Innovative Plant-Based Nutraceuticals: Enhancing Iron Bioavailability to Address Iron Deficiency Anaemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Development of Nutritional Formula
2.1.1. Literature Review
2.1.2. Preparation of Plant Foodstuffs and Nutraceuticals
2.2. Chemical Analysis of Plant-Based Foodstuffs and Nutraceuticals
2.2.1. Phenolic Profile Determination
2.2.2. Total Phytic Acid Content
2.2.3. Total Iron Content
2.3. Antioxidant Potential of Plant-Based Foodstuffs and Nutraceuticals
2.3.1. DPPH Assay
2.3.2. FRAP Assay
2.4. Experimental Animals
2.4.1. In Vivo Study on the Effects of the Nutraceuticals on Iron Bioavailability from Oral Iron Supplementation When Applied Simultaneously
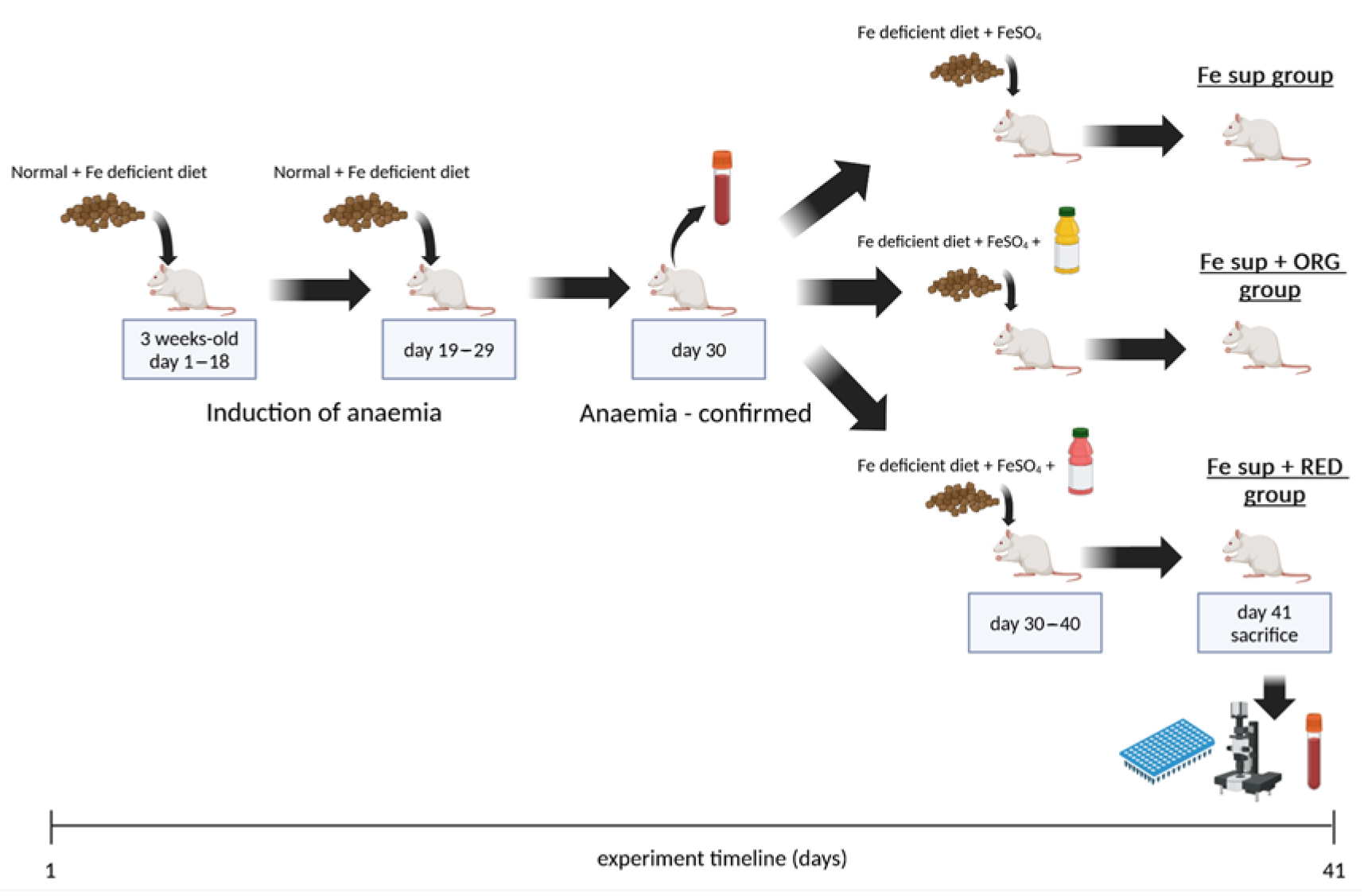
2.4.2. In Vivo Study on the Effects of the Nutraceutical ORG on Iron Bioavailability
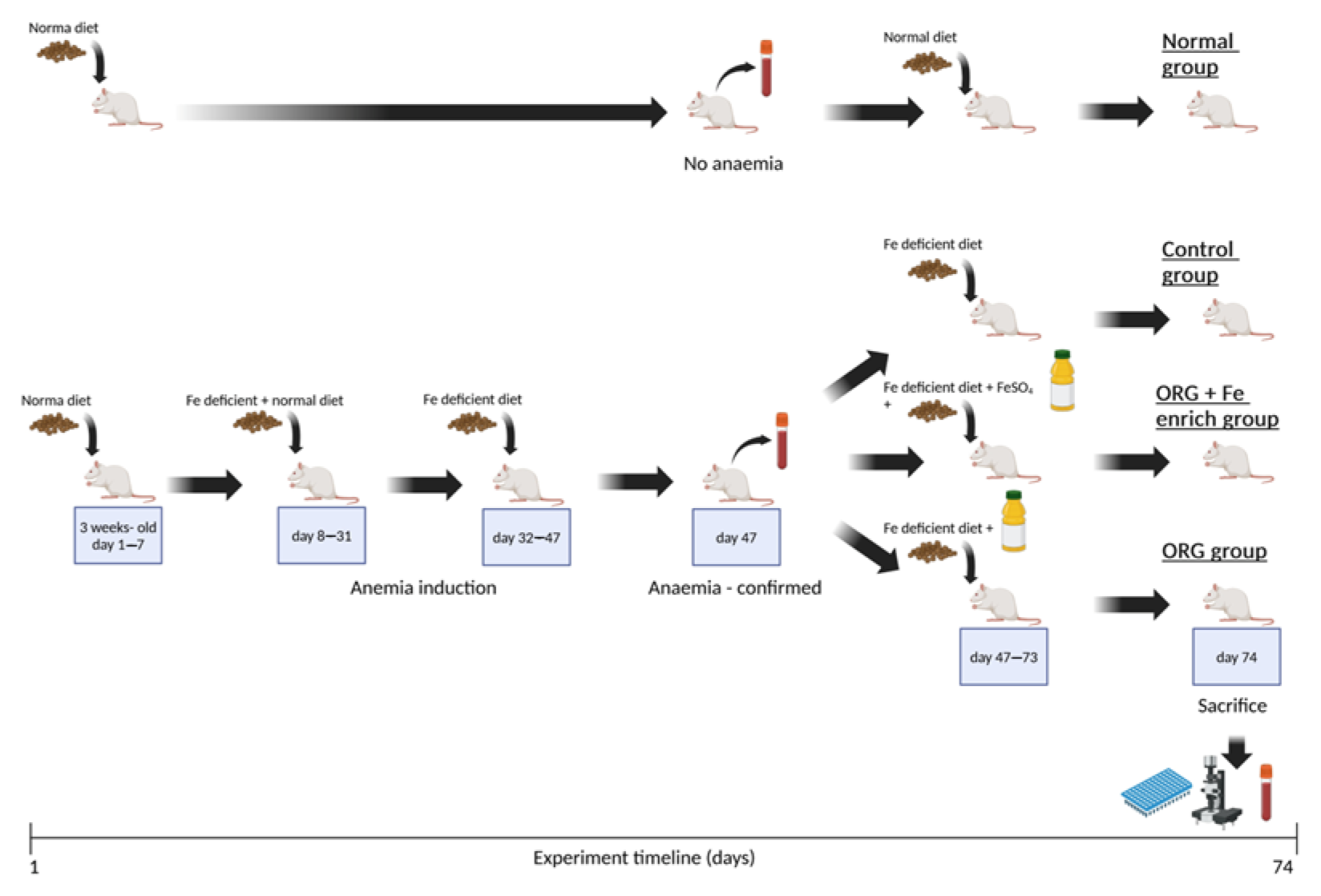
2.5. Analysis of Blood Samples
2.6. Histopathological Analysis
2.7. Non-Haem Tissue Iron
2.8. RNA Extraction and RT-PCR
2.9. Statistics
3. Results
3.1. Design of Nutritional Formula and Nutraceuticals
| Compound | Plant-Based Foodstuffs | Nutraceuticals | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WPB ng/g | BEE ng/mL | KIW ng/mL | PIN ng/mL | BSB ng/g | MEL ng/mL | CIN ng/g | HON ng/g | ORG ng/mL | RED ng/mL | |
| Phenolic acids | ||||||||||
| p-Hydroxybenzoic acid | <LoQ b | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 12,808.04 ± 514.25 | 3645.60 ± 177.32 | 123.33 ± 5.56 a c | 41.38 ± 2.78 b |
| Cinnamic acid | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 494,341.61 ± 2833.43 | <LoQ | 1597.42 ± 47.47 | <LoQ |
| Protocatechuic acid | 14.93 ± 0.38 | <LoQ | 18.99 ± 1.74 | <LoQ | 11.68 ± 0.52 | <LoQ | 137,959.64 ± 866.64 | 568.76 ± 28.32 | 461.21 ± 42.83 a | 54.44 ± 0.60 b |
| Gentisic acid | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 371.35 ± 5.13 | <LoQ | <LoQ | <LoQ |
| p-Coumaric acid | <LoQ | 12.56 ± 1.07 | 7.97 ± 0.74 | <LoQ | 13.87 ± 0.98 | 33.81 ± 0.77 | 2778.11 ± 289.13 | 944.87 ± 36.11 | 179.50 ± 9.47 a | 28.67 ± 2.07 b |
| o-Coumaric acid | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 9208.65 ± 675.49 | <LoQ | 21.89 ± 1.55 | <LoQ |
| Gallic acid | <LoQ | <LoQ | <LoQ | 27.63 ± 1.52 | <LoQ | <LoQ | 2734.83 ± 125.74 | 382.64 ± 32.86 | 26.84 ± 2.25 a | 23.41 ± 1.12 b |
| Vanillic acid | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 222.23 ± 13.83 | 5394.00 ± 478.74 | <LoQ | <LoQ | 168.82 ± 11.76 |
| Caffeic acid | 95.06 ± 3.78 | 17.42 ± 1.58 | 73.42 ± 6.78 | <LoQ | <LoQ | <LoQ | 319.40 ± 25.24 | 950.55 ± 48.29 | 355.73 ± 32.10 b | 1427.64 ± 52.11 a |
