A Comparative Study on the Interaction Performance of the Striped Flea Beetle with Different Fungal Entomopathogens
Abstract
1. Introduction
2. Methods and Materials
2.1. Strain of Entomopathogenic Fungi and Cultivation
2.2. Striped Flea Beetle and Rearing
2.3. Bioassay of Entomopathogenic Fungi Bioactivity to Striped Flea Beetle Adult
2.4. Observation on the Performance of Striped Flea Beetle Infected by Entomopathogenic Fungi
2.4.1. Adults Infected by Entomopathogenic Fungi
2.4.2. Larvae Infected by Entomopathogenic Fungi
3. Results
3.1. Virulence of the Entomopathogenic Fungi to the Striped Flea Beetle Adults
3.2. Morphology of the Infection Process of Entomopathogenic Fungi to Striped Flea Beetle
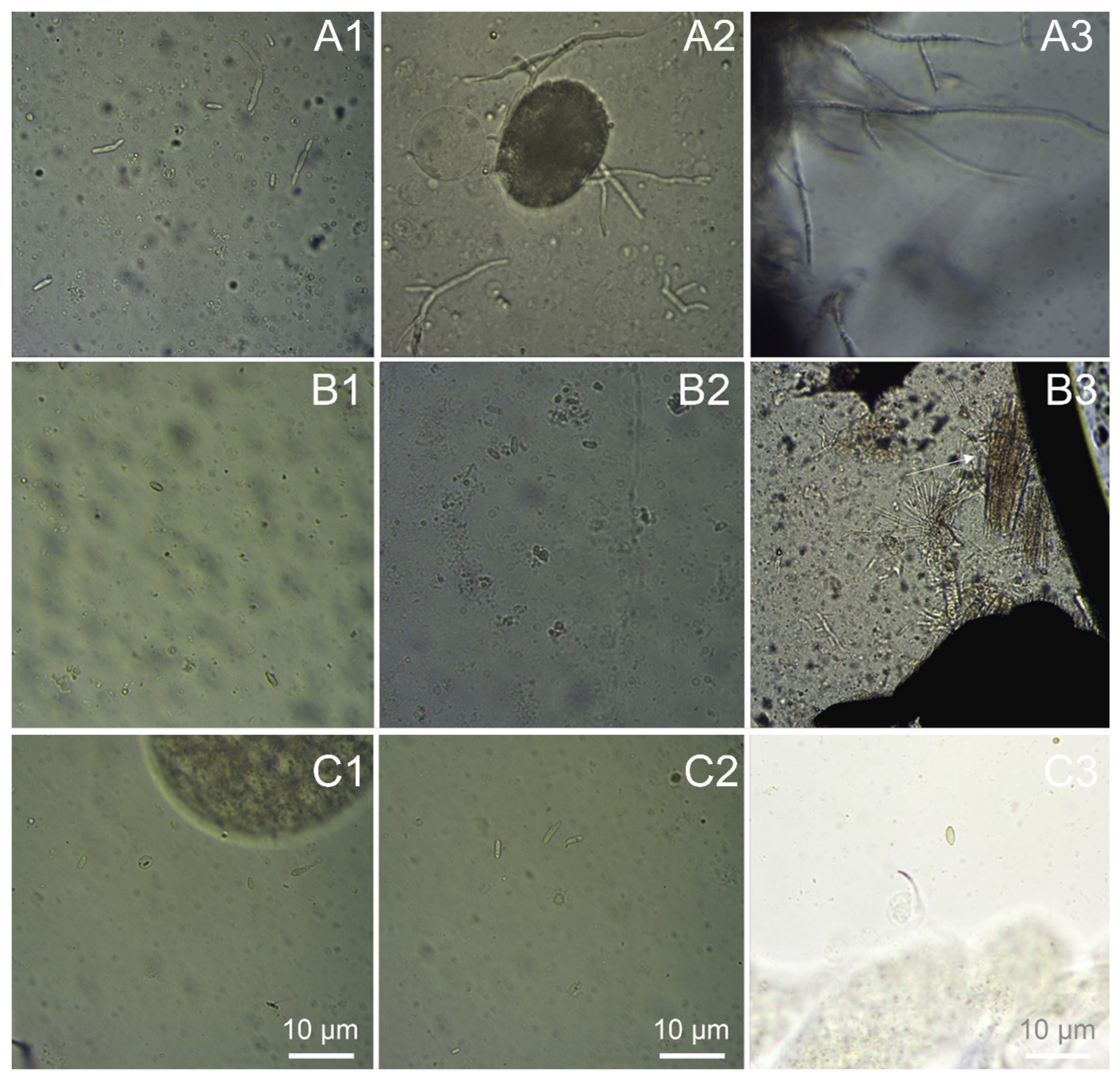
3.2.1. Attachment and Penetration of Entomopathogenic Fungi on the Striped Flea Beetle Adult’s Cuticle
3.2.2. Symptoms of Striped Flea Beetle Adults Infected by Entomopathogenic Fungi
3.2.3. Anatomical Hemocoel of Striped Flea Beetle Adults Infected by Entomopathogenic Fungi
3.2.4. Characteristics of Striped Flea Beetle Larvae Infected by Entomopathogenic Fungi
3.2.5. Anatomical Hemocoel of Striped Flea Beetle Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rousseau, M.; LeSage, L. Earliest North American occurrence of Phyllotreta striolata (Coleoptera: Chrysomelidae) from Québec, Canada. Can. Entomol. 2016, 148, 476–478. [Google Scholar] [CrossRef]
- Li, Z.Y.; Costamagna, A.C.; Beran, F.; You, M.S. Biology, ecology, and management of flea beetles in Brassica crops. Annu. Rev. Entomol. 2024, 69, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Mittapelly, P.; Guelly, K.N.; Hussain, A.; Cárcamo, H.A.; Soroka, J.J.; Vankosky, M.A.; Hegedus, D.D.; Tansey, J.A.; Costamagna, A.C.; Gavloski, J.; et al. Flea beetle (Phyllotreta spp.) management in spring-planted canola (Brassica napus L.) on the northern Great Plains of North America. Glob. Change Biol. Bioenergy 2024, 16, e13178. [Google Scholar] [CrossRef]
- Raghavendra, K.V.; Rekha, B.; Ramesh, K.B.; Felix, K.T.; Chander, S. Integrated pest management strategy for striped flea beetle, Phyllotreta striolata infesting radish (Raphanus sativus). Indian J. Agric. Sci. 2023, 93, 1308–1313. [Google Scholar] [CrossRef]
- Kong, L.; Xu, J.; Shen, W.; Zhang, S.; Xu, Z.; Zhu, K.Y. Development and evaluation of RNA microsphere-based RNAi approaches for managing the striped flea beetle (Phyllotreta striolata), a globally destructive pest of Cruciferae crops. Pest. Manag. Sci. 2025, 81, 1529–1538. [Google Scholar] [CrossRef]
