Demographic and Sleep Study Factors Influencing Short-Term Adherence to Positive Airway Pressure Therapy in Obstructive Sleep Apnea
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Demographic Data
2.3. Sleep Study Data
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OSA | Obstructive sleep apnea |
| PAP | Positive airway pressure |
| AHI | Apnea–hypopnea index |
| BMI | Body mass index |
| ESS | Epworth sleepiness scale |
| PSQI | Pittsburgh sleep quality index |
| TST | Total sleep time |
| SE | Sleep efficiency |
| RERA | Respiratory effort-related arousal |
| RDI | Respiratory disturbance index |
| ODI | Oxygen desaturation index |
| ORs | Odds ratios |
| CIs | Confidence intervals |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
| REM | Rapid eye movement |
| SDB | Sleep-disordered breathing |
References
- Epstein, L.J.; Kristo, D.; Strollo, P.J., Jr.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar] [PubMed]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2019, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Strollo, P.J., Jr.; Rogers, R.M. Obstructive sleep apnea. N. Engl. J. Med. 1996, 334, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.E.; Issa, F.G.; Berthon-Jones, M.; Eves, L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981, 1, 862–865. [Google Scholar] [CrossRef]
- Kakkar, R.K.; Berry, R.B. Positive airway pressure treatment for obstructive sleep apnea. Chest 2007, 132, 1057–1072. [Google Scholar] [CrossRef]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea With Positive Airway Pressure: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J. Clin. Sleep Med. 2019, 15, 301–334. [Google Scholar] [CrossRef]
- Karimi, M.; Hedner, J.; Häbel, H.; Nerman, O.; Grote, L. Sleep apnea-related risk of motor vehicle accidents is reduced by continuous positive airway pressure: Swedish Traffic Accident Registry data. Sleep 2015, 38, 341–349. [Google Scholar] [CrossRef]
