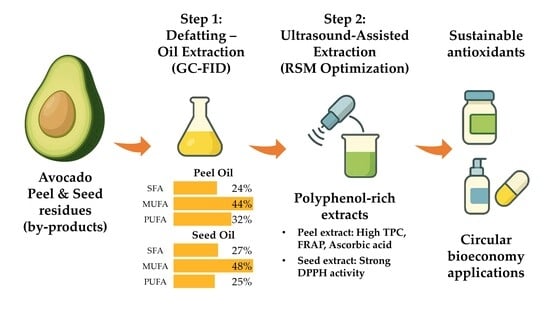
Optimization of Polyphenol-Rich Extracts from Defatted Avocado Peel and Seed Residues Using Ultrasound-Assisted RSM: Antioxidant Potential and Valorization Prospects
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Avocado Fruit Handling
2.3. Defatting and Lipophilic Analysis
2.3.1. Defatting Procedure
2.3.2. Fatty Acid Profile
2.3.3. Antioxidant Activity of Oils
2.3.4. Oxidative Stability Indices
2.3.5. Lipophilic Pigments
2.4. Experimental Design
2.5. Extraction of Polar Compounds from Defatted Residues
2.6. Determination of Antioxidant Parameters
2.6.1. Measurement of Total Polyphenolic Content (TPC)
2.6.2. Measurement of Ferric Reducing Antioxidant Power (FRAP)
2.6.3. Measurement of DPPH Radical Scavenging Activity
2.6.4. Measurement of Ascorbic Acid Content
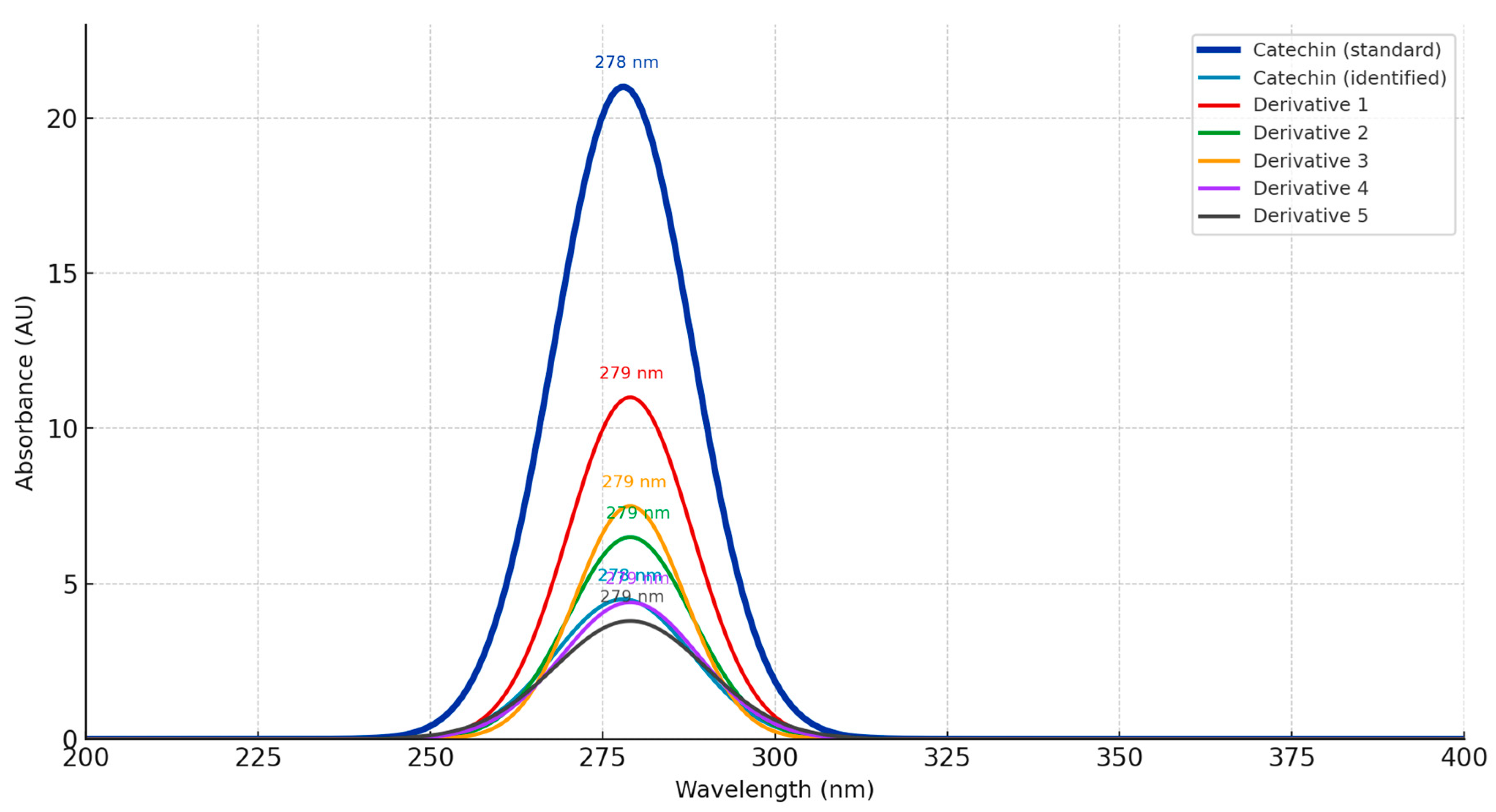
2.6.5. HPLC Analysis
2.7. Color Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Peel and Seed Oils Analysis
3.1.1. Fatty Acid Composition
3.1.2. Oxidative Stability and Antioxidant Activity
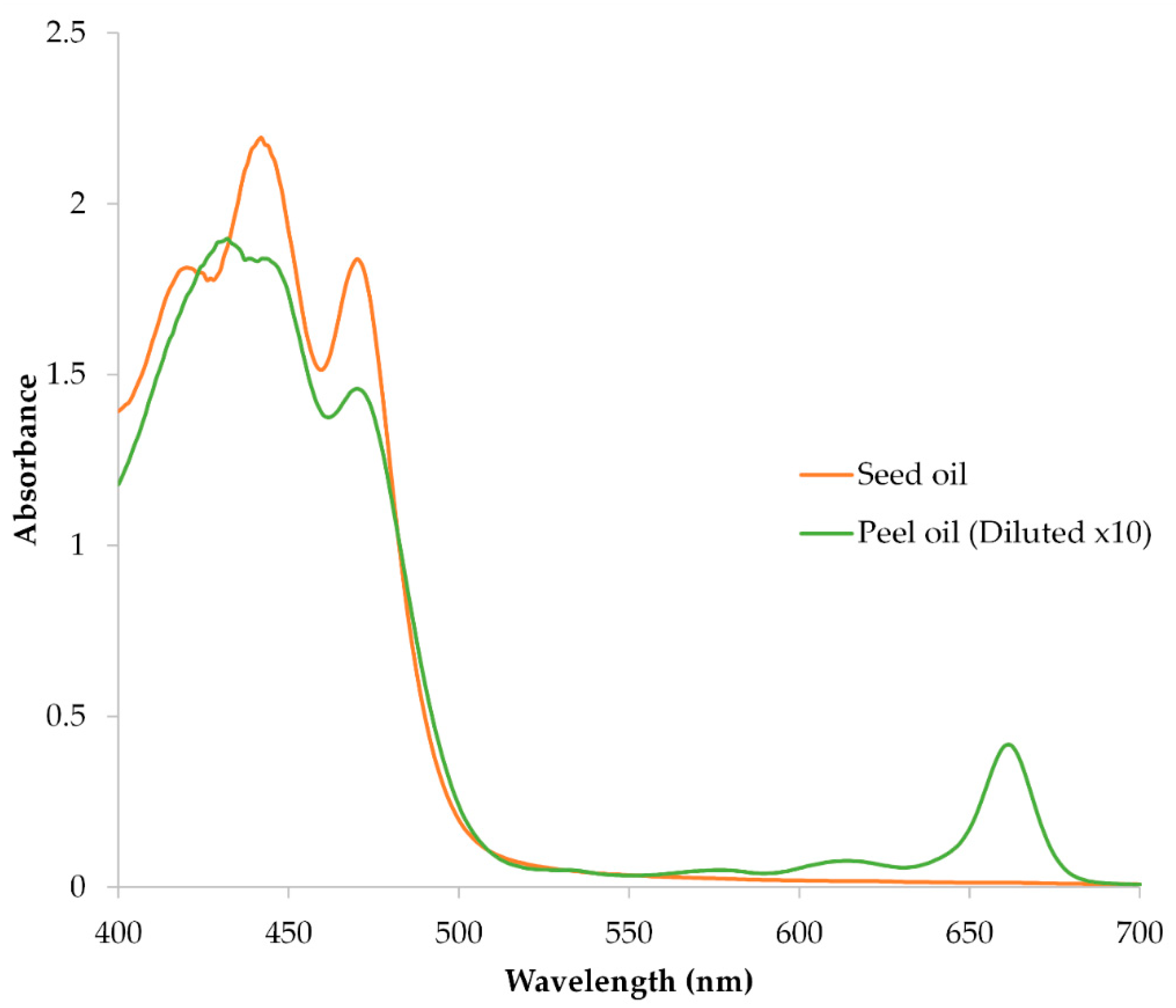
3.1.3. Pigment Composition and Spectral Characteristics
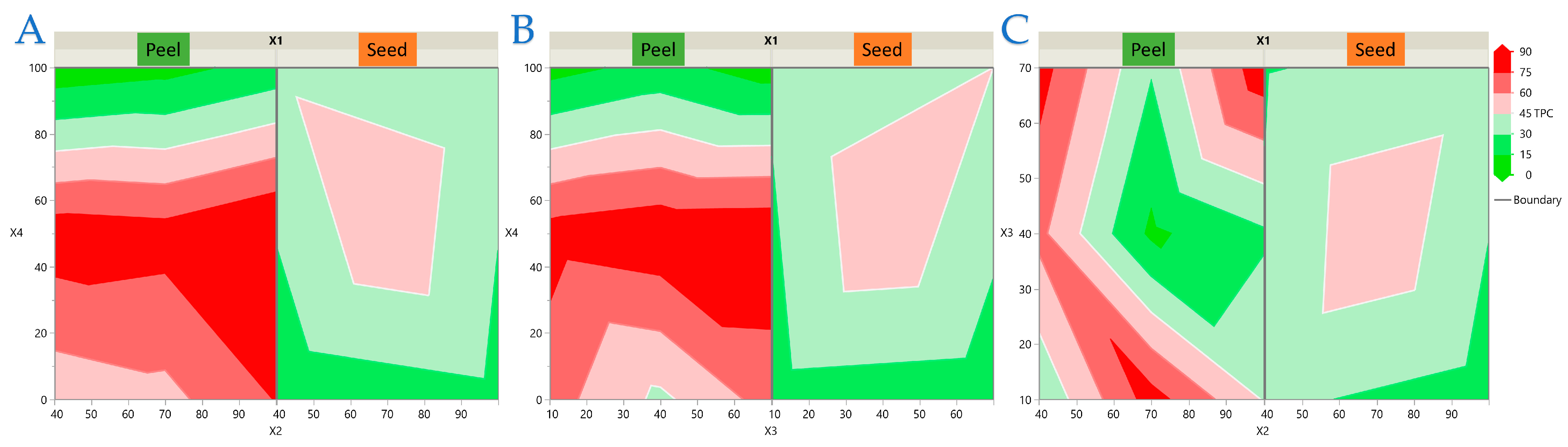
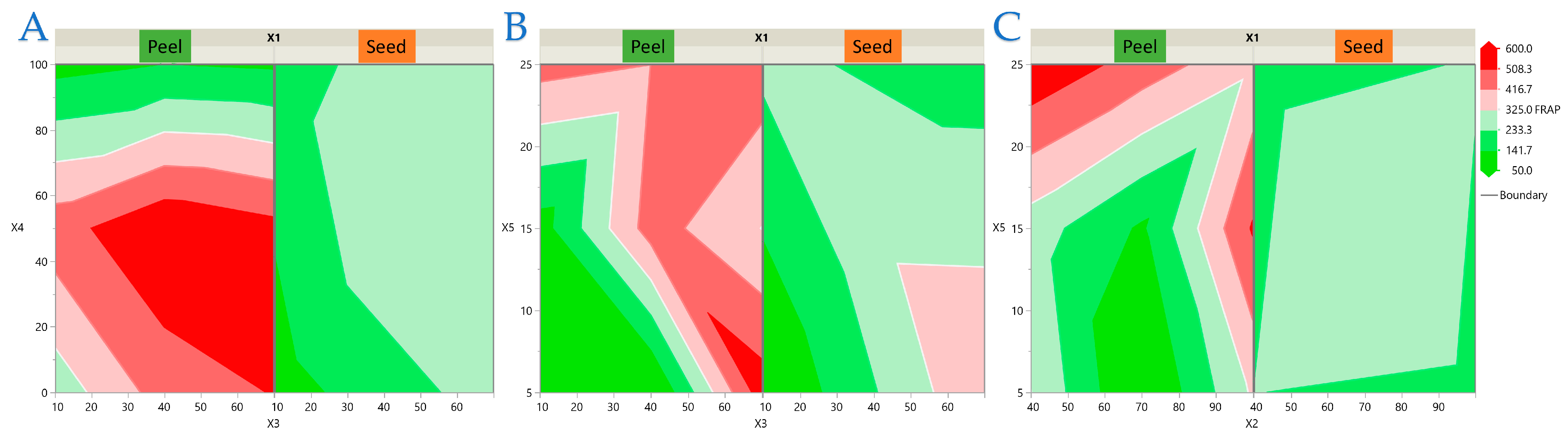
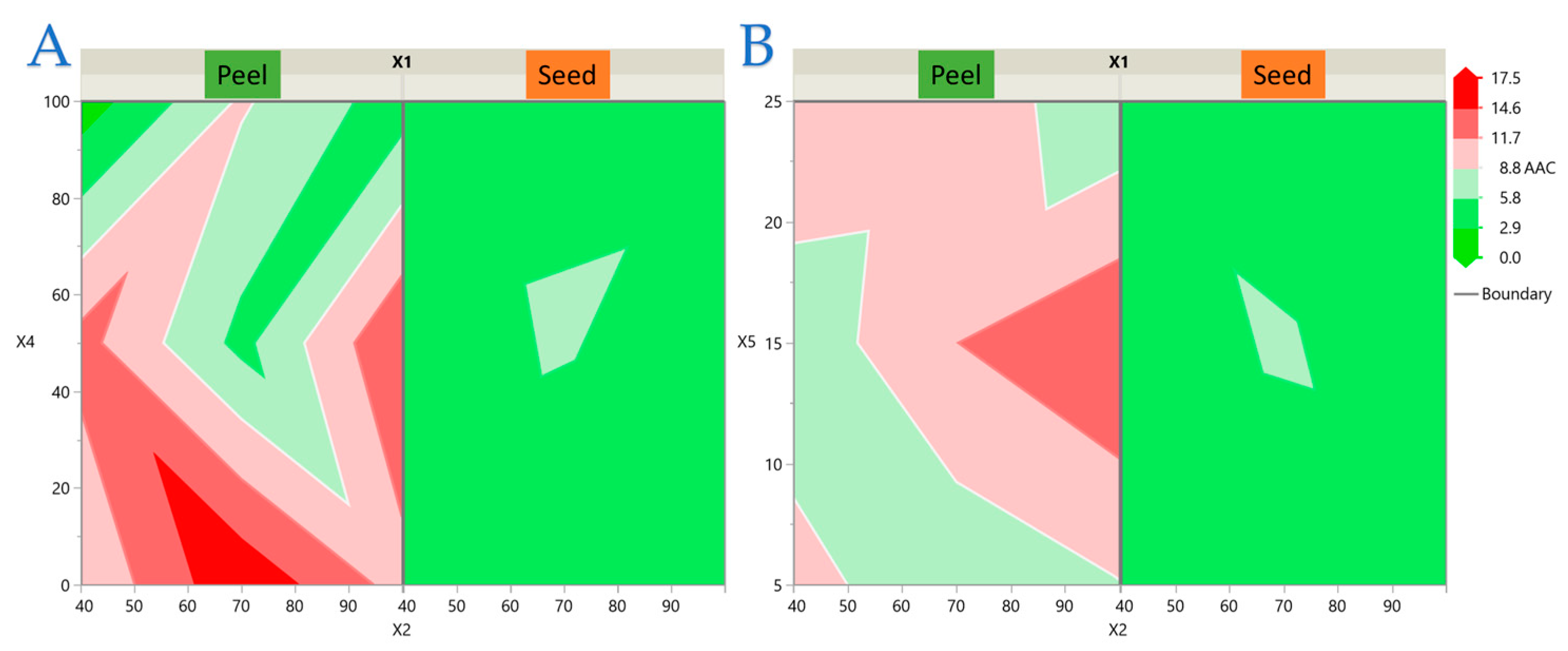
3.2. Experimental Results of the RSM Design
3.3. Model Analysis
