Abstract
Diffuse lipomatosis of the thyroid (DLT) is an uncommon condition where mature fat cells infiltrate the thyroid gland, disrupting its normal structure. Although rare, it typically manifests as neck enlargement or symptoms of compression, including breathing difficulties, trouble swallowing, and voice changes, which can complicate diagnosis. This report presents a case of a 61-year-old woman with DLT, who exhibited a multinodular goiter and progressive neck swelling, and examines 53 additional cases from the existing literature. The analysis indicates that DLT is frequently misdiagnosed because of its similarities with other thyroid disorders. The precise mechanism underlying its development remains uncertain, but theories include oxygen deprivation in tissues, developmental abnormalities, and disruptions in fat metabolism. Surgical removal is the preferred treatment, especially for patients experiencing symptoms, and has shown favorable long-term outcomes. Additional studies should aim to elucidate the exact cause of DLT and enhance diagnostic precision, particularly in distinguishing it from other fat-containing thyroid lesions such as amyloid goiter and adenolipomas. A deeper understanding of this condition will inform better treatment approaches and enhance patient outcomes for this uncommon but significant thyroid disorder.
1. Introduction
Diffuse lipomatosis of the thyroid or DLT is characterized by infiltration of the parenchyma of the gland and replacement of the otherwise typical anatomical structure with mature adipose cells. It is not commonly found among other pathological conditions of the thyroid but nonetheless presents an interesting phenomenon. Although the thyroid is closely connected to other mesodermal structures during embryogenesis, contributing to fat deposition in areas around the cervical blood vessels in the subcapsular region of the anterior thyroid region, the presence of significant amounts of adipocytes within the thyroid stroma is rare [1,2,3,4]. Diffuse adipose tissue causing enlargement is often found in the thymus, parathyroid glands, pancreas, and salivary glands, as a result of atrophy-related parenchymal fatty metaplasia, whereas thyrolipoma is the most common condition in the thyroid [5,6,7]. Adenolipomas of the thyroid or thyrolipomas are well-circumscribed admixtures of adipose and follicular cells surrounded by a fibrous capsule consisting of a mixed neoplasm that is mesenchymo-epithelial [4]. Both thyrolipomas and diffuse lipomatosis are benign lesions of the gland, with the latter being more frequently associated with escalating growth clinically manifesting with compression symptoms from nearby organs such as dyspnea, hoarseness, dysphagia, and overall swelling. The enlargement observed in acquired and congenital goiters may also be caused by fat invasion rather than follicular proliferation [8,9]. The exact pathophysiological mechanism responsible for the diffuse nature of the fatty infiltration of the thyroid remains unclear. Here, we present an indigenous case and review the literature on DLT.
2. Case Report
Our case involves a 61-year-old woman who was referred to our department for a total thyroidectomy. She had been diagnosed with a substernal multinodular goiter two years previously, and in the last six months, she gradually developed neck swelling, dyspnea, and dysphagia. Her medical history showed that she had chronic renal failure of unknown etiology, and she had been undergoing hemodialysis for the last year. She was also diagnosed with hypertension, diabetes mellitus, and obesity [BMI (body mass index): 37]. Upon clinical examination, a substantial thyroid mass with a soft consistency was palpated. Her thyroid function was normal [TSH (Thyroid Stimulating Hormone): 0.46 pmol/L, FT4 (Free Thyroxine): 20.09 pmol/L, FT3 (Free Triiodothyronine): 3.1 pmol/L)] and she tested negative for serum thyroperoxidase (anti-TPO) and thyroglobulin antibodies (anti-TG). Ultrasonography findings indicated an enlarged thyroid gland with dispersed hyperechoic regions. In accordance, CT (computed tomography) confirmed the presence of an enlarged gland showing low attenuation and septations. Computed tomography revealed that the thyroid had invaded the entire cervical area and the upper mediastinum, compressing the surrounding tissue (Figure 1). Thyroid scintigraphy with Tc99m (Technetium-99m) revealed low uptake of radioactivity in the entire gland, suggesting the possibility of subacute thyroiditis. The patient underwent total thyroidectomy. The operation lasted for 2 h and proved to be an arduous challenge. Intraoperatively, the thyroid was friable upon touch, its capsule was very thin, and the gland was soft–fatty in texture, similar to a lipoma (Figure 2). Following surgery, the patient experienced no complications and was released two days later. Histopathological examination revealed fatty infiltration of the thyroid gland and degeneration of follicles. Immunohistochemical staining for thyroglobulin (TG) and thyroid transcription factor-1 (TTF1) verified the existence of a few colloid-filled thyroid follicles, whereas positive S100 staining demonstrated the preponderance of adipose tissue within the gland. (Figure 3). Therefore, the patient was diagnosed with diffuse thyroid lipomatosis. Two years after surgical treatment, the patient died because of complications associated with kidney failure.
