Green Tea Catechins Mitigate Hepatocyte Ferroptosis Through Attenuation of Oxidative Stress and Improvement of Antioxidant Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Green Tea Extract
2.3. HPLC-DAD Analysis of Catechins and Caffeine
2.4. Determination of Total Phenolic Content
2.5. Total Flavonoid Content
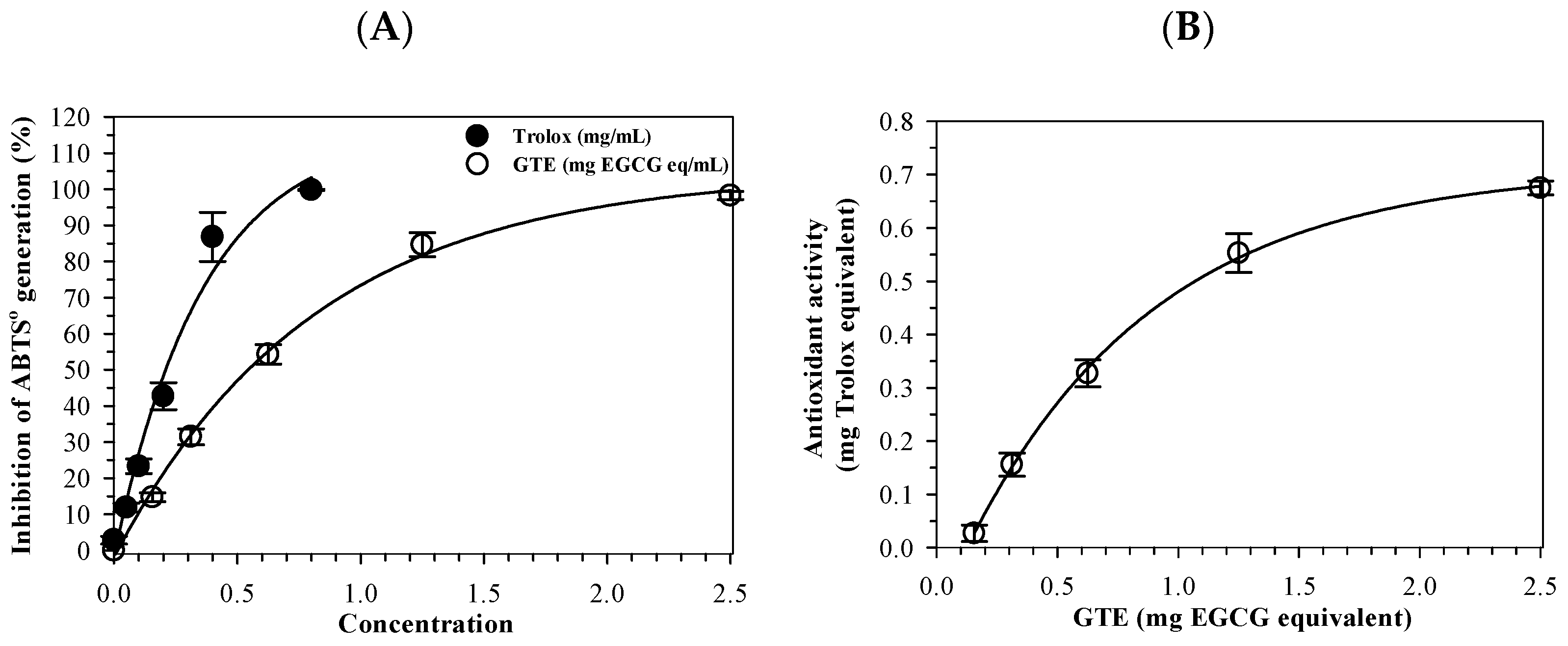
2.6. Assay of Antioxidant Activity
2.7. Cytotoxicity Test
2.8. Investigation of Liver Ferroptosis
2.8.1. Hepatocyte Culture and Iron Loading
2.8.2. Treatment of Iron-Loaded Huh7 Cells
2.8.3. Assay of Cellular LIP
2.8.4. Measurement of Cellular ROS
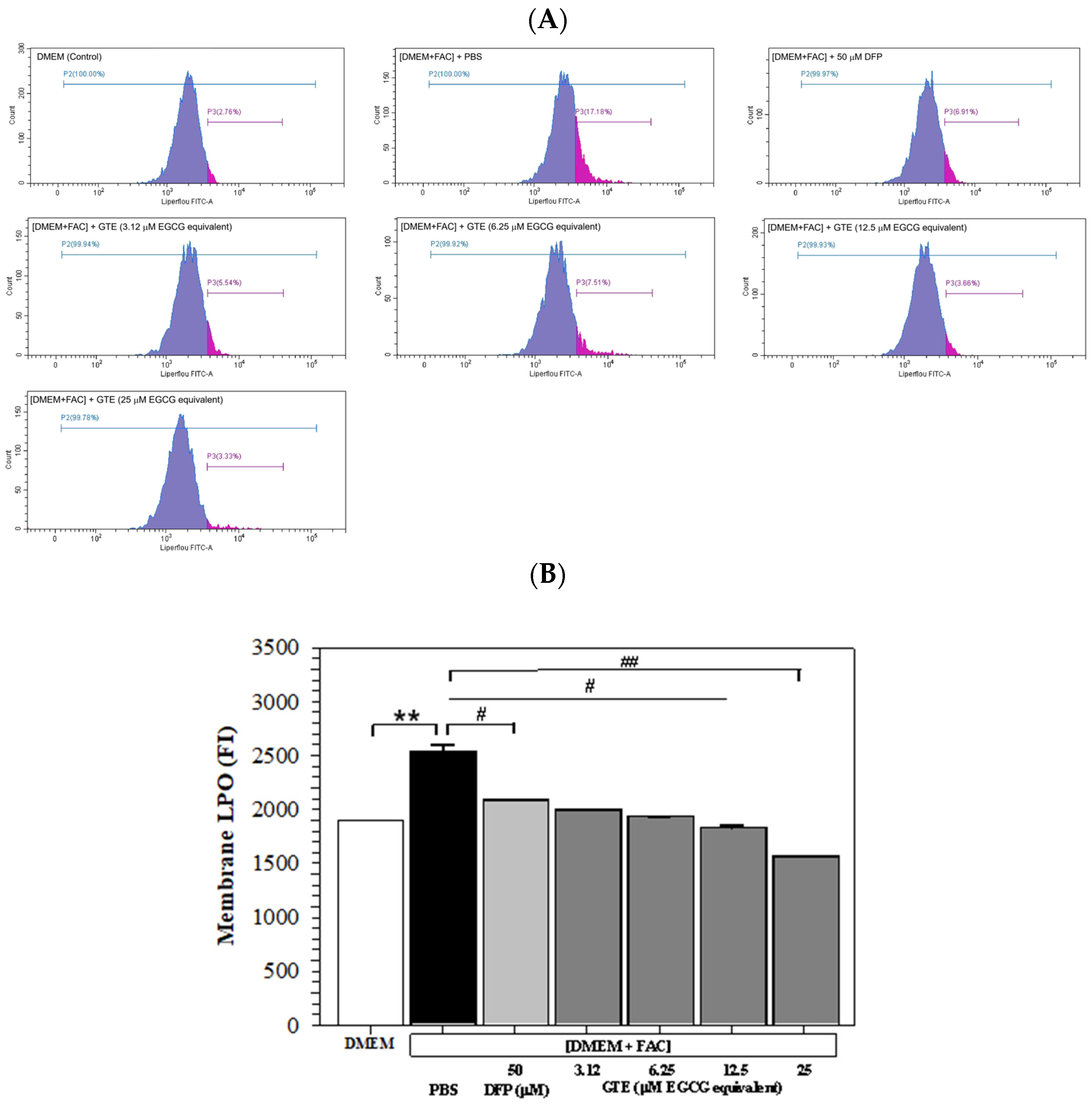
2.8.5. Analysis of Membrane LPO
2.8.6. Determination of Cellular GSH Content
2.8.7. Assay of GPX-4 Activity
2.8.8. Measurement of Protein Content
2.8.9. Identifying Phenolics and Their Metabolites with HPLC-MS
2.9. Statistical Analysis
3. Results
3.1. Chemical Compositions of GTE
3.2. Antioxidant Activity of GTE
3.3. Cytotoxicity of GTE
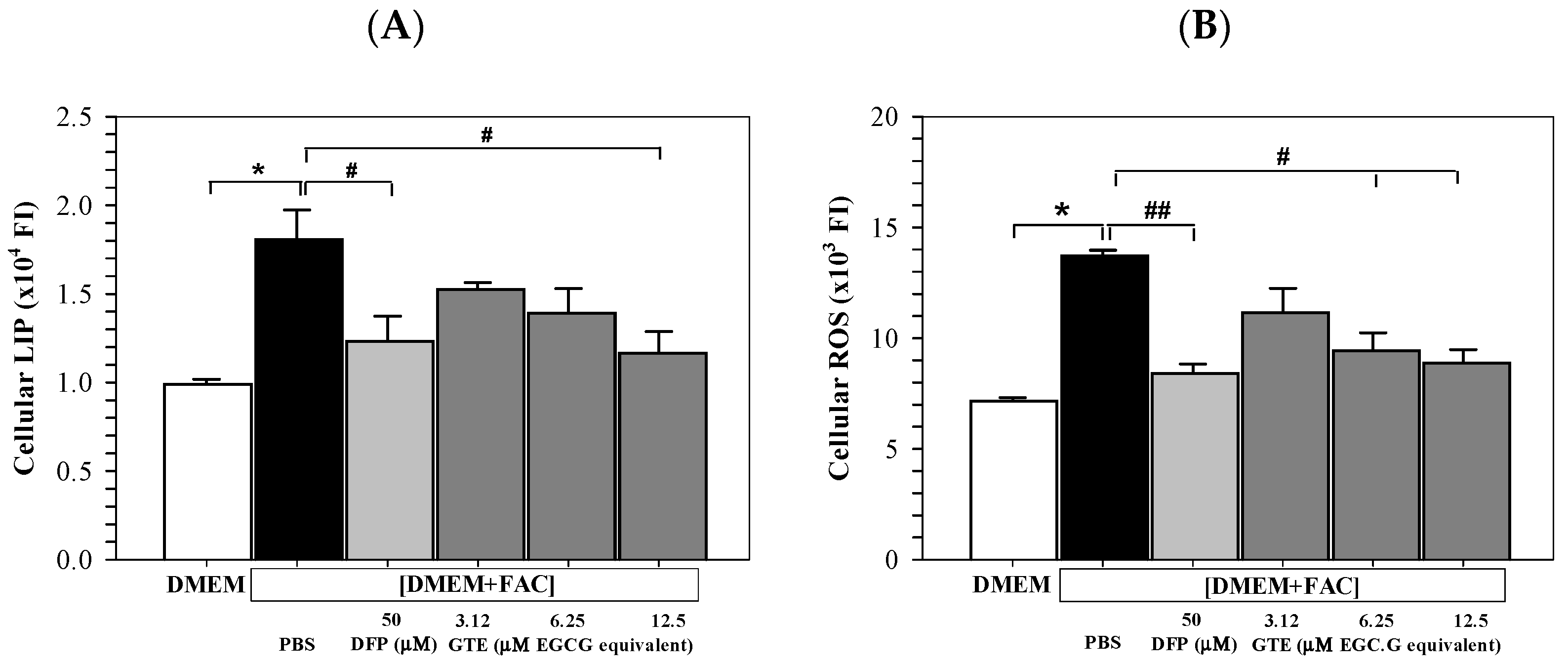
3.4. Cellular LIP and ROS Content
3.5. Membrane LPO Content
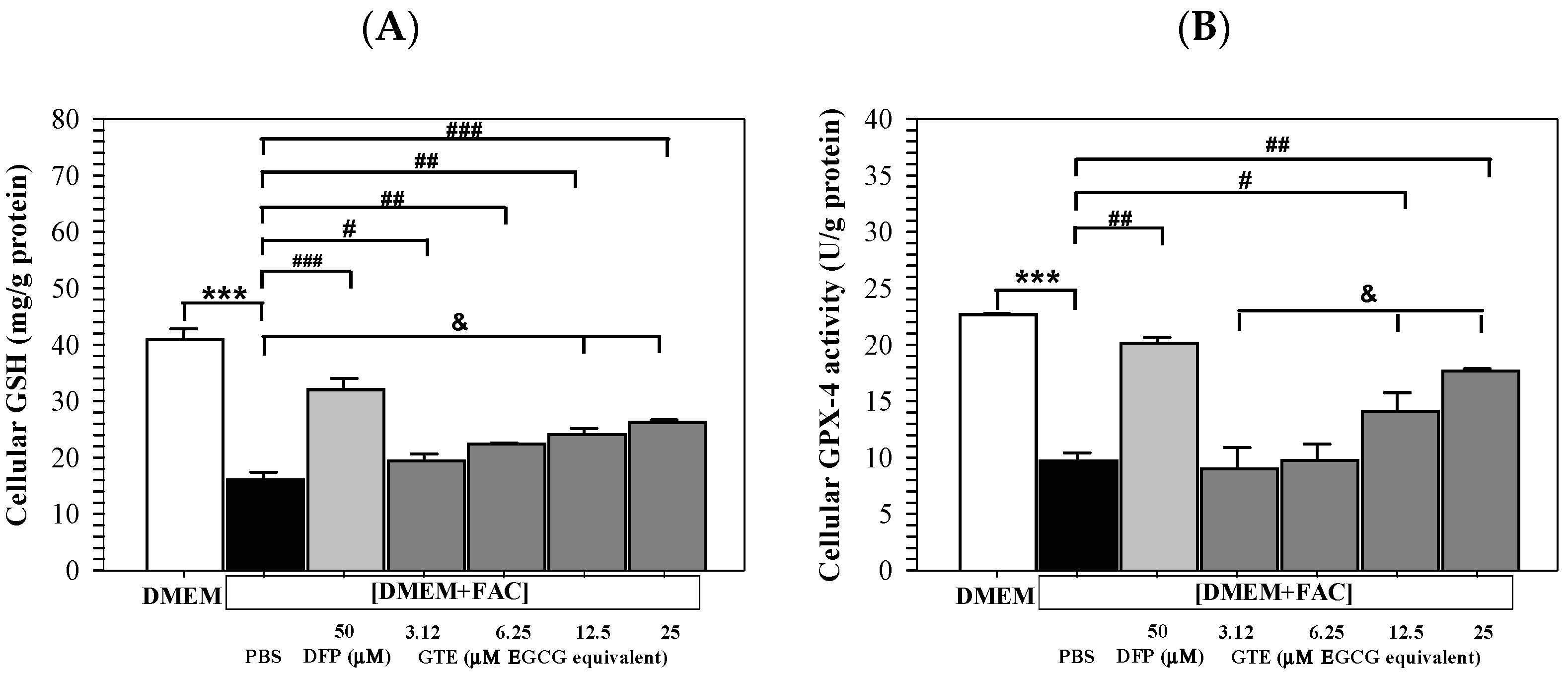
3.6. Cellular GSH
3.7. Catechins and Their Metabolites in Culture Medium and Cell Lysate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | L-ascorbic acid |
| ABTS | 2,2-Azino-bis-(3-methylbenzothiazoline-6-sulfonic acid |
| ABTS•+ | ABTS radical |
| ACSL4 | Acyl CoA synthetase long chain family member 4 |
| AIPH | 2,2-Azobis-[2-(2-imidazolin-2-yl)propane]dihydrochloride |
| ANOVA | Analysis of variance |
| BSA | Bovine serum albumin |
| C | Catechin |
| CF | Caffeine |
| CV | Coefficient of variation |
| CoQ10 | Coenzyme Q10 |
| DAD | Diode array detector |
| DCFH-DA | 2′,7′-Dichlorohydrofluorescein diacetate |
| DFP | Deferiprone |
| DI | Deionized water |
| DMEM | Dulbecco’s modified Eagle medium |
| [DMEM + FAC] | Iron-loaded DMEM |
| DMSO | Dimethyl sulfoxide |
| DTNB | 5,5-Dithio-bis (2-nitrobenzoic acid) |
| EC | Epicatechin |
| ECG | Epicatechin 3-gallate |
| EGC | Epigallocatechin |
| EGCG | Epigallocatechin-3-gallate |
| ERA | Erastin |
| FAC | Ferrous ammonium citrate |
| FAS | Ferrous ammonium sulfate |
| FBS | Fetal bovine serum |
| FO | FerroOrange |
| Fe2+ | Ferrous ion |
| Fer-1 | Ferrostatin 1 |
| FI | Fluorescence intensity |
| FTH/L | Ferritin heavy-chain and light-chain gene |
| GA | Gallic acid |
| GAE | Gallic acid equivalent |
| GCG | Gallocatechin gallate |
