Analyses of Antioxidative Response in Tomato (Solanum lycopersicum L.) Grown with Biochar and PGPMs
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Growth and Biochar Functionalization
2.2. Plant Treatment and Sample Collection
2.3. Soil Analysis
2.3.1. Determination of Soil pH and Electrical Conductivity (EC)
2.3.2. Active Carbon (POXC) and Soil Respiration
2.3.3. Total Soil Enzymatic Activity
2.4. Morphological, Physiological, and Biochemical Analysis in Plants
2.4.1. Cellular Respiration and Lipid Peroxidation
2.4.2. Photosynthetic Activity
2.4.3. Proline Content in Leaves
2.4.4. Hydrogen Peroxide Content
2.4.5. Quantification of Ascorbic Acid
2.4.6. Extraction in Methanol
2.4.7. Antioxidant Activity: DPPH Assay
2.4.8. Antioxidant Activity: ABTS Assay
2.4.9. Total Phenolic Content (TPC)
2.4.10. Protein Extraction and Quantification
2.4.11. Determination of SOD Activity
2.4.12. Determination of POD, CAT, and APX Activities
2.4.13. Determination of Total Soluble Sugars
2.5. Statistical Analysis
3. Results and Discussion
3.1. Influence of Biochar–PGPM Combination on Soil Microbial Activity
3.2. Yield of Roots and Above-Ground Parts
3.3. Roots to Shoots Redox Responses and Oxidative Stress Indicators
3.4. Antioxidant Modulation in Roots and Leaves
3.5. Integrated Multivariate Insights into Plant–Soil System: A Decryption Key
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, R.; Jiang, F.; Liu, Y.; Yu, X.; Song, X.; Wu, Z.; Cammarano, D. Environmental Changes Impact on Vegetables Physiology and Nutrition—Gaps between Vegetable and Cereal Crops. Sci. Total Environ. 2024, 933, 173180. [Google Scholar] [CrossRef] [PubMed]
- Heikonen, S.; Heino, M.; Jalava, M.; Siebert, S.; Viviroli, D.; Kummu, M. Climate Change Threatens Crop Diversity at Low Latitudes. Nat. Food 2025, 6, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Schreinemachers, P.; Simmons, E.B.; Wopereis, M.C.S. Tapping the Economic and Nutritional Power of Vegetables. Glob. Food Secur. 2018, 16, 36–45. [Google Scholar] [CrossRef]
- González Guzmán, M.; Cellini, F.; Fotopoulos, V.; Balestrini, R.; Arbona, V. New Approaches to Improve Crop Tolerance to Biotic and Abiotic Stresses. Physiol. Plant 2022, 174, e13547. [Google Scholar] [CrossRef]
- Chaudhary, S.; Devi, P.; HanumanthaRao, B.; Jha, U.C.; Sharma, K.D.; Prasad, P.V.V.; Kumar, S.; Siddique, K.H.M.; Nayyar, H. Physiological and Molecular Approaches for Developing Thermotolerance in Vegetable Crops: A Growth, Yield and Sustenance Perspective. Front. Plant Sci. 2022, 13, 878498. [Google Scholar] [CrossRef]
- Erika, C.; Griebel, S.; Naumann, M.; Pawelzik, E. Biodiversity in Tomatoes: Is It Reflected in Nutrient Density and Nutritional Yields Under Organic Outdoor Production? Front. Plant Sci. 2020, 11, 589692. [Google Scholar] [CrossRef]