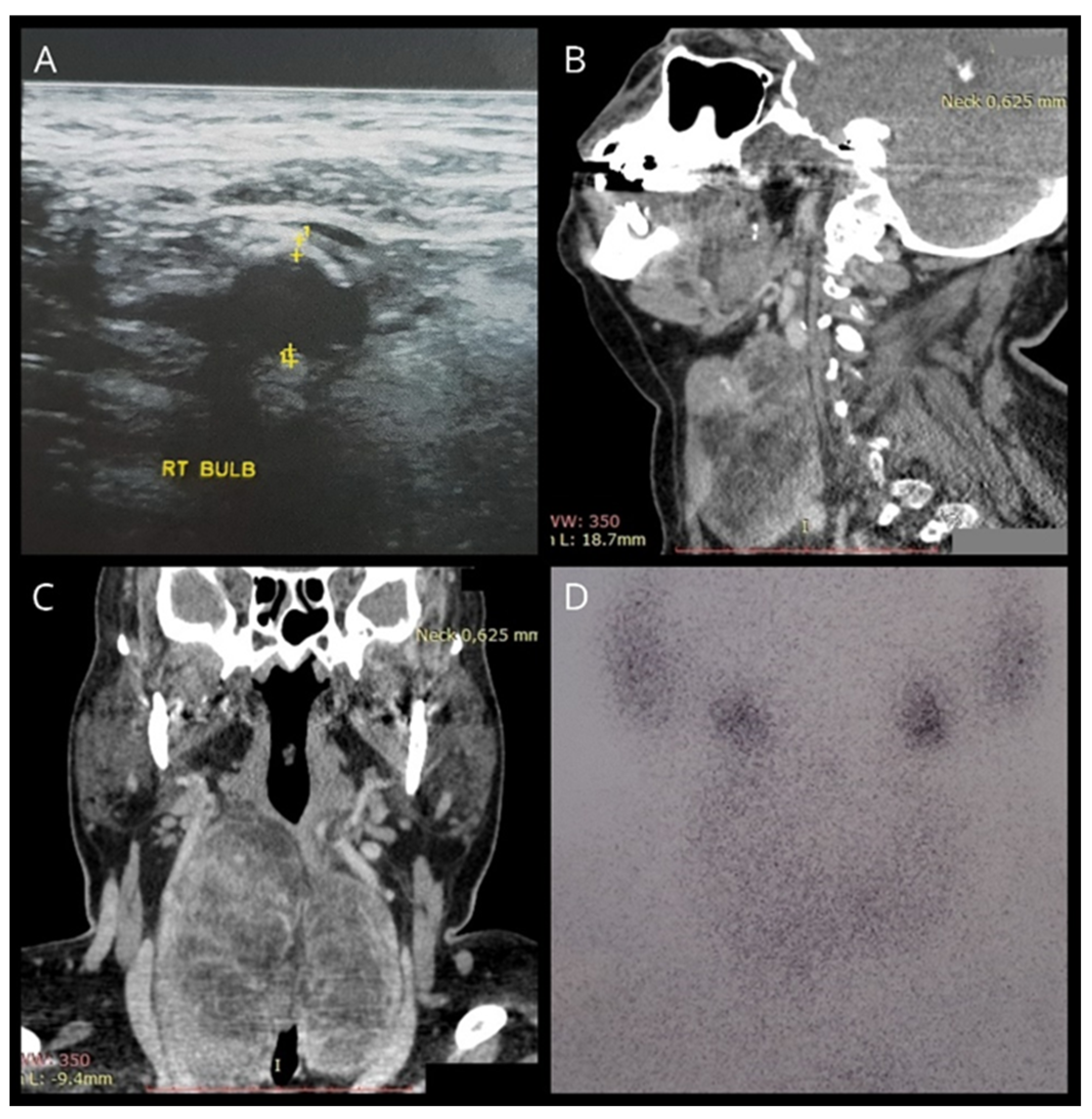
Figure 1.
(A) Ultrasonography showing a hyperechogenic thyroid gland. (B,C) CT findings indicating a diffusely enlarged thyroid with hypodense areas and intrathoracic extensions. (D) Tc99m scintigraphy showing heterogeneous uptake of the isotope with diffuse hypofixation.
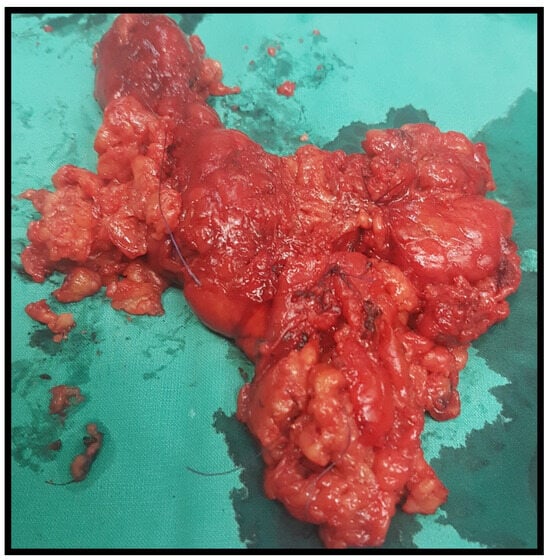
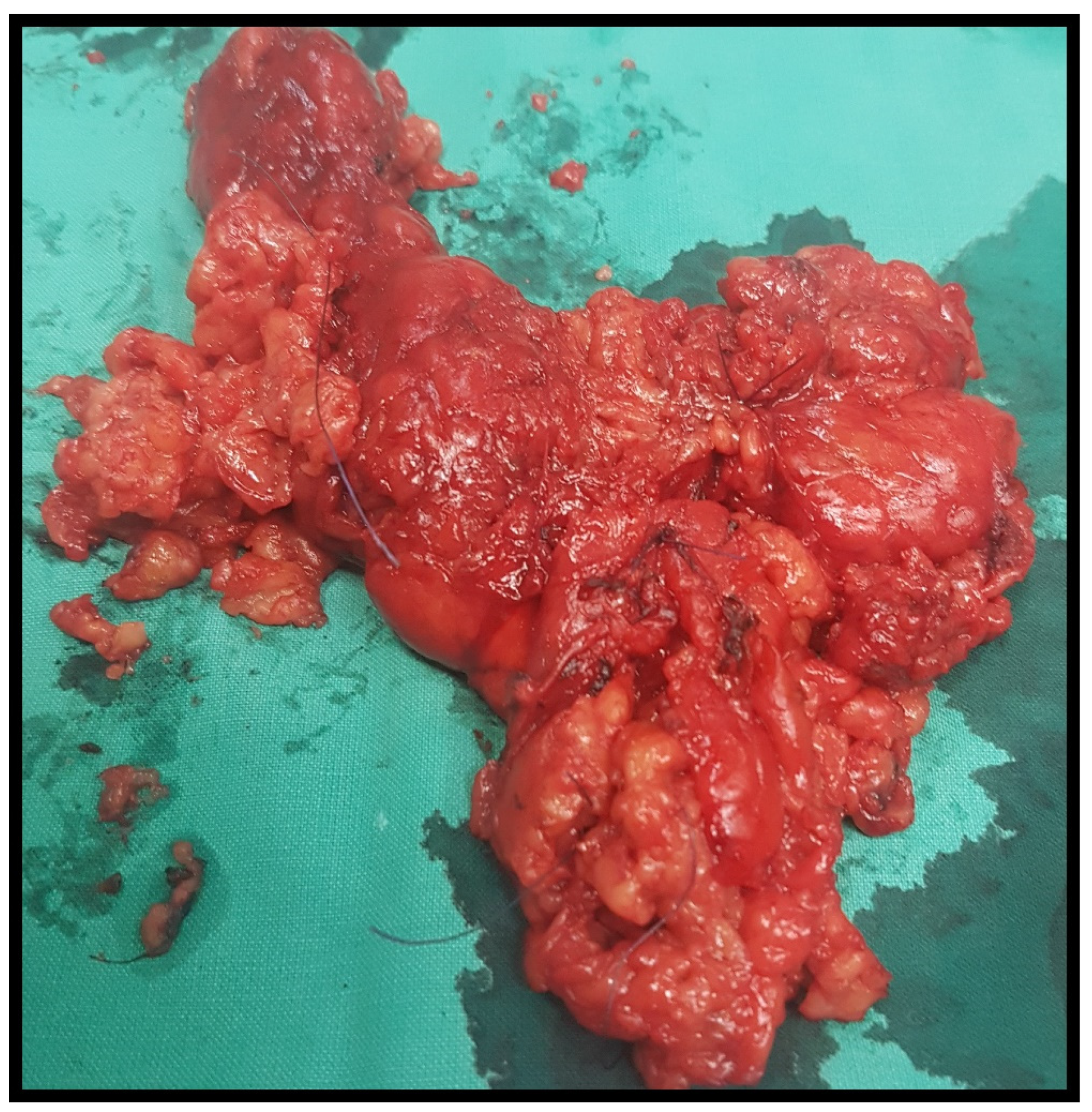
Figure 2.
