Molecular Insights into the Genesis of Heat Hardening in Marine Bivalves
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
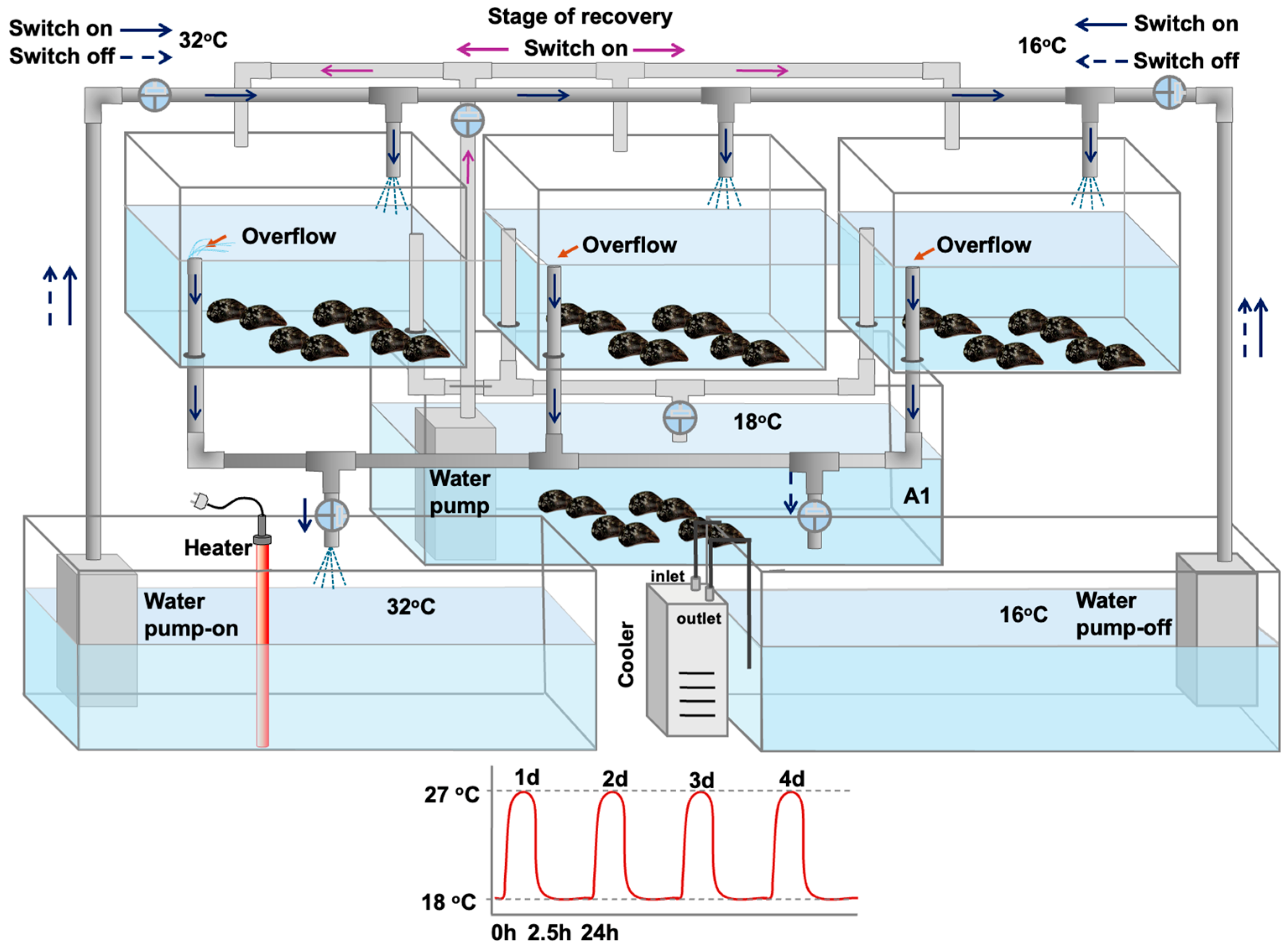
2.2. Experimental Design
2.2.1. Laboratory Exposure—Hardening Process
2.2.2. Tissue Collection
2.3. Determination of Gene Expression at the mRNA Level
2.4. Statistics
3. Results
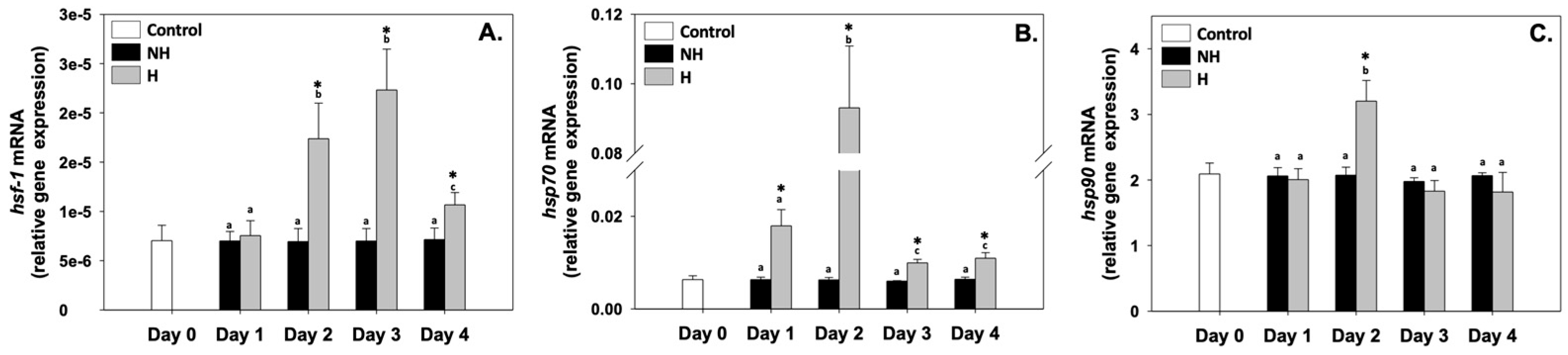
3.1. Heat Shock Response
3.2. Intermediate Metabolism
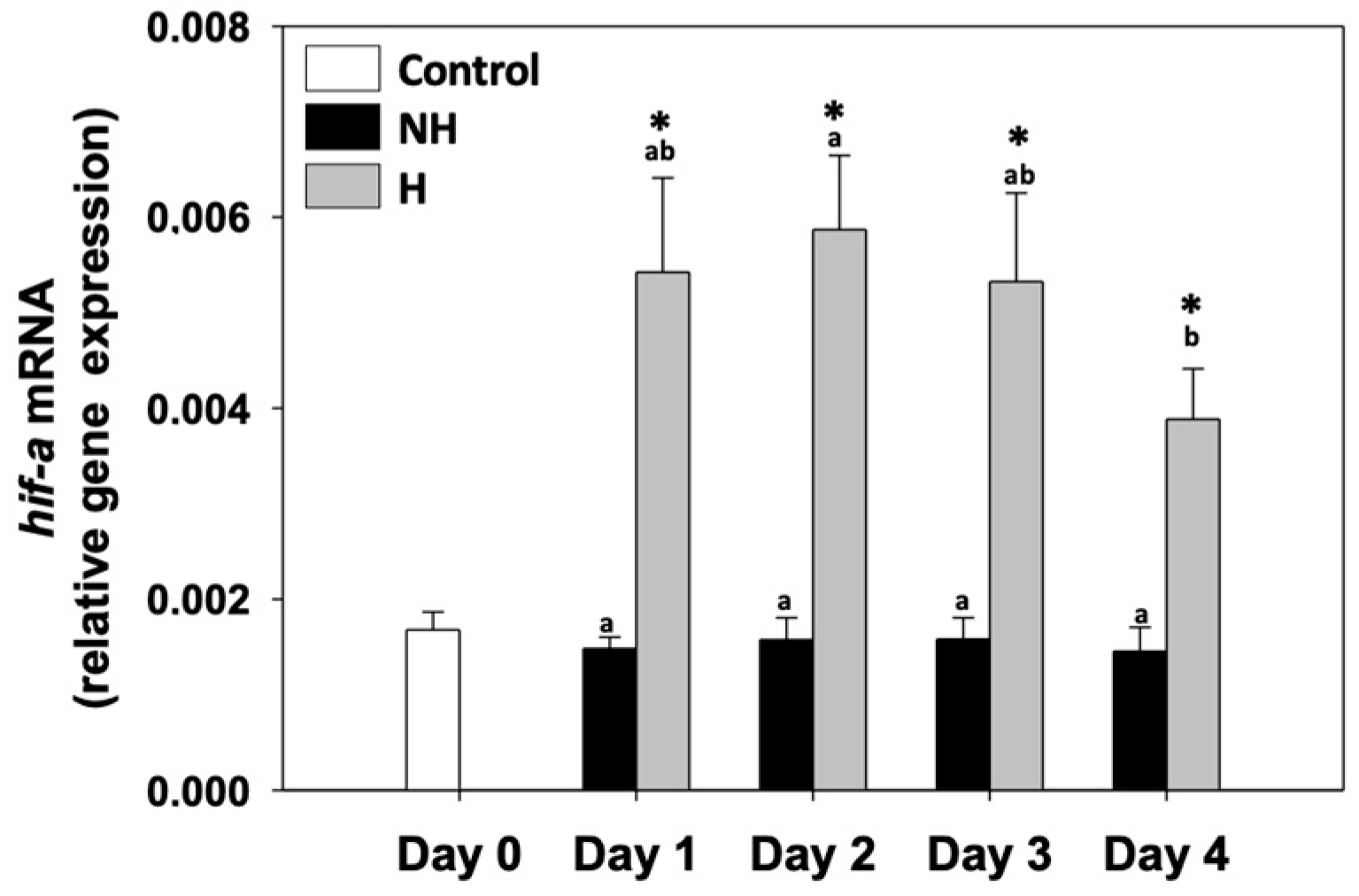
3.3. Hypoxia
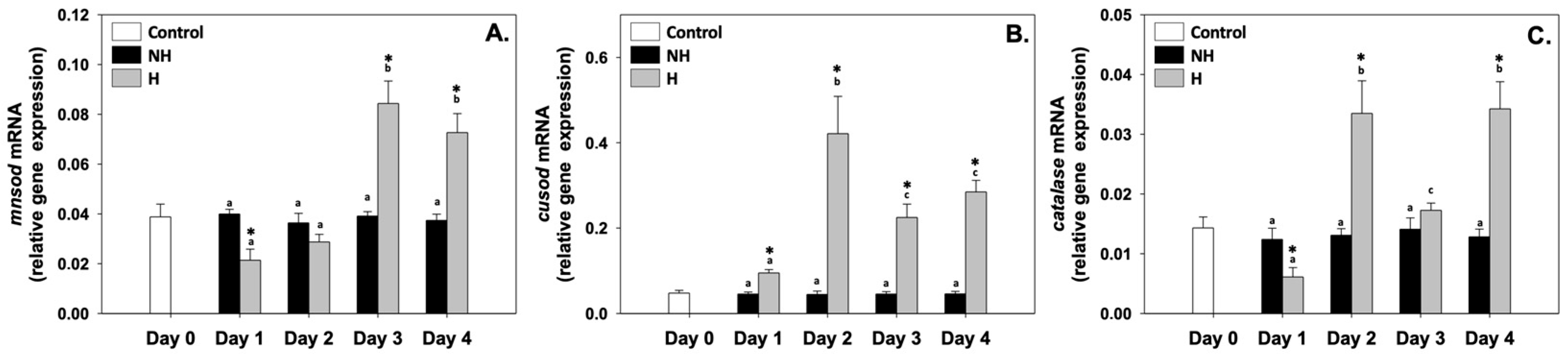
3.4. Antioxidant Defense
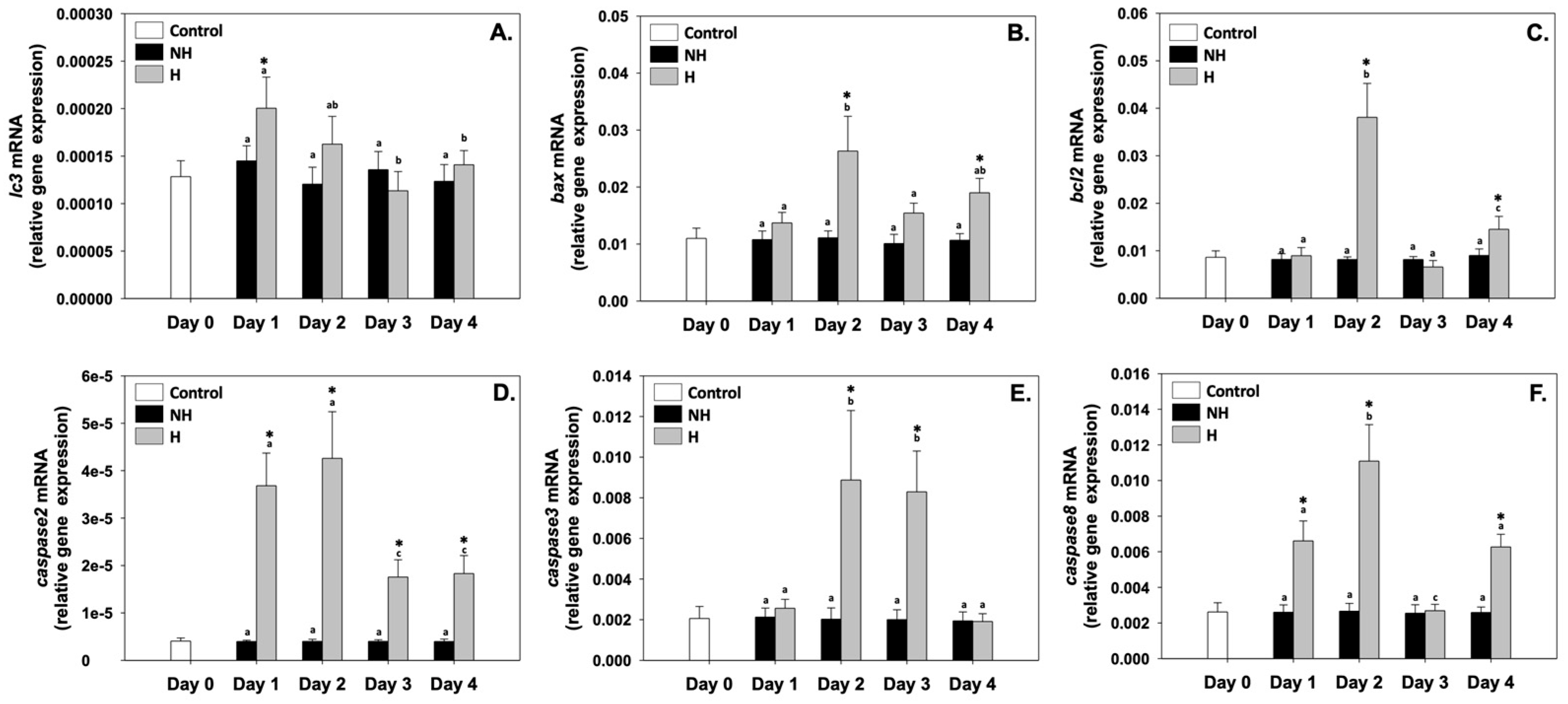
3.5. Autophagy and Apoptosis
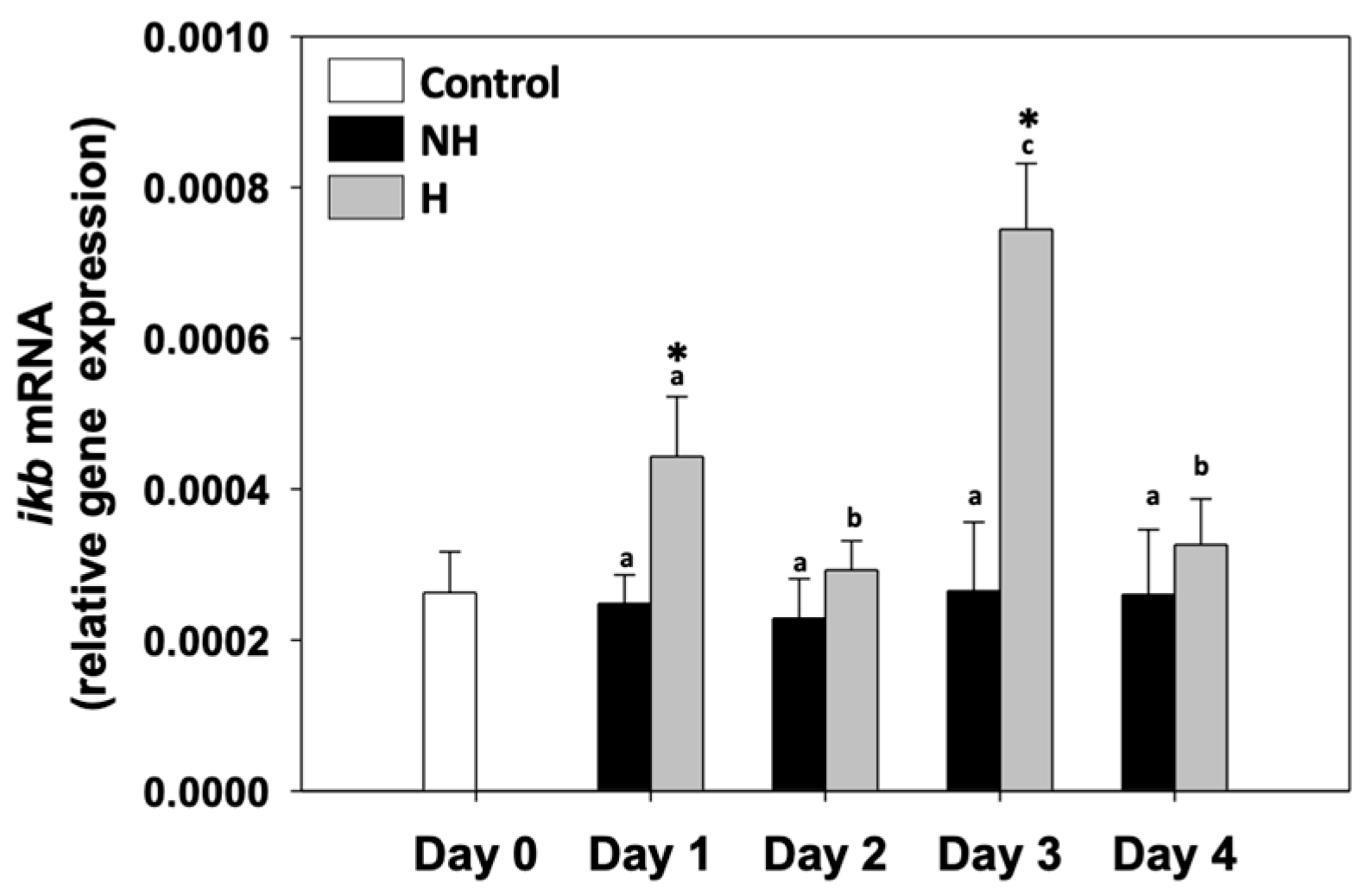
3.6. Inflammation
3.7. Multivariate Analysis
4. Discussion
4.1. Heat Shock Response and Proteostasis
4.2. Intermediary Metabolism: Coordinating Energy Supply
4.3. Hypoxia Signaling: Oxygen Sensing Under Elevated Metabolism
4.4. Antioxidant Defense: Redox Homeostasis
4.5. Autophagy and Apoptosis: Cellular Quality Control
4.6. Inflammation: Integrating Cellular Responses
4.7. Integrative Perspective: Stress Memory as a Biochemical Network
4.8. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ETS | Electron transfer system |
| TCA | Tricarboxylic acid |
| ROS | Reactive oxygen species |
| Hsps | Heat shock proteins |
| CTM | Critical thermal maximum |
| H | Hardened |
| NH | Non-hardened |
| ANOVA | Analysis of variance |
| SD | Standard deviation |
| hsf-1 | Heat shock factor 1 |
| hsp70 | Heat shock protein 70 |
| hsp90 | Heat shock protein 90 |
| atpase6 | ATPase 6 |
| cox1 | Cytochrome c oxidase I |
| nadh | NADH dehydrogenase |
| pk | Pyruvate kinase |
| cs | Citrate synthase |
| hif1-a | Hypoxia-induced factor 1α |
| mnsod | Mn superoxide dismutase |
| cusod | Cu superoxide dismutase |
