Abstract
In the presented study a total of 104 samples of herbal material (herbage of thyme, savory, sage, rock rose, marjoram, horsetail, oregano, basil; seeds of flax; roots of liquorice, valerian and lovage, flowers of coneflower and camomile and fruits of fennel and caraway) were analysed for the content of 250 pesticides. Residues of 16 pesticides were identified in 72.1% of the analysed herbal samples. In 11 of the analysed samples of thyme herbage and in one sample of basil herbage concentrations exceeding the maximum allowable levels were demonstrated. Residues of the identified substances were detected most frequently in samples of thyme (66.34%), compared to the other groups of analysed herbal material where the percentage share of samples containing the compounds sought was at the level of approximately 20%.
1. Introduction
The use of herbs for medicinal purposes has a very long history and still constitutes a high proportion of the traditional therapeutic methods in use all over the world. The World Health Organisation (WHO) estimated that approximately 80% of the world’s population uses herbal products for therapeutic purposes, most frequently in the form of extracts from plants or of the active components of plants []. Commercialisation of herbal products is related to their extensive application in various production sectors, such as: food, pharmaceuticals, nutraceuticals, herbal agents, diet supplements, perfumes and fragrances, cosmetics or aromatising products [,].
In recent years, the international market noted a doubled demand for medicinal plants. According to the WHO the current demand for plant-derived medical products amounts to 14 thousand million dollars a year, and by the year 2050 it will have increased to five billion dollars []. The observed growth of interest in the use of herbal products in developed countries is the result of the assumption that “natural” implies “harmless”. However, with the increasing popularity and global expansion of the market of herbal materials, the safety of herbal products is becoming a serious problem relating to public health [].
In view of the above, the demand for herbs creates the interest of the agricultural branch. The production of herbs in Poland has a well-established tradition. The contribution of the Polish production of herbs in the European production is approximately 30%. Polish agriculture has the largest area of herbal cultivations in Europe (30,000 hectares), and the number of producers oscillates around the level of 20,000 farms annually []. Currently, about 70 species of herb plants are grown. The Polish herbal industry uses about 130 species of plants from a natural collection (10,000 tons of collected raw material) and about 60 species from crops (50,000 tons). In Poland, 29 original varieties of herbs are grown. The owner and breeder of 22 of them is the Institute of Natural Fibers and Medicinal Plants in Poznań. The vast majority of the material produced is purchased by the processing of companies, and the average purchase is 20,000 tons per year [,]. In Poland, the following raw materials are most often obtained from lowland positions: thyme herb, lemon balm leaf, peppermint leaf and herb, valerian root and rhizome, chamomile basket, plantain leaf, fennel fruit, sage leaf, cumin fruit, marigold flower and milk thistle fruit. In the mountainous part of Poland, mainly roots are produced: angelica, purple coneflower, lovage and marshmallow.
On average, 10% of Polish farms grow herbs, but one half of the total declare interest in such cultivations, which indicates a high production potential. Farmers consider herbs to be economically attractive crop plants, under conditions of guaranteed purchasing and good prices []. Therefore, the cultivation of herbal plants is a prospective direction for agricultural activity and constitutes an important supplement to the income of Polish farmers. Intensification of cultivation and a focus on obtaining high yields of good quality crop plants often results in contamination of the products with the chemical agents used in agriculture. Therefore, it is now extremely important to analyse foods of plant origin for chemical contaminants, including pesticide residues. Plant protection agents are commonly used in all sectors of agricultural production to control pests, as well as to improve yields and yield quality. They are also applied post-harvest, to extend the period of storage and to maintain desired product properties []. Caring for food safety for consumers, many countries have implemented programmes for the monitoring and official control of food regarding pesticide content, in accordance with the maximum allowable levels of these compounds [].
To ensure correct and reliable results of determination of pesticide residues in food of plant origin, including samples of herbal products, it is important to apply specific and selective analytical methods which currently include the QuEChERS (Quick Easy Cheap Effective Rugged Safe) method, combined with the techniques of gas chromatography (GC) and high performance liquid chromatography (HPLC) with mass spectrometry detector MS and tandem mass spectrometry (MS/MS) [,,,]. There is little published data on residues of plant-protection products in herbal raw materials produced in Poland. As reported by Malinowska and Jankowski [] and Dyjak et al. [], preparations containing active substances such as azoxystrobin, diphenoconazole chlorpyrifos-ethyl, diphenyloamine and tebuconazole have, to date, been used on crops in Poland.