- Gielen, R.; Ude, K.; Kaasik, A.; Poldmaa, K.; Teder, T.; Tammaru, T. Entomopathogenic fungi as mortality agents in insect populations: A review. Ecol. Evol. 2024, 14, e70666. [Google Scholar] [CrossRef]
- Panwar, N.; Szczepaniec, A. Endophytic entomopathogenic fungi as biological control agents of insect pests. Pest. Manag. Sci 2024, 80, 6033–6040. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Wang, J.J. The registration situation and use of mycopesticides in the world. J. Fungi 2023, 9, 940. [Google Scholar] [CrossRef]
- Rajput, M.; Sajid, M.S.; Rajput, N.A.; George, D.R.; Usman, M.; Zeeshan, M.; Iqbal, O.; Bhutto, B.; Atiq, M.; Rizwan, H.M.; et al. Entomopathogenic fungi as alternatives to chemical acaricides: Challenges, opportunities and prospects for sustainable tick control. Insects 2024, 15, 1017. [Google Scholar] [CrossRef]
- Wang, B.; Kang, Q.J.; Lu, Y.Z.; Bai, L.Q.; Wang, C.S. Unveiling the biosynthetic puzzle of destruxins in Metarhizium species. Proc. Natl. Acad. Sci. USA 2016, 113, E4756. [Google Scholar] [CrossRef]
- Ying, S.H. Subcellular biochemistry and biology of filamentous entomopathogenic fungi. Adv. Appl. Microbiol. 2024, 129, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yuan, W.; Guan, X.; Hu, Q. Biodiversity of Isaria in soil and its activity against Phyllotreta striolata. J. South China Agric. Univ. 2021, 42, 75–82. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, W.J.; He, R.K.; Pu, X.H.; Hu, Q.B.; Weng, Q.F. Screening of fungal strains and formulations of Metarhizium anisopliae to control Phyllotreta striolata in Chinese flowering cabbage. Insects 2023, 14, 567. [Google Scholar] [CrossRef]
- Nagalingam, T.; Costamagna, A.C. Two methods for rearing the striped flea beetle Phyllotreta striolata (Coleoptera: Chrysomelidae) under laboratory conditions. Can. Entomol. 2019, 151, 677–683. [Google Scholar] [CrossRef]
- Yao, L.; Pu, X.; Wu, Y.; Zhang, K.; Berestetskiy, A.; Hu, Q.; Weng, Q. An optimized bioassay system for the striped flea beetle, Phyllotreta striolata. Insects 2025, 16, 510. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data processing system (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Abrar, A.; Abbas, M.; Mehmood, S.; Ghani, N.; Fatima, A.; Shahzadi, R. Scanning electron microscopy for identification of local strain of Aspergillus parasiticus and its larvicidal efficacy against Aedes aegypti and non-target toxicity testing on fingerlings of Hypophthalmichthys molitrix. Microsc. Res. Tech. 2022, 85, 3187–3192. [Google Scholar] [CrossRef]
