Cognitive Training Combined with Multifocal tDCS over the Reading Network Improves Reading Performance: A Case of Severe Dyslexia
Abstract
1. Introduction
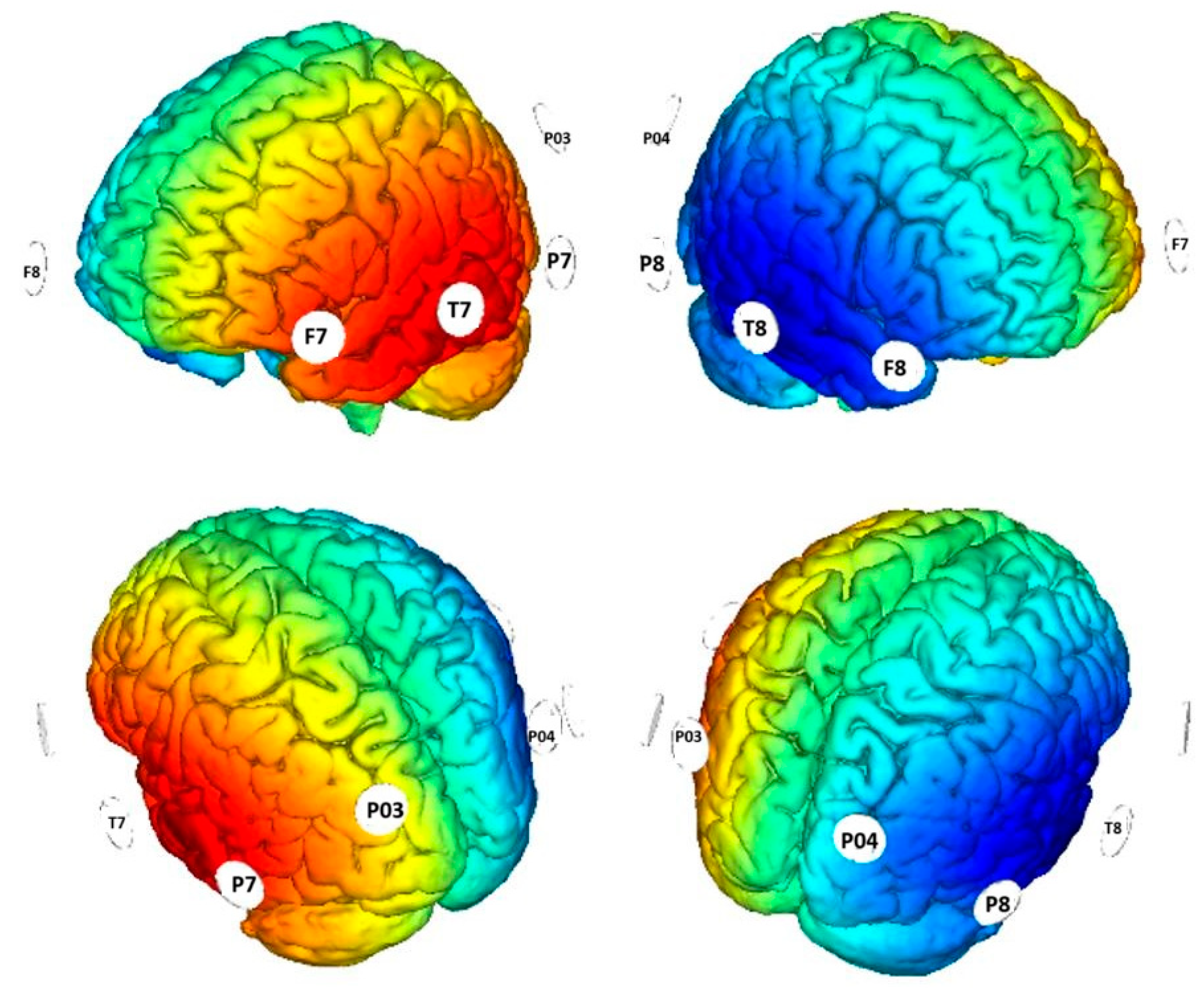
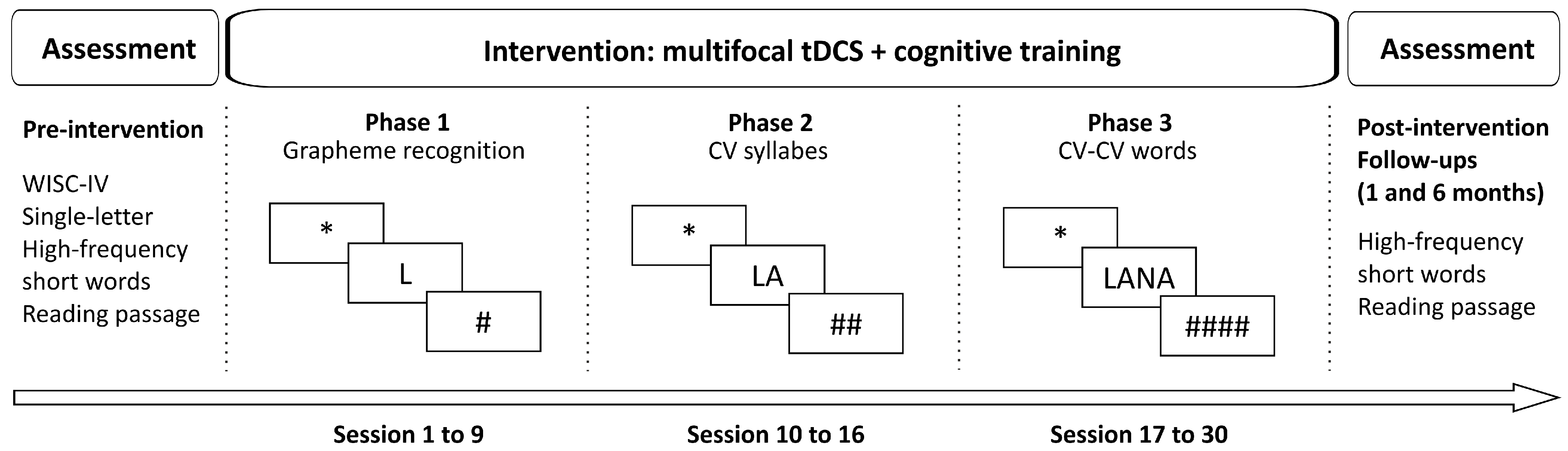
2. Materials and Methods
3. Descriptives Results
- Single Grapheme Training
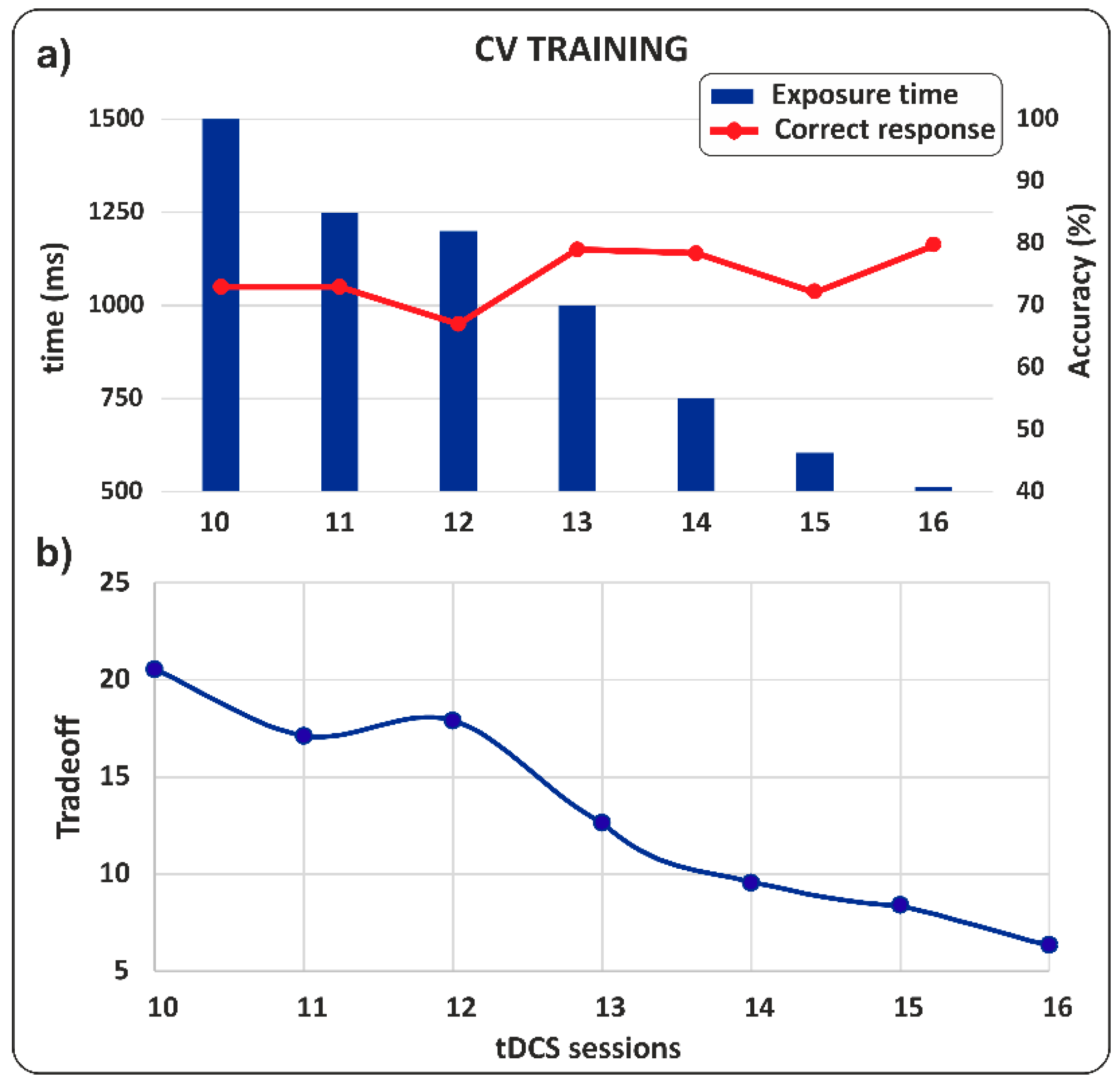
- CV Training
- CV-CV word training
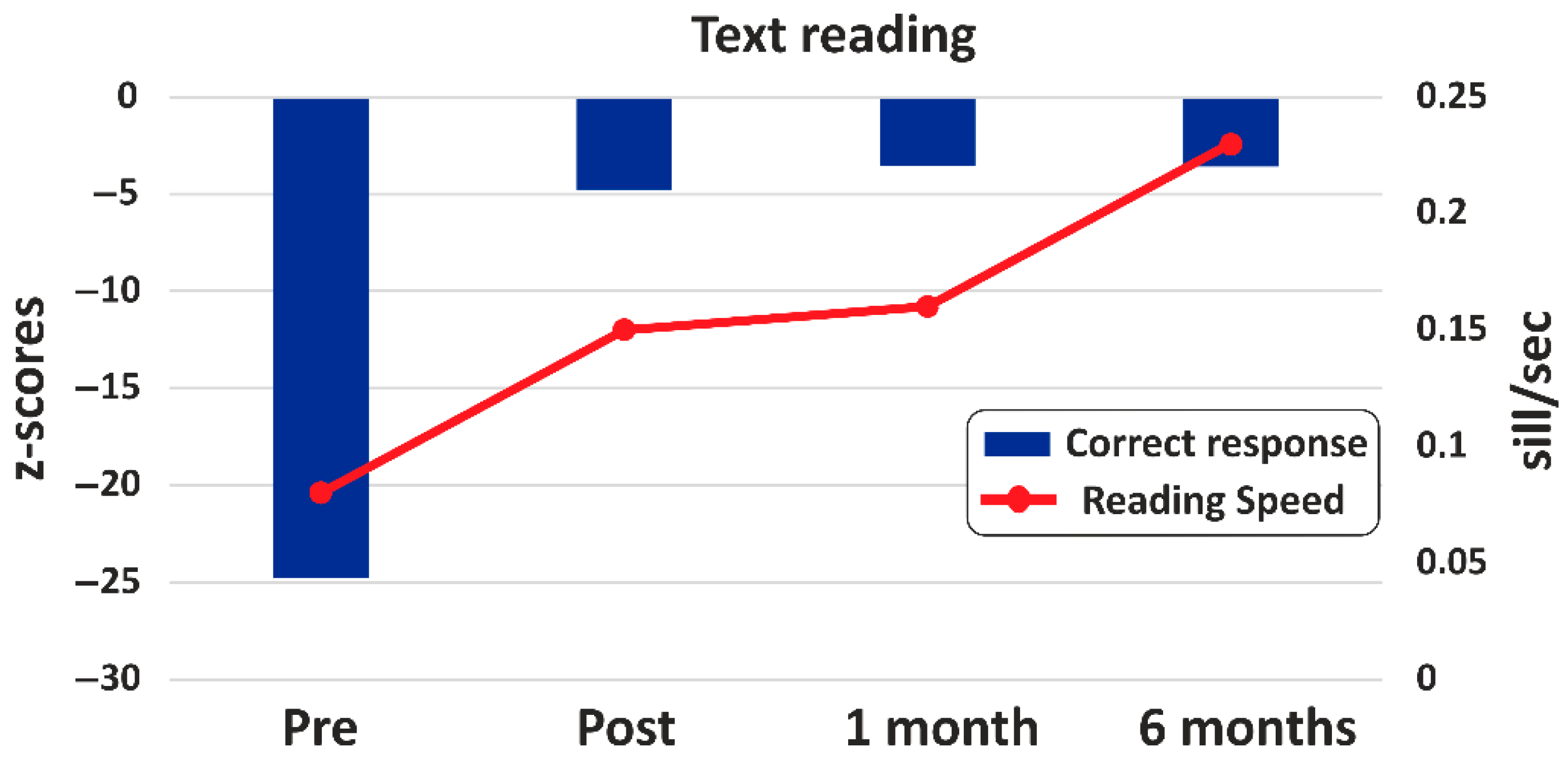
- Text Reading
- High-frequency short words
4. Comments
5. General Discussion
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galuschka, K.; Ise, E.; Krick, K.; Schulte-Korne, G. Effectiveness of treatment approaches for children and adolescents with reading disabilities: A meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e89900. [Google Scholar] [CrossRef]
- Łuniewska, M.; Chyl, K.; Dębska, A.; Kacprzak, A.; Plewko, J.; Szczerbiński, M.; Szewczyk, J.; Grabowska, A.; Jednoróg, K. Neither action nor phonological video games make dyslexic children read better. Sci. Rep. 2018, 8, 549. [Google Scholar] [CrossRef]
- Franceschini, S.; Trevisan, P.; Ronconi, L.; Bertoni, S.; Colmar, S.; Double, K.; Facoetti, A.; Gori, S. Action video games improve reading abilities and visual-to-auditory attentional shifting in English-speaking children with dyslexia. Sci. Rep. 2017, 7, 5863. [Google Scholar] [CrossRef] [PubMed]
- Lawton, T. Improving dorsal stream function in dyslexics by training figure/ground motion discrimination improves attention, reading fluency, and working memory. Front. Hum. Neurosci. 2016, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Tressoldi, P.E.; Stella, G.; Faggella, M. The development of reading speed in Italians with dyslexia: A longitudinal study. J. Learn. Disabil. 2001, 34, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Wanzek, J.; Vaughn, S.; Roberts, G.; Fletcher, J.M. Efficacy of a reading intervention for middle school students identified with learning disabilities. Except. Child. 2011, 78, 73–87. [Google Scholar] [CrossRef]