- Durán-Cantolla, J.; Aizpuru, F.; Montserrat, J.M.; Ballester, E.; Terán-Santos, J.; Aguirregomoscorta, J.I.; Gonzalez, M.; Lloberes, P.; Masa, J.F.; De La Peña, M.; et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: Randomised controlled trial. BMJ 2010, 341, c5991. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Wen, F.; He, Z.; Niu, W.; Ren, C.; Li, N.; Wang, Q.; Ren, Y.; Liang, C. Does continuous positive airway pressure therapy benefit patients with coronary artery disease and obstructive sleep apnea? A systematic review and meta-analysis. Clin. Cardiol. 2021, 44, 1041–1049. [Google Scholar] [CrossRef]
- Babu, A.R.; Herdegen, J.; Fogelfeld, L.; Shott, S.; Mazzone, T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch. Intern. Med. 2005, 165, 447–452. [Google Scholar] [CrossRef]
- Beninati, W.; Harris, C.D.; Herold, D.L.; Shepard, J.W., Jr. The effect of snoring and obstructive sleep apnea on the sleep quality of bed partners. Mayo Clin. Proc. 1999, 74, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Parish, J.M.; Lyng, P.J. Quality of life in bed partners of patients with obstructive sleep apnea or hypopnea after treatment with continuous positive airway pressure. Chest 2003, 124, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.Y.; Lee, S.H. Compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Sleep Med. Res. 2020, 11, 7–14. [Google Scholar] [CrossRef]
- Mehrtash, M.; Bakker, J.P.; Ayas, N. Predictors of Continuous Positive Airway Pressure Adherence in Patients with Obstructive Sleep Apnea. Lung 2019, 197, 115–121. [Google Scholar] [CrossRef]
- Billings, M.E.; Auckley, D.; Benca, R.; Foldvary-Schaefer, N.; Iber, C.; Redline, S.; Rosen, C.L.; Zee, P.; Kapur, V.K. Race and residential socioeconomics as predictors of CPAP adherence. Sleep 2011, 34, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Simon-Tuval, T.; Reuveni, H.; Greenberg-Dotan, S.; Oksenberg, A.; Tal, A.; Tarasiuk, A. Low socioeconomic status is a risk factor for CPAP acceptance among adult OSAS patients requiring treatment. Sleep 2009, 32, 545–552. [Google Scholar] [CrossRef]
- Weaver, T.E.; Maislin, G.; Dinges, D.F.; Younger, J.; Cantor, C.; McCloskey, S.; Pack, A.I. Self-efficacy in sleep apnea: Instrument development and patient perceptions of obstructive sleep apnea risk, treatment benefit, and volition to use continuous positive airway pressure. Sleep 2003, 26, 727–732. [Google Scholar] [CrossRef]
- Kohler, M.; Smith, D.; Tippett, V.; Stradling, J.R. Predictors of long-term compliance with continuous positive airway pressure. Thorax 2010, 65, 829–832. [Google Scholar] [CrossRef]