| lc3b | Microtubule-associated protein 1 light chain 3 beta |
| bax | Bcl-2-associated X protein |
| bcl | B-cell lymphoma 2 |
| ikb | Inhibitor of kappa B |
| PCA | Principal component analysis |
| HSR | Heat shock response |
| DAMPs | Damage-associated molecular patterns |
References
- Bilyk, K.T.; Evans, C.W.; DeVries, A.L. Heat hardening in Antarctic notothenioid fishes. Polar Biol. 2012, 35, 1447–1451. [Google Scholar] [CrossRef]
- Bowler, K. Acclimation, heat shock and hardening. J. Therm. Biol. 2005, 30, 125–130. [Google Scholar] [CrossRef]
- Precht, H. Temperature and Life; Precht, H., Christophersen, J., Hensel, H., Larcher, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1973; pp. 302–348. [Google Scholar]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. 2016, 91, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, E.A.; Shadel, G.S. Alternative mitochondrial fuel extends lifespan. Cell Metab. 2012, 15, 417–418. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Calabrese, E.J. A global environmental health perspective and optimisation of stress. Sci. Total Environ. 2020, 704, 135263. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Galluzzi, L.; Kroemer, G. Hormesis, cell death and aging. Aging 2011, 3, 821–828. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Hormesis: A general biological principle. Chem. Res. Toxicol. 2022, 35, 547–549. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Lucassen, M.; Storch, D. Metabolic biochemistry: Its role in thermal tolerance and in the capacities of physiological and ecological function. Fish Physiol. 2005, 22, 79–154. [Google Scholar] [CrossRef]
- Kalmar, B.; Greensmith, L. Induction of heat shock proteins for protection against oxidative stress. Adv. Drug Deliv. Rev. 2009, 61, 310–318. [Google Scholar] [CrossRef]
