The Role of VibraPlus on Fatigue in Multiple Sclerosis Patients: A Randomized Controlled Trial
Abstract
1. Introduction
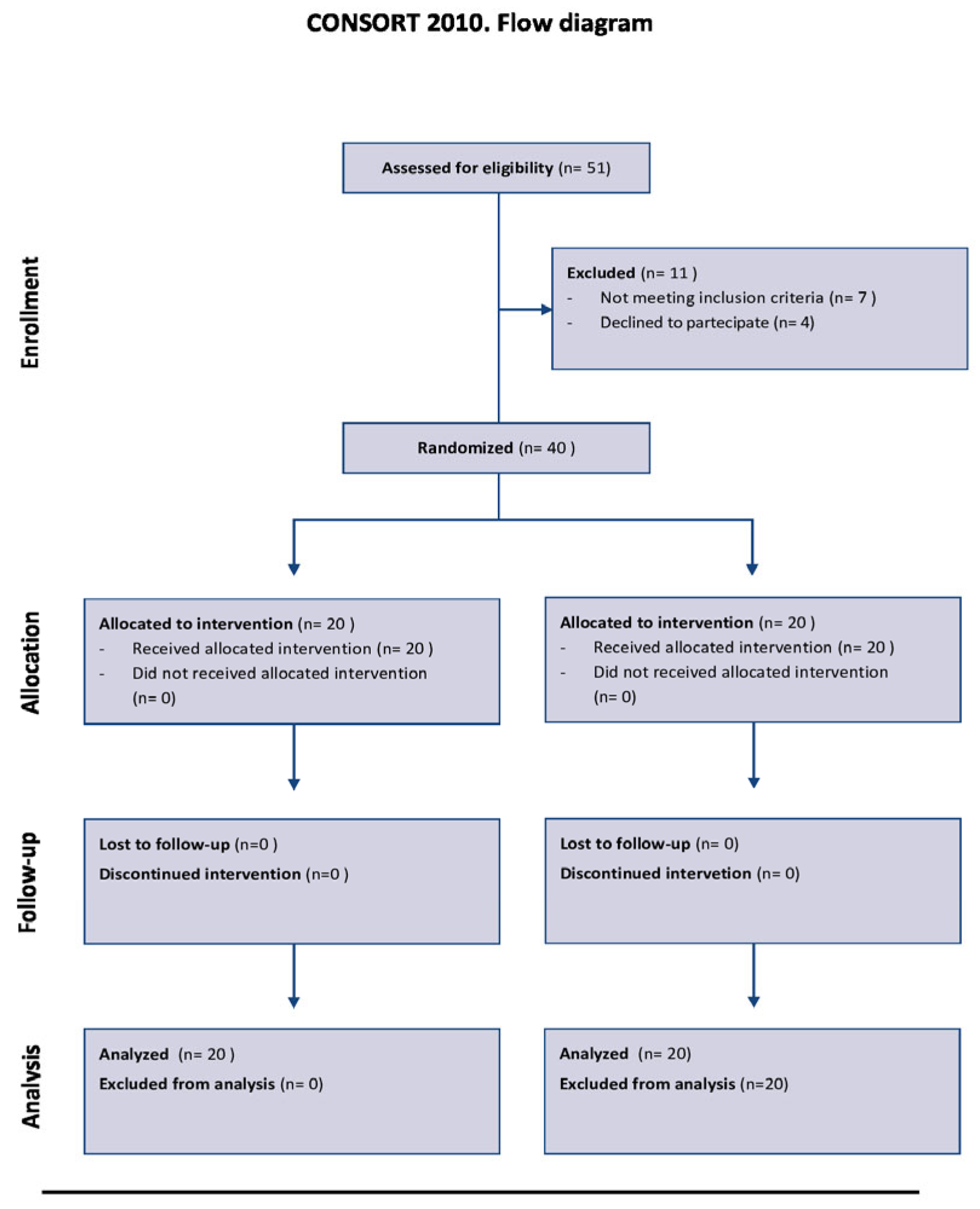
2. Materials and Methods
2.1. Study Design and Population
2.2. Procedures and Device
2.3. Outcome Measures
- -
- Berg Balance Scale (BBS) consists of 14 mobility tasks, with tasks varying in levels of difficulty. Tasks are divided into 3 domains, namely seated balance, standing balance, and dynamic balance. Each activity is assessed on a Likert scale of 5 points with a maximum score of 56. A total score below 45 is associated with a higher risk of falls [31].
- -
- The 6-Minute Walk Test (6MWT) assesses the distance covered in 6 min and provides information about endurance and cardiorespiratory function and evaluates the response to therapeutic treatments [32].
