Abstract
Antimicrobial resistance (AMR) is a serious threat to public health due to the lack of effective drugs to combat infectious diseases, which generates the need to search for new antimicrobial substances. In this study, the potential of soil as a source of antimicrobial-producing bacteria (APB) was investigated and the importance of the connection between education and science was emphasized, using service-learning methodologies. Sixty-one soil samples were collected, and 1220 bacterial isolates were recovered. Eighteen of these isolates showed antimicrobial activity against at least 1 of the 12 indicator bacteria tested (including multidrug-resistant and relevant pathogens). The 18 APB were identified by MALDI-TOF and 6 different genera (Bacillus, Brevibacillus, Lysinobacillus, Peribacillus, Streptomyces, and Advenella) and 10 species were identified. The 18 APB were tested for antifungal activity against four phytopathogenic fungi (Botritis cynerea, Lecanicillium fungicola, Trichoderma harzianum, and Cladobotryum mycophilum). Moreover, the antibiotic susceptibility of APB was tested using the disk-diffusion method as well as their β-hemolytic activity (important safety criteria for potential future applications). A total of 10 of the 18 APB were able to inhibit at least 50% of indicator bacteria tested, including methicillin-resistant Staphylococcus aureus (MRSA), among others. A total of 4 of the 18 APB (3 Bacillus pumilus and 1 Bacillus altitudinis) showed inhibitory activity against two of the four fungal pathogens tested (B. cinerea and L. fungicola), as well as against 5–7 of the 12 bacterial pathogen indicators; these 4 isolates showed susceptibility to the antibiotics tested and lacked β-hemolytic activity and were considered promising APB for use as potential biocontrol agents. In addition, one Brevibacillus laterosporus strain had activity against 83% of indicator bacteria tested including Escherichia coli, MRSA and other methicillin-resistant staphylococci, as well as vancomycin-resistant enterococci (but not against fungi). These results show that soil is a source of APB with relevant antibacterial and antifungal activities, and also emphasize the importance of education and science to raise public awareness of the AMR problem and the strategies to control it.
1. Introduction
Antimicrobial resistance (AMR) represents an increasing challenge for global public health due to the lack of effective drugs to combat infectious diseases, leading to the need to look for new antimicrobial substances [1]. The constant movement of microorganisms between plants, animals, and humans is key for maintaining the good health of all organisms within an ecosystem [2,3].
Soils are very important for global health and represent a huge reservoir for the immense diversity of microorganisms on our planet [4,5], and serve as a site for the exchange of substances between these microbial communities and plants [6,7]. The organisms most commonly found in soil are bacteria, which play an important role in these processes of exchange and transformation of substances and materials (decomposition of organic matter, transformation of soil nutrients, and regulation of soil fertility) [8,9,10]. Bacteriocins are bioactive peptides with antimicrobial properties that are ribosomally synthesized by a wide range of bacteria as a defense mechanism against other microorganisms with which they coexist in their ecosystem [11,12,13]. These peptides allow bacteria to increase their chances of adaptation in a hostile environment and, if the mechanisms involved can be understood, they are an excellent alternative for dealing with AMR [13,14,15].
For several years, the massive use of chemical pesticides has been causing harmful effects on the environment, especially on soils [16]. The search for ecological methods that are both environmentally friendly and economically feasible is currently the goal of many researchers [17]. Soil bacteria, which are constantly competing for limited resources, represent a very good starting point for the search for new molecules with antimicrobial activity and biocontrol potential [7,18]. Bacteria of the genera Bacillus and Brevibacillus are widespread in nature, mainly in soil, and have been found to produce many peptides with antibacterial and antifungal activities. Their use as biocontrol agents could control plant diseases or pests caused by bacteria, fungi, and viruses, as well as pathogenic yeasts and protozoa [19,20].
Moreover, the search for new antimicrobial substances is extended to society through educational citizen science projects, such as MicroMundo. The MicroMundo service-learning educational project teaches knowledge of soil microbiota through the search of antimicrobial substances while trying to raise awareness about the AMR problem [21]. MicroMundo is integrated into a global citizen science project in AMR called “Tiny Earth” [22], originally implemented in 2012, in the USA with “Small World Initiative” designation [23]. We highlight the benefits of global collaboration against AMR and advocate for participation in the OneHealth initiative to address and mitigate the challenges posed by this silent pandemic [24,25].
We have previous experience in the development of the MicroMundo project in the region of La Rioja (Spain) in 2019 [26] and 2020–2022 [27]. After the success achieved in those years in terms of citizen participation and awareness of the AMR problem, we continued the project in 2023, looking not only for antimicrobial-producing bacteria (APB), but also for those of interest as potential biocontrol agents. This study demonstrates the relevant link between science and education and the benefits of implementing service-learning methodologies with new students (secondary school, Masters in Education, and PhD students) in different educational institutions. Therefore, the present work aimed to search for APB (putative producers) from soils at new sampling points, and to determine their potential as antibacterial and antifungal biocontrol agents.
