Effect of Diabetes Mellitus on Survival and Complication Rates of Tooth-Supported Fixed Dental Prostheses (FDPs): Long-Term Clinical Evaluation
Abstract
1. Introduction
2. Materials and Methods
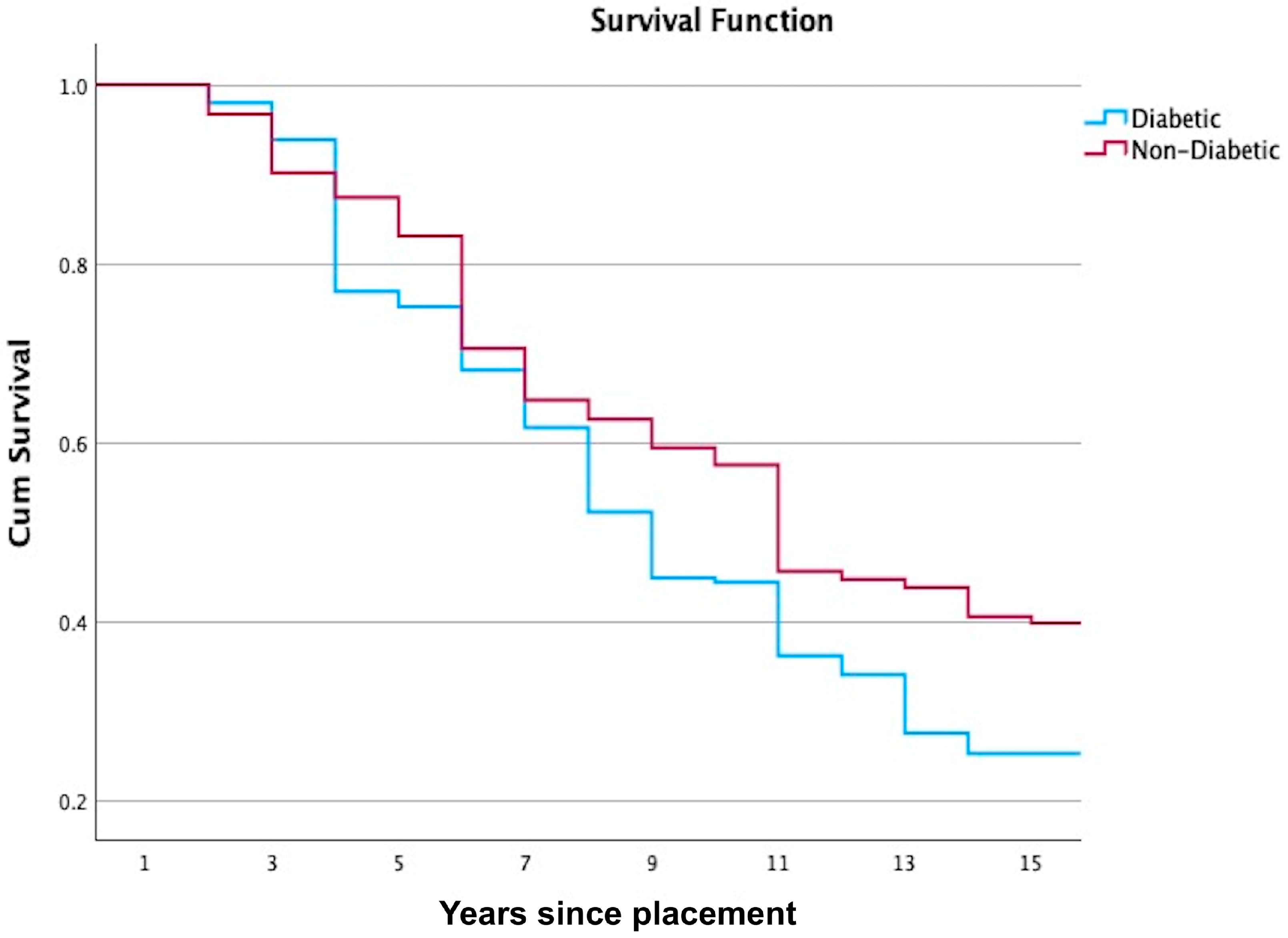
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FDPs | Fixed Dental Prostheses |
| OHI-S | Oral Hygiene Index |
| DM | Diabetes mellitus |
References
- Fu, X.; Jin, L.; Ma, P.; Fan, Z.; Wang, S. Allogeneic Stem Cells From Deciduous Teeth in Treatment for Periodontitis in Miniature Swine. J. Periodontol. 2014, 85, 845–851. [Google Scholar] [CrossRef]
- Alwithanani, N. Periodontal diseases and diabetes mellitus: A systematic review. J. Pharm. Bioallied Sci. 2023, 15, S54–S63. [Google Scholar] [CrossRef]
- Ojo, O.A.; Ibrahim, H.S.; Rotimi, D.E.; Ogunlakin, A.D.; Ojo, A.B. Diabetes mellitus: From molecular mechanism to pathophysiology and pharmacology. Med. Nov. Technol. Devices 2023, 19, 100247. [Google Scholar] [CrossRef]
- Surlari, Z.; Ciurcanu, O.E.; Budala, D.G.; Butnaru, O.; Luchian, I. An update on the interdisciplinary dental care approach for geriatric diabetic patients. Geriatrics 2023, 8, 114. [Google Scholar] [CrossRef]
- Persson, G.R. Diabetes and Periodontal Disease: An Update for Health Care Providers. Diabetes Spectr. 2011, 24, 195–198. [Google Scholar] [CrossRef]
- Naujokat, H.; Kunzendorf, B.; Wiltfang, J. Dental implants and diabetes mellitus—A systematic review. Int. J. Implant Dent. 2016, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Ahmadinia, A.R.; Rahebi, D.; Mohammadi, M.; Ghelichi-Ghojogh, M.; Jafari, A.; Esmaielzadeh, F.; Rajabi, A. Association between type 2 diabetes (T2D) and tooth loss: A systematic review and meta-analysis. BMC Endocr. Disord. 2022, 22, 100. [Google Scholar] [CrossRef] [PubMed]
- Mauri-Obradors, E.; Estrugo-Devesa, A.; Jané-Salas, E.; Viñas, M.; López-López, J. Oral manifestations of Diabetes Mellitus. A systematic review. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e586. [Google Scholar] [CrossRef] [PubMed]
- Ringholm, L.; Stougaard, E.B.; Nørgaard, S.K.; Damm, P.; Mathiesen, E.R. Diabetes Management During Breastfeeding in Women with Type 1 Diabetes. Curr. Diabetes Rep. 2020, 20, 34. [Google Scholar] [CrossRef]
- Shiferaw, A.; Alem, G.; Tsehay, M.; Kibret, G.D. Dental caries and associated factors among diabetic and nondiabetic adult patients attending Bichena Primary Hospital’s Outpatient Department. Front. Oral Health 2022, 3, 938405. [Google Scholar] [CrossRef]
- Borgnakke, W.S.; Anderson, P.F.; Shannon, C.; Jivanescu, A. Is there a relationship between oral health and diabetic neuropathy? Curr. Diab. Rep. 2015, 15, 93. [Google Scholar] [CrossRef]