| 3,4-Dimethoxycinnamic acid | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 2156.78 ± 134.52 | <LoQ | <LoQ |
| Ferulic acid | 90.98 ± 6.33 | 4638.78 ± 383.12 | <LoQ | <LoQ | <LoQ | <LoQ | 3285.53 ± 140.67 | <LoQ | 118.69 ± 8.44 a | 122.39 ± 11.13 a |
| Syringic acid | <LoQ | 976.67 ± 57.76 | <LoQ | <LoQ | <LoQ | <LoQ | 6745.86 ± 334.56 | <LoQ | 45.76 ± 1.87 b | 50.13 ± 2.48 a |
| 5-O-caffeoylquinic acid (chlorogenic acid) | 10,075.24 ± 831.08 | 43.58 ± 3.18 | 47.76 ± 3.69 | 27.97 ± 2.12 | 59.32 ± 2.52 | <LoQ | 1663.97 ± 45.88 | 333.01 ± 22.21 | 4679.01 ± 62.64 a | 10,812.91 ± 105.22 b |
| Flavonoids | ||||||||||
| Catechin | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 49,249.49 ± 2589.33 | <LoQ | <LoQ | <LoQ |
| Epicatechin | <LoQ | <LoQ | 2537.28 ± 218.30 | <LoQ | <LoQ | <LoQ | 16,008.53 ± 136.99 | <LoQ | <LoQ | 239.62 ±17.20 |
| Kaempferol 3-O-glucoside | <LoQ | <LoQ | 82.29 ± 7.33 | 43.54 ± 2.33 | <LoQ | <LoQ | 749.08 ± 6.31 | <LoQ | 7.02 ± 0.61 a | 20.72 ± 0.72 b |
| Chrysoeriol | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 210.74 ± 2.07 | <LoQ |
| Quercetin 3-O-glucoside | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 617.85 ± 21.99 | <LoQ | <LoQ | 33.38 ± 0.39 |
| Quercetin 3-O-galactoside | <LoQ | <LoQ | 133.42 ± 11.76 | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ |
| Rutin | 14.13 ± 1.12 | <LoQ | 265.62 ± 18.76 | <LoQ | <LoQ | <LoQ | 153.81 ± 11.52 | 127.53 ± 10.64 | 302.19 ± 4.14 a | 659.78 ± 12.44 a |
| Apigenin 3-O-glucoside | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 91.15 ± 7.67 | <LoQ | <LoQ | <LoQ |
| Appin | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 84.89 ± 7.58 | <LoQ | <LoQ | <LoQ |
| Quercitrin | <LoQ | <LoQ | 996.26 ± 38.76 | <LoQ | <LoQ | <LoQ | 4611.08 ± 34.56 | <LoQ | 14.66 ± 1.66 a | 344.16 ± 3.02 b |
| Naringenin | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 599.08 ± 21.98 | 147.75 ± 9.35 | <LoQ | <LoQ |
| Coumarines | ||||||||||
| Umbelliferone | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 139.23 ± 2.21 | <LoQ | <LoQ | <LoQ |
| Skopoletin | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 397.81 ± 31.44 | <LoQ | <LoQ | 32.29 ± 2.41 |
| Aesculetin | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 146.56 ± 9.45 | <LoQ | 77.01 ± 4.38 |
| Lignane | ||||||||||
| Secoisolariciresinol | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | 4468.53 ± 256.44 | <LoQ | <LoQ | <LoQ |
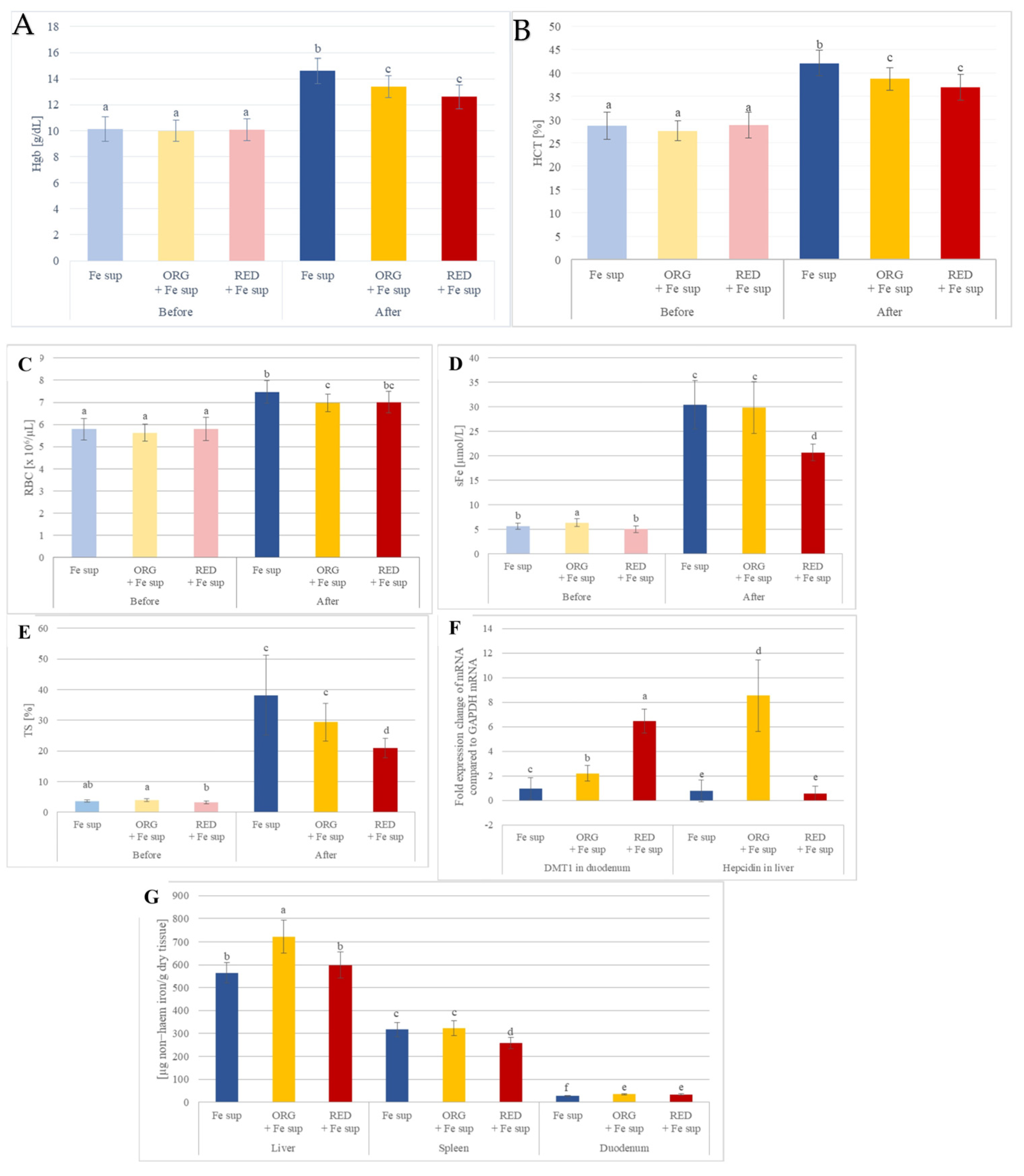
3.2. Effects of Nutraceuticals on the In Vivo Iron Bioavailability from Oral Iron Supplementation When Applied Simultaneously
3.3. Effects of Nutraceutical ORG on In Vivo Iron Bioavailability