- Falcone, V.A.; Damiani, M.F.; Quaranta, V.N.; Capozzolo, A.; Resta, O. Polysomnograph chart view by patients: A new educational strategy to improve CPAP adherence in sleep apnea therapy. Respir. Care 2014, 59, 193–198. [Google Scholar] [CrossRef]
- Neuzeret, P.C.; Morin, L. Impact of different nasal masks on CPAP therapy for obstructive sleep apnea: A randomized comparative trial. Clin. Respir. J. 2017, 11, 990–998. [Google Scholar] [CrossRef]
- Park, D.Y.; Cho, J.H.; Jung, Y.G.; Choi, J.H.; Kim, D.K.; Kim, S.W.; Kim, H.J.; Kim, H.Y.; Park, S.K.; Park, C.S.; et al. Clinical Practice Guideline: Clinical Efficacy of Nasal Surgery in the Treatment of Obstructive Sleep Apnea. Clin. Exp. Otorhinolaryngol. 2023, 16, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Kwak, K.H.; Lee, Y.J.; Lee, J.Y.; Cho, J.H.; Choi, J.H. The Effect of Pharyngeal Surgery on Positive Airway Pressure Therapy in Obstructive Sleep Apnea: A Meta-Analysis. J. Clin. Med. 2022, 11, 6443. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J.; Pang, L.; Wang, Y.; Ma, G.; Liao, W. Telemonitor care helps CPAP compliance in patients with obstructive sleep apnea: A systemic review and meta-analysis of randomized controlled trials. Ther. Adv. Chronic Dis. 2020, 11, 2040622320901625. [Google Scholar] [CrossRef] [PubMed]
- DeVettori, G.; Troxel, W.M.; Duff, K.; Baron, K.G. Positive airway pressure adherence among patients with obstructive sleep apnea and cognitive impairment: A narrative review. Sleep Med. 2023, 111, 28–35. [Google Scholar] [CrossRef]
- Andrade, A.G.; Bubu, O.M.; Varga, A.W.; Osorio, R.S. The relationship between obstructive sleep apnea and Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 64, S255–S270. [Google Scholar] [CrossRef]
- Chasens, E.R.; Pack, A.I.; Maislin, G.; Dinges, D.F.; Weaver, T.E. Claustrophobia and adherence to CPAP treatment. West. J. Nurs. Res. 2005, 27, 307–321. [Google Scholar] [CrossRef]
- Edinger, J.D.; Carwile, S.; Miller, P.; Hope, V.; Mayti, C. Psychological status, syndromatic measures, and compliance with nasal CPAP therapy for sleep apnea. Percept. Mot. Ski. 1994, 78, 1116–1118. [Google Scholar] [CrossRef]