- Ozygit, I.I.; Can, H.; Uyanik, O.L.; Yalcin, I.E.; Demir, G. Fruit Mineral Nutrient Contents of Field and Greenhouse Grown Tomatoes and Comparison with Standard Values. Not. Bot. Horti Agrobot. Cluj Napoca 2024, 52, 13479. [Google Scholar] [CrossRef]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2021, 10, 45. [Google Scholar] [CrossRef]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef]
- Carucci, F.; Gagliardi, A.; Giuliani, M.M.; Gatta, G. Irrigation Scheduling in Processing Tomato to Save Water: A Smart Approach Combining Plant and Soil Monitoring. Appl. Sci. 2023, 13, 7625. [Google Scholar] [CrossRef]
- Khan, Q.; Wang, Y.; Xia, G.; Yang, H.; Luo, Z.; Zhang, Y. Deleterious Effects of Heat Stress on the Tomato, Its Innate Responses, and Potential Preventive Strategies in the Realm of Emerging Technologies. Metabolites 2024, 14, 283. [Google Scholar] [CrossRef] [PubMed]
- Hubab, M.; Lorestani, N.; Al-Awabdeh, R.A.M.; Shabani, F. Climate Change-Driven Shifts in the Global Distribution of Tomato and Potato Crops and Their Associated Bacterial Pathogens. Front. Microbiol. 2025, 16, 1520104. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, R.; Ceylan, R.F.; Özkan, B. Global Tomato Production: Price Sensitivity and Policy Impact in Mexico, Türkiye, and the United States. Horticulturae 2025, 11, 84. [Google Scholar] [CrossRef]
- Fiodor, A.; Singh, S.; Pranaw, K. The Contrivance of Plant Growth Promoting Microbes to Mitigate Climate Change Impact in Agriculture. Microorganisms 2021, 9, 1841. [Google Scholar] [CrossRef]
- Afshar, M.; Mofatteh, S. Biochar for a Sustainable Future: Environmentally Friendly Production and Diverse Applications. Results Eng. 2024, 23, 102433. [Google Scholar] [CrossRef]
- Allohverdi, T.; Mohanty, A.K.; Roy, P.; Misra, M. A Review on Current Status of Biochar Uses in Agriculture. Molecules 2021, 26, 5584. [Google Scholar] [CrossRef]
- Kabir, E.; Kim, K.H.; Kwon, E.E. Biochar as a Tool for the Improvement of Soil and Environment. Front. Environ. Sci. 2023, 11, 1324533. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Fang, H.; Liu, K.; Li, D.; Peng, X.; Zhang, W.; Zhou, H. Long-Term Effects of Inorganic Fertilizers and Organic Manures on the Structure of a Paddy Soil. Soil Tillage Res. 2021, 213, 105137. [Google Scholar] [CrossRef]
- Aloo, B.N.; Tripathi, V.; Makumba, B.A.; Mbega, E.R. Plant Growth-Promoting Rhizobacterial Biofertilizers for Crop Production: The Past, Present, and Future. Front. Plant Sci. 2022, 13, 1002448. [Google Scholar] [CrossRef]