2. Results
2.1. Evaluation of the Antibacterial Activity in the First Screening
A total of 61 soil samples were analyzed during the MicroMundo project, and 1220 bacterial isolates (20 isolates/sample) were obtained and subjected to a first screening for antimicrobial activity. Among them, 52 isolates (4.2%) showed potential inhibitory capacity in the initial school-level screening against two indicator bacteria (E. coli and S. epidermidis). The identification by MALDI-TOF of 51 of the 52 putative APB (1 isolate could not be identified) revealed that 42 were Gram-positive bacteria (22 species and 11 genera) and the other 9 isolates were Gram-negative bacteria (8 species and 4 genera) (Figure 1A). Overall, Bacillus was the most abundant genus of potential APB (34.6%). The following microbial diversity was detected: Bacillus (18 isolates), Peribacillus (7), Pseudomonas (6), Lysinibacillus (5), Paenarthrobacter (3), Paenibacillus (2), Advenella (1), Brevibacillus (1), Escherichia (1), Exiguobacterium (1), Psychrobacillus (1), Scandinavium (1), Staphylococcus (1), and Streptomyces (1) (Figure 1A).
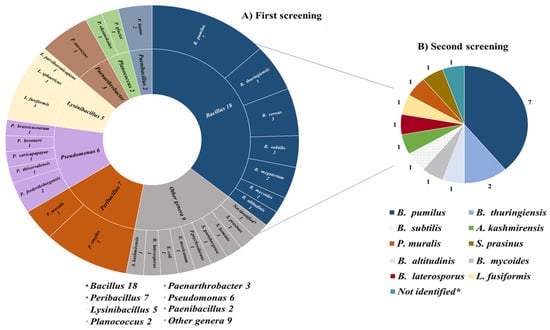
Figure 1.
Genus and species identification of (A) the 51 potential APB obtained in the first screening; (B) the 18 APB verified in the second screening. * One of the isolates could not be identified by MALDI-TOF.
2.2. Verification of Antibacterial Activity in the Second Screening
Subsequently, these 52 isolates were subjected to a second screening in the laboratory of the University of La Rioja. Thus, the antimicrobial activity was evaluated against 12 indicator bacteria using the Spot-on-Lawn method [27] and after rigorous repetitions, 18 out of the 52 bacteria isolated were finally selected because of their clear antimicrobial activity against at least one indicator bacteria and were considered as APB (Figure 1B and Table 1).

Table 1.
Antibacterial activity profile of the 18 APB against the 12 indicator bacteria tested (in green: positive; in red: negative).
The APB were considered highly effective producers if they showed activity against more than 50% of the indicators tested, as was shown for six of the isolates: B. laterosporus X9433 (83%); B. pumilus X9430, X4969, and X9475 (58%); and Bacillus thuringiensis X4968 and X4970 (58%) (Table 1). All of these isolates inhibited methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus pseudintermedius (MRSP) indicator isolates, and five of them also inhibited Escherichia coli. Four additional APB inhibited 50% of indicator bacterial isolates.
The most susceptible indicator bacteria to the action of the APB were as follows: Staphylococcus delphini (78% inhibition), MRSP (78%), Micrococcus luteus (78%), methicillin-susceptible S. pseudintermedius MSSP (72%), MRSA (61%), and methicillin-resistant Staphylococcus epidermidis (MRSE) (61%) (Table 1).
Interestingly, five APB showed antimicrobial activity against the Gram-negative indicator E. coli (B. laterosporus X9433; B. pumilus X9430, X9469, and X9475; and Bacillus subtilis X9429). Moreover, Brevibacillus laterosporus X9433 showed antimicrobial activity against 10 out of the 12 indicator bacteria (83%) and was the only isolate able to inhibit Listeria monocytogenes, a very important food-borne pathogen, as well as to inhibit vancomycin-resistant Enterococcus isolates (Table 1).
2.3. Evaluation of the Antifungal Activity
To evaluate the potential of the 18 selected APB as biocontrol agents, four fungal pathogens were considered in this study. Figure 2 shows the results of the analysis for the determination of the antifungal activity of the 18 APB against the four fungi seeded in Czapek–Dox agar plates (Condalab, Madrid, Spain).
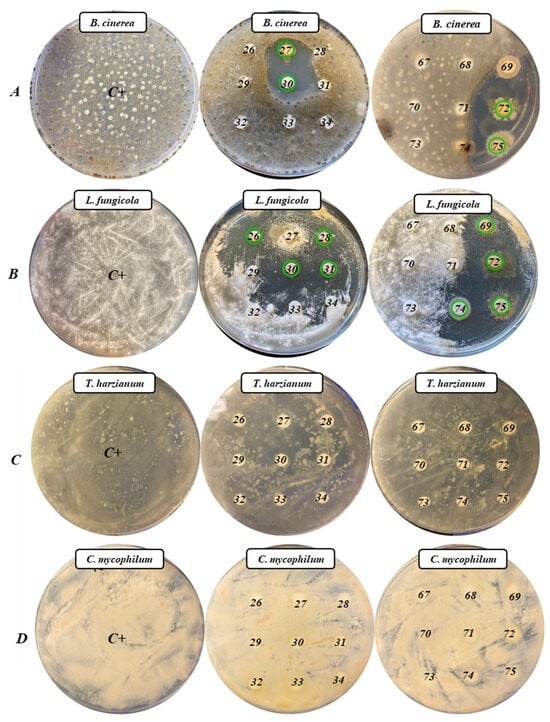
Figure 2.
