Deciphering the Structural, Textural, and Electrochemical Properties of Activated BN-Doped Spherical Carbons
Abstract
1. Introduction
2. Experimental
2.1. Starting Materials
2.2. Experimental Procedure
2.3. Electrochemical Characterization
3. Results and Discussion
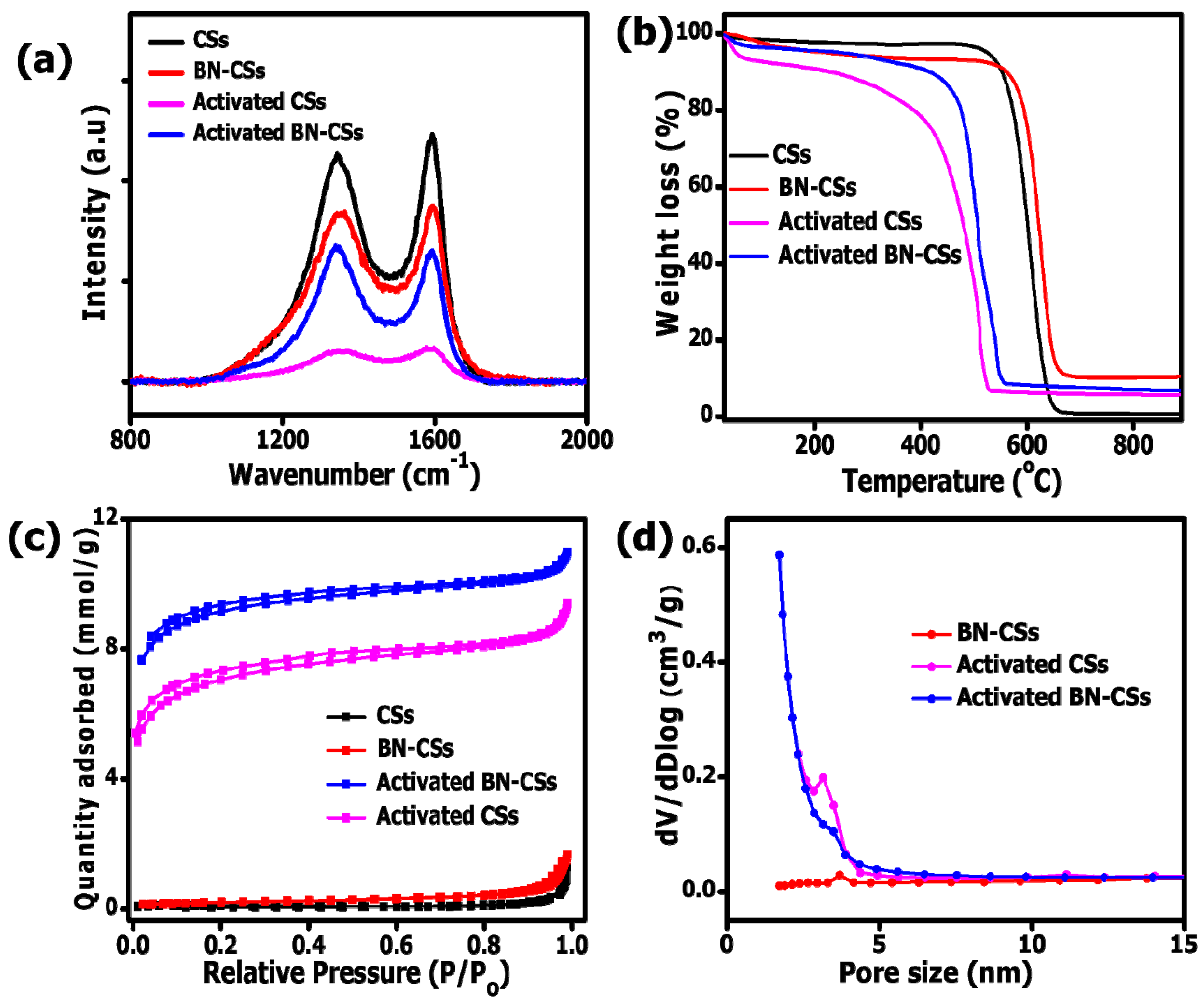
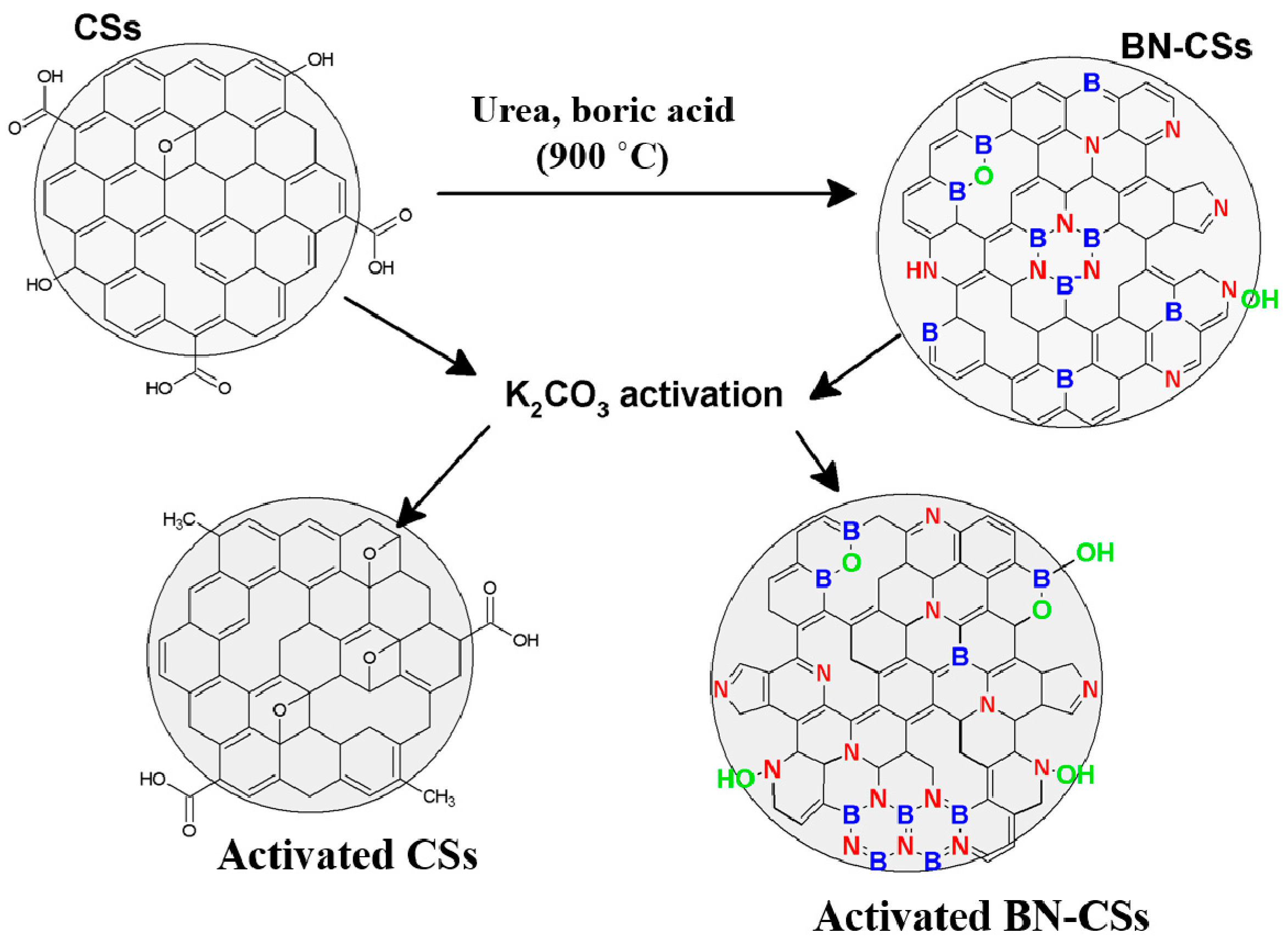
3.1. Morphological, Structural, and Textural Properties
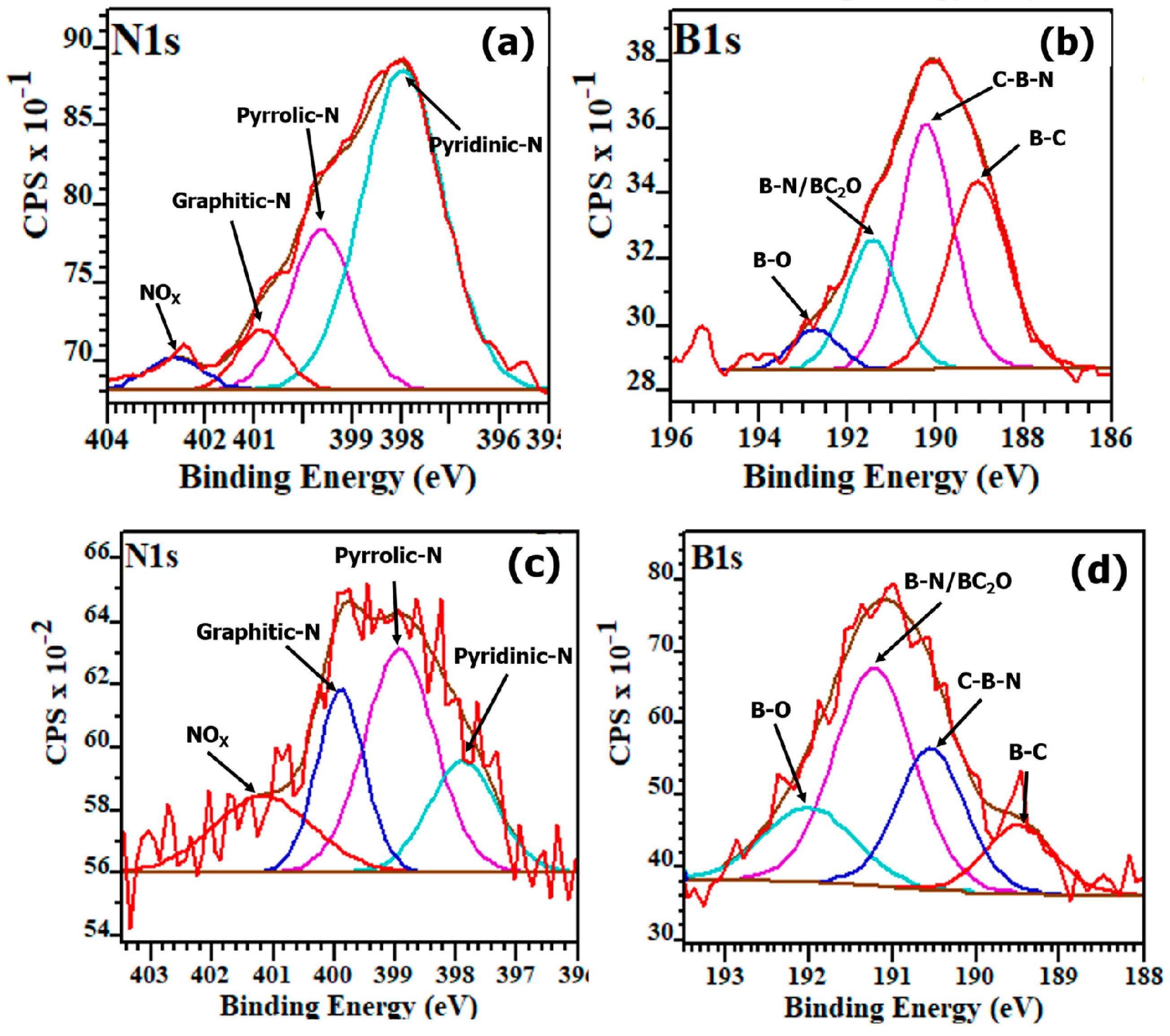
3.2. Compositional Analysis Using XPS
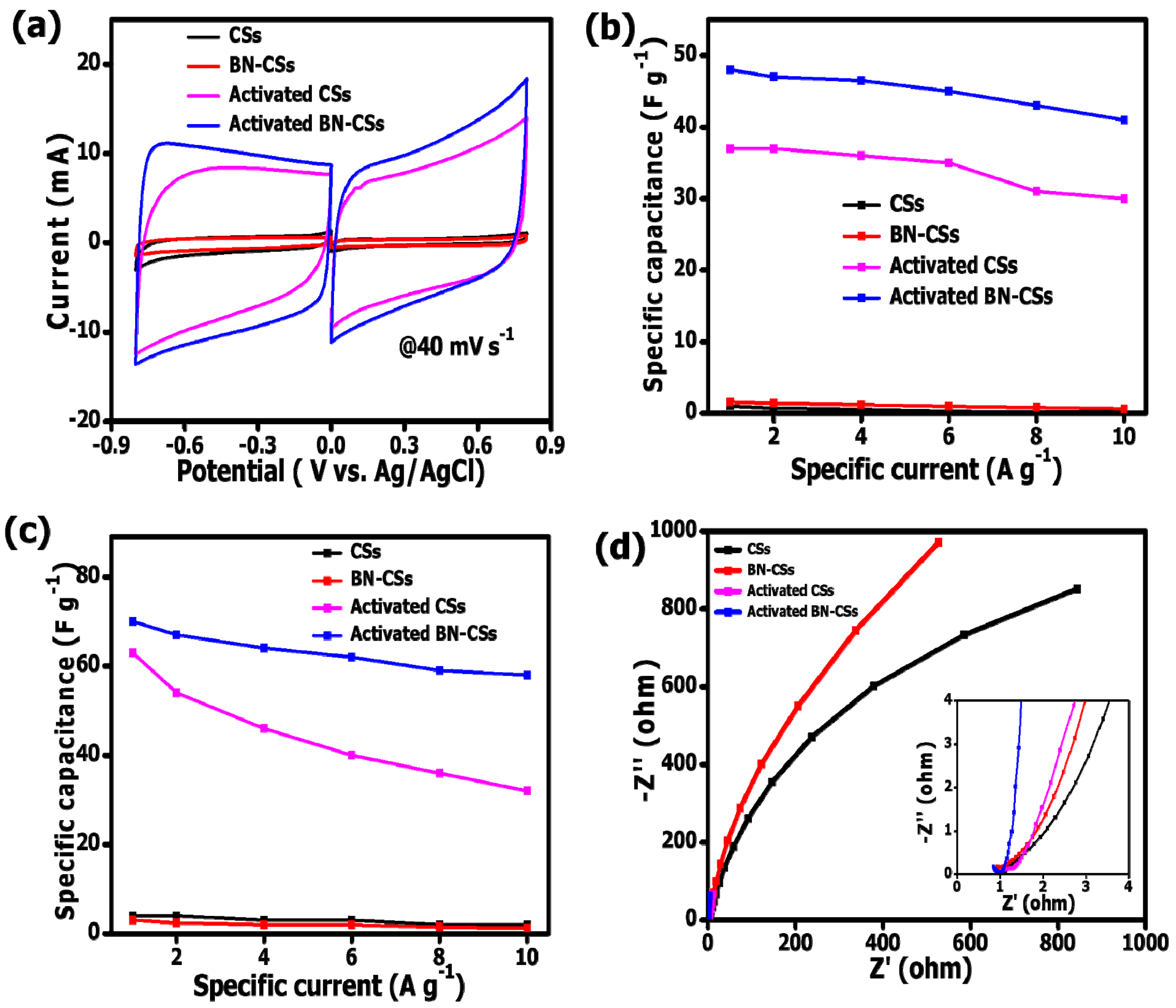
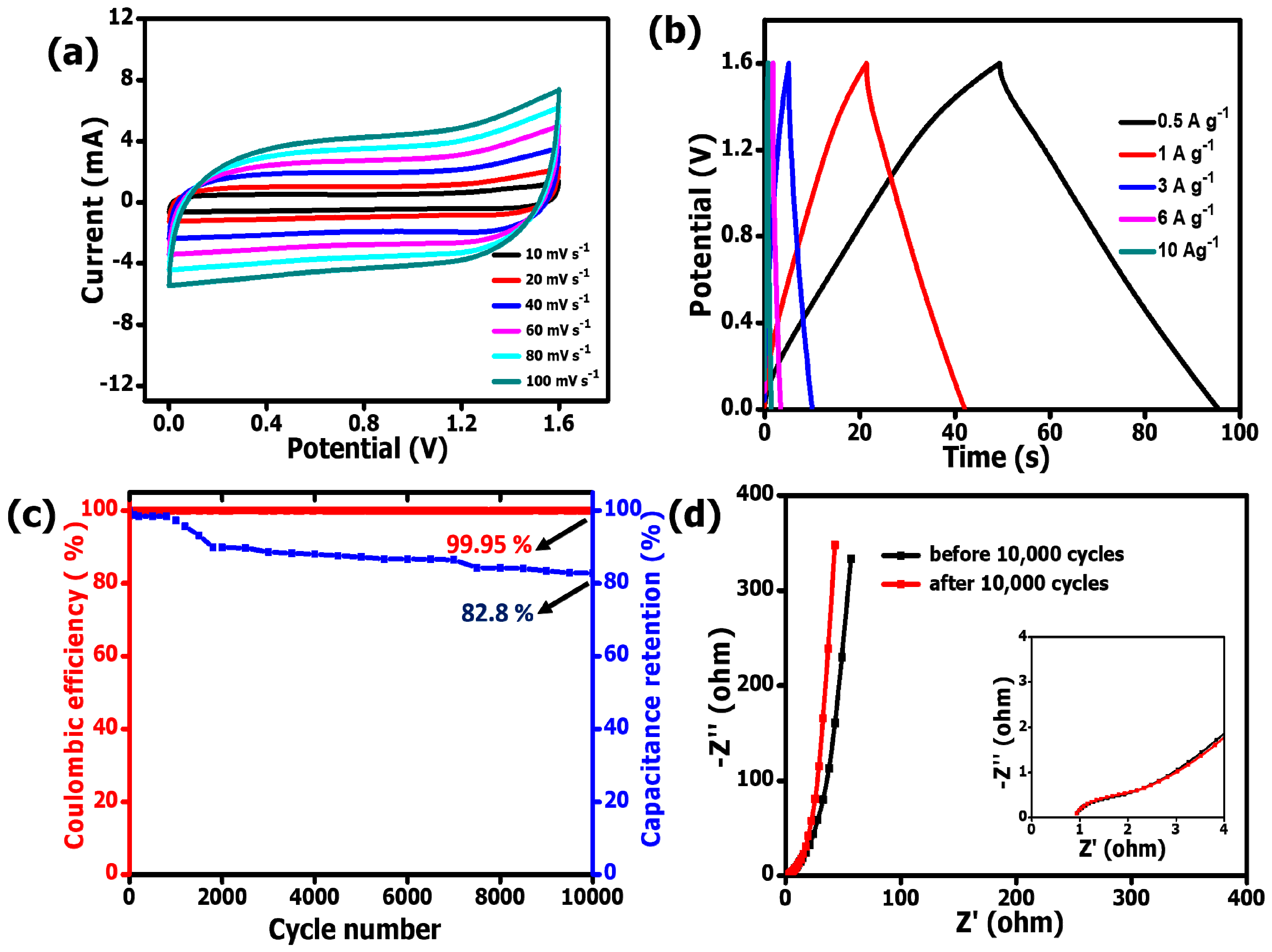
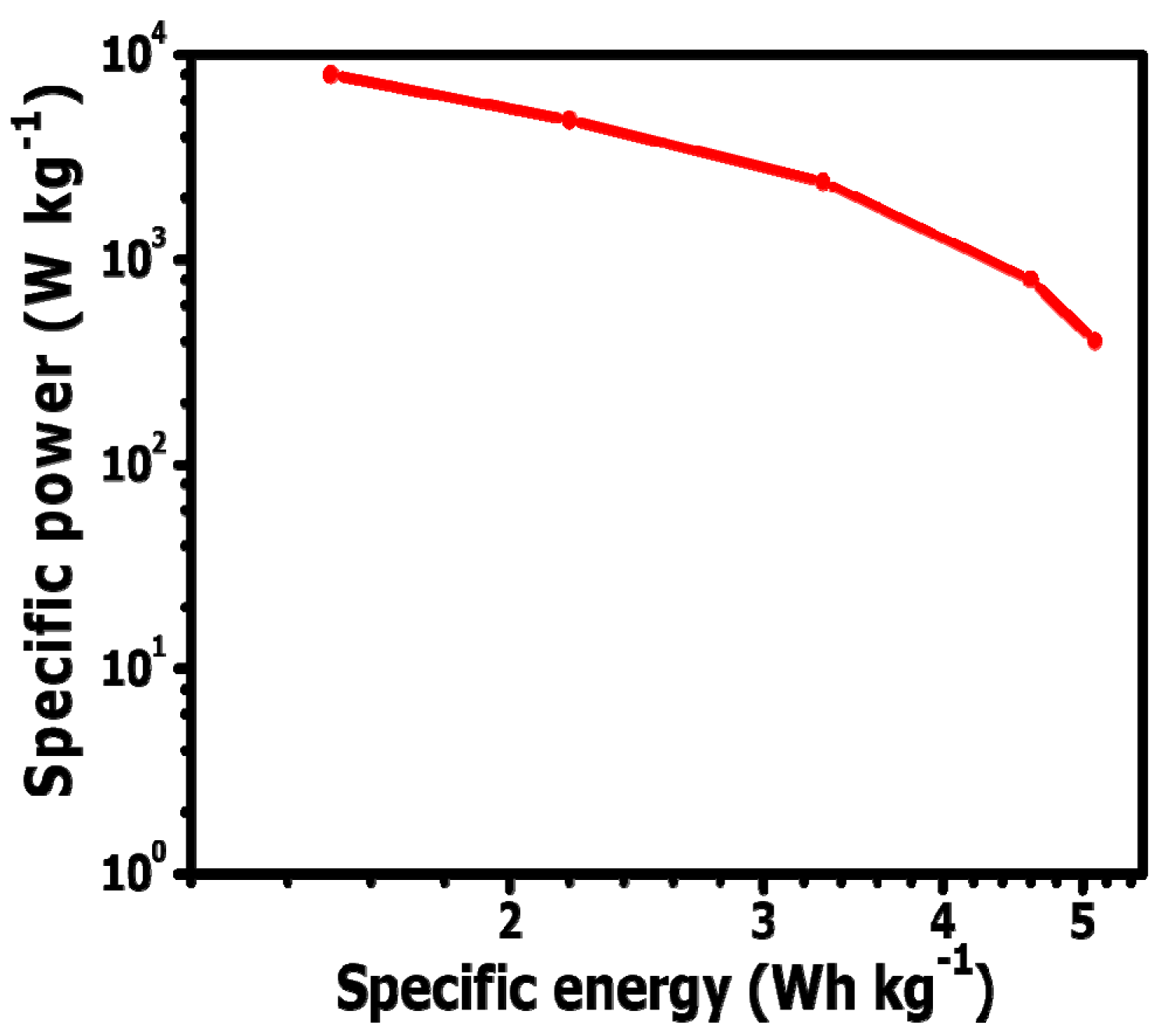
3.3. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Stein, A.; Wang, Z.; Fierke, M.A. Functionalization of Porous Carbon Materials with Designed Pore Architecture. Adv. Mater. 2009, 21, 265–293. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Carbon nanomaterials for high-performance supercapacitors. Mater. Today 2013, 16, 272–280. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, D.; Sun, Z.; Deng, Y.; Xia, Y.; Zhao, D. A Controllable Synthesis of Rich Nitrogen-Doped Ordered Mesoporous Carbon for CO2 Capture and Supercapacitors. Adv. Funct. Mater. 2013, 23, 2322–2328. [Google Scholar] [CrossRef]
- Momodu, D.; Madito, M.; Barzegar, F.; Bello, A.; Khaleed, A.; Olaniyan, O. Activated carbon derived from tree bark biomass with promising material properties for supercapacitors. J. Solid State Electrochem. 2017, 21, 859–872. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, M.; Gan, L.; Ma, X.; Zhu, D.; Xu, Z. Ultramicroporous Carbon Nanoparticles for the High-Performance Electrical Double-Layer Capacitor Electrode. Energy Fuels 2014, 28, 1561–1568. [Google Scholar] [CrossRef]
- Inada, M.; Enomoto, N.; Hojo, J.; Hayashi, K. Structural analysis and capacitive properties of carbon spheres prepared by hydrothermal carbonization. Adv. Powder Technol. 2017, 28, 884–889. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Gao, C.; Hsu, W.K.; Zhu, Y.; Huczko, A.; Bystrzejewski, M. Large-scale synthesis and characterization of carbon spheres prepared by direct pyrolysis of hydrocarbons. Carbon 2005, 43, 1944–1953. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Mhlanga, S.D.; Coville, N.J. Carbon spheres. Mater. Sci. Eng. R Rep. 2010, 70, 1–28. [Google Scholar] [CrossRef]