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BEE | beetroot cold-pressed juice |
| BSB | butternut squash boiled |
| CIN | cinnamon powder |
| Dcytb | duodenal cytochrome B |
| DL | below detection limit |
| DMT1 | divalent metal transporter 1 |
| FPN | ferroportin |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| H&E | hematoxylin and eosin |
| HCT | haematocrit |
| Hgb | haemoglobin |
| HON | acacia honey |
| HPF | high power fields |
| IDA | iron deficiency anaemia |
| IP | intraperitoneal injection |
| KIW | kiwi cold-pressed juice |
| LoQ | below instrument quantification limit |
| MCH | mean corpuscular haemoglobin |
| MCHC | mean corpuscular haemoglobin concentration |
| MCV | mean corpuscular volume |
| MEL | melon cold-pressed juice |
| ORG | final food product, named orange smoothie |
| PIN | pineapple cold-pressed juice |
| PLT | platelet count |
| RBC | red blood cells |
| RDW | red cell distribution width |
| RED | final food product, named red smoothie |
| SEM | standard error of the mean |
| TIBC | total iron-binding capacity |
| TS | transferrin saturation |
| UIBC | unsaturated iron-binding capacity |
| WBC | white blood cell |
| WHO | World Health Organisation |
| WPB | white potato boiled |
References
- WHO. The Global Prevalence of Anaemia in 2011; WHO Document Production Services: Geneva, Switzerland, 2015. [Google Scholar]
- WHO. Accelerating Anaemia Reduction. A Comprehensive Framework for Action; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Lozoff, B.; Beard, J.; Connor, J.; Barbara, F.; Georgieff, M.; Schallert, T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006, 64, S34–S91. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Lee, A.C.; Kozuki, N.; Lawn, J.E.; Cousens, S.; Blencowe, H.; Ezzati, M.; Bhutta, Z.A.; Marchant, T.; Willey, B.A.; et al. CHERG Small-for-Gestational-Age-Preterm Birth Working Group. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: A pooled country analysis. Lancet 2013, 382, 417–425. [Google Scholar] [CrossRef]
- Stangret, A.; Wnuk, A.; Szewczyk, G.; Pyzlak, M.; Szukiewicz, D. Maternal hemoglobin concentration and hematocrit values may affect fetus development by influencing placental angiogenesis. J. Matern. Fetal Neonatal Med. 2017, 30, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.M.G.; Gomes-Filho, I.S.; Silva, R.B.; Pereira, P.P.S.; Mata, F.A.F.D.; Lyrio, A.O.; Souza, E.S.; Cruz, S.S.; Pereira, M.G. Maternal Anemia and Low Birth Weight: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.M.G.; Gomes-Filho, I.S.; Batista, J.E.T.; Orrico, G.S.; Porto, E.C.L.; Cruz Pimenta, R.M.; Dos Santos Conceição, S.; Brito, S.M.; Ramos, M.S.X.; Sena, M.C.F.; et al. Maternal anemia and birth weight: A prospective cohort study. PLoS ONE 2019, 14, e0212817. [Google Scholar] [CrossRef]
