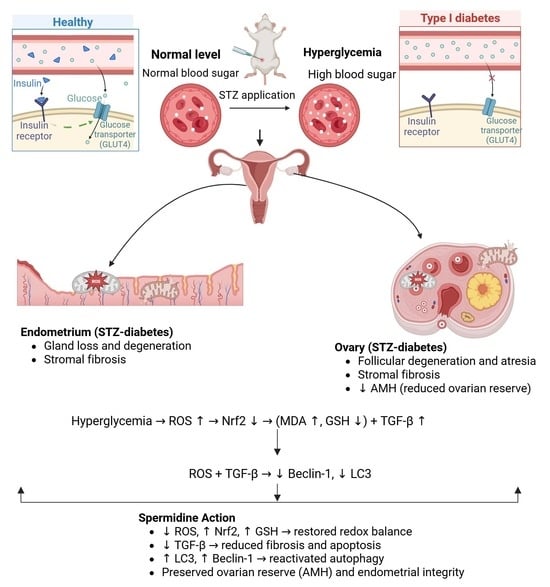
Protective Role of Spermidine Against Diabetes-Induced Ovarian and Endometrial Injury via LC3 and Beclin-1 Modulation
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Experimental Protocol
2.2.1. STZ-Induced Diabetes
2.2.2. Experimental Groups
2.2.3. Sample Collection
2.2.4. Blood Glucose Measurement
2.3. Histopathological Evaluation
2.4. Measurement of Plasma AMH Levels
2.5. Biochemical Analysis of Ovarian and Uterine Tissues
2.5.1. Tissue Preparation
2.5.2. Determination of Autophagy- and Fibrosis-Related Proteins (LC3, Beclin-1, TGF-β)
2.5.3. Measurement of Ovarian Nrf2 Levels
2.5.4. Determination of Tissue Glutathione (GSH) Levels
2.5.5. Determination of Antioxidant Enzyme Levels
2.5.6. Assessment of Lipid Peroxidation (MDA Levels)
2.6. Statistical Analysis
3. Results
3.1. Blood Glucose Levels (mg/dL)
3.2. Histopathological Scores and Plasma AMH Levels
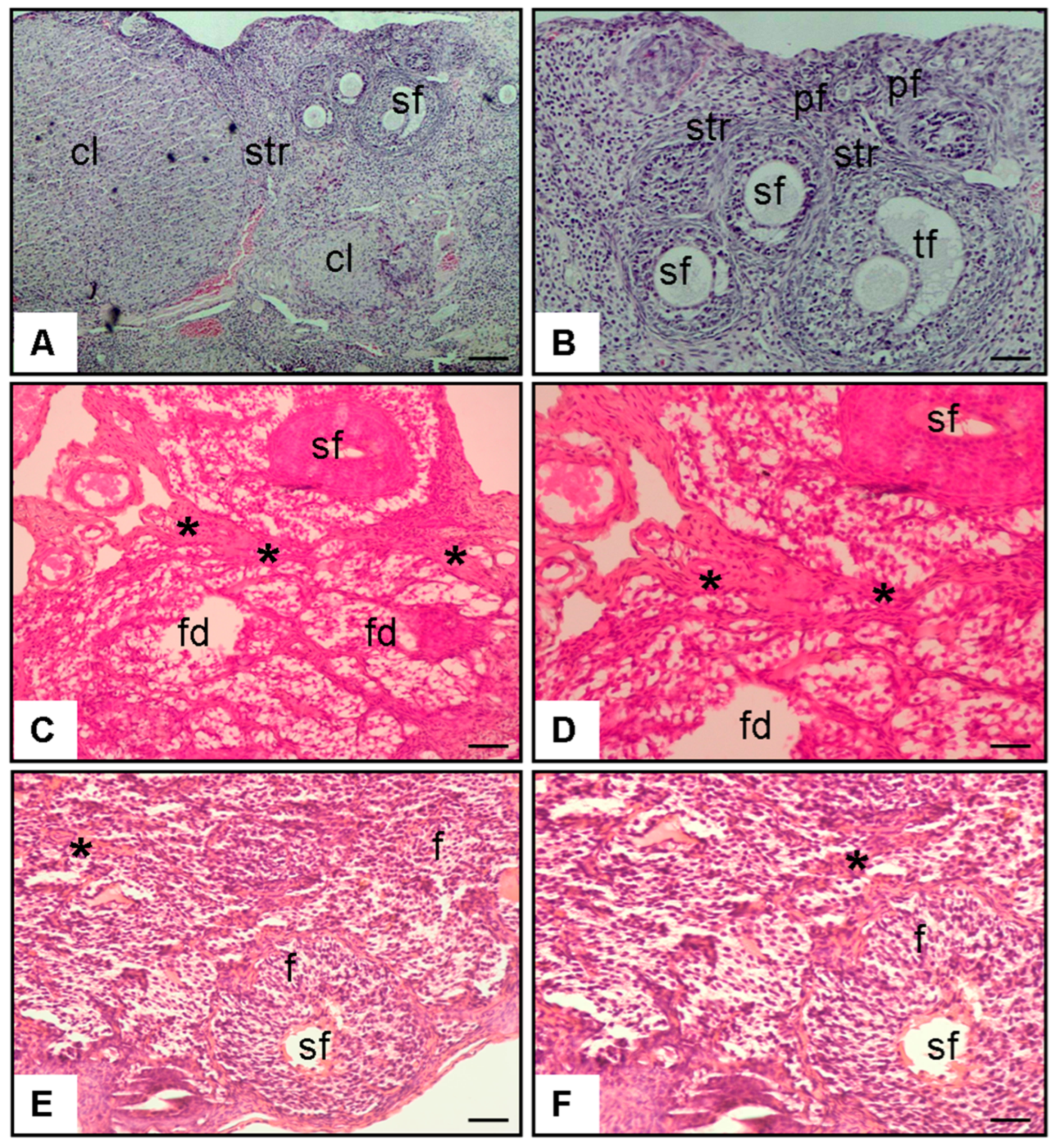
3.2.1. Figure 4 (Histopathology–Ovary, H&E Staining)
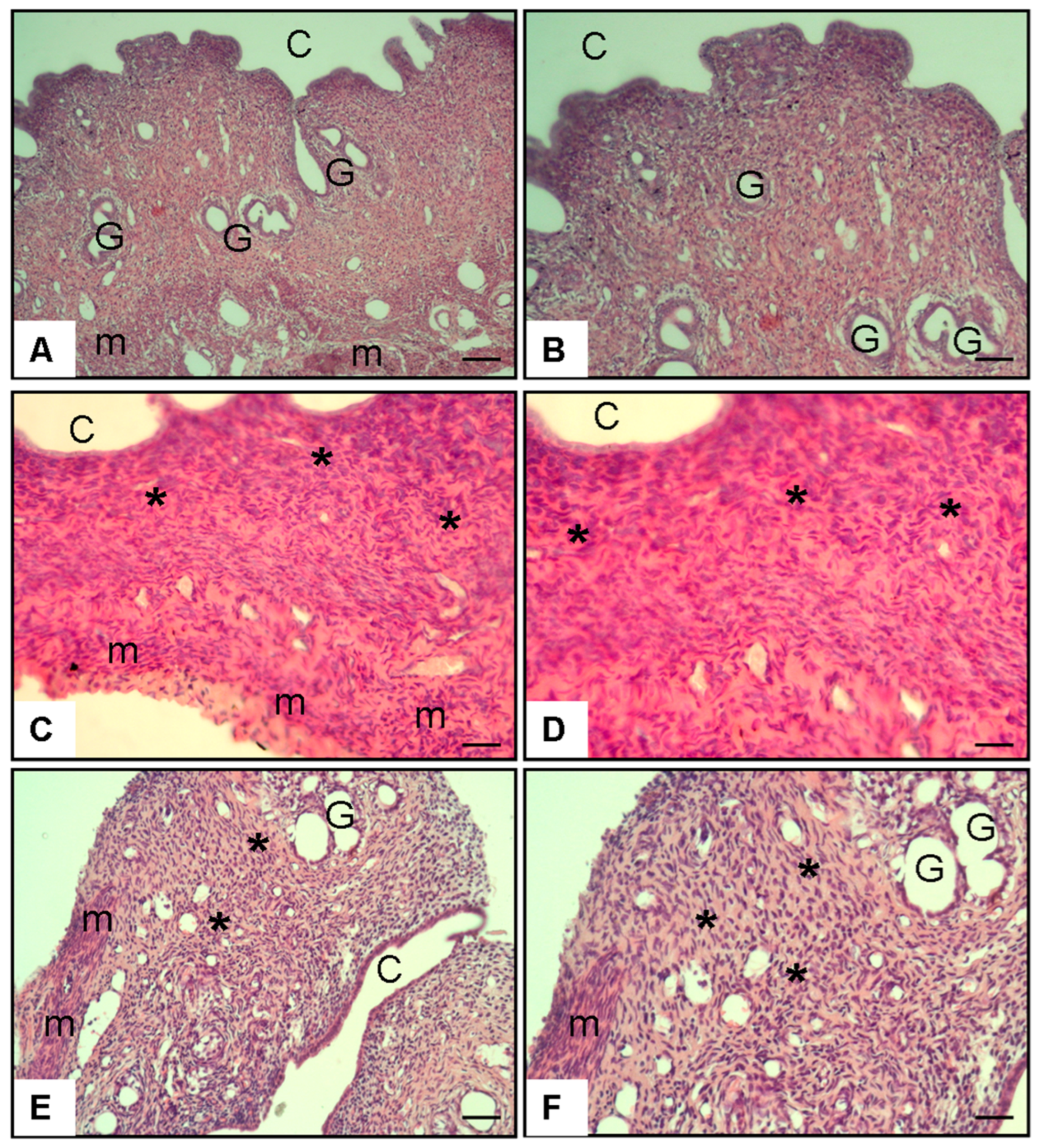
3.2.2. Figure 5 (Histopathology–Uterus, H&E Staining)
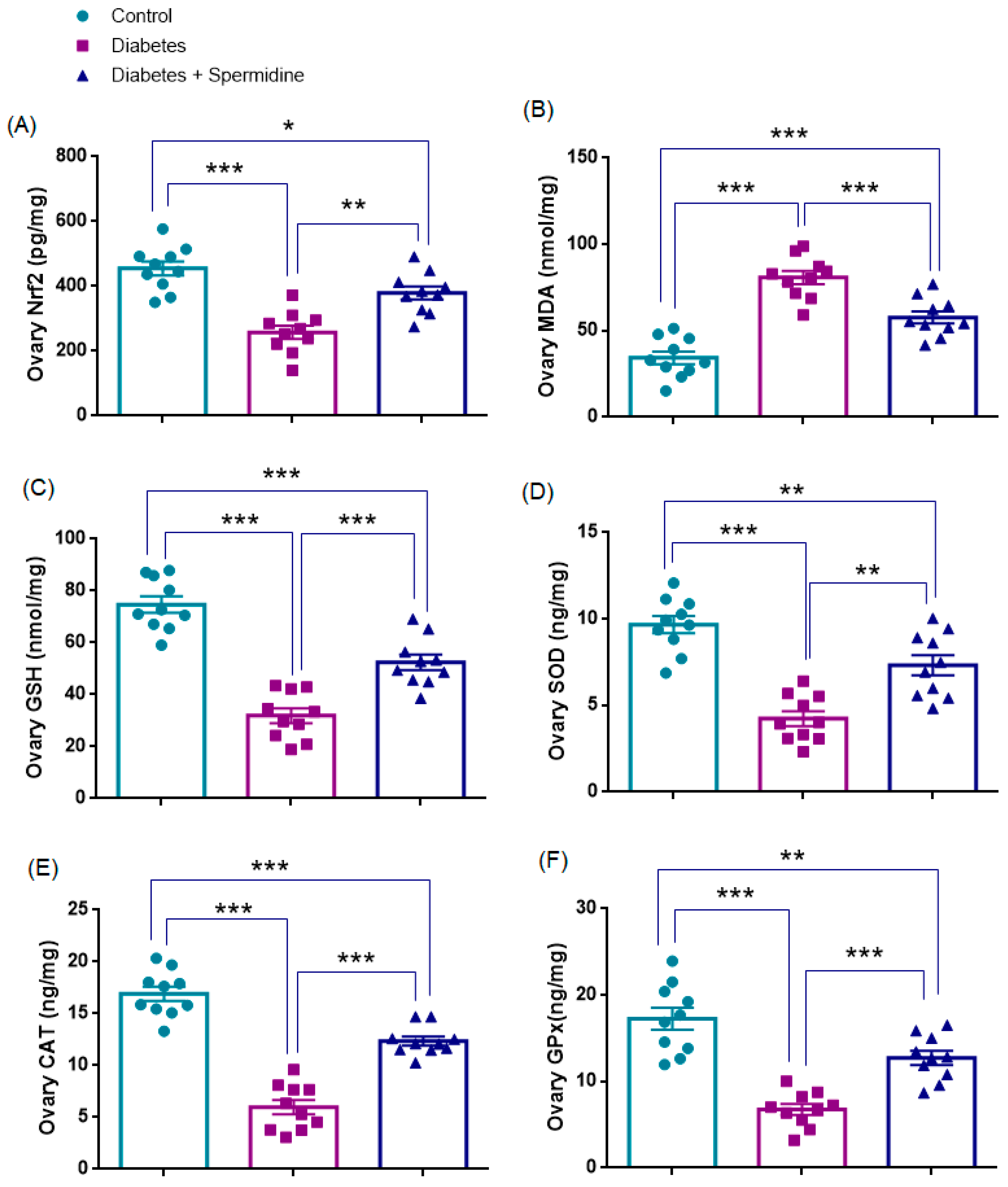
3.3. Oxidative Stress Parameters in Ovarian Tissue
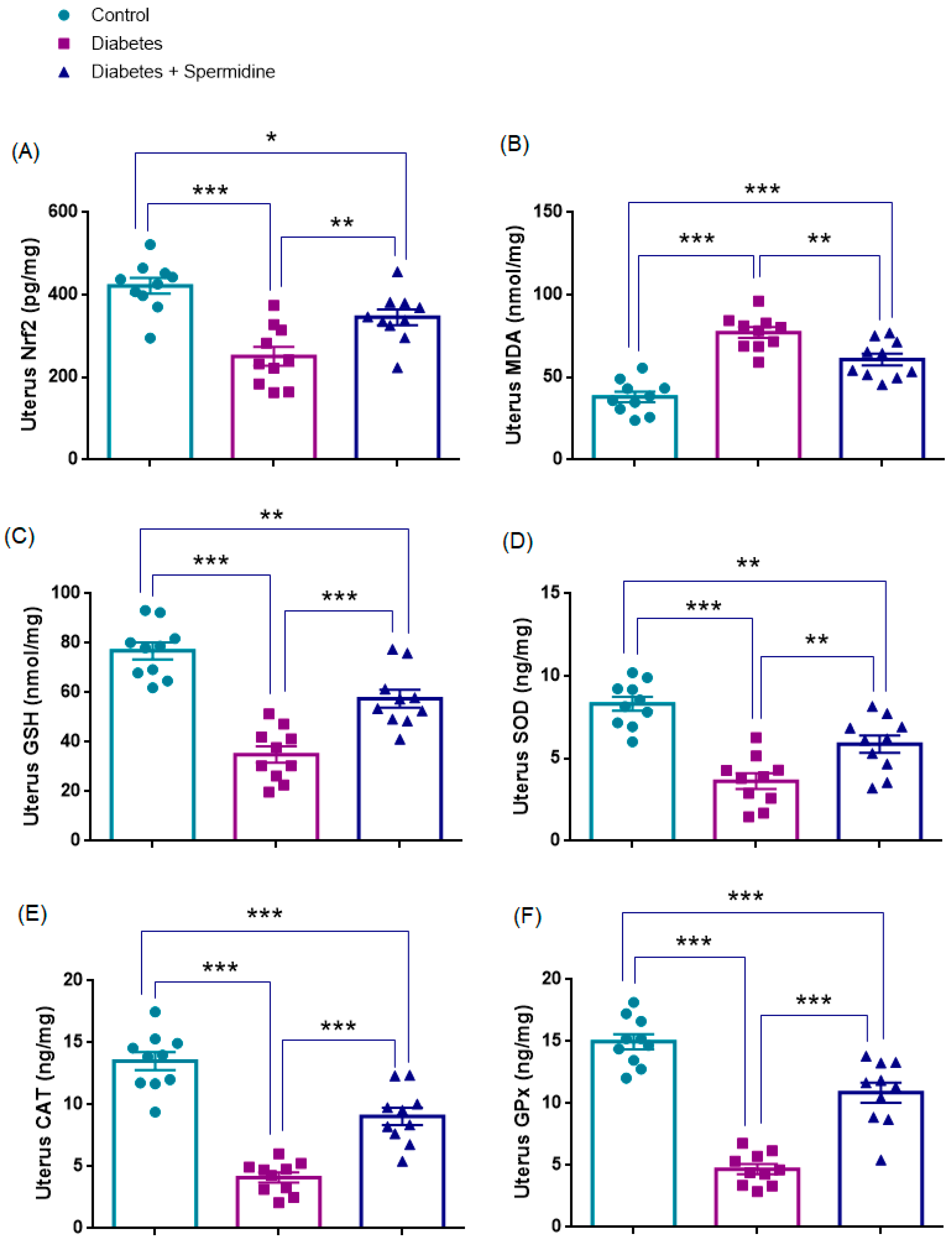
3.4. Oxidative Stress Parameters in Uterine Tissue
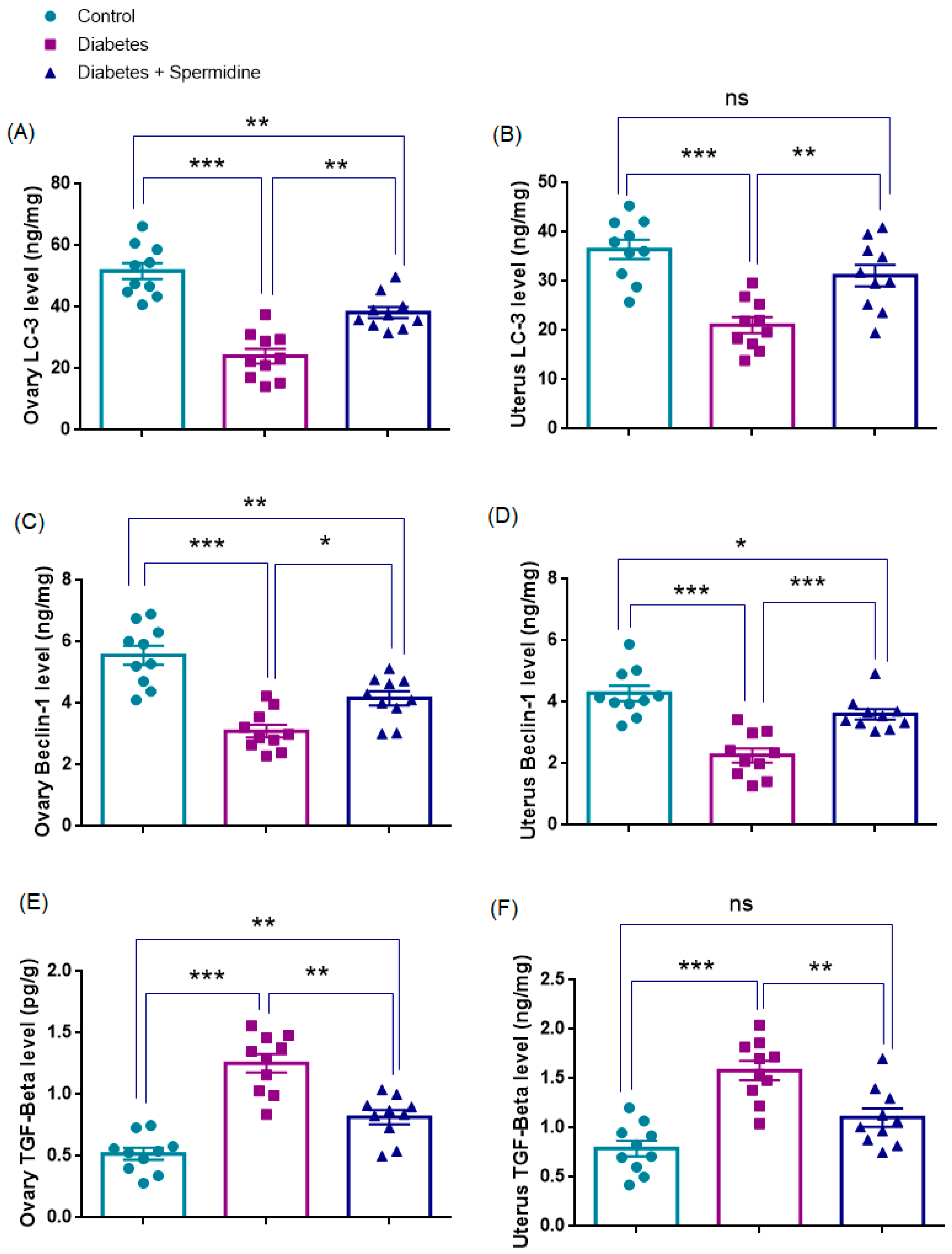
3.5. Autophagy- and Fibrosis-Related Parameters in Ovarian Tissue
3.6. Autophagy- and Fibrosis-Related Parameters in Uterine Tissue
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaimi, M.; Michalopoulou, O.; Stefanaki, K.; Kazakou, P.; Vasileiou, V.; Psaltopoulou, T.; Paschou, S.A. Gonadal dysfunction in women with diabetes mellitus. Endocrine 2024, 85, 461–472. [Google Scholar] [CrossRef]
- Ali, E.M.; Abdallah, H.I.; El-Sayed, S.M. Histomorphological, VEGF and TGF-β immunoexpression changes in the diabetic rats’ ovary and the potential amelioration following treatment with metformin and insulin. J. Mol. Histol. 2020, 51, 287–305. [Google Scholar] [CrossRef]