- Pollak, E.; Levy, N.; Eliad, L.; Salitra, G.; Soffer, A.; Aurbach, D. Review on Engineering and Characterization of Activated Carbon Electrodes for Electrochemical Double Layer Capacitors and Separation Processes. Isr. J. Chem. 2008, 48, 287–303. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Shrestha, L.K.; Hossain, M.S.A.; Alothman, Z.A.; Yamauchi, Y. Activated Porous Carbon Spheres with Customized Mesopores through Assembly of Diblock Copolymers for Electrochemical Capacitor. ACS Appl. Mater. Interfaces 2017, 9, 18986–18993. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kim, S.; Heo, M.S.; Kim, J.E.; Suh, H.; Kim, I. Easy Synthesis of Hierarchical Carbon Spheres with Superior Capacitive Performance in Supercapacitors. Langmuir 2013, 29, 12266–12274. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, R.; Dou, Y.; Wu, Z.; Huang, T.; Feng, D. Synthesis of mesoporous carbon spheres with a hierarchical pore structure for the electrochemical double-layer capacitor. Carbon 2011, 49, 1248–1257. [Google Scholar] [CrossRef]
- Bello, A.; Barzegar, F.; Momodu, D.; Dangbegnon, J.; Taghizadeh, F.; Manyala, N. Symmetric supercapacitors based on porous 3D interconnected carbon framework. Electrochim. Acta 2015, 151, 386–392. [Google Scholar] [CrossRef]
- Kwon, T.; Nishihara, H.; Itoi, H.; Yang, Q.-H.; Kyotani, T. Enhancement Mechanism of Electrochemical Capacitance in Nitrogen-/Boron-Doped Carbons with Uniform Straight Nanochannels. Langmuir 2009, 25, 11961–11968. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, L.; Tian, C.; Tan, T.; Xie, Y.; Shi, K. Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv. 2012, 2, 4498. [Google Scholar] [CrossRef]
- Kim, C.; Kim, K.; Moon, J.H. Highly N-doped microporous carbon nanospheres with high energy storage and conversion efficiency. Sci. Rep. 2017, 14400. [Google Scholar] [CrossRef]
- Hao, J.; Wang, J.; Qin, S.; Liu, D.; Li, Y.; Lei, W. B/N co-doped carbon nanosphere frameworks as high-performance electrodes for supercapacitor. J. Mater. Chem. A 2018, 8053–8058. [Google Scholar] [CrossRef]