- Limones, A.; Molinero-Mourelle, P.; Azevedo, L.; Romeo-Rubio, M.; Correia, A.; Gomez-Polo, M. Zirconia-ceramic versus metal-ceramic posterior multiunit tooth-supported fixed dental prostheses: A systematic review and meta-analysis of randomized controlled trials. J. Am. Dent. Assoc. 2020, 151, 230–238.e7. [Google Scholar] [CrossRef]
- Sailer, I.; Balmer, M.; Hüsler, J.; Hämmerle, C.H.F.; Känel, S.; Thoma, D.S. 10-year randomized trial (RCT) of zirconia-ceramic and metal-ceramic fixed dental prostheses. J. Dent. 2018, 76, 32–39. [Google Scholar] [CrossRef]
- Dragomir, L.P.; Nicolae, F.-M.; Gheorghe, D.N.; Popescu, D.M.; Dragomir, I.M.; Boldeanu, L.; Boldeanu, V.M.; Popescu, M.R. The Influence of Fixed Dental Prostheses on the Expression of Inflammatory Markers and Periodontal Status—Narrative Review. Medicina 2023, 59, 941. [Google Scholar] [CrossRef]
- Greene, J.C.; Vermillion, J.R. THE SIMPLIFIED ORAL HYGIENE INDEX. J. Am. Dent. Assoc. 1964, 68, 7–13. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Lamster, I.B.; Lalla, E.; Borgnakke, W.S.; Taylor, G.W. The relationship between oral health and diabetes mellitus. J. Am. Dent. Assoc. 2008, 139, 19S–24S. [Google Scholar] [CrossRef]
- Mealey, B.L.; Ocampo, G.L. Diabetes mellitus and periodontal disease. Periodontol. 2000 2007, 44, 127–153. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Xiao, E.; Graves, D.T. Diabetes mellitus related bone metabolism and periodontal disease. Int. J. Oral Sci. 2015, 7, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Huang, X.; Zhou, X.; Li, M.; Ren, B.; Peng, X.; Cheng, L. Influence of Dental Prosthesis and Restorative Materials Interface on Oral Biofilms. Int. J. Mol. Sci. 2018, 19, 3157. [Google Scholar] [CrossRef]
- Kurtzman, G.M.; Horowitz, R.A.; Johnson, R.; Prestiano, R.A.; Klein, B.I. The systemic oral health connection: Biofilms. Medicine 2022, 101, e30517. [Google Scholar] [CrossRef]
- Alenezi, A.; Aloqayli, S. Technical complications with tooth-supported fixed dental prostheses (FDPs) of different span lengths: An up to 15-year retrospective study. BMC Oral Health 2023, 23, 393. [Google Scholar] [CrossRef]
- Alfadhli, R.; Alshammari, Y.; Baig, M.R.; Omar, R. Clinical outcomes of single crown and 3-unit bi-layered zirconia-based fixed dental prostheses: An upto 6- year retrospective clinical study: Clinical outcomes of zirconia FDPs. J. Dent. 2022, 127, 104321. [Google Scholar] [CrossRef]
- Sailer, I.; Makarov, N.A.; Thoma, D.S.; Zwahlen, M.; Pjetursson, B.E. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: Single crowns (SCs). Dent. Mater. 2015, 31, 603–623. [Google Scholar] [CrossRef]
- Alsterstål-Englund, H.; Moberg, L.-E.; Petersson, J.; Smedberg, J.-I. A retrospective clinical evaluation of extensive tooth-supported fixed dental prostheses after 10 years. J. Prosthet. Dent. 2021, 125, 65–72. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin. Oral Implants Res. 2012, 23 (Suppl. 6), 22–38. [Google Scholar] [CrossRef]