- Wolff, U. Effects of a Randomized Reading Intervention Study Aimed at 9-Year-Olds: A 5-Year Follow-up. Dyslexia 2016, 22, 85–100. [Google Scholar] [CrossRef]
- Wanzek, J.; Roberts, G. Reading interventions with varying instructional emphases for fourth graders with reading difficulties. Learn. Disabil. Q. 2012, 35, 90–101. [Google Scholar] [CrossRef]
- Denton, C.A.; Tolar, T.D.; Fletcher, J.M.; Barth, A.E.; Vaughn, S.; Francis, D.J. Effects of tier 3 intervention for students with persistent reading difficulties and characteristics of inadequate responders. J. Educ. Psychol. 2013, 105, 633–648. [Google Scholar] [CrossRef]
- Christodoulou, J.A.C.A.; Murtagh, J.; Chang, P.; Lin, J.; Guarino, A.J.; Hook, P.; Gabrieli, J.D. Impact of intensive summer reading intervention for children with reading disabilities and difficulties in early elementary school. J. Learn. Disabil. 2015, 50, 115–127. [Google Scholar] [CrossRef]
- Falth, L.; Gustafson, S.; Tjus, T.; Heimann, M.; Svensson, I. Computer-assisted interventions targeting reading skills of children with reading disabilities—A longitudinal study. Dyslexia 2013, 19, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Miciak, J.; Roberts, G.; Taylor, W.P.; Solis, M.; Ahmed, Y.; Vaughn, S.; Fletcher, J.M. The effects of one versus two years of intensive reading intervention implemented with late elementary struggling readers. Learn. Disabil. Res. Pract. 2018, 33, 24–36. [Google Scholar] [CrossRef]