Thyroid specimen after total thyroidectomy.
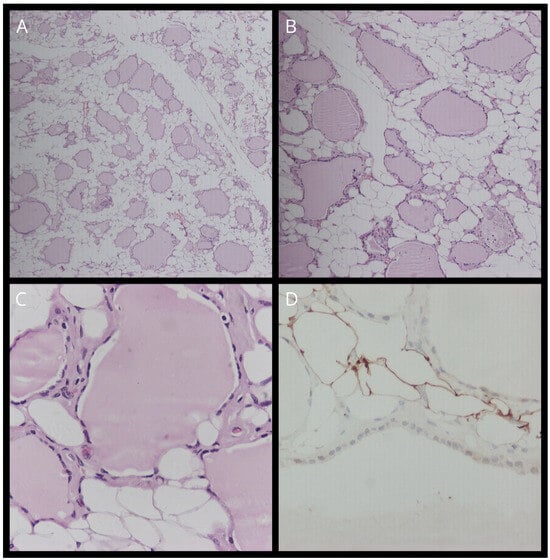
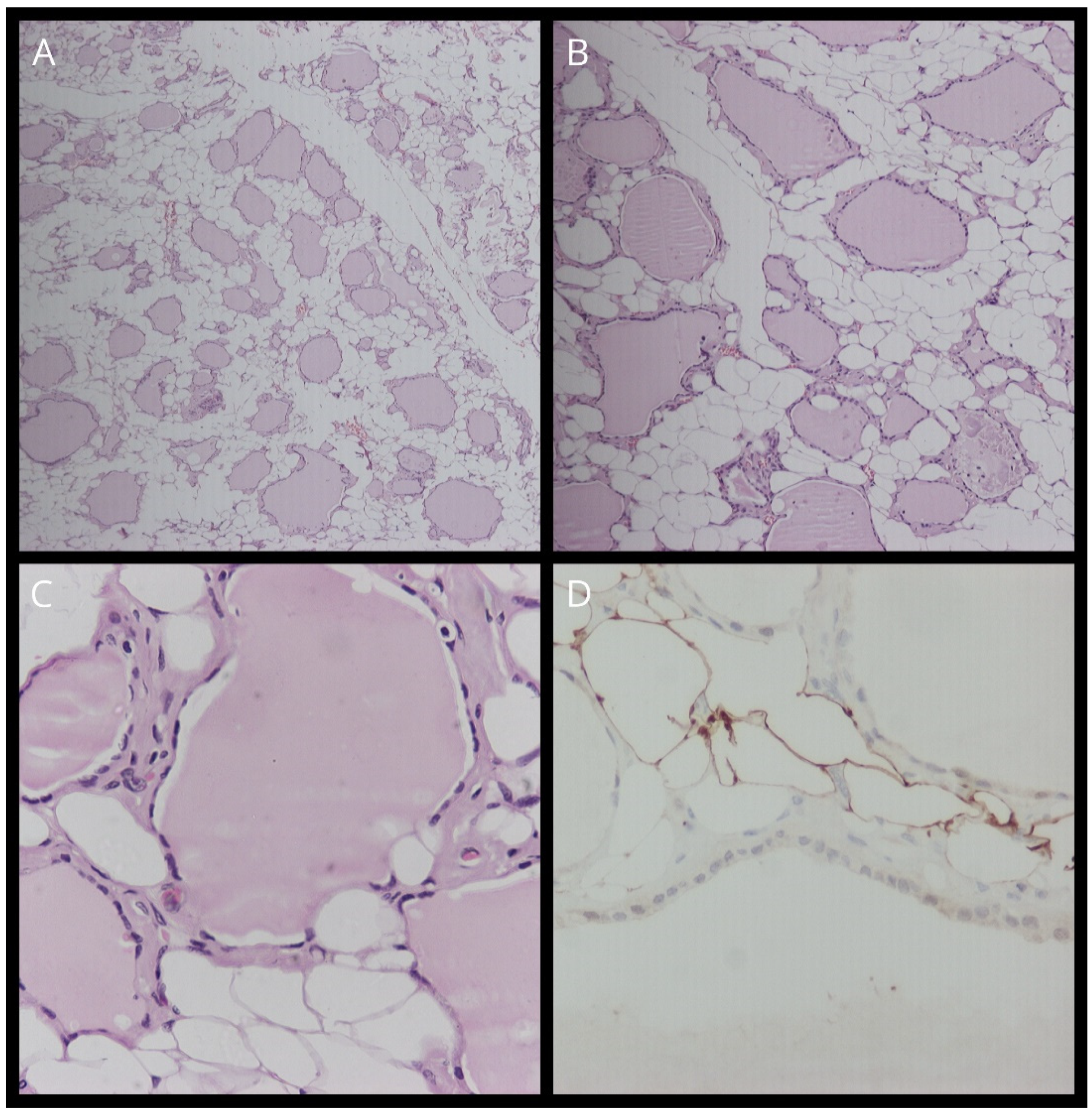
Figure 3.
(A) Staining with hematoxylin–eosin (H&E) (magnification, ×10). (B) Staining with hematoxylin–eosin (H&E) (magnification, ×40). (C) Staining with hematoxylin–eosin (H&E) (magnification, ×100). (D) TTF-1 staining.