- -
- The Modified Borg Dyspnea Scale (MBS) is a numerical score rated from 0 to 10 used to measure dyspnea as reported by the patient during intense exercise [33].
- -
- Modified Timed Up and Go (TUG) with bilateral turns provides an observational approach to gait assessment and can help to predict the risk of falls. The test assesses the time to rise from a chair, walk 3 m, turn around, and then sit down again. It has been suggested that a cut-off point of 13.5 s may identify individuals with an increased risk of falls [34].
- -
- The 10-Meter Walk Test is a performance measure used to assess gait speed in meters per second over a short distance. The total time taken to walk 6 m is recorded [35].
- -
- The Expanded Disability Status Scale (EDSS) measures the level of disability in MS patients with a range scale from 0 to 10. The initial levels from 1.0 to 4.5 refer to individuals with a high degree of walking ability, from 5.0 to 9.5, pertain to the loss of walking ability [36].
- -
- The Fatigue Severity Scale (FSS) assesses the impact of perceived fatigue on a patient. The instrument consists of Likert scales from 1 to 7 (1 completely agree, 7 completely disagree) [37].
- -
- The Modified Fatigue Impact Scale (MFIS) assesses perceived fatigue and the impact on physical, social, and cognitive levels [38].
- -
- The Fatigue Scale for Motor and Cognitive Functions (FSMC) is a scale composed of 20 items, with 10 items related to cognitive fatigue (FSMC cog) and 10 items related to motor fatigue (FSMC mot). The instrument consists of Likert scales from 1 to 5 points. The total possible score ranges from 20 to 100 points. A total score of ≥43 is classified as mild fatigue, ≥53 as moderate fatigue, and ≥63 as severe fatigue [39].
2.4. Gait Analysis
3. Statistical Analysis
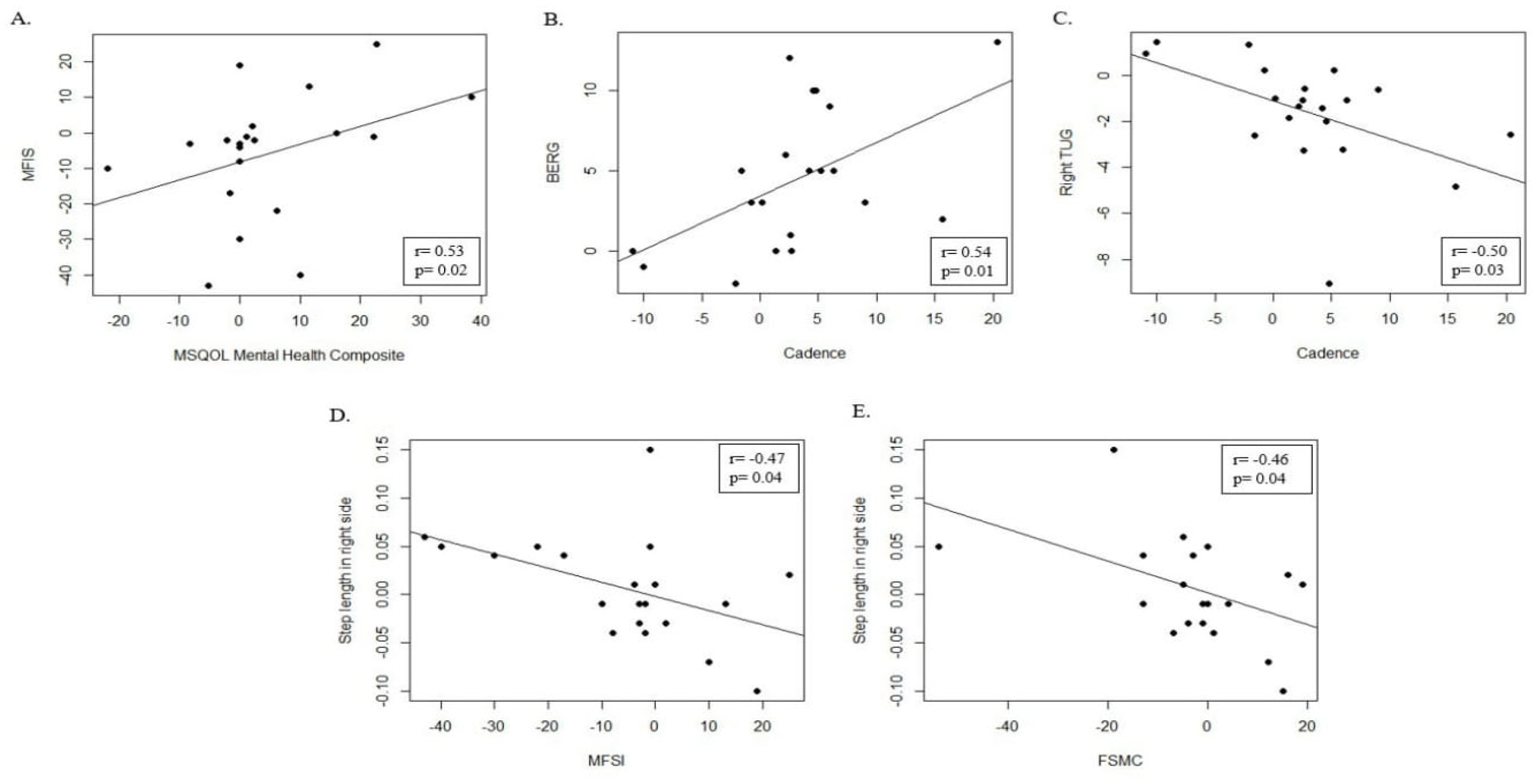
4. Results
4.1. Motor Outcome
4.2. Gait Analysis Outcome
4.3. Cognitive Outcome (BRB-N)
4.4. Quality of Life Outcome (MSQOL-54)
5. Discussion
Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MS | Multiple sclerosis |
| EG | Experimental group |
| CG | Control group |
| TNF | Tumor necrosis factor |
| WBV | Whole-body vibration |
| FMV | Focal muscle vibration |
| QoL | Quality of life |
| RRMS | Relapsing remitting MS |
References
- Howard, J.; Trevick, S.; Younger, D.S. Epidemiology of Multiple Sclerosis. Neurol. Clin. 2016, 34, 919–939. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.; Goldman, M.D. Epidemiology and Pathophysiology of Multiple Sclerosis. Continuum 2022, 28, 988–1005. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a028936. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Pathogenic Mechanisms Associated with Different Clinical Courses of Multiple Sclerosis. Front. Immunol. 2019, 9, 3116. [Google Scholar] [CrossRef]
- Broch, L.; Simonsen, C.S.; Flemmen, H.Ø.; Berg-Hansen, P.; Skardhamar, Å.; Ormstad, H.; Celius, E.G. High prevalence of fatigue in contemporary patients with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2021, 7, 205521732199982. [Google Scholar] [CrossRef]