- Fathi Azar, E.; Mirzaie, H.; Oftadeh Balani, S.; Hejazi-Shirmard, M. Effects of transcranial electrical stimulation on academic and cognitive skills in individuals with specific learning disabilities: A systematic review. Neuroscience 2025, 576, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Auvichayapat, N.; Auvichayapat, P. Transcranial direct current stimulation in treatment of child neuropsychiatric disorders: Ethical considerations. Front. Hum. Neurosci. 2022, 16, 842013. [Google Scholar] [CrossRef]
- Costanzo, F.; Rossi, S.; Varuzza, C.; Varvara, P.; Vicari, S.; Menghini, D. Long-lasting improvement following tDCS treatment combined with a training for reading in children and adolescents with dyslexia. Neuropsychologia 2019, 130, 38–43. [Google Scholar] [CrossRef]
- Battisti, A.; Lazzaro, G.; Costanzo, F.; Varuzza, C.; Rossi, S.; Vicari, S.; Menghini, D. Effects of a short and intensive transcranial direct current stimulation treatment in children and adolescents with developmental dyslexia: A crossover clinical trial. Front. Psychol. 2022, 13, 986242. [Google Scholar] [CrossRef]
- Lazzaro, G.; Costanzo, F.; Varuzza, C.; Rossi, S.; De Matteis, M.E.; Vicari, S.; Menghini, D. Individual differences modulate the effects of tDCS on reading in children and adolescents with dyslexia. Sci. Stud. Read. 2021, 25, 470–485. [Google Scholar] [CrossRef]
- Costanzo, F.; Varuzza, C.; Rossi, S.; Sdoia, S.; Varvara, P.; Oliveri, M.; Koch, G.; Vicari, S.; Menghini, D. Reading changes in children and adolescents with dyslexia after transcranial direct current stimulation. Neuroreport 2016, 27, 295–300. [Google Scholar] [CrossRef]