Antifungal activity of the 18 APB in Czapek–Dox Agar plates ((A): B. cinerea; (B): L. fungicola; (C): T. harzianum; and (D): C. mycophilum). Green circles mark the isolates with positive activity. Isolates tested were 26: X9426; 27: X9427; 28: X9428; 29: X9429; 30: X9430; 31: X9431; 32: X9432; 33: X9433; 34: X9434; 67: X9467; 68: X9468; 69: X9469; 70: X9470; 71: X9471; 72: X9472; 73: X9473; 74: X9474; 75: X9475.
In the plates inoculated with B. cinerea (Figure 2A), it was observed that isolates B. pumilus X9427, X9430, and X9475 and B. altitudinis X9472 showed strong antifungal activity against this pathogen. Furthermore, in the plates inoculated with L. fungicola (Figure 2B), the isolates B. pumilus X9426, X9428, X9430, X9431, X9469, X9474, and X9475, B. altitudinis X9472, and L. fusiformis X9474 also showed clear antifungal activity. For plates inoculated with the pathogens T. harzianum and C. mycophilum, there was no antifungal activity by any of the 18 APB (Figure 2C,D).
2.4. Safety Assessment of the Antimicrobial-Producing Isolates
2.4.1. Antibiotic Susceptibility Testing of the APB
Antibiotic susceptibility testing was performed for the 15 APB of the genera Bacillus Brevibacillus, Lysinibacillus, and Peribacillus, using the breakpoints of Bacillus (EUCAST 2023). The remaining three APB isolates belong to the genera Streptomyces and Advenella (including also isolate X4973, which was not identified by MALDI-TOF), for which breakpoints could not be found in the guidelines and therefore, their antibiotic susceptibility was not analyzed. Table 2 shows the inhibition halos (mm) around the disc of the tested APB. As it is shown, all these bacteria showed susceptibility to all the antibiotics tested, except for B. laterosporus X9433 to ERY15 and B. pumilis X9430 to CLI2.

Table 2.
Inhibition halos (mm) of the antibiotics tested in the 15 APB (EUCAST 2023 breakpoints for Bacillus spp.).
2.4.2. β-Hemolytic Activity of the Antimicrobial-Producing Isolates
A total of 8 APB (3 B. pumilus, 1 B. laterosporus, 1 S. prasinus, 1 P. muralis, 1 A. kashmirensis and 1 B. altitudinis) lacked β-hemolytic activity, while the remaining 10 antimicrobial-producing isolates showed either weak (n = 5) or strong (n = 5) β-hemolytic activity (Table 3).

Table 3.
β-Hemolytic activity of the 18 antimicrobial-producing isolates.
3. Material and Methods
3.1. Study Area and Sampling
MicroMundo was implemented in the region of La Rioja (Spain) in 2023 through two phases of practical training involving the University of La Rioja and five secondary education institutions. The first phase of the project was carried out at the University itself and was conducted by a qualified professor who trained secondary education Masters’ students (n = 22), doctoral students (n = 4), and other biology teachers (n = 9). During the training sessions, the methodology and logistics of the planned sampling points in the different geographical locations of the region were discussed. The second phase of the project was carried out in secondary schools and involved postgraduate students, secondary school teachers, and 177 secondary school students. A total of 61 groups were formed, and each of them analyzed one soil sample.
3.2. First School-Level Screening during the MicroMundo Project
The 61 soil samples were first diluted and plated onto tryptic soy agar plates (Condalab, Madrid, Spain) for selection of bacterial colonies (20 isolates/sample) according to the methodology proposed by Robredo et al. [26]. The antimicrobial activity of the 20 isolates obtained from each soil sample was tested, using Gram-positive Staphylococcus epidermidis C2663 and Gram-negative Escherichia coli C408 as indicator bacteria. The indicator bacteria were resuspended in saline and plated onto tryptic soy agar plates in a lawn pattern. Microbial isolates to be tested for the production of antimicrobial activity were then transferred using a sterile toothpick. After 24 h of incubation, the plates were evaluated by the students and the isolates with potential inhibitory activity (presence of inhibition halos) were selected and sent to the university for further verification and characterization.
3.3. Bacterial Identification
Matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI-TOF) was used to identify the isolates with potential antimicrobial activity in the first screening (n = 52). The recommended standard protein extraction protocol for the commercial device from Bruker Daltonics, Germany, was followed (MALDI-TOF Biotyper®, Bruker, Billerica, MA, USA).
3.4. Evaluation of Antibacterial Activity
Second Screening of Antibacterial Activity Using the Spot-on-Lawn Method
The selected isolates that showed potential antibacterial activity in the first school-level screening (n = 52) were subjected to further analysis and characterization at the university. To achieve this, the isolates were tested against 12 indicator bacteria including multidrug-resistant and relevant pathogens.