- Mahmud, A.A.; Upadhyay, S.K.; Srivastava, A.K.; Bhojiya, A.A. Biofertilizers: A Nexus between Soil Fertility and Crop Productivity under Abiotic Stress. Curr. Res. Environ. Sustain. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Khosravi, H.; Khoshru, B.; Nosratabad, A.F.; Mitra, D. Exploring the Landscape of Biofertilizers Containing Plant Growth-Promoting Rhizobacteria in Iran: Progress and Research Prospects. Curr. Res. Microb. Sci. 2024, 7, 100268. [Google Scholar] [CrossRef]
- Nosheen, S.; Ajmal, I.; Song, Y. Microbes as Biofertilizers, a Potential Approach for Sustainable Crop Production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Yadav, A.; Yadav, A.N.; Suman, A.; Ahluwalia, A.S.; Saxena, A.K. Minerals Solubilizing and Mobilizing Microbiomes: A Sustainable Approach for Managing Minerals’ Deficiency in Agricultural Soil. J. Appl. Microbiol. 2022, 133, 1245–1272. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Jia, B.; Tao, L.; Li, H.; Wang, J.; Yuan, Z.; Sun, X.; Yao, Y. Four Decades of Bacillus Biofertilizers: Advances and Future Prospects in Agriculture. Microorganisms 2025, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, R.; Hussain, A.; Sabir, A.; Ben Fekih, I.; Ditta, A.; Xing, S. Role of Biochar and Plant Growth Promoting Rhizobacteria to Enhance Soil Carbon Sequestration—A Review. Environ. Monit. Assess. 2019, 191, 251. [Google Scholar] [CrossRef]
- Guan, T.K.; Wang, Q.Y.; Li, J.S.; Yan, H.W.; Chen, Q.J.; Sun, J.; Liu, C.J.; Han, Y.Y.; Zou, Y.J.; Zhang, G.Q. Biochar Immobilized Plant Growth-Promoting Rhizobacteria Enhanced the Physicochemical Properties, Agronomic Characters and Microbial Communities during Lettuce Seedling. Front. Microbiol. 2023, 14, 1218205. [Google Scholar] [CrossRef] [PubMed]
- Tabacchioni, S.; Passato, S.; Ambrosino, P.; Huang, L.; Caldara, M.; Cantale, C.; Hett, J.; Del Fiore, A.; Fiore, A.; Schlüter, A.; et al. Identification of Beneficial Microbial Consortia and Bioactive Compounds with Potential as Plant Biostimulants for a Sustainable Agriculture. Microorganisms 2021, 9, 426. [Google Scholar] [CrossRef]
- Moebius-Clune, B.N.; Moebius-Clune, D.J.; Gugino, B.K.; Idowu, O.J.; Schindelbeck, R.R.; Ristow, A.J.; van Es, H.M.; Thies, J.E.; Shayler, H.A.; McBride, M.B.; et al. Comprehensive Assessment of Soil Health—The Cornell Framework; Standard Operating Procedures; Cornell University: Geneva, NY, USA, 2016. [Google Scholar]
- Adam, G.; Duncan, H. Development of a Sensitive and Rapid Method for the Measurement of Total Microbial Activity Using Fluorescein Diacetate (FDA) in a Range of Soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- ISO 11063:2020; Soil Quality—Direct Extraction of Soil DNA. International Organization for Standardization: Geneva, Switzerland, 2020.