- Symington, E.A.; Baumgartner, J.; Malan, L.; Wise, A.J.; Ricci, C.; Zandberg, L.; Smuts, C.M. Maternal iron-deficiency is associated with premature birth and higher birth weight despite routine antenatal iron supplementation in an urban South African setting: The NuPED prospective study. PLoS ONE 2019, 14, e0221299. [Google Scholar] [CrossRef]
- Haas, J.D.; Brownlie, T. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J. Nutr. 2001, 131, 676S–690S. [Google Scholar] [CrossRef]
- Horton, S.; Ross, J. The economics of iron deficiency. Food Policy 2003, 28, 51–75, Erratum in Food Policy 2007, 32, 141–143. [Google Scholar] [CrossRef]
- Strauss, W.E.; Auerbach, M. Health-related quality of life in patients with iron deficiency anemia: Impact of treatment with intravenous iron. Patient Relat. Outcome Meas. 2018, 9, 285–298. [Google Scholar] [CrossRef]
- Kinyoki, D.; Osgood-Zimmerman, A.E.; Bhattacharjee, N.V.; Hay, S.I.; Local Burden of Disease Anaemia Collaborators. Anemia prevalence in women of reproductive age in low- and middle-income countries between 2000 and 2018. Nat. Med. 2021, 27, 1761–1782. [Google Scholar] [CrossRef]
- WHO. Global Nutrition Target 2025: Anaemia Policy Brief (WHO/NMH/NHD/14.4); WHO: Geneva, Switzerland, 2014. [Google Scholar]
- WHO/UNICEF. WHO/UNICEF Discussion Paper, The Extension of the 2025 Maternal, Infant and Young Child Nutrition Targets to 2030; WHO/UNICEF: Geneva, Switzerland, 2021. [Google Scholar]
- Christian, P. Anemia in women—An intractable problem that requires innovative solutions. Nat. Med. 2021, 27, 1675–1677. [Google Scholar] [CrossRef]
- GBD 2021 Anaemia Collaborators. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990-2021: Findings from the Global Burden of Disease Study 2021. Lancet Haematol. 2023, 10, e713–e734, Erratum in Lancet Haematol. 2023, 10, e796. Erratum in Lancet Haematol. 2024, 11, e10. [Google Scholar] [CrossRef]
- Kurtoglu, E.; Ugur, A.; Baltaci, A.K.; Undar, L. Effect of Iron Supplementation on Oxidative Stress and Antioxidant Status in Iron-Deficiency Anemia. Biol. Trace Elem. Res. 2003, 96, 117–123. [Google Scholar] [CrossRef]
- Yoo, J.-H.; Maeng, H.-Y.; Sun, Y.-K.; Kim, Y.-A.; Park, D.-W.; Park, T.S.; Lee, S.T.; Choi, J.-R. Oxidative Status in Iron-Deficiency Anemia. J. Clin. Lab. Anal. 2009, 23, 319–323. [Google Scholar] [CrossRef]
