Abstract
The fall armyworm, Spodoptera frugiperda, is a serious invasive pest of the family Noctuidae (Lepidoptera) that poses a significant threat to global crop production, with poaceae crops being particularly affected. Previous studies have indicated that, as a voracious insect, the fall armyworm possesses the potential for food source diversification. However, to date, limited research has been conducted on whether plants other than maize (Zea mays L.) and rice (Oryza sativa L.) can serve as potential food resources for the pest. In Yunnan Province, China, the distribution ranges of the fall armyworm and Ficus plants show a significant degree of overlap. Ficus species, including the widely distributed Ficus microcarpa L. f., commonly grow within or near cornfields. Our previous field studies have documented instances of fall armyworms in cornfields exhibiting feeding behavior on F. microcarpa. In this study, maize and F. microcarpa were selected as food resources for fall armyworms to compare larval feeding preferences, development time, survival rate, and reproductive capacity. The results demonstrated that when both maize and F. microcarpa were available simultaneously, fall armyworm larvae consumed both plant species. Further analysis revealed that larvae feeding on F. microcarpa exhibited a significantly longer developmental period from the third stage to pupation (14.08 ± 0.44 d) compared to those feeding on maize (9.21 ± 0.14 d). Moreover, the pupae size, pupae weight, and egg count were reduced by approximately 10%, 30%, and 30%, respectively, in larvae that fed on F. microcarpa. Despite these physiological challenges, our research findings indicated that, despite F. microcarpa not being the primary food source for fall armyworms under natural conditions, fall armyworms feeding on F. microcarpa were still capable of completing the life cycle from the third instar to the second generation when relying solely on F. microcarpa. Therefore, it is crucial to strengthen the observation and monitoring of fall armyworm populations feeding on F. microcarpa and implement targeted control strategies according to specific circumstances, thereby preventing F. microcarpa from acting as a potential host.
1. Introduction
The fall armyworm (FAW), Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), is a globally significant invasive pest that originated in the tropical and subtropical regions of the Americas [1,2,3]. This species is characterized by its broad host range, high adult feeding capacity, strong migratory potential, and remarkable reproductive ability [4,5]. It was first reported outside its native continent, Africa, in 2016 [6]. In 2018, it was first detected in Asia and subsequently exhibited rapid expansion across many Asian countries that offer favorable ecological conditions for its establishment [7]. By the end of 2018, it had migrated to Yunnan Province in southern China via Myanmar and subsequently dispersed to 27 provinces in China, posing a significant threat to the production safety of food crops in China [8]. Yunnan Province is the first province in China to experience invasion by the fall armyworm, as its climate closely resembles that of the pest’s native habitat. This climatic similarity enables the year-round reproduction of the fall armyworm in Yunnan [9]. Moreover, considering that Yunnan is the province with the richest biodiversity in China, it may encounter significant threats to its biodiversity due to the severe infestation caused by the fall armyworm.
Previous field investigations and DNA sequencing analyses have confirmed that the fall armyworm populations in Yunnan Province predominantly exhibit characteristics of the ‘maize strain’ [10]. Although the ‘maize strain’ of fall armyworm predominantly favors maize as its primary host, under natural conditions, its feeding range may extend beyond the currently documented species. Specific herbaceous plants, vines, and woody plants might serve as potential alternative hosts for fall armyworm [11,12]. A study has suggested that banana (Musa nana Lour.) may potentially serve as a host for fall armyworm; however, this investigation did not examine whether fall armyworm would preferentially feed on or lay eggs on bananas in the presence of both maize and banana [11]. A report from Brazil estimated that 76 families and 353 plant species might serve as potential hosts for the fall armyworm [5]. The potential plant hosts in this report include Ficus carica. However, this result is based purely on speculation and lacks empirical support. In China, Ficus species are primarily distributed in tropical and subtropical areas. Yunnan Province is notable as the province with the richest diversity of Ficus plants in China, hosting more than a hundred species, which constitute over 70% of the Ficus species found in China [13]. During our initial field investigations, we documented Ficus trees growing both around and within cornfields across the tropical and subtropical regions of Yunnan Province. Furthermore, we observed fall armyworm larvae infesting the leaves of Ficus microcarpa. Previous studies have demonstrated that when preferred host plants are either harvested or unavailable, the larvae of fall armyworm can shift to adjacent plants to sustain their feeding activities [14]. However, the fact that an insect larva feeds on a particular plant does not necessarily indicate that the insect is capable of completing its entire life cycle, encompassing growth, development, and reproduction, on that plant [15]. For instance, the fall armyworm can successfully complete its life cycle on plants such as maize (Zea mays L.), wheat (Triticum aestivum L.), and rice (Oryza sativa L.) [16]. Conversely, for other plants like broccoli (Brassica oleracea var. italica Plenck) and cabbage (Brassica oleracea var. capitata Linnaeus), although they are also consumed by fall armyworm larvae [14], there is currently no empirical evidence to confirm the duration required for the pest to complete its life cycle on these hosts.
A variety of factors, such as host plant characteristics and population density, the abundance of natural enemies, and climatic conditions, collectively influence fluctuations in insect populations [17,18,19,20,21]. However, the life history traits of herbivorous insects are highly sensitive to the nutritional composition of their host plants. By consuming host plants, herbivorous insects acquire essential nutrients such as amino acids, proteins, and carbohydrates, which are crucial for their growth and reproduction [22]. The nutritional resources provided by different plant species exhibit substantial variation, which profoundly influences insect growth and reproduction [23,24]. For example, Spodoptera litura Fabricius larvae reared on cauliflower showed significantly higher larval survival rates, pupal weights, eclosion rates, mean fecundity, and egg hatchability compared to those fed on pea (Pisum sativum L.) and T. aestivum [25]. Insects typically exhibit differential oviposition preferences for various host plants, often favoring nutrient-rich hosts that promote the growth and development of their offspring [26,27,28]. Nevertheless, existing research on insect oviposition and feeding preferences remains limited, and our comprehension of these behaviors in polyphagous insects is still substantially limited.
