Microencapsulation Enhances the Biological Potential, Bioaccessibility, and Intracellular Oxidative Status of Guava Phenolic Extracts
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Methods
- Production of microencapsulated extracts and initial characterization: The soluble phenolic fraction from guava pulp and waste powders was obtained and microencapsulated. The microencapsulated extracts were characterized for their physicochemical properties, total phenolic and flavonoid contents, antiradical capacity and ferric reducing ability, α-glucosidase and pancreatic lipase inhibition, oxidative protection to LDL-cholesterol and supercoiled DNA, and phenolic profile by HPLC-UV-MS-TOF. Results were compared with unencapsulated extracts obtained from the same sources.
- Simulated gastrointestinal (GI) digestion of microencapsulated extracts: Microencapsulated extracts and their unencapsulated counterparts were subjected to an in vitro GI model comprising oral, gastric, small intestine, and large intestine stages. The phenolic bioaccessibility (%) was assessed by comparing the total phenolics released from microencapsulated extracts during each digestion phase with the output of their unencapsulated counterparts. The antiradical and ferric reducing capacity were also measured at each digestion stage. Finally, the bioaccessible fraction (small intestine digesta) of each extract was assessed for α-glucosidase and pancreatic lipase inhibition, as well as oxidative protection to LDL-cholesterol and supercoiled DNA.
- Impact of microencapsulated extract on the redox status of human cell lines: The microencapsulated extract showing the best performance on the previous parts was chosen for testing in Caco-2 and HeLa cells. The extract was incubated with each cell line at multiple concentrations for a short period and evaluated for cytotoxicity (MTT assay) and cellular antioxidant activity (CAA).
2.2.1. Preparation of Guava Powders
2.2.2. Extraction of Soluble Phenolic Compounds
2.2.3. Microencapsulation of Phenolic Extracts
2.2.4. Physicochemical Characterization of Microencapsulated Extracts
Moisture Content
Hygroscopicity
Particle Size and Polydispersity Index
2.2.5. Bioactive Characterization of Microencapsulated Extracts
Total Phenolic and Total Flavonoid Content
- Determination of core and surface phenolics
Antioxidant Activity
- 2,2-Diphenyl-1-picrylhydrazyl (DPPH) scavenging capacity
- 2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) scavenging capacity
- Ferric reducing antioxidant power (FRAP)
Biochemical Assays
- α-Glucosidase and pancreatic lipase inhibitory activity
- Inhibition of cupric ion-induced human low-density lipoprotein (LDL) peroxidation
- Inhibition of hydroxyl radical-induced supercoiled DNA strand scission
Identification and Quantification of Phenolic Compounds by High Performance Liquid Chromatography with Ultraviolet Detection Coupled with Time-of-Flight Mass Spectrometry (HPLC-UV-MS-TOF)
2.2.6. In Vitro Simulated Gastrointestinal (GI) Digestion
- Oral: One gram of extract was mixed with 75 U/mL α-amylase and 0.75 mM calcium chloride dissolved in 0.01 M PBS (pH 7.4). The pH was adjusted to 6.5 with a pH meter (FisherBrand, AB315, Waltham, MA, USA) using 5M HCl and 5M NaOH solutions. The mixture was incubated at 37 °C for 10 min under constant stirring (200 rpm). Incubation conditions (except for time) was the same for all digestion stages.
- Gastric: The orally digested pellet was combined with 2000 U/mL pepsin and 0.075 M calcium chloride diluted in PBS. The pH was adjusted to 2 and incubation lasted for 2 h.
- Small intestine: The gastric-digested pellet was mixed with 100 U/mL pancreatin and 10 mM bile salt containing 0.3 mM calcium chloride dissolved in PBS. The pH was adjusted to 7.4 and incubation lasted for 3 h.
- Large intestine: The intestinally digested pellet was combined with 30 μL of Viscozyme-L enzyme blend (cellulase, hemicellulose, arabanase, β-glucanase, and xylanase) in PBS. The pH was adjusted to 4 and incubation lasted for 16 h.