| GPX-4 | Glutathione peroxidase 4 |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GSSG | Oxidized glutathione |
| GTE | Green tea extract |
| H2DCFH-DA | 2′,7′-Dichlorohydrofluorescein diacetate reduced form |
| HEPES | Hydroxyethyl piperazine ethane sulfonic acid |
| HFE | High Fe2+ gene |
| 4-HNE | 4-Hydroxynonenal |
| Huh7 | Human hepatocellular carcinoma |
| IC50 | Half-maximal inhibitory concentration |
| ip | Intraperitoneal |
| LIP | Labile iron pool |
| Lipro-1 | Liproxstatin-1 |
| LOD | Limit of detection |
| LPO | Lipid hydroperoxides |
| MDA | Malondialdehyde |
| MPA | m-phosphoric acid |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NAC | N-acetylcysteine |
| NAFLD | Nonalcoholic fatty liver disease |
| NO3 | Peroxynitrite |
| NRF-2 | Nuclear factor erythroid 2-related factor 2 |
| OD | Optical density |
| PB | Phosphate buffer |
| PBS | Phosphate-buffered saline |
| PTFE | Polytetrafluoroethylene |
| QE | Quercetin equivalent |
| ROS | Reactive oxygen species |
| RSL-3 | RAS-selective lethal 3 |
| SD | Standard deviation |
| SEM | Standard error of the means |
| SI | Serum iron |
| SLC3A2 | Solute carrier family 3 member A2 |
| SLC7A11 | Solute carrier family 7 member 11 |
| TBA | Thiobarbituric acid |
| TEAC | Trolox equivalent antioxidant capacity |
| TFC | Total flavonoid content |
| TIBC | Total iron–binding capacity |
| TPC | Total phenolic content |
| TR | Retention time |
| Trolox | 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid |
| TS | Transferrin saturation |
| v/v | Volume by volume |
| w/v | Weight by volume |
| -xCT | Cystine/glutamate transporter |
| ε | Molar extinction coefficient |
| λ | Wavelength |
| • | Radical |
References
- Srichairatanakool, S.; Ounjaijean, S.; Thephinlap, C.; Khansuwan, U.; Phisalpong, C.; Fucharoen, S. Iron-chelating and free-radical scavenging activities of microwave-processed green tea in iron overload. Hemoglobin 2006, 30, 311–327. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Ning, K.; Lu, K.; Chen, Q.; Guo, Z.; Du, X.; Riaz, F.; Feng, L.; Fu, Y.; Yin, C.; Zhang, F.; et al. Epigallocatechin gallate protects mice against methionine-choline-deficient-diet-induced nonalcoholic steatohepatitis by improving gut microbiota to attenuate hepatic injury and regulate metabolism. ACS Omega 2020, 5, 20800–20809. [Google Scholar] [CrossRef]