- Yadav, K.; Mishra, O.P.; Khandhadiya, K.; Mishra, S.P.; Mishra, A.; Singh, A.; Agrawal, R.K.; Sanghvi, N.; Mishra, A. Markers of Oxidative Stress in Children with Iron Deficiency Anemia. Pediatr. Hematol. Oncol. J. 2024, 9, 9–14. [Google Scholar] [CrossRef]
- Galloway, R.; Dusch, E.; Elder, L.; Achadi, E.; Grajeda, R.; Hurtado, E.; Favin, M.; Kanani, S.; Marsaban, J.; Meda, N.; et al. Women’s perceptions of iron deficiency and anemia prevention and control in eight developing countries. Soc. Sci. Med. 2002, 55, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Cancelo-Hidalgo, M.J.; Castelo-Branco, C.; Palacios, S.; Haya-Palazuelos, J.; Ciria-Recasens, M.; Manasanch, J.; Pérez-Edo, L. Tolerability of different oral iron supplements: A systematic review. Curr. Med. Res. Opin. 2013, 29, 291–303. [Google Scholar] [CrossRef]
- Fouelifack, F.Y.; Sama, J.D.; Sone, C.E. Assessment of adherence to iron supplementation among pregnant women in the Yaounde gynaeco-obstetric and paediatric hospital. Pan Afr. Med. J. 2019, 34, 211. [Google Scholar] [CrossRef] [PubMed]
- Akarsu, S.; Demır, H.; Selek, S.; Oguzoncul, F. Iron Deficiency Anemia and Levels of Oxidative Stress Induced by Treatment Modality. Pediatr. Int. 2013, 55, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Lesjak, M.; Hoque, R.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.; Sharp, P.A. Quercetin inhibits intestinal iron absorption and ferroportin transporter expression in vivo and in vitro. PLoS ONE 2014, 9, e102900. [Google Scholar] [CrossRef]
- Lesjak, M.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.S. Quercetin inhibits intestinal non-haem iron absorption by regulating iron metabolism genes in the tissues. Eur. J. Nutr. 2019, 58, 743–753. [Google Scholar] [CrossRef]
- Petry, N.; Egli, I.; Gahutu, J.B.; Tugirimana, P.L.; Boy, E.; Hurrell, R. Phytic acid concentration influences iron bioavailability from biofortified beans in Rwandese women with low iron status. J. Nutr. 2014, 144, 1681–1687, Erratum in J. Nutr. 2015, 145, 1973. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Opportunities for plant-derived enhancers for iron, zinc, and calcium bioavailability: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 652–685. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.; Egli, I.; Campion, B.; Nielsen, E.; Hurrell, R. Genetic reduction of phytate in common bean (Phaseolus vulgaris L.) seeds increases iron absorption in young women. J. Nutr. 2013, 143, P1219–P1224. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.H.R.; Thankachan, P.; Hatakayama, M.; Hiremath, N.; Moretti, D.; Nanjareddy, Y.A.; Thumilan, M.B.; Ravikumar, R.L.; Phadnis, S.; Bose, B.; et al. A Natural Low Phytic Acid Finger Millet Accession Significantly Improves Iron Bioavailability in Indian Women. Front. Nutr. 2022, 8, 791392. [Google Scholar] [CrossRef]