- Burkill, S.; Montgomery, S.; Kockum, I.; Piehl, F.; Strid, P.; Hillert, J.; Alfredsson, L.; Olsson, T.; Bahmanyar, S. The association between multiple sclerosis and pain medications. Pain 2019, 160, 424–432. [Google Scholar] [CrossRef]
- Chisari, C.G.; Sgarlata, E.; Arena, S.; D’Amico, E.; Toscano, S.; Patti, F. An update on the pharmacological management of pain in patients with multiple sclerosis. Expert Opin. Pharmacother. 2020, 21, 2249–2263. [Google Scholar] [CrossRef]
- Kim, E.S. Fampridine Prolonged Release: A Review in Multiple Sclerosis Patients with Walking Disability. Drugs 2017, 77, 1593–1602. [Google Scholar] [CrossRef]
- Halabchi, F.; Alizadeh, Z.; Sahraian, M.A.; Abolhasani, M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017, 17, 185. [Google Scholar] [CrossRef]
- Rooney, S.; Wood, L.; Moffat, F.; Paul, L. Prevalence of fatigue and its association with clinical features in progressive and non-progressive forms of Multiple Sclerosis. Mult. Scler. Relat. Disord. 2019, 28, 276–282. [Google Scholar] [CrossRef]
- Vienažindytė, I.; Cesarskaja, J.; Vaičiulytė, D.; Balnytė, R.; Matijošaitis, V. Do prodrome symptoms influence multiple sclerosis disease course and severity? Med. Hypotheses 2022, 165, 110888. [Google Scholar] [CrossRef]
- Yusuf, F.L.A.; Wijnands, J.M.; Kingwell, E.; Zhu, F.; Evans, C.; Fisk, J.D.; Zhao, Y.; Sutherland, J.M.; Patrick, D.M.; Marrie, R.A.; et al. Fatigue, sleep disorders, anaemia and pain in the multiple sclerosis prodrome. Mult. Scler. J. 2021, 27, 290–302. [Google Scholar] [CrossRef]
- Karatepe, A.G.; Kaya, T.; Günaydn, R.; Demirhan, A.; Çe, P.; Gedizlioğlu, M. Quality of life in patients with multiple sclerosis. Int. J. Rehabil. Res. 2011, 34, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Langeskov-Christensen, M.; Bisson, E.J.; Finlayson, M.L.; Dalgas, U. Potential pathophysiological pathways that can explain the positive effects of exercise on fatigue in multiple sclerosis: A scoping review. J. Neurol. Sci. 2017, 373, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Heine, M.; van de Port, I.; Rietberg, M.B.; van Wegen, E.E.; Kwakkel, G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst. Rev. 2015, 2015, CD009956. [Google Scholar] [CrossRef]
- Heine, M.; Verschuren, O.; Hoogervorst, E.L.; van Munster, E.; Hacking, H.G.; Visser-Meily, A.; Twisk, J.W.; Beckerman, H.; de Groot, V.; Kwakkel, G.; et al. Does aerobic training alleviate fatigue and improve societal participation in patients with multiple sclerosis? A randomized controlled trial. Mult. Scler. 2017, 23, 1517–1526. [Google Scholar] [CrossRef]
- Englund, S.; Piehl, F.; Kierkegaard, M. High-intensity resistance training in people with multiple sclerosis experiencing fatigue: A randomised controlled trial. Mult. Scler. Relat. Disord. 2022, 68, 104106. [Google Scholar] [CrossRef]