As the invasion of the fall armyworm poses a significant threat to agricultural production and food security, China has implemented an integrated series of monitoring and management strategies. These strategies incorporate a variety of approaches, such as physical control measures, chemical interventions, and biological control techniques, all aimed at effectively mitigating the adverse effects of this pest on agricultural productivity [12]. Currently, the control of fall armyworm in Yunnan Province, China, is predominantly focused on cornfields. However, with the intensification of agricultural management, this pest, which has a broad host range, may sustain its population by shifting to alternative host plants, thereby posing new challenges to crop protection strategies. In Yunnan Province, there is a notable spatial overlap in the distribution of the fall armyworm and F. microcarpa (Figure 1). Combined with our previous field observations, this finding indicates that the fall armyworm might feed on F. microcarpa. Therefore, we carried out a comparative analysis of the feeding and oviposition preferences of the fall armyworm for maize and F. microcarpa and examined the impacts of these two plants on the growth and reproduction of the fall armyworm. These findings will deepen our understanding of the biological characteristics of the fall armyworm and offer valuable scientific insights for effectively managing this voracious pest.
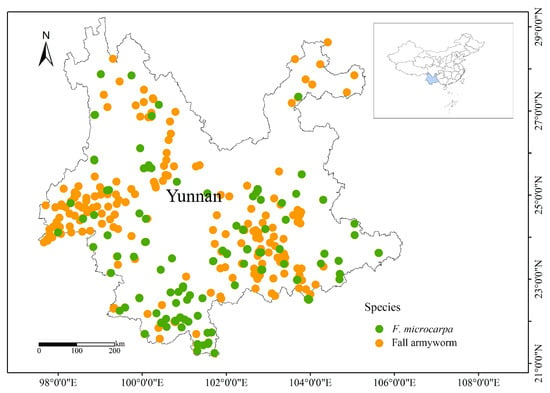
Figure 1.
The distribution of fall armyworm and F. microcarpa in Yunnan Province, China.
2. Materials and Methods
2.1. Experimental Insects
Fall armyworms were collected from a cornfield in Yunnan Province, China, and subsequently transported to the laboratory in glass bottle (diameter = 8.4 cm, high = 12 cm) for further breeding and experimentation. Fall armyworm was identified by morphological characteristics. The insects were reared in an artificial climate chamber under controlled conditions of 27 ± 1 °C, 60–70% relative humidity, and a photoperiod of 14:10 h (light/dark). Larvae were reared in plastic boxes (25 cm × 15 cm × 8 cm) specifically designed for fall armyworm breeding and fed with an artificial diet [29]. Adults were maintained in rearing cages and provided with a 10% honey–water solution. After several generations of controlled breeding, both adult insects and larvae at the required developmental stages were used for subsequent experiments.
2.2. Experimental Plants
The F. microcarpa and maize used in the experiment were obtained through artificial cultivation. Seeds of F. microcarpa were collected from Xishuangbanna, Yunnan Province, China, while the maize variety employed was ‘Nongkenuo 336’, with seeds sourced from the Chinese Academy of Agricultural Sciences in Beijing. The soil mixture for cultivating F. microcarpa and maize was formulated based on prior experimental investigations, with the optimal ratio of peat soil/vermiculite/perlite set at 10:10:1 (by mass). Furthermore, plants were irrigated every three days with a nutrient solution comprising nitrogen, phosphorus, and potassium fertilizers in a ratio of N:P:K = 1:3:2, ensuring an adequate nutrient supply to support optimal growth.
Both F. microcarpa and maize were cultivated in over 100 pots each to ensure an adequate supply of experimental materials. During the experiment, the potted F. microcarpa and maize were meticulously maintained under controlled conditions to ensure optimal plant health. When maize reached the 6–8 leaf stage and F. microcarpa had been cultivated for approximately one year, these plants were subsequently utilized in experiments evaluating the feeding and oviposition choice behaviors of the fall armyworm.
2.3. Experimental Methods
2.3.1. Comparative Analysis of the Growth and Reproduction of Fall Armyworms Feeding on Maize and F. microcarpa
Through preliminary experiments, we observed that first- and second-instar larvae exhibited extremely high mortality rates and were too fragile to handle for controlled experiments. Consequently, we initiated our experiments with third-instar larvae, which display a more stable physiological state and significantly lower mortality rates, as well as greater mobility. Third-instar larvae from the same cohort were individually reared in cylindrical containers (diameter = 7 cm, height = 3 cm) with ventilation apertures on the upper surface. These cylindrical containers with larvae were deposited in an artificial climate chamber under controlled conditions of 27 ± 1 °C, 60–70% relative humidity, and a photoperiod of 14:10 h (light/dark). Each treatment group consisted of 60 replicates. Observations were conducted daily between 10:00 and 12:00 to systematically monitor larval growth, development, survival status, and developmental stages, which were meticulously documented. Pupal weights were measured with an electronic balance (Ohaus Corporation, New Jersey, USA), and their length and width were measured with a digital vernier caliper (Guilin Guanglu Measuring Instrument Co., Ltd., Guilin, China) on the second day of larval pupation. Furthermore, key developmental parameters, including the duration of each larval instar, larval survival rate, pupation rate, and adult emergence rate, were calculated. Based on these measurements, the growth index (GI), standardized growth index (SGI), and fitness index were subsequently derived [30]. Throughout the experiment, fresh maize and F. microcarpa leaves were replaced every 1–2 days to feed the fall armyworm larvae, and rearing containers were kept clean to ensure optimal growth conditions (Figure 2B).