- Guo, H.; Gao, Q. Boron and nitrogen co-doped porous carbon and its enhanced properties as supercapacitor. J. Power Sources 2009, 186, 551–556. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, L.-Z.; Zhou, M.-Q.; Guan, H.; Qiao, S.; Antonietti, M. Nitrogen-Containing Hydrothermal Carbons with Superior Performance in Supercapacitors. Adv. Mater. 2010, 22, 5202–5206. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ning, G.; Qi, C.; Li, J.; Ma, X.; Xu, C.; Li, Y.; Zhang, X.; Gao, J. An Advanced Lithium Ion Battery Based on a Sulfur-Doped Porous Carbon Anode and a Lithium Iron Phosphate Cathode. Electrochim. Acta 2016, 190, 141–149. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Puziy, A.M.; Poddubnaya, O.I.; Suárez-García, F.; Tascón, J.M.D.; Lu, G.Q. Highly Stable Performance of Supercapacitors from Phosphorus-Enriched Carbons. J. Am. Chem. Soc. 2009, 131, 5026–5027. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Jindal, V.K. Designing band gap of graphene by B and N dopant atoms. RSC Adv. 2013, 3, 802–812. [Google Scholar] [CrossRef]
- Panchakarla, L.S.; Subrahmanyam, K.S.; Saha, S.K.; Govindaraj, A.; Krishnamurthy, H.R.; Waghmare, U.V. Synthesis, Structure, and Properties of Boron- and Nitrogen-Doped Graphene. Adv. Mater. 2009, 21. [Google Scholar] [CrossRef]
- Dou, S.; Huang, X.; Ma, Z.; Wu, J.; Wang, S. A simple approach to the synthesis of BCN graphene with high capacitance. Nanotechnology 2015, 26, 45402. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Xu, Y.; Mu, S.; Li, W. The effect of nitrogen and/or boron doping on the electrochemical performance of non-caking coal-derived activated carbons for use as supercapacitor electrodes. New Carbon Mater. 2017, 32, 442–450. [Google Scholar] [CrossRef]
- Zhang, M.; He, C.; Liu, E.; Zhu, S.; Shi, C.; Li, J. Activated Carbon Nanochains with Tailored Micro-Meso Pore Structures and Their Application for Supercapacitors. J. Phys. Chem. C 2015, 119, 21810–21817. [Google Scholar] [CrossRef]
- Suleman, M.; Othman, M.A.R.; Hashmi, S.A.; Kumar, Y.; Deraman, M.; Omar, R. Activated graphene oxide/reduced graphene oxide electrodes and low viscous sulfonium cation based ionic liquid incorporated flexible gel polymer electrolyte for high rate supercapacitors. J. Alloys Compd. 2017, 695, 3376–3392. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.; Stoller, M.D.; Zhu, Y.; Ji, H.; Murali, S. Highly Conductive and Porous Activated Reduced Graphene Oxide Films for High-Power Supercapacitors. Nano Lett. 2012, 12, 1806–1812. [Google Scholar] [CrossRef]
- Kim, T.; Jung, G.; Yoo, S.; Suh, K.S.; Ruoff, R.S. Activated Graphene-Based Carbons as Supercapacitor Electrodes with Macro- and Mesopores. ACS Nano 2013, 7, 6899–6905. [Google Scholar] [CrossRef] [PubMed]
