Abstract
This study aimed to investigate the effects of the oat hay feeding method and compound probiotics (CMP) on the growth, health, serum antioxidant and immune indicators, rumen fermentation, and bacteria community of dairy calves from 3 to 5 months of age. Forty-eight female Holstein calves (80 ± 7 days of age, 93.71 ± 5.33 kg BW) were selected and randomly divided into four groups. A 2 × 2 factorial design was adopted for the experiment, with the factors of the oat hay feeding method (fed as free-choice or 16.7% in the diet) and compound probiotics (CMP) inclusion (0.15% or 0%) in the pelleted starter. The results showed that, compared with giving oat hay as free-choice, feeding a diet of 16.7% oat hay increased the pelleted starter intake at 1–84 d (p < 0.05), with an average daily gain (ADG) at 61–84 d (p = 0.02); adding CMP to the pelleted starter did not significantly affect body weight, and reduced the fecal index (p < 0.05). Feeding 16.7% oat hay increased the concentration of IgA, IgG, and IgM (p < 0.01), while adding CMP increased the catalase (p < 0.01) and decreased the concentration of malondialdehyde (p < 0.01) in serum. Feeding 16.7% oat hay increased the ruminal concentration of propionic acid (p < 0.05) and isobutyric acid (p = 0.08), and decreased the ruminal pH (p = 0.08), the concentration of acetic acid (p < 0.05), and the ratio of acetic acid to propionic acid (p < 0.01). Feeding 16.7% oat hay reduced the relative abundance of ruminal Firmicutes, Unidentified-Bacteria, Actinobacteria, Prevotella, NK4A214-group, Olsenella, and Actinobacteriota (p < 0.05); adding CMP increased the relative abundance of ruminal Prevotella, Rikenellaceae-RC9-gut-group, Ruminococcus, NK4A214-group, and Ruminococcus (p < 0.05), and decreased the abundance of Desulfobacterora, Prevotella-7, and Erysipelotricaceae-UCG-002 (p < 0.05). In conclusion, feeding a diet of 16.7% oat hay increased the pelleted starter intake and average daily gain, while slightly reducing the ruminal pH values; adding CMP to the pelleted starter resulted in reduced diarrhea incidence, increased serum antioxidant capacity and immunity, as well as ruminal richness and diversity of microorganisms in dairy calves from 3 to 5 months of age.
1. Introduction
The health and growth performance of calves are not only related to the economic benefits of producers, but also related to the stable growth of dairy herds and the sustainable development of dairy farms [1]. Research has shown that early nutritional regulation and feeding management can improve the growth, development, and health of calves [2]. Feeding high-level starter is helpful to rumen development, as the carbohydrates in it can produce butyric acid and propionic acid [3]. However, excessive readily fermentable carbohydrates can cause a rapid decrease in ruminal pH, leading to ruminal acidosis [4]. Studies have shown that providing hay may help to avoid ruminal and hindgut acidosis in calves [5]. Adequate structural fiber in calves’ diet is crucial for stimulating rumen papilla development, chewing activity, and saliva secretion, which are necessary for healthy rumen and gut function [6]. Therefore, it is suggested that a certain amount of hay should be supplemented in calves’ diet. Some studies have investigated the effects of hay restriction on calves, but the results are inconsistent. Nemati et al. [7] provided 25% alfalfa hay for calves of 7–10 weeks, and their growth rate reached 900 g/d. Meta-analysis showed that calves fed with high-level forage gained more weight on average than calves fed with low-level forage [8]. Further research is needed to determine whether restricting hay feeding can affect the growth and development, gastrointestinal health, antioxidant and immune status of weaned calves.
There are complex microbial communities in the digestive tract of animals, and the health of the animals is highly correlated with the microbial communities in the gut [9]. Previously, antibiotics were widely used to prevent and treat gastrointestinal infections in livestock. However, antibiotic resistance had a long-term impact and caused damage to intestinal flora [10,11]. In 2014, the International Scientific Association for Probiotics and Prebiotics (ISAPP) emphasized the importance of probiotics in improving animal viability [12]. Microecological preparation includes probiotics, prebiotics, and synbiotics, which have beneficial effects on the host when applied in sufficient amounts [13]. After weaning, the digestive physiological function of the developing calves changes sharply. Many factors such as diet composition, growth, and development at this stage directly affect future productivity [14]. Probiotic supplementation in this period is helpful to reduce the exposure of developing rumen to harsh conditions, and to reduce the adverse effects on health and growth caused by weaning stress [15]. Since the beneficial effects of probiotics are strain dependent, it has been suggested that combinations of different probiotic strains may be more effective than single-strain probiotics [16].