- Thephinlap, C.; Ounjaijean, S.; Khansuwan, U.; Fucharoen, S.; Porter, J.B.; Srichairatanakool, S. Epigallocatechin-3-gallate and epicatechin-3-gallate from green tea decrease plasma non-transferrin bound iron and erythrocyte oxidative stress. Med. Chem. 2007, 3, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Wang, L.; Yang, P.; Liu, Z.; Rajput, S.A.; Hassan, M.; Qi, D. Epigallocatechin gallate and glutathione attenuate aflatoxin B1-induced acute liver injury in ducklings via mitochondria-mediated apoptosis and the Nrf2 signalling pathway. Toxins 2022, 14, 876. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Darley-Usmar, V.; Zhang, J. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol. 2014, 2, 82–90. [Google Scholar] [CrossRef]
- Koonyosying, P.; Kongkarnka, S.; Uthaipibull, C.; Svasti, S.; Fucharoen, S.; Srichairatanakool, S. Green tea extract modulates oxidative tissue injury in beta-thalassemic mice by chelation of redox iron and inhibition of lipid peroxidation. Biomed. Pharmacother. 2018, 108, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Upanan, S.; Pangjit, K.; Uthaipibull, C.; Fucharoen, S.; McKie, A.T.; Srichairatanakool, S. Combined treatment of 3-hydroxypyridine-4-one derivatives and green tea extract to induce hepcidin expression in iron-overloaded β-thalassemic mice. Asian Pac. J. Trop. Biomed. 2015, 5, 1010–1017. [Google Scholar] [CrossRef]
- Kose, T.; Vera-Aviles, M.; Sharp, P.A.; Latunde-Dada, G.O. Curcumin and (−)- epigallocatechin-3-gallate protect murine MIN6 pancreatic beta-cells against iron toxicity and erastin-induced ferroptosis. Pharmaceuticals 2019, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sun, X.; Chen, Y.; Deng, Y.; Qian, K. Epigallocatechin gallate attenuated non-alcoholic steatohepatitis induced by methionine- and choline-deficient diet. Eur. J. Pharmacol. 2015, 761, 405–412. [Google Scholar] [CrossRef]
- Noman, A.M.; Sultan, M.T.; Mazhar, A.; Baig, I.; Javaid, J.; Hussain, M.; Imran, M.; Alsagaby, S.A.; Al Abdulmonem, W.; Mujtaba, A.; et al. Anticancer molecular mechanisms of epigallocatechin gallate: An updated review on clinical trials. Food Sci. Nutr. 2025, 13, e70735. [Google Scholar] [CrossRef] [PubMed]
- Al-Basher, G.I. Green tea activity and iron overload induced molecular fibrogenesis of rat liver. Saudi J. Biol. Sci. 2019, 26, 531–540. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Wang, H.; Helliwell, K.; You, X. Isocratic elution system for the determination of catechins, caffeine and gallic acid in green tea using HPLC. Food Chem. 2000, 68, 115–121. [Google Scholar] [CrossRef]