- Petry, N. Polyphenols and low iron bioavailability. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zabadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; Chapter 24; pp. 311–322. [Google Scholar]
- Petry, N.; Egli, I.; Zeder, C.; Walczyk, T.; Hurrell, R. Polyphenols and Phytic Acid Contribute to the Low Iron Bioavailability from Common Beans in Young Women. J. Nutr. 2010, 140, 1977–1982. [Google Scholar] [CrossRef]
- Disler, P.B.; Lynch, S.R.; Charlton, R.W.; Torrance, J.D.; Bothwell, T.H.; Walker, R.B.; Mayet, F. The Effect of Tea on Iron Absorption. Gut 1975, 16, 193–200. [Google Scholar] [CrossRef]
- Siegenberg, D.; Baynes, R.D.; Bothwell, T.H.; Macfarlane, B.J.; Lamparelli, R.D.; Car, N.G.; MacPhail, P.; Schmidt, U.; Tal, A.; Mayet, F. Ascorbic acid prevents the dose-dependent inhibitory effects of polyphenols and phytates on nonheme-iron absorption. Am. J. Clin. Nutr. 1991, 53, 537–541. [Google Scholar] [CrossRef]
- Deng, J.; Ramelli, L.; Li, P.Y.; Eshaghpour, A.; Li, A.; Schuenemann, G.; Crowther, M.A. Efficacy of Vitamin C with Fe Supplementation in Patients with Iron Deficiency Anemia: A Systematic Review and Meta-Analysis. Blood Vessel. Thromb. Hemost. 2024, 1, 100023. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.; Haytowitz, D. USDA Database for the Flavonoid Content of Selected Foods, Release 3.3 (March 2018); Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA: Beltsville, MD, USA, 2022. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database J. Biol. Databases Curation 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Haytowitz, D.B.; Jaspreet, A.K.C.; Pehrsson, P.R.; Roseland, J.M.; Wu, X.; Khan, M.; Li, Y.; Nickle, M.; Nguyen, Q.; Patterson, K.; et al. Composition of Foods: Raw, Processed, Prepared; USDA National Nutrient Database for Standard Reference, Legacy (2018); Documentation and User Guidez; USDA ARS, Beltswile Human Nutrition Research Center, Nutrient Data Laboratory: Beltsville, MD, USA, 2019. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Orčić, D.; Anačkov, G.; Balog, K.; Francišković, M.; Mimica-Dukić, N. Juniperus sibirica Burgsdorf. as a novel source of antioxidant and anti-inflammatory agents. Food Chem. 2011, 124, 850–856. [Google Scholar] [CrossRef]
- Orčić, D.; Francišković, M.; Bekvalac, K.; Svirčev, E.; Beara, I.; Lesjak, M.; Mimica-Dukić, N. Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef]
- No. 41/2009-191; Law on Animal Welfare. Official Gazette of Republic of Serbia: Belgrade, Serbia, 2009.