W(12.6 + 0.120X2 − 0.286X4)
0.271X3X5 + W(44.7 − 55.6 − 1.59X4)
0.208X3X5 − 0.0874X4X5 + W(259.0 − 3.03X2 + 0.637X3 − 1.72X4 + 3.93X5)
W(1.09 + 0.0511X3 − 0.0299X4)
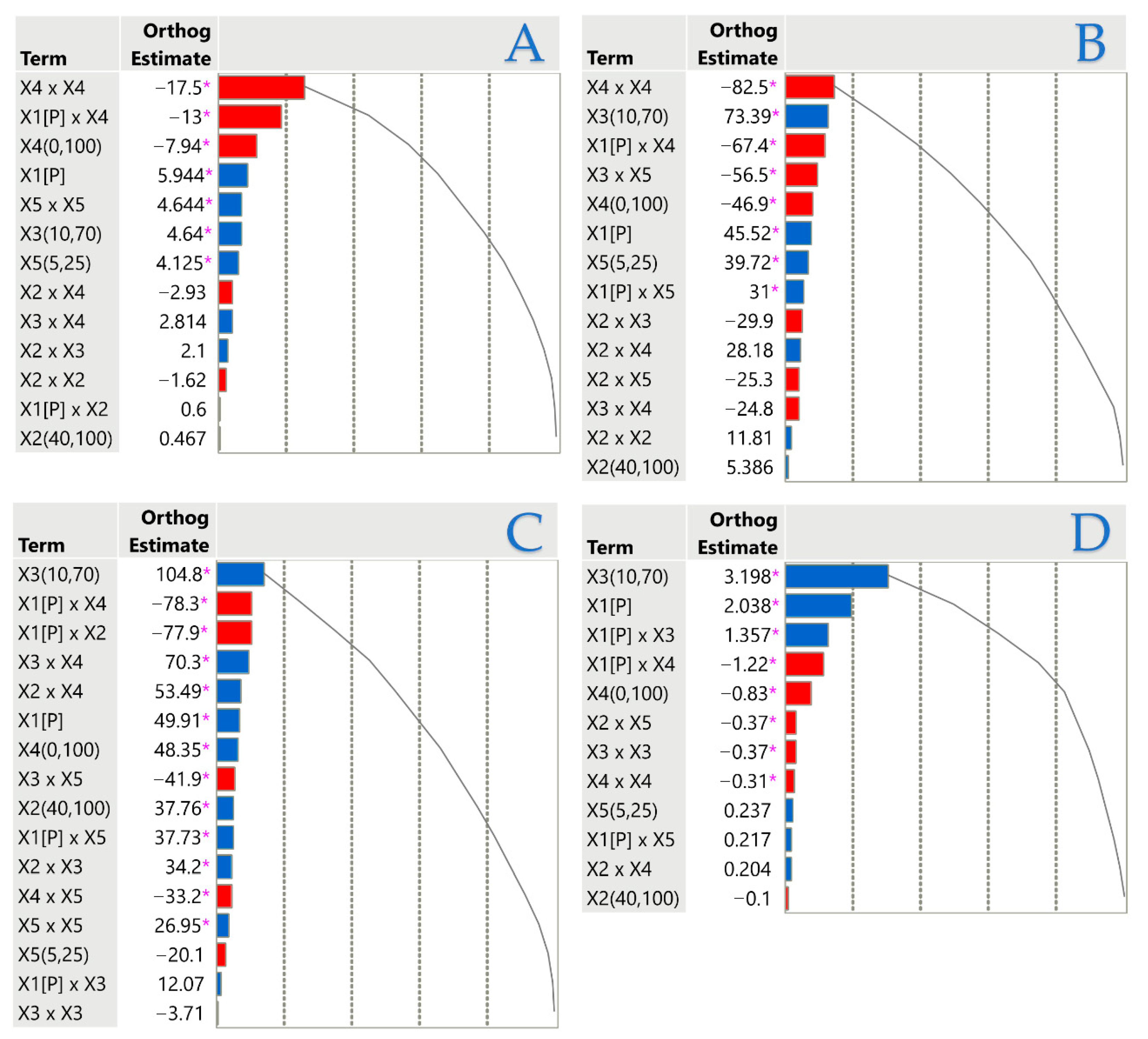
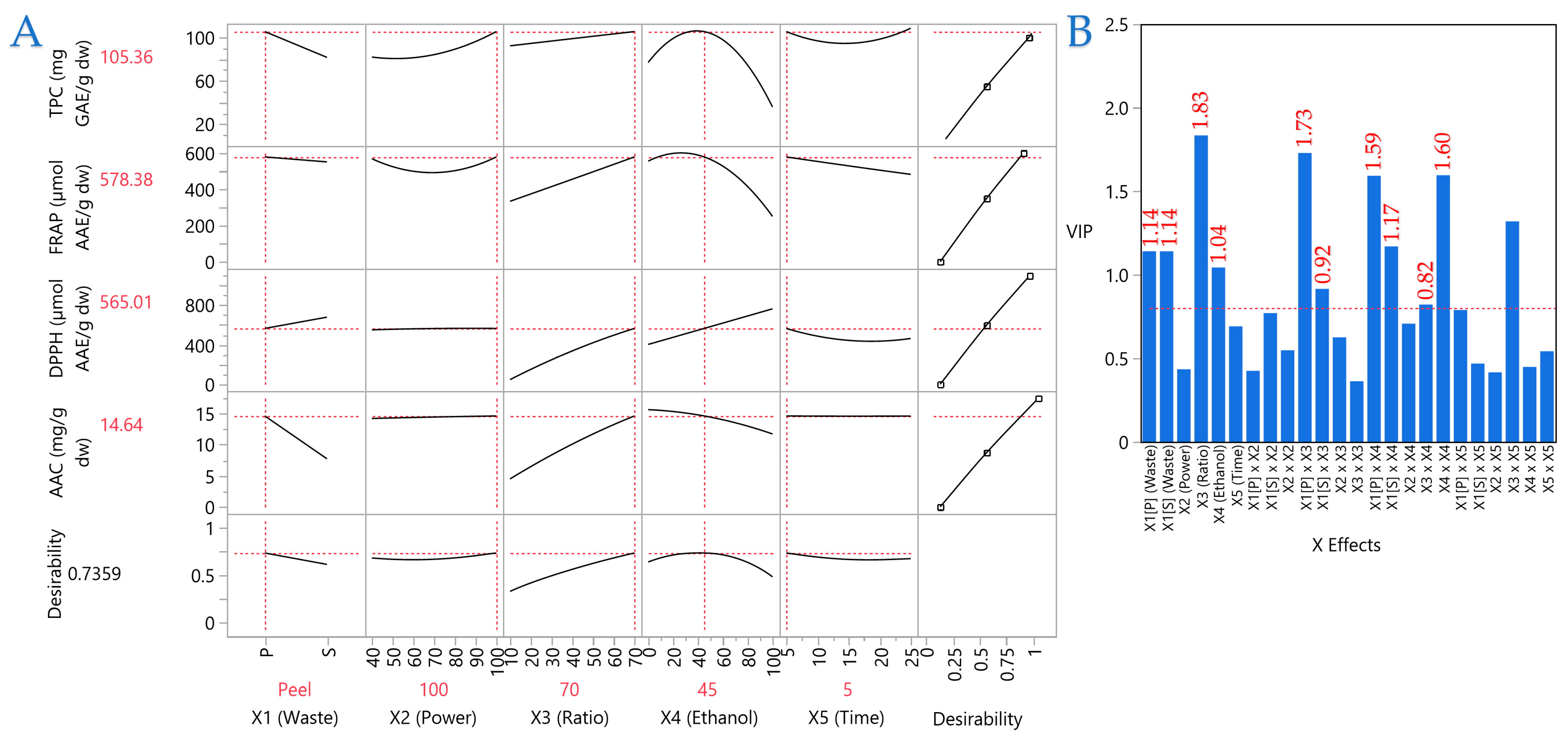
3.4. Impact of Extraction Parameters on Antioxidant Assays Through Pareto Plot Analysis
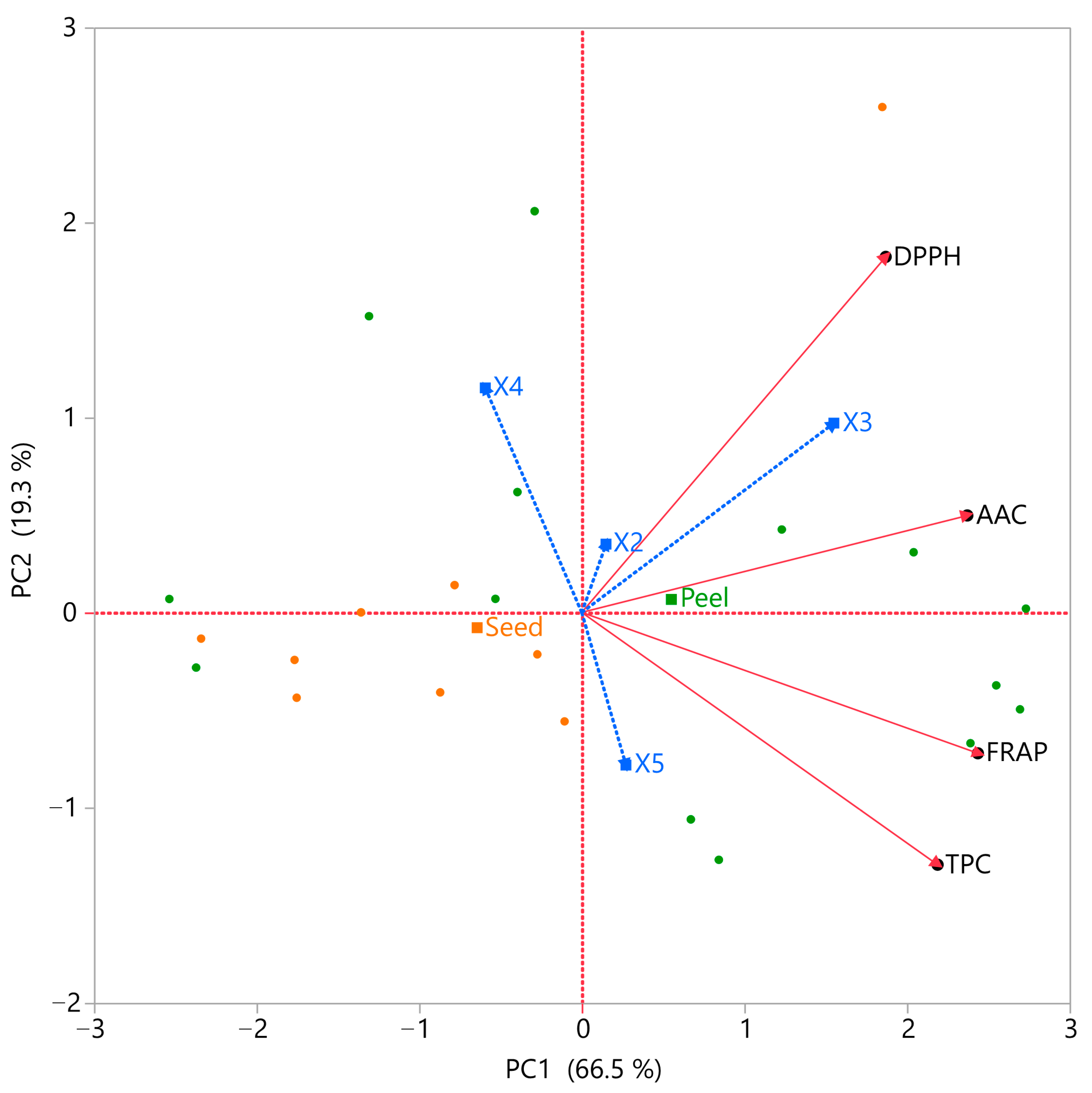
3.5. Principal Component Analysis (PCA) and Multivariate Component Analysis (MCA)
3.6. Partial Least Squares (PLS) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAC | Ascorbic Acid Content |
| AAE | Ascorbic Acid Equivalents |
| ANOVA | Analysis of Variance |
| AP | Avocado Peel |
| AS | Avocado Seed |
| CD | Conjugated Dienes |
| CT | Conjugated Trienes |
| DAD | Diode Array Detector |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| dw | Dry Weight |
| FAME | Fatty Acid Methyl Esters |
| FRAP | Ferric Reducing Antioxidant Power |
| GAE | Gallic Acid Equivalents |
| GC-FID | Gas Chromatography with Flame Ionization Detector |
| HPLC | High-Performance Liquid Chromatography |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| MUFA | Monounsaturated Fatty Acids |
| PCA | Principal Component Analysis |
| PLS | Partial Least Squares |
| PUFA | Polyunsaturated Fatty Acids |
| PV | Peroxide Value |
| ROS | Reactive Oxygen Species |
| RSM | Response Surface Methodology |