According to the above literature, there is still controversy regarding the optimal hay level and feeding method in the diet of weaned dairy calves. In addition, further research is needed to determine whether there is a corresponding interaction between hay feeding methods and the addition of probiotics to the pelleted starter. Therefore, in this study, we hypothesized that restricted feeding of oat hay and compound probiotic supplementation can improve the growth performance and health of weaned calves. Based on these hypotheses, this study aimed to examine the effect and the mechanism of oat hay restriction and compound probiotics on growth, health, serum antioxidant and immune indicators, rumen fermentation, and bacteria communities of dairy calves from 3 to 5 months of age.
2. Materials and Methods
2.1. Animals and Experimental Design
The experiment was conducted from November to February at the experimental dairy farm of the South China Agricultural University (Hezhou, China). In this experiment, 48 weaned female Holstein calves (80 ± 7 days of age, 93.71 ± 5.33 kg of initial body weight; mean ± SE) were selected, and randomly divided into four treatment groups (n = 12). The study lasted for 84 days. Before the experimental period, all calves were fed the chopped oat hay and pelleted starter as free-choice. In the trial period, a 2 × 2 factorial design was adopted for the experiment, with the factors of the oat hay feeding method and compound probiotic (CMP) product inclusion in the pelleted starter. Dietary treatments were as follows: (1) chopped oat hay and pelleted starter fed as free-choice (F); (2) chopped oat hay and pelleted starter fed as free-choice, with 0.15% CMP added to the pelleted starter on an air dry basis (FP); (3) diet with 16.7% chopped oat hay and 83.3% pelleted starter on an air dry basis (L); (4) diet with 16.7% chopped oat hay and 83.3% pelleted starter, with 0.15% CMP added to the pelleted starter (LP). In order to facilitate feeding, weaned calves on scaled dairy farms in South China are usually fed with a 1:4 ratio of hay to pelleted starter. After calculation, the proportion of hay was 16.7%, and that of pelleted starter was 83.3%. The CMP product used in this experiment contained inactivated Lactobacillus acidophilus 107 cell count/g, Bacillus subtilis 107 colony-forming unit [CFU]/g, and Aspergillus oryzae 107 u/g, provided by Bioforte Biotechnology (Shenzhen) Co., Ltd. (Shenzhen, China). The ingredients and nutritional composition of the pelleted starter are listed in Table 1. The oat hay was cut into 3–4 cm lengths for feeding, and the nutritional components are listed in Table 1. The calves were fed with pelleted starter and oat hay twice daily at 8:00 am and 15:00 pm. The pelleted starter and oat hay were fed separately in two troughs in each pen. The pens were bedded with bamboo chaff and were refreshed every day. Manure was removed daily to keep the pens clean and dry. No animals were sick or were treated for sicknesses or vaccinated during the trial. The calves had free access to water. During the study, the temperature of the pens was 10–17 °C and the air humidity was 70–75%.

Table 1.
Nutrient composition of pelleted starter and oat hay (% of DM) a.
2.2. Feed Intake and Growth Performance
During the trial period, the feed amount of pelleted starter and oat hay for each group of experimental calves was recorded daily, and the residual feed was collected and weighed before each morning feeding to calculate the intake of pelleted starter and oat hay. All the calves were weighed and recorded on day 0, day 30, day 60, and day 84 of the trial period before morning feeding.
Pelleted starter and oat hay were collected every 10 days, dried in an oven (Model 2000; Experimental Mill, Beijing, China) at 65 °C for 48 h, then ground through a 1 mm screen using a Wiley mill (standard model 4; Arthur H. Thomas Co., Philadelphia, PA, USA). The dry matter (DM), crude protein (CP), ether extract (EE), calcium, and phosphorus were determined according to the Association of Official Analytical Chemists method [17]. The neutral detergent fiber (NDF) and acid detergent fiber (ADF) content were determined using the ANKOM A-200i fiber analyzer (Ankom Technology Corp., Fairport, NY, USA) according to the method of Van Soest et al. [18]. Starch was determined by colorimetry on a Synergy H1 Microplate Reader (BioTeck, San Leandro, CA, USA) [19].