- Kierkegaard, M.; Lundberg, I.E.; Olsson, T.; Johansson, S.; Ygberg, S.; Opava, C.; Holmqvist, L.W.; Piehl, F. High-intensity resistance training in multiple sclerosis—An exploratory study of effects on immune markers in blood and cerebrospinal fluid, and on mood, fatigue, health-related quality of life, muscle strength, walking and cognition. J. Neurol. Sci. 2016, 362, 251–257. [Google Scholar] [CrossRef]
- Afrasiabifar, A.; Karami, F.; Najafi Doulatabad, S. Comparing the effect of Cawthorne–Cooksey and Frenkel exercises on balance in patients with multiple sclerosis: A randomized controlled trial. Clin. Rehabil. 2018, 32, 57–65. [Google Scholar] [CrossRef]
- Brichetto, G.; Piccardo, E.; Pedullà, L.; Battaglia, M.A.; Tacchino, A. Tailored balance exercises on people with multiple sclerosis: A pilot randomized, controlled study. Mult. Scler. J. 2015, 21, 1055–1063. [Google Scholar] [CrossRef]
- Tramontano, M.; Martino Cinnera, A.; Manzari, L.; Tozzi, F.F.; Caltagirone, C.; Morone, G.; Pompa, A.; Grasso, M.G. Vestibular rehabilitation has positive effects on balance, fatigue and activities of daily living in highly disabled multiple sclerosis people: A preliminary randomized controlled trial. Restor. Neurol. Neurosci. 2018, 36, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.; Geroin, C.; Picelli, A.; Munari, D.; Waldner, A.; Tamburin, S.; Marchioretto, F.; Smania, N. Robot-assisted vs. sensory integration training in treating gait and balance dysfunctions in patients with multiple sclerosis: A randomized controlled trial. Front. Hum. Neurosci. 2014, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Saggini, R.; Ancona, E.; Supplizi, M.; Barassi, C.S.; Carmignano, S.; Bellomo, R. Effect of Two Different Rehabilitation Training with a Robotic Gait System in Body Weight Support and a Proprioceptive Sensory-motor Exercises on Unstable Platforms in Rehabilitation of Gait and Balance Impairment and Fatigue in Multiple Sclerosis. Int. J. Phys. Med. Rehabil. 2017, 5, 2. [Google Scholar] [CrossRef]
- Moggio, L.; de Sire, A.; Marotta, N.; Demeco, A.; Ammendolia, A. Vibration therapy role in neurological diseases rehabilitation: An umbrella review of systematic reviews. Disabil. Rehabil. 2022, 44, 5741–5749. [Google Scholar] [CrossRef]
- Uszynski, M.K.; Purtill, H.; Donnelly, A.; Coote, S. Comparing the effects of whole-body vibration to standard exercise in ambulatory people with Multiple Sclerosis: A randomised controlled feasibility study. Clin. Rehabil. 2016, 30, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Pollock, R.D.; Provan, S.; Martin, F.C.; Newham, D.J. The effects of whole body vibration on balance, joint position sense and cutaneous sensation. Eur. J. Appl. Physiol. 2011, 111, 3069–3077. [Google Scholar] [CrossRef]
- Rittweger, J. Vibration as an exercise modality: How it may work, and what its potential might be. Eur. J. Appl. Physiol. 2010, 108, 877–904. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Spiegazione ed Elaborazione: Linee guida aggiornate per il reporting di trial randomizzati a gruppi paralleli. Evidence 2012, 4, e1000024. [Google Scholar]