- Budhiraja, R.; Kushida, C.A.; Nichols, D.A.; Walsh, J.K.; Simon, R.D.; Gottlieb, D.J.; Quan, S.F. Impact of randomization, clinic visits, and medical and psychiatric cormorbidities on continuous positive airway pressure adherence in obstructive sleep apnea. J. Clin. Sleep Med. 2016, 12, 333–341. [Google Scholar] [CrossRef]
- Drager, L.F.; Malhotra, A.; Yan, Y.; Pépin, J.-L.; Armitstead, J.P.; Woehrle, H.; Nunez, C.M.; Cistulli, P.A.; Benjafield, A.V.; Group, m. Adherence with positive airway pressure therapy for obstructive sleep apnea in developing vs. developed countries: A big data study. J. Clin. Sleep Med. 2021, 17, 703–709. [Google Scholar] [CrossRef]
- Weaver, T.E.; Grunstein, R.R. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proc. Am. Thorac. Soc. 2008, 5, 173–178. [Google Scholar] [CrossRef]
- Bae, M.R.; Lee, Y.H.; Lee, S.W.; Chung, S.; Chung, Y.S. Positive Airway Pressure Therapy Compliance in Patients With Comorbid Insomnia and Sleep Apnea. Clin. Exp. Otorhinolaryngol. 2024, 17, 116–121. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Choi, J.H. Positive airway pressure prescription and management for patients with obstructive sleep apnea. J. Rhinol. 2020, 27, 73–82. [Google Scholar] [CrossRef]
- Troester, M.M.; Quan, S.F.; Berry, R.B.; Plante, D.T.; Abreu, A.R.; Alzoubaidi, M.; Bandyopadhyay, A.; DelRosso, L.; Ebben, M.; Kwon, Y.; et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 3; American Academy of Sleep Medicine: Darien, IL, USA, 2023. [Google Scholar]
- Kashyap, R.; Hock, L.M.; Bowman, T.J. Higher prevalence of smoking in patients diagnosed as having obstructive sleep apnea. Sleep Breath. 2001, 5, 167–172. [Google Scholar] [CrossRef]
- McArdle, N.; Devereux, G.; Heidarnejad, H.; Engleman, H.M.; Mackay, T.W.; Douglas, N.J. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am. J. Respir. Crit. Care Med. 1999, 159, 1108–1114. [Google Scholar] [CrossRef]
- Russo-Magno, P.; O’Brien, A.; Panciera, T.; Rounds, S. Compliance with CPAP therapy in older men with obstructive sleep apnea. J. Am. Geriatr. Soc. 2001, 49, 1205–1211. [Google Scholar] [CrossRef]