- Price, C.S.V.; Campbell, H.; Pope, T.W. Assessing the potential of biopesticides to control the cabbage stem flea beetle Psylliodes chrysocephala. Pest. Manag. Sci. 2024, 80, 2471–2479. [Google Scholar] [CrossRef]
- Reddy, G.V.; Tangtrakulwanich, K.; Miller, J.H.; Ophus, V.L.; Prewett, J. Sustainable management tactics for control of Phyllotreta cruciferae (Coleoptera: Chrysomelidae) on Canola in Montana. J. Econ. Entomol. 2014, 107, 661–666. [Google Scholar] [CrossRef]
- King, R.; Buer, B.; Davies, T.G.E.; Ganko, E.; Guest, M.; Hassani-Pak, K.; Hughes, D.; Raming, K.; Rawlings, C.; Williamson, M.; et al. The complete genome assemblies of 19 insect pests of worldwide importance to agriculture. Pestic. Biochem. Physiol. 2023, 191, 105339. [Google Scholar] [CrossRef]
- Rohlfs, M.; Churchill, A.C.L. Fungal secondary metabolites as modulators of interactions with insects and other arthropods. Fungal Genet. Biol. 2011, 48, 23–34. [Google Scholar] [CrossRef]
- Tong, S.; Li, M.L.; Keyhani, N.O.; Liu, Y.; Yuan, M.; Lin, D.M.; Jin, D.; Li, X.B.; Pei, Y.; Fan, Y.H. Characterization of a fungal competition factor: Production of a conidial cell-wall associated antifungal peptide. PLoS Pathog. 2020, 16, e1008518. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, H.; Pang, s.; Yin, X.; Cao, B.; Huang, J.; Xu, X.; Weng, Q.; Hu, Q. Destruxin a inhibits the hemocytin-mediated hemolymph immunity of host insects to facilitate Metarhizium infection infection. Cell Rep. 2024, 43, 113686. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.M.; Song, S.X.; Wang, C.S. Metarhizium robertsi. Trends Parasitol. 2024, 4, 192–193. [Google Scholar] [CrossRef]
- Wang, D.; Xing, P.X.; Diao, H.L.; Zhou, W.W.; Li, X.W.; Zhang, L.J.; Ma, R.Y. Pathogenicity characteristics of the entomopathogenic fungus Cordyceps javanica ij-tg19 to Acyrthosiphon pisum. Biocontrol 2023, 68, 447–458. [Google Scholar] [CrossRef]
- Yin, F.; Hu, L.; Li, Z.; Yang, X.; Kendra, P.E.; Hu, Q. Effects of destruxin a on hemocytes of the domestic silkworm, Bombyx mori. Front. Microbiol. 2023, 14, 1210647. [Google Scholar] [CrossRef]
- Xing, P.; Mao, R.; Zhang, G.; Li, Y.; Zhou, W.; Diao, H.; Ma, R. Secondary metabolites in Cordyceps javanica with insecticidal potential. Pestic. Biochem. Physiol. 2024, 204, 106076. [Google Scholar] [CrossRef]
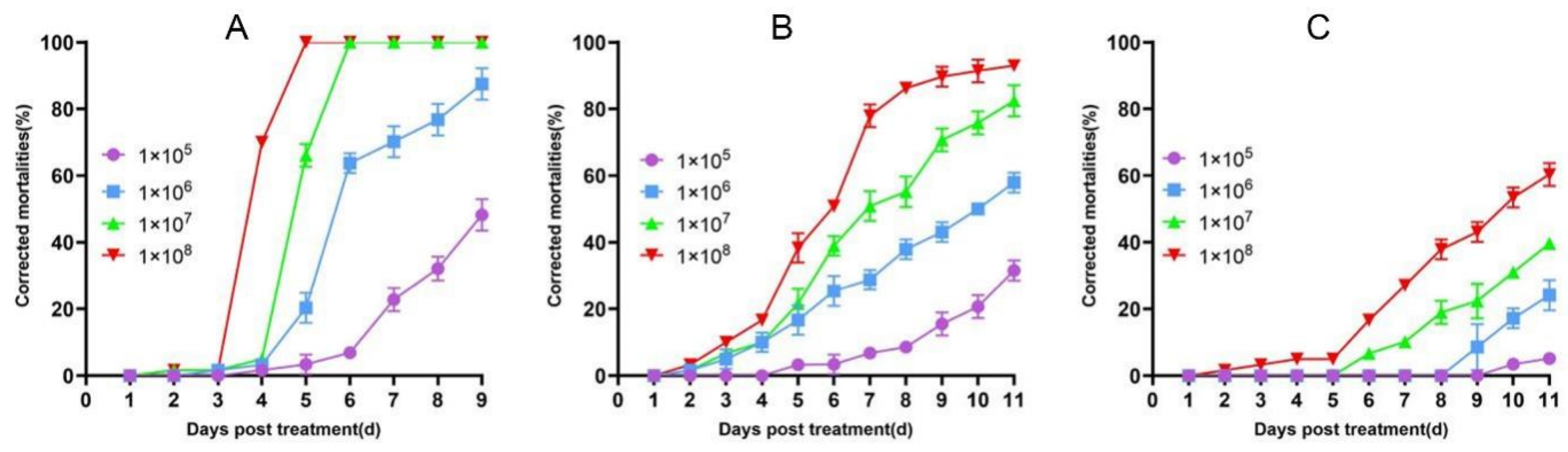