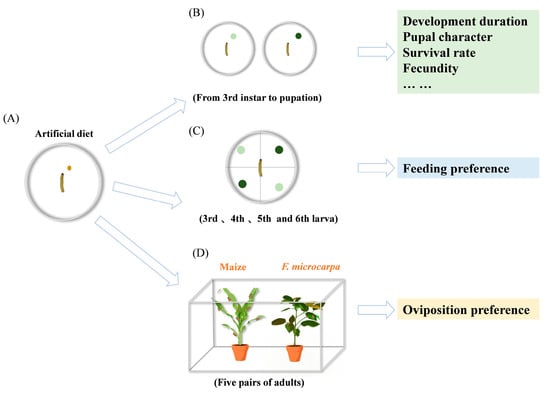
Figure 2.
(A) First- and second-instar fall armyworm larvae were reared on an artificial diet. (B) The effects of feeding on maize and F. microcarpa leaves on the growth and reproduction of fall armyworms were recorded starting from the 3rd instar. (C) Feeding preference experiment for fall armyworm between maize and F. microcarpa. (D) Oviposition preference experiment for fall armyworm between maize and F. microcarpa.
A one-day-old adult female and male pair of fall armyworms were put into a plastic bucket (diameter = 8 cm, height = 11 cm) for mating. Each container was covered with gauze and contained absorbent cotton soaked in a 10% (w/v) honey solution at the bottom as a food source for adults. The honey solution was replenished every 1–2 days to maintain a consistent food supply. Each adult pair represented one replicate, with nine replicates per treatment. Egg masses were collected daily, and the total number of eggs per unit mass was recorded until all females had died. After egg hatching, the number of hatched larvae was counted daily to determine the hatchability rate.
Growth index = pupation rate/larval development time;
Standard growth index = pupal weight/larval development time;
Fitness index = (pupation rate × pupal weight)/(larval development time × pupal stage);
Hatching rate (%) = (total hatching number/total spawning number) × 100%.
2.3.2. Investigation into the Feeding Preferences of Fall Armyworm for F. microcarpa and Maize
A choice assay experiment was performed to understand the feeding preference of the fall armyworm on two host plants. Larvae from the 3rd to 6th instars were starved for 6 h before the experiment [31]. A Petri dish (diameter = 15 cm) was divided into four equal sections, and filter paper moistened with 2 mL of distilled water was placed inside to maintain humidity. Leaves from the middle portion of maize plants and fully expanded leaves from F. microcarpa were selected, cut into leaf discs (d = 10 mm), and evenly distributed across the sections of the Petri dish, with five leaf discs of each plant species positioned in two opposing sections (totaling ten leaf discs per species per Petri dish). One larva was placed at the center of each Petri dish, and its feeding choice was recorded after 1 h (Figure 2C). The experiment was replicated 30 times for each larval instar to ensure statistical reliability.
Feeding preferences (%) = (larvae feeding on the leaves of specific plant species/total larvae) × 100%.
2.3.3. Investigation into the Oviposition Preferences of Fall Armyworm for F. microcarpa and Maize
Maize and F. microcarpa seedlings with similar height and leaf area were selected for the experiment. One pot of each plant species was evenly arranged in a rearing cage (60 cm × 40 cm × 40 cm). A cotton ball soaked in a 10% honey–water solution was placed at the center of the cage to provide nutritional supplementation for adult moths. Newly emerged, mated female fall armyworm adults were introduced into the center of the cage. The number of eggs laid on maize and F. microcarpa plants was recorded daily; then we removed the eggs after counting them, and the oviposition preference index between the two plant species was calculated. Data collection continued until all female adults had died, with 30 replicates of adult individuals used in the experiment (Figure 2D).
Oviposition preferences (%) = (number of eggs laid on the leaves of specific plant species/total number of eggs) × 100%.
2.3.4. Data Analysis
The Kaplan–Meier survival analysis and the log-rank test were used to analyze larval survival rates of the fall armyworm. The overall pupation rate, eclosion rate, and hatching rate were analyzed using chi-square tests. The Shapiro–Wilk test was used to test the normality of the data. For adult oviposition preferences and pupal length, width, fresh weight, developmental time, and feeding preferences at different ages, independent-sample t-tests were applied for data conforming to a normal distribution, while Mann–Whitney U tests were used for data that did not conform to a normal distribution. The results are presented as mean ± standard error (SE). Statistical significance was defined as p < 0.05. All statistical analyses were conducted using R software (R version 4.2.2), and graphs were generated using Origin 2023.
3. Results
3.1. The Differences in the Growth and Reproduction of Fall Armyworms When Feeding on Maize and F. microcarpa
Under laboratory rearing conditions, fall armyworm larvae that fed on F. microcarpa leaves developed normally to the pupal stage (Figure 3). The survival curves of larvae that fed on maize and F. microcarpa leaves showed similar trends. Specifically, 75.00% of larvae that fed on maize and 73.33% of larvae that fed on F. microcarpa successfully reached the reproductive stage, with no significant difference between the two groups (χ2 = 0.23, p = 0.63). Early-instar larvae exhibited relatively high mortality rates. The survival rates of larvae feeding on maize stabilized on the 11th day, while those feeding on F. microcarpa stabilized on the 16th day. By these respective time points, the larvae had largely completed pupation (Figure 4).
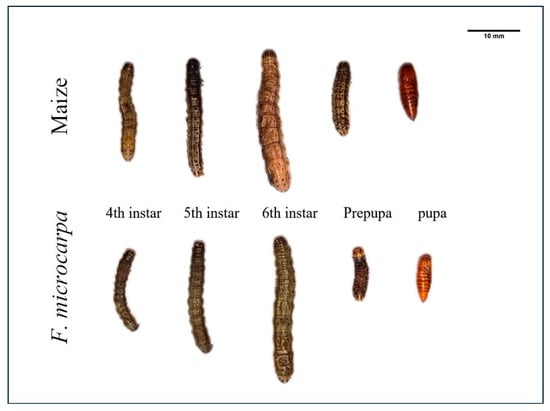
Figure 3.
Fall armyworm larvae and pupae feeding on maize and F. microcarpa.