2.2.7. Cell Culture and Sample Dilution
2.2.8. Cell Viability
2.2.9. Intracellular Oxidative Status
2.2.10. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Characterization of Microencapsulated Extracts
3.2. Estimation of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
3.3. Phenolic Composition
3.4. Antiradical and Reducing Activity
3.5. Effect of Microencapsulated Guava Extracts on Biomarkers of Metabolic Diseases
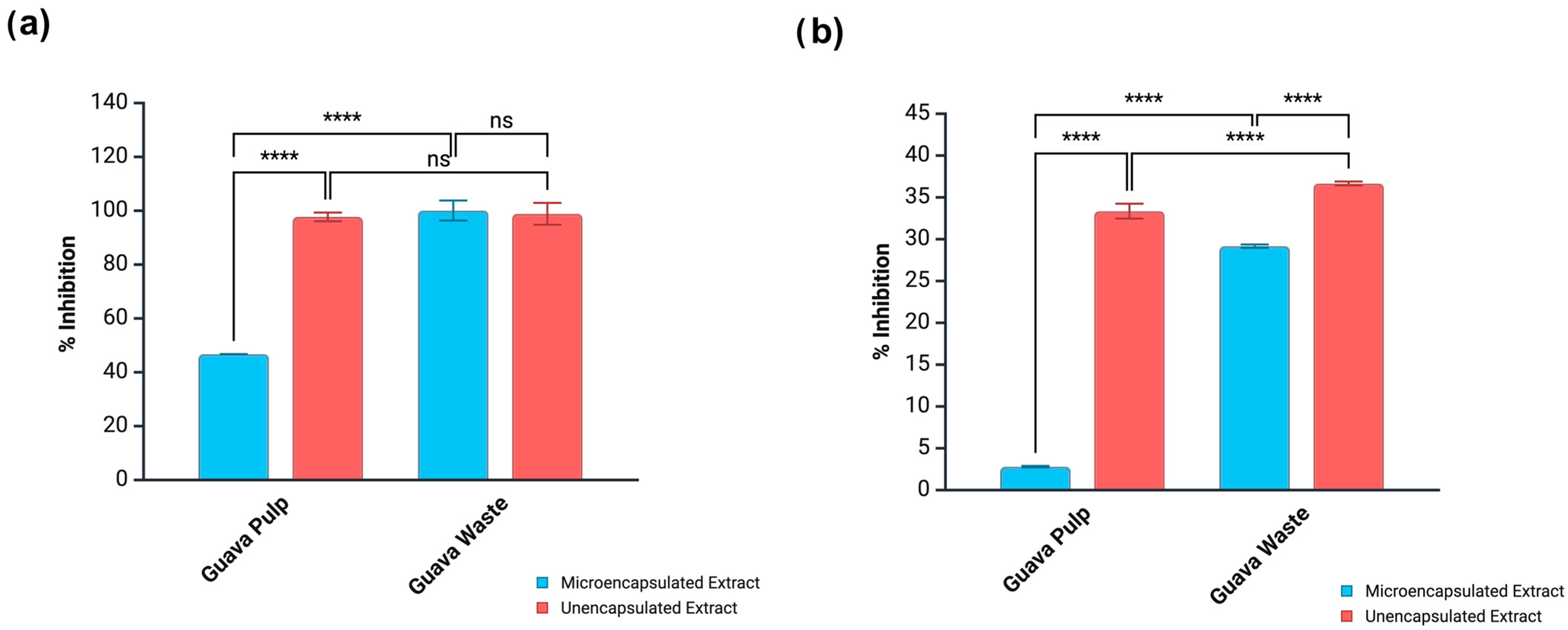
3.5.1. Inhibition of Metabolic Enzymes
3.5.2. Suppression of Oxidative Damage to LDL-Cholesterol and Supercoiled DNA
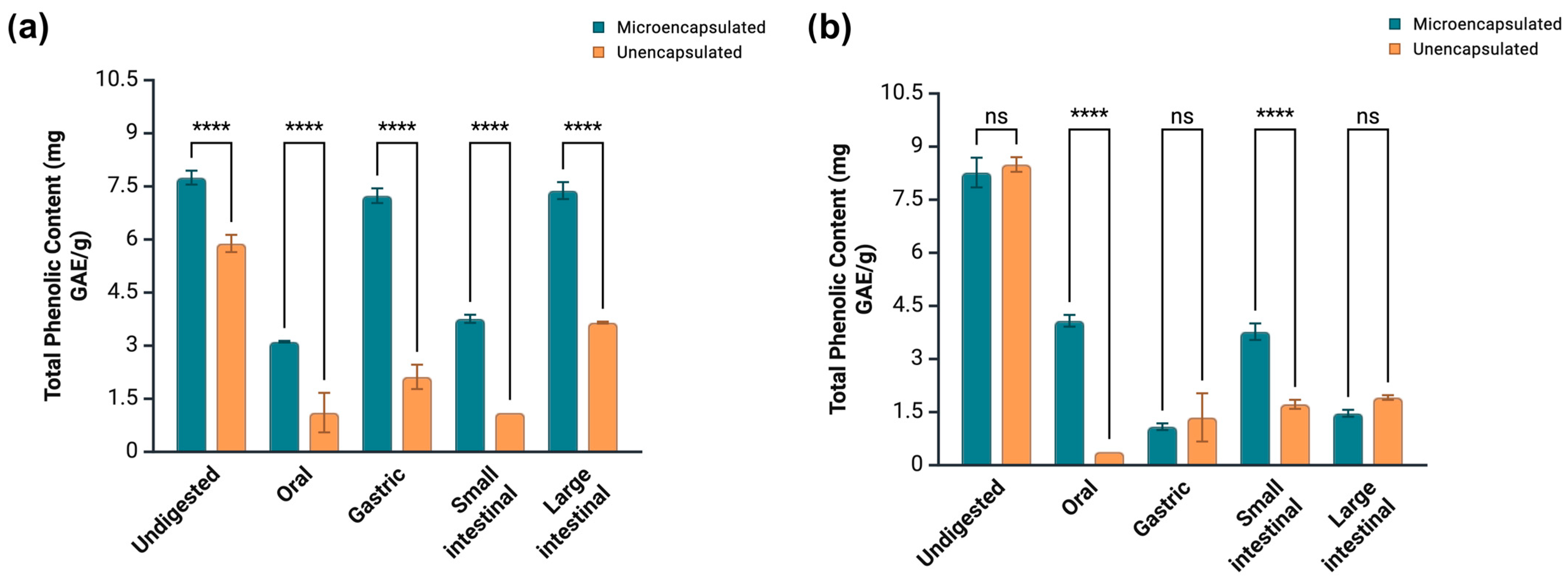
3.6. Impact of Microencapsulation on the Phenolic Bioaccessibility of Guava Extracts
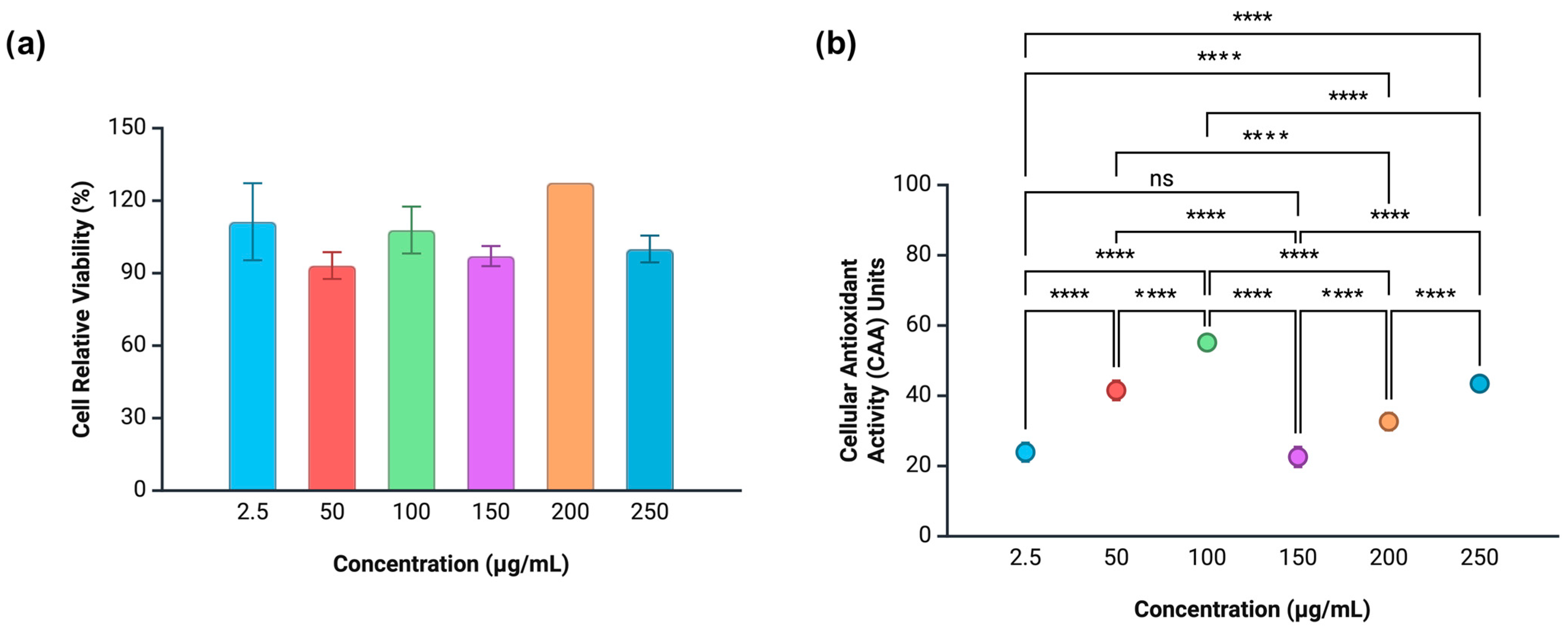
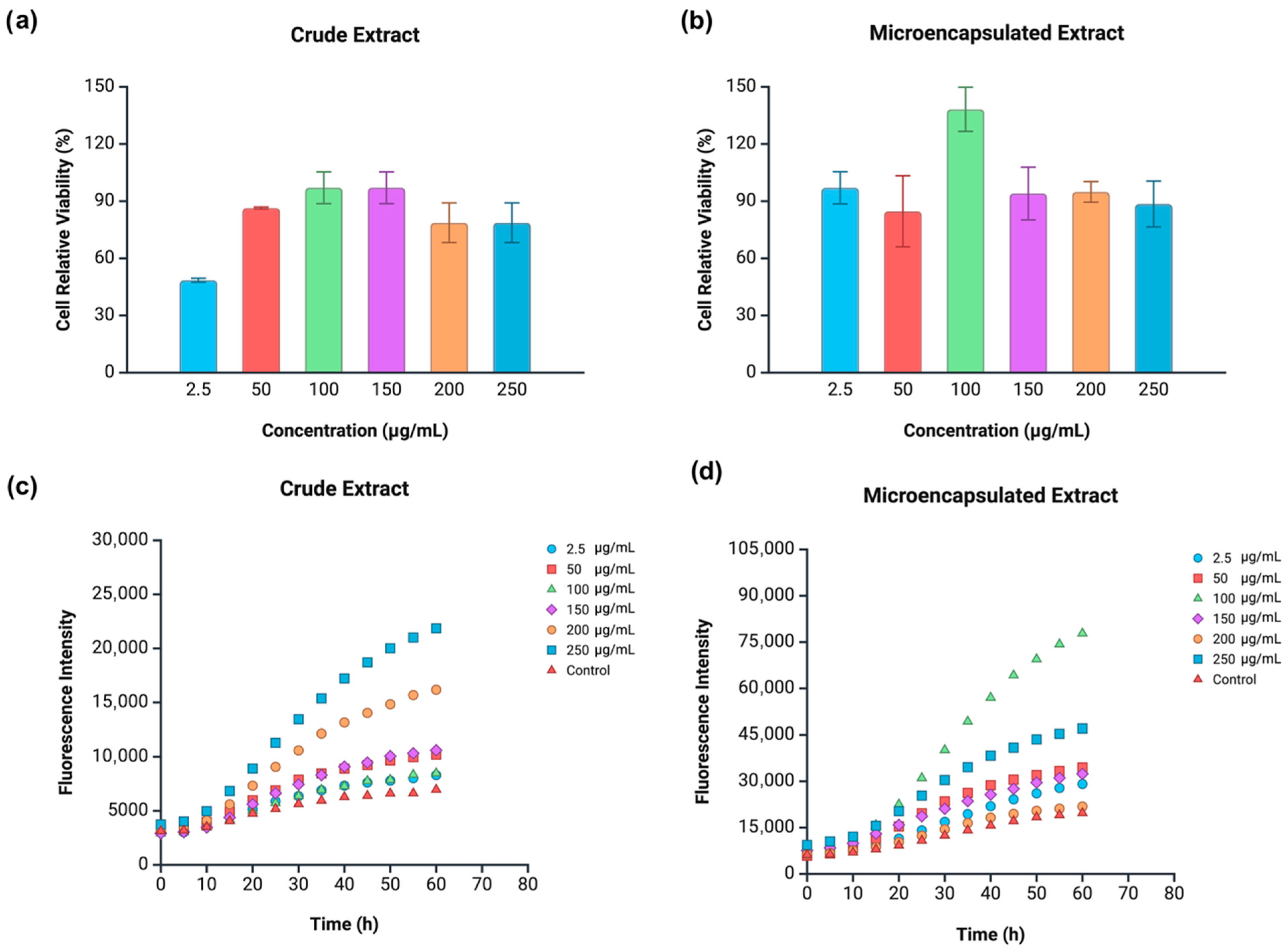
3.7. Cytotoxicity and Intracellular Antioxidant Activity
3.7.1. Caco-2 Cells
3.7.2. HeLa Cells
4. Cross-Assay Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AAE | Ascorbic acid equivalent |
| CE | Catechin equivalent |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FRAP | Ferric reducing antioxidant power |
| GAE | Gallic acid equivalent |
| GI | Gastrointestinal |
| ROS | Reactive oxygen species |
| TE | Trolox equivalent |
| TFC | Total flavonoid content |
| TPC | Total phenolic content |
References
- Angulo-López, J.E.; Flores-Gallegos, A.C.; Torres-León, C.; Ramírez-Guzmán, K.N.; Martínez, G.A.; Aguilar, C.N. Guava (Psidium guajava L.) fruit and valorization of industrialization by-products. Processes 2021, 9, 1075. [Google Scholar] [CrossRef]
- Danielski, R.; Shahidi, F. Guava processing waste: Biological activity profile of a natural and sustainable source of phenolic antioxidants. Food Biosci. 2023, 56, 103294. [Google Scholar] [CrossRef]
- Souza, H.A.; Parent, S.É.; Rozane, D.E.; Amorim, D.A.; Modesto, V.C.; Natale, W.; Parent, L.E. Guava waste to sustain guava (Psidium guajava) agroecosystem: Nutrient “balance” concepts. Front. Plant Sci. 2016, 7, 1252. [Google Scholar] [CrossRef]