| SD | Standard Deviation |
| SFA | Saturated Fatty Acids |
| TE | Trolox Equivalents |
| TPC | Total Polyphenolic Content |
| TPTZ | 2,4,6-Tris(2-pyridyl)-s-triazine |
| UAE | Ultrasound-Assisted Extraction |
| UV-Vis | Ultraviolet-Visible Spectroscopy |
| VIP | Variable Importance in Projection |
Appendix A
| Polyphenolic Compound | Equation (Linear) | R2 | RT (min) | UVmax (nm) | LOD (mg/L) | LOQ (mg/L) |
|---|---|---|---|---|---|---|
| 3-Hydroxytyrosol | y = 183,448.37x – 251,422.04 | 0.997 | 14.552 | 278 | 2.87 | 8.69 |
| Catechin | y = 11,920.78x − 128.19 | 0.997 | 20.933 | 278 | 2.54 | 7.71 |
| Chlorogenic acid | y = 50,320.40x − 23,038.36 | 0.994 | 21.947 | 325 | 3.67 | 11.11 |
| Vanillic acid | y = 28,178.39x + 15,571.90 | 0.999 | 23.900 | 270 | 2.31 | 6.99 |
| Homovanillic acid | y = 18,843.08x + 6856.98 | 0.999 | 24.458 | 279 | 1.18 | 3.59 |
| Epicatechin | y = 142,099.00x + 4705.94 | 0.999 | 25.821 | 278 | 0.19 | 0.58 |
| Luteolin-7-glucoside | y = 34,875.94x − 16,827.36 | 0.999 | 36.949 | 347 | 1.28 | 3.89 |
References
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Kyriakopoulou, E.; Tsakni, A.; Korakidis, E.; Mpekoulis, G.; Kalliampakou, K.I.; Polanska, M.; Van Impe, J.F.M.; Tsakali, E.; Houhoula, D.; Vassilaki, N. Evaluation of Polyphenolic Compounds Common in Greek Medicinal Plants for Their Antioxidant Effects and Antiviral Activity Against Dengue and Yellow Fever Viruses. Antioxidants 2025, 14, 1103. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- ‘Aqilah, N.M.N.; Rovina, K.; Felicia, W.X.L.; Vonnie, J.M. A Review on the Potential Bioactive Components in Fruits and Vegetable Wastes as Value-Added Products in the Food Industry. Molecules 2023, 28, 2631. [Google Scholar] [CrossRef]
- Marianne, L.-C.; Lucía, A.-G.; De Jesús, M.-S.M.; Eric Leonardo, H.-M.; Mendoza-Sánchez, M. Optimization of the Green Extraction Process of Antioxidants Derived from Grape Pomace. Sustain. Chem. Pharm. 2024, 37, 101396. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of High Added-Value Components from Food Wastes: Conventional, Emerging Technologies and Commercialized Applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Mantiniotou, M.; Athanasiadis, V.; Kalompatsios, D.; Lalas, S.I. Optimization of Carotenoids and Other Antioxidant Compounds Extraction from Carrot Peels Using Response Surface Methodology. Biomass 2025, 5, 3. [Google Scholar] [CrossRef]
- Pereira, T.C.; Souza, V.P.; Padilha, A.P.F.; Duarte, F.A.; Flores, E.M. Trends and Perspectives on the Ultrasound-Assisted Extraction of Bioactive Compounds Using Natural Deep Eutectic Solvents. Curr. Opin. Chem. Eng. 2025, 47, 101088. [Google Scholar] [CrossRef]