- Romero-Anaya, A.J.; Ouzzine, M.; Lillo-Ródenas, M.A.; Linares-Solano, A. Spherical carbons: Synthesis, characterization and activation processes. Carbon 2014, 68, 296–307. [Google Scholar] [CrossRef]
- Bedin, K.C.; Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Almeida, V.C. KOH-activated carbon prepared from sucrose spherical carbon: Adsorption equilibrium, kinetic and thermodynamic studies for Methylene Blue removal. Chem. Eng. J. 2016, 286, 476–484. [Google Scholar] [CrossRef]
- Härmas, M.; Thomberg, T.; Romann, T.; Jänes, A.; Lust, E. Carbon for Energy Storage Derived from Granulated White Sugar by Hydrothermal Carbonization and Subsequent Zinc Chloride Activation. J. Electrochem. Soc. 2017, 164, A1866–A1872. [Google Scholar] [CrossRef]
- Yang, X.; Xia, H.; Liang, Z.; Li, H.; Yu, H. Monodisperse Carbon Nanospheres with Hierarchical Porous Structure as Electrode Material for Supercapacitor. Nanoscale Res. Lett. 2017, 12, 550. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Fuertes, A.B. A Green Approach to High-Performance Supercapacitor Electrodes: The Chemical Activation of Hydrochar with Potassium Bicarbonate. ChemSusChem 2016, 9, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Moyo, B.; Momodu, D.; Fasakin, O.; Bello, A.; Dangbegnon, J.; Manyala, N. Electrochemical analysis of nanoporous carbons derived from activation of polypyrrole for stable supercapacitors. J. Mater. Sci. 2018, 53, 5229–5241. [Google Scholar] [CrossRef]
- Kim, H.; Fortunato, M.E.; Xu, H.; Bang, J.H.; Suslick, K.S. Carbon Microspheres as Supercapacitors. J. Phys. Chem. C 2011, 115, 20481–20486. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Cui, C.; Fan, H.; Wang, R. Heteroatom-doped Carbon Spheres from Hierarchical Hollow Covalent Organic Framework Precursors for Metal-Free Catalysis. ChemSusChem 2017, 10, 4921–4926. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiong, Y.; Yu, T.; Zhu, P.; Yan, X.; Wang, Z. Boron and nitrogen co-doped porous carbon with a high concentration of boron and its superior capacitive behavior. Carbon 2017, 113, 266–273. [Google Scholar] [CrossRef]
- Bolouri, K.S.; Amouroux, J. Reactor design and energy concepts for a plasma process of acetylene black production. Plasma Chem. Plasma Process. 1986, 6, 335–348. [Google Scholar] [CrossRef]