- Kumar, K.; Ahmed, N.; Jan, S.; Thakur, P.; Chauhan, D.; Kaur, J. Guava wastes and by-products: Chemistry, processing, and utilization. In Handbook of Fruit Wastes and By-Products, 1st ed.; Muzaffar, K., Sofi, S.A., Mir, S.A., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 99–112. [Google Scholar]
- Danielski, R.; Shahidi, F. Effect of simulated gastrointestinal digestion on the phenolic composition and biological activities of guava pulp and processing by-products. Food Chem. 2025, 465, 142080. [Google Scholar] [CrossRef]
- Shahidi, F.; Peng, H. Bioaccessibility and bioavailability of phenolic compounds. J. Food Bioact. 2018, 4, 11–68. [Google Scholar] [CrossRef]
- Huang, K.; Yuan, Y.; Baojun, X. A critical review on the microencapsulation of bioactive compounds and their application. Food Rev. Int. 2023, 39, 2594–2634. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Methodologies for simulation of gastrointestinal digestion of different controlled delivery systems and further uptake of encapsulated bioactive compounds. Trends Food Sci. Technol. 2021, 114, 510–520. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Iwamoto, S.; Yamauchi, R. Microencapsulation: A review of applications in the food and pharmaceutical industries. Rev. Agric. Sci. 2016, 4, 56–65. [Google Scholar] [CrossRef]
- Aragones, G.; Danesi, F.; Del Rio, D.; Mena, P. The importance of studying cell metabolism when testing the bioactivity of phenolic compounds. Trends Food Sci. Technol. 2017, 69, 230–242. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Pashazadeh, H.; Zannou, O.; Ghellam, M.; Koca, I.; Galanakis, C.M.; Aldawoud, T.M. Optimization and encapsulation of phenolic compounds extracted from maize waste by freeze-drying, spray-drying, and microwave-drying using maltodextrin. Foods 2021, 10, 1396. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists International; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of grape (Vitis labrusca var. Bordo) skin phenolic extract using gum Arabic, polydextrose, and partially hydrolyzed guar gum as encapsulating agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vit. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Kitts, D.D.; Wijewickreme, A.N.; Hu, C. Antioxidant properties of a North American ginseng extract. Mol. Cell. Biochem. 2000, 203, 1–10. [Google Scholar] [CrossRef]
- Lima, R.S.; Ferreira, S.R.S.; Vitali, L.; Block, J.M. May the superfruit red guava and its processing waste be a potential ingredient in functional foods? Food Res. Int. 2019, 115, 451–459. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.; Zhang, H.Y. Estimation of scavenging activity of phenolic compounds using the ABTS•+ assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Scientific methodology: Determination of total antioxidant activity in fruits by scavenging of ABTS+ free radical. Embrapa’s Online Tech. Commun. 2007, 128, 1679–6535. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Shahidi, F. Antioxidant potential of date (Phoenix dactylifera L.) seed protein hydrolysates and carnosine in food and biological systems. J. Agric. Food Chem. 2015, 63, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Danielski, R.; Kumari, S.; Sarkar, S.; Berry, M.D.; Shahidi, F. Polyphenols from unconventional fruit by-products protect human epithelial intestinal cells from oxidative damage. Food Biosci. 2024, 62, 105302. [Google Scholar] [CrossRef]
- Kellett, M.E.; Greenspan, P.; Pegg, R.B. Modification of the cellular antioxidant activity (CAA) assay to study phenolic antioxidants in a Caco-2 cell line. Food Chem. 2018, 244, 359–363. [Google Scholar] [CrossRef]
- Silva, P.I.; Stringheta, P.C.; Teófilo, R.F.; De Oliveira, I.R.N. Parameter optimization for spray-drying microencapsulation of jaboticaba (Myrciaria jaboticaba) peel extracts using simultaneous analysis of responses. J. Food Eng. 2013, 117, 538–544. [Google Scholar] [CrossRef]