- Lavalle, S.; Masiello, E.; Iannella, G.; Magliulo, G.; Pace, A.; Lechien, J.R.; Calvo-Henriquez, C.; Cocuzza, S.; Parisi, F.M.; Favier, V. Unraveling the complexities of oxidative stress and inflammation biomarkers in obstructive sleep apnea syndrome: A comprehensive review. Life 2024, 14, 425. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, E.J.; Kim, K.W.; Choi, J.; Kwon, S.Y.; Lee, H.M.; Kim, T.H.; Lee, S.H.; Shin, C.; Lee, S.H. Optimal continuous positive airway pressure level in korean patients with obstructive sleep apnea syndrome. Clin. Exp. Otorhinolaryngol. 2010, 3, 207–211. [Google Scholar] [CrossRef]
- He, S.; Cistulli, P.A.; de Chazal, P. A review of novel oximetry parameters for the prediction of cardiovascular disease in obstructive sleep apnoea. Diagnostics 2023, 13, 3323. [Google Scholar] [CrossRef]
- Van Ryswyk, E.; Anderson, C.S.; Antic, N.A.; Barbe, F.; Bittencourt, L.; Freed, R.; Heeley, E.; Liu, Z.; Loffler, K.A.; Lorenzi-Filho, G. Predictors of long-term adherence to continuous positive airway pressure in patients with obstructive sleep apnea and cardiovascular disease. Sleep 2019, 42, zsz152. [Google Scholar] [CrossRef]
- Campos-Rodriguez, F.; Martinez-Garcia, M.A.; Reyes-Nunez, N.; Caballero-Martinez, I.; Almeida-Gonzalez, C.V.; Catalan-Serra, P.; Pena-Grinan, N. Long-term continuous positive airway pressure compliance in females with obstructive sleep apnoea. Eur. Respir. J. 2013, 42, 1255–1262. [Google Scholar] [CrossRef]
- Law, M.; Naughton, M.; Ho, S.; Roebuck, T.; Dabscheck, E. Depression may reduce adherence during CPAP titration trial. J. Clin. Sleep Med. 2014, 10, 163–169. [Google Scholar] [CrossRef] [PubMed]
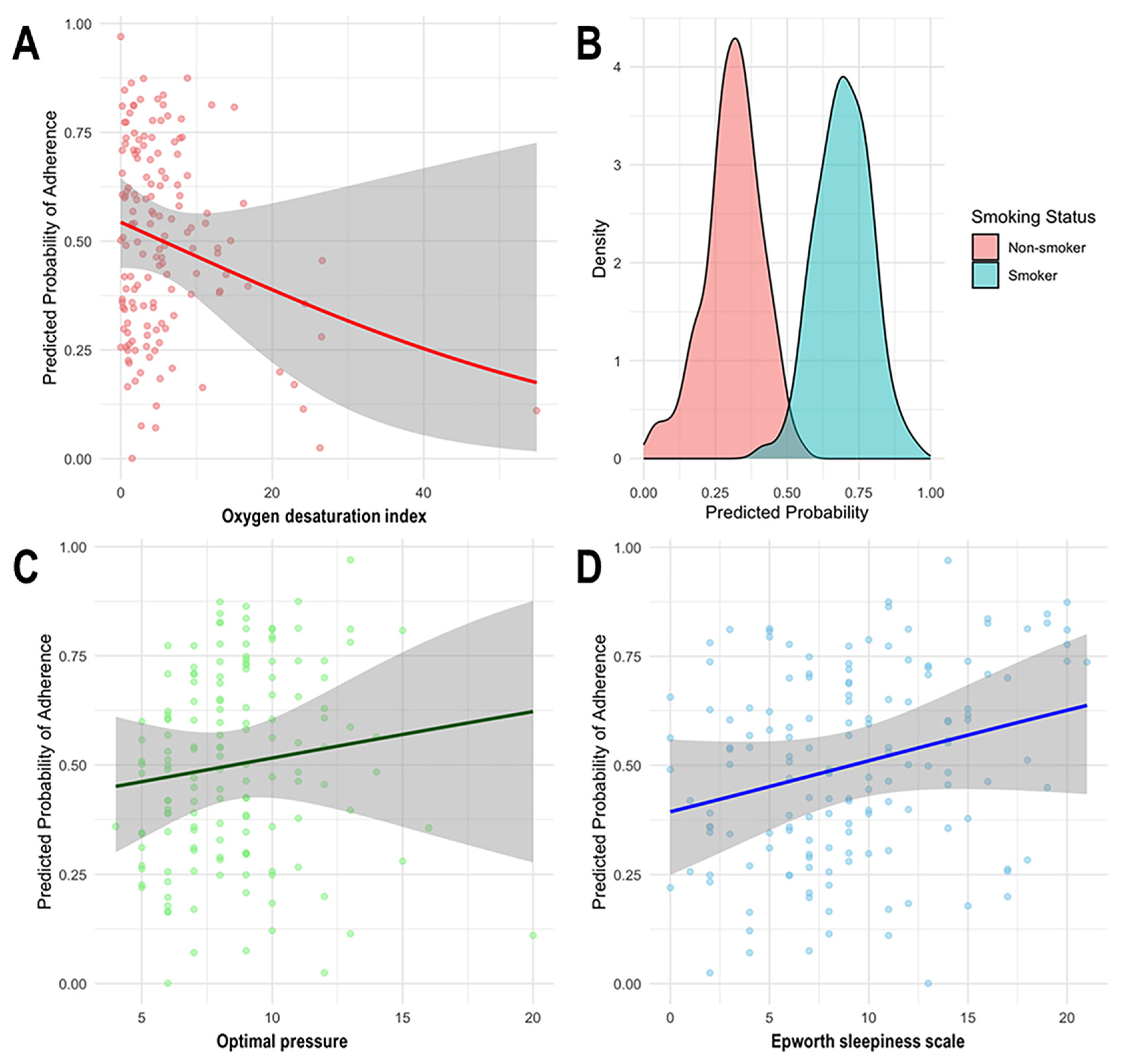
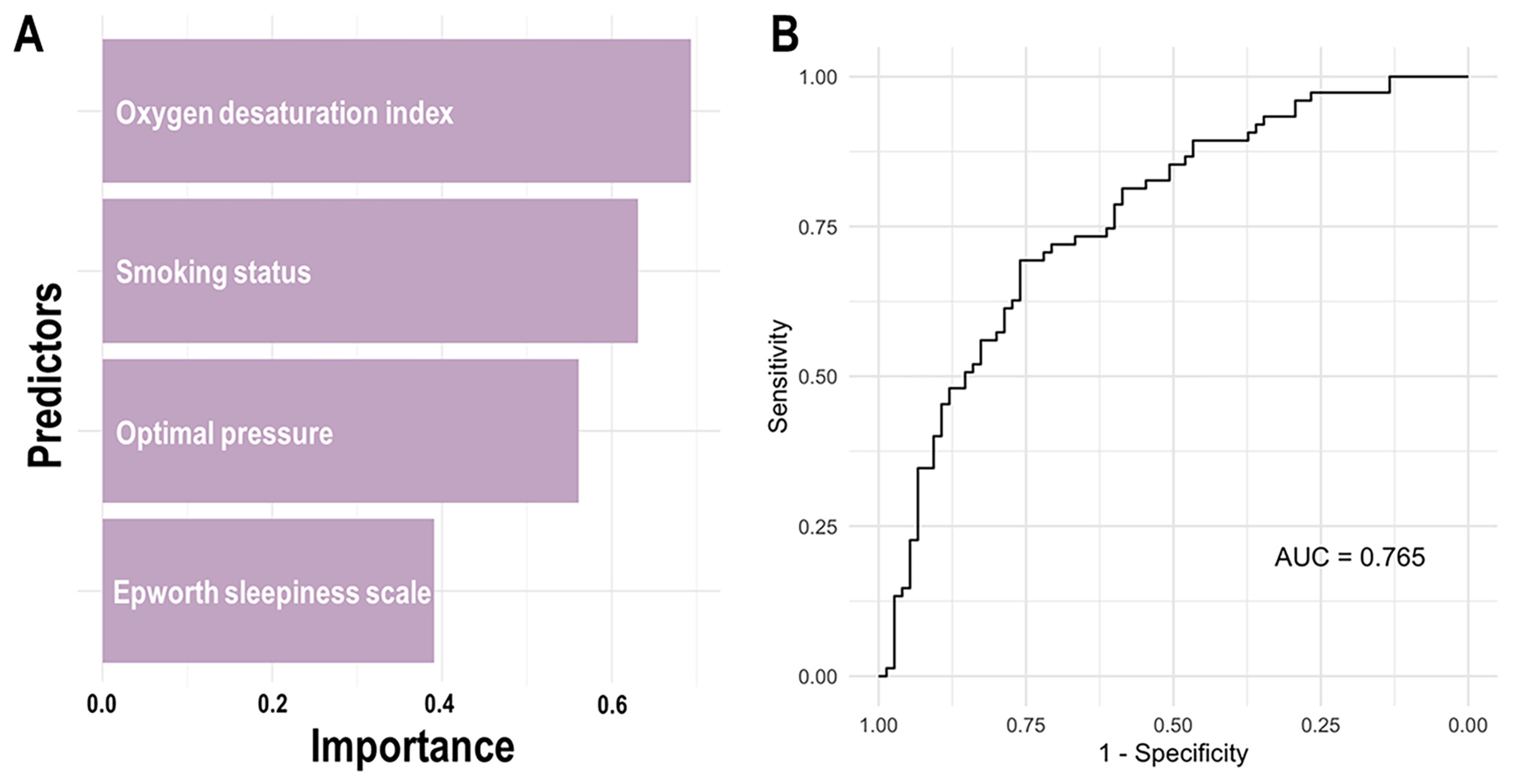
| Characteristics | Adherence Group (n = 75) | Non-adherence Group (n = 75) |
|---|---|---|
| Demographic data | ||
| Age (years) | 49.91 ± 14.25 | 49.21 ± 14.47 |
| Gender (male:female) | 60:15 | 59:16 |
| Body mass index (kg/m2) | 29.09 ± 4.69 | 28.83 ± 5.54 |
| Smoking status (yes:no) | 35:40 | 58:17 |
| ESS | 9.75 ± 5.06 | 8.55 ± 5.06 |
| PSQI | 5.81 ± 2.45 | 5.36 ± 2.23 |
| Polysomnographic data | ||
| TST (minutes) | 341.64 ± 42.73 | 344.93 ± 45.25 |
| Sleep efficiency (%) | 81.69 ± 11.86 | 81.07 ± 12.34 |
| Respiratory arousal index | 32.79 ± 22.32 | 27.32 ± 23.19 |
| Spontaneous arousal index | 5.95 ± 10.18 | 5.14 ± 5.30 |
