Experimental and Clinical Approaches to Preventing Aminoglycoside-Induced Ototoxicity: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Experimental Evidence from in Vitro and in Vivo Studies
3.2. Clinical Evidence: Interventional Studies and Practice Changes
3.3. Future Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAPD | Continuous ambulatory peritoneal dialysis |
| NAC | N-acetyl-L-cysteine |
| OAEs | Otoacoustic emissions |
| ROS | Reactive oxygen species |
| SAHA | Suberoylanilide hydroxamic acid |
References
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef]
- Begg, E.J.; Barclay, M.L. Aminoglycosides-50 years on. Brit. J. Clin. Pharmacol. 1995, 39, 597–603. [Google Scholar]
- Atassi, G.; Medernach, R.; Scheetz, M.; Nozick, S.; Rhodes, N.J.; Murphy-Belcaster, M.; Murphy, K.R.; Alisoltani, A.; Ozer, E.A.; Hauser, A.R. Genomics of Aminoglycoside Resistance in Pseudomonas aeruginosa Bloodstream Infections at a United States Academic Hospital. Microbiol. Spectr. 2023, 11, e0508722. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, F. Characterization of Resistance to Aminoglycosides in Methicillin-Resistant Staphylococcus aureus Strains Isolated From a Tertiary Care Hospital in Tehran, Iran. Jundishapur J. Microbiol. 2016, 9, e29237. [Google Scholar] [CrossRef] [PubMed]
- Fàbrega, A.; Vila, J. Yersinia enterocolitica: Pathogenesis, virulence and antimicrobial resistance. Enferm. Infecc. Microbiol. Clin. 2012, 30, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kassinger, S.J.; van Hoek, M.L. Genetic Determinants of Antibiotic Resistance in Francisella. Front. Microbiol. 2021, 12, 644855. [Google Scholar] [CrossRef] [PubMed]
- Rivetti, S.; Romano, A.; Mastrangelo, S.; Attinà, G.; Maurizi, P.; Ruggiero, A. Aminoglycosides-Related Ototoxicity: Mechanisms, Risk Factors, and Prevention in Pediatric Patients. Pharmaceuticals 2023, 16, 1353. [Google Scholar] [CrossRef] [PubMed]
- Leitner, M.G.; Halaszovich, C.R.; Oliver, D. Aminoglycosides inhibit KCNQ4 channels in cochlear outer hair cells via depletion of phosphatidylinositol(4,5)bisphosphate. Mol. Pharmacol. 2011, 79, 51–60. [Google Scholar] [CrossRef]
- Esterberg, R.; Linbo, T.; Pickett, S.B.; Wu, P.; Ou, H.C.; Rubel, E.W.; Raible, D.W. Mitochondrial calcium uptake underlies ROS generation during aminoglycoside-induced hair cell death. J. Clin. Investig. 2016, 126, 3556–3566. [Google Scholar] [CrossRef]
- Qian, Y.; Guan, M.X. Interaction of aminoglycosides with human mitochondrial 12S rRNA carrying the deafness-associated mutation. Antimicrob. Agents Chemother. 2009, 53, 4612–4618. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ Clin. Res. 2021, 372, n71. [Google Scholar]
- Zadrożniak, M.; Szymański, M.; Łuszczki, J.J. N-Acetyl-L-cysteine Affects Ototoxicity Evoked by Amikacin and Furosemide Either Alone or in Combination in a Mouse Model of Hearing Threshold Decrease. Int. J. Mol. Sci. 2023, 24, 7596. [Google Scholar] [CrossRef]
- Kim, Y.R.; Baek, J.I.; Lee, K.Y.; Kim, U.K. Berberine chloride protects cochlear hair cells from aminoglycoside-induced ototoxicity by reducing the accumulation of mitochondrial reactive oxygen species. Free Radic. Biol. Med. 2023, 204, 177–183. [Google Scholar] [CrossRef]
- Zong, Y.; Chen, F.; Li, S.; Zhang, H. (-)-Epigallocatechin-3-gallate (EGCG) prevents aminoglycosides-induced ototoxicity via anti-oxidative and anti-apoptotic pathways. Int. J. Pediatr. Otorhinolaryngol. 2021, 150, 110920. [Google Scholar] [CrossRef]