- Abbe Maleyki, M.J., Jr.; Azrina, A.; Amin, I. Assessment of antioxidant capacity and phenolic content of selected commercial beverages. Malays. J. Nutr. 2007, 13, 149–159. [Google Scholar]
- Petry, R.D.; Ortega, G.G.; Silva, W.B. Flavonoid content assay: Influence of the reagent concentration and reaction time on the spectrophotometric behavior of the aluminium chloride--flavonoid complex. Pharmazie 2001, 56, 465–470. [Google Scholar]
- Pellegrini, N.; Del Rio, D.; Colombi, B.; Bianchi, M.; Brighenti, F. Application of the 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) radical cation assay to a flow injection system for the evaluation of antioxidant activity of some pure compounds and beverages. J. Agric. Food Chem. 2003, 51, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Dey, S.K.; Saha, C. Protective effect of black tea extract during chemotherapeutic drug induced oxidative damage on normal lymphocytes in comparison with cancerous K562 cells. Int. J. Sci. Eng. Res. 2014, 5, 437–447. [Google Scholar]
- Su, Y.; Cheng, R.; Zhang, J.; Qian, J.; Diao, C.; Ran, J.; Zhang, H.; Li, L. Interferon-alpha2b gene-modified human bone marrow mesenchymal stem cells inhibit hepatocellular carcinoma by reducing the Notch1 levels. Life Sci. 2015, 143, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Shariati, L.; Modaress, M.; Khanahmad, H.; Hejazi, Z.; Tabatabaiefar, M.A.; Salehi, M.; Modarressi, M.H. Comparison of different methods for erythroid differentiation in the K562 cell line. Biotechnol. Lett. 2016, 38, 1243–1250. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Saric, A.; Nicolas, G.; Sureau, F.; Petit, P.X. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019, 5, 150. [Google Scholar] [CrossRef]
- Li, J.; Koonyosying, P.; Korsieporn, W.; Paradee, N.; Hutachok, N.; Xu, H.; Ma, Y.; Chuljerm, H.; Srichairatanakool, S. Deferiprone-resveratrol hybrid attenuates iron accumulation, oxidative stress, and antioxidant defenses in iron-loaded human Huh7 hepatic cells. Front. Mol. Biosci. 2024, 11, 1364261. [Google Scholar] [CrossRef]
- Paradee, N.; Yimcharoen, T.; Utama-Ang, N.; Settakorn, K.; Chuljerm, H.; Srichairatanakool, S.; Koonyosying, P. Phytochemical analysis and anti-lipid accumulation effects of pulsed electric field (PEF)-processed black rice and green tea extracts in oleic acid-induced hepatocytes. Food Sci. Nutr. 2025, 13, e70329. [Google Scholar] [CrossRef]