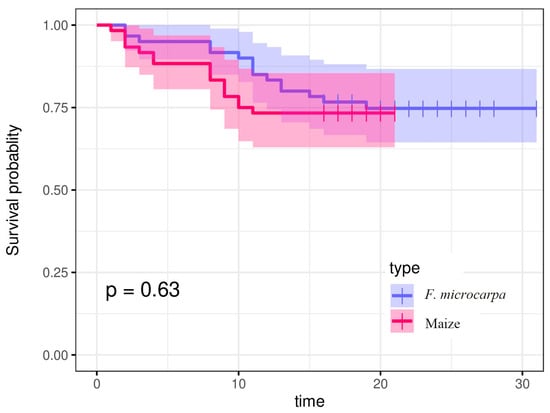
Figure 4.
The survival rate of fall armyworms feeding on maize and F. microcarpa.
During larval development, there were no significant differences in the developmental duration of the third instar (Z = 0.51, p = 0.61), fourth instar (Z = −0.80, p = 0.42), or prepupal stage (Z = −1.13, p = 0.26) between larvae reared on F. microcarpa and maize. However, larvae that fed on F. microcarpa exhibited significantly longer developmental durations in both the fifth instar and sixth instar compared to those fed maize (fifth instar: Z = −6.70, p < 0.001; sixth instar: Z = −6.70, p < 0.001). Specifically, the developmental times for larvae fed F. microcarpa were 1.94 and 3.33 times longer than those fed maize during the fifth instar and sixth instars, respectively. In general, the developmental duration from third-instar larva to pupation was significantly prolonged for fall armyworm larvae feeding on F. microcarpa compared to those feeding on maize (Z = −7.19, p < 0.001). Further analysis indicated that the growth index, standardized growth index, and fitness index of larvae feeding on F. microcarpa were significantly lower than those feeding on maize (growth index: Z = −3.76, p < 0.001; standardized growth index: Z = −8.02, p < 0.001; fitness index: Z = −5.97, p < 0.001). There were no significant differences in pupation rate, pupal development time, or eclosion rate during the pupal developmental stage (pupation rate: χ2 = 0.96, p = 0.33; pupal development time: Z = −0.21, p = 0.84; eclosion rate: χ2 = 0.67, p = 0.41). However, female adult fall armyworms that fed on different host plants exhibited variations in fecundity. Specifically, females reared on maize demonstrated significantly higher fecundity (F = 4.81, df = 16, p < 0.001) and hatching rates (χ2 = 176.93, p < 0.001) compared to those reared on F. microcarpa (Table 1).

Table 1.
Effects of feeding on F. microcarpa on the growth and reproduction of fall armyworm. Mean ± SE values followed by different letters indicate significant differences within the same column of data (p < 0.05).
3.2. Comparison of the Characteristics of Fall Armyworm Pupae Feeding on Maize and F. microcarpa
The research results demonstrated that the pupal width, pupal length, and pupal weight of fall armyworm larvae feeding on maize were significantly greater than those of larvae feeding on F. microcarpa (pupal width: F = 9.60, df = 98, p < 0.001; pupal length: Z = −5.00, p < 0.001; pupal weight: Z = −6.92, p < 0.001). Specifically, the values for larvae feeding on maize were 1.15 times, 1.09 times, and 1.41 times higher than those for larvae feeding on F. microcarpa, respectively (Figure 5).
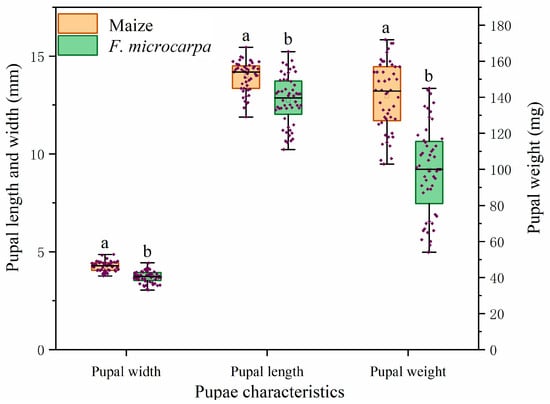
Figure 5.
Differences in pupal characteristics of fall armyworm larvae feeding on maize and F. microcarpa. Data represent means ± SE, and different letters above the bars indicate significant differences (p < 0.05).
3.3. Feeding Preferences of Fall Armyworm on Maize and F. microcarpa
When both maize and F. microcarpa are available, the third- to sixth-instar larvae of the fall armyworm demonstrate a significantly stronger preference for maize over F. microcarpa (third-instar larvae: Z = −2.02, p < 0.05; fourth-instar larvae: Z = −4.24, p < 0.05; fifth-instar larvae: Z = −2.02, p < 0.05; sixth-instar larvae: Z = −2.02, p < 0.05). Nevertheless, approximately 20% of larvae across all instars still opted to feed on F. microcarpa as their food source (Figure 6).
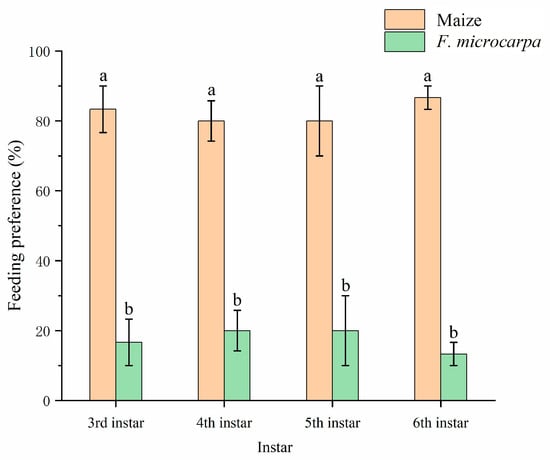
Figure 6.
Feeding preference of fall armyworm at different instars for maize and F. microcarpa. Data represent means ± SE, and different letters above the bars indicate significant differences (p < 0.05).