- Dirain, C.O.; Ng, M.R.A.V.; Milne-Davies, B.; Joseph, J.K.; Antonelli, P.J. Evaluation of Mitoquinone for Protecting Against Amikacin-Induced Ototoxicity in Guinea Pigs. Otol. Neurotol. 2018, 39, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Miman, M.C.; Ozturan, O.; Iraz, M.; Erdem, T.; Olmez, E. Amikacin ototoxicity enhanced by Ginkgo biloba extract (EGb 761). Hear. Res. 2002, 169, 121–129. [Google Scholar] [CrossRef]
- El-Anwar, M.W.; Abdelmonem, S.; Nada, E.; Galhoom, D.; Abdelsameea, A.A. Protective effect of pentoxifylline on amikacin-induced ototoxicity. Ear Nose Throat J. 2018, 97, E8–E12. [Google Scholar] [CrossRef] [PubMed]
- El-Anwar, M.W.; Abdelmonem, S.; Nada, E.; Galhoom, D.; Abdelsameea, A.A. Cilostazol Effect on Amikacin-Induced Ototoxicity: An Experimental Study. Audiol. Neuro-Otol. 2016, 21, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Kitcher, S.R.; Kirkwood, N.K.; Camci, E.D.; Wu, P.; Gibson, R.M.; Redila, V.A.; Simon, J.A.; Rubel, E.W.; Raible, D.W.; Richardson, G.P.; et al. ORC-13661 protects sensory hair cells from aminoglycoside and cisplatin ototoxicity. JCI Insight 2019, 4, e126764. [Google Scholar] [CrossRef]
- Zadrożniak, M.; Szymański, M.; Luszczki, J.J. Effects of Methionine and Glutathione on Acute Ototoxicity Induced by Amikacin and Furosemide in an Animal Model of Hearing Threshold Decrease. Biomedicines 2025, 13, 1476. [Google Scholar] [CrossRef]
- Zadrożniak, M.; Szymański, M.; Luszczki, J.J. Vitamin C alleviates ototoxic effect caused by coadministration of amikacin and furosemide. Pharmacol. Rep. 2019, 71, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.O.; Kim, J.S. Protective Effects of Deferoxamine on Vestibulotoxicity in Gentamicin-Induced Bilateral Vestibulopathy Rat Model. Front. Neurol. 2021, 12, 650752. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, L.; Liu, J.; Dodel, R.C.; Farlow, M.R.; Du, Y. Minocycline prevents gentamicin-induced ototoxicity by inhibiting p38 MAP kinase phosphorylation and caspase 3 activation. Neuroscience 2005, 131, 513–521. [Google Scholar] [CrossRef]
- Cortada, M.; Levano, S.; Bodmer, D. Brimonidine Protects Auditory Hair Cells from in vitro-Induced Toxicity of Gentamicin. Audiol. Neuro-Otol. 2017, 22, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Cortada, M.; Wei, E.; Jain, N.; Levano, S.; Bodmer, D. Telmisartan Protects Auditory Hair Cells from Gentamicin-Induced Toxicity in vitro. Audiol. Neuro-Otol. 2020, 25, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, B.; Lou, X.; Olze, H.; Haupt, H.; Szczepek, A.J. In vitro protection of auditory hair cells by salicylate from the gentamicin-induced but not neomycin-induced cell loss. Neurosci. Lett. 2012, 506, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.W.; Bas, E.; Gupta, C.; Selman, Y.; Eshraghi, A.; Telischi, F.F.; Van De Water, T.R. Otoprotective properties of mannitol against gentamicin induced hair cell loss. Otol. Neurotol. 2014, 35, e187–e194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, J. Baicalin attenuates gentamicin-induced cochlear hair cell ototoxicity. J. Appl. Toxicol. 2019, 39, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Sekulic-Jablanovic, M.; Wright, M.B.; Petkovic, V.; Bodmer, D. Pioglitazone Ameliorates Gentamicin Ototoxicity by Affecting the TLR and STAT Pathways in the Early Postnatal Organ of Corti. Front. Cell. Neurosci. 2020, 14, 566148. [Google Scholar] [CrossRef]
- Wu, K.; Wang, B.; Cao, B.; Ma, W.; Zhang, Y.; Cheng, Y.; Hu, J.; Gao, Y. Protective role of pyrroloquinoline quinone against gentamicin induced cochlear hair cell ototoxicity. J. Appl. Toxicol. 2024, 44, 235–244. [Google Scholar] [CrossRef]
- Ojano-Dirain, C.P.; Antonelli, P.J.; Le Prell, C.G. Mitochondria-targeted antioxidant MitoQ reduces gentamicin-induced ototoxicity. Otol. Neurotol. 2014, 35, 533–539. [Google Scholar] [CrossRef]