- Eurtronik SRL. User Manual; Eurtronik SRL: Milan, Italy, 2024. [Google Scholar]
- Tataranu, L.G.; Rizea, R.E. Neuroplasticity and Nervous System Recovery: Cellular Mechanisms, Therapeutic Advances, and Future Prospects. Brain Sci. 2025, 15, 400. [Google Scholar] [CrossRef]
- Miranda, N.; Tiu, T.K. Berg Balance Testing. 2023 Feb 17. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Enright, P.L. The six-minute walk test. Respir. Care 2003, 48, 783–785. [Google Scholar]
- Bausewein, C.; Farquhar, M.; Booth, S.; Gysels, M.; Higginson, I.J. Measurement of breathlessness in advanced disease: A systematic review. Respir. Med. 2007, 101, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar]
- Watson, M.J. Refining the Ten-metre Walking Test for Use with Neurologically Impaired People. Physiotherapy 2002, 88, 386–397. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis. Neurology 1983, 33, 1444. [Google Scholar] [CrossRef]
- Krupp, L.B. The Fatigue Severity Scale. Arch. Neurol. 1989, 46, 1121. [Google Scholar] [CrossRef]
- Mills, R.J.; Young, C.A.; Pallant, J.F.; Tennant, A. Rasch analysis of the Modified Fatigue Impact Scale (MFIS) in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Penner, I.K.; Raselli, C.; Stöcklin, M.; Opwis, K.; Kappos, L.; Calabrese, P. The Fatigue Scale for Motor and Cognitive Functions (FSMC): Validation of a new instrument to assess multiple sclerosis-related fatigue. Mult. Scler. 2009, 15, 1509–1517. [Google Scholar] [CrossRef]
- Boringa, J.B.; Lazeron, R.H.; Reuling, I.E.; Adèr, H.J.; Pfennings, L.E.; Lindeboom, J.; de Sonneville, L.M.; Kalkers, N.F.; Polman, C.H. The Brief Repeatable Battery of Neuropsychological Tests: Normative values allow application in multiple sclerosis clinical practice. Mult. Scler. J. 2001, 7, 263–267. [Google Scholar] [CrossRef]
- Heiskanen, S.; Meriläinen, P.; Pietilä, A. Health-related quality of life-testing the reliability of the MSQOL-54 instrument among MS patients. Scand. J. Caring Sci. 2007, 21, 199–206. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B. Rehabilitation in Multiple Sclerosis: A Systematic Review of Systematic Reviews. Arch. Phys. Med. Rehabil. 2017, 98, 353–367. [Google Scholar] [CrossRef]
- Motl, R.W.; Sandroff, B.M.; Kwakkel, G.; Dalgas, U.; Feinstein, A.; Heesen, C.; Feys, P.; Thompson, A.J. Exercise in patients with multiple sclerosis. Lancet Neurol. 2017, 16, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Alashram, A.R.; Padua, E.; Annino, G. Effects of Whole-Body Vibration on Motor Impairments in Patients with Neurological Disorders. Am. J. Phys. Med. Rehabil. 2019, 98, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Chanou, K.; Gerodimos, V.; Karatrantou, K.; Jamurtas, A. Whole-body vibration and rehabilitation of chronic diseases: A review of the literature. J. Sports Sci Med. 2012, 11, 187–200. [Google Scholar] [PubMed]