Studies aimed at the determination of the presence of pesticides in herbal materials from agricultural production are important in their quality evaluation. These provide information on a given group of agricultural materials over a specific period of time, with emphasis on continuous improvement of that status which also, on the other hand, is affected by the relevant legislative regulations.
In view of the above, the objective of this study was to estimate the content of pesticide residues in various herbal materials originating from cultivations in the eastern part of Poland.
2. Materials and Methods
2.1. Experimental Material
The research material consisted of samples of unprocessed plant products collected at random from farms situated in the eastern part of Poland, in the period of 2016–2017. The minimum weight of a sample was 3 kg. The total number of samples was 104: 74 samples of thyme herbage, eight samples of flax seed, three samples of root of liquorice, three samples of herbage of savory, two samples of herbage of common sage, two samples of root of valerian, two samples of herbage of rock rose, two samples of flower of coneflower, and one sample each of herbage of marjoram, horsetail, oregano, basil, flower of camomile, fruit of fennel and caraway and root of lovage.
2.2. Chemicals
High-purity pesticide standards (250) were used for testing (98%–99%, Dr. Ehrenstorfer GmbH, Augsburg, Niemcy; ChemService, West Chester, PA, USA): 2,4,5-T, 2,4-D, 2,4-DB, 3,5-dichloroaniline, 3-hydroxycarbofuran, abamectin, acephate, acetamiprid, acrinathrin, alachlor, aldicarb, aldicarb sulfoxide, aldicarb sulphone, ametryn, amitraz, atrazine, azinophos-ethyl, azinophos-methyl, azoxystrobin, benfuracarb, bentazon, benzoylprop ethyl, bifenazate, bromacil, bromoxynil, bromuconazole, buprofezine, butoxycarboxin, CAP, carbaryl, carbendazim, carbetamide, arbofuran, carbosulfan, carboxin, chlorantraniliprole, chloridazon, chlorotoluron, chlorpyrifos, chlorsulfuron, clofentezine, clomazone, clothianidin, coumaphos, cyanazine, cyanofenphos, cycloate, cymoxanil, cyphenothrin, cyprofuram, DEF, demeton-s-methyl, demeton-S-methylsulphon, desethyl atrazin, desisopropyl atrazin, desmedipham, desmetryn, diafenthiuron, dialifos, diazinon, dicamba, dichlofluanid, dichloprop (2.4-DP), diclorvos, dicrotophos, diflubenzuron, dimefuron, dimethachlor, dimethenamide, dimethoate, dimethomorph, diniconazole, diphenamide, diphenylamine, disulfoton, ditalimfos, diuron, DMF, dodine, epoxiconazole, etaconazole, ethiofencarb, ethirimol, ethofenprox, etoxazole, etrimphos, fenamidon, fenamiphos, fenazaquin, fenbuconazole, fenhexamid, fenoxap-p-ethyl, fenoxycarb, fenpropimorph, fenpyroximate, fenthion, fenthion sulfon, fenuron, fipronil, flazasulfuron, florosulam, fluazifop, fluazifop-p-butyl, fluazinam, fludioxonil, flufenacet, flufenoxuron, fluometuron, fluroxypyr, flurtamon, fluthiacet methyl, flutriafol, fonofos, fosthiazate, fuberidazol, furathiocarb, halfenprox, haloxyfop, haloxyfop methyl, haloxyfop-2-ethoxyethyl, heptenophos, hexaflumuron, hexazinone, hexythiazox, imazalil, imazamox, imazapyr, imidacloprid, indoxacarb, ioxynil, iprodione, iprovalicarb, isazofos, isocarbamide, isomethiozin, isoproturon, isoxaflutole, lenacil, linuron, lufenuron, malaoxon, malathion, MCPA, MCPB, MCPP mecoprop, mecarbam, mepanipyrim, metalaxyl, metalaxyl-M, metamitron, metazachlor, metconazol, methabenzthiazuron, methacrifos, methamidophos, methidathion, methiocarb, methoprotryne, methoxyfenozide, metobromuron, metolachlor, metolachlor S, metosulam, metoxuron, metrafenon, monocrotophos, monolinuron, monuron, myclobutanil, nicosulfuron, nitenpyram, norflurazon, novaluron, omethoate, oxamyl, oxycarboxin, oxydemethon methyl, paraoxon ethyl, paraoxon methyl, parathion ethyl, pebulat, penconazole, pencycuron, phenkapton, phenmedipham, phenothrin, phenthoate, phorate, phosalone, phosmet, phosphamidon, phoxim, picoxystrobin, pirimicarb, pirimiphos methyl, prochloraz, profenofos, prometryn, propamocarb, propanil, propaquizafop, prophos, prosulfuron, pyraclostrobin, pyraflufen ethyl, pyridaphenthion, pyridate, pyrimiphos ethyl, pyriproxyfen, quinmerac, quizalofop-p-ethyl, resmethrine, rimsulfuron, sebuthylazin, sethoxydim, siltiopham, simazine, simetryn, spinosad A, spinosad D, spirotetramat, spiroxamin, sulfotep, sulprofos, tebuconazole, tebufenozide, tebufenpyrad, tebutam, teflubenzuron, tepraloxydim, terbucarb, terbumeton, terbuthialzine desethyl, terbuthylazine, tetramethrin, thiabendazole, thiacloprid, thiamethoxam, thiodicarb, thiophanate methyl, tolclofos methyl, tolylfluanid, triadimefon, tri-allate, triamiphos, triazophos, trichlorofon, triclopyr, trifloxystrobin, triflumuron, triforine.