- Karasawa, T.; Wang, Q.; David, L.L.; Steyger, P.S. Calreticulin binds to gentamicin and reduces drug-induced ototoxicity. Toxicol. Sci. 2011, 124, 378–387. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jankauskas, S.S.; Plotnikov, E.Y.; Morosanova, M.A.; Pevzner, I.B.; Zorova, L.D.; Skulachev, V.P.; Zorov, D.B. Mitochondria-targeted antioxidant SkQR1 ameliorates gentamycin-induced renal failure and hearing loss. Biochem. Biokhimiia 2012, 77, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.; Lee, Y.; Hong, S.; Han, E.; Park, S.; Baek, H.W.; Kim, H.J.; Rah, Y.C.; Choi, J. In vivo and in vitro evaluation of the protective effects of osthole against ototoxicity using the zebrafish model and HEI-OC1 cell line. Neurotoxicology 2025, 110, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Unal, O.F.; Ghoreishi, S.M.; Ataş, A.; Akyürek, N.; Akyol, G.; Gürsel, B. Prevention of gentamicin induced ototoxicity by trimetazidine in animal model. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Lautermann, J.; McLaren, J.; Schacht, J. Glutathione protection against gentamicin ototoxicity depends on nutritional status. Hear. Res. 1995, 86, 15–24. [Google Scholar] [CrossRef]
- Uzun, L.; Balbaloglu, E.; Akinci, H. Garlic-supplemented diet attenuates gentamicin-induced ototoxicity: An experimental study. Ann. Otol. Rhinol. Laryngol. 2012, 121, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Draz, E.I.; Abdin, A.A.; Sarhan, N.I.; Gabr, T.A. Neurotrophic and antioxidant effects of silymarin comparable to 4-methylcatechol in protection against gentamicin-induced ototoxicity in guinea pigs. Pharmacol. Rep. 2015, 67, 317–325. [Google Scholar] [CrossRef]
- Turan, M.; Ciğer, E.; Arslanoğlu, S.; Börekci, H.; Önal, K. Could edaravone prevent gentamicin ototoxicity? An experimental study. Hum. Exp. Toxicol. 2017, 36, 123–127. [Google Scholar] [CrossRef]
- Nordang, L.; Anniko, M. Nitro-L-arginine methyl ester: A potential protector against gentamicin ototoxicity. Acta Oto-Laryngol. 2005, 125, 1033–1038. [Google Scholar] [CrossRef]
- Kahya, V.; Ozucer, B.; Dogan, R.; Meric, A.; Yuksel, M.; Gedikli, O.; Ozturan, O. Pomegranate extract: A potential protector against aminoglycoside ototoxicity. J. Laryngol. Otol. 2014, 128, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.; Boney, R.; Ordoobadi, A.J.; Sommers, T.F.; Trapani, J.G.; Coffin, A.B. Natural Bizbenzoquinoline Derivatives Protect Zebrafish Lateral Line Sensory Hair Cells from Aminoglycoside Toxicity. Front. Cell. Neurosci. 2016, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Smouha, E.E.; Qiu, D.; Li, F.; Johnson, F.; Luft, B. Flavanoid of Drynaria fortunei protects against gentamicin ototoxicity. Phytother. Res. 2004, 18, 609–614. [Google Scholar] [CrossRef]
- Shi, L.; An, Y.; Wang, A.; Gao, Q.; Yang, Y. The protective effect of Salvia miltiorrhiza on gentamicin-induced ototoxicity. Am. J. Otolaryngol. 2014, 35, 171–179. [Google Scholar] [CrossRef]
- Hong, S.; Han, E.; Park, S.; Hyun, K.; Lee, Y.; Baek, H.W.; Kim, H.J.; Rah, Y.C.; Choi, J. Protective Effects of (-)-Butaclamol Against Gentamicin-Induced Ototoxicity: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2025, 26, 4201. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Sergi, B.; Ferraresi, A.; Paludetti, G.; Troiani, D. alpha-Tocopherol protective effects on gentamicin ototoxicity: An experimental study. Int. J. Audiol. 2004, 43, 166–171. [Google Scholar] [CrossRef]
- Francis, S.P.; Kramarenko, I.I.; Brandon, C.S.; Lee, F.S.; Baker, T.G.; Cunningham, L.L. Celastrol inhibits aminoglycoside-induced ototoxicity via heat shock protein 32. Cell Death Dis. 2011, 2, e195. [Google Scholar] [CrossRef] [PubMed]