- Directive 2010/63/EU of the European Parliament and of the Council. In Off. J. Eur. Union; 2010; Volume 276, pp. 33–76. Available online: http://data.europa.eu/eli/dir/2010/63/oj (accessed on 27 October 2025).
- Torrance, J.D.; Bothwell, T.H. Tissue iron stores. In Methods in Hematology; Cook, J.D., Ed.; Churchill Livingstone: New York, NY, USA, 1980; pp. 90–115. [Google Scholar]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef]
- Ning, S.; Zeller, M.P. Management of iron deficiency. Hematology Am. Soc. Hematol. Educ. Program. 2019, 2019, 315–322. [Google Scholar] [CrossRef]
- National Institute of Health. Available online: https://ods.od.nih.gov/factsheets/Iron-Consumer/ (accessed on 15 November 2023).
- Burgos, G.; Liria, R.; Zeder, C.; Kroon, P.A.; Hareau, G.; Penny, M.; Dainty, J.; Al-Jaibaji, O.; Boy, E.; Mithen, R.; et al. Total iron absorbed from iron-biofortified potatoes is higher than that from nonbiofortified potatoes: A randomized trial using stable iron isotopes in women from the Peruvian highlands. J. Nutr. 2023, 153, 1710–1717. [Google Scholar] [CrossRef]
- FoodData Central, U.S. Departement of Agriculture. Available online: https://fdc.nal.usda.gov/fdc-app.html#/?component=1089 (accessed on 15 November 2023).
- Hynes, M.J.; O’Coinceanainn, M. The Kinetics and Mechanisms of Reactions of Iron(III) with Caffeic Acid, Chlorogenic Acid, Sinapic Acid, Ferulic Acid and Naringin. J. Inorg. Biochem. 2004, 98, 1457–1464. [Google Scholar] [CrossRef]
- Kose, T.; Sharp, P.A.; Latunde-Dada, G.O. Phenolic Acids Rescue Iron-Induced Damage in Murine Pancreatic Cells and Tissues. Molecules 2023, 28, 4084. [Google Scholar] [CrossRef] [PubMed]
- Živanović, N.; Lesjak, M.; Simin, N.; Srai, S.K.S. Beyond Mortality: Exploring the Influence of Plant Phenolics on Modulating Ferroptosis—A Systematic Review. Antioxidants 2024, 13, 334. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Zoller, H.; Theurl, I.; Koch, R.O.; McKie, A.T.; Vogel, W.; Weiss, G. Duodenal cytochrome b and hephaestin expression in patients with iron deficiency and hemochromatosis. Gastroenterology 2003, 125, 746–754. [Google Scholar] [CrossRef]
- Muckenthaler, M.; Galy, B.; Hentze, M.W. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 2008, 28, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S.; Minotti, G.; Cairo, G. Iron regulatory proteins: From molecular mechanisms to drug development. Antioxid. Redox Signal 2010, 13, 1593–1616. [Google Scholar] [CrossRef]
- Viatte, L.; Vaulont, S. Hepcidin, the iron watcher. Biochimie 2009, 91, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Angeles-Agdeppa, I.; Magsadia, C.R.; Capanzana, M.V. Fortified juice drink improved iron and zinc status of schoolchildren. Asia Pac. J. Clin. Nutr. 2011, 20, 535–543. [Google Scholar] [PubMed]
- Xie, T.; Zaidi, H. Age-dependent small-animal internal radiation dosimetry. Mol. Imaging 2013, 12, 364–375. [Google Scholar] [PubMed]
- Taconic Biosciences. Control Data of BrlHan: WIST@Tac (GALAS) Rat; Taconic Biosciences: Albany, NY, USA, 2003. [Google Scholar]
- Charles River Laboratories. Clinical laboratory Parameters for Crl:WI (Han); Charles River Laboratories: Wilmington, MA, USA, 2008. [Google Scholar]
- Moshtaghie, M.; Malekpouri, P.; Dinko, M.R.; Moshtaghie, A.A. Changes in serum parameters associated with iron metabolism in male rat exposed to lead. J. Physiol. Biochem. 2013, 69, 297–304. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Huang, Q.; Liu, C.; Jia, S.; Wang, Y.; An, F.; Song, H. Effectiveness of AOS-iron on iron deficiency anemia in rats. RSC Adv. 2019, 9, 5053–5063. [Google Scholar] [CrossRef]