2.3. Diarrhea Incidence
The calves’ feces were scored before morning feeding each day from day 31 to day 60 of the experiment. Briefly, a standard scoring procedure (1 = normal feces; 2 = semi-formed feces; 3 = loose feces; and 4 = watery feces) were used by two researchers who were blinded to the experimental groups. Diarrhea was recorded when the calf feces score was ≥3. The onset and duration of diarrhea were recorded. The diarrhea rate was calculated according to the procedure described by Sun et al. [20], and the formula was given as: diarrhea rate = (number of calves with diarrhea × days of diarrhea)/(total number of calves × examined days) × 100. The fecal consistency index (FCI) proposed by Marcondes et al. [21] was employed. The FCI was calculated at different stages of the experiment to judge the softness versus hardness of feces, as follows:
In the formula, dE1, dE2, dE3, and dE4 represent the days when the feces score was 1, 2, 3, and 4, respectively. Td represents the test evaluation days.
2.4. Blood Sampling and Analysis
Before the morning feeding on the 84th day of the trial period, 10 mL of blood was collected from the jugular vein of all calves, and the blood samples were left standing for 30 min and centrifuged at 3000 r/min for 15 min. The serum was collected into a 1.5 mL centrifuge tube, and stored at −20 °C for further analyses. The level of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), total antioxidant capacity (T-AOC), catalase (CAT), and malondialdehyde (MDA) in the serum were detected with kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The level of immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM) in the serum were determined by the enzyme-linked immunosorbent assay (ELISA) method using an enzyme-labeled analyzer (Rayto, Shenzhen, China).
2.5. Rumen Sampling and Analysis
Before the morning feeding on the 84th day of the trial period, 60 mL rumen fluid samples were collected from each calf with a rumen fluid oral collector (Kelibo A1164K, Wuhan, China). The samples were filtered through four layers of gauze, and the pH was immediately measured with a pH meter (Sartorius, PB-10, Gottingen, Germany). The concentration of ammoniacal nitrogen (NH3-N) was determined using the colorimetric method [22]. The content of volatile fatty acids (VFA) was quantified using a high-performance gas chromatograph (Aglient 7890B, Santa Clara, CA, USA) with an HP-INNOWax capillary column (30.0 m × 320 μm × 0.5 μm) and FID detector.
Extraction of the total DNA from rumen samples was performed using the CTAB method, according to the instructions provided with the commercial DNA extraction kit (Tiangen Biochemical Technology Company, Beijing, China). Selection of the V4 region of the 16SrDNA gene was conducted by PCR amplification. The universal primers were 515F (5’-GTGCCAGCMGCCGCGGTAA-3’-3’) and 806R (5’-GGACTACHVGG GTWTCTAAT-3’). All PCR reactions were carried out in 30 µL reactions with 15 µL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs, Beijing, China), 0.2 µM of forward and reverse primers, and about 10 ng of template DNA. The PCR products were sequenced by equimolar paired-end sequencing on the Illumina Novaseq 6000 platform (Personal Biotechnology Co., Ltd., Shanghai, China). Sequences analysis was performed with Uparse software (Uparse v7.0.1001) [23]. Sequences with ≥97% similarity were assigned to the same OTUs. A representative sequence for each OTU was screened for further annotation. For each representative sequence, the Silva Database [24] was used to annotate taxonomic information based on Mothur algorithm. In order to study the phylogenetic relationships of different OTUs, and the difference of the dominant species in different samples (groups), multiple sequence alignments were conducted using the MUSCLE software (Version 3.8.31) [25]. OTU abundance information was normalized using a standard of sequence numbers corresponding to the sample with the fewest sequences.
The α- and β-diversity of the bacterial communities were calculated with the QIIME (Version 1.7.0) software and the “vegan” package in R (Version 2.15.3) software [26], and β-diversity was analyzed with the “vegan” package in R software(version 4.2.1). The function of the bacterial community was predicted from the PICRUST database [27]. Linear discriminant analysis (LDA) effect size (LEfSe) and functional prediction of the microflora were carried out using an online tool (http://huttenhower.sph.harvard.edu/galaxy/) accessed on 15 September 2022, and LDA scores >2 and p < 0.05 were selected as cutoffs. All sequencing and analysis were performed by Novogene, Beijing, China.