- Zallocchi, M.; Hati, S.; Xu, Z.; Hausman, W.; Liu, H.; He, D.Z.; Zuo, J. Characterization of quinoxaline derivatives for protection against iatrogenically induced hearing loss. JCI Insight 2021, 6, e141561. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Gao, W.Q. Concanavalin A protects hair cells against gentamicin ototoxicity in rat cochlear explant cultures. J. Neurobiol. 1999, 39, 29–40. [Google Scholar] [CrossRef]
- Kuang, X.; Sun, Y.; Wang, Z.; Zhou, S.; Liu, H. A mitochondrial targeting tetrapeptide Bendavia protects lateral line hair cells from gentamicin exposure. J. Appl. Toxicol. 2018, 38, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, Y.; He, Q.; Zhang, Y.; Han, L.; Jin, M.; Liu, T.; Liu, K.; Sun, C. A new active peptide from Neptunea arthritica cumingii exerts protective effects against gentamicin-induced sensory-hair cell injury in zebrafish. Drug Chem. Toxicol. 2022, 45, 161–169. [Google Scholar] [CrossRef]
- Quan, Y.; Xia, L.; Shao, J.; Yin, S.; Cheng, C.Y.; Xia, W.; Gao, W.Q. Adjudin protects rodent cochlear hair cells against gentamicin ototoxicity via the SIRT3-ROS pathway. Sci. Rep. 2015, 5, 8181. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Chen, X.; Zhang, P.Z.; Shi, Z.T.; Wen, L.T.; Qiu, J.H.; Chen, F.Q. Histone deacetylase inhibitor sodium butyrate attenuates gentamicin-induced hearing loss in vivo. Am. J. Otolaryngol. 2015, 36, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, N.K.; O’Reilly, M.; Derudas, M.; Kenyon, E.J.; Huckvale, R.; van Netten, S.M.; Ward, S.E.; Richardson, G.P.; Kros, C.J. d-Tubocurarine and Berbamine: Alkaloids That Are Permeant Blockers of the Hair Cell’s Mechano-Electrical Transducer Channel and Protect from Aminoglycoside Toxicity. Front. Cell. Neurosci. 2017, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zong, T.; Liu, J.; Wang, D.; Gong, K.; Yin, H.; Zhang, W.; Xu, T.; Yang, R. Rutin Attenuates Gentamycin-induced Hair Cell Injury in the Zebrafish Lateral Line via Suppressing STAT1. Mol. Neurobiol. 2024, 61, 9548–9561. [Google Scholar] [CrossRef] [PubMed]
- Kucharava, K.; Sekulic-Jablanovic, M.; Horvath, L.; Bodmer, D.; Petkovic, V. Pasireotide protects mammalian cochlear hair cells from gentamicin ototoxicity by activating the PI3K-Akt pathway. Cell Death Dis. 2019, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Ji, H.M.; Kim, S.J.; Kil, S.H.; Lee, J.N.; Kwak, S.; Choe, S.K.; Park, R. Fenofibrate exerts protective effects against gentamicin-induced toxicity in cochlear hair cells by activating antioxidant enzymes. Int. J. Mol. Med. 2017, 39, 960–968. [Google Scholar] [CrossRef][Green Version]
- Niwa, K.; Matsunobu, T.; Kurioka, T.; Kamide, D.; Tamura, A.; Tadokoro, S.; Satoh, Y.; Shiotani, A. The beneficial effect of Hangesha-shin-to (TJ-014) in gentamicin-induced hair cell loss in the rat cochlea. Auris Nasus Larynx 2016, 43, 507–513. [Google Scholar] [CrossRef]
- Liu, H.Y.; Chi, F.L.; Gao, W.Y. Taurine attenuates aminoglycoside ototoxicity by inhibiting inducible nitric oxide synthase expression in the cochlea. Neuroreport 2008, 19, 117–120. [Google Scholar] [CrossRef]
- Fang, J.; Wu, H.; Zhang, J.; Mao, S.; Shi, H.; Yu, D.; Chen, Z.; Su, K.; Xing, Y.; Dong, H.; et al. A reduced form of nicotinamide riboside protects the cochlea against aminoglycoside-induced ototoxicity by SIRT1 activation. Biomed. Pharmacother. 2022, 150, 113071. [Google Scholar] [CrossRef]
- Layman, W.S.; Williams, D.M.; Dearman, J.A.; Sauceda, M.A.; Zuo, J. Histone deacetylase inhibition protects hearing against acute ototoxicity by activating the Nf-κB pathway. Cell Death Discov. 2015, 1, 15012. [Google Scholar] [CrossRef]