- Krause, A.; Lee, K.; König, D.; Faist, M.; Freyler, K.; Gollhofer, A.; Ritzmann, R. Six weeks of whole-body vibration improves fine motor accuracy, functional mobility and quality of life in people with multiple sclerosis. PLoS ONE 2022, 17, e0270698. [Google Scholar] [CrossRef]
- Bazett-Jones, D.M.; Finch, H.W.; Dugan, E.L. Comparing the effects of various whole-body vibration accelerations on counter-movement jump performance. J. Sports Sci. Med. 2008, 7, 144–150. [Google Scholar]
- de Sá-Caputo, D.D.C.; Taiar, R.; Bernardo-Filho, M. Whole-body vibration exercise as an intervention to improve musculoskeletal performance. In Physical Therapy Effectiveness; IntechOpen: London, UK, 2019. [Google Scholar]
- Zhang, Y.; Xu, P.; Deng, Y.; Duan, W.; Cui, J.; Ni, C.; Wu, M. Effects of vibration training on motor and non-motor symptoms for patients with multiple sclerosis: A systematic review and meta-analysis. Front. Aging Neurosci. 2022, 14, 960328. [Google Scholar] [CrossRef]
- Su, Y.-C.; Chang, S.-F. Effects of Whole-Body Vibration Training on Improving Physical Function, Cognitive Function, and Sleep Quality for Older People with Dynapenia in Long-Term Care Institutions: A Randomized Controlled Study. Appl. Sci. 2024, 14, 6830. [Google Scholar] [CrossRef]
- Gil-González, I.; Martín-Rodríguez, A.; Conrad, R.; Pérez-San-Gregorio, M.Á. Quality of life in adults with multiple sclerosis: A systematic review. BMJ Open 2020, 10, e041249. [Google Scholar] [CrossRef]
- Latimer-Cheung, A.E.; Pilutti, L.A.; Hicks, A.L.; Martin Ginis, K.A.; Fenuta, A.M.; MacKibbon, K.A.; Motl, R.W. Effects of Exercise Training on Fitness, Mobility, Fatigue, and Health-Related Quality of Life Among Adults with Multiple Sclerosis: A Systematic Review to Inform Guideline Development. Arch. Phys. Med. Rehabil. 2013, 94, 1800–1828.e3. [Google Scholar] [CrossRef]
- Bueno, I.C.; Ramos-Campo, D.J.; Rubio-Arias, J. A Effects of whole-body vibration training in patients with multiple sclerosis: A systematic review. Neurol. (Engl. Ed.) 2018, 33, 534–548. [Google Scholar] [CrossRef]
- Berrigan, L.I.; Fisk, J.D.; Patten, S.B.; Tremlett, H.; Wolfson, C.; Warren, S.; Fiest, K.M.; McKay, K.A.; Marrie, R.A.; Blanchard, J.; et al. Health-related quality of life in multiple sclerosis. Neurology 2016, 86, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, N.G. Impact of Walking Impairment in Multiple Sclerosis. Patient Patient-Centered Outcomes Res. 2011, 4, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Vanner, E.A.; Block, P.; Christodoulou, C.C.; Horowitz, B.P.; Krupp, L.B. Pilot study exploring quality of life and barriers to leisure-time physical activity in persons with moderate to severe multiple sclerosis. Disabil. Health J. 2008, 1, 58–65. [Google Scholar] [CrossRef] [PubMed]
| EG | CG | p-Value | ||
|---|---|---|---|---|
| BERG | T0 | 42.8 ± 9.0 | 48.5 (39.3–54.0) | 0.31 |