- Mei, H.; Zhao, L.; Li, W.; Zheng, Z.; Tang, D.; Lu, X.; He, Y. Inhibition of ferroptosis protects House Ear Institute-Organ of Corti 1 cells and cochlear hair cells from cisplatin-induced ototoxicity. J. Cell. Mol. Med. 2020, 24, 12065–12081. [Google Scholar] [CrossRef] [PubMed]
- Amer, J.; Goldfarb, A.; Fibach, E. Flow cytometric measurement of reactive oxygen species production by normal and thalassaemic red blood cells. Eur. J. Haematol. 2003, 70, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Tang, D.; Zhao, L.; Li, W.; Han, J.; Hu, B.; Nie, G.; He, Y. Liproxstatin-1 protects Hair cell-like HEI-OC1 cells and cochlear hair cells against neomycin ototoxicity. Oxidative Med. Cell. Longev. 2020, 2020, 1782659. [Google Scholar] [CrossRef] [PubMed]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef]
- Ernst, O.; Zor, T. Linearization of the bradford protein assay. J. Vis. Exp. 2010, e1918. [Google Scholar] [CrossRef]
- Hutachok, N.; Angkasith, P.; Chumpun, C.; Fucharoen, S.; Mackie, I.J.; Porter, J.B.; Srichairatanakool, S. Anti-platelet aggregation and anti-cyclooxygenase activities for a range of coffee extracts (Coffea arabica). Molecules 2020, 26, 10. [Google Scholar] [CrossRef]
- Penarrieta, J.M.; Alvarado, J.A.; Akesson, B.; Bergenstahl, B. Total antioxidant capacity and content of flavonoids and other phenolic compounds in canihua (Chenopodium pallidicaule): An Andean pseudocereal. Mol. Nutr. Food Res. 2008, 52, 708–717. [Google Scholar] [CrossRef]
- Quesada, I.M.; Bustos, M.; Blay, M.; Pujadas, G.; Ardevol, A.; Salvado, M.J.; Blade, C.; Arola, L.; Fernandez-Larrea, J. Dietary catechins and procyanidins modulate zinc homeostasis in human HepG2 cells. J. Nutr. Biochem. 2011, 22, 153–163. [Google Scholar] [CrossRef]
- Hamden, K.; Carreau, S.; Marki, F.A.; Masmoudi, H.; El Feki, A. Positive effects of green tea on hepatic dysfunction, lipid peroxidation and antioxidant defence depletion induced by cadmium. Biol. Res. 2008, 41, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, A.; Tan, L.; Tang, D.; Chen, W.; Lai, X.; Gu, K.; Chen, J.; Chen, D.; Tang, Q. Epigallocatechin-3-gallate alleviates liver oxidative damage caused by iron overload in mice through inhibiting ferroptosis. Nutrients 2023, 15, 1993. [Google Scholar] [CrossRef]
- Tang, G.; Xu, Y.; Zhang, C.; Wang, N.; Li, H.; Feng, Y. Green tea and epigallocatechin gallate (EGCG) for the management of nonalcoholic fatty liver diseases (NAFLD): Insights into the role of oxidative stress and antioxidant mechanism. Antioxidants 2021, 10, 1076. [Google Scholar] [CrossRef]