- Javed, F.; Romanos, G.E. Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: A systematic literature review. J. Periodontol. 2009, 80, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Pjetursson, B.E.; Lang, N.P.; Chan, E.S.Y. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. Clin. Oral Implant Res. 2004, 15, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Hawthan, M.A.; Chrcanovic, B.R.; Larsson, C. Long-term retrospective clinical study of tooth-supported fixed partial dentures: A multifactorial analysis. J. Prosthodont. Res. 2023, 67, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Aloqayli, S.; Alsalhi, H.; Alenezi, A. Clinical Outcomes and Complication Rates of Endodontically Treated Teeth with Fixed Dental Prostheses: A Retrospective Study. Dent. J. 2025, 13, 42. [Google Scholar] [CrossRef]
- Alenezi, A.; Alkhudhayri, O.; Altowaijri, F.; Aloufi, L.; Alharbi, F.; Alrasheed, M.; Almutairi, H.; Alanazi, A.; Yehya, M.; Al Asmari, D. Secondary caries in fixed dental prostheses: Long-term clinical evaluation. Clin. Exp. Dent. Res. 2023, 9, 249–257. [Google Scholar] [CrossRef]
- Eskow, C.C.; Oates, T.W. Dental implant survival and complication rate over 2 years for individuals with poorly controlled type 2 diabetes mellitus. Clin. Implant. Dent. Relat. Res. 2017, 19, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Jokstad, A. Secondary caries and microleakage. Dent. Mater. 2016, 32, 11–25. [Google Scholar] [CrossRef]
- Scurria, M.S.; Bader, J.D.; Shugars, D.A. Meta-analysis of fixed partial denture survival: Prostheses and abutments. J. Prosthet. Dent. 1998, 79, 459–464. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Brägger, U.; Lang, N.P.; Zwahlen, M. Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns (SCs). Clin. Oral Implants Res. 2007, 18 (Suppl. 3), 97–113. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47, S20–S42. [Google Scholar] [CrossRef]
- Almusawi, M.A.; Gosadi, I.; Abidia, R.; Almasawi, M.; Khan, H.A. Potential risk factors for dental caries in Type 2 diabetic patients. Int. J. Dent. Hyg. 2018, 16, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.W.; Borgnakke, W.S. Periodontal disease: Associations with diabetes, glycemic control and complications. Oral Dis. 2008, 14, 191–203. [Google Scholar] [CrossRef] [PubMed]
- De Backer, H.; Van Maele, G.; Decock, V.; Van den Berghe, L. Long-term survival of complete crowns, fixed dental prostheses, and cantilever fixed dental prostheses with posts and cores on root canal-treated teeth. Int. J. Prosthodont. 2007, 20, 229–234. [Google Scholar]