| T1 | 47.5 (43.8–52.2) | 48.5 (39.3–54.0) | 0.91 | |
| p | 0.0002 * | NA | ||
| 10M | T0 | 7.7 ± 3.3 | 6.1 (5.5–9.1) | 0.69 |
| T1 | 6.0 (4.8–9.2) | 6.1 (5.5–9.1) | 0.34 | |
| p | 0.11 | NA | ||
| TUG Right | T0 | 14.3 ± 5.7 | 8.2 (7.6–12.5) | 0.04 * |
| T1 | 11.9 ± 4.2 | 8.2 (7.6–12.5) | 0.15 | |
| p | 0.007 * | NA | ||
| TUG Left | T0 | 14.8 ± 6.1 | 7.9 (7.1–12.1) | 0.02 * |
| T1 | 13.0 ± 5.6 | 7.9 (7.1–12.1) | 0.09 | |
| p | 0.02 * | NA | ||
| BORG | T0 | 3.4 ± 2.0 | 3.6 ± 3.0 | 0.83 |
| T1 | 3.0 (1.8–4.4) | 3.6 ± 3.0 | 0.88 | |
| p | 0.94 | NA | ||
| MFIS | T0 | 51.9 ± 14.9 | 38.7 ± 23.4 | 0.04* |
| T1 | 46.0 ± 17.5 | 40.5 ± 22.8 | 0.27 | |
| p | 0.15 | 0.33 | ||
| FSMC | T0 | 71.8 ± 17.0 | 66.4 ± 22.5 | 0.4 |
| T1 | 68.9 ± 18.2 | 66.4 ± 22.5 | 0.7 | |
| p | 0.41 | NA | ||
| FSS | T0 | 48.7 ± 10.8 | 41.6 ± 12.1 | 0.06 |
| T1 | 46.6 ± 10.7 | 41.6 ± 12.1 | 0.17 | |
| p | 0.23 | NA | ||
| 6 MIN | T0 | 263.6 ± 115.4 | 351.0 (232.5–378.5) | 0.2 |
| T1 | 285.9 ± 125.1 | 351.0 (232.5–378.5) | 0.52 | |
| p | 0.006 * | 0.37 | ||
| EDSS | T0 | 5.5 (4.4–6.0) | 3.5 (3.5–4.1) | 0.01 * |
| T1 | 5.3 (4.4–5.6) | 3.5 (3.5–4.1) | 0.01 * | |
| p | 0.59 | 1 | ||
| VAS fatigue | T0 | 7.7 ± 1.3 | 7.0 (6.0–7.0) | 0.02 * |
| T1 | 6.5 ± 1.90 | 7.0 (6.0–7.0) | 0.73 | |
| p | 0.001 * | NA |
| EG | CG | p-Value | ||
|---|---|---|---|---|
| SRT-LTS | T0 | 26.2 ± 15.5 | 28.4 ± 10.7 | 0.61 |
| T1 | 34.7 ± 16.6 | 28.1 ± 13.6 | 0.18 | |
| p | 0.002 * | 0.93 | ||
| SRT-CLTR | T0 | 21.4 ± 16.4 | 23.6 ± 9.6 | 0.62 |
| T1 | 26.7 ± 16.7 | 23.7 ± 5.5 | 0.44 | |
| p | 0.04 * | 0.96 | ||
| SPART | T0 | 15.9 ± 5.0 | 19.0 ± 4.3 | 0.04 * |
| T1 | 18.1 ± 6.1 | 19.7 ± 4.5 | 0.35 | |
| p | 0.03 * | 0.37 | ||
| SDMT | T0 | 33.0 ± 12.4 | 34.9 ± 13.4 | 0.64 |
| T1 | 36.3 ± 13.3 | 35.0 ± 12.7 | 0.75 | |
| p | 0.02 * | 0.95 | ||
| PASAT-3 | T0 | 31.2 ± 13.3 | 40.0 (16.4–46.5) | 0.68 |
| T1 | 36.6 ± 16.8 | 40.0 (16.4–46.5) | 0.6 | |
| p | 0.11 | 0.18 | ||
| PASAT-2 | T0 | 27.2 ± 15.4 | 25.9 ± 15.1 | 0.78 |
| T1 | 31.9 ± 14.8 | 26.5 ± 14.2 | 0.25 | |
| p | 0.07 | 0.03 * | ||
| SRT-D | T0 | 6.1 ± 3.0 | 7.8 ± 2.2 | 0.04 * |
| T1 | 6.6 ± 3.0 | 7.9 ± 2.0 | 0.12 | |
| p | 0.22 | 0.81 | ||
| SPART-D | T0 | 5.9 ± 2.6 | 6.9 ± 2.2 | 0.19 |
| T1 | 7.2 (3.9–9.04) | 7.1 ± 1.9 | 0.63 | |
| p | 0.05 | 0.1 | ||
| WLG | T0 | 21.1 ± 5.9 | 20.7 ± 5.9 | 0.84 |
| T1 | 21.8 ± 5.8 | 19.9 (18.9–25.1) | 0.61 | |
| p | 0.34 | 0.37 |
| EG | CG | p-Value | ||
|---|---|---|---|---|
| MSQOL Physical Composite | T0 | 90.8 ± 42.1 | 81.8 (53.7–91.3) | 0.32 |
| T1 | 95.3 ± 37.5 | 74.1 (50.0–82.8) | 0.02 * | |
| p | 0.47 | 0.06 | ||
| Physical Function | T0 | 6.4 ± 4.7 | 9.8 (4.3–13.8) | 1 |
| T1 | 8.4 ± 4.3 | 8.8 ± 5.6 | 0.77 | |
| p | 0.12 | 0.34 | ||
| Health Perception | T0 | 5.1 ± 3.3 | 8.5 ± 4.4 | 0.01 * |
| T1 | 6.4 ± 2.9 | 7.7 (5.3–10.2) | 0.08 | |
| p | 0.11 | 0.33 | ||
| Energy/Fatigue | T0 | 4.4 ± 2.8 | 7.2 (5.5–14.4) | 0.003 * |
| T1 | 4.6 ± 2.3 | 5.8 (4.8–6.9) | 0.06 | |