- de Souza, V.B.; Thomazini, M.; de Carvalho Balieiro, J.C.; Fávaro-Trindade, C.S. Effect of spray drying on the physicochemical properties and color stability of the powdered pigment obtained from vinification byproducts of the Bordo grape (Vitis labrusca). Food Bioprod. Process. 2015, 93, 39–50. [Google Scholar] [CrossRef]
- Oxley, J. Overview of microencapsulation process technologies. In Microencapsulation in the Food Industry, 2nd ed; Sobel, R., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 35–46. [Google Scholar]
- Abd Ghani, A.; Adachi, S.; Shiga, H.; Neoh, T.L.; Adachi, S.; Yoshii, H. Effect of different dextrose equivalents of maltodextrin on oxidation stability in encapsulated fish oil by spray drying. Biosci. Biotechnol. Biochem. 2017, 81, 705–711. [Google Scholar] [CrossRef]
- Comunian, T.A.; Drusch, S.; Brodkorb, A. Advances of plant-based structured food delivery systems on the in vitro digestibility of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2022, 62, 6485–6504. [Google Scholar] [CrossRef]
- Ahmadian, Z.; Niazmand, R.; Pourfarzad, A. Microencapsulation of saffron petal phenolic extract: Their characterization, in vitro gastrointestinal digestion, and storage stability. J. Food Sci. 2019, 84, 2745–2757. [Google Scholar] [CrossRef]
- Zymone, K.; Benetis, R.; Trumbeckas, D.; Baseviciene, I.; Trumbeckaite, S. Different effects of quercetin glycosides and quercetin on kidney mitochondrial function—Uncoupling, cytochrome C reducing and antioxidant activity. Molecules 2022, 27, 6377. [Google Scholar] [CrossRef]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Antioxidant, alpha-glucosidase inhibition activities, in silico molecular docking and pharmacokinetics study of phenolic compounds from native australian fruits and spices. Antioxidants 2023, 12, 254. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Oteiza, P.I. Proanthocyanidins at the gastrointestinal tract: Mechanisms involved in their capacity to mitigate obesity-associated metabolic disorders. Crit. Rev. Food Sci. Nutr. 2024, 64, 220–240. [Google Scholar] [CrossRef]