3. Review
The first description of DLT in the medical literature was by Dhayagude [10] in 1942. Since then, we identified another 53 cases of thyroid lipomatosis including our own. We systematically researched MEDLINE, Embase, Scopus, and Web of Science for all articles involving thyroid lipomatosis cases. We included only reports where the gland was almost entirely and diffusely infiltrated by fat. Based on our criteria, we identified common characteristics among the above reports including sex, age, symptoms, thyroid function, renal status, initial diagnosis, treatment, histopathological features, and follow-up. Nine of the patients (16%) were described by Ge [1], two in 2016 by Bell [11], and, more recently, three cases by Celik [8]. The average age was 50 years (range, 11–78 years), and there was no significant sex predilection between males (47%) and females (53%). The most significant clinical feature was local compression (60%), which involved dysphagia, dysphonia/hoarseness, dyspnea, and neck swelling (46.7%), whereas one-third of the patients did not show any symptoms. Thyroid function was uncompromised in most patients (66%), and only a few patients had hypothyroidism (12.7%) or hyperthyroidism (21.3%). What appeared to be of notice was the fact that 40% of patients suffered from renal failure, with most of them being affected by secondary amyloidosis. Amyloid goiters are found more frequently in secondary amyloidosis than in primary amyloidosis and are commonly misdiagnosed as thyroid carcinoma [12]. In terms of diagnostic imaging, the most frequently employed techniques were thyroid ultrasound—U/S (72%), CT scans (67.4%), scintigraphy (23.2%), and magnetic resonance imaging—MRI (16.2%). Many patients were initially diagnosed with goiters (64.6%), either diffuse or multinodular, and one patient reported an amyloid goiter (2.1%), while a diagnosis of DLT was recently proposed in eight reports (16.7%). Among the reports that mentioned a treatment method, total thyroidectomy was the predominant choice (47.9%). Only one patient with a preliminary diagnosis of DLT was discharged without treatment, and partial thyroidectomy was an alternative, especially in patients with unilateral nodular disease. The average thyroid weight was 232.16 g (range 15–700 g). All cases revealed that the thyroid was almost entirely composed of mature adipose cells, and in cases of amyloid infiltration, the confirmation consisted of positive Congo red or thioflavin staining. Fat deposits that vary in amount are frequently found in amyloid goiter [7]. Amyloid infiltration was observed in almost half of patients (44.8%). We only included reports in which the gland was almost completely or in most parts infiltrated by fat tissue regardless of amyloid deposition, fibrosis, or lymphocyte aggregates. Only a minority of reports (43.4%) included follow-up information, and most (78.3%) had an uncomplicated post-operative course ranging from 1 day to 12 months. One patient reported respiratory difficulty 3 years after the first evaluation, three others died of complications, two because of renal insufficiency and the other after developing metastases in the residual thyroid lobe. Finally, a DLT case with concurrent secondary amyloidosis was revealed via the patient’s autopsy. Table 1 summarizes the characteristics of the DLT in these patients (Table 1).

Table 1.
Summary of thyroid lipomatosis case reports.
4. Discussion
Numerous attempts have been made to explain the etiology of fatty infiltration in the thyroid gland. According to Dhayagude [10], fat deposits in colloid goiters may arise from the degeneration of follicular tissue due to damage such as hemorrhage, fibrosis, infarction, calcification, or cystic degeneration. In his textbook “The borderland of Embryology and Pathology”, Willis [53] describes an adenolipoma and explains that its presence could be a result of the metaplastic formation of fat. In amyloid goiters, it is presumed that adipose tissue is formed from stromal metaplasia of fibroblasts as a result of senile involution or tissue hypoxia [54]. Trites [55] hypothesized that some factors may have affected the primitive foregut during embryogenesis, causing the formation of mixed embryonic tumors and explaining fatty infiltration in congenital goiters. In various lipomas, it has been suggested that a disturbance in lipid metabolism could promote the accumulation of fat tissue [56], whereas others have argued that fat accumulation does not seem to depend on general factors such as obesity [57]. New research suggests that individuals with a BMI exceeding 25 are more likely to develop steatosis and have increased fat deposits between follicles in their thyroid glands. In certain instances, the amount of fat accumulation appeared to increase in proportion to the BMI value [58,59]. Obesity-related fat accumulation can be detected through the use of HAM56 (macrophages monoclonal antibody 56) immunostaining. This technique identifies macrophages that encircle senescent adipocytes, serving as a marker of late-stage obesity [60]. Chevsky et al. [14] proposed that adipose tissue might be incorporated into the thyroid during embryogenesis, along with striated muscle, before the gland capsule is formed. Schroder [4] postulated that DLT is associated with “displaced nests of embryonic structures, calling the entity “choristomatous adiposity”. A recent theory that attempts to explain the pathophysiology of the disease suggests that somatic mutations leading to the loss of succinate dehydrogenase-subunit B (SDHB) expression may play a role. Immunohistological staining revealed the loss of this protein in cells from DLT tissue, resulting in the deregulation of the mitochondrial respiration process and interference with lipid metabolism. This could result in a decrease in fatty acid oxidation, explaining the attenuation of fat in the gland and the replacement of normal follicles [6]. All of these theories could be plausible because they explain different aspects of infiltration, either regarding its diffuse presence in congenital and acquired goiters or the limitation of fatty deposition in certain regions of the gland.
Amyloidosis can either lead to or result from chronic kidney failure. The two main types of amyloidosis are primary, which stems from plasma cell disorders and is characterized by an increase in immunoglobulin light chains, and secondary (or reactive), which is linked to long-term inflammation, infections, and cancers. Both forms can potentially cause chronic renal failure due to amyloid accumulation. On the other hand, hemodialysis-associated amyloidosis, typically occurring after about ten years of treatment, is related to the ineffective filtration of β2 microglobulin during dialysis, leading to its buildup as Aβ2M amyloid protein. All these types of amyloidosis are connected to kidney failure. Although rare, the abnormal deposition of amyloids may also result in an amyloid goiter [61,62].