- Mutlu, A.K.; Tüfekci, K.K.; Kaplan, S. The protective effect of curcumin on the diabetic uterus: Quantitative and qualitative evaluation. Tissue Cell 2025, 95, 102852. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.; Ma, Q.; Sun, C. The role of autophagy in fibrosis: Mechanisms, progression and therapeutic potential. Int. J. Mol. Med. 2025, 55, 61. [Google Scholar] [CrossRef]
- Behmanesh, M.A.; Poormoosavi, S.M.; Mahmoodi-kouhi, A.; Najafzadehvarzi, H. Pistacia atlantica’s effect on ovary damage and oxidative stress in streptozotocin-induced diabetic rats. JBRA Assist. Reprod. 2021, 25, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, H.; Zeng, Y.; Yang, C.; Zhang, R.; Lund, A.K.; Zhang, M. Spermidine as a Potential Protective Agents Against Poly (I: C)-Induced Immune Response, Oxidative Stress, Apoptosis, and Testosterone Decrease in Yak Leydig Cells. Int. J. Mol. Sci. 2025, 26, 2753. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Guo, Y.; Niu, C.; Long, S.; Jiang, Y.; Wang, Z.; Kang, B. Exploration of the antioxidant effect of spermidine on the ovary and screening and identification of differentially expressed proteins. Int. J. Mol. Sci. 2023, 24, 5793. [Google Scholar] [CrossRef]
- Baek, A.R.; Hong, J.; Song, K.S.; Jang, A.S.; Kim, D.J.; Chin, S.S.; Park, S.W. Spermidine attenuates bleomycin-induced lung fibrosis by inducing autophagy and inhibiting endoplasmic reticulum stress (ERS)-induced cell death in mice. Exp. Mol. Med. 2020, 52, 2034–2045. [Google Scholar] [CrossRef]
- Niu, C.; Jiang, D.; Guo, Y.; Wang, Z.; Sun, Q.; Wang, X.; Kang, B. Spermidine suppresses oxidative stress and ferroptosis by Nrf2/HO-1/GPX4 and Akt/FHC/ACSL4 pathway to alleviate ovarian damage. Life Sci. 2023, 332, 122109. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Li, S.; Liu, M.; Lu, Y.; He, M.; Zheng, L. Non-linear associations of serum spermidine with type 2 diabetes mellitus and fasting plasma glucose: A cross-sectional study. Front. Nutr. 2024, 11, 1393552. [Google Scholar] [CrossRef]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Madeo, F. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef]