Standard solutions of pesticide in acetonitrile, with concentration of approximately 1000 mg/L were prepared. Next, standard solutions of a mixture of pesticides in acetonitrile, with concentration of about 35 mg/L, were prepared for each of the compounds. Working standard solutions were prepared by diluting the standard mixtures of pesticide solutions with acetonitrile. All standard solutions were stored at temperature lower than −20 °C.
2.3. Pesticides Analysis
The content of pesticide residues in the analysed samples was assayed at the Central Agroecological Laboratory of the University of Life Sciences in Lublin, following a modified procedure developed in accordance with the standard PN-EN 15662 [], with the use of the QuEChERS method combined with LC–MS/MS analysis. The procedure applied in the study was approved by the Polish Centre of Accreditation (PCA 1375).
2.4. Preparation of Samples
Portions of about 3 kg of plant material were suitably mixed to obtained uniform material, and then samples of approximately 100 g were collected and homogenised. The obtained homogenisate was transferred in suitable amounts to 50 mL test tubes. In the case of dry matrices, the samples were moistened to the level of about 95%.
The next step was the addition, to the homogenisate, of 10 mL of acetonitrile (Merck) and 100 µL of internal standard of triphenylphosphate (Merck) (10 µg/mL) assayed in the mode of positive ionisation and 100 µL of internal standard of bis-nitrophenyl urea (Merck) (10 µg/mL) assayed in the mode of negative ionisation as an internal standard. The test tube was shaken vigorously for 1 min. Next, a mixture of salts QuECheRS Mix I (Agilent Technologies, Santa Clara, CA, USA) was added, and the tube was shaken again for 1 min and centrifuged for 5 min (3000 rpm). The obtained extract was purified by adding the mixture of salts QuEChERS Mix II (Agilent Technologies Santa Clara, CA, USA), while in the case of samples containing chlorophyll, the mixture QuEChERS Mix III (Agilent Technologies, Santa Clara, CA, USA) was additionally added, and the tube was shaken again for 1 min, and then centrifuged for 5 min (3000 rpm). The extract prepared in this manner was transferred to the autosampler vial and subjected to chromatographic analysis.
2.5. HPLC–MS/MS Analysis
A Shimadzu Prominence/20 series HPLC system (Shimadzu, Tokyo, Japan) and AB SCIEX 4000 QTRAP® LC–MS/MS system with Turbo V source (Foster City, California, USA) were used for LC–MS/MS analysis. The HPLC system was equipped with a LC-20 AD binary pump, a SIL-20 AC autosampler, a DGU-20A5 online degasser and a CTO-20A column oven. Nitrogen with a purity of at least 99% generated from a Peak Scientific nitro en generator (Billerica, MA, USA) was used in the ESI source and the collision cell. Analysis was performed using a 4.6 × 100 mm × 5 µm Agilent ZORBAX Eclipse XDB C18 column with a 10 µL injection. The column temperature was constant at 40 °C. A mobile phase gradient of water with 5 mM ammonium acetate and methanol with 5 mM ammonium formate and flow rate of 0.5 mL/min were used. The mobile phase was composed of HPLC-grade water containing 5 mM ammonium acetate (eluant A) and HPLC-grade methanol containing 5 mM ammonium acetate (eluant B). The gradient elution was performed as follows: 0–0.1 min: 20% B; 0.1–1 min: 20%–45% B; 1–9 min: 45%–80% B; 9–19 min: 80%–100% B, 19–20 min: 100% B; 20–21 min: 100%–20% B; 21–24 min: 20% B. A flow rate of 0.5 mL/min and an injection volume of 15 mL were used in the LC–MS/MS system.