- Zhu, Y.; Luan, Y.; Zhao, Y.; Liu, J.; Duan, Z.; Ruan, R. Current Technologies and Uses for Fruit and Vegetable Wastes in a Sustainable System: A Review. Foods 2023, 12, 1949. [Google Scholar] [CrossRef]
- Rao, M.V.; Sengar, A.S.; Rawson, A. Ultrasonication-A Green Technology Extraction Technique for Spices: A Review. Trends Food Sci. Technol. 2021, 116, 975–991. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Ali Redha, A.; Salauddin, M.; Harahap, I.A.; Rupasinghe, H.P.V. Factors Affecting the Extraction of (Poly)Phenols from Natural Resources Using Deep Eutectic Solvents Combined with Ultrasound-Assisted Extraction. Crit. Rev. Anal. Chem. 2025, 55, 139–160. [Google Scholar] [CrossRef]
- Nyakang’i, C.O.; Marete, E.; Ebere, R.; Arimi, J.M. Physicochemical Properties of Avocado Seed Extract Model Beverages and Baked Products Incorporated with Avocado Seed Powder. Int. J. Food Sci. 2023, 2023, 6860806. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Domínguez-Avila, J.A.; Yahia, E.M.; Belmonte-Herrera, B.H.; Wall-Medrano, A.; Montalvo-González, E.; González-Aguilar, G.A. Avocado Fruit and By-Products as Potential Sources of Bioactive Compounds. Food Res. Int. 2020, 138, 109774. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Association of the United States. Available online: http://www.fao.org/faostat/en/ (accessed on 27 March 2024).