- Usatiuc, L.O.; Pop, R.M.; Adrian, S.; Pârvu, M.; Țicolea, M.; Uifălean APârvu, A.E. Multitargeted Effects of Plantago ovata Ethanol Extract in Experimental Rat Streptozotocin-Induced Diabetes Mellitus and Letrozole-Induced Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2025, 26, 4712. [Google Scholar] [CrossRef]
- Eisa, N.M.; Elshaer, S.S.; Bakry, S.; Abdelzaher, O.F.; Eldesoky, N.A.R. Placental extract augments mesenchymal stem cells in pancreatic tissue regeneration: A new insight into diabetes treatment. Tissue Cell 2025, 95, 102883. [Google Scholar] [CrossRef]
- Anderson, J.G.; Ramadori, G.; Ioris, R.M.; Galie, M.; Berglund, E.D.; Coate, K.C.; Fujikawa, T.; Pucciarelli, S.; Moreschine, B.; Amci, A.; et al. Enhanced insulin sensitivity in skeletal muscle and liver by physiological overexpression of SIRT6. Mol. Metab. 2015, 4, 846–856. [Google Scholar] [CrossRef]
- Mendez, J.D.; Balderas, F.L. Inhibition by L-arginine and spermidine of hemoglobin glycation and lipid peroxidation in rats with induced diabetes. Biomed. Pharmacother. 2006, 60, 26–31. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Fu, Z. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut. Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, J.; Li, R.; Yu, Z.; Yuan, W.; Gao, H.; Febg, W.; Gu, C.; Sun, Z.; Zheng, L. Association between serum spermidine and TyG index: Results from a cross-sectional study. Nutrients 2022, 14, 3847. [Google Scholar] [CrossRef]
- Mehrabianfar, P.; Dehghani, F.; Karbalaei, N.; Mesbah, F. The effects of metformin on stereological and ultrastructural features of the ovary in streptozotocin-induced diabetes adult rats: An experimental study. Int. J. Reprod. Biomed. 2020, 18, 651–666. [Google Scholar] [CrossRef]
- Artunc-Ulkumen, B.; Pala, H.G.; Pala, E.E.; Yavasoglu, A.; Yigitturk, G.; Erbas, O. Exenatide improves ovarian and endometrial injury and preserves ovarian reserve in streptozocin induced diabetic rats. Gynecol. Endocrinol. 2015, 31, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Ryu, S.B.; Park, Y.I.; Ahn, K.; Lee, S.N.; Nam, J.H. Diabetes mellitus induces vaginal tissue fibrosis by TGF-beta 1 expression in the rat model. J. Sex Marital Ther. 2001, 27, 577–587. [Google Scholar] [CrossRef]
