules 2021, 26, 2354. [Google Scholar] [CrossRef] [PubMed]
- Bekker, T.F.; Kaiser, C.; vd Merwe, R.; Labuschagne, N. In-Vitro inhibition of mycelial growth of several phytopathogenic fungi by soluble potassium silicate. S. Afr. J. Plant Soil 2006, 23, 169–172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).