- Humli, V.; Szepesy, J.; Zsilla, G.; Miklya, I.; Timár, J.; Szabó, S.I.; Polony, G.; Gáborján, A.; Halmos, G.B.; Dunkel, P.; et al. Protective Effect of Selegiline (R-deprenyl) in Aminoglycoside-Induced Hearing Loss. Neurochem. Res. 2025, 50, 200. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, S.A.; Kirkegaard, M.; Nyengaard, J.R. 2,3-Dihydroxybenzoic acid attenuates kanamycin-induced volume reduction in mouse utricular type I hair cells. Hear. Res. 2006, 212, 99–108. [Google Scholar] [CrossRef]
- Polony, G.; Humli, V.; Andó, R.; Aller, M.; Horváth, T.; Harnos, A.; Tamás, L.; Vizi, E.S.; Zelles, T. Protective effect of rasagiline in aminoglycoside ototoxicity. Neuroscience 2014, 265, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, J.; Li, H.; Li, Z.; Chen, M.; Ma, S.; Shen, R.; Lou, X. Berberine protects against neomycin-induced ototoxicity by reducing ROS generation and activating the PI3K/AKT pathway. Neurosci. Lett. 2023, 817, 137518. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.W.; Cheng, H.L.; Su, T.R.; Yang, J.J.; Su, C.C. Cichoric Acid May Play a Role in Protecting Hair Cells from Ototoxic Drugs. Int. J. Mol. Sci. 2022, 23, 6701. [Google Scholar] [CrossRef] [PubMed]
- Nassauer, L.; Schott, J.W.; Harre, J.; Warnecke, A.; Morgan, M.; Galla, M.; Schambach, A. The caspase-inhibitor Emricasan efficiently counteracts cisplatin- and neomycin-induced cytotoxicity in cochlear cells. J. Mol. Med. 2022, 102, 1163–1174. [Google Scholar] [CrossRef]
- Cui, C.; Liu, D.; Qin, X. Attenuation of streptomycin ototoxicity by tetramethylpyrazine in guinea pig cochlea. Otolaryngol.–Head Neck Surg. 2015, 152, 904–911. [Google Scholar] [CrossRef]
- Sha, S.H.; Qiu, J.H.; Schacht, J. Aspirin to prevent gentamicin-induced hearing loss. N. Engl. J. Med. 2006, 354, 1856–1857. [Google Scholar] [CrossRef]
- Behnoud, F.; Davoudpur, K.; Goodarzi, M.T. Can aspirin protect or at least attenuate gentamicin ototoxicity in humans? Saudi Med. J. 2009, 30, 1165–1169. [Google Scholar]
- Kharkheli, E.; Kevanishvili, Z.; Maglakelidze, T.; Davitashvili, O.; Schacht, J. Does vitamin E prevent gentamicin-induced ototoxicity? Georgian Med. News 2007, 146, 14–17. [Google Scholar]
- Feldman, L.; Efrati, S.; Eviatar, E.; Abramsohn, R.; Yarovoy, I.; Gersch, E.; Averbukh, Z.; Weissgarten, J. Gentamicin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney Int. 2007, 72, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Vural, A.; Koçyiğit, İ.; Şan, F.; Eroğlu, E.; Ketenci, İ.; Ünal, A.; Tokgöz, B.; Ünlü, Y. Long-Term Protective Effect of N-Acetylcysteine against Amikacin-Induced Ototoxicity in End-Stage Renal Disease: A Randomized Trial. Perit. Dial. Int. 2018, 38, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, I.; Vural, A.; Unal, A.; Sipahioglu, M.H.; Yucel, H.E.; Aydemir, S.; Yazici, C.; İlhan Sahin, M.; Oymak, O.; Tokgoz, B. Preventing amikacin related ototoxicity with N-acetylcysteine in patients undergoing peritoneal dialysis. Eur. Arch. Oto-Rhino-Laryngol. Head Neck Surg. 2015, 272, 2611–2620. [Google Scholar] [CrossRef]
- Tokgoz, B.; Ucar, C.; Kocyigit, I.; Somdas, M.; Unal, A.; Vural, A.; Sipahioglu, M.; Oymak, O.; Utas, C. Protective effect of N-acetylcysteine from drug-induced ototoxicity in uraemic patients with CAPD peritonitis. Nephrol. Dial. Transplant. 2011, 26, 4073–4078. [Google Scholar] [CrossRef] [PubMed]
- Ardıç, F.N.; Tümkaya, F.; Aykal, K.; Çabuk, B. Selective Window Application of Gentamicin+ Dexamethasone in Meniere’s Disease. J. Int. Adv. Otol. 2017, 13, 243–246. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services, National Institutes of Health, National Library of Medicine, National Center for Biotechnology Information. Available online: https://clinicaltrials.gov (accessed on 2 November 2025).