- Kupnik, K.; Primožič, M.; Kokol, V.; Knez, Ž.; Leitgeb, M. Enzymatic, Antioxidant, and Antimicrobial Activities of Bioactive Compounds from Avocado (Persea Americana L.) Seeds. Plants 2023, 12, 1201. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.; Garcia, P.; Quitral, V.; Vasquez, K.; Parra-Ruiz, C.; Reyes-Farias, M.; Garcia-Diaz, D.F.; Robert, P.; Encina, C.; Soto-Covasich, J. Pulp, Leaf, Peel and Seed of Avocado Fruit: A Review of Bioactive Compounds and Healthy Benefits. Food Rev. Int. 2021, 37, 619–655. [Google Scholar] [CrossRef]
- Akan, S. Phytochemicals in Avocado Peel and Their Potential Uses. Food Health 2021, 7, 138–149. [Google Scholar] [CrossRef]
- Kautsar, D.B.; Rois, M.F.; Faizah, N.; Widiyastuti, W.; Nurtono, T.; Setyawan, H. Antioxidant and Antimicrobial Agents from Avocado (Persea Americana) Seed Extract Encapsulated in Gum Arabic through Spray Drying Method. Period. Polytech. Chem. Eng. 2023, 67, 161–171. [Google Scholar] [CrossRef]
- Nascimento, A.P.S.; Duarte, M.E.M.; Rocha, A.P.T.; Barros, A.N. Valorization of Avocado (Persea Americana) Peel and Seed: Functional Potential for Food and Health Applications. Antioxidants 2025, 14, 1032. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, V.; Kalompatsios, D.; Mantiniotou, M.; Lalas, S.I. Investigation into the Reduction of Palm Oil in Foods by Blended Vegetable Oils through Response Surface Methodology and Oxidative Stability Tests. Antioxidants 2024, 13, 929. [Google Scholar] [CrossRef] [PubMed]
- International Dairy Federation. Anhydrous Milkfat: Determination of Peroxide Value (IDF Standard 74A:1991); IDF: Brussels, Belgium, 1991.
- Scott, K.J. Detection and Measurement of Carotenoids by UV/VIS Spectrophotometry. Curr. Protoc. Food Anal. Chem. 2001, F2.2.1–F2.2.10. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and Program to Determine Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents. In Advances in Photosynthesis Research: Proceedings of the VIth International Congress on Photosynthesis, Brussels, Belgium, August 1–6, 1983 Volume 2; Sybesma, C., Ed.; Springer: Dordrecht, The Netherlands, 1984; pp. 9–12. ISBN 978-94-017-6368-4. [Google Scholar]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Mantiniotou, M.; Athanasiadis, V.; Liakos, K.G.; Bozinou, E.; Lalas, S.I. Artificial Intelligence and Extraction of Bioactive Compounds: The Case of Rosemary and Pressurized Liquid Extraction. Processes 2025, 13, 1879. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Mantiniotou, M.; Kalompatsios, D.; Makrygiannis, I.; Alibade, A.; Lalas, S.I. Evaluation of Antioxidant Properties of Residual Hemp Leaves Following Optimized Pressurized Liquid Extraction. AgriEngineering 2025, 7, 1. [Google Scholar] [CrossRef]
- Mantiniotou, M.; Athanasiadis, V.; Kalompatsios, D.; Bozinou, E.; Ntourtoglou, G.; Dourtoglou, V.G.; Lalas, S.I. Atmospheric Room Temperature Plasma as a Green Pretreatment Strategy for Enhanced Phytochemical Extraction from Moringa Oleifera Leaves. Foods 2025, 14, 3233. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Palaiogiannis, D.; Makrygiannis, I.; Bozinou, E.; Lalas, S.I. Evaluation of the Efficacy and Synergistic Effect of α- and δ-Tocopherol as Natural Antioxidants in the Stabilization of Sunflower Oil and Olive Pomace Oil during Storage Conditions. Int. J. Mol. Sci. 2023, 24, 1113. [Google Scholar] [CrossRef]
- Sandmann, G. Antioxidant Protection from UV-and Light-Stress Related to Carotenoid Structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef]
- Flieger, J.; Raszewska-Famielec, M.; Radzikowska-Büchner, E.; Flieger, W. Skin Protection by Carotenoid Pigments. Int. J. Mol. Sci. 2024, 25, 1431. [Google Scholar] [CrossRef]
- Foppen, F.H. Tables for the Identification of Carotenoid Pigments. Chromatogr. Rev. 1971, 14, 133–298. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Estévez, M. Avocado By-Products as Inhibitors of Color Deterioration and Lipid and Protein Oxidation in Raw Porcine Patties Subjected to Chilled Storage. Meat Sci. 2011, 89, 166–173. [Google Scholar] [CrossRef]
- Hefzalrahman, T.; Morsi, M.K.S.; Morsy, N.F.S.; Hammad, K.S.M. Application of Enzyme and Ultrasound Assisted Extraction of Polyphenols from Avocado (Persea Americana Mill.) Peel as Natural Antioxidants. Acta Sci. Pol. Technol. Aliment. 2022, 21, 129–138. [Google Scholar] [CrossRef]
- Lyu, X.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Phenolic Compounds Profiling and Their Antioxidant Capacity in the Peel, Pulp, and Seed of Australian Grown Avocado. Antioxidants 2023, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, M.; Dhana Rangesh Kumar, V.; Nithya, T.G.; Danya, U. Assessment of Antioxidant, Antibacterial Activity and Phytoactive Compounds of Aqueous Extracts of Avocado Fruit Peel from Ethiopia. Int. J. Pept. Res. Ther. 2020, 26, 1549–1557. [Google Scholar] [CrossRef]