- Akman, L.; Erbas, O.; Akdemir, A.; Yavasoglu, A.; Taskiran, D.; Kazandi, M. Levetiracetam ameliorates ovarian function in streptozotocin-induced diabetic rats. Gynecol. Endocrinol. 2015, 31, 657–662. [Google Scholar] [CrossRef]
- Codner, E.; Merino, P.M. Tena-Sempere, Female reproduction and type 1 diabetes: From mechanisms to clinical findings. Hum. Reprod. Update 2012, 18, 568–585. [Google Scholar] [CrossRef]
- Mattsson, K.; Nilsson-Condori, E.; Elmerstig, E.; Vassard, D.; Schmidt, L.; Ziebe, S.; Jöud, A. Fertility outcomes in women with pre-existing type 2 diabetes-a prospective cohort study. Fertil. Steril. 2021, 116, 505–513. [Google Scholar] [CrossRef]
- Yang, W.; Lin, C.; Zhang, M.; Lv, F.; Zhu, X.; Han XJi, L. Assessment of ovarian reserve in patients with type 1 diabetes: A systematic review and meta-analysis. Endocrine 2022, 77, 205–212. [Google Scholar] [CrossRef]
- Kim, J.; Kyriazi, H.; Greene, D.A. Normalization of Na(+)-K(+)-ATPase activity in isolated membrane fraction from sciatic nerves of streptozocin-induced diabetic rats by dietary myo-inositol supplementation in vivo or protein kinase C agonists in vitro. Diabetes 1991, 40, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef]
- Aihara, S.; Torisu, K.; Uchida, Y.; Imazu, N.; Nakano, T.; Kitazono, T. Spermidine from arginine metabolism activates Nrf2 and inhibits kidney fibrosis. Commun. Biol. 2023, 6, 676. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhu, L.; Li, W.; Li, Z.; Lu, X.; Hua, W.; Zhou, Y.; Gu, Y.; Zhu, M. PG545 alleviates diabetic retinopathy by promoting retinal Müller cell autophagy to inhibit the inflammatory response. Biochem. Biophys. Res. Commun. 2020, 531, 452–458. [Google Scholar] [CrossRef]