- European Medicines Agency. Available online: https://euclinicaltrials.eu/search-for-clinical-trials (accessed on 2 November 2025).

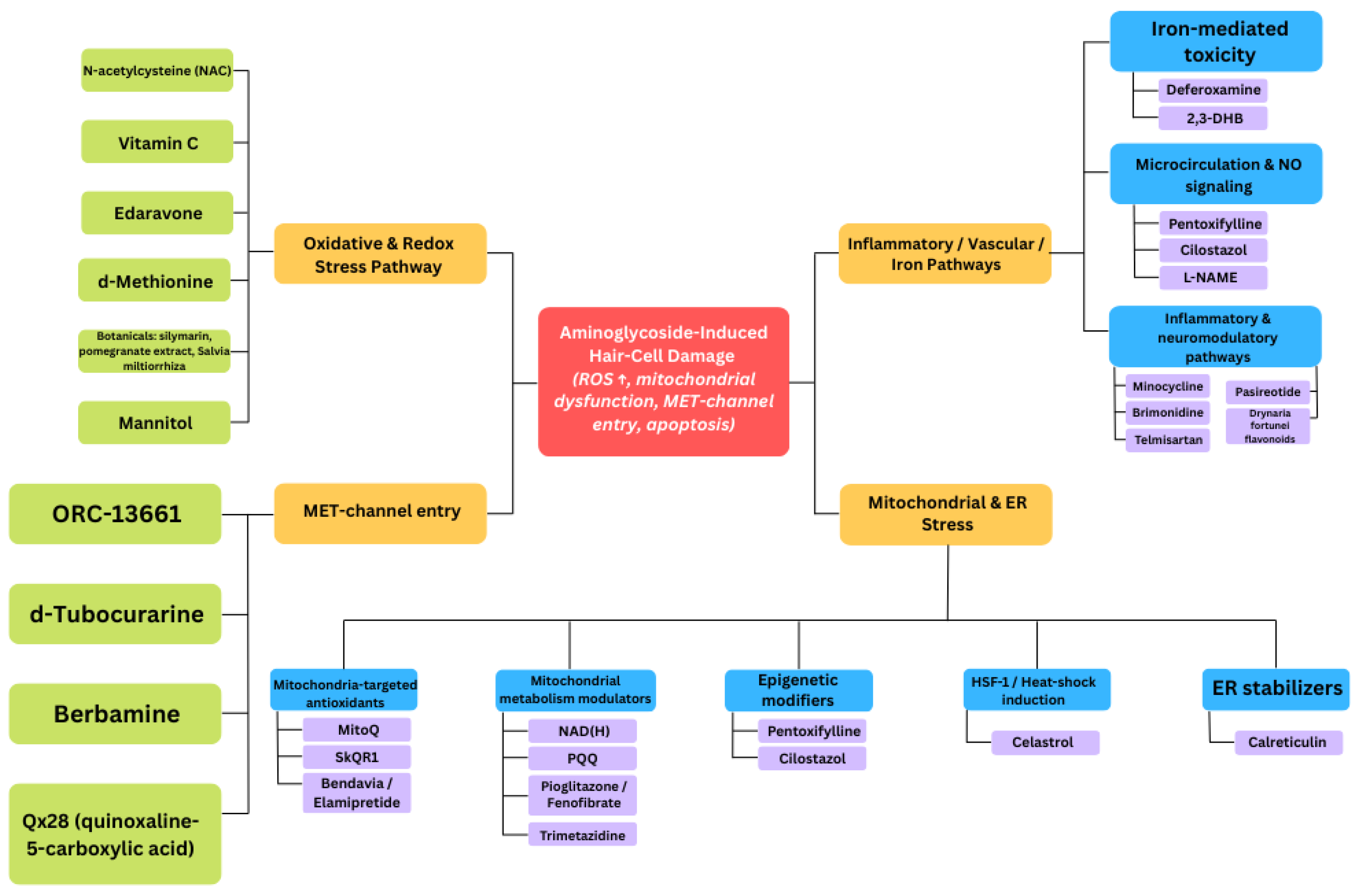
| Tested Compound | Aminoglycoside Antibiotic | Experimental Model | Potential Otoprotective Effect | Reference |
|---|---|---|---|---|
| N-acetyl-L-cysteine | Amikacin | Albino Swiss outbred mice | yes | [12] |
| Berberine chloride | Amikacin | Mice | yes | [13] |
| (−)-Epigallocatechin-3-gallate | Amikacin | Zebrafish AB wild-type | yes | [14] |
| Mitoquinone | Amikacin | Guinea pigs | no | [15] |
| N-acetyl-L-cysteine | Amikacin (+furosemide) | Albino Swiss outbred mice | yes | [12] |
| Ginkgo biloba | Amikacin | Sprague Dawley rats | no (enhance) | [16] |
| Pentoxifylline | amikacin | Wistar rats | yes | [17] |
| Cilostazol | amikacin | Wistar rats | yes | [18] |
| ORC-13661 | amikacin | Zebrafish, CD-1 mice | yes | [19] |
| Methionine | amikacin (+furosemide) | Albino Swiss outbred mice | yes | [20] |
| Glutathione | amikacin (+furosemide) | Albino Swiss outbred mice | no | [20] |
| Vitamin C | amikacin (+furosemide) | Albino Swiss outbred mice | yes | [21] |
| Deferoxamine | gentamicin | Sprague Dawley rats | yes | [22] |
| Minocycline | gentamicin | Sprague Dawley rats | yes | [23] |
| Brimonidine | gentamicin | Wistar rat pups | yes | [24] |
| Telmisartan | gentamicin | Wistar rat pups | yes | [25] |
| Berberine chloride | gentamicin | Mice | yes | [13] |
| Salicylate | gentamicin | Wistar rat pups | yes | [26] |
| Mannitol | gentamicin | Rats | yes | [27] |
| Baicalin | gentamicin | HEI-OC1 cell line | yes | [28] |
| (−)-Epigallocatechin-3-gallate | gentamicin | Zebrafish AB wild-type | yes | [14] |
| Pioglitazone | gentamicin | C57BL/6N mouse pups | yes | [29] |
| Pyrroloquinoline quinone | gentamicin | HEI-OC1 cell line | yes | [30] |
| Mitoquinone | gentamicin | Guinea pigs | yes | [31] |
| Calreticulin | gentamicin | HEI-OC1 cells | yes | [32] |
| SkQR1 | gentamicin | Rats | yes | [33] |
| Osthole | gentamicin | HEI-OC1 cell line, zebrafish | yes | [34] |