The bacteria were cultured on brain heart infusion agar plates (Condalab, Madrid, Spain) and incubated at 37 °C for 24 h to obtain a pure culture. To prepare the test plates, a suspension of the indicator strain was prepared in a 3 mL sterile saline solution to give a turbidity of 0.5 MacFarland. This suspension was spread onto solid tryptic soy agar plates (supplemented with 0.3% yeast extract) using a sterile swab (Condalab, Madrid, Spain).
A fresh solid culture of each isolate being tested for antimicrobial activity was spotted onto the agar plates seeded with the indicator strains using a sterile toothpick. The plates were incubated at 37 °C for 24 h to allow for the evaluation of inhibition zones. If a bacterial isolate showed a clear and distinct inhibition zone against at least 1 of the 12 indicator strains, it was classified as an antimicrobial-producing bacteria (APB).
3.5. Evaluation of Antifungal Activity
The antifungal activity of the APB obtained in the second screening (n = 18) was evaluated against four fungi considered to be relevant phytopathogens: (a) Botrytis cinerea, which is involved in gray rot in grapevines; (b) Lecanicillium fungicola and (c) Trichoderma harzianum, which are two pathogenic fungi of the white mushroom (Agaricus bisporus); and (d) Cladobotryum mycophilum, which is the causal agent of cobwebs in mushroom crops. These fungi are responsible for significant losses in the agricultural sector.
3.5.1. Preparation of the Inoculum (Conidial Suspension)
A culture of each fungus was prepared on potato dextrose agar plates (Condalab, Madrid, Spain) for 7 days at 25 °C. Each plate was washed with 10 mL of sterile distilled water, and then scraped with a seeding loop to release the conidia. This suspension was filtered with a sterile Miracloth Merk polypropylene mesh filter (20–25 µm pore diameter) to remove any mycelial fragments. The conidial concentration of the suspension was measured with a hemocytometer and adjusted with sterile distilled water to the required concentration (7.5 × 103 spores/mL).
3.5.2. Seeding of the Conidial Suspension
A 25 µL volume of the conidial suspension of each of the four fungi was seeded in triplicate on Czapek–Dox agar plates (Condalab, Madrid, Spain) using a Drigalsky loop. This medium was chosen because it was the most suitable for the growth of both fungi and APB.
3.5.3. Inoculation of Antimicrobial-Producing Isolates
Three plates were inoculated with each fungus, and later, 18 sterile discs were placed in two of these plates seeded with 10 µL of an inoculum of the 18 APB (concentration of 2 McFarland). The third plate was used as a fungal growth control (C+), and no bacterial strain was inoculated. The results were measured after 7 days of incubation at 25 °C.
3.6. Safety Assessment of Antimicrobial-Producing Isolates
3.6.1. Antibiotic Susceptibility Testing
Antibiotic susceptibility testing using the disk diffusion method was performed on the APB corresponding to genera for which do exist breakpoints in international committees. In this sense, the European Committee on Antimicrobial Susceptibility Testing guidelines (EUCAST, 2023) were followed for interpreting the results for Bacillus spp. and related genera (Brevibacillus, Lysinibacillus, and Penibacillus); the APB of the remaining genera were not tested for antibiotic susceptibility testing due to lack of standard breakpoints. For this assay, a standard inoculum of 0.5 McFarland of the bacteria was applied to the surface of Muller–Hinton plates (Condalab, Madrid, Spain). Disks of the antibiotics (OXOID) were placed on the agar surface. After an incubation period of 24 h at 37 °C, the diameter of the inhibition zone around the disc was measured. The antibiotics tested were as follows (abbreviation and dose in mg): imipenem (IMP10), erythromycin (ERY15), clindamycin (CLI2), ciprofloxacin (CIP5), and linezolid (LZD10).
3.6.2. β-Hemolytic Activity Test
This test was used to evaluate the ability of the APB (n = 18) to exhibit β-hemolytic activity. During the test, the isolated bacteria were spotted with sterile toothpicks on blood agar plates (Condalab, Madrid, Spain) and were incubated under appropriate conditions. Bacterial colonies were observed for the presence or absence of complete hemolysis (beta-hemolysis). This assay was conducted with the objective of determining their suitability as potential bioprotective agents in agriculture.
4. Discussion
This research carried out extensive soil sampling thanks to citizen collaboration. A total of 1220 bacteria were isolated, of which, 18 APB were identified, representing 1.5% of the total recovered isolates. These results are similar to those carried out during the former study conducted by Fernández-Fernández et al. 2022 [27], in which only 1.2% of total tested isolates were selected as potential antimicrobial producers.