The mass spectrometer was operated using an electrospray ionization (ESI) source in the positive and negative mode. ESI parameters were as follows: ion spray voltage 5.5 kV (ESI+) and −4.5 kV (ESI−), source temperature 600 °C, curtain gas (nitrogen) 35 psi, ion source gas “1” 50 psi, ion source gas “2” 65 psi, collision gas (nitrogen) 5 psi. ESI–MS/MS was operated in scheduled multiple reaction monitoring mode (MRM), in both positive and negative polarities, by scanning two precursor/product ion transitions for each target analyte.
3. Results
The analyses of pesticide residues in samples of herbal material covered 74 samples of thyme herbage, eight samples of flux seed, three samples of root of liquorice, three samples of herbage of savory, two samples of herbage of common sage, two samples of root of valerian, two samples of herbage of rock rose, two samples of flower of coneflower, and one sample each of herbage of marjoram, horsetail, oregano, basil, flower of camomile, fruit of fennel and caraway and root of lovage. The analyses demonstrated that among the 104 analysed samples pesticide residues were detected in 75 samples (72.1%), while in 29 samples (27.9%) no presence of those substances was found. In 11 analysed samples of thyme herbage and in one sample of basil herbage, in which the presence of the sought compounds was identified, their levels exceeded the maximum allowable concentrations. The occurrence of the analysed contaminants in the particular kinds of analysed samples of herbal material is presented in Table 1.

Table 1.
Number of samples with and without detected pesticides residues for each analysed herb sample.
Among the samples in which pesticide residues were identified, the presence of a single such substance was detected in 24 samples. Residues of two or more pesticides were found in 51 samples (68%). The presence of two pesticides was noted in a total of 18 samples (24%), in 11 samples the presence of three pesticides was found (14.67%), and in 12 and nine samples, respectively, the presence of four and five pesticides was detected (16% and 12%). One of the analysed samples contained a combination of six identified compounds (Figure 1).
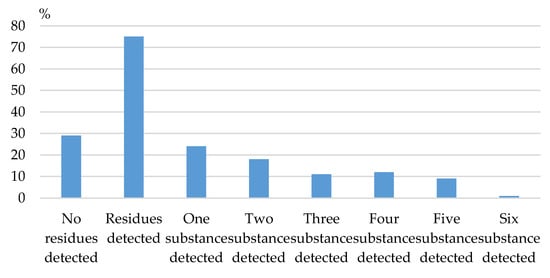
Figure 1.
Distribution of samples with detected pesticide residues.
Co-occurrence of pesticide residues was noted in 48 samples of thyme herbage (64%) and in one sample of herbage of basil, flower of coneflower, fruit of caraway, flax seed, and root of valerian and lovage (1.3%) (Table 2). In the case of the analysed samples of herbal material the most-frequently-detected combination was that of a fungicide and a herbicide (azoxystrobin and linuron)—34 samples (45.3%), a combination of two fungicides and a herbicide (azoxystrobin, carbendazim and linuron)—detected in 16 samples (21.3%), and combinations of two fungicides with two herbicides (azoxystrobin, linuron, metalaxyl and metalaxyl M)—found in eight samples (10.7%). In seven of the analysed samples of herbal material (9.3%) the presence of a combination of two fungicides with three herbicides was detected (azoxystrobin, carbendazim, linuron, metalaxyl and metalaxyl M), while in three samples (4%)—the combination of a fungicide, a herbicide and an insecticide (azoxystrobin, linuron and dimethoate) was detected—Table 2.

Table 2.
Pesticide residue concentrations in examined food samples.
In the analysed samples the presence of residues of a total of 16 pesticides was detected. The most-frequently identified ones were azoxystrobin—detected in 59 samples (78.7%), linuron—assayed in 36 samples (48%), carbendazim—detected in 29 samples (38.7%), metalaxyl and metalaxyl M—found in 21 samples (28%) and dimethoate—detected in five samples (6.7%). The frequency of occurrence of the detected pesticides is presented in Figure 2. From among 250 compounds sought in the presented experiment, the presence of 16 pesticides (6.4%) was detected in the analysed samples, which means that no presence of 234 pesticides from the examined group of plant protection agents was found.