| Trimetazidine | gentamicin | Albino Swiss mice | yes | [35] |
| Glutathione | gentamicin | Guinea pigs | yes (only in animals in a state of nutrient deficiency) | [36] |
| Garlic | gentamicin | Wistar rats | yes | [37] |
| Silymarin | gentamicin | Guinea pigs | yes | [38] |
| 4-Methylcatechol | gentamicin | Guinea pigs | no | [38] |
| Edaravone | gentamicin | guinea pigs | yes | [39] |
| Nitro-L-arginine methyl ester | gentamicin | Sprague Dawley rats | yes (only in high-frequency range) | [40] |
| Pomegranate | gentamicin | Wistar rats | yes | [41] |
| Bizbenzoquinoline | gentamicin | Zebrafish | yes | [42] |
| Flavanoid fraction from Drynaria fortunei | gentamicin | Guinea pigs | yes | [43] |
| Salvia miltiorrhiza | gentamicin | Guinea pigs | yes | [44] |
| (-)-Butaclamol | gentamicin | Zebrafish, HEI-OC1 line | yes | [45] |
| alpha-Tocopherol | gentamicin | Albino guinea pigs | yes | [46] |
| Celastrol | gentamicin | CBA/J, Hsf-1−/−, HSP70.1/3−/−mice | yes | [47] |
| Quinoxaline-5-carboxylic acid (Qx28) | gentamicin | Zebrafish | yes | [48] |
| Concanavalin A | gentamicin | Rats | yes | [49] |
| Bendavia | gentamicin | Zebrafish | yes | [50] |
| Peptide from Netunea arthritica cumingii | gentamicin | Zebrafish | yes | [51] |
| Adjudin | gentamicin | Sprague Dawley rats, C57BL/6J mice | yes | [52] |
| Sodium butyrate | gentamicin | Guinea pigs | yes | [53] |
| d-Tubocurarine and Berbamine | gentamicin | Zebrafish, CD-1 mice | yes | [54] |
| Rutin | gentamicin | Zebrafish | yes | [55] |
| Pasireotide | gentamicin | C57BL/6N mice | yes | [56] |
| Fenofibrate | gentamicin | Sprague Dawley rats | yes | [57] |
| Hangesha-shin-to (TJ-014) | gentamicin | Rats | yes | [58] |
| Taurine | gentamicin (+furosemide) | Guinea pigs | yes | [59] |
| ORC-13661 | gentamicin (+furosemide) | Zebrafish, CD-1 mice | yes | [19] |
| Dihydronicotinamide riboside | kanamycin (+furosemide) | C57BL/6J mice | yes | [60] |
| Suberoylanilide hydroxamic acid (SAHA) | kanamycin (+furosemide) | FVB/NJ and C57BL/6J wild-type mice | yes | [61] |
| Berberine chloride | kanamycin | Mice | yes | [13] |