- Subramaniyan, V.; Hanim, Y.U. Role of pancreatic lipase inhibition in obesity treatment: Mechanisms and challenges towards current insights and future directions. Int. J. Obes. 2025, 49, 492–506. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Alvarez-Parrilla, E.; Díaz-Sánchez, Á.G.; Rosa, L.D.L.; Núñez-Gastélum, J.A.; Vazquez-Flores, A.A.; Gonzalez-Aguilar, G.A. In vitro inhibition of pancreatic lipase by polyphenols: A kinetic, Fluorescence spectroscopy and molecular docking study. Food Technol. Biotechnol. 2017, 55, 519–530. [Google Scholar] [CrossRef]
- Ahmadi, A.; Jamialahmadi, T.; Sahebkar, A. Polyphenols and atherosclerosis: A critical review of clinical effects on LDL oxidation. Pharmacol. Res. 2022, 184, 106414. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Ibarz, R.; Fernandes, J.M.; Pinheiro, A.C.; Botelho, C.; Rocha, C.M.; Teixeira, J.A.; Martín-Belloso, O. Encapsulated pine bark polyphenolic extract during gastrointestinal digestion: Bioaccessibility, bioactivity and oxidative stress prevention. Foods 2021, 10, 328. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Y.; Wan, Y.; Zou, Q.; Shen, L.; Fu, G.; Gong, E.S. Effect of pretreatments of camellia seeds on the quality, phenolic profile, and antioxidant capacity of camellia oil. Front. Nutr. 2022, 9, 1023711. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Damas, A.M.; Martins, P.; Rocha, F. Study of the inhibition effect on the microencapsulated enzyme β-galactosidase. Environ. Eng. Manag. J. 2012, 11, 1923–1930. [Google Scholar] [CrossRef]
- Mahmutović, L.; Sezer, A.; Bilajac, E.; Hromić-Jahjefendić, A.; Uversky, V.N.; Glamočlija, U. Polyphenol stability and bioavailability in cell culture medium: Challenges, limitations and future directions. Int. J. Biol. Macromol. 2024, 279, 135232. [Google Scholar] [CrossRef] [PubMed]