- Pagano, L.; Carlo, S.; Lepore, G.O.; Bonanni, V.; Zizic, M.; Pollastri, S.; Margheri, S.; Orsilli, J.; Puri, A.; Villani, M.; et al. Mechanistic Understanding of Iron Oxide Nanobiotransformation in Zea Mays: A Combined Synchrotron-Based, Physiological and Molecular Approach. Environ. Sci. Nano 2025, 12, 4107–4121. [Google Scholar] [CrossRef]
- Marmiroli, M.; Mussi, F.; Pagano, L.; Imperiale, D.; Lencioni, G.; Villani, M.; Zappettini, A.; White, J.C.; Marmiroli, N. Cadmium Sulfide Quantum Dots Impact Arabidopsis Thaliana Physiology and Morphology. Chemosphere 2020, 240, 124856. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, B. Measurements of Proline and Malondialdehyde Content and Antioxidant Enzyme Activities in Leaves of Drought Stressed Cotton. Bio Protoc. 2016, 6, e1913. [Google Scholar] [CrossRef]
- Marmiroli, M.; Mussi, F.; Gallo, V.; Gianoncelli, A.; Hartley, W.; Marmiroli, N. Combination of Biochemical, Molecular, and Synchrotron-Radiation-Based Techniques to Study the Effects of Silicon in Tomato (Solanum lycopersicum L.). Int. J. Mol. Sci. 2022, 23, 15837. [Google Scholar] [CrossRef]
- Jagota, S.K.; Dani, H.M. A New Calorimetric Technique for the Estimation of Vitamin C Using Folin Phenol Reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Mingle, C.E.; Newsome, A.L. An Amended Potassium Persulfate ABTS Antioxidant Assay Used for Medicinal Plant Extracts Revealed Variable Antioxidant Capacity Based upon Plant Extraction Process. BioRxiv 2020. [Google Scholar]
- Ernst, O.; Zor, T. Linearization of the Bradford Protein Assay. J. Vis. Exp. 2010, 12, 1918. [Google Scholar] [CrossRef]
- Abedi, T.; Pakniyat, H. Antioxidant Enzyme Changes in Response to Drought Stress in Ten Cultivars of Oilseed Rape (Brassica napus L.). Czech J. Genet. Plant Breed. 2010, 46, 27–34. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Water Stress-Induced Abscisic Acid Accumulation Triggers the Increased Generation of Reactive Oxygen Species and up-Regulates the Activities of Antioxidant Enzymes in Maize Leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef]
- Shakir, S.K.; Irfan, S.; Akhtar, B.; Rehman, S.U.; Daud, M.K.; Taimur, N.; Azizullah, A. Pesticide-Induced Oxidative Stress and Antioxidant Responses in Tomato (Solanum lycopersicum) Seedlings. Ecotoxicology 2018, 27, 919–935. [Google Scholar] [CrossRef]