| EPF | LC-P Equation (y = A + Bx) and Significant Test | LC50 (95% Confidence Interval, ×106 Spores/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept (A) | Slope (B) | SE | R | χ2 | DF | p | ||||
| BbPs01 | 5 dpt | −4.3438 | 1.416 | 0.1682 | 0.9444 | 3.3075 | 2 | 0.1913 | 39.68 | (27.07–59.69) |
| 6 dpt | −7.3098 | 2.1267 | 0.2871 | 0.9843 | 1.2909 | 2 | 0.5244 | 6.14 | (3.64–8.83) | |
| MrCb01 | 6 dpt | 0.1077 | 0.6883 | 0.1327 | 0.9472 | 5.1407 | 2 | 0.0765 | 128.11 | 5 (9.81–529.55) |
| 10 dpt | −0.2144 | 0.8878 | 0.1180 | 0.9553 | 5.4021 | 2 | 0.0671 | 7.47 | (4.04–12.18) | |
| 11 dpt | 0.4470 | 0.8179 | 0.1180 | 0.9641 | 2.9985 | 2 | 0.2233 | 3.69 | 1 (0.54–6.72) | |
| IjH6102 | 10 dpt | 0.3774 | 0.6422 | 0.1224 | 0.9353 | 6.1430 | 2 | 0.0464 | 157.61 | (70.01–719.66) |
| 11 dpt | 0.4499 | 0.6610 | 0.1158 | 0.9409 | 6.1127 | 2 | 0.0471 | 76.46 | (39.65–217.80) | |
| EPF Concentration (spores/mL) | LT-P Equation (y = A + Bx) and Significant Test | LT50 (95% Confidence Interval, ×106 Spores/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept (A) | Slope (B) | SE | R | χ2 | DF | p | ||||
| BbPs01 | 1 × 105 | −1.2446 | 6.5669 | 0.9046 | 0.9934 | 0.8238 | 4 | 0.9352 | 8.93 | (8.16–10.34) |
| MrCb01 | 1 × 106 | 1.7713 | 3.4676 | 0.7606 | 0.9927 | 0.3112 | 4 | 0.9891 | 8.53 | (7.78–9.46) |
| 1 × 107 | 1.1181 | 4.6183 | 0.7890 | 0.9905 | 0.7423 | 4 | 0.9460 | 6.93 | (6.12–7.48) | |
| IjH6102 | 1 × 108 | / | / | / | / | / | / | / | >11 d | |
| Observed Item | BbPs01 | MrCb01 | IjH6102 | ||
|---|---|---|---|---|---|
| Adults | EPF adhesion | Germination of conidia | 12 hpt | 18 hpt | 12 hpt |
| Mucus secretion | 12 hpt | 12 hpt | 12 hpt | ||
| Penetration of germ-tube | 12–18 hpt | / | 18 hpt | ||
| Appressoria formation | 24 hpt | 24 hpt | / | ||
| Symptoms | Self-grooming | Increase | Increase | Increase | |
| Appetite decrease | 3 dpt | 3–4 dpt | 4–5 dpt | ||
| Typical symptoms | Inactivity, slow movement with a balanced style | Tremors of antennae and feet, generalized convulsions, inactivity, and body imbalance with leg stiffness | Lameness and leg weakness, imbalance, slight body convulsions | ||
| Hemocoel | Early stage | In 1–3 dpt, massive hyphal bodies | In 2–4 dpt, a few blastospores; no hyphal bodies | In 2–4 dpt, a few blastospores; no hyphal bodies | |
| Mid-stage | In 2–4 dpt, mycelia invade tissues and organs | In 3–5 dpt, no mycelia | In 4–6 dpt, no mycelia | ||
| Late stage | In 4 dpt, massive mycelia, tissues, and organs are easy to break and deconstruct | In 5- dpt, a few blastospores, muscles loose, and dissociation | In 6- dpt, a few blastospores but no other abnormalities | ||