- Mutuma, B.K.; Matsoso, B.J.; Ranganathan, K.; Keartland, J.M.; Wamwangi, D.; Coville, N.J. Generation of radical species in CVD grown pristine and N-doped solid carbon spheres using H2 and Ar as carrier gases. RSC Adv. 2017, 7, 21187–21195. [Google Scholar] [CrossRef]
- Matsoso, B.J.; Ranganathan, K.; Mutuma, B.K.; Lerotholi, T.; Jones, G.; Coville, N.J. Time-dependent evolution of the nitrogen configurations in N-doped graphene films. RSC Adv. 2016, 6, 106914–106920. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Z.; Wang, J.; Ma, J.; Zhang, M.; Liu, L. Solvothermal One-Step Synthesis of Ni–Al Layered Double Hydroxide/Carbon Nanotube/Reduced Graphene Oxide Sheet Ternary Nanocomposite with Ultrahigh Capacitance for Supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 5443–5454. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.C.; Wang, Z.L. On Accretion of Nanosize Carbon Spheres. J. Phys. Chem. 1996, 100, 5163–5165. [Google Scholar] [CrossRef]
- Singh, M.; Wal, R.V.; Singh, M.; Wal, R.L.V. Nanostructure Quantification of Carbon Blacks. C 2018, 5, 2. [Google Scholar] [CrossRef]
- Lahaye, J.; Ehrburger-Dolle, F. Mechanisms of carbon black formation. Correlation with the morphology of aggregates. Carbon 1994, 32, 1319–1324. [Google Scholar] [CrossRef]
- Pantea, D.; Darmstadt, H.; Kaliaguine, S.; Roy, C. Heat-treatment of carbon blacks obtained by pyrolysis of used tires. Effect on the surface chemistry, porosity and electrical conductivity. J. Anal. Appl. Pyrolysis 2003, 67, 55–76. [Google Scholar] [CrossRef]
- Coville, N.J.; Mhlanga, S.D.; Nxumalo, E.N.; Shaikjee, A. A review of shaped carbon nanomaterials. S. Afr. J. Sci. 2011, 107, 1–15. [Google Scholar] [CrossRef]
- Wang, Z.L.; Kang, Z.C. Pairing of pentagonal and heptagonal carbon rings in the growth of nanosize carbon spheres synthesized by a mixed-valent oxide-catalytic carbonization process. J. Phys. Chem. 1996, 100, 17725–17731. [Google Scholar] [CrossRef]
- Wigmans, T.; Elfring, R.; Moulijn, J.A. On the mechanism of the potassium carbonate catalysed gasification of activated carbon: The influence of the catalyst concentration on the reactivity and selectivity at low steam pressures. Carbon 1983, 21, 1–12. [Google Scholar] [CrossRef]