| Selegiline | kanamycin | CD-1 mice | [62] | |
| Celastrol | kanamycin | CBA/J, Hsf-1−/−, HSP70.1/3−/−mice | yes | [47] |
| 2,3-Dihydroxybenzoic acid | kanamycin | Albino mice of the strain Balb/cA | yes | [63] |
| Rasagiline | kanamycin | CD-1 male mice | yes | [64] |
| Salicylate | neomycin | Wistar rat pups | no | [26] |
| Celastrol | neomycin | CBA/J, Hsf-1−/−, HSP70.1/3−/−mice | yes | [47] |
| Berberine | neomycin | Mice | yes | [65] |
| Bizbenzoquinoline | neomycin | Zebrafish | yes | [42] |
| ORC-13661 | neomycin | Zebrafish, CD-1 mice | yes | [19] |
| Quinoxaline-5-carboxylic acid (Qx28) | neomycin | Zebrafish | yes | [48] |
| Cichoric Acid | neomycin | Zebrafish | yes | [66] |
| Sodium thiosulfate | neomycin | HEI-OC1, phoenix auditory cells and primary rat SGN cultures | no | [67] |
| Emricasan | neomycin | HEI-OC1, phoenix auditory cells and primary rat SGN cultures | yes | [67] |
| d-Tubocurarine and Berbamine | neomycin | Zebrafish, CD-1 mice | yes | [54] |
| Tetramethylpyrazine | streptomycin | Guinea pigs | yes | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zadrożniak, M.; Biskupski, M.; Szymański, M.; Łuszczki, J.J. Experimental and Clinical Approaches to Preventing Aminoglycoside-Induced Ototoxicity: A Scoping Review. Antioxidants 2025, 14, 1467. https://doi.org/10.3390/antiox14121467
Zadrożniak M, Biskupski M, Szymański M, Łuszczki JJ. Experimental and Clinical Approaches to Preventing Aminoglycoside-Induced Ototoxicity: A Scoping Review. Antioxidants. 2025; 14(12):1467. https://doi.org/10.3390/antiox14121467
Chicago/Turabian StyleZadrożniak, Marek, Maciej Biskupski, Marcin Szymański, and Jarogniew J. Łuszczki. 2025. "Experimental and Clinical Approaches to Preventing Aminoglycoside-Induced Ototoxicity: A Scoping Review" Antioxidants 14, no. 12: 1467. https://doi.org/10.3390/antiox14121467
APA StyleZadrożniak, M., Biskupski, M., Szymański, M., & Łuszczki, J. J. (2025). Experimental and Clinical Approaches to Preventing Aminoglycoside-Induced Ototoxicity: A Scoping Review. Antioxidants, 14(12), 1467. https://doi.org/10.3390/antiox14121467