Imaging techniques are a common diagnostic tool in the investigation of diffuse goiter and are the presumed initial diagnosis in most cases. After extensive investigation, only nine reports assumed that the gland was infiltrated by fat tissue. Ultrasonographic findings most commonly indicate parenchymal heterogeneity [26], gland enlargement, and cystic or solid nodules with septations [25,28,41]. Computed tomography (CT) and fine-needle aspiration (FNA) biopsy are the gold standard for the early diagnosis of disease [25]. Tomographic findings consisted of an enlarged gland with low attenuation (−30 to −70 Hounsfield units) and heterogeneity in the parenchyma with a few areas showing hyperattenuation, probably indicating the presence of normal thyroid tissue [25,28,31,34,39]. In cases where compressing symptoms were present, invasion of the retropharyngeal space, expansion to the thoracic inlet, and compression of nearby organs such as the trachea or esophagus, were found [11,28]. Overall, almost all reports noted a low tomographic density of the thyroid stoma, but it was not low enough to be certain of fatty infiltration [11,34]. Lately, MRI (magnetic resonance imaging) has been more frequently used to confirm findings of low density in the stroma and increased signals with fat suppression in T1 and T2 sequences [40,44]. Scintigraphy with Tc99m or I123 (Iodine-123) showed heterogeneity in radioactive uptake, occasionally indicating the presence of cold nodules [26,36]. Finally, FNA cytology or biopsy was performed in ambivalent cases expressing infiltration of the gland with fatty tissue, either in the background or predominantly. Fine needle aspiration is only reliable when samples are obtained from areas of fat infiltration. In other cases, the results can be highly variable, potentially suggesting anything from a colloid cyst to a possible follicular carcinoma [41,46,51]. However, clear guidelines for the definitive diagnosis of thyroid lipomatosis are eclipsed, and almost none of the above cases reached a diagnosis until after pathological findings were released [41]. A definitive diagnosis can be achieved after thyroidectomy if the pathological report describes diffuse gland infiltration [11].
The differential diagnoses include non-neoplastic fat-containing lesions such as adenolipoma, DLT, amyloid goiter, lymphocytic thyroiditis, heterotopic nests of adipose cells, parathyroid lipoma, or intrathyroid thymic tissue, and neoplastic lesions such as lipid-rich cell adenoma, liposarcoma encapsulated papillary carcinoma or anaplastic carcinoma. Adenolipoma can be easily differentiated from diffuse lipomatosis by its well-defined, encapsulated appearance along with the simultaneous admixture with proliferated thyroid follicles [25,26,40]. An amyloid goiter is usually found in systemic amyloidosis, either primary or secondary, and its pathognomonic characteristic of positive Congo red or crystal violet staining makes the diagnosis relatively straightforward [5,26]. However, there are cases where both amyloid and fat cell depositions were found in equal amounts or amyloid deposits were scarce amidst a thyroid stroma mainly composed of fat cells [26]. As there is no certainty regarding the criteria for the differential diagnosis of amyloid goiters with fatty infiltration from thyroid lipomatosis, we only considered cases where there was a clear predominance of fat tissue over amyloid proteins. Accumulation of various amounts of fat cells is a common finding in amyloid goiters because tissue hypoxia caused by a gradual increase in amyloid could drive fibroblasts to differentiate into fat cells, as previously stated [22]. Lymphocytic thyroiditis is associated with extensive infiltration of the stroma by lymphocytes, whereas heterotopic nests can be only found in the subcapsular regions of the gland [3,25,57]. The intimate embryologic origin of the thyroid with the parathyroid glands and thymus may be the cause of parathyroid lipomas, with characteristic cytoplasmic glycogen deposits, and ectopic thymic tissue [1,5]. Lipid-rich follicular adenomas can be distinguished by the presence of follicles with aggravated intracytoplasmic lipid formation, circular nuclei, and vesicular morphology [3,35]. Liposarcoma is a rare, aggressive neoplasm that usually expands rapidly beyond the thyroid capsule. Finally, there have been a few cases in which papillary carcinoma was found within diffusely enlarged goiters infiltrated with fat. Carcinomas can be identified via immunostaining for p53, CD68 (Cluster of Differentiation 68), HBME-1 (Human Bone Marrow Endothelium Marker-1), and CK19 (Cytokeratin 19) [63,64,65]. In one case, the histopathological features and immunohistochemical profile of the tumor were evident, and adipose cells were occasionally found in small amounts within the stroma [31].
The swift enlargement of the thyroid and its tendency to press on surrounding structures necessitates distinguishing it from anaplastic cancer and Riedel’s thyroiditis. In most instances, imaging techniques can differentiate between lipomatosis and anaplastic cancer, guiding appropriate diagnosis and treatment strategies [31]. It is noteworthy that invasive fibrous thyroiditis, though rare, should be considered, particularly as it shares alarming symptoms like difficulty swallowing and breathing, and exhibits similar CT findings. Nevertheless, supplementary imaging methods such as ultrasound and MRI can typically discern between these conditions, as Riedel’s thyroiditis presents hypoechoic and low signal density characteristics due to fibrotic tissue infiltration. While FNA biopsy or cytology can probably differentiate thyroid lipomatosis from the aforementioned conditions, only an open surgical biopsy can definitively distinguish between anaplastic carcinoma and Riedel’s thyroiditis [66,67,68].
A distinguishing feature of diffuse thyroid lipomatosis is the infiltration and replacement of what would be an otherwise normal thyroid stroma by mature adipose cells [33]. Macroscopically, the gland has a pale yellow-brown color and a soft and friable texture. In most cases, the gland size exceeded the normal weight (10–20 g), the lobes were enlarged, and when cut, they had a lobular appearance [5,11,31]. Schroder et al. [4] noted that the gland may resemble a congenital goiter because of its progressive growth during the first decade of life. Microscopic analysis revealed that mature adipocytes lacking encapsulation had replaced normal thyroid follicles [5,11,42,54]. The fat tissue is lobulated by strands of fibrous tissue. The remaining indigenous cells were clustered in random amounts and were scattered throughout the fatty stroma [54]. The follicles were lined with cuboidal epithelium and exhibited colloid accumulation [31]. Infiltration of fat with scarce lymphocytes was observed in a few cases, whereas the deposition of pink unshaped material was found around the remnants of the follicular tissue [5,11]. Only in instances of systemic amyloidosis was this discovery described; in these cases, the amyloid A protein was identified through immunohistochemical staining and validated by apple-green birefringence during polarized microscopy using Congo red staining [11]. Papillary carcinoma coincided with DLT in three cases and was described as a localized group of follicles surrounded by fat, with oval cells and enlarged ground glass nuclei. Immunohistochemical positivity for thyroglobulin and TTF-1 confirmed the diagnosis [3,69].