- Hintao, J.; Teanpaisan, R.; Chongsuvivatwong, V.; Dahlen, G.; Rattarasarn, C. Root surface and coronal caries in adults with type 2 diabetes mellitus. Community Dent. Oral Epidemiol. 2007, 35, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Laouali, N.; El Fatouhi, D.; Aguayo, G.; Balkau, B.; Boutron-Ruault, M.-C.; Bonnet, F.; Fagherazzi, G. Type 2 diabetes and its characteristics are associated with poor oral health: Findings from 60,590 senior women from the E3N study. BMC Oral Health 2021, 21, 315. [Google Scholar] [CrossRef] [PubMed]
| Group | Number of FDPs (%) | Number of FDPs (%) with Sign of Biological Complications | p-Value | Number of FDPs (%) with Sign of Technical Complications | p-Value | Total Number of FDPs (%) with Complication | (95% CI) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Gender | Male | 381 (33.9%) | 227 (59.6%) | 0.002 | 22 (5.8%) | 0.486 | 244 (64.0%) | 59–69% | 0.002 |
| Female | 744 (66.1%) | 370 (49.7%) | 51 (6.9%) | 404 (54.3%) | 51–58% | ||||
| Medical condition | Diabetic | 305 (27.1%) | 178 (58.4%) | 0.03 | 23 (7.5%) | 0.382 | 198 (64.9%) | 60–70% | 0.002 |
| Non-diabetic | 820 (72.9%) | 419 (51.1%) | 50 (6.1%) | 450 (54.9%) | 51–58% | ||||
| Location | Maxilla | 666 (59.2%) | 341 (51.2%) | 0.131 | 45 (6.8%) | 0.66 | 372 (55.9%) | 52–60% | 0.154 |
| Mandible | 459 (40.8%) | 256 (55.8%) | 28 (6.1%) | 276 (60.1%) | 56–65% | ||||
| Oral hygiene | Good | 85 (7.6%) | 11 (12.9%) | <0.001 | 11 (12.9%) | <0.001 | 19 (22.4%) | 13–31% | <0.001 |
| Fair | 430 (38.2%) | 146 (34.0%) | 38 (8.8%) | 176 (40.9%) | 36–46% | ||||
| Poor | 610 (54.2%) | 440 (72.1%) | 24 (3.9%) | 453 (74.3%) | 71–78% | ||||
| FPD design | Veneer | 21 (1.9%) | 0 (0.0%) | <0.001 | 4 (19.0%) | 0.002 | 4 (19.0%) | 1–37% | <0.001 |
| Single crown | 627 (55.7%) | 298 (47.5%) | 27 (4.3%) | 317 (50.6%) | 47–54% | ||||
| Splinted crown | 43 (3.8%) | 28 (65.1%) | 2 (4.7%) | 30 (69.8%) | 55–84% | ||||
| Cantilever bridge | 23 (2.0%) | 17 (73.9%) | 1 (4.3%) | 18 (78.3%) | 60–96% | ||||
| Conventional bridge | 411 (36.5%) | 254 (61.8%) | 39 (9.5%) | 279 (67.9%) | 63–72% | ||||
| Type of material | Full ceramic | 90 (8.0%) | 45 (50.0%) | <0.001 | 2 (2.2%) | 0.097 | 47 (52.2%) | 42–63% | <0.001 |
| Zirconia | 176 (15.6%) | 45 (25.6%) | 16 (21.9%) | 47 (26.7%) | 20–33% | ||||
| PFM | 859 (76.4%) | 507 (59.0%) | 55 (6.4%) | 554 (64.5%) | 61–68% | ||||
| Total | 1125 | 597 (53.1%) | 73 (6.5%) | 648 (57.6%) | |||||
| Interval Start Time | Number Entering Interval | Number Withdrawing During Interval | Number Exposed to Risk | Number of Terminal Events | Proportion Surviving | Cumulative Proportion Surviving at End of Interval | Std. Error |
|---|---|---|---|---|---|---|---|
| 0 | 305 | 0 | 305 | 0 | 1.00 | 1.00 | 0.00 |
| 1 | 305 | 12 | 299 | 6 | 0.98 | 0.98 | 0.01 |