| p | 0.73 | 0.03 * | ||
| Role Physical Limitation | T0 | 3.0 (0.0–9.0) | 6.0 (2.3–12.0) | 0.17 |
| T1 | 0.0 (0.0–12.0) | 6.5 (2.5–12.0) | 0.09 | |
| p | 0.62 | 0.07 | ||
| Pain | T0 | 5.5 ± 3.3 | 6.1 (4.1–7.6) | 0.51 |
| T1 | 6.2 ± 3.1 | 5.9 (4.1–7.4) | 0.71 | |
| p | 0.28 | 1 | ||
| Sexual Functions | T0 | 52.8 (34.5–80.0) | 18.3 (7.7–38.8) | 0.003 * |
| T1 | 53.2 (38.2–80.0) | 14.0 (6.7–38.8) | 0.004 * | |
| p | 1 | 0.58 | ||
| Social Functions | T0 | 6.7 ± 3.4 | 7.5 (5.8–10.0) | 0.21 |
| T1 | 8.5 (4.7–9.9) | 8.0 (5.8–10.0) | 0.38 | |
| p | 0.51 | 1 | ||
| Physical Health Distress | T0 | 5.1 ± 3.6 | 8.3 (5.2–11.0) | 0.03 * |
| T1 | 5.4 ± 3.3 | 7.1 ± 3.4 | 0.12 | |
| p | 0.5 | 1 | ||
| MSQOL Mental Composite | T0 | 46.2 ± 24.4 | 50.8 ± 12.8 | 0.46 |
| T1 | 50.9 ± 20.7 | 52.1 ± 14.0 | 0.84 | |
| p | 0.12 | 0.58 | ||
| Emotional Health Distress | T0 | 6.4 ± 4.5 | 10.0 (5.4–11.9) | 0.06 |
| T1 | 6.9 ± 4.1 | 10.0 (5.4–12.6) | 0.08 | |
| p | 0.47 | 1 | ||
| General Quality of Life | T0 | 7.6 ± 2.8 | 8.7 (7.2–10.3) | 0.09 |
| T1 | 8.7 ± 3.2 | 8.7 (7.2–10.5) | 0.41 | |
| p | 0.17 | 0.37 | ||
| Emotional Well-being | T0 | 13.3 ± 7.5 | 9.6 (7.7–12.0) | 0.09 |
| T1 | 14.5 (11.3–20.0) | 11.8 ± 3.1 | 0.06 | |
| p | 0.08 | 0.18 | ||
| Role Emotional Limitation | T0 | 7.9 (0.0–24.0) | 10.4 (0.0–16.0) | 0.7 |
| T1 | 11.9 (0.0–24.0) | 12.4 /(1.0–14.5) | 0.48 | |
| p | 0.25 | 0.06 | ||
| Cognitive Functions | T0 | 8.6 ± 4.1 | 10.5 (8.6–12.0) | 0.61 |
| T1 | 8.0 ± 4.2 | 11.3 (9.0–12.0) | 0.04 * | |
| p | 0.35 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formica, C.; Latella, D.; Bonanno, L.; Lombardo Facciale, A.; Paladina, G.; Leo, A.; Pergolizzi, L.; Fonti, B.; Quartarone, A.; Cellini, R.; et al. The Role of VibraPlus on Fatigue in Multiple Sclerosis Patients: A Randomized Controlled Trial. J. Clin. Med. 2025, 14, 3990. https://doi.org/10.3390/jcm14113990
Formica C, Latella D, Bonanno L, Lombardo Facciale A, Paladina G, Leo A, Pergolizzi L, Fonti B, Quartarone A, Cellini R, et al. The Role of VibraPlus on Fatigue in Multiple Sclerosis Patients: A Randomized Controlled Trial. Journal of Clinical Medicine. 2025; 14(11):3990. https://doi.org/10.3390/jcm14113990
Chicago/Turabian StyleFormica, Caterina, Desirée Latella, Lilla Bonanno, Antonino Lombardo Facciale, Giuseppe Paladina, Antonino Leo, Luca Pergolizzi, Bartolo Fonti, Angelo Quartarone, Roberta Cellini, and et al. 2025. "The Role of VibraPlus on Fatigue in Multiple Sclerosis Patients: A Randomized Controlled Trial" Journal of Clinical Medicine 14, no. 11: 3990. https://doi.org/10.3390/jcm14113990
APA StyleFormica, C., Latella, D., Bonanno, L., Lombardo Facciale, A., Paladina, G., Leo, A., Pergolizzi, L., Fonti, B., Quartarone, A., Cellini, R., & Calabrò, R. S. (2025). The Role of VibraPlus on Fatigue in Multiple Sclerosis Patients: A Randomized Controlled Trial. Journal of Clinical Medicine, 14(11), 3990. https://doi.org/10.3390/jcm14113990