3.4. Oviposition Preferences of Fall Armyworm Feeding on Maize and F. microcarpa
When both maize and F. microcarpa were available, female fall armyworm adults exhibited a significant oviposition preference for maize (F = 3.48, df = 4, p < 0.05). Nevertheless, 18% of the eggs were still deposited on F. microcarpa leaves. The mean number of eggs laid on maize (mean ± SE = 804 ± 162.43) was significantly greater than that on F. microcarpa (mean ± SE = 173 ± 81.00).
The oviposition peak of the fall armyworm feeding on maize occurred on the second day (mean ± SE = 1154.00 ± 276.06), whereas the oviposition peak for the fall armyworm feeding on F. microcarpa was observed on the fifth day (Mean ± SE = 241.67 ± 120.86). The oviposition for F. microcarpa then gradually declined until ceasing entirely on the ninth day when the adults died (Figure 7).
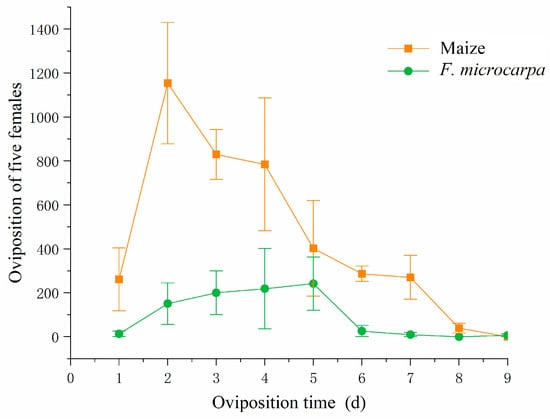
Figure 7.
Daily variation in the fecundity of fall armyworm feeding on maize and F. microcarpa leaves.
4. Discussion
Fall armyworm is a highly migratory and voracious pest that poses significant threats to agricultural production [32]. Studies have shown that its migration patterns, colonization abilities, and overwintering survival are closely linked to its host adaptability [31]. In China, the fall armyworm, a major agricultural pest first detected in 2019, has rapidly spread to most provinces within 2–3 years. This pest is known to feed on a wide variety of plants, including key economic crops such as soybean (Glycine max (L.) Merr.), tomato (Solanum lycopersicum L.), and cotton (Gossypium hirsutum L.), as well as other crops and field weeds like Cyperus rotundus L. [14,33]. As a result, it presents a significant threat to China’s food security and ecological balance [8]. Several studies have indicated that, as a polyphagous and voracious pest, the fall armyworm might possess the potential to shift to alternative hosts. However, in China, investigations into the host-shifting behavior of the fall armyworm remain relatively limited. The few existing studies exhibit certain constraints, and none have incorporated the active host selection behavior of the fall armyworm into their research scope. Yunnan Province, as the first province in China to be invaded by the fall armyworm, exhibits climatic conditions that are highly conducive to the pest’s growth and reproduction. Moreover, Yunnan is renowned for possessing the richest biodiversity in China, with a greater diversity of plant species compared to other provinces of China [34]. This extensive biodiversity provides the fall armyworm with multiple potential food sources. In nature, there have been several reports documenting the host transfer of agricultural and forestry pests. For example, polyphagous pests such as Empoasca onukii Matsuda, Caloptilia theivora Walsingham, and Buzura suppressaria Guenee successfully adapted to tea trees as a new host following a period of feeding adjustment, eventually becoming dominant species on tea plants [35]. Identifying potential hosts of key agricultural and forestry pests is crucial for effective pest management. This study implies that fall armyworm larvae can potentially complete their life cycle, including both growth and reproduction, on F. microcarpa. Additionally, the significant overlap between the distribution areas of F. microcarpa and the fall armyworm in Yunnan Province indicates a high likelihood of the fall armyworm utilizing this plant species as a host for completing either part or all of its life cycle.
Existing research has shown that host plant species are a critical determinant of the growth and reproduction of herbivorous insects [19,36]. The larval development time of herbivores is widely regarded as a key metric for evaluating their adaptability to specific host plants [37]. Typically, polyphagous insects exhibit shorter developmental periods when feeding on their preferred or optimal host plants compared to less suitable alternatives [33,38]. For example, Tuta absoluta Meyrick demonstrates a markedly reduced larval development period when feeding on its optimal host plant S. lycopersicum, in contrast to its slower development on crops such as Solanum tuberosum L. and Solanum melongena L. [39]. In this study, we observed that the development time of fall armyworm larvae fed on F. microcarpa was significantly longer than that of larvae fed on maize, which is consistent with previous findings regarding the relationship between food types and the development time of fall armyworm [40]. This phenomenon can likely be attributed to the physiological adaptation mechanisms of phytophagous insects to their optimal host plants during long-term evolution. For example, the secretion of detoxification metabolic enzymes and salivary oxidases not only enhances the digestion and absorption of nutrients from host plants but also aids in the degradation of toxic substances present in these plants [41]. Our comprehensive analysis of the fundamental growth parameters of the larvae reveals that the growth index, standard growth index, and fitness index of the fall armyworm feeding on F. microcarpa are significantly lower than those of the fall armyworm feeding on maize. These findings suggest that the fall armyworm demonstrates stronger adaptation to maize as a host plant compared to F. microcarpa. In addition to the developmental duration of the larval stage as a determinant of insect fitness, pupal length, width, and weight, reproductive capacity, and hatchability rate also serve as critical indicators of insect fitness [33,42]. Some researchers argue that pupal weight serves as a reliable indicator of successful insect development, with larger pupae conferring competitive advantages during subsequent developmental stages [43,44]. Additionally, pupal weight can reflect the adaptability of herbivorous insects to their host plants. To some extent, there is a positive correlation between female adult fecundity and pupal weight: higher pupal quality suggests that the food source is more favorable for the growth and reproduction of the insect, thus facilitating population expansion [37,45,46]. The findings of this study further demonstrate that fall armyworms feeding on maize exhibit significantly greater pupal weights and higher oviposition rates, thereby confirming that maize serves as an optimal host for fall armyworms. Additionally, our research reveals that the pupal weight of fall armyworms feeding on F. microcarpa is comparable to that of fall armyworms feeding on cotton leaves, as reported in previous studies. Given that maize, cotton, and sorghum (Sorghum bicolor L.) are the primary food sources for the maize strain of fall armyworms, these results suggest that if pupal weight is used as a metric, F. microcarpa could potentially serve as a viable alternative food source for fall armyworms [33].