- Nasr, M.A.; Dovbeshko, G.I.; Bearne, S.L.; El-Badri, N.; Matta, C.F. Heat shock proteins in the “hot” mitochondrion: Identity and putative roles. BioEssays 2019, 41, 1900055. [Google Scholar] [CrossRef]
- Georgoulis, I.; Feidantsis, K.; Giantsis, I.A.; Kakale, A.; Bock, C.; Pörtner, H.O.; Sokolova, I.M.; Michaelidis, B. Heat hardening enhances mitochondrial potential for respiration and oxidative defence capacity in the mantle of thermally stressed Mytilus galloprovincialis. Sci. Rep. 2021, 11, 17098. [Google Scholar] [CrossRef]
- Georgoulis, I.; Bock, C.; Lannig, G.; Pörtner, H.O.; Feidantsis, K.; Giantsis, I.A.; Sokolova, I.M.; Michaelidis, B. Metabolic remodeling caused by heat hardening in the Mediterranean mussel Mytilus galloprovincialis. J. Exp. Biol. 2022, 225, jeb244795. [Google Scholar] [CrossRef]
- Georgoulis, I.; Bock, C.; Lannig, G.; Pörtner, H.O.; Sokolova, I.M.; Feidantsis, K.; Giantsis, I.A.; Michaelidis, B. Heat hardening enhances metabolite-driven thermoprotection in the Mediterranean mussel Mytilus galloprovincialis. Front. Physiol. 2023, 14, 1244314. [Google Scholar] [CrossRef]
- Georgoulis, I.; Giantsis, I.A.; Michaelidis, B.; Feidantsis, K. Heat Hardening Ameliorates Apoptotic and Inflammatory Effects Through Increased Autophagy in Mussels. Mar. Biotechnol. 2024, 26, 1271–1286. [Google Scholar] [CrossRef]
- Georgoulis, I.; Giantsis, I.A.; Michaelidis, B.; Papadopoulos, D.K.; Lattos, A.; Katselis, G.; Feidantsis, K. From the laboratory to the field: Heat hardening shields and enhances Mediterranean mussels’ physiological performance against global warming. Mar. Pollut. Bull. 2025, 221, 118569. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Schmelz, E.A. Immunological “memory” in the induced accumulation of nicotine in wild tobacco. Ecology 1996, 77, 236–246. [Google Scholar] [CrossRef]