- Sadeghi, S.; Delphan, M.; Shams, M.; Esmaeili, F.; Shanaki-Bavarsad, M.; Shanaki, M. The high-intensity interval training (HIIT) and curcumin supplementation can positively regulate the autophagy pathway in myocardial cells of STZ-induced diabetic rats. BMC Res. Notes 2023, 16, 21. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, S.S.; Han, Z.; Han, F.; Chang, Y.P.; Yang, Y.; Chen, L.M. Triptolide restores autophagy to alleviate diabetic renal fibrosis through the miR-141-3p/PTEN/Akt/mTOR pathway. Mol. Ther. Nucleic Acids 2017, 9, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Wang, L.; Wang, P.; Xue, Y.; Li, X.; Chen, C. Autophagy impairment mediated by S-nitrosation of ATG4B leads to neurotoxicity in response to hyperglycemia. Autophagy 2017, 13, 1145–1160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X.; Wang, Y.; Guo, Y. reticulum stress and autophagy are involved in adipocyte-induced fibrosis in hepatic stellate cells. Mol. Cell. Biochem. 2021, 476, 2527–2538. [Google Scholar] [CrossRef]
- Jia, Q.; Yang, R.; Mehmood, S.; Li, Y. Epigallocatechin-3-gallate attenuates myocardial fibrosis in diabetic rats by activating autophagy. Exp. Biol. Med. 2022, 247, 1591–1600. [Google Scholar] [CrossRef]
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef]
- Yue, F.; Li, W.; Zou, J.; Jiang, X.; Xu, G.; Huang, H.; Liu, L. Spermidine Prolongs Lifespan and Prevents Liver Fibrosis and Hepatocellular Carcinoma by Activating MAP1S-Mediated Autophagy. Cancer Res. 2017, 77, 2938–2951. [Google Scholar] [CrossRef]
- Singh, K.K.; Lovren, F.; Pan, Y.; Quan, A.; Ramadan, A.; Matkar, P.N.; Ehsan, M.; Sandhu, P.; Mantella, L.E.; Gupta, N.; et al. The essential autophagy gene ATG7 modulates organ fibrosis via regulation of endothelial-to-mesenchymal transition. J. Biol. Chem. 2015, 290, 2547–2559. [Google Scholar] [CrossRef]
- Lu, C.; Yang, Y.; Zhu, Y.; Lv, S.; Zhang, J. An intervention target for myocardial fibrosis: Autophagy. BioMed Res. Int. 2018, 2018, 6215916. [Google Scholar] [CrossRef]
- Hu, F.; Yang, D.; Qian, B.; Fan, S.; Zhu, Q.; Ren, H.; Li, X.; Zhai, B. The exogenous delivery of microRNA-449b-5p using spermidine-PLGA nanoparticles efficiently decreases hepatic injury. RSC Adv. 2019, 9, 35135–35144. [Google Scholar] [CrossRef]