In addition to examining the “passive” adaptation of fall armyworms to different host plants, we also explored their “active” selection of host plants. Previous studies have demonstrated that the oviposition and feeding preferences of pests are critical factors for understanding their ecological and evolutionary interactions with host plants, as well as for developing effective plant protection strategies [47]. Insect oviposition preferences are significantly influenced by host plant characteristics [48]. It is widely acknowledged that adult phytophagous insects select host plants for oviposition that provide optimal conditions for the growth and development of their offspring [27,28]. The results of this study demonstrated that when the two plants had equal opportunities to be selected, approximately 80% of the eggs were laid on maize, while about 20% were deposited on the leaves of F. microcarpa, and these eggs successfully hatched into larvae. This confirms the potential of F. microcarpa as a host plant from the perspective of oviposition preferences. Studies have shown that most of the insect larvae of Noctuidae have strong activity and can independently select host plants. It is particularly noteworthy that the third-instar larvae of the fall armyworm exhibit greater mobility compared to younger larvae. These larvae employ a dispersal mechanism known as ballooning, wherein they attach silk threads to plants and are subsequently carried by the wind to new locations with more abundant food resources [49]. Therefore, in addition to the oviposition preference experiment, we also carried out the feeding preference experiment of the larvae. The results showed that about 80% of the larvae of fall armyworm chose to feed on maize, but about 20% of the larvae still fed on the leaves of F. microcarpa. This result also suggests that although maize is the most suitable host for fall armyworm, this voracious insect is still likely to feed on F. microcarpa when the most suitable food resources are lacking.
In general, our study revealed no significant differences in pupation rate, eclosion rate, and prepupal and pupal development time between fall armyworms that fed on F. microcarpa and those that fed on maize. However, regarding key indicators such as growth index and fitness, individuals that fed on maize exhibited superior performance compared to those that fed on F. microcarpa. When both host plants were available, some fall armyworms still preferred F. microcarpa for feeding and oviposition. This indicates that fall armyworms may utilize F. microcarpa as an alternative host when the optimal host is unavailable or scarce. For example, fall armyworms are very likely to use F. microcarpa as a temporary ‘shelter’ to complete either a full or partial developmental cycle or reproduction after maize has been harvested or after fields have been heavily treated with pesticides. This allows the fall armyworm population to persist. Given the extensive distribution of F. microcarpa across tropical and subtropical regions of China, including Yunnan Province, its large population size, and significant spatial overlap with maize-growing areas, this study emphasizes the need for vigilance regarding the potential transfer of fall armyworm to F. microcarpa, particularly in proximity to cornfields. When F. microcarpa plants are present within or near cornfields, it is advisable to incorporate targeted control measures for these plants into integrated pest management (IPM) strategies. Such integration aims to prevent F. microcarpa from functioning as a ‘shelter’ that supports the proliferation of fall armyworm populations.
In our study, we performed an exploratory analysis of the correlation between Ficus plants and the fall armyworm, indicating that Ficus plants may potentially serve as host plants for the fall armyworm. It should be noted that the current work did not address the attraction effects of plant volatile organic compounds on the fall armyworm, nor did it systematically investigate the impacts of different host plant metabolites on fall armyworm development. Future research could investigate the relationship between various Ficus species and the fall armyworm more comprehensively by employing a wider array of diversified methodologies. Such research would not only enhance our understanding of the ecological adaptation mechanisms of the fall armyworm, but also establish a critical foundation for formulating scientifically sound and precise management strategies.
5. Conclusions
This study analyzed the potential of F. microcarpa as a host plant for the fall armyworm. The results demonstrated that fall armyworm individuals feeding on F. microcarpa exhibited slightly inferior performance compared to those feeding on maize across multiple parameters, including larval development, pupal stage, and reproductive capacity, as well as feeding and oviposition preferences. However, the fall armyworm may be able to complete part of its lifecycle or its entire life cycle when reared on F. microcarpa. This suggests that under conditions of high population density or scarcity of preferred hosts, this pest may shift to feeding on F. microcarpa, thereby sustaining its population. These findings provide critical insights for comprehensively understanding the ecological adaptation mechanisms of the fall armyworm and formulating targeted control strategies.