| Total arousal index | 42.13 ± 19.93 | 38.10 ± 20.85 |
| Stage N1 (% of TST) | 28.87 ± 17.16 | 25.67 ± 14.60 |
| Stage N2 (% of TST) | 42.38 ± 15.65 | 45.54 ± 14.81 |
| Stage N3 (slow-wave sleep) (% of TST) | 4.89 ± 6.65 | 4.25 ± 5.73 |
| Stage R (REM sleep) (% of TST) | 15.91 ± 6.11 | 16.08 ± 5.47 |
| AHI (events/hour of TST) | 43.56 ± 23.19 | 37.26 ± 24.06 |
| Supine AHI | 52.31 ± 24.55 | 47.84 ± 27.18 |
| RERA index | 3.84 ± 3.13 | 5.98 ± 7.70 |
| RDI (events/hour of TST) | 47.39 ± 21.55 | 42.58 ± 22.31 |
| ODI (events/hour of TST) | 40.59 ± 23.52 | 36.29 ± 23.95 |
| PAP titration data | ||
| TST (minutes) | 356.65 ± 43.02 | 347.81 ± 51.09 |
| Sleep efficiency (%) | 84.57 ± 9.66 | 81.31 ± 13.39 |
| Total arousal index | 19.82 ± 10.31 | 20.64 ± 12.78 |
| Stage N1 (% of TST) | 15.01 ± 8.89 | 15.20 ± 8.84 |
| Stage N2 (% of TST) | 51.10 ± 13.05 | 50.58 ± 12.76 |
| Stage N3 (slow-wave sleep) (% of TST) | 7.14 ± 7.26 | 6.60 ± 7.54 |
| Stage R (REM sleep) (% of TST) | 20.69 ± 5.76 | 17.96 ± 7.43 |
| AHI (events/hour of TST) | 5.27 ± 5.62 | 7.19 ± 9.91 |
| Supine AHI | 5.52 ± 5.76 | 7.24 ± 9.69 |
| RERA index | 1.43 ± 1.60 | 1.31 ± 1.42 |
| RDI (events/hour of TST) | 6.68 ± 5.49 | 8.48 ± 10.00 |
| ODI (events/hour of TST) | 4.91 ± 4.76 | 6.33 ± 8.64 |
| Optimal pressure | 8.69 ± 2.34 | 8.40 ± 2.87 |
| Dependent Variable | Independent Variables | B | SE | OR | 95% CI | p |
|---|---|---|---|---|---|---|
| Adherence | (Intercept) | −7.815 | 3.567 | 0.000 | 0.000–0.439 | 0.028 * |
| Smoking status | −1.200 | 0.456 | 0.301 | 0.123–0.736 | 0.009 ** | |
| ESS | 0.091 | 0.045 | 1.096 | 1.004–1.198 | 0.042 * | |
| PSQI | 0.127 | 0.091 | 1.135 | 0.949–1.357 | 0.164 | |
| TST † | −0.005 | 0.009 | 0.995 | 0.977–1.012 | 0.552 | |
| Sleep efficiency † | 0.037 | 0.034 | 1.038 | 0.970–1.111 | 0.277 | |
| Spontaneous ArI † | 0.037 | 0.031 | 1.038 | 0.976–1.103 | 0.233 | |
| N1 sleep † | 0.013 | 0.022 | 1.013 | 0.970–1.057 | 0.557 | |
| N2 sleep † | −0.006 | 0.025 | 0.994 | 0.947–1.043 | 0.808 | |
| N3 sleep † | 0. 063 | 0.044 | 1.066 | 0.978–1.161 | 0.147 | |
| REM sleep † | 0.007 | 0.040 | 1.007 | 0.931–1.090 | 0.858 | |
| RERA index † | −0.162 | 0.068 | 0.851 | 0.745–0.971 | 0.017 * | |
| TST ‡ | 0.002 | 0.006 | 1.002 | 0.990–1.014 | 0.733 | |