It is important to ensure the absence of acquired antibiotic resistance mechanisms or virulence factors in the strains that are candidates for potential biocontrol agents [28]. Therefore, in this work, antibiotic susceptibility tests were carried out on 15 of the 18 APB, using the breakpoints for Bacillus spp., and demonstrated that they were susceptible to the antibiotics tested with a few exceptions. In addition, eight of them lacked β-hemolytic activity, including isolates with high inhibitory activity against multidrug-resistant (MDR) bacteria (B. laterosporus) and isolates with activity against fungi (B. pumilus and B. altitudinis), which makes them promising candidates for potential future applications. The members of Bacillus and Brevibacillus genera obtained in this research are known for their ability to produce a variety of bioactive compounds with different activities such as antibacterial [29,30], antifungal [16,31,32,33,34,35,36,37,38], antiviral [39,40,41], and pest control properties [42,43].
Bacillus spp. has been widely used to control plant diseases such as wilt in tomato [36], tobacco [39], and banana [37], which are all caused by fungi, as well as bacterial wilt in tobacco [38]. Members of the genus Bacillus have been most commonly chosen to prepare bioformulations with a positive impact on soil health as well as plant growth and health [44,45,46]. Regarding B. laterosporus, Chen et al. 2017 [47] made the first report on the effective biocontrol of potato common scab (an economically important disease caused by Streptomyces spp.) using B. laterosporus. Li et al. 2021 [48] demonstrated that the application of the strain B. laterosporus, used for effective control of potato common scab, successfully modified the composition and function of soil bacterial community in the tuberosphere and rhizosphere.
Bacillus subtilis is currently considered one of the most promising microorganisms in sustainable agriculture [49] and it has been reported as a growth promoter and has activity against a wide variety of plant pathogens [50], although in our study, the only strain of Bacillus subtilis had β-hemolytic activity and was not a good candidate. Lahlali et al. 2013 [51] have reported the successful application of the biofungicide Serenade (B. subtilis), which was effective against infection of canola by Plasmodiophora brassicae. It has been demonstrated that B. subtilis, isolated from the rhizosphere of tomato plants, inhibited the growth of Fusarium oxysporum and R. solanacearum, the main phytopathogens that hinder this type of greenhouse crop [44].
However, we have detected three isolates of B. pumilus with potential as biocontrol agents, the same species successfully used by Dai et al. in 2021 [52] to inhibit the growth of Sphaeropsis sapinea, the pathogen responsible for pine shoot blight disease.
In our study, B. laterosporus X9433 is particularly remarkable for its broad spectrum of action against 10 of the 12 indicator bacteria (83%) including MRSA, MRSE, MRSP, L. monocytogenes, E. coli, and vancomycin-resistant Enterococcus isolates, although it did not show antifungal activity. This strain will be studied by whole-genome sequencing in future assays to determine whether it possesses genes encoding brevibacillin (antimicrobial lipopeptide effective against multidrug-resistant strains) [32,53,54,55,56,57].
Authors such as Zhao et al. 2021 [55] succeeded in identifying, purifying, and characterizing a brevibacillin 2V from B. laterosporus with strong antimicrobial activity against E. faecium, E. faecalis, and MRSA, while Yang et al. 2018 [57] studied the antimicrobial mechanism of action of brevibacillin against sensitive S. aureus strains, although further studies are still needed to better understand its antimicrobial capacity. In addition, Le Han et al. 2022 [32] isolated strains of B. halotolerans and B. laterosporus from marine sediment samples that produced thermostable and pH-tolerant antifungal compounds, which inhibited the growth of relevant pathogens such as Alternaria alternata, Candida albicans, Cladosporium sp., Trichophyton rubrum, Trichosporon pullulans, and F. oxysporum. The data obtained in this study corroborate the antibacterial capacity of B. laterosporus, which has also been previously detected in soil samples by our research group [27].
In summary, the B. pumilus X9430, X9469, and X9475, B. subtilis X9429 and B. laterosporus X9433 isolates recovered in this study showed activity against the Gram-negative indicator E. coli, and four of them also inhibited MRSA, MRSE, and MRSP (except B. subtilis X9429). Moreover, the isolates B. pumilus X9430 and B. altitudinis X9472 expressed antifungal activity against B. cinerea and B. pumilus X9426, X9430, and X9431 showed significant antimicrobial activity against L. fungicola.
5. Conclusions
This study highlights the threat of AMR and the need to find new solutions from a “One Health” perspective. The antimicrobial activity of selected soil bacteria was evaluated to identify potential biocontrol agents. A total of 3 of the 18 APB were considered excellent candidates for further studies to fully determine their potential as biocontrol agents, as they met the eligibility criteria established in this study (lack of β-hemolytic activity and absence of resistance to the tested antibiotics): (a) B. pumilus X9430 and (b) B. altitudinis X4972 (which inhibited three methicillin-resistant Staphylococcus indicator bacteria and two fungal pathogens); (c) B. laterosporus X9433 (with strong antibacterial activity against MDR bacteria). These results underscore the potential of soil bacteria as part of the solution to AMR and the importance of community engagement in the fight against this global health challenge.
Author Contributions
M.S.P.-H., R.F.-F., B.R. and C.T. designed the study; M.S.P.-H., R.F.-F. and B.R., performed the experiments; M.S.P.-H., R.F.-F., C.T. and B.R. performed the first analysis of the data and prepared the draft of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the project PID2019-106158RB-I00 of the MCIN/AEI/10.13039/501100011033. Mario Sergio Pino-Hurtado has a Predoctoral Contract FPI from the University of La Rioja (FPI-UR) and Rosa Fernández-Fernández has a Predoctoral Fellowship FPU from the Ministry of Science, Innovation and Universities of Spain (FPU18/05438).