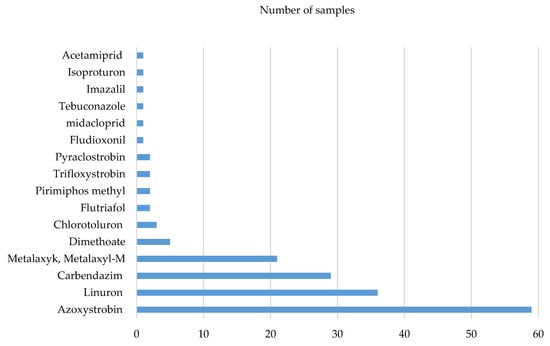
Figure 2.
Pesticide occurrence frequency in analysed samples.
In 11 analysed samples of thyme herbage the maximum allowable concentration levels were exceeded for five identified active substances—isoproturon (assayed value of 0.19 mg/kg at maximum residue level (MRL)—0.05 mg/kg), carbeddazim (assayed values of 0.68; 0.52; 0.30; 0.37; 1.7; 0.92; 0.89 and 0.14 mg/kg at MRL—0.1 mg/kg), chlorotoluron (assayed values of 0.041 and 0.048 mg/kg at MRL—0.02 mg/kg), dimethoate (assayed value of 0.21 mg/kg at MRL—0.02 mg/kg) and flutriafol (assayed value of 0.10 mg/kg at MRL—0.02 mg/kg)—Table 2.
4. Discussion
In the presented study the percentage of samples of herbal material containing pesticide residues (72%) correlates with results obtained by other authors (Table 3). In a study on fresh herbs and vegetables purchased at local supermarkets it was demonstrated that 91.7% of the samples contained residues of plant protection agents []. In a monitoring study on Egyptian herbs, vegetables and fruits in the aspect of the content of identified substances [], pesticide residues were found in 73% of the analysed samples, while in a later study, in 54.55% of all analysed samples []. The results of this study find support also in another study on the estimation of the content of pesticide residues in samples of herbs and spices, in which their authors noted the presence of contaminants in 59% of the samples []. Similar results concerning the content of contaminants in samples of herbs were obtained by Simèon et al. [] in a study on herbal material used in animal feeds, and also by Di Bella et al. [] in a study on Italian and Tunisian herbs, in which contamination with those substances was at the level of 63% and 55% of the analysed samples, respectively. Analysis of pesticide residues in Chinese medicines prepared on the base of herbal materials [,,,] showed that from 72.8% to 97.5% of the analysed samples contained at least one pesticide, while in later reports [,,,] from 36.73% to 100% of the analysed samples of herbs contained residues of active substances.

Table 3.
Summary of the most-frequently-detected pesticides in different herbs samples reported in the literature.
The number of detected pesticide residues in the presented study varied from one to six compounds in a single sample—Table 2. The highest percentage was that of samples containing from one to two identified compounds, at 32% and 24%, respectively, while a significantly smaller number of samples were found in the group containing from three to five pesticide residues—12% to 16%, with a single sample representing the presence of six active compounds. The results of the presented study support earlier reports on research on herbs, in which the numbers of compounds detected in individual samples varied from one to nine [,]. In the study on Egyptian herbs, fruits and vegetables, 43.18% of the samples were contaminated with residues of one substance, 6.06% with residues of two pesticides, and 5.3% with resides of more than two contaminants. Among the analysed samples of herbs, three samples contained residues of four pesticides, and one sample of marjoram, residues of five different active substances [].
Among the 74 samples of thyme analysed in this study, only five of them (27.88%) did not contain any residues of plant protection agents. The remaining 69 analysed samples (93.24%) contained pesticides, which is in support of the studies by Reinhold et al. [] and by Di Bella et al. [], who demonstrated similar levels of pesticide residues in the analysed samples of that herbal species, at 82% and 50%, respectively. In our study, the compounds identified most frequently in the analysed samples of thyme were azoxystrobin, linuron, carbendazim, metalaxyl and metalaxyl M, and dimethoate, while in the studies by other authors, cymoxanil, dimethoate and tebuconazole [,].