Total thyroidectomy via a transverse horizontal cervical incision appears to be an appropriate therapeutic option for patients with symptomatic swelling [35]. Caution should be exercised in view of the softness and friability of the thyroid gland to avoid extensive intra-operative bleeding. Mobilization of the gland should be performed meticulously, with minimal traction during detachment and identification of the adjacent laryngeal nerves and parathyroid glands [25]. Hemithyroidectomy was also performed on some occasions where the findings could not exclude malignancy. A subsequent histopathological evaluation confirmed the diagnosis of diffuse thyroid lipomatosis. Biopsy samples may have been obtained from areas with scarce or no fat content [42]. Our review indicates that there should be a surgical attempt to assuage the patient only in cases in which compression symptoms are present. If the patient is asymptomatic and DLT is confirmed via imaging techniques or biopsy, the patient should be discharged and follow-up should be advised in the next few months to re-estimate the extent of the disease [40]. Owing to the underlying pathophysiological mechanisms of the disease, in cases where DLT is confirmed before or during surgery, the potential presence of ectopic thyroid tissue should be investigated [47]. Finally, in cases with thyroid swelling and hyperactivity indicated by scintigraphy, a CT scan should be ordered to examine the consistency of the gland. In the case of a possible DLT diagnosis, radioiodine ablation therapy can be dismissed altogether and replaced directly by total thyroidectomy [34].
5. Conclusions
Diffuse lipomatosis of the thyroid gland is a rare but fascinating condition that involves the replacement of normal thyroid tissue with fatty infiltration. Our review of the literature and presented case report highlight the clinical features, diagnostic challenges, and treatment options associated with this condition. Our results indicate that surgical intervention should be considered in symptomatic patients as it has been shown to lead to positive long-term results in most cases. Further research is needed to better understand the underlying mechanisms of DLT, which will enable the development of more accurate diagnostic and therapeutic approaches and improve patient outcomes.
Author Contributions
A.E.: Methodology (equal), formal analysis (lead), investigation (lead), writing—original draft (lead), and writing—review and editing (equal). M.K.: Resources (equal), writing—review and editing (equal), and project administration (supporting). K.P.-P.: Resources (equal), supervision (supporting), and writing—review and editing (equal). N.M.: Conceptualization (lead), methodology (equal), supervision (lead), writing—review and editing (equal), and project administration (lead). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All relevant data are within this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ge, Y.; Luna, M.A.; Cowan, D.F.; Truong, L.D.; Ayala, A.G. Thyrolipoma and thyrolipomatosis: 5 case reports and historical review of the literature. Ann. Diagn. Pathol. 2009, 13, 384–389. [Google Scholar] [CrossRef]
- Sanuvada, R.; Chowhan, A.; Rukmangadha, N.; Patnayak, R.; Yootla, M.; Amancharla, L. Thyrolipomatosis: An inquisitive rare entity. Gland. Surgery 2014, 3, E6–E9. [Google Scholar] [CrossRef] [PubMed]
- Nandyala, H.S.; Madapuram, S.; Yadav, M.; Katamala, S.K. Diffuse lipomatosis of the thyroid gland with papillary microcarcinoma: Report of a rare entity. Indian J. Pathol. Microbiol. 2015, 58, 348–350. [Google Scholar] [CrossRef]
- Schroder, S.; Bocker, W.; Hüsselmann, H.; Dralle, H. Adenolipoma (Thyrolipoma) of the Thyroid Gland Report of Two Cases and Review of Literature. Virchows Archiv A 1984, 404, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Dombale, V.D.; Javalgi, A.P. Symmetric Diffuse Lipomatosis of the Thyroid Gland. J. Clin. Diagn. Res. 2011, 5, 867–868. Available online: www.jcdr.net (accessed on 11 April 2023).
- Lau, E.; Freitas, P.; Costa, J.; Batista, R.; Máximo, V.; Coelho, R.; Matos-Lima, L.; Eloy, C.; Carvalho, D. Loss of mitochondrial SDHB expression: What is its role in diffuse thyroid lipomatosis? Horm. Metab. Res. 2015, 47, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Daboin, K.P.; Ochoa-Perez, V.; Luna, M.A. Adenolipomas of the head and neck: Analysis of 6 cases. Ann. Diagn. Pathol. 2006, 10, 72–76. [Google Scholar] [CrossRef]
- Çelik, Z.E. Mature Fat Containing Thyroid Lesions. Eur. J. Gen. Med. 2015, 12, 353–355. [Google Scholar] [CrossRef]
- Citgez, B.; Uludag, M.; Yetkin, G.; Akgun, I.; Yener, S.; Polat, N.; Isgör, A. Amyloid goiter with diffuse lipomatosis. World J. Endocr. Surg. 2011, 3, 97–99. [Google Scholar] [CrossRef]
- Dhayagude, R.G. Case report: Massive fatty infiltration in a colloid goiter. Arch. Pathol. 1942, 33, 357–360. [Google Scholar]
- Bell, S.; Sosa, G.A.; del Valle Jaen, A.; Russo Picasso, M.F. Thyroid lipomatosis in a 36-year-old patient with rheumatoid arthritis and a kidney transplant. Endocrinol. Diabetes Metab. Case Rep. 2016, 2016, 160007. [Google Scholar] [CrossRef] [PubMed]
- Munzinger, U. Amyloid Goiter. Schweiz. Med. Wochenschr. 1974, 104, 1131–1136. [Google Scholar] [PubMed]
- Simard, L.C. Une nouvelle forme de goître: La scléro-lympho-lipomatose thyroidiènne. Union. Med. Can. 1945, 74, 884–991. [Google Scholar]