Institutional Review Board Statement
In this study, each participating teacher worked with their regular students and the data collected were completely anonymous. In any case, the participants were duly informed, and the parents or legal guardians signed an informed consent form that followed the international ethical regulations of the Helsinki protocol, including a description of the research and its objectives.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
We thank to the voluntary participation of the Master’s students, and secondary school teachers and students that allowed us to carry out the sampling for this study. Special thanks to Susana Sanz of the University of La Rioja for the provided protocols and materials to carry out the experiments with fungi.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- Baquero, F.; Martínez, J.L.; Lanza, V.F.; Rodríguez-Beltrán, J.; Galán, J.C.; San Millán, A.; Cantón, R.; Coque, T.M. Evolutionary Pathways and Trajectories in Antibiotic Resistance. Clin. Microbiol. Rev. 2021, 34, e0005019. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Banerjee, S.; Van der Heijden, M.G.A. Soil microbiomes and One Health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- Schloss, P.D.; Handelsman, J. Toward a Census of Bacteria in Soil. PLoS Comput. Biol. 2006, 2, e92. [Google Scholar] [CrossRef]
- Fan, K.; Weisenhorn, P.; Gilbert, J.A.; Chu, H. Wheat rhizosphere harbors a less complex and more stable microbial co-occurrence pattern than bulk soil. Soil Biol. Biochem. 2018, 125, 51–260. [Google Scholar] [CrossRef]
- Klibi, N.; Ben Slimen, N.; Fhoula, I.; López, M.; Ben Slama, K.; Daffonchio, D.; Boudabous, A.; Torres, C.; Ouzari, H. Genotypic Diversity, Antibiotic Resistance and Bacteriocin Production of Enterococci Isolated from Rhizospheres. Microbes Environ. 2012, 27, 533–537. [Google Scholar] [CrossRef]
- Oburger, E.; Gruber, B.; Wanek, W.; Watzinger, A.; Stanetty, C.; Schindlegger, Y.; Hann, S.; Schenkeveld, W.D.C.; Kraemer, S.M.; Puschenreiter, M. Microbial decomposition of 13C- labeled phytosiderophores in the rhizosphere of wheat: Mineralization dynamics and key microbial groups involved. Soil Biol. Biochem. 2016, 98, 196–207. [Google Scholar] [CrossRef]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Reddy, B.V.B.; Kallifidas, D.; Kim, J.H.; Charlop-Powers, Z.; Feng, Z.; Brady, S.F. Natural Product Biosynthetic Gene Diversity in Geographically Distinct Soil Microbiomes. Appl. Environ. Microbiol. 2012, 78, 3744–3752. [Google Scholar] [CrossRef]
- Riley, M.A.; Wertz, J.E. Bacteriocins: Evolution, Ecology, and Application. Annu. Rev. Microbiol. 2002, 56, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Yount, N.Y.; Weaver, D.C.; de Anda, J.; Lee, E.Y.; Lee, M.W.; Wong, G.C.L.; Yeaman, M.R. Discovery of Novel Type II Bacteriocins Using a New High-Dimensional Bioinformatic Algorithm. Front. Immunol. 2020, 1, 1873. [Google Scholar] [CrossRef] [PubMed]
- Twomey, E.; Hill, C.; Field, D.; Begley, M. Recipe for Success: Suggestions and Recommendations for the Isolation and Characterisation of Bacteriocins. Int. J. Microbiol. 2021, 2021, 9990635. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef] [PubMed]
- Telhig, S.; Ben Said, L.; Torres, C.; Rebuffat, S.; Zirah, S.; Fliss, I. Evaluating the Potential and Synergetic Effects of Microcins against Multidrug-Resistant Enterobacteriaceae. Microbiol. Spectr. 2022, 10, e0275221. [Google Scholar] [CrossRef] [PubMed]
- Asaka, O.; Shoda, M. Biocontrol of Rhizoctonia solani Damping-Off of Tomato with Bacillus subtilis RB14. Appl. Environ. Microbiol. 1996, 62, 4081–4085. [Google Scholar] [CrossRef]
- Zhou, L.; Song, C.; Li, Z.; Kuipers, O.P. Antimicrobial activity screening of rhizosphere soil bacteria from tomato and genome-based analysis of their antimicrobial biosynthetic potential. BMC Genom. 2021, 22, 29. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and Implementation of Biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Valderrama, M.J.; González-Zorn, B.; Calvo de Pablo, P.; Díez-Orejas, R.; Fernández-Acero, T.; Gil-Serna, J.; de Juan, L.; Martín, H.; Molina, M.; Navarro-García, F.; et al. Educating in antimicrobial resistance awareness: Adaptation of the Small World Initiative program to service-learning. FEMS Microbiol. Lett. 2018, 365, fny161. [Google Scholar] [CrossRef]
- Tiny Earth. Available online: https://tinyearth.wisc.edu/ (accessed on 25 September 2023).