Author Contributions
Conceptualization, Y.Z. (Yuan Zhang) and C.C.; methodology, Y.Z. (Yuan Zhang) and Z.L. (Zongbo Li); writing—original draft preparation, C.C. and Y.Z. (Yuan Zhang); writing—review and editing, Y.Z. (Yuan Zhang), Y.W. and Y.Z. (Yana Zhou); provided the plant materials, C.C.; performed the experiments, C.C., Y.W., Y.Z. (Yana Zhou), and Z.L. (Zhu Liu); analyzed the data C.C. and Y.W.; prepared the figures and tables, C.C., Y.W., and Z.L. (Zhu Liu). All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from the National Natural Science Foundation of China (32160296, 32260719, 31560116), the Fundamental Research Program of Yunnan Province, China (202401AT070265, 202401BD070001-111), the Young Top-Notch Talent of Yunnan Outstanding Talent Program (XDYCQNRC-2022-0207), and the First Class Forestry Academic Subject in Yunnan Province (523003).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors give thanks to the Yunnan Academy of Biodiversity for kindly providing the experimental equipment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Spark, A.N. A Review of the Biology of the Fall Armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Kebede, M.; Shimalis, T. Out-break, Distribution and Management of fall armyworm, Spodoptera frugiperda J.E. Smith in Africa: The Status and Prospects. Am. Res. J. Agric. 2019, 4, 43. [Google Scholar]
- Kenis, M. Prospects for classical biological control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in invaded areas using parasitoids from the Americas. J. Econ. Entomol. 2023, 116, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Todd, E.L.; Poole, R.W. Keys and illustrations for the armyworm moths of the noctuid genus Spodoptera Guenée from the western hemisphere. Ann. Entomol. Soc. Am. 1980, 73, 722–738. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef]
- Sisay, B.; Simiyu, J.; Malusi, P.; Likhayo, P.; Mendesil, E.; Elibariki, N.; Wakgari, M.; Ayalew, G.; Tefera, T. First report of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), natural enemies from Africa. J. Appl. Entomol. 2018, 142, 800–804. [Google Scholar] [CrossRef]
- Wu, Q.L.; He, L.M.; Shen, X.J.; Jiang, Y.Y.; Liu, J.; Hu, G.; Wu, K.M. Estimation of the Potential Infestation Area of Newly-invaded Fall Armyworm Spodoptera Frugiperda in the Yangtze River Valley of China. Insects 2019, 10, 298. [Google Scholar] [CrossRef]
- Huang, Y.R.; Dong, Y.Y.; Huang, W.J.; Ren, B.Y.; Deng, Q.Y.; Shi, Y.; Bai, J.; Ren, Y.; Geng, Y.; Ma, H.Q. Overwintering Distribution of Fall Armyworm (Spodoptera frugiperda) in Yunnan, China, and Influencing Environmental Factors. Insects 2020, 11, 805. [Google Scholar] [CrossRef]
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Zhou, S.C.; Qin, Y.X.; Wang, X.Y.; Zheng, X.L.; Lu, W. Fitness of the fall armyworm Spodoptera frugiperda to a new host plant, banana (Musa nana Lour.). Chem. Biol. Technol. Agric. 2022, 9, 78. [Google Scholar] [CrossRef]
- Xue, J.B.; Chen, Y.; Kong, X.Y.; Jia, R.Z.; Jiang, X.Q.; Guo, J.Y.; Guo, Y.L.; Yang, Y. The Potential Threats of Spodoptera frugiperda on Six Economic Tree Species in the Tropical Region. Forests 2024, 15, 701. [Google Scholar] [CrossRef]
- Yu, X.; Deng, L.L. Study on the distribution of Ficus resources in Yunnan Province. J. Hubei Minzu Univ. (Nat. Sci. Ed.) 2017, 35, 176–181. [Google Scholar] [CrossRef]
- Nurkomar, I.; Trisnawati, D.W.; Fahmi, F.; Buchori, D. Survival, Development, and Fecundity of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) on Various Host Plant Species and Their Implication for Pest Management. Insects 2023, 14, 629. [Google Scholar] [CrossRef] [PubMed]
- Zalucki, M.P.; Daglish, G.J.; Firempong, S.; Twine, P. The biology and ecology of Heliothis-armigera (Hübner) and Heliothis-punctigera Wallengren (Lepidoptera, Noctuidae) in Australia: What Do We Know? Aust. J. Zool. 1986, 34, 779–814. [Google Scholar] [CrossRef]
- Han, S.P.; Zhou, Y.Y.; Wang, D.; Qin, Q.J.; Song, P.; He, Y.Z. Impact of host plants on biological characteristics and Vg/VgR expression of Spodoptera frugiperda. J. Pest Sci. 2023, 96, 1569–1577. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Zanuncio, T.V.; Zanuncio, J.C.; Medeiros, A.G.B. Species richness and fluctuation of defoliator Lepidoptera populations in Brazilian plantations of Eucalyptus grandis as affected by plant age and weather factors. For. Ecol. Manag. 2000, 137, 179–184. [Google Scholar] [CrossRef]
- Kajita, Y.; Evans, E.W. Alfalfa fields promote high reproductive rate of an invasive predatory lady beetle. Biol. Invasions. 2010, 12, 2293–2302. [Google Scholar] [CrossRef]
- Saeed, S.; Sayyed, A.H.; Ahmad, I. Effect of host plants on life-history traits of Spodoptera exigua (Lepidoptera: Noctuidae). J. Pest Sci. 2010, 83, 165–172. [Google Scholar] [CrossRef]
- Moanaro; Choudhary, J.S. Influence of weather parameters on population dynamics of thrips and mites on summer season cowpea in Eastern Plateau and Hill region of India. J. Agrometeorol. 2016, 18, 296–299. [Google Scholar] [CrossRef]
- Elsensohn, J.E.; Schal, C.; Burrack, H.J. Plasticity in Oviposition Site Selection Behavior in Drosophila suzukii (Diptera: Drosophilidae) in Relation to Adult Density and Host Distribution and Quality. J. Econ. Entomol. 2021, 114, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Umbanhowar, J.; Hastings, A. The impact of resource limitation and the phenology of parasitoid attack on the duration of insect herbivore outbreaks. Theor. Popul. Biol. 2002, 62, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Malekera, M.J.; Dhungana, S.K.; Sharma, S.R.; Lee, K.Y. Impact of Rice and Potato Host Plants Is Higher on the Reproduction than Growth of Corn Strain Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 256. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Zhang, B.; Zhuang, M.M.; Ren, M.F.; Li, D.Q.; Yan, H.W.; Long, J.M.; Jiang, X.L. Preference and performance of the fall armyworm, Spodoptera frugiperda, on six cereal crop species. Entomol. Exp. Appl. 2023, 171, 492–501. [Google Scholar] [CrossRef]