- Dvořáčková, H.; Dvořáček, J.; Záhora, J.; Šimečková, J. Biochar Alone Did Not Increase Microbial Activity in Soils from a Temperate Climate That Had Long-Term Acidity Stress. Agriculture 2022, 12, 941. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Bai, M.X.; Situ, G.M.; Li, S.H.; Wu, Q.F.; Liang, C.F.; Qin, H.; Chen, J.H. Effects of Combined Application of Biochar with Organic Amendments on Enzyme Activity and Microbial Metabolic Function of Carbon Sources in Infertile Red Soil. Ying Yong Sheng Tai Xue Bao 2022, 33, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Kolton, M.; Graber, E.R.; Tsehansky, L.; Elad, Y.; Cytryn, E. Biochar-Stimulated Plant Performance Is Strongly Linked to Microbial Diversity and Metabolic Potential in the Rhizosphere. New Phytol. 2017, 213, 1393–1404. [Google Scholar] [CrossRef]
- Hagemann, N.; Joseph, S.; Schmidt, H.P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W.; et al. Organic Coating on Biochar Explains Its Nutrient Retention and Stimulation of Soil Fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef]
- Qiu, H.; Hu, Z.; Liu, J.; Zhang, H.; Shen, W. Effect of Biochar on Labile Organic Carbon Fractions and Soil Carbon Pool Management Index. Agronomy 2023, 13, 1385. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A. Soil Enzyme Activity Response under the Amendment of Different Types of Biochar. Agronomy 2022, 12, 569. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.; Wang, J. The Effect of Chemical Fertilizer on Soil Organic Carbon Renewal and CO2 Emission—A Pot Experiment with Maize. Plant Soil 2012, 353, 85–94. [Google Scholar] [CrossRef]
- Premalatha, R.P.; Poorna Bindu, J.; Nivetha, E.; Malarvizhi, P.; Manorama, K.; Parameswari, E.; Davamani, V. A Review on Biochar’s Effect on Soil Properties and Crop Growth. Front. Energy Res. 2023, 11, 1092637. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Du, B.; Li, H. Effect of Biochar Applied with Plant Growth-Promoting Rhizobacteria (PGPR) on Soil Microbial Community Composition and Nitrogen Utilization in Tomato. Pedosphere 2021, 31, 872–881. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T.; et al. The Potential of Biochar as a Microbial Carrier for Agricultural and Environmental Applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. Hydrogen Peroxide Priming Modulates Abiotic Oxidative Stress Tolerance: Insights from ROS Detoxification and Scavenging. Front. Plant Sci. 2015, 6, 141857. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Liao, W. Hydrogen Peroxide Signaling in Plant Development and Abiotic Responses: Crosstalk with Nitric Oxide and Calcium. Front. Plant Sci. 2016, 7, 177967. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Gill, S.S.; Corpas, F.J.; Ortega-Villasante, C.; Hernandez, L.E.; Tuteja, N.; Sofo, A.; Hasanuzzaman, M.; Fujita, M. Editorial: Recent Insights Into the Double Role of Hydrogen Peroxide in Plants. Front. Plant Sci. 2022, 13, 843274. [Google Scholar] [CrossRef]