- Lazzaro, G.; Bertoni, S.; Menghini, D.; Costanzo, F.; Franceschini, S.; Varuzza, C.; Ronconi, L.; Battisti, A.; Gori, S.; Facoetti, A.; et al. Beyond Reading Modulation: Temporo-Parietal tDCS Alters Visuo-Spatial Attention and Motion Perception in Dyslexia. Brain Sci. 2021, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Mirahadi, S.S.; Nitsche, M.A.; Pahlavanzadeh, B.; Mohamadi, R.; Ashayeri, H.; Abolghasemi, J. Reading and phonological awareness improvement accomplished by transcranial direct current stimulation combined with phonological awareness training: A randomized controlled trial. Appl. Neuropsychol. Child 2023, 12, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Heth, I.; Lavidor, M. Improved reading measures in adults with dyslexia following transcranial direct current stimulation treatment. Neuropsychologia 2015, 70, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, V.; Mohammadkhani, G.; Alaghband Rad, J.; Mousavi, S.Z.; Khalili, M.E. Modulation of auditory temporal processing, speech in noise perception, auditory-verbal memory, and reading efficiency by anodal tDCS in children with dyslexia. Neuropsychologia 2022, 177, 108427. [Google Scholar] [CrossRef] [PubMed]
- Im, C.; Seo, H.; Jun, S.C. Geometrical variation’s influence on the effects of stimulation may be important in the conventional and multi-array tDCS–comparison of electrical fields computed. IEEE Access 2018, 7, 8557–8569. [Google Scholar] [CrossRef]
- Price, C.J. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage 2012, 62, 816–847. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Kronbichler, M.; Richlan, F. Dyslexic brain activation abnormalities in deep and shallow orthographies: A meta-analysis of 28 functional neuroimaging studies. Hum. Brain Mapp. 2016, 37, 2676–2699. [Google Scholar] [CrossRef] [PubMed]