- Bruce, T.J.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- Jaskiewicz, M.; Conrath, U.; Peterhänsel, C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 2011, 12, 50–55. [Google Scholar] [CrossRef]
- Yakovlev, I.A.; Asante, D.K.; Fossdal, C.G.; Junttila, O.; Johnsen, Ø. Differential gene expression related to an epigenetic memory affecting climatic adaptation in Norway spruce. Plant Sci. 2011, 180, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, V.H. Critical thermal maxima in salamanders. Physiol. Zool. 1961, 34, 92–125. [Google Scholar] [CrossRef]
- Feidantsis, K.; Giantsis, I.A.; Vratsistas, A.; Makri, S.; Pappa, A.Z.; Drosopoulou, E.; Anestis, A.; Mavridou, E.; Exadactylos, A.; Vafidis, D.; et al. Correlation between intermediary metabolism, HSP gene expression, and oxidative stress-related proteins in long-term thermal-stressed Mytilus galloprovincialis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R264–R281. [Google Scholar] [CrossRef]
- Falfushynska, H.; Piontkivska, H.; Sokolova, I.M. Effects of intermittent hypoxia on cell survival and inflammatory responses in the intertidal marine bivalves Mytilus edulis and Crassostrea gigas. J. Exp. Biol. 2020, 223, jeb217026. [Google Scholar] [CrossRef]
- Woo, S.; Denis, V.; Won, H.; Shin, K.; Lee, G.; Lee, T.-K.; Yum, S. Expressions of oxidative stress-related genes and antioxidant enzyme activities in Mytilus galloprovincialis (Bivalvia, Mollusca) exposed to hypoxia. Zool. Stud. 2013, 52, 15. [Google Scholar] [CrossRef]
- Giannetto, A.; Maisano, M.; Cappello, T.; Oliva, S.; Parrino, V.; Natalotto, A.; De Marco, G.; Fasulo, S. Effects of oxygen availability on oxidative stress biomarkers in the Mediterranean mussel Mytilus galloprovincialis. Mar. Biotechnol. 2017, 19, 614–626. [Google Scholar] [CrossRef]