Azoxystrobin, so often identified in the presented studies, is a systemic fungicide commonly used in agriculture. It is a broad-spectrum substance. protecting plants against fungal diseases (Oomycetes, Ascomycetes, Basidiomycetes and Deuteromycetes). Its fungicidal activity results from the inhibition of mitochondrial respiration in fungi. This is achieved by the prevention of electron transfer between cytochrome b and cytochrome c. Because of its novel mode of action, azoxystrobin is effective against pathogens which have developed reduced sensitivity to other fungicides []. Linuron is a herbicide from the group of urea derivatives. It is characterized by soil action and is recommended for controlling dicotyledonous weeds. Linuron penetrates into the plant mainly through the roots and to a lesser extent through the leaves. It moves in xylem and causes disturbances of photosynthesis []. Linuron was withdrawn from use in 2018.
Numerical data contained in research reports concerning the observed presence of various pesticide residues in herbal samples is presented in Figure 3. Analysing the frequency of occurrence of the particular active substances in the analysed group of herbs, the most-frequently-detected pesticides were: hexachlorobenzene (16%), chlorpyrifos (16%), quintozene, (12%), carbendazim (12%), malathion (12%), dimethoate (8%), tebuconazole (8%), profenos (8%), benzene hexachloride—α-BHC (8%) and pentachloronitrobenzene—PCNB (8%). Two of those—carbendazim and dimethoate—were also among the most-frequently-identified pesticides in the presented study—Figure 2.
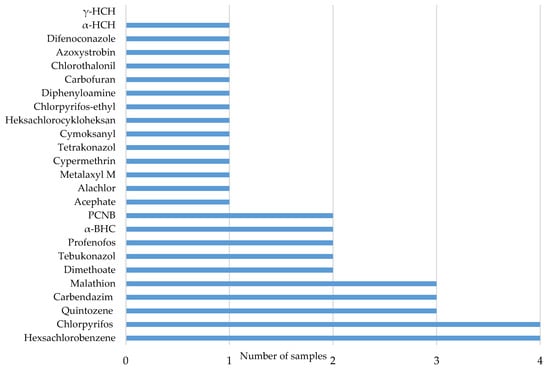
Figure 3.
The most-frequently-detected compounds in fruit and vegetable samples (see literature reports in Table 3).
In the group of analysed herbal samples, the sought compounds were detected most frequently in samples of thyme (66.34%). In the group of 69 analysed samples of that material, in which the presence of pesticide residues was identified, in 11 samples (15.9%) the maximum allowable residue concentration levels were exceeded. This confirms the results of a study by Reinholds et al. [], in which the concentrations of pesticide residues in 10% of samples of oregano and in 46% of samples of thyme were above the allowable levels. The results of the presented study are in conformance with earlier reports on studies on herbs, in which the percentage of samples with exceeded maximum permitted concentration of pesticide residues varied from 6% to 18% [,,,,].
Summing up the results of the presented study, we should emphasise that 72.1% of samples of herbal material from cultivations in the eastern part of Poland contained pesticide residues. In 11.5% of those, the maximum allowable concentrations of residue were exceeded. Pesticide residues were detected the most frequently in samples of thyme (ca. 66 %), compared to the other groups of analysed herbs, where the percentage of samples containing the sought compounds was at the level of approximately 20%. Special attention should be paid to possible contamination of analysed samples with azoxystrobin, linuron and carbendazim. Analysed samples of thyme contained the largest number and diversity of identified pesticide residues, compared to the remaining samples, which raises concern about the quality of those food components. The results of the study emphasise the importance of monitoring of pesticide residues in herbs, especially in the case of thyme which was identified as the most contaminated matrices in that group of products. The percentage share of samples containing pesticide residues in thyme was decidedly at the highest level.
5. Conclusions
In relation to the growing importance of the use of herbal plants in various domains of human life, studies in the area of analysis of pesticide residues play a significant role in the estimation of quality of that raw material. The results obtained indicate that the occurrence of pesticide residues in the analysed products from Polish cultivations cannot be considered a serious threat to human and animal health. However, the observed instances of exceeded maximum allowable concentrations of the residues in a small number of samples indicate the necessity of permanent monitoring of the content of pesticide residues in herbal matrices. To ensure high quality of food and safety of consumer health, efforts should also be aimed at the implementation of strict regulations concerning the maximum allowable concentrations of those compounds that are of key importance, with a view to the reduction of potential risk involved in the use of herbal products.