- Moreno-Fernandez, J.; Díaz-Castro, J.; Alférez, M.J.M.; López-Aliaga, I. Iron deficiency and neuroendocrine regulators of basal metabolism, body composition and energy expenditure in rats. Nutrients 2019, 11, 631. [Google Scholar] [CrossRef]
- Gravesen, E.; Hofman-Bang, J.; Mace, M.L.; Lewin, E.; Olgaard, K. High dose intravenous iron, mineral homeostasis and intact FGF23 in normal and uremic rats. BMC Nephrol. 2013, 14, 281. [Google Scholar] [CrossRef]

| Plant-Based Foodstuffs | Nutraceuticals | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WPB | BEE | KIW | PIN | BSB | MEL | CIN | HON | ORG | RED | |
| Total phenolics (µg gallic acid equivalents/g of plant foods or mL of juice/smoothie) | 259.09 ± 13.98 | 683.45 ± 34.65 | 315.07 ± 2.16 | 538.60 ± 34.53 | 112.87 ± 9.70 | 180.66 ± 13.04 | (36.56 ± 2.26) × 103 | 191.22 ± 2.61 | 360.06 ± 5.04 b b | 988.42 ± 43.65 a |
| Total flavonoids (µg quercetin equivalents/g of plant foods or mL of juice/smoothie) | 68.26 ± 3.66 | 109.33 ± 7.28 | 6.17 ± 0.57 | <4.28 | 102.20 ± 4.99 | 243.17 ± 10.56 | 7184.82 ± 655.33 | <30.61 | 4.63 ± 0.10 b | 6.16 ± 0.67 a |
| Total phytic acid (mg phytic acid/g of plant foods or mL of juice/smoothie) | 0.43 ± 0.03 | <DL c | 0.07 ± 0.00 | 0.60 ± 0.05 | 0.58 ± 0.03 | 0.76 ± 0.05 | 2.77 ± 0.18 | 2.35 ± 0.22 | 0.16 ± 0.01 a | 0.07 ± 0.00 b |
| Total iron (µg iron/g of plant foods or mL of juice/smoothie) | 5.66 ± 0.52 | 2.98 ± 0.26 | 4.20 ± 0.26 | 4.31 ± 0.26 | 2.47 ± 0.24 | 1.73 ± 0.17 | 259.11 ± 23.25 | 2.41 ± 0.16 | 5.24 ± 0.47 a | 3.51 ± 0.16 b |
| DPPH assay (mM Trolox equivalents/g of plant foods or mL of juice/smoothie) | 0.23 ± 0.00 | 1.07 ± 0.16 | 0.18 ± 0.01 | 0.67 ± 0.01 | 0.15 ± 0.00 | 0.35 ± 0.03 | 42.84 ± 2.64 | N/A | 0.37 ± 0.04 b | 0.50 ± 0.02 a |
| FRAP assay (mM Trolox equivalents/g of plant foods or mL of juice/smoothie) | 0.18 ± 0.01 | 3.53 ± 0.11 | 0.44 ± 0.03 | 0.78 ± 0.03 | 0.10 ± 0.01 | 0.61 ± 0.02 | 160.59 ± 2.65 | 0.12 ± 0.01 | 0.31 ± 0.02 b | 0.41 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Živanović, N.; Mijatović Jovin, V.; Andrejić Višnjić, B.; Pintać Šarac, D.; Ćujić, D.; Simin, N.; Lesjak, M. Innovative Plant-Based Nutraceuticals: Enhancing Iron Bioavailability to Address Iron Deficiency Anaemia. Antioxidants 2025, 14, 1335. https://doi.org/10.3390/antiox14111335
Živanović N, Mijatović Jovin V, Andrejić Višnjić B, Pintać Šarac D, Ćujić D, Simin N, Lesjak M. Innovative Plant-Based Nutraceuticals: Enhancing Iron Bioavailability to Address Iron Deficiency Anaemia. Antioxidants. 2025; 14(11):1335. https://doi.org/10.3390/antiox14111335
Chicago/Turabian StyleŽivanović, Nemanja, Vesna Mijatović Jovin, Bojana Andrejić Višnjić, Diandra Pintać Šarac, Danica Ćujić, Nataša Simin, and Marija Lesjak. 2025. "Innovative Plant-Based Nutraceuticals: Enhancing Iron Bioavailability to Address Iron Deficiency Anaemia" Antioxidants 14, no. 11: 1335. https://doi.org/10.3390/antiox14111335
APA StyleŽivanović, N., Mijatović Jovin, V., Andrejić Višnjić, B., Pintać Šarac, D., Ćujić, D., Simin, N., & Lesjak, M. (2025). Innovative Plant-Based Nutraceuticals: Enhancing Iron Bioavailability to Address Iron Deficiency Anaemia. Antioxidants, 14(11), 1335. https://doi.org/10.3390/antiox14111335