- Chesky, V.E.; Dreese, W.C.; Hellwig, C.A. Adenolipomatosis of the thyroid: A new type of goiter. Surgery 1953, 34, 38–45. [Google Scholar]
- Bielicki, F.; Dawiskiba, E.; Kasprzak, A.; Kawecki, K.; Zagrobelny, Z. [Struma lipomatosa]. Pol. Tyg. Lek. 1968, 23, 2018–2019. [Google Scholar]
- Dalforno, S.; Donna, A. Lipomatosi diffusa della tiroide (Struma lipomatose). Cancro 1969, 22, 613–617. [Google Scholar]
- Asirwatham, J.E.; Barcos, M.; Shimaoka, K. Hamartomatous adiposity of thyroid gland. J. Med. 1979, 10, 197–206. [Google Scholar]
- Simha, M.R.; Doctor, V.M. Adenolipomatosis of the thyroid gland. Indian J. Cancer 1983, 20, 215–217. [Google Scholar]
- Téllez, R.; Le Cerf, P.; Araos, F.; Michaud, P. Diffuse fatty infiltration of the thyroid gland associated to amyloidosis in a patient with chronic renal failure. Rev. Med. Chil. 1996, 124, 1251–1255. [Google Scholar]
- Paoletti, H.; Tourrette, J.; Terrier, J.; Colineau, X.; Thiebaut, C.; Dussaut, J.P.; Pujol, A.; Muyard, B.; Nun, P.; Solacroup, J.C. [Diffuse thyroid lipomatosis]. J. Radiol. 1997, 78, 1291–1294. [Google Scholar]
- Arslan, A.; Lent Alíç, B.; Kemal Uzunlar, A.; Seyin Büyü, H.; Sarí, I. Diffuse lipomatosis of thyroid gland. Auris Nasus Larynx 1999, 26, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Himmetoglu, C.; Yamak, S.; Tezel, G.G. Diffuse fatty infiltration in amyloid goiter. Pathol. Int. 2007, 57, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Di Scioscio, V.; Loffreda, V.; Feraco, P.; Luccaroni, R.; Palena, L.M.; Balbi, T.; Zompatori, M. Diffuse lipomatosis of thyroid gland. J. Clin. Endocrinol. Metab. 2008, 93, 8–9. [Google Scholar] [CrossRef]
- Gupta, R.; Arora, R.; Sharma, A.; Dinda, A. Diffuse lipomatosis of the thyroid gland: A pathologic curiosity. Indian J. Pathol. Microbiol. 2009, 52, 215. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, P.V.; Kumar, R.; Ragavan, M.; Ramakrishna, B.A. Diffuse lipomatosis of thyroid with hyperthyroidism. J. Postgrad. Med. 2010, 56, 35–36. [Google Scholar] [CrossRef]
- Gonulalan, G.; Esen, H.; Mehmet, E.; Cakir, M. Thyroid Lipomatosis. Intern. Med. 2012, 51, 3383–3385. [Google Scholar] [CrossRef]
- Jacques, T.A.; Stearns, M.P. Diffuse lipomatosis of the thyroid with amyloid deposition. J. Laryngol. Otol. 2013, 127, 426–428. [Google Scholar] [CrossRef]
- Lo, R.; Donaldson, C. Diffuse Lipomatosis of the Thyroid Gland. Ultrasound Q. 2013, 29, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Pardal, J.; Máximo, V.; Gonçalves, F.; Eloy, C. Diffuse lipomatosis of thyroid: A case report. Virchows Archiv. 2013, 463, 155. [Google Scholar]
- Liyanaarachchi, N.; Lim, A.; Donaldson, E. Diffuse lipomatosis and amyloid deposition of the thyroid gland associated with poorly differentiated/insular carcinoma of the thyroid: Report of a rare entity. Pathology 2016, 48, S74. [Google Scholar] [CrossRef][Green Version]
- Ben Gamra, O.; Romdhane, N.; Nefzaoui, S.; Mahjoubi, M.; Abid, W.; Koubaa, W.; Hariga, I.; Chadli, A.; Mbarek, C.C. Diffuse lipomatosis of the thyroid gland. Egypt. J. Ear Nose Throat Allied Sci. 2016, 17, 167–169. [Google Scholar] [CrossRef]
- Kumar, R.; Bhargava, A.; Jaiswal, G. A Case Report on Radiologic Findings of Thyrolipomatosis: A Rare Fat Containing Lesion diffusely Infiltrating throughout the Thyroid Gland. J. Kathmandu Med. Coll. 2016, 5, 71–73. [Google Scholar] [CrossRef][Green Version]
- Ishida, M.; Kashu, I.; Morisaki, T.; Takenobu, M.; Moritani, S.; Uemura, Y.; Tsuta, K. Thyrolipomatosis: A case report with review of the literature. Mol. Clin. Oncol. 2017, 6, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Harisankar, C.N.B. A Rare Case of Thyrolipomatosis Presenting with Latent Hyperthyroidism. Indian J. Nucl. Med. 2018, 33, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, D.M.; Addas, F.A.; Alghanmi, N.M.; Marzouki, H.Z.; Merdad, M.A. An enlarged goiter presenting with a rare diffuse lipomatosis of the thyroid gland. Am. J. Case Rep. 2018, 19, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; El Amine, R.; Bouziane, C. Diffuse Lipomatosis of Thyroid—Case Report. Surg. Sci. 2018, 9, 469–473. [Google Scholar] [CrossRef]
- López-Muñoz, B.; Greco Bermúdez, L.; Marín-Jiménez, D.; de la Fuente, M.F.S.; Capparelli, A.F.; Martínez, I.M.; Corredor, S.S. An Unusual Amyloid Goiter in a 48-Year-Old Woman with Rheumatoid Arthritis, Secondary Amyloidosis and Renal Failure. Case Rep. Endocrinol. 2019, 2019, 4291486. [Google Scholar] [CrossRef]
- Stanaway, A.; Lam, T. Consecutive cases of thyrolipomatosis and thymolipoma: A case report. ANZ J. Surg. 2019, 89, 614–616. [Google Scholar] [CrossRef]
- Ravinder, K.; Abhishek, B.; Gagan, J. Thyrolipomatosis: A Rare Fat Containing Lesion diffusely Infiltrating Throughout the Thyroid Gland. J. Assoc. Physicians India 2019, 67, 77. [Google Scholar]
- Aggarwal, A.; Goyal, A.; Kandasamy, D. Diffuse Thyroid Lipomatosis—A Rare Image. Indian J. Surg. 2020, 82, 1310–1311. [Google Scholar] [CrossRef]
- Cavaco, D.R.; Alves Rafael, A.; Cabrera, R.; Vilar, H.; Leite, V. Case Report: A Rare Association of Diffuse Thyroid Lipomatosis with Amyloid Deposition. Eur. Thyroid. J. 2021, 10, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Martí-Fernández, R.; Cassinello-Fernández, N.; Palomares-Casasús, S.; Gómez-Adrián, J.C.; Ferrández-Izquierdo, A. Diffuse Lipomatosis of the Thyroid Gland. Am. Surg. 2021, 89, 2092–2093. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, S.; Hammami, B.; Boudaouara, O.; Boudawara, T.; Kallel, S.; Charfeddine, I. [Association of thyrolipoma and thyrolipomatosis: A case report]. Ann. Pathol. 2021, 41, 326–329. [Google Scholar] [CrossRef]