- Schroeder, S.; Mörtl, D.; Schauer, A.; Krüger, D.; Sinner, F.; Madeo, F. Spermidine supplementation enhances angiogenic capacity in senescent endothelial cells and improves ischemia-induced neovascularization in aged mice. Sci. Rep. 2023, 13, 35447. [Google Scholar]
- Grasso, D.; Sacchetti, M.L.; Bruno, L.; Lo, R.A.; Iovanna, J.L.; Gonzalez, C.D.; Vaccaro, M.I. Autophagy and VMP1 expression are early cellular events in experimental diabetes. Pancreatology 2009, 9, 81–88. [Google Scholar] [CrossRef]
- Xu, X.; Kobayashi, S.; Chen, K.; Timm, D.; Volden, P.; Huang, Y.; Liang, Q. Diminished autophagy limits cardiac injury in mouse models of type 1 diabetes. J. Biol. Chem. 2013, 288, 18077–18092. [Google Scholar] [CrossRef]
- Liu, H.Y.; Han, J.; Cao, S.Y.; Hong, T.; Zhuo, D.; Shi, J.; Cao, W. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: Inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J. Biol. Chem. 2009, 284, 31484–31492. [Google Scholar] [CrossRef]
- Deshpande, S.; Abdollahi, M.; Wang, M.; Lanting, L.; Kato, M.; Natarajan, R. Reduced autophagy by a microRNA-mediated signaling cascade in diabetes-induced renal glomerular hypertrophy. Sci. Rep. 2018, 8, 6954. [Google Scholar] [CrossRef]
- Sadasivan, S.K.; Vasamsetti, B.; Singh, J.; Marikunte, V.V.; Oommen, A.M.; Jagannath, M.R.; Rao, R.P. Exogenous administration of spermine improves glucose utilization and decreases bodyweight in mice. Eur. J. Pharmacol. 2014, 729, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Ma, D.; Luo, M.; Tan, Y.P.; Zhong, O.; Tian, G.; Lei, X.C. Effect of spermidine on ameliorating spermatogenic disorders in diabetic mice via regulating glycolysis pathway. Reprod. Biol. Endocrinol. 2022, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lu, X.; Zhang, X.; Shao, X.; Wang, Y.; Chen, J.; Zhao, B.; Li, S.; Xu, C.; Wei, C. Exogenous spermine attenuates myocardial fibrosis in diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress and the canonical Wnt signaling pathway. Cell Biol. Int. 2020, 44, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Andreollo, N.A.; Santos, E.F.D.; Araújo, M.R.; Lopes, L.R. Rat’s age versus human’s age: What is the relationship? ABCD. Arq. Bras. Cir. Dig. 2012, 25, 49–51. [Google Scholar] [CrossRef]