- Pugh, K.R.; Mencl, W.E.; Jenner, A.R.; Katz, L.; Frost, S.J.; Lee, J.R.; Shaywitz, S.E.; Shaywitz, B.A. Neurobiological studies of reading and reading disability. J. Commun. Disord. 2001, 32, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Richlan, F.; Kronbichler, M.; Wimmer, H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2009, 30, 3299–3308. [Google Scholar] [CrossRef] [PubMed]
- Shaywitz, B.A.; Shaywitz, S.E.; Pugh, K.R.; Mencl, W.E.; Fulbright, R.K.; Skudlarski, P.; Constable, R.T.; Marchione, K.E.; Fletcher, J.M.; Lyon, G.R.; et al. Disruption of posterior brain Systems for Reading in children with developmental dyslexia. Biol. Psychiatry 2002, 52, 101–110. [Google Scholar] [CrossRef]
- Dehaene, S.; Cohen, L. The unique role of the visual word form area in reading. Trends Cogn Sci. 2011, 15, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J.; Devlin, J.T. The myth of the visual word form area. NeuroImage 2003, 19, 473–481. [Google Scholar] [CrossRef]
- Kearns, D.M.; Hancock, R.; Hoeft, F.; Pugh, K.R.; Frost, S.J. The neurobiology of dyslexia. Teach. Except. Child. 2019, 51, 175–188. [Google Scholar] [CrossRef]
- Turker, S.; Hartwigsen, G. Exploring the neurobiology of reading through non-invasive brain stimulation: A review. Cortex 2021, 141, 497–521. [Google Scholar] [CrossRef] [PubMed]
- Vandermosten, M.; Hoeft, F.; Norton, E.S. Integrating MRI brain imaging studies of pre-reading children with current theories of developmental dyslexia: A review and quantitative meta-analysis. Curr. Opin. Behav. Sci. 2016, 10, 155–161. [Google Scholar] [CrossRef]
- Turker, S. Exploring the neurofunctional underpinnings of developmental dyslexia: A review focussing on dyslexic children. In The Talking Species; Luef, E., Marin, M., Eds.; Uni Graz Press: Graz, Austria, 2018; p. 495. [Google Scholar]
- Richlan, F.; Kronbichler, M.; Wimmer, H. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage 2011, 56, 1735–1742. [Google Scholar] [CrossRef]