- Ding, S.B.; Chu, X.L.; Jin, Y.X.; Jiang, J.J.; Zhao, X.; Yu, M. Epigallocatechin gallate alleviates high-fat diet-induced hepatic lipotoxicity by targeting mitochondrial ROS-mediated ferroptosis. Front. Pharmacol. 2023, 14, 1148814. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jia, L.; Li, X.; Zhang, K.; Wang, X.; He, Y.; Hao, M.; Rayman, M.P.; Zhang, J. Prooxidant activity-based guideline for a beneficial combination of (−)-epigallocatechin-3-gallate and chlorogenic acid. Food Chem. 2022, 386, 132812. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhu, K.; Liu, Z.; Huang, J. Prooxidant effects of epigallocatechin-3-gallate in health benefits and potential adverse effect. Oxidative Med. Cell. Longev. 2020, 2020, 9723686. [Google Scholar] [CrossRef]
- Su, Y.; Zeng, Y.; Zhou, M.; Liao, M.; Qin, P.; Wu, R.; Han, J.; Liang, X.; Wang, Z.; Jiang, J.; et al. Natural polyphenol-mediated Iihibition of ferroptosis alleviates oxidative damage and inflammation in acute liver injury. Biomater. Res. 2025, 29, 1067. [Google Scholar] [CrossRef] [PubMed]
- Hino, K.; Nishina, S.; Yanatori, I. Ferroptosis: Biology and role in liver disease. J. Gastroenterol. 2025, 60, 1339–1361. [Google Scholar] [CrossRef]
- Peleman, C.; Hellemans, S.; Veeckmans, G.; Arras, W.; Zheng, H.; Koeken, I.; Van San, E.; Hassannia, B.; Walravens, M.; Kayirangwa, E.; et al. Ferroptosis is a targetable detrimental factor in metabolic dysfunction-associated steatotic liver disease. Cell Death Differ. 2024, 31, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Ballotin, V.R.; Bigarella, L.G.; Brandao, A.B.M.; Balbinot, R.A.; Balbinot, S.S.; Soldera, J. Herb-induced liver injury: Systematic review and meta-analysis. World J. Clin. Cases 2021, 9, 5490–5513. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Samavat, H.; Dostal, A.M.; Wang, R.; Torkelson, C.J.; Yang, C.S.; Butler, L.M.; Kensler, T.W.; Wu, A.H.; Kurzer, M.S.; et al. Effect of green tea supplements on liver enzyme elevation: Results from a randomized intervention study in the United States. Cancer Prev. Res. 2017, 10, 571–579. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Jiang, X. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. J. Agric. Food Chem. 2008, 56, 2694–2701. [Google Scholar] [CrossRef]