| 2 | 287 | 6 | 284 | 12 | 0.96 | 0.94 | 0.01 |
| 3 | 269 | 40 | 249 | 45 | 0.82 | 0.77 | 0.03 |
| 4 | 184 | 7 | 181 | 4 | 0.98 | 0.75 | 0.03 |
| 5 | 173 | 7 | 170 | 16 | 0.91 | 0.68 | 0.03 |
| 6 | 150 | 3 | 149 | 14 | 0.91 | 0.62 | 0.03 |
| 7 | 133 | 5 | 131 | 20 | 0.85 | 0.52 | 0.03 |
| 8 | 108 | 3 | 107 | 15 | 0.86 | 0.45 | 0.03 |
| 9 | 90 | 1 | 90 | 1 | 0.99 | 0.44 | 0.03 |
| 10 | 88 | 3 | 87 | 16 | 0.82 | 0.36 | 0.03 |
| 11 | 69 | 2 | 68 | 4 | 0.94 | 0.34 | 0.03 |
| 12 | 63 | 1 | 63 | 12 | 0.81 | 0.27 | 0.03 |
| 13 | 50 | 3 | 49 | 4 | 0.92 | 0.25 | 0.03 |
| 14 | 43 | 0 | 43 | 0 | 1.00 | 0.25 | 0.03 |
| 15 | 43 | 0 | 43 | 3 | 0.93 | 0.23 | 0.03 |
| Interval Start Time | Number Entering Interval | Number Withdrawing During Interval | Number Exposed to Risk | Number of Terminal Events | Proportion Surviving | Cumulative Proportion Surviving at End of Interval | Std. Error |
|---|---|---|---|---|---|---|---|
| 0 | 820 | 0 | 820 | 0 | 1.00 | 1.00 | 0.00 |
| 1 | 820 | 50 | 795 | 26 | 0.97 | 0.97 | 0.01 |
| 2 | 744 | 49 | 720 | 49 | 0.93 | 0.90 | 0.01 |
| 3 | 646 | 43 | 625 | 19 | 0.97 | 0.87 | 0.01 |
| 4 | 584 | 29 | 570 | 28 | 0.95 | 0.83 | 0.01 |
| 5 | 527 | 38 | 508 | 77 | 0.85 | 0.71 | 0.02 |
| 6 | 412 | 15 | 405 | 33 | 0.92 | 0.65 | 0.02 |
| 7 | 364 | 10 | 359 | 12 | 0.97 | 0.63 | 0.02 |
| 8 | 342 | 15 | 335 | 17 | 0.95 | 0.59 | 0.02 |
| 9 | 310 | 4 | 308 | 10 | 0.97 | 0.57 | 0.02 |
| 10 | 296 | 31 | 281 | 58 | 0.79 | 0.46 | 0.02 |
| 11 | 207 | 6 | 204 | 4 | 0.98 | 0.45 | 0.02 |
| 12 | 197 | 4 | 195 | 4 | 0.98 | 0.44 | 0.02 |
| 13 | 189 | 3 | 188 | 14 | 0.93 | 0.41 | 0.02 |
| 14 | 172 | 2 | 171 | 3 | 0.98 | 0.40 | 0.02 |
| 15 | 167 | 24 | 155 | 28 | 0.82 | 0.33 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alenezi, A. Effect of Diabetes Mellitus on Survival and Complication Rates of Tooth-Supported Fixed Dental Prostheses (FDPs): Long-Term Clinical Evaluation. J. Clin. Med. 2025, 14, 5673. https://doi.org/10.3390/jcm14165673
Alenezi A. Effect of Diabetes Mellitus on Survival and Complication Rates of Tooth-Supported Fixed Dental Prostheses (FDPs): Long-Term Clinical Evaluation. Journal of Clinical Medicine. 2025; 14(16):5673. https://doi.org/10.3390/jcm14165673
Chicago/Turabian StyleAlenezi, Ali. 2025. "Effect of Diabetes Mellitus on Survival and Complication Rates of Tooth-Supported Fixed Dental Prostheses (FDPs): Long-Term Clinical Evaluation" Journal of Clinical Medicine 14, no. 16: 5673. https://doi.org/10.3390/jcm14165673
APA StyleAlenezi, A. (2025). Effect of Diabetes Mellitus on Survival and Complication Rates of Tooth-Supported Fixed Dental Prostheses (FDPs): Long-Term Clinical Evaluation. Journal of Clinical Medicine, 14(16), 5673. https://doi.org/10.3390/jcm14165673