- Sengupta, P. A Scientific Review of Age Determination for a Laboratory Rat: How old is it in comparison with Human age? Biomed. Int. 2012, 2, 81–89. [Google Scholar]
- Wirth, M.; Schwarz, C.; Benson, G.; Horn, N.; Buchert, R.; Lange, C.; Flöel, A. Effects of spermidine supplementation on cognition and biomarkers in older adults with subjective cognitive decline (SmartAge)—Study protocol for a randomized controlled trial. Alzheimer’s Res. Ther. 2019, 11, 36. [Google Scholar] [CrossRef] [PubMed]

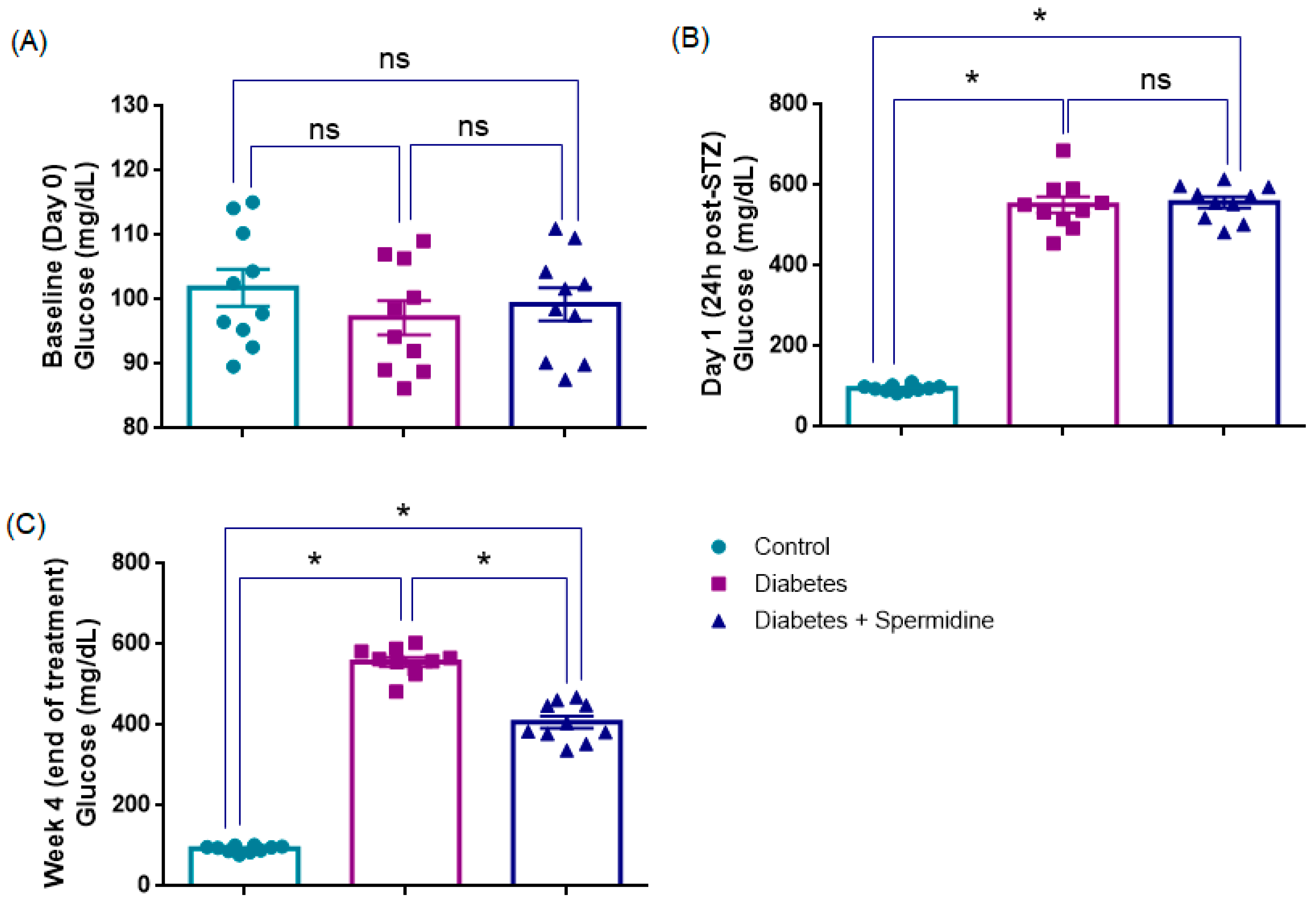

| Control | Diabetes | Diabetes + Spermidine | |
|---|---|---|---|
| Baseline blood glucose (mg/dL, Day 0) | 102 ± 3 | 97 ± 2 | 99 ± 2 |
| Blood glucose at 24 h post-STZ (mg/dL) | 96 ± 2 | 550 ± 20 * | 557 ± 14 |
| Blood glucose at Week 4 (mg/dL, end of treatment) | 93 ± 2 | 557 ± 13 * | 407 ± 15 ### |
| Control Group | Diabetes Group | Diabetes + Spermidine Group | |
|---|---|---|---|
| Endometrial gland degeneration score | 0 [0–0] | 3 [2–3] * | 1 [0.75–1.25] ### |
| Endometrial stromal fibrosis score | 0 [0–0] | 2.5 [2–3] * | 0 [0–1] ### |
| Ovarian follicle degeneration score | 0 [0–0] | 2 [1–2.25] * | 1 [1–1.25] # |
| Ovarian stromal fibrosis score | 0 [0–0] | 1 [1–2] * | 0.5 [0–1] # |
| Plasma AMH (ng/mL) | 2.79 ± 0.2 | 1.29 ± 0.1 * | 1.93 ± 0.1 ## |
| Parameter (Unit) | Control | Diabetes | Diabetes + Spermidine |
|---|---|---|---|
| Ovary Nrf2 (pg/mg) | 454.82 ± 21.83 | 257.99 ± 20.50 * | 379.51 ± 20.15 ## |
| Ovary MDA (nmol/mg) | 34.31 ± 3.64 | 80.77 ± 3.83 * | 58.71 ± 4.07 ### |
| Ovary GSH (nmol/mg) | 74.7 ± 3.2 | 31.9 ± 2.9 * | 52.4 ± 2.9 ### |
| Ovary SOD (ng/mg) | 9.67 ± 0.49 | 4.23 ± 0.42 * | 7.32 ± 0.58 ## |
| Ovary CAT (ng/mg) | 16.91 ± 0.68 | 5.98 ± 0.73 * | 12.36 ± 0.44 ### |
| Ovary GPx (ng/mg) | 17.27 ± 1.27 | 6.76 ± 0.64 * | 12.73 ± 0.82 ### |
| Parameter (Unit) | Control | Diabetes | Diabetes + Spermidine |
|---|---|---|---|