- Saeed, R.; Sayyed, A.H.; Shad, S.A.; Zaka, S.M. Effect of different host plants on the fitness of diamond-back moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Prot. 2010, 29, 178–182. [Google Scholar] [CrossRef]
- Berdegue, M.; Trumble, J.T. Effects of plant chemical extracts and physical characteristics of Apium graveolens and Chenopodium murale on host choice by Spodoptera exigua larvae. Entomol. Exp. Appl. 1996, 78, 253–262. [Google Scholar] [CrossRef]
- Pöykkö, H. Females and larvae of a geometrid moth, Cleorodes lichenaria, prefer a lichen host that assures shortest larval period. Environ. Entomol. 2006, 35, 1669–1676. [Google Scholar] [CrossRef]
- Refsnider, J.M.; Janzen, F.J. Putting Eggs in One Basket: Ecological and Evolutionary Hypotheses for Variation in Oviposition-Site Choice. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 39–57. [Google Scholar] [CrossRef]
- Wu, T.; Cao, D.H.; Liu, Y.; Yu, H.; Fu, D.Y.; Ye, H.; Xu, J. Mating-Induced Common and Sex-Specific Behavioral, Transcriptional Changes in the Moth Fall Armyworm (Spodoptera frugiperda, Noctuidae, Lepidoptera) in Laboratory. Insects 2023, 14, 209. [Google Scholar] [CrossRef]
- Zheng, L.; Tan, M.T.; Yan, S.C.; Jiang, D. Cadmium exposure-triggered growth retardation in Hyphantria cunea larvae involves disturbances in food utilization and energy metabolism. Ecotoxicol. Environ. Saf. 2023, 256, 114886. [Google Scholar] [CrossRef]
- Qi, X.W.; Hong, L.; Chen, J.; Liang, Y.Y. Fitness and cold tolerance of Spodoptera frugiperda fed on corn and two winter crops. J. Appl. Entomol. 2023, 148, 49–56. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Goergen, G.; Tounou, K.A.; Agboka, K.; Koffi, D.; Meagher, R.L. Analysis of strain distribution, migratory potential, and invasion history of fall armyworm populations in northern Sub-Saharan Africa. Sci. Rep. 2018, 8, 3710. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; He, P.Y.; Zhang, Y.Y.; Liu, T.X.; Jing, X.F.; Zhang, S.Z. The Population Growth of Spodoptera frugiperda on Six Cash Crop Species and Implications for Its Occurrence and Damage Potential in China. Insects 2020, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.S.; Chen, J.H.; Deng, T.; Sun, H. Plant diversity in Yunnan: Current status and future directions. Plant Divers. 2020, 42, 281–291. [Google Scholar] [CrossRef]
- Chen, Z.M. Chemical Ecology of Tea Pests; Shanghai Scientific & Technical Publishers: Shanghai, China, 2013. [Google Scholar]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Juárez, M.L.; Schöfl, G.; Vera, M.T.; Vilardi, J.C.; Murúa, M.G.; Willink, E.; Hänniger, S.; Heckel, D.G.; Groot, A.T. Population structure of Spodoptera frugiperda maize and rice host forms in South America: Are they host strains? Entomol. Exp. Appl. 2014, 152, 182–199. [Google Scholar] [CrossRef]
- Altaf, N.; Idrees, A.; Ullah, M.I.; Arshad, M.; Afzal, A.; Afzal, M.; Rizwan, M.; Li, J. Biotic Potential Induced by Different Host Plants in the Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 921. [Google Scholar] [CrossRef]
- Negi, S.; Sharma, P.L.; Sharma, K.C.; Verma, S.C. Effect of host plants on developmental and population parameters of invasive leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Phytoparasitica 2018, 46, 213–221. [Google Scholar] [CrossRef]
- Guo, J.F.; Zhang, M.D.; Gao, Z.P.; Wang, D.J.; He, K.L.; Wang, Z.Y. Comparison of larval performance and oviposition preference of Spodoptera frugiperda among three host plants: Potential risks to potato and tobacco crops. Insect Sci. 2021, 28, 602–610. [Google Scholar] [CrossRef]
- Tian, D.L.; Peiffer, M.; Shoemaker, E.; Tooker, J.; Haubruge, E.; Francis, F.; Luthe, D.S.; Felton, G.W. Salivary Glucose Oxidase from Caterpillars Mediates the Induction of Rapid and Delayed-Induced Defenses in the Tomato Plant. PLoS ONE 2012, 7, e36168. [Google Scholar] [CrossRef]
- Scott Brown, A.S.; Simmonds, M.S.J.; Blaney, W.M. Relationship between nutritional composition of plant species and infestation levels of thrips. J. Chem. Ecol. 2002, 28, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- William, E.M. Extrinsic effects on fecundity-maternal weight relations in capital-breeding Lepidoptera. J. Lepid. Soc. 2005, 59, 143–160. Available online: https://biostor.org/reference/113793 (accessed on 4 September 2024).
- Eck, D.J.; Shaw, R.G.; Geyer, C.J.; Kingsolver, J.G. An integrated analysis of phenotypic selection on insect body size and development time. Evolution 2015, 69, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Leuck, D.B.; Perkins, W.D. A Method of Estimating Fall Armyworm progeny reduction when evaluating control achieved by host-plant resistance. J. Econ. Entomol. 1972, 65, 482–483. [Google Scholar] [CrossRef]
- Takahashi, C.G.; Kalns, L.L.; Bernal, J.S. Plant defense against fall armyworm in micro-sympatric maize (Zea mays ssp. mays) and Balsas teosinte (Zea mays ssp. parviglumis). Entomol. Exp. Appl. 2012, 145, 191–200. [Google Scholar] [CrossRef]
- Agelopoulos, N.; Birkett, M.A.; Hick, A.J.; Hooper, A.M.; Pickett, J.A.; Pow, E.M.; Smart, L.E.; Smiley, D.W.M.; Wadhams, L.J.; Woodcock, C.M. Exploiting semiochemicals in insect control. Pestic. Sci. 1999, 55, 225–235. [Google Scholar] [CrossRef]
- De Groote, H.; Kimenju, S.C.; Munyua, B.; Palmas, S.; Kassie, M.; Bruce, A. Spread and impact of fall armyworm (Spodoptera frugiperda J.E. Smith) in maize production areas of Kenya. Agric. Ecosyst. Environ. 2020, 292, 106804. [Google Scholar] [CrossRef]
- Hellmann, J.J. The effect of an environmental change on mobile butterfly larvae and the nutritional quality of their hosts. J. Anim. Ecol. 2002, 71, 925–936. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).