- Woo, S.; Jeon, H.Y.; Kim, S.R.; Yum, S. Differentially displayed genes with oxygen depletion stress and transcriptional responses in the marine mussel, Mytilus galloprovincialis. Comp. Biochem. Physiol. D 2011, 6, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, A.; Maisano, M.; Cappello, T.; Oliva, S.; Parrino, V.; Natalotto, A.; De Marco, G.; Barberi, C.; Romeo, O.; Mauceri, A.; et al. Hypoxia-Inducible Factor α and Hif-prolyl Hydroxylase Characterization and Gene Expression in Short-Time Air-Exposed Mytilus galloprovincialis. Mar. Biotechnol. 2015, 17, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yuan, Z.; Wu, H.; Liu, F.; Zhao, J. Molecular characterization of a manganese superoxide dismutase and copper/zinc superoxide dismutase from the mussel Mytilus galloprovincialis. Fish Shellfish. Immunol. 2013, 34, 1345–1351. [Google Scholar] [CrossRef]
- Papadopoulos, D.K.; Michaelidis, B.; Giantsis, I.A. Cell death and antioxidant responses in Mytilus galloprovincialis under heat stress: Evidence of genetic loci potentially associated with thermal resilience. PLoS ONE 2025, 20, e0321682. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670. [Google Scholar] [CrossRef]
- Tomanek, L. Variation in the heat shock response and its implication for predicting the effect of global climate change on species’ biogeographical distribution ranges and metabolic costs. J. Exp. Biol. 2010, 213, 971–979. [Google Scholar] [CrossRef]
- Böttinger, L.; Guiard, B.; Oeljeklaus, S.; Kulawiak, B.; Zufall, N.; Wiedemann, N.; Warscheid, B.; van der Laan, M.; Becker, T. A complex of Cox4 and mitochondrial Hsp70 plays an important role in the assembly of the cytochrome c oxidase. Mol. Biol. Cell 2013, 24, 2609–2619. [Google Scholar] [CrossRef]