- Braud, L.; Peyre, L.; de Sousa, G.; Armand, M.; Rahmani, R.; Maixent, J.M. Effect of brewing duration on the antioxidant and hepatoprotective abilities of tea phenolic and alkaloid compounds in a t-BHP oxidative stress-induced rat hepatocyte model. Molecules 2015, 20, 14985–15002. [Google Scholar] [CrossRef]
- Roth, M.; Timmermann, B.N.; Hagenbuch, B. Interactions of green tea catechins with organic anion-transporting polypeptides. Drug Metab. Dispos. 2011, 39, 920–926. [Google Scholar] [CrossRef]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Meng, X.; Sang, S.; Zhu, N.; Lu, H.; Sheng, S.; Lee, M.J.; Ho, C.T.; Yang, C.S. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chem. Res. Toxicol. 2002, 15, 1042–1050. [Google Scholar] [CrossRef]
- Settakorn, K.; Hantrakool, S.; Petiwathayakorn, T.; Hutachok, N.; Tantiworawit, A.; Charoenkwan, P.; Chalortham, N.; Chompupoung, A.; Paradee, N.; Koonyosying, P.; et al. A randomized placebo-controlled clinical trial of oral green tea epigallocatechin 3-gallate on erythropoiesis and oxidative stress in transfusion-dependent beta-thalassemia patients. Front. Mol. Biosci. 2023, 10, 1248742. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Doll, S.; Conrad, M. Iron and ferroptosis: A still ill-defined liaison. IUBMB Life 2017, 69, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Hu, F.; Feng, H.; Linkermann, A.; Min, W.; Stockwell, B.R. Determination of the subcellular localization and mechanism of action of ferrostatins in suppressing ferroptosis. ACS Chem. Biol. 2018, 13, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Kwon, S.J.; Hong, J.; Yang, C.S. Salivary hydrogen peroxide produced by holding or chewing green tea in the oral cavity. Free Radic. Res. 2007, 41, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Yin, O.Q.; Shi, X.; Chow, M.S. Reliable and specific high-performance liquid chromatographic method for simultaneous determination of loratadine and its metabolite in human plasma. J. Chromatogr. B 2003, 796, 165–172. [Google Scholar] [CrossRef]
- Lippmann, J.; Petri, K.; Fulda, S.; Liese, J. Redox modulation and induction of ferroptosis as a new therapeutic strategy in hepatocellular carcinoma. Transl. Oncol. 2020, 13, 100785. [Google Scholar] [CrossRef]
- Raza, H.; John, A. In vitro protection of reactive oxygen species-induced degradation of lipids, proteins and 2-deoxyribose by tea catechins. Food Chem. Toxicol. 2007, 45, 1814–1820. [Google Scholar] [CrossRef]