- Linkersdörfer, J.; Lonnemann, J.; Lindberg, S.; Hasselhorn, M.; Fiebach, C.J. Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: An ALE meta-analysis. PLoS ONE 2012, 7, e43122. [Google Scholar] [CrossRef]
- Giraud, A.-L.; Ramus, F. Neurogenetics and auditory processing in developmental dyslexia. Curr. Opin. Neurobiol. 2013, 23, 37–42. [Google Scholar] [CrossRef]
- Devoto, F.; Carioti, D.; Danelli, L.; Berlingeri, M. A meta-analysis of functional neuroimaging studies on developmental dyslexia across European orthographies: The ADOD model. Lang. Cogn. Neurosci. 2022, 37, 285–314. [Google Scholar] [CrossRef]
- Yan, X.; Jiang, K.; Li, H.; Wang, Z.; Perkins, K.; Cao, F. Convergent and divergent brain structural and functional abnormalities associated with developmental dyslexia. eLife 2021, 10, e69523. [Google Scholar] [CrossRef]
- Cao, F.; Bitan, T.; Booth, J.R. Reading development in children: Interactions between neural systems. Dev. Neuropsychol. 2018, 43, 209–222. [Google Scholar]
- Waldie, K.E.; Haigh, C.E. Dyslexia: Role of the right hemisphere. Dyslexia 2013, 19, 68–83. [Google Scholar]
- Morken, F.; Helland, T.; Hugdahl, K.; Specht, K. Reading in dyslexia across literacy development: A longitudinal study of effective connectivity. NeuroImage 2017, 144, 92–100. [Google Scholar] [CrossRef]
- Naghavi, A.; Dadgar, H.; Daraei, G.; Modarreszadeh, A. On the Effects of Non-Invasive Brain Stimulation Techniques on Developmental Dyslexia: A Systematic Review of Randomized Controlled Trials. Iran. J Psychiatry 2025, 20, 209–222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Starstim®: Wireless Multichannel Transcranial Current Stimulator. Neuroelectrics: Barcelona, Spain. Available online: https://www.neuroelectrics.com.