- Hu, J.; Chen, B.; Li, Z. Thermal plasticity is related to the hardening response of heat shock protein expression in two Bactrocera fruit flies. J. Insect Physiol. 2014, 67, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Somero, G.N. Proteins and temperature. Annu. Rev. Physiol. 1995, 57, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Hochachka, P.W.; Somero, G.N. Biochemical Adaptation: Mechanism and Process in Physiological Evolution; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Movafagh, S.; Crook, S.; Vo, K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: New developments in an old debate. J. Cell Biochem. 2015, 116, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Somero, G.N. The cellular stress response and temperature: Function, regulation, and evolution. J. Exp. Zool. A. 2020, 333, 379–397. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.D.; Caron, A.W.; Bourget, L.; Denis-Larose, C.; Massie, B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell Biol. 1997, 17, 5317–5327. [Google Scholar] [CrossRef]
- Kelly, S.; Zhang, Z.J.; Zhao, H.; Xu, L.; Giffard, R.G.; Sapolsky, R.M.; Yenari, M.A.; Steinberg, G.K. Gene transfer of HSP72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: Influence of Bcl-2. Ann. Neurol. 2002, 52, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Beere, H.M. ‘The stress of dying’: The role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 2004, 117, 2641–2651. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef]
- Deretic, V.; Levine, B. Autophagy balances inflammation in innate immunity. Autophagy 2018, 14, 243–251. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. 2008, 3, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Schmeisser, K. Mitohormesis: Promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose-Response 2014, 12, 288–341. [Google Scholar] [CrossRef] [PubMed]