| Sample | Moisture (%) | Hygroscopicity (%) | Particle Size (µm) | Polydispersity Index | Encapsulation Efficiency (%) * | Payload (%) |
|---|---|---|---|---|---|---|
| Guava Pulp | 10.1 ± 0.01 a | 16.2 ± 0.5 a | 4.0 ± 1.1 a | 0.134 ± 0.01 b | 89.5 ± 7.1 a | 51.0 ± 4.5 a |
| Guava Waste | 8.9 ± 1.0 b | 15.3 ± 1.6 b | 3.1 ± 0.01 b | 0.733 ± 0.01 a | 79.3 ± 0.4 b | 26.0 ± 2.2 b |
| Phenolic Compound | [M-H]− (m/z) | RT (min) | MS2 Ion Fragments | GP-M | GP-U | GW-M | GW-U |
|---|---|---|---|---|---|---|---|
| Phenolic acids | |||||||
| trans-Cinnamic acid + | 147 | 8.6 | 126, 137 | 25.01 ± 1.2 c | - | 32.45 ± 6.3 b | 23.37 ± 2.3 a |
| Protocatechuic acid + | 153 | 6.8 | 138 | - | - | tr | 19.49 ± 2.9 b |
| Ferulic acid + | 193 | 10.3 | 133, 173 | 15.64 ± 0.1 d | - | 18.14 ± 5.0 d | - |
| Sinapic acid + | 223 | 1.067 | 162 | 11.11 ± 0.5 e | - | - | - |
| Ellagic acid + | 300 | 11.2 | 187, 263 | 24.11 ± 2.3 c | 46.29 ± 4.4 a | 26.15 ± 0.9 c | - |
| 1-O-(4-Coumaroyl)-glucose | 325 | 7.7 | 163 | - | - | 3.773 ± 0.3 g | - |
| p-Coumaroyl malonyldihexoside | 411 | 3.0 | 137, 205, 251, 375 | 44.95 ± 6.2 a | - | 49.69 ± 7.8 a,b | - |
| Flavonoids | |||||||
| Pinocembrin | 255 | 11.3 | 112, 159 | - | - | - | - |
| (+)-Catechin + | 289 | 8.8 | 245 | tr | - | - | - |
| Quercetin + | 301 | 10.8 | 113, 127, 137, 139, 159, 183, 217 | - | - | 4.540 ± 0.7 f | - |
| Epicatechin gallate | 457 | 6.6 | 355, 411 | - | - | 14.82 ± 1.4 e | - |
| Ellagitannin | |||||||
| Ellagic acid derivative | 389 | 13.6 | 300 | 29.61 ± 1.2 b | 21.12 ± 1.5 b | 52.20 ± 7.7 a | - |
| Proanthocyanidin | |||||||
| B-type proanthocyanidin trimer | 897 | 12.6 | 137, 249, 339, 448.3, 554, 746, 840 | - | - | tr | - |
| Total (μg/g) | 175.5 | 437.7 | 201.8 | 210.98 | |||
| Sample | DPPH Scavenging Activity (μmol TE/g) | ABTS Scavenging Activity (μmol AAE/g) | FRAP (μmol AAE/g) |
|---|---|---|---|
| Guava Pulp | |||
| Crude extract | 17.1 ± 5 a | 89.4 ± 1 a | 77.0 ± 0.2 c |
| Microencapsulated extract | 8.53 ± 0.5 b | 71.6 ± 2 c | 98.9 ± 0.8 b |
| Guava Waste | |||