| Sleep efficiency ‡ | −0.023 | 0.029 | 0.976 | 0.992–1.034 | 0.412 | |
| Total ArI ‡ | 0.061 | 0.038 | 1.063 | 0.988–1.145 | 0.102 | |
| N1 sleep ‡ | 0.007 | 0. 039 | 1.007 | 0.932–1.145 | 0.851 | |
| N2 sleep ‡ | 0.037 | 0.027 | 1.038 | 0.983–1.095 | 0.176 | |
| N3 sleep ‡ | 0.032 | 0.041 | 1.032 | 0.953–1.118 | 0.433 | |
| REM sleep ‡ | 0.106 | 0.044 | 1.112 | 1.020–1.213 | 0.016 * | |
| ODI ‡ | −0.136 | 0.046 | 0.873 | 0.798–0.956 | 0.003 ** | |
| Optimal pressure | 0.232 | 0.114 | 1.261 | 1.008–1.577 | 0.042 * |
| Dependent Variable | Independent Variables | B | SE | OR | 95% CI | p |
|---|---|---|---|---|---|---|
| Adherence | (Intercept) | −1.95 | 1.018 | 0.142 | 0.018–1.003 | 0.055 |
| Smoking status | −1.32 | 0.407 | 0.267 | 0.116–0.580 | 0.001 ** | |
| ESS | 0.08 | 0.038 | 1.080 | 1.004–1.166 | 0.042 * | |
| RERA index † | −0.11 | 0.056 | 0.900 | 0.802–0.994 | 0.057 | |
| ODI ‡ | −0.10 | 0.041 | 0.906 | 0.829–0.975 | 0.015 * | |
| REM sleep ‡ | 0.05 | 0.028 | 1.048 | 0.992–1.109 | 0.097 | |
| Optimal pressure ‡ | 0.21 | 0.099 | 1.240 | 1.007–1.119 | 0.029 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.H.; Lee, Y.; Shin, S.; Ha, T.K.; Suh, S. Demographic and Sleep Study Factors Influencing Short-Term Adherence to Positive Airway Pressure Therapy in Obstructive Sleep Apnea. J. Clin. Med. 2025, 14, 3988. https://doi.org/10.3390/jcm14113988
Choi JH, Lee Y, Shin S, Ha TK, Suh S. Demographic and Sleep Study Factors Influencing Short-Term Adherence to Positive Airway Pressure Therapy in Obstructive Sleep Apnea. Journal of Clinical Medicine. 2025; 14(11):3988. https://doi.org/10.3390/jcm14113988
Chicago/Turabian StyleChoi, Ji Ho, Yeji Lee, Sungkyoung Shin, Tae Kyoung Ha, and Sooyeon Suh. 2025. "Demographic and Sleep Study Factors Influencing Short-Term Adherence to Positive Airway Pressure Therapy in Obstructive Sleep Apnea" Journal of Clinical Medicine 14, no. 11: 3988. https://doi.org/10.3390/jcm14113988
APA StyleChoi, J. H., Lee, Y., Shin, S., Ha, T. K., & Suh, S. (2025). Demographic and Sleep Study Factors Influencing Short-Term Adherence to Positive Airway Pressure Therapy in Obstructive Sleep Apnea. Journal of Clinical Medicine, 14(11), 3988. https://doi.org/10.3390/jcm14113988