- Brunoni, A.R.; Amadera, J.; Berbel, B.; Volz, M.S.; Rizzerio, B.G.; Fregni, F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int. J. Neuropsychopharmacol. 2011, 14, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Orsini, A.; Pezzuti, L.; Picone, L. Wechsler Intelligence Scale for Chíldren, 4th ed.; APA PsycTests: Washington, DC, USA, 2012. (In Italian) [Google Scholar]
- Cornoldi, C.; Carretti, B. Prove MT-3-Clinica. Batteria per la Valutazione Delle Abilità di Lettura e Comprensione del Testo, 3rd ed.; Giunti OS: Firenze, Italy, 2016. [Google Scholar]
- Bonifacci, P.; Candria, L.; Contento, S. ALCE. Batteria per la Valutazione Delle Abilità Linguistiche e Comunicative; Erickson: Trento, Italy, 2014. [Google Scholar]
- Sartori, G.; Job, R.; Tressoldi, P.E. DDE-2: Batteria per la Valutazione Della Dislessia e Della Disortografia Evolutiva—2; Giunti Organizzazioni Speciali: Firenze, Italy, 2007. [Google Scholar]
- Zoccolotti, P.; De Luca, M.; Di Filippo, G.; Judica, A.; Martelli, M. Reading development in an orthographically regular language: Effects of length, frequency, lexicality and global processing ability. Read. Writ. 2009, 22, 1053–1079. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Andreou, G.; Argatzopoulou, A. Unraveling ADHD Through Eye-Tracking Procedures: A Scoping Review. J. Atten. Disord. 2025, 29, 10870547251344731. [Google Scholar] [CrossRef]
- Christou, A.I.; Tsermentseli, S.; Drigas, A. The Role of Mobile Games and Environmental Factors in Improving Learning and Metacognitive Potential of Young Students. Int. J. Interact. Mob. Technol. 2023, 17, 67–84. [Google Scholar] [CrossRef]


| Task | Index of Performance | Pre-Treatment | Post-Treatment | One-Month Follow-Up | Six-Month Follow-Up |
|---|---|---|---|---|---|
| High-frequency short words | Accuracy | 60 | 10 | 30 | 20 |
| Speed | 0.09 | 0.26 | 0.20 | 0.15 | |
| Text reading | Accuracy | 93 | 21 | 16 | 16 |
| Speed | 0.08 | 0.15 | 0.16 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Filippo, G.; Bonuomo, M.; Ravizza, M.; Velardi, A.; Perri, R.L. Cognitive Training Combined with Multifocal tDCS over the Reading Network Improves Reading Performance: A Case of Severe Dyslexia. J. Clin. Med. 2025, 14, 5671. https://doi.org/10.3390/jcm14165671
Di Filippo G, Bonuomo M, Ravizza M, Velardi A, Perri RL. Cognitive Training Combined with Multifocal tDCS over the Reading Network Improves Reading Performance: A Case of Severe Dyslexia. Journal of Clinical Medicine. 2025; 14(16):5671. https://doi.org/10.3390/jcm14165671
Chicago/Turabian StyleDi Filippo, Gloria, Marika Bonuomo, Martina Ravizza, Andrea Velardi, and Rinaldo Livio Perri. 2025. "Cognitive Training Combined with Multifocal tDCS over the Reading Network Improves Reading Performance: A Case of Severe Dyslexia" Journal of Clinical Medicine 14, no. 16: 5671. https://doi.org/10.3390/jcm14165671
APA StyleDi Filippo, G., Bonuomo, M., Ravizza, M., Velardi, A., & Perri, R. L. (2025). Cognitive Training Combined with Multifocal tDCS over the Reading Network Improves Reading Performance: A Case of Severe Dyslexia. Journal of Clinical Medicine, 14(16), 5671. https://doi.org/10.3390/jcm14165671