Recently, organic farming has been proposed in the cultivation of herbs. It involves the use of crop rotation and appropriate cultivation techniques and selection of resistant species and varieties. Organic farming rules exclude the possibility of using synthetic pesticides. Therefore, monitoring research may be relevant to determine how trends in herb production change. In organic farming, only biological plant protection products are allowed, e.g., natural pathogen antagonists. A negative result relative to the presence of plant protection products in such crops confirms the use of other plant protection methods. In conventional cultivation, pesticide residues in herbs are also determined by improper use by farmers (use of excessive amounts and non-compliance with waiting periods). Unfortunately, available studies indicate that MRLs were exceeded in herbs from conventional crops. In addition, the problem of using plant protection products not authorized in Poland is also noticeable. Therefore, research into the assessment of pesticide residues is of particular importance for improving the quality of herbal products.
Funding
This research received no external funding.
Conflicts of Interest
The author declares that she has no conflicts of interest to disclose.
References
- Shaban, N.S.; Abdou, K.A.; Hassan, N.E.-H.Y. Impact of toxic heavy metals and pesticide residues in herbal products. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 102–106. [Google Scholar] [CrossRef]
- Kosalec, I.; Cvek, J.; Tomić, S. Contaminants of medicinal herbs and herbal products. Arch. Ind. Hyg. Toxicol. 2009, 60, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, V.; Basak, B.B.; Varghese, T.S.; Saha, A. Residues and contaminants in medicinal herbs—A review. Phytochem. Lett. 2015, 14, 67–78. [Google Scholar] [CrossRef]
- WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residue; WHO: Geneva, Switzerland, 2007.
- Spychalski, G. Determinants of growing herbs in polish agriculture. Herba Pol. 2014, 59, 5–18. [Google Scholar] [CrossRef][Green Version]
- Forycka, A.; Buchwald, W. Badania zasobów naturalnych roślin leczniczych objętych w Polsce ochroną prawną. Herba Pol. 2008, 54, 81–112. [Google Scholar]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Kowalska, G.; Kowalski, R. Pestycydy—zakres i ryzyko stosowania, korzyści i zagrożenia. Praca przeglądowa. Ann. Hortic. 2019, 29, 5–25. [Google Scholar] [CrossRef]
- Perez-Parada, A.; Colazzo, M.; Besil, N.; Dellacassa, E.; Cesio, V.; Heinzen, H.; Amadeo, R. Pesticide Residues in Natural Products with Pharmaceutical Use: Occurrence, Analytical Advances and Perspectives. In Pesticides in the Modern World—Trends in Pesticides Analysis; IntechOpen Limited: London, UK, 2011; pp. 357–390. [Google Scholar]
- Nantia, E.A.; Moreno-González, D.; Manfo, F.P.T.; Gámiz-Gracia, L.; García-Campaña, A.M. QuEChERS-based method for the determination of carbamate residues in aromatic herbs by UHPLC-MS/MS. Food Chem. 2017, 216, 334–341. [Google Scholar] [CrossRef]
- Tripathy, V.; Saha, A.; Kumar, J. Detection of pesticides in popular medicinal herbs: A modified QuEChERS and gas chromatography–mass spectrometry based approach. J. Food Sci. Technol. 2017, 54, 458–468. [Google Scholar] [CrossRef]
- Kowalska, G.; Kowalski, R. Badania pozostałości pestycydów w żywności pochodzenia roślinnego przy użyciu metody QuEChERs i technik chromatograficznych GC i HPLC z detektorem spektrometrii mas MS i MS/MS. Agron. Sci. 2019, 74, 99–112. [Google Scholar] [CrossRef]
- Malinowska, E.; Jankowski, K. Pesticide residues in some herbs growing in agricultural areas in Poland. Environ. Monit. Assess. 2015, 187, 775. [Google Scholar] [CrossRef] [PubMed]
- Dyjak, K.; Michota-Katulska, E.; Zegan, M. Pilotażowe badania pozostałości pestycydów w wybranych świeżych ziołach i warzywach przyprawowych zakupionych w krajowych supermarketach. Zywn. Nauk. Technol. Jakosc/Food. Sci. Technol. Qual. 2017, 24, 126–138. [Google Scholar]
- Pn-En 15662 Foods of Plant Origin—Determination of Pesticide Residues Using Gc-Ms and/or Lc-Ms/Ms Following Acetonitrile Extraction/Partitioning and Cleanup by Dispersive Spe—Quechers-Method; BRITISH STANDARD: London, UK, 2008.