- Vinha, A.; Moreira, J.; Barreira, S. Physicochemical Parameters, Phytochemical Composition and Antioxidant Activity of the Algarvian Avocado (Persea Americana Mill.). J. Agric. Sci. 2013, 5, 100. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of Catechins and Their Applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Prakash, M.; Basavaraj, B.V.; Chidambara Murthy, K.N. Biological Functions of Epicatechin: Plant Cell to Human Cell Health. J. Funct. Foods 2019, 52, 14–24. [Google Scholar] [CrossRef]
- Abate, M.; Pisanti, S.; Caputo, M.; Citro, M.; Vecchione, C.; Martinelli, R. 3-Hydroxytyrosol Promotes Angiogenesis In Vitro by Stimulating Endothelial Cell Migration. Int. J. Mol. Sci. 2020, 21, 3657. [Google Scholar] [CrossRef] [PubMed]
- Selka, A.; Ndongou Moutombi, F.J.; Cormier, M.; Touaibia, M. Phenethyl Esters and Amide of Ferulic Acid, Hydroferulic Acid, Homovanillic Acid, and Vanillic Acid: Synthesis, Free Radicals Scavenging Activity, and Molecular Modeling as Potential Cholinesterases Inhibitors. Molbank 2020, 2020, M1151. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef]
- Razola-Díaz, M.d.C.; De Montijo-Prieto, S.; Aznar-Ramos, M.J.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Effect of Lactic Acid Bacteria Fermentation on the Polar Compounds Content with Antioxidant and Antidiabetic Activity of Avocado Seed Extracts. Fermentation 2023, 9, 420. [Google Scholar] [CrossRef]
- Trujillo-Mayol, I.; Casas-Forero, N.; Pastene-Navarrete, E.; Lima Silva, F.; Alarcón-Enos, J. Fractionation and Hydrolyzation of Avocado Peel Extract: Improvement of Antibacterial Activity. Antibiotics 2021, 10, 23. [Google Scholar] [CrossRef] [PubMed]



| Independent Variables | Coded Units | Type | Coded Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Waste | X1 | Categorical | Peel | - | Seed |
| Ultrasonic power (E, %) | X2 | Continuous | 40 | 70 | 100 |
| Liquid-to-solid ratio (R, mL/g) | X3 | Continuous | 10 | 40 | 70 |
| Ethanol concentration (C, % v/v) | X4 | Continuous | 0 | 50 | 100 |
| Extraction time (t, min) | X5 | Continuous | 5 | 15 | 25 |
| Fatty Acid | Peel (%) | Seed (%) |
|---|---|---|
| C10:0 (Decanoic) | n.d. | 0.11 ± 0.00 * |
| C12:0 (Lauric) | 3.17 ± 0.19 * | 0.17 ± 0.01 |
| C14:0 (Myristic) | 0.34 ± 0.01 * | 0.07 ± 0.00 |
| C16:0 (Palmitic) | 19.38 ± 0.79 | 20.73 ± 0.48 |
| C18:0 (Stearic) | 0.96 ± 0.04 | 4.70 ± 0.15 * |
| C20:0 (Arachidic) | n.d. | 0.05 ± 0.00 * |
| C22:0 (Behenic) | 0.23 ± 0.01 * | 0.03 ± 0.00 |
| C24:0 (Lignoceric) | 0.20 ± 0.01 | 0.94 ± 0.04 * |
| ∑ SFA | 24.28 ± 1.06 | 26.80 ± 0.69 * |
| C16:1 (Palmitoleic) | 1.68 ± 0.05 * | n.d. |
| C18:1 (Oleic) | 42.05 ± 3.11 | 48.09 ± 2.31 |
| C22:1 (Erucic) | 0.05 ± 0.00 * | n.d. |
| ∑ MUFA | 43.78 ± 3.16 | 48.09 ± 2.31 |
| C18:2 (Linoleic, ω-6) | 21.88 ± 1.53 | 24.04 ± 0.87 |
| C18:3 (α-Linolenic, ω-3) | 9.72 ± 0.70 * | 0.61 ± 0.02 |
| ∑ PUFA | 31.60 ± 2.23 * | 24.65 ± 0.88 |
| ∑ UFA | 75.38 ± 5.40 | 72.74 ± 3.19 |
| PUFA:SFA | 1.30 ± 0.04 * | 0.92 ± 0.01 |
| MUFA:PUFA | 1.39 ± 0.00 | 1.95 ± 0.02 * |
| ω-6/ω-3 | 2.25 ± 0.00 * | 39.43 ± 1.54 * |
| Parameter | Peel | Seed |
|---|---|---|
| DPPH (mmol TEAC/kg oil) | 15.49 ± 0.91 | 14.97 ± 0.66 |
| PV (mmol H2O2/kg oil) | 16.95 ± 0.76 * | 13.50 ± 0.27 |
| CD (mmol/kg oil) | 33.56 ± 2.52 * | 11.51 ± 0.51 |
| CT (mmol/kg oil) | 10.52 ± 0.36 * | 1.67 ± 0.13 |
| Sample | Assigned Pigment | λmax (nm) | %III/II | Concentration (mg/kg Oil) |
|---|---|---|---|---|
| Seed oil | Lutein | 420, 442, 470 | 83.80% | 29.2 ± 2.16 |
| Peel oil | α-Carotene | 432, 470 | n.d. | 253.1 ± 15.95 |
| Peel oil | Chlorophyll a | 661 | — | 209.4 ± 9.76 |
| Design Point | Independent Variables | Actual Responses * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Waste (X1) | Power (X2, %) | Ratio (X3, mL/g) | Ethanol (X4, % v/v) | Time (X5, min) | TPC | FRAP | DPPH | AAC | |
| 1 | Seed | 70 | 40 | 50 | 15 | 53.84 | 275.03 | 235.1 | 6.18 |
| 2 | Seed | 40 | 70 | 0 | 5 | 17.18 | 407.16 | 54.21 | 5.82 |
| 3 | Peel | 40 | 10 | 100 | 15 | 3.72 | 77.69 | 160.59 | 1.9 |
| 4 | Seed | 100 | 10 | 100 | 25 | 32.01 | 369.81 | 291.93 | 2.72 |
| 5 | Peel | 40 | 40 | 0 | 15 | 43.83 | 546.7 | 389.09 | 12.73 |
| 6 | Peel | 40 | 70 | 50 | 5 | 71.87 | 568.65 | 586 | 14.81 |
| 7 | Seed | 70 | 40 | 50 | 15 | 53.79 | 272.46 | 243.16 | 6.43 |
| 8 | Peel | 70 | 70 | 100 | 15 | 10.2 | 130.45 | 488.92 | 11.36 |
| 9 | Peel | 100 | 40 | 100 | 25 | 18.17 | 203.12 | 401.18 | 8.08 |
| 10 | Seed | 100 | 70 | 100 | 5 | 41.05 | 408.65 | 1103.76 | 8.09 |
| 11 | Peel | 70 | 70 | 0 | 25 | 51.26 | 449.8 | 480.74 | 17.19 |
| 12 | Peel | 40 | 10 | 0 | 5 | 58.8 | 68.32 | 327.66 | 5.59 |
| 13 | Peel | 40 | 40 | 50 | 25 | 88.4 | 529.99 | 422.69 | 10.68 |
| 14 | Seed | 100 | 10 | 0 | 5 | 26.22 | 95.02 | 96.69 | 2.45 |
| 15 | Seed | 70 | 40 | 50 | 15 | 51.18 | 257.28 | 230.27 | 6.02 |
| 16 | Seed | 40 | 10 | 0 | 25 | 25.78 | 149.16 | 105.78 | 2.94 |
| 17 | Peel | 100 | 70 | 50 | 15 | 103.14 | 566.51 | 420.87 | 13.78 |
| 18 | Seed | 100 | 70 | 0 | 25 | 28.89 | 174.59 | 136.52 | 4.67 |
| 19 | Peel | 100 | 10 | 100 | 5 | 11.53 | 93.69 | 53.58 | 2.02 |