- Ansabayeva, A.; Makhambetov, M.; Rebouh, N.Y.; Abdelkader, M.; Saudy, H.S.; Hassan, K.M.; Nasser, M.A.; Ali, M.A.A.; Ebrahim, M. Plant Growth-Promoting Microbes for Resilient Farming Systems: Mitigating Environmental Stressors and Boosting Crops Productivity—A Review. Horticulturae 2025, 11, 260. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The Influence of Plant Growth-Promoting Rhizobacteria in Plant Tolerance to Abiotic Stress: A Survival Strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef]
- Graziano, S.; Caldara, M.; Gullì, M.; Bevivino, A.; Maestri, E.; Marmiroli, N. A Metagenomic and Gene Expression Analysis in Wheat (T. durum) and Maize (Z. mays) Biofertilized with PGPM and Biochar. Int. J. Mol. Sci. 2022, 23, 10376. [Google Scholar] [CrossRef]
- Lewoyehu, M.; Amare, M. Comparative Evaluation of Analytical Methods for Determining the Antioxidant Activities of Honey: A Review. Cogent Food Agric. 2019, 5, 1685059. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-Evaluation of the 2,2-Diphenyl-1-Picrylhydrazyl Free Radical (DPPH) Assay for Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef]
- Wang, G.; Hu, C.; Zhou, J.; Liu, Y.; Cai, J.; Pan, C.; Wang, Y.; Wu, X.; Shi, K.; Xia, X.; et al. Systemic Root-Shoot Signaling Drives Jasmonate-Based Root Defense against Nematodes. Curr. Biol. 2019, 29, 3430–3438.e4. [Google Scholar] [CrossRef] [PubMed]
- Ralmi, N.H.A.A.; Khandaker, M.M.; Mohd, K.S.; Majrashi, A.; Fallatah, A.M.; Badaluddin, N.A.; Yusoff, N.; Mahmud, K.; Saifuddin, M.; Osman, N.; et al. Influence of Rhizopheric H2O2 on Growth, Mineral Absorption, Root Anatomy and Nematode Infection of Ficus Deltoidea. Agronomy 2021, 11, 704. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Sharma, N.; Mahawar, L.; Mishra, A.; Albrectsen, B.R. Microbial Contributions to Plant Growth and Stress Tolerance: Mechanisms for Sustainable Plant Production. Plant Stress 2025, 17, 100966. [Google Scholar] [CrossRef]
- Supriya, L.; Dake, D.; Woch, N.; Gupta, P.; Gopinath, K.; Padmaja, G.; Muthamilarasan, M. Sugar Sensors in Plants: Orchestrators of Growth, Stress Tolerance, and Hormonal Crosstalk. J. Plant Physiol. 2025, 307, 154471. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Saini, S.; Lohani, S.; Khati, P.; Rani, V. PGPR-Mediated Mitigation of Biotic and Abiotic Stress in Plants. In Advanced Microbial Technology for Sustainable Agriculture and Environment; Academic Press: Cambridge, MA, USA, 2023; pp. 199–227. [Google Scholar] [CrossRef]
- Mehmood, N.; Saeed, M.; Zafarullah, S.; Hyder, S.; Rizvi, Z.F.; Gondal, A.S.; Jamil, N.; Iqbal, R.; Ali, B.; Ercisli, S.; et al. Multifaceted Impacts of Plant-Beneficial Pseudomonas Spp. in Managing Various Plant Diseases and Crop Yield Improvement. ACS Omega 2023, 8, 22296. [Google Scholar] [CrossRef]