- Kim, S.-K.; Jung, E.; Goodman, M.D.; Schweizer, K.S.; Tatsuda, N.; Yano, K. Self-Assembly of Monodisperse Starburst Carbon Spheres into Hierarchically Organized Nanostructured Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2015, 7, 9128–9133. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, T.; Yamazato, M.; Higa, A.; Toguchi, M. Raman Analysis of trans-Polyacetylene Chains in Hydrogenated Amorphous Carbon Films. Jpn. J. Appl. Phys. 2007, 46, 756–760. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 75414. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef]
- Matsoso, B.J.; Ranganathan, K.; Mutuma, B.K.; Lerotholi, T.; Jones, G.; Coville, N.J. Synthesis and characterization of boron carbon oxynitride films with tunable composition using methane, boric acid and ammonia. New J. Chem. 2017, 41, 9497–9504. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef]
- Petit, C.; Kante, K.; Bandosz, T.J. The role of sulfur-containing groups in ammonia retention on activated carbons. Carbon 2010, 48, 654–667. [Google Scholar] [CrossRef]
- Shruthi, T.K.; Kumar, M.S.; Arjunan, M.; Pratap, A.; Chandrasekaran, N. Graphene oxide aided structural tailoring of 3-D N-doped amorphous carbon network for enhanced energy storage. RSC Adv. 2015, 5, 93423–93432. [Google Scholar] [CrossRef]
- Burgess, C.G.V.; Everett, D.H.; Nuttall, S. Adsorption hysteresis in porous materials. Pure Appl. Chem. 1989, 61, 1845–1852. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Ozaki, J.; Kimura, N.; Anahara, T.; Oya, A. Preparation and oxygen reduction activity of BN-doped carbons. Carbon 2007, 45, 1847–1853. [Google Scholar] [CrossRef]
- Han, W.-Q.; Brutchey, R.; Tilley, T.D.; Zettl, A. Activated Boron Nitride Derived from Activated Carbon. Nano Lett. 2004, 4, 173–176. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, G.; Guo, Z.; Li, X.; Sun, D.; Yu, X. Boron and nitrogen co-doped porous carbon nanotubes webs as a high-performance anode material for lithium ion batteries. Int. J. Hydrog. Energy. 2016, 41, 14252–14260. [Google Scholar] [CrossRef]
- Hulicova, D.; Yamashita, J.; Soneda, Y.; Hatori, H.; Kodama, M. Supercapacitors prepared from melamine-based carbon. Chem. Mater. 2005, 17, 1241–1247. [Google Scholar] [CrossRef]
- Mérel, P.; Tabbal, M.; Chaker, M.; Moisa, S.; Margot, J. Direct evaluation of the sp3 content in diamond-like-carbon films by XPS. Appl. Surf. Sci. 1998, 136, 105–110. [Google Scholar] [CrossRef]