| Larvae | Symptoms | Typical | Pink body | Melanization in cuticle and spiracle | “dendritic” melanization |
| Melanization | ~10% insects melanized; small melanized spots on the cuticle and spiracle; the melanized area is small and unchanged | ~30% larvae melanized; ~20% larvae melanized on spiracle; and the melanized area obviously increases gradually | ~15% larvae with “dendritic” melanization; the melanized area obviously increases till 2–3 days before death | ||
| Movement before death | Slowly moving since an early stage | Slowly moving, no other abnormal movement | Slowly moving; no other abnormal movement | ||
| Hemocoel | Early stage | At 1–3 dpt, massive hyphal bodies and no melanization | At 1–4 dpt, a few blastospores and no melanization | At 1–5 dpt, mycelia penetrating tracheae and forming a melanized capsule | |
| Mid-stage | At 4–6 dpt, massive mycelia and melanized clumps | At 4–7 dpt, melanization occurred but no mycelia | At 5–9 dpt, “dendritic” melanization in tracheae; no mycelia | ||
| Late stage | At 6 dpt, mycelia invade tissues and organs and grow on the body surface | At 7 dpt, a few blastospores, but no mycelia in the hemocoel | At 9 dpt, mycelia penetrating tracheae | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, X.; Hu, X.; Zhang, K.; Berestetskiy, A.; Dubovik, V.; Hu, Q.; Weng, Q. A Comparative Study on the Interaction Performance of the Striped Flea Beetle with Different Fungal Entomopathogens. Agriculture 2025, 15, 1188. https://doi.org/10.3390/agriculture15111188
Pu X, Hu X, Zhang K, Berestetskiy A, Dubovik V, Hu Q, Weng Q. A Comparative Study on the Interaction Performance of the Striped Flea Beetle with Different Fungal Entomopathogens. Agriculture. 2025; 15(11):1188. https://doi.org/10.3390/agriculture15111188
Chicago/Turabian StylePu, Xinhua, Xiangyu Hu, Ke Zhang, Alexander Berestetskiy, Vsevolod Dubovik, Qiongbo Hu, and Qunfang Weng. 2025. "A Comparative Study on the Interaction Performance of the Striped Flea Beetle with Different Fungal Entomopathogens" Agriculture 15, no. 11: 1188. https://doi.org/10.3390/agriculture15111188
APA StylePu, X., Hu, X., Zhang, K., Berestetskiy, A., Dubovik, V., Hu, Q., & Weng, Q. (2025). A Comparative Study on the Interaction Performance of the Striped Flea Beetle with Different Fungal Entomopathogens. Agriculture, 15(11), 1188. https://doi.org/10.3390/agriculture15111188