- Saewong, T.; Ounjaijean, S.; Mundee, Y.; Pattanapanyasat, K.; Fucharoen, S.; Porter, J.B.; Srichairatanakool, S. Effects of green tea on iron accumulation and oxidative stress in livers of iron-challenged thalassemic mice. Med. Chem. 2010, 6, 57–64. [Google Scholar] [CrossRef]
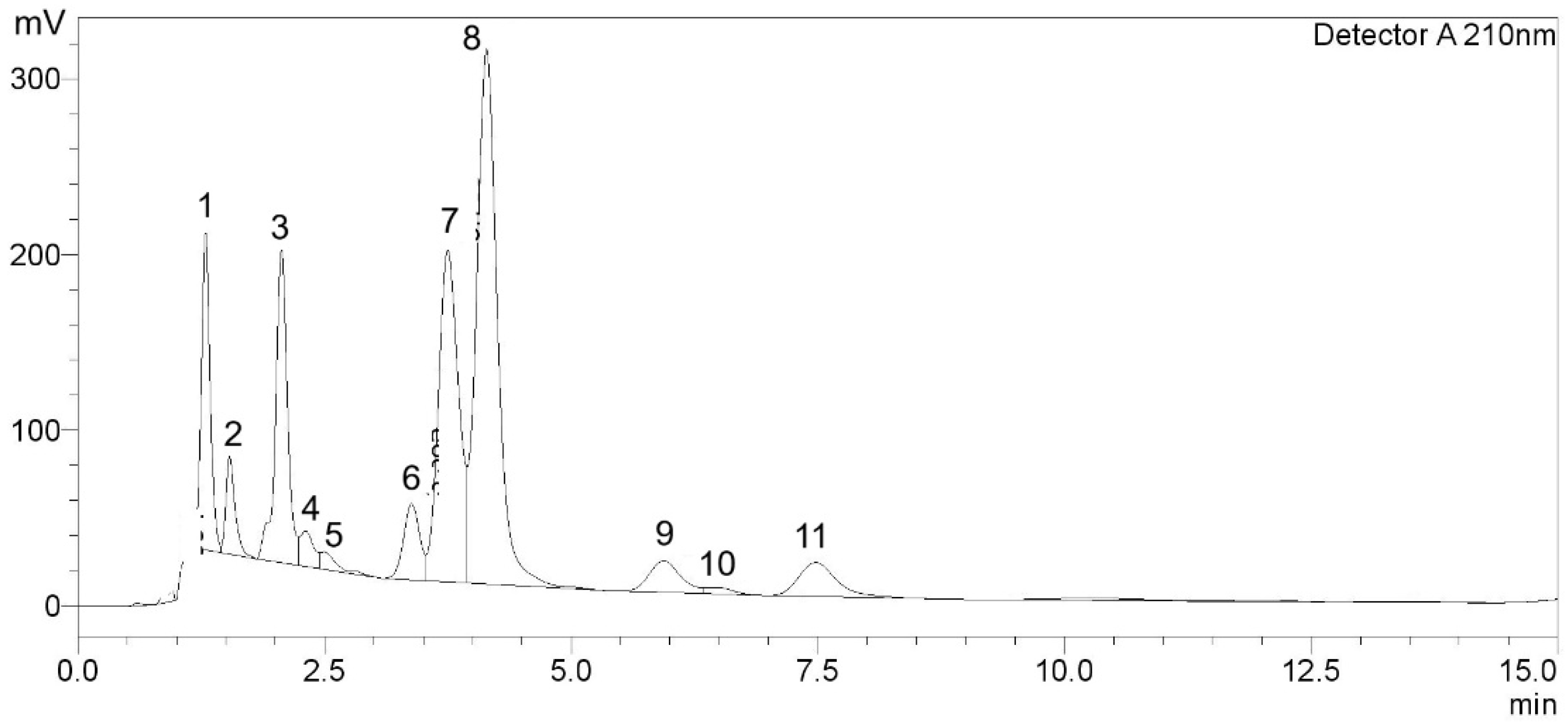

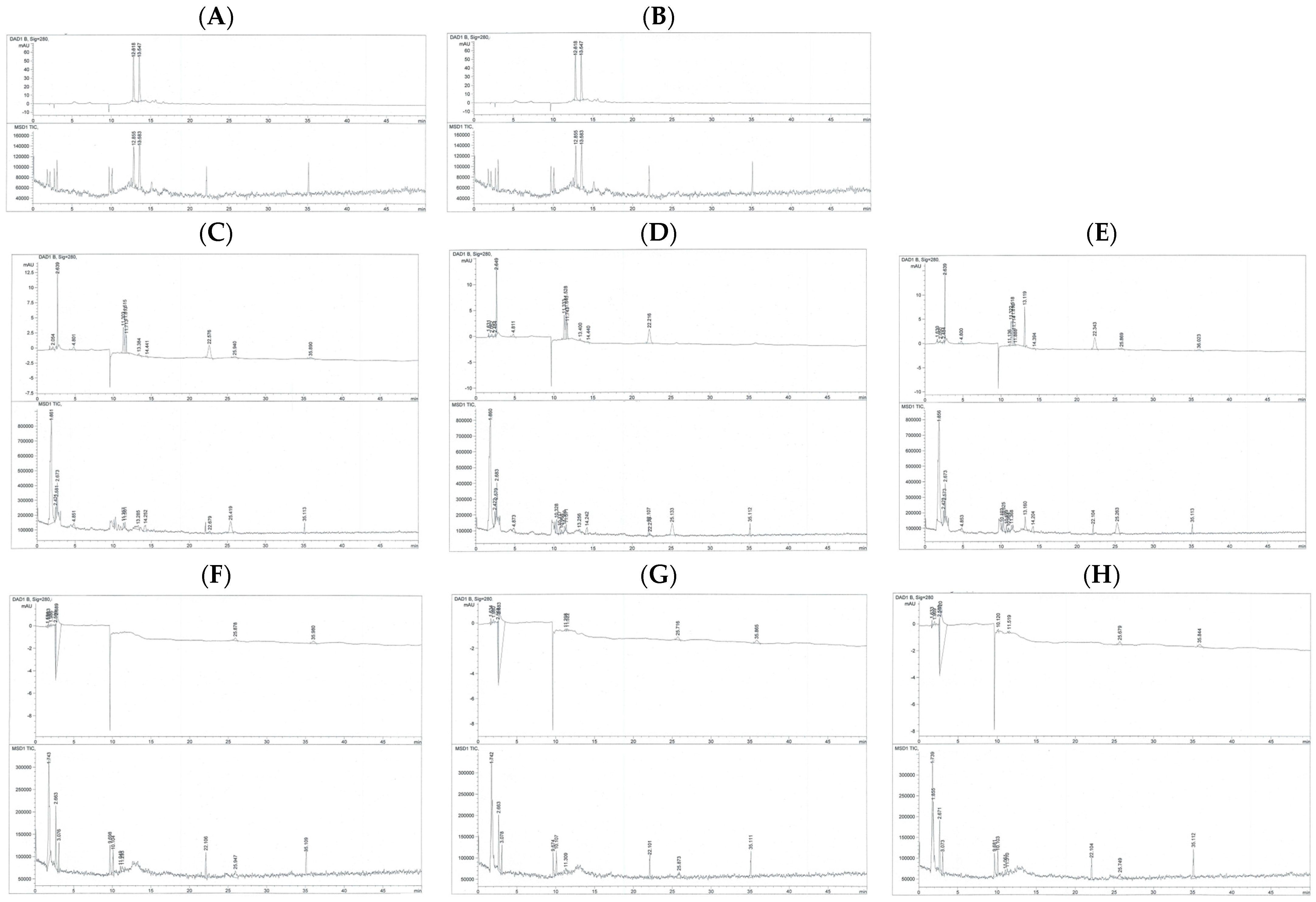
| Peak | TR (min) | PA | PH | Compounds | Amount (mg/g) |
|---|---|---|---|---|---|
| 1 | 1.297 | 1,128,089 | 180,566 | GA | 7.96 ± 0.31 |
| 2 | 1.537 | 358,332 | 55,712 | GCG | 3.36 ± 0.20 |
| 3 | 2.064 | 1,494,302 | 177,913 | Unknown | - |
| 4 | 2.310 | 198,745 | 20,126 | C | ND |
| 5 | 2.495 | 101,230 | 9911 | Unknown | - |
| 6 | 3.385 | 490,797 | 43,292 | EC | 1.24 ± 0.02 |
| 7 | 3.750 | 2,704,511 | 188,897 | EGCG | 8.38 ± 0.18 |
| 8 | 4.142 | 4,523,257 | 304,418 | CF | 31.89 ± 0.51 |
| 9 | 5.939 | 407,201 | 18,064 | Unknown | - |
| 10 | 6.465 | 71,298 | 3769 | Unknown | - |
| 11 | 7.478 | 524,542 | 19,375 | ECG | 2.50 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koonyosying, P.; Tharanon, W.; Pairojthanachai, K.; Samakarn, Y.; Meejak, K.; Paradee, N.; Kerdto, O.; Yubo, S.; Zhong, Y.; Srichairatanakool, S. Green Tea Catechins Mitigate Hepatocyte Ferroptosis Through Attenuation of Oxidative Stress and Improvement of Antioxidant Systems. Antioxidants 2025, 14, 1483. https://doi.org/10.3390/antiox14121483
Koonyosying P, Tharanon W, Pairojthanachai K, Samakarn Y, Meejak K, Paradee N, Kerdto O, Yubo S, Zhong Y, Srichairatanakool S. Green Tea Catechins Mitigate Hepatocyte Ferroptosis Through Attenuation of Oxidative Stress and Improvement of Antioxidant Systems. Antioxidants. 2025; 14(12):1483. https://doi.org/10.3390/antiox14121483
Chicago/Turabian StyleKoonyosying, Pimpisid, Wit Tharanon, Kavee Pairojthanachai, Yanisa Samakarn, Kornkan Meejak, Narisara Paradee, Onsaya Kerdto, Suphatta Yubo, Yanping Zhong, and Somdet Srichairatanakool. 2025. "Green Tea Catechins Mitigate Hepatocyte Ferroptosis Through Attenuation of Oxidative Stress and Improvement of Antioxidant Systems" Antioxidants 14, no. 12: 1483. https://doi.org/10.3390/antiox14121483
APA StyleKoonyosying, P., Tharanon, W., Pairojthanachai, K., Samakarn, Y., Meejak, K., Paradee, N., Kerdto, O., Yubo, S., Zhong, Y., & Srichairatanakool, S. (2025). Green Tea Catechins Mitigate Hepatocyte Ferroptosis Through Attenuation of Oxidative Stress and Improvement of Antioxidant Systems. Antioxidants, 14(12), 1483. https://doi.org/10.3390/antiox14121483