| Gene | Sequence (5′-3′) | Accession Number | Reference |
|---|---|---|---|
| atpase6 F | 5′-GGTTGTCCGTTAATCCTTGTG-3′ | NC006886 | Georgoulis et al. [15] |
| atpase6 R | 5′-AACCAACCCACTACCAACTC-3′ | ||
| caspase2 F | 5′-ACAAGTGCAGATGCTGTGTTG-3′ | HQ424449.1 | Falfushynska et al. [23] |
| caspase2 F | 5′-ACACCTCTCACATTGTCGGC-3′ | ||
| caspase3 F | 5′-ACGACAGCTAGTTCACCAGG-3′ | HQ424453.1 | Falfushynska et al. [23] |
| caspase3 R | 5′-CCACCAGAAGAGGAGTTCCG-3′ | ||
| caspase8 F | 5′-AATGTCGGTACCCCACGATG-3′ | HQ424450.1 | Falfushynska et al. [23] |
| caspase8 R | 5′-CGTGTATGAACCATGCCCCT-3′ | ||
| bcl-2 F | 5′-CGGTGGTTGGCAAGGATTTG-3′ | KC545829.1 | Falfushynska et al. [23] |
| bcl-2 R | 5′-CGCCATTGCGCCTATTACAC-3′ | ||
| bax F | 5′-TAACTGGGGACGTGTAGGCA-3′ | KC545830.1 | Falfushynska et al. [23] |
| bax R | 5′-CCAGGGGGCGACATAATCTG-3′ | ||
| ikB F | 5′-TGTCATTTGCCGATTCTACGA-3′ | MglkB2_Fs | The present study |
| ikB R | 5′-GGCTCCATTCCTCCTTAGTG-3′ | qlkB2MgR | |
| cox1 F | 5′-GTGTCTTCTTATGGGTCTG-3′ | FJ890849 | Woo et al. [24] |
| cox1 R | 5′-GCTATAAACATGCTTTCTCC-3′ | ||
| nadh F | 5′-TGGTGTTTTCCTCTACACTC-3′ | FJ549901 | Woo et al. [24] |
| nadh R | 5′-AGGGTCTTATTACCCGCACT-3′ | ||
| hsp70 F | 5′-CGGAGGCAAGCCAAAACTAC-3′ | AB180909.1 | Giannetto et al. [25] |
| hsp70 R | 5′-AGCCTCGGCAGTTTCTTTCA-3′ | ||
| hsp90 F | 5′-GGTTGCTGATAAAGTAGTTG-3′ | AJ586906.3 | Woo et al. [26] |
| hsp90 R | 5′-ATTCAGTCTGGTCTTCTTTC-3′ | ||
| hsf-1 F | 5′-TGGGTAACGGAGCAGCAGA-3′ | XM034457664.2 | The present study |
| hsf-1 R | 5′-CATGGGTGGTAGGTTGGATAAG-3′ | ||
| hif-a F | 5′-TGCTAAATACCTTGGCATCTCA-3′ | KP185351.1 | Giannetto et al. [27] |