- Dí, J.; Paolicelli, G.; Ferrer, S.; Comin, F. Separation of the sp 3 and sp 2 components in the C 1s photoemission spectra of amorphous carbon films, 1996. Phys. Rev. B 1996, 54, 8064. [Google Scholar]
- Kang, Y.; Chu, Z.; Zhang, D.; Li, G.; Jiang, Z.; Cheng, H. Incorporate boron and nitrogen into graphene to make BCN hybrid nanosheets with enhanced microwave absorbing properties. Carbon 2013, 61, 200–208. [Google Scholar] [CrossRef]
- Gao, S.; Chen, Y.; Fan, H.; Wei, X.; Hu, C.; Luo, H. Large scale production of biomass-derived N-doped porous carbon spheres for oxygen reduction and supercapacitors. J. Mater. Chem. A 2014, 2, 3317. [Google Scholar] [CrossRef]
- Jansen, R.J.J.; van Bekkum, H. XPS of nitrogen-containing functional groups on activated carbon. Carbon 1995, 33, 1021–1027. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, C.B.; Zhang, S.; Zhang, D.M.; Shen, Q.; Zhang, L.M. Effect of nitrogen pressure on structure and optical properties of pulsed laser deposited BCN thin films. Surf. Coat. Technol. 2010, 204, 1863–1867. [Google Scholar] [CrossRef]
- Enterría, M.; Pereira, M.F.R.; Martins, J.I.; Figueiredo, J.L. Hydrothermal functionalization of ordered mesoporous carbons: The effect of boron on supercapacitor performance. Carbon 2015, 95, 72–83. [Google Scholar] [CrossRef]
- Kim, C.K.; Choi, I.T.; Kang, S.H.; Kim, H.K. Anchovy-derived nitrogen and sulfur co-doped porous carbon materials for high-performance supercapacitors and dye-sensitized solar cells. RSC Adv. 2017, 7, 35565–35574. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839. [Google Scholar] [CrossRef]
- Hou, S.; Xu, X.; Wang, M.; Xu, Y.; Lu, T.; Yao, Y. Carbon-incorporated Janus-type Ni2P/Ni hollow spheres for high performance hybrid supercapacitors. J. Mater. Chem. A 2017, 5, 19054–19061. [Google Scholar] [CrossRef]
- Zeiger, M.; Jäckel, N.; Mochalin, V.N.; Presser, V. Review: Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 3172–3196. [Google Scholar] [CrossRef]
- Lee, W.; Moon, J.H. Monodispersed N-Doped Carbon Nanospheres for Supercapacitor Application. ACS Appl. Mater. Interfaces 2014, 6, 13968–13976. [Google Scholar] [CrossRef]
- Portet, C.; Yushin, G.; Gogotsi, Y. Electrochemical performance of carbon onions, nanodiamonds, carbon black and multiwalled nanotubes in electrical double layer capacitors. Carbon 2007, 45, 2511–2518. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520. [Google Scholar] [CrossRef]