- Badiaa, O.; Abdelhakim, H.; Yssaad, R.; Topcuoglu, B. Effect of Heavy Metal (Copper and Zinc) on Proline, Polyphenols and Flavonoids Content of Tomato (Lycopersicon esculentum Mill.). Plant Arch 2020, 20, 2125–2137. [Google Scholar]
- Shi, Y.; Pang, X.; Liu, W.; Wang, R.; Su, D.; Gao, Y.; Wu, M.; Deng, W.; Liu, Y.; Li, Z. SlZHD17 Is Involved in the Control of Chlorophyll and Carotenoid Metabolism in Tomato Fruit. Hortic. Res. 2021, 8, 259. [Google Scholar] [CrossRef]
- Zou, Q.; Zhao, L.; Guan, L.; Chen, P.; Zhao, J.; Zhao, Y.; Du, Y.; Xie, Y. The Synergistic Interaction Effect between Biochar and Plant Growth-Promoting Rhizobacteria on Beneficial Microbial Communities in Soil. Front. Plant Sci. 2024, 15, 1501400. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil Beneficial Bacteria and Their Role in Plant Growth Promotion: A Review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Tattini, M.; Gori, A.; Beatriz, L.; Nascimento, S.; Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative Water Content, Proline, and Antioxidant Enzymes in Leaves of Long Shelf-Life Tomatoes under Drought Stress and Rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The Importance of the Microbiome of the Plant Holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Marmiroli, M.; Caldara, M.; Pantalone, S.; Malcevschi, A.; Maestri, E.; Keller, A.A.; Marmiroli, N. Building a risk matrix for the safety assessment of wood derived biochars. Sci. Total Environ. 2022, 839, 156265. [Google Scholar] [CrossRef]
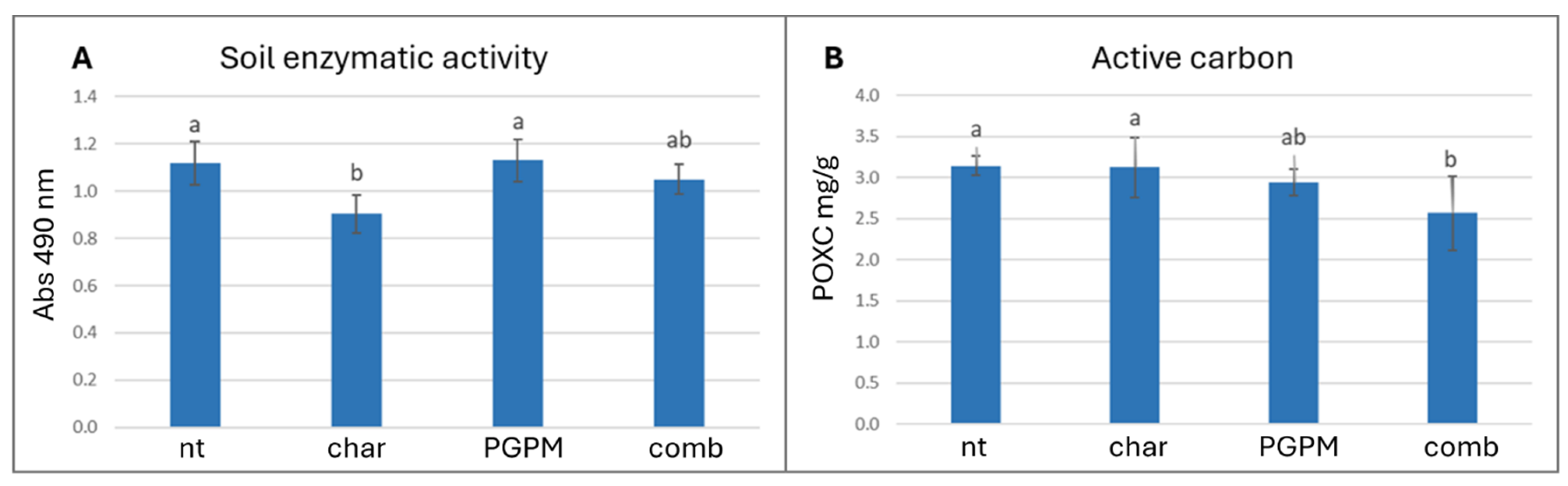

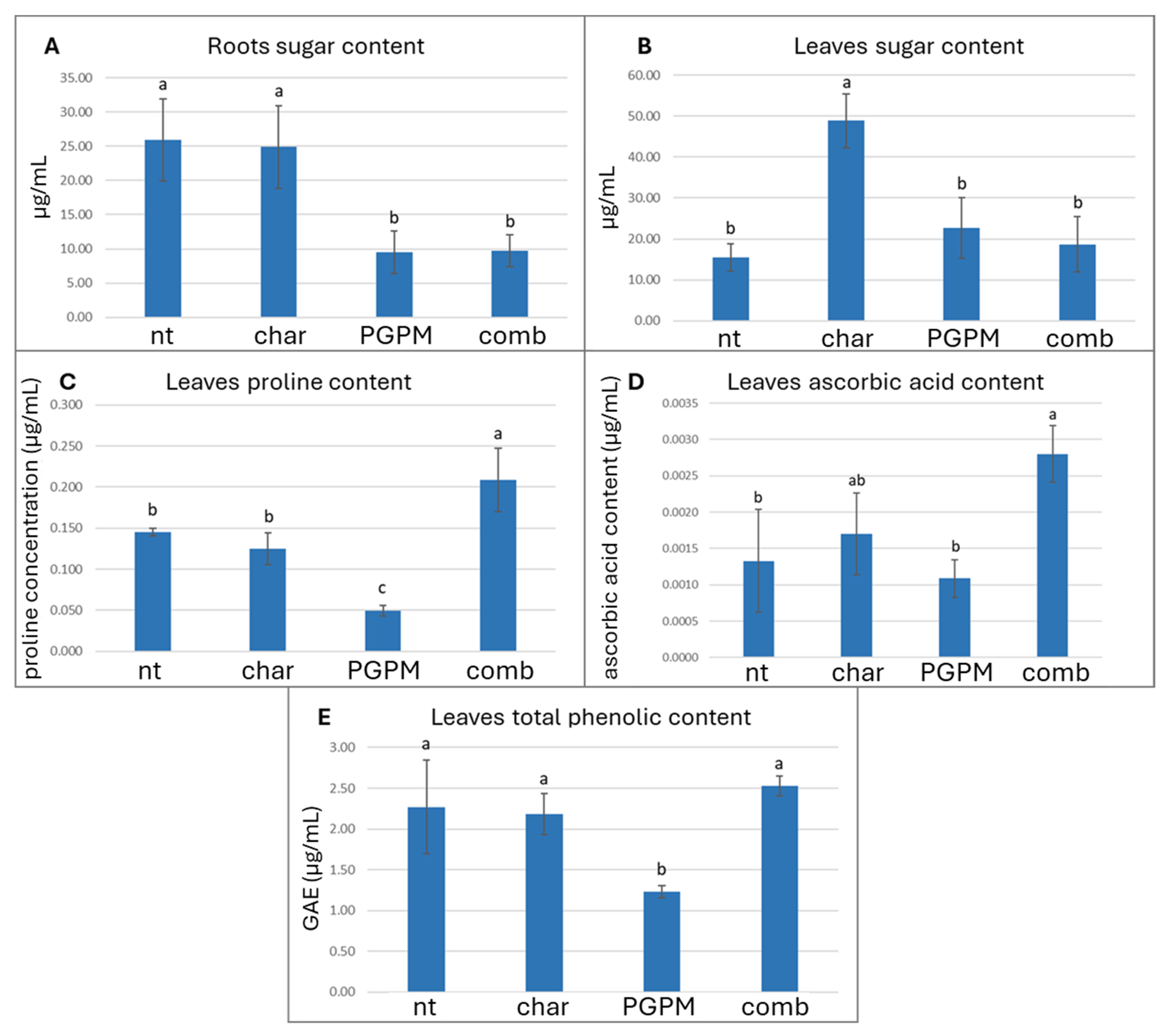


| Soil Samples | Untreated (nt) | Biochar (char) | PGPM | Biochar + PGPM (comb) | p-Value |
|---|---|---|---|---|---|
| Electrical Conductivity (µS/cm2) | 2817.2 ±1405.5 | 2647.2 ±1365.9 | 2166.9 ±612.7 | 2607.5 ±1125.2 | 0.91 |
| pH | 7.53 ±0.20 | 7.35 ±0.74 | 7.51 ±0.12 | 7.51 ±0.34 | 0.95 |
| Soil respiration (mg CO2) | 8.91 ±0.47 | 7.61 ±1.78 | 8.53 ±1.14 | 9.30 ±0.13 | 0.33 |
| Weight | Untreated (nt) | Biochar (char) | PGPM | Biochar + PGPM (comb) | p-Value |
|---|---|---|---|---|---|
| Roots fresh weight (g) | 12.5 ±2.78 | 8.0 ±0.56 | 11.0 ±1.41 | 6.0 ±0.74 | 0.45 |
| Shoots fresh weight leaves and stems (g) | 275.0 ±35.36 | 212.5 ±95.46 | 232.5 ±10.61 | 255.0 ±24.04 | 0.68 |
| Shoots dry weight leaves and stems (g) | 26.04 ±8.78 | 23.05 ±0.47 | 28.50 ±5.71 | 21.71 ±1.28 | 0.61 |
| Oxidative Stress Parameters | Untreated (nt) | Biochar (char) | PGPM | Biochar + PGPM (comb) | p-Value |
|---|---|---|---|---|---|
| Roots lipidic peroxidation−MDA assay (ng/mL) | 1.15 ±0.13 | 1.15 ±0.27 | 1.25 ±0.25 | 1.17 ±0.15 | 0.91 |