- Campion, T.; Maity, A.; Ali, S.; Richards, P.; Adams, A. Concurrent thyrolipomatosis and thymolipoma in a patient with myasthenia gravis: A case report and review of the literature. Ann. R. Coll. Surg. Engl. 2021, 103, 212–215. [Google Scholar] [CrossRef]
- Xhemalaj, D.; Xhardo, E.; Gradica, F.; Lisha, L. Diffuse Lipomatosis of Thyroid Gland. Case Report and Review of Literature. Diagn. Pathol. 2022, 7, 285. [Google Scholar] [CrossRef]
- Morado da Silva, E.M.; Ferreira, R.A.d.C.; Lozada, A.R.C.; Duarte, J.M.S. A 54-Year-Old Woman with Papillary Thyroid Carcinoma Associated with Secondary Amyloid Goiter and Thyroid Lipomatosis. Am. J. Case Rep. 2022, 23, e938156-1–e938156-4. [Google Scholar] [CrossRef]
- Kesici, U.; Karatepe, Y.K.; Isceviren, B. Concurrence of Thyrolipoma-tosis with Hyperthyroidism and Ectopic Thyroid Tissue. J. Coll. Physicians Surg. Pak. 2022, 32, 1231–1232. [Google Scholar] [CrossRef]
- Kawai, C.; Miyao, M.; Kotani, H.; Minami, H.; Abiru, H.; Hamayasu, H.; Yamamoto, A.; Tamaki, K. Systemic amyloidosis with amyloid goiter: An autopsy report. Leg. Med. 2023, 60, 102167. [Google Scholar] [CrossRef]
- Paz-Ibarra, J.; Concepción-Zavaleta, M.; Mendoza-Quispe, D.; Suárez-Rojas, J.; del Sur, L.U.C.; Fabián, K.R.; Deutz-Gómez, D.; Quiroz-Aldave, J.; Peralta, J.S.; Arróspide, T.A. Coexistence of thyrolipomatosis and tongue squamous cell carcinoma: A case report. touchREVIEWS Endocrinol. 2023, 19, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, S.; Saleem, A.; Alhajri, O.; Alozairi, O. Thyrolipoma presentation as a huge multinodular goiter; A case report of an extremely rare entity. Int. J. Surg. Case Rep. 2023, 112, 108936. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.M.; Ruiz-Santillan, M.A.; Force, B.K.; Gaba, R. A Case of Diffuse Thyroid Lipomatosis with Amyloid Deposits Presenting with Thyrotoxicosis. JCEM Case Rep. 2024, 2, luae030. [Google Scholar] [CrossRef]
- George, D.M.; Shah, S.N. Diffuse Thyroid Lipomatosis and Amyloid Goiter With Incidental Papillary Thyroid Carcinoma: A Rare Case Report. Cureus 2024, 16, e57896. [Google Scholar] [CrossRef] [PubMed]
- Willis, R.A. The Borderland of Embryology and Pathology; Butterworth &, Co.: London, UK, 1958. [Google Scholar]
- Schroder, S.; Boeker, W. Lipomatous Lesions of the Thyroid Gland: A Review. Appl. Pathol. 1985, 3, 140–149. [Google Scholar] [PubMed]
- Trites, A.E.W. Thyrolipoma Thyrolipoma, Thymolipoma and Pharyngeal Lipoma: A Syndrome. Canad Med. Ass J. 1966, 95, 1254–1259. [Google Scholar] [PubMed]
- Gellhorn, A.; Marks, P.A. The composition and biosynthesis of lipids in human adipose tissues. J. Clin. Investig. 1961, 40, 925–932. [Google Scholar] [CrossRef]
- Derienzo, D.; Truong, L. Thyroid Neoplasms Containing Mature Fat: A Report of Two Cases and Review of the Literature. Mod. Pathol. 1989, 2, 506–510. [Google Scholar] [PubMed]
- Lee, M.H.; Lee, J.U.; Joung, K.H.; Kim, Y.K.; Ryu, M.J.; Lee, S.E.; Kim, S.J.; Chung, H.K.; Choi, M.J.; Chang, J.Y.; et al. Thyroid dysfunction associated with follicular cell steatosis in obese male mice and humans. Endocrinology 2015, 156, 1181–1193. [Google Scholar] [CrossRef]
- Basolo, A.; Poma, A.M.; Giannini, R.; Ceccarini, G.; Pelosini, C.; Fierabracci, P.; Castany, M.U.; Genzano, S.B.; Ambrosini, C.E.; Materazzi, G.; et al. Histological pattern and gene expression profiling of thyroid tissue in subjects with obesity. J. Endocrinol. Investig. 2022, 45, 413–423. [Google Scholar] [CrossRef]
- Martyniak, K.; Masternak, M.M. Changes in adipose tissue cellular composition during obesity and aging as a cause of metabolic dysregulation. Exp. Gerontol. 2017, 94, 59–63. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Hawkins, P.N. Pathophysiology and treatment of systemic amyloidosis. Nat. Rev. Nephrol. 2013, 9, 574–586. [Google Scholar] [CrossRef]
- Villa, F.; Dionigi, G.; Tanda, M.L.; Rovera, F.; Boni, L. Amyloid goiter. Int. J. Surg. 2008, 6 (Suppl. S1), S16–S18. [Google Scholar] [CrossRef]
- Sung, Y.N.; Kim, D.; Kim, J. p53 immunostaining pattern is a useful surrogate marker for TP53 gene mutations. Diagn. Pathol. 2022, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cho, S.W.; Min, H.S.; Kim, K.M.; Yeom, G.J.; Kim, E.Y.; Lee, K.E.; Yun, Y.G.; Park, D.J.; Park, Y.J. The Expression of Tumor-Associated Macrophages in Papillary Thyroid Carcinoma. Endocrinol. Metab. 2013, 28, 192. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.; Ezzat, S.; Freeman, J.L.; Rosen, I.B.; Asa, S.L. Immunohistochemical Diagnosis of Papillary Thyroid Carcinoma. Mod. Pathol. 2001, 14, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.Y.; Shin, J.H. Description and comparison of the sonographic characteristics of poorly differentiated thyroid carcinoma and anaplastic thyroid carcinoma. J. Ultrasound Med. 2016, 35, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, J.V. Riedel’s thyroiditis: A clinical review. J. Clin. Endocrinol. Metab. 2011, 96, 3031–3041. [Google Scholar] [CrossRef]
- Zala, A.; Berhane, T.; Christofer Juhlin, C.; Calissendorff, J.; Falhammar, H. Riedel thyroiditis. J. Clin. Endocrinol. Metab. 2020, 105, E3469–E3481. [Google Scholar] [CrossRef]
- Kuk, M.; Kuo, C.J.; Nguyen, V.H.; Chen, C.C. Synchronous thyrolipoma and papillary thyroid carcinoma: A rare but significant event. Diagnostics 2021, 11, 1334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).