- Small World Initiative. Available online: http://www.smallworldinitiative.org/ (accessed on 25 September 2023).
- Singh, K.S.; Anand, S.; Dholpuria, S.; Sharma, J.K.; Blankenfeldt, W.; Shouche, Y. Antimicrobial resistance dynamics and the One-Health strategy: A review. Environ. Chem. Lett. 2021, 19, 2995–3007. [Google Scholar] [CrossRef]
- Davis, E.; Sloan, T.; Aurelius, K.; Barbour, A.; Bodey, E.; Clark, B.; Dennis, C.; Drown, R.; Fleming, M.; Humbert, A.; et al. Antibiotic discovery throughout the Small World Initiative: A molecular strategy to identify biosynthetic gene clusters involved in antagonistic activity. Microbiologyopen 2017, 6, 435. [Google Scholar] [CrossRef]
- Robredo, B.; Fernández-Fernández, R.; Torres, C. Antimicrobial resistance as a nexus between teaching and research. J. Biol. Educ. 2021, 57, 856–872. [Google Scholar] [CrossRef]
- Fernández-Fernández, R.; Robredo, B.; Navajas, E.; Torres, C. Citizen Contribution for Searching for Alternative Antimicrobial Activity Substances in Soil. Antibiotics 2022, 12, 57. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Huang, B.; Jia, H.; Han, X.; Gou, J.; Huang, C.; Wang, J.; Wei, J.; Wang, J.; Zhang, C. Effects of biocontrol Bacillus and fermentation bacteria additions on the microbial community, functions and antibiotic resistance genes of prickly ash seed oil meal-biochar compost. Bioresour. Technol. 2021, 340, 125668. [Google Scholar] [CrossRef]
- Dimkić, I.; Janakiev, T.; Petrović, M.; Degrassi, G.; Fira, D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms—A review. Physiol. Mol. Plant Pathol. 2022, 117, 101754. [Google Scholar] [CrossRef]
- Silo-Suh, L.A.; Lethbridge, B.J.; Raffel, S.J.; He, H.; Clardy, J.; Handelsman, J. Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW85. Appl. Environ. Microbiol. 1994, 60, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Jiang, L.; Thu Tran, T.N.; Muhammad, N.; Kim, S.-G.; Tran Pham, V.P.; Ng, Y.J.; Khoo, K.S.; Chew, K.W.; Phuong Nguyen, T.D. Whole-genome analysis and secondary metabolites production of a new strain Brevibacillus halotolerans 7WMA2: A potential biocontrol agent against fungal pathogens. Chemosphere 2022, 307, 136004. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, X.; Xiao, C.; Wang, W.; Zhao, X.; Sui, J.; Sa, R.; Guo, T.L.; Liu, X. Antifungal activity of Brevibacillus laterosporus JX-5 and characterization of its antifungal components. World J. Microbiol. Biotechnol. 2015, 31, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, C.; Novinscak, A.; Cantin, G.; Joly, D.L.; Filion, M. Biocontrol Activity of Bacillus spp. and Pseudomonas spp. Against Botrytis cinerea and Other Cannabis Fungal Pathogens. Phytopathology 2022, 112, 549–560. [Google Scholar] [CrossRef]
- Cui, L.; Yang, C.; Wei, L.; Li, T.; Chen, X. Isolation and identification of an endophytic bacteria Bacillus velezensis 8-4 exhibiting biocontrol activity against potato scab. Biol. Control 2020, 141, 104156. [Google Scholar] [CrossRef]
- Abdallah, R.A.B.; Stedel, C.; Garagounis, C.; Nefzi, A.; Jabnoun-Khiareddine, H.; Papadopoulou, K.K.; Daami-Remadi, M. Involvement of lipopeptide antibiotics and chitinase genes and induction of host defense in suppression of Fusarium wilt by endophytic Bacillus spp. in tomato. Crop Prot. 2017, 99, 45–58. [Google Scholar] [CrossRef]
- Fu, L.; Penton, C.R.; Ruan, Y.; Shen, Z.; Xue, C.; Li, R.; Shen, Q. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol. Biochem. 2017, 104, 39–48. [Google Scholar] [CrossRef]
- Wu, B.; Wang, X.; Yang, L.; Yang, H.; Zeng, H.; Qiu, Y.; Wang, C.; Yu, J.; Li, J.; Xu, D.; et al. Effects of Bacillus amyloliquefaciens ZM9 on bacterial wilt and rhizosphere microbial communities of tobacco. Appl. Soil Ecol. 2016, 103, 2653. [Google Scholar] [CrossRef]
- Lian, L.; Xie, L.; Zheng, L.; Lin, Q. Induction of systemic resistance in tobacco against Tobacco mosaic virus by Bacillus spp. Biocontrol. Sci. Technol. 2011, 21, 281–292. [Google Scholar] [CrossRef]
- Ara, I. Antiviral activities of streptomycetes against tobacco mosaic virus (TMV) in Datura plant: Evaluation of different organic compounds in their metabolites. Afr. J. Biotechnol. 2012, 11, 2130–2138. [Google Scholar]