| Leaves lipidic peroxidation − MDA assay (ng/mL) | 10.1 ±2.2 | 10.9 ±0.6 | 10.2 ±0.9 | 12.5 ±1.3 | 0.16 |
| Leaves H2O2 content (µM) | 3.95 ±0.33 | 4.55 ±0.99 | 4.13 ±0.19 | 3.45 ±0.62 | 0.40 |
| Leaves cellular respiration − TTC assay (Abs λ = 530 nm) | 0.26 ±0.036 | 0.23 ±0.028 | 0.22 ±0.052 | 0.27 ±0.086 | 0.65 |
| Antioxidants Parameters | Untreated (nt) | Biochar (char) | PGPM | Biochar + PGPM (comb) | p-Value |
|---|---|---|---|---|---|
| Chlorophyll A content (mg/g FW) | 0.50 ±0.004 | 0.53 ±0.029 | 0.55 ±0.030 | 0.51 ±0.024 | 0.11 |
| Chlorophyll B content (mg/g FW) | 0.54 ±0.091 | 0.69 ±0.068 | 0.72 ±0.052 | 0.75 ±0.104 | 0.06 |
| Carotenoids content (mg/g FW) | 0.164 ±0.009 | 0.174 ±0.004 | 0.178 ±0.009 | 0.169 ±0.008 | 0.21 |
| Leaves total protein content (BSA µg/mL) | 392.03 ±28.74 | 438.13 ±83.61 | 387.57 ±38.26 | 470.62 ±33.08 | 0.22 |
| Leaves SOD activity (I%) | 54.77 ±13.79 | 52.29 ±4.44 | 50.56 ±3.88 | 55.89 ±5.22 | 0.84 |
| Leaves POD activity (U/mL) | 0.096 ±0.018 | 0.127 ±0.033 | 0.109 ±0.036 | 0.115 ±0.032 | 0.68 |
| Leaves CAT activity (U/mL) | 0.205 ±0.085 | 0.086 ±0.047 | 0.108 ±0.013 | 0.103 ±0.029 | 0.36 |
| Leaves APX activity (U/mL) | 0.272 ±0.071 | 0.539 ±0.111 | 0.545 ±0.250 | 0.401 ±0.128 | 0.18 |
| Roots total protein content (BSA µg/mL) | 223.11 ±50.95 | 225.45 ±18.34 | 247.83 ±23.98 | 251.82 ±9.58 | 0.56 |
| Roots SOD activity (I%) | 45.01 ±3.00 | 47.70 ±2.55 | 44.31 ±3.63 | 48.90 ±6.18 | 0.50 |
| Roots POD activity (U/mL) | 0.634 ±0.10 | 0.661 ±0.11 | 0.705 ±0.20 | 0.739 ±0.08 | 0.78 |
| Roots CAT activity (U/mL) | 0.043 ±0.012 | 0.063 ±0.040 | 0.048 ±0.031 | 0.124 ±0.099 | 0.33 |
| Roots APX activity (U/mL) | 0.925 ±0.080 | 0.896 ±0.338 | 0.921 ±0.171 | 1.354 ±0.291 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlo, S.; Trazza, M.; Pagano, L.; Marmiroli, M. Analyses of Antioxidative Response in Tomato (Solanum lycopersicum L.) Grown with Biochar and PGPMs. Antioxidants 2025, 14, 1482. https://doi.org/10.3390/antiox14121482
Carlo S, Trazza M, Pagano L, Marmiroli M. Analyses of Antioxidative Response in Tomato (Solanum lycopersicum L.) Grown with Biochar and PGPMs. Antioxidants. 2025; 14(12):1482. https://doi.org/10.3390/antiox14121482
Chicago/Turabian StyleCarlo, Silvia, Marta Trazza, Luca Pagano, and Marta Marmiroli. 2025. "Analyses of Antioxidative Response in Tomato (Solanum lycopersicum L.) Grown with Biochar and PGPMs" Antioxidants 14, no. 12: 1482. https://doi.org/10.3390/antiox14121482
APA StyleCarlo, S., Trazza, M., Pagano, L., & Marmiroli, M. (2025). Analyses of Antioxidative Response in Tomato (Solanum lycopersicum L.) Grown with Biochar and PGPMs. Antioxidants, 14(12), 1482. https://doi.org/10.3390/antiox14121482