- Dogheim, S.M.; Ashraf, E.M.M.; Alla, S.A.G.; Khorshid, M.A.; Fahmy, S.M. Pesticides and heavy metals levels in Egyptian leafy vegetables and some aromatic medicinal plants. Food Addit. Contam. 2004, 21, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Farag, R.S.; Abdel Latif, M.S.; Abd El-Gawad, A.E.; Dogheim, S.M. Monitoring of pesticide residues in some Egyptian herbs, fruits and vegetables. Int. Food Res. J. 2011, 18, 659–665. [Google Scholar]
- Reinholds, I.; Pugajeva, I.; Bavrins, K.; Kuckovska, G.; Bartkevics, V. Mycotoxins, pesticides and toxic metals in commercial spices and herbs. Food Addit. Contam. Part B 2017, 10, 5–14. [Google Scholar] [CrossRef]
- Simeon, F.M.; Coralie, A.; Clara, L.; Johann, H.; Sylvain, K. Pesticide residues in botanics used in feed additives: Focusing on wild vs. cultivable plants. In sed in feed additives: Focusing on wild vs. cultivable plants. In Proceedings of the Proceedings of the 4th World Congress on Civil, Structural, and Environmental Engineering (CSEE’19), Rome, Italy, 7–9 April 2019; p. ICEPTP 130. [Google Scholar]
- Di Bella, G.; Potortì, A.G.; Ben Tekaya, A.; Beltifa, A.; Ben Mansour, H.; Sajia, E.; Bartolomeo, G.; Naccari, C.; Dugo, G.; Lo Turco, V. Organic contamination of Italian and Tunisian culinary herbs and spices. J. Environ. Sci. Heal. Part B 2019, 54, 345–356. [Google Scholar] [CrossRef]
- Leung, K.S.-Y.; Chan, K.; Chan, C.-L.; Lu, G.-H. Systematic evaluation of organochlorine pesticide residues in Chinese materia medica. Phyther. Res. 2005, 19, 514–518. [Google Scholar] [CrossRef]
- Sun, N.; Hao, L.; Xue, J.; Jin, H.; Tian, J.; Lin, R. Multi-residue analysis of 18 organochlorine pesticides in 10 traditional chinese medicines by gas chromatography (GC). J. Health Sci. 2007, 53, 464–469. [Google Scholar] [CrossRef][Green Version]
- Rai, V.; Kakkar, P.; Singh, J.; Misra, C.; Kumar, S.; Mehrotra, S. Toxic metals and organochlorine pesticides residue in single herbal drugs used in important ayurvedic formulation—‘Dashmoola’. Environ. Monit. Assess. 2008, 143, 273–277. [Google Scholar] [CrossRef]
- Xue, J.; Hao, L.; Peng, F. Residues of 18 organochlorine pesticides in 30 traditional Chinese medicines. Chemosphere 2008, 71, 1051–1055. [Google Scholar] [CrossRef]
- Harris, E.S.J.; Cao, S.; Littlefield, B.A.; Craycroft, J.A.; Scholten, R.; Kaptchuk, T.; Fu, Y.; Wang, W.; Liu, Y.; Chen, H.; et al. Heavy metal and pesticide content in commonly prescribed individual raw Chinese Herbal Medicines. Sci. Total Environ. 2011, 409, 4297–4305. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Song, Y.; Wang, Y. Rapid simultaneous determination of multiple pesticide residues in traditional Chinese medicines using programmed temperature vaporizer injection-fast gas chromatography coupled with mass spectrometry. J. Sep. Sci. 2011, 34, 3372–3382. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Tong, Y.; Xue, J.; Liu, D.; Wu, X. Multi-residual pesticide monitoring in commercial Chinese herbal medicines by gas chromatography–triple quadrupole tandem mass spectrometry. Food Anal. Methods 2014, 7, 135–145. [Google Scholar] [CrossRef]
- Chen, L.; Song, F.; Liu, Z.; Zheng, Z.; Xing, J.; Liu, S. Multi-residue method for fast determination of pesticide residues in plants used in traditional chinese medicine by ultra-high-performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2012, 1225, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Abou-Arab, A.A.; Abou Donia, M. Pesticide residues in some Egyptian spices and medicinal plants as affected by processing. Food Chem. 2001, 72, 439–445. [Google Scholar] [CrossRef]
- Hewitt, H.G. Strobilurins. In Fungicides in Crop Protection; CAB International: New York, NY, USA, 1998; pp. 128–129. [Google Scholar]
- Correia, N.M.; Carvalho, A.D.F. Selectivity of linuron herbicide for carrot when sprayed in post-emergence. Planta Daninha 2017, 35, 7–8. [Google Scholar] [CrossRef][Green Version]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).