- Abdelkhalek, A.; Aseel, D.G.; Király, L.; Künstler, A.; Moawad, H.; Al-Askar, A.A. Induction of Systemic Resistance to Tobacco mosaic virus in Tomato through Foliar Application of Bacillus amyloliquefaciens Strain TBorg1 Culture Filtrate. Viruses 2022, 14, 1830. [Google Scholar] [CrossRef]
- Antil, S.; Kumar, R.; Pathak, D.V.; Kumari, A. Recent advances in utilizing bacteria as biocontrol agents against plant parasitic nematodes emphasizing Meloidogyne spp. Biol. Control 2023, 183, 105244. [Google Scholar] [CrossRef]
- Valtierra-de-Luis, D.; Villanueva, M.; Berry, C.; Caballero, P. Potential for Bacillus thuringiensis and Other Bacterial Toxins as Biological Control Agents to Combat Dipteran Pests of Medical and Agronomic Importance. Toxins 2020, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Yu, X.; Liang, X.; Liu, Y.; Borriss, R.; Liu, Y. Addition of plant-growth-promoting Bacillus subtilis PTS-394 on tomato rhizosphere has no durable impact on composition of root microbiome. BMC Microbiol. 2017, 17, 131. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.L.; Jiao, X.Y.; Fan, F.F.; Wang, J.S.; Guo, J.; Dong, E.W.; Wang, L.G.; Shen, X.M. Effect of continuous sorghum cropping on the rhizosphere microbial community and the role of Bacillus amyloliquefaciens in altering the microbial composition. Plant Growth Regul. 2019, 89, 299–308. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Yang, X.; Li, C.; Wang, L.; Feng, J.; Chen, S.; Li, X.; Yang, Y. Effects of integrated biocontrol on bacterial wilt and rhizosphere bacterial community of tobacco. Sci. Rep. 2021, 11, 2653. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, M.; Wang, J.; Lv, D.; Ma, Y.; Zhou, B.; Wang, B. Biocontrol effects of Brevibacillus laterosporus AMCC100017 on potato common scab and its impact on rhizosphere bacterial communities. Biol. Control 2017, 106, 89–98. [Google Scholar] [CrossRef]
- Li, C.; Shi, W.; Wu, D.; Tian, R.; Wang, B.; Lin, R.; Zhou, B.; Gao, Z. Biocontrol of potato common scab by Brevibacillus laterosporus BL12 is related to the reduction of pathogen and changes in soil bacterial community. Biol. Control 2021, 153, 104496. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhao, D.L.; Shen, L.L.; Jing, C.L.; Zhang, C.S. Application and Mechanisms of Bacillus subtilis in Biological Control of Plant Disease. In Role of Rhizospheric Microbes in Soil; Springer: Singapore, 2018; pp. 225–250. [Google Scholar]
- Hashem, A.; Tabassum, B.; Fathi, E.; Allah, A. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Lahlali, R.; Peng, G.; Gossen, B.D.; McGregor, L.; Yu, F.Q.; Hynes, R.K.; Hwang, S.F.; McDonald, M.R.; Boyetchko, S.M. Evidence that the Biofungicide Serenade (Bacillus subtilis) Suppresses Clubroot on Canola via Antibiosis and Induced Host Resistance. Phytopathology 2013, 103, 245–254. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, X.Q.; Wang, Y.H.; Zhu, M.L. Biocontrol potential of Bacillus pumilus HR10 against Sphaeropsis shoot blight disease of pine. Biol. Control 2021, 152, 104458. [Google Scholar] [CrossRef]
- Eveno, M.; Belguesmia, Y.; Bazinet, L.; Gancel, F.; Fliss, I.; Drider, D. In silico analyses of the genomes of three new bacteriocin-producing bacteria isolated from animal’s faeces. Arch. Microbiol. 2021, 203, 205–217. [Google Scholar] [CrossRef]
- Yang, X.; Huang, E.; Yesil, M.; Xiaoli, L.; Dudley, E.G.; Yousef, A.E. Draft Genome Sequence of Brevibacillus laterosporus OSY-I1, a Strain That Produces Brevibacillin, Which Combats Drug-Resistant Gram-Positive Bacteria. Genome Announc. 2017, 5, e01093-17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, X.; Shukla, R.; Kumar, R.; Weingarth, M.; Breukink, E.; Kuipers, O.P. Brevibacillin 2V, a Novel Antimicrobial Lipopeptide With an Exceptionally Low Hemolytic Activity. Front. Microbiol. 2021, 12, 693725. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, E.; Yousef, A.E. Brevibacillin, a cationic lipopeptide that binds to lipoteichoic acid and subsequently disrupts cytoplasmic membrane of Staphylococcus aureus. Microbiol. Res. 2017, 195, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yousef, A.E. Antimicrobial peptides produced by Brevibacillus spp.: Structure, classification and bioactivity: A mini review. World J. Microbiol. Biotechnol. 2018, 34, 57. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).