| hif-a R | 5′-GCTCTCCAAACGGCAATGTA-3′ | ||
| pk F | 5′-GACATGRTTTTYGCSTCCTTCA-3′ | XM022456018 | Georgoulis et al. [13] |
| pk R | 5′-TCATCATCTTCTGKGCVAGGAA-3′ | ||
| cs F | 5′-AACCACGTGACGACCATGCTGAA-3′ | LOC143064983 | The present study |
| cs R | 5′-TCCTCGTAGACATGCTCCCA-3′ | ||
| mnsod F | 5′-GATGCAGCAGTAGCAGTCCA-3′ | JN863295.1 | Wang et al. [28] |
| mnsod R | 5′-GTAGGCATGCTCCCAGACAT-3′ | ||
| cusod F | 5′-AGGCGCAATCCATTTGTTAC-3′ | JN863295.1 | Wang et al. [28] |
| cusod R | 5′-CATGCCTTGTGTGAGCATCT-3′ | ||
| catalase F | 5′-CTCTGACCGTGGAACCCCTGA-3′ | AY743716.2 | Giannetto et al. [25] |
| catalase R | 5′-ATCACGGATGGCATAATCTGGA-3′ | ||
| lc3b F | 5′-CCWCAAGARCTCTCYATGTC-3′ | XM_011417532.3 | Papadopoulos et al. [29] |
| lc3b R | 5′-TCYTGTGAKGCATAWGTCAT-3′ | XM_063570666.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgoulis, I.; Giantsis, I.A.; Michaelidis, B.; Kouniakis, A.; Feidantsis, K. Molecular Insights into the Genesis of Heat Hardening in Marine Bivalves. Antioxidants 2025, 14, 1468. https://doi.org/10.3390/antiox14121468
Georgoulis I, Giantsis IA, Michaelidis B, Kouniakis A, Feidantsis K. Molecular Insights into the Genesis of Heat Hardening in Marine Bivalves. Antioxidants. 2025; 14(12):1468. https://doi.org/10.3390/antiox14121468
Chicago/Turabian StyleGeorgoulis, Ioannis, Ioannis A. Giantsis, Basile Michaelidis, Athanasios Kouniakis, and Konstantinos Feidantsis. 2025. "Molecular Insights into the Genesis of Heat Hardening in Marine Bivalves" Antioxidants 14, no. 12: 1468. https://doi.org/10.3390/antiox14121468
APA StyleGeorgoulis, I., Giantsis, I. A., Michaelidis, B., Kouniakis, A., & Feidantsis, K. (2025). Molecular Insights into the Genesis of Heat Hardening in Marine Bivalves. Antioxidants, 14(12), 1468. https://doi.org/10.3390/antiox14121468