- Portet, C.; Taberna, P.; Simon, P.; Laberty-Robert, C. Modification of Al current collector surface by sol–gel deposit for carbon–carbon supercapacitor applications. Electrochim. Acta 2004, 49, 905–912. [Google Scholar] [CrossRef]
- Zhang, A.; Cao, S.; Zhao, Y.; Zhang, C.; Chen, A. Facile one-pot hydrothermal synthesis of particle-based nitrogen-doped carbon spheres and their supercapacitor performance. New J. Chem. 2018, 42, 6903. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Ahmed, B.; Hedhili, M.N.; Anjum, D.H.; Alshareef, H.N. Effect of Postetch Annealing Gas Composition on the Structural and Electrochemical Properties of Ti2CTx MXene Electrodes for Supercapacitor Applications. Chem. Mater. 2015, 27, 5314–5323. [Google Scholar] [CrossRef]
- Gao, Y.; Shen, Y.; Qian, M.; Nan, X.; Redepenning, J.; Goodman, P. Chemical activation of carbon nano-onions for high-rate supercapacitor electrodes. Carbon 2012, 51, 52–58. [Google Scholar] [CrossRef]


| Material | D Band Position (cm−1) | G Band Position (cm−1) | ID/IG Ratio |
|---|---|---|---|
| CSs | 1344 | 1594 | 0.91 |
| BN-CSs | 1354 | 1590 | 0.97 |
| Activated CSs | 1343 | 1598 | 0.98 |
| Activated BN-CSs | 1340 | 1596 | 1.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutuma, B.K.; Matsoso, B.J.; Momodu, D.; Oyedotun, K.O.; Coville, N.J.; Manyala, N. Deciphering the Structural, Textural, and Electrochemical Properties of Activated BN-Doped Spherical Carbons. Nanomaterials 2019, 9, 446. https://doi.org/10.3390/nano9030446
Mutuma BK, Matsoso BJ, Momodu D, Oyedotun KO, Coville NJ, Manyala N. Deciphering the Structural, Textural, and Electrochemical Properties of Activated BN-Doped Spherical Carbons. Nanomaterials. 2019; 9(3):446. https://doi.org/10.3390/nano9030446
Chicago/Turabian StyleMutuma, Bridget K., Boitumelo J. Matsoso, Damilola Momodu, Kabir O. Oyedotun, Neil J. Coville, and Ncholu Manyala. 2019. "Deciphering the Structural, Textural, and Electrochemical Properties of Activated BN-Doped Spherical Carbons" Nanomaterials 9, no. 3: 446. https://doi.org/10.3390/nano9030446
APA StyleMutuma, B. K., Matsoso, B. J., Momodu, D., Oyedotun, K. O., Coville, N. J., & Manyala, N. (2019). Deciphering the Structural, Textural, and Electrochemical Properties of Activated BN-Doped Spherical Carbons. Nanomaterials, 9(3), 446. https://doi.org/10.3390/nano9030446