| 20 | Peel | 100 | 10 | 0 | 25 | 67.32 | 371.43 | 278.81 | 5.33 |
| 21 | Seed | 40 | 70 | 100 | 25 | 51.04 | 265.98 | 240.95 | 7.95 |
| 22 | Seed | 70 | 40 | 50 | 15 | 52.65 | 263.33 | 282.13 | 6.16 |
| 23 | Peel | 100 | 70 | 0 | 5 | 78.97 | 514.63 | 423.45 | 15.59 |
| 24 | Seed | 40 | 10 | 100 | 5 | 36.57 | 67.29 | 102.85 | 2.48 |
| 25 | Peel | 70 | 10 | 50 | 25 | 90.86 | 602.39 | 207.89 | 4.87 |
| 26 | Peel | 70 | 40 | 100 | 5 | 17.41 | 61.61 | 404 | 5.54 |
| Term/Source | TPC | FRAP | DPPH | AAC |
|---|---|---|---|---|
| Model (overall) | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** |
| X1 − Waste | 0.0008 ** | 0.0085 ** | 0.0042 ** | <0.0001 *** |
| X2 − Power | 0.0973 (ns) | 0.828 (ns) | 0.0077 ** | 0.154 (ns) |
| X3 − Ratio | 0.1962 (ns) | 0.0011 ** | <0.0001 *** | <0.0001 *** |
| X4 − Ethanol concentration | 0.0041 ** | 0.0268 * | 0.0003 *** | 0.0001 *** |
| X5 − Time | 0.4163 (ns) | 0.067 (trend) | 0.047 * | 0.176 (ns) |
| X1 × X2 | 0.0733 (trend) | – | 0.0002 *** | – |
| X1 × X3 | – | – | 0.222 (ns) | <0.0001 *** |
| X1 × X4 | <0.0001 *** | 0.0007 *** | 0.0002 *** | <0.0001 *** |
| X1 × X5 | – | 0.053 (trend) | 0.025 * | 0.083 (trend) |
| X2 × X3 | 0.053 (trend) | 0.067 (trend) | 0.012 * | – |
| X2 × X4 | 0.0838 (trend) | 0.233 (ns) | 0.018 * | 0.046 * |
| X2 × X5 | – | 0.062 (trend) | – | 0.011 * |
| X3 × X4 | 0.0693 (trend) | 0.0227 * | 0.0002 *** | – |
| X3 × X5 | – | 0.0018 ** | 0.0045 ** | – |
| X4 × X5 | – | – | 0.027 * | – |
| X22 | 0.0315 * | 0.0333 * | – | – |
| X32 | – | – | 0.280 (ns) | 0.106 (trend) |
| X42 | <0.0001 *** | 0.0008 *** | – | 0.039 * |
| X52 | 0.0087 ** | – | 0.048 * | – |
| Lack of fit | 0.0043 * | 0.0015 * | 0.048 * | 0.0177 * |
| R2/Adj. R2 | 0.961/0.919 | 0.934/0.850 | 0.972/0.924 | 0.989/0.979 |
| Response | Waste (X1) | Power (X2, %) | Ratio (X3, mL/g) | Ethanol (X4, % v/v) | Time (X5, min) | Desirability | Maximum Predicted Response |
|---|---|---|---|---|---|---|---|
| TPC | Peel | 100 | 70 | 39 | 5 | 0.9465 | 105.98 ± 12.94 |
| FRAP | Peel | 40 | 70 | 20 | 24 | 0.9458 | 673.89 ± 141 |
| DPPH | Seed | 100 | 70 | 100 | 5 | 0.8776 | 1071.31 ± 131.16 |
| AAC | Peel | 40 | 70 | 0 | 25 | 0.9919 | 17.9 ± 1.18 |
| Responses | TPC | FRAP | DPPH | AAC |
|---|---|---|---|---|
| TPC | – | 0.738 | 0.308 | 0.530 |
| FRAP | – | 0.425 | 0.688 | |
| DPPH | – | 0.587 | ||
| AAC | – |
| Response | Waste (X1) | Power (X2, %) | Ratio (X3, mL/g) | Ethanol (X4, % v/v) | Time (X5, min) | PLS Regression | Desirability | Experimental Values |
|---|---|---|---|---|---|---|---|---|
| TPC | Peel | 100 | 70 | 45 | 5 | 105.36 | 0.7359 | 87.79 ± 5 |
| FRAP | 578.38 | 741.4 ± 43.93 | ||||||
| DPPH | 565.01 | 616.07 ± 13.94 | ||||||
| AAC | 14.64 | 10.8 ± 0.66 |
| No | Polyphenolic Compound | mg/L Extract | mg/g Dry Weight |
|---|---|---|---|
| 1 | 3-Hydroxytyrosol | 8.46 ± 0.61 | 0.34 ± 0.02 |
| 2 | Catechin | 15.54 ± 0.5 | 0.62 ± 0.02 |
| 3 | Chlorogenic acid | 41.48 ± 1.62 | 1.65 ± 0.06 |
| 4 | Vanillic acid | 30.05 ± 1.11 | 1.2 ± 0.04 |
| 5 | Homovanillic acid | 8.88 ± 0.64 | 0.35 ± 0.03 |
| 6 | Epicatechin | 6.18 ± 0.16 | 0.25 ± 0.01 |
| 7 | Catechin derivative 1 | 55.34 ± 3.27 | 2.21 ± 0.13 |
| 8 | Catechin derivative 2 | 29.38 ± 1.62 | 1.17 ± 0.06 |
| 9 | Catechin derivative 3 | 40.18 ± 1 | 1.6 ± 0.04 |
| 10 | Catechin derivative 4 | 12.42 ± 0.32 | 0.5 ± 0.01 |
| 11 | Catechin derivative 5 | 7.76 ± 0.51 | 0.31 ± 0.02 |
| 12 | Luteolin-7-glucoside | 7.34 ± 0.35 | 0.29 ± 0.01 |
| Total identified | 263.03 ± 11.71 | 10.48 ± 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrantonaki, A.; Theodoropoulou, V.-I.; Mantiniotou, M.; Athanasiadis, V.; Bozinou, E.; Lalas, S.I. Optimization of Polyphenol-Rich Extracts from Defatted Avocado Peel and Seed Residues Using Ultrasound-Assisted RSM: Antioxidant Potential and Valorization Prospects. Antioxidants 2025, 14, 1293. https://doi.org/10.3390/antiox14111293
Petrantonaki A, Theodoropoulou V-I, Mantiniotou M, Athanasiadis V, Bozinou E, Lalas SI. Optimization of Polyphenol-Rich Extracts from Defatted Avocado Peel and Seed Residues Using Ultrasound-Assisted RSM: Antioxidant Potential and Valorization Prospects. Antioxidants. 2025; 14(11):1293. https://doi.org/10.3390/antiox14111293
Chicago/Turabian StylePetrantonaki, Argyro, Vasiliki-Ioanna Theodoropoulou, Martha Mantiniotou, Vassilis Athanasiadis, Eleni Bozinou, and Stavros I. Lalas. 2025. "Optimization of Polyphenol-Rich Extracts from Defatted Avocado Peel and Seed Residues Using Ultrasound-Assisted RSM: Antioxidant Potential and Valorization Prospects" Antioxidants 14, no. 11: 1293. https://doi.org/10.3390/antiox14111293
APA StylePetrantonaki, A., Theodoropoulou, V.-I., Mantiniotou, M., Athanasiadis, V., Bozinou, E., & Lalas, S. I. (2025). Optimization of Polyphenol-Rich Extracts from Defatted Avocado Peel and Seed Residues Using Ultrasound-Assisted RSM: Antioxidant Potential and Valorization Prospects. Antioxidants, 14(11), 1293. https://doi.org/10.3390/antiox14111293