| Crude extract | 6.73 ± 0.5 c | 75.5 ± 0.4 c | 106.6 ± 1 a |
| Microencapsulated extract | 8.21 ± 1 b | 82.5 ± 3 b | 97.9 ± 0.8 b |
| Sample | Digestion Stage | DPPH Scavenging Activity (μmol TE/g) | ABTS Scavenging Activity (μmol AAE/g) | FRAP (μmol AAE/g) |
|---|---|---|---|---|
| Microencapsulated Guava Pulp Extract | Oral | 27.4 ± 5 b | 29.6 ± 0.1 d | 10.8 ± 0.2 b |
| Gastric | 33.1 ± 1 a | 40.8 ± 0.1 c | 12.2 ± 2 b | |
| Small intestine | 6.69 ± 0.4 c | 193.4 ± 3 a | 7.28 ± 0.3 c | |
| Large intestine | 25.7 ± 1 b | 135.5 ± 2 b | 19.0 ± 2 a | |
| Microencapsulated Guava Waste Extract | Oral | 35.7 ± 0.2 a | 31.2 ± 2 d | 10.5 ± 0.1 b |
| Gastric | 33.3 ± 3 a | 42.5 ± 0.4 c | 11.9 ± 0.2 a | |
| Small intestine | 12.6 ± 3 c | 198.7 ± 3 a | 11.4 ± 0.4 a,b | |
| Large intestine | 19.4 ± 2 b | 131.2 ± 2 b | 12.1 ± 0.5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danielski, R.; Kumari, S.; Kakumani, P.K.; Shahidi, F. Microencapsulation Enhances the Biological Potential, Bioaccessibility, and Intracellular Oxidative Status of Guava Phenolic Extracts. Antioxidants 2025, 14, 1334. https://doi.org/10.3390/antiox14111334
Danielski R, Kumari S, Kakumani PK, Shahidi F. Microencapsulation Enhances the Biological Potential, Bioaccessibility, and Intracellular Oxidative Status of Guava Phenolic Extracts. Antioxidants. 2025; 14(11):1334. https://doi.org/10.3390/antiox14111334
Chicago/Turabian StyleDanielski, Renan, Sarika Kumari, Pavan Kumar Kakumani, and Fereidoon Shahidi. 2025. "Microencapsulation Enhances the Biological Potential, Bioaccessibility, and Intracellular Oxidative Status of Guava Phenolic Extracts" Antioxidants 14, no. 11: 1334. https://doi.org/10.3390/antiox14111334
APA StyleDanielski, R., Kumari, S., Kakumani, P. K., & Shahidi, F. (2025). Microencapsulation Enhances the Biological Potential, Bioaccessibility, and Intracellular Oxidative Status of Guava Phenolic Extracts. Antioxidants, 14(11), 1334. https://doi.org/10.3390/antiox14111334