| Uterus Nrf2 (pg/mg) | 421.97 ± 19.16 | 250.9 ± 22.91 * | 345.5 ± 19.22 ## |
| Uterus MDA (nmol/mg) | 38.12 ± 3.16 | 77.17 ± 3.28 * | 60.84 ± 3.57 ## |
| Uterus GSH (nmol/mg) | 76.83 ± 3.42 | 34.95 ± 3.37 * | 57.55 ± 3.67 ### |
| Uterus SOD (ng/mg) | 8.31 ± 0.42 | 3.63 ± 0.47 * | 5.87 ± 0.52 ## |
| Uterus CAT (ng/mg) | 13.50 ± 0.73 | 4.11 ± 0.40 * | 9.05 ± 0.70 ### |
| Uterus GPx(ng/mg) | 14.98 ± 0.61 | 4.69 ± 0.40 * | 10.88 ± 0.81 ### |
| Parameter (Unit) | Control | Diabetes | Diabetes + Spermidine |
|---|---|---|---|
| Ovary LC3 (ng/mg) | 51.8 ± 2.6 | 24.0 ± 2.4 * | 38.2 ± 1.8 ## |
| Ovary Beclin-1 (ng/mg) | 5.6 ± 0.3 | 3.1 ± 0.2 * | 4.2 ± 0.2 # |
| Ovary TGF-β (pg/g) | 0.52 ± 0.05 | 1.25 ± 0.08 * | 0.82 ± 0.06 ## |
| Uterus LC3 (ng/mg) | 36.5 ± 2 | 21.1 ± 1.6 * | 31.2 ± 2.2 ## |
| Uterus Beclin-1 (ng/mg) | 4.3 ± 0.2 | 2.3 ± 0.2 * | 3.6 ± 0.2 ### |
| Uterus TGF-β (pg/g) | 0.79 ± 0.08 | 1.58 ± 0.09 * | 1.10 ± 0.09 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbaş, B.; Dinç, G.; Akbaş, A.; Hacım, N.A.; Ercan, G.; Aygün, H.; Erbaş, O. Protective Role of Spermidine Against Diabetes-Induced Ovarian and Endometrial Injury via LC3 and Beclin-1 Modulation. Antioxidants 2025, 14, 1294. https://doi.org/10.3390/antiox14111294
Akbaş B, Dinç G, Akbaş A, Hacım NA, Ercan G, Aygün H, Erbaş O. Protective Role of Spermidine Against Diabetes-Induced Ovarian and Endometrial Injury via LC3 and Beclin-1 Modulation. Antioxidants. 2025; 14(11):1294. https://doi.org/10.3390/antiox14111294
Chicago/Turabian StyleAkbaş, Bakiye, Gülseren Dinç, Ahmet Akbaş, Nadir Adnan Hacım, Gülçin Ercan, Hatice Aygün, and Oytun Erbaş. 2025. "Protective Role of Spermidine Against Diabetes-Induced Ovarian and Endometrial Injury via LC3 and Beclin-1 Modulation" Antioxidants 14, no. 11: 1294. https://doi.org/10.3390/antiox14111294
APA StyleAkbaş, B., Dinç, G., Akbaş, A., Hacım, N. A., Ercan, G., Aygün, H., & Erbaş, O. (2025). Protective Role of Spermidine Against Diabetes-Induced Ovarian and Endometrial Injury via LC3 and Beclin-1 Modulation. Antioxidants, 14(11), 1294. https://doi.org/10.3390/antiox14111294