2.6. Statistical Analysis
All results were analyzed using the MIXED procedure of SAS (version 9.4, SAS Institute Inc., Cary, NC, USA), and Tukey was used for multiple comparisons. The following model was used for statistical analysis:
where Yij = dependent variable, μ = population mean, Oi = feeding effect of oat hay, Pj = effect of CMP, OPij = interaction effect of oat hay and CMP, and εij = random residual. The outliers were processed based on the absolute studentized residual values >3. The threshold of significance was set at p < 0.05; trends were declared at 0.05 < p < 0.10.
Yij = μ + Oi + Pj + OPij + εij
3. Results
3.1. Intake and Growth Performance
In the current study, compared with the calves fed oat hay as free-choice, feeding 16.7% oat hay increased the pelleted starter and starch intake of calves at 31–60 d, 61–84 d, and 1–84 d (p < 0.05), the protein intake at 31–60 d (p < 0.05), and the average daily gain (ADG) at 61–84 d (p = 0.02); however, the oat hay intake decreased at 31–60 d, 61–84 d, and 1–84 d, the NDF intake at 61–84 d and 1–84 d (p < 0.05) (Table 2). Feeding CMP increased the oat hay intake at 1–30 d, and the protein and starch intake at 61–84 d (p = 0.05); however, the pelleted starter intake decreased at 61–84 d, the total feed intake at 61–84 d, and the NDF intake at 1–30 d and 61–84 d (p < 0.05), but did not significantly affect the body weight and daily gain of calves. In addition, the interaction between the oat hay feeding method and the CMP on the daily gain of calves at 31–60 d and 61–84 d, the pelleted starter intake at 1–30 d and 31–60 d, the oat hay intake at 1–30 d, the total feed intake at 1–30 d, the protein and starch intake at 1–30 d and 31–60 d, and the NDF intake at 1–30 d were observed (p < 0.05).

Table 2.
Effect of dietary treatments on the growth performance of dairy calves.
3.2. Diarrhea Incidence
According to Table 3, the oat hay feeding method had no effect on the diarrhea rate and fecal index of calves, and the addition of CMP to the pelleted starter significantly reduced the fecal index of calves (p < 0.05). There was no interaction between the oat hay feeding method and CMP.

Table 3.
Effects of dietary treatments on the diarrhea incidence in dairy calves.
3.3. Serum Antioxidant and Immune Indicators
According to Table 4, compared with the calves fed oat hay as free-choice, feeding 16.7% oat hay tended to decrease the serum concentration of GSH-Px (p = 0.08), and increased the serum content of IgA, IgG, and IgM significantly (p < 0.01). The addition of CMP to pelleted starter significantly increased the serum content of CAT (p < 0.01) and decreased the content of MDA (p < 0.01). The oat hay feeding method and CMP had interactive effects on the concentration of SOD (p = 0.05), CAT (p = 0.06), and MDA (p = 0.01) in serum.

Table 4.
Effects of dietary treatments on the serum antioxidant and immune indexes in dairy calves.
3.4. Rumen Fermentation Parameters
According to Table 5, compared with the calves fed oat hay as free-choice, feeding 16.7% oat hay increased the concentration of propionic acid (p < 0.05) and isovaleric acid (p = 0.08) in the rumen, and decreased the ruminal pH (p = 0.08), the concentration of acetic acid (p < 0.05), and the ratio of acetic acid to propionic acid (p < 0.01). Adding CMP to the pelleted starter did not significantly affect the rumen fermentation parameters of the calves. There was no interaction between the oat hay feeding method and CMP on the rumen fermentation parameters.

Table 5.
Effects of dietary treatments on the rumen fermentation parameters in dairy calves.
3.5. Rumen Bacteria Community
According to Figure 1A, there are 1593 OTUs shared by the four groups, which is 47.65% of the total. The number and proportion of OTUs in each experimental group were 2462 and 73.65% in the F group, 2246 and 67.19% in the FP group, 2265 and 67.75% in the L group, and 2520 and 75.38% in the LP group. There were 213, 169, 183, and 193 unique OTUs in the F, FP, L, and LP groups, respectively. According to Principal Coordinates Analysis (PCoA, Figure 1B), the first and second principal coordinates explained 20.18% and 10.00% of the variations, respectively. The samples from the calves fed oat hay as free-choice and 16.7% oat hay are far from each other, indicating that different oat hay feeding methods have a large impact on the rumen bacterial flora composition. The L group and LP group are partly overlapped, but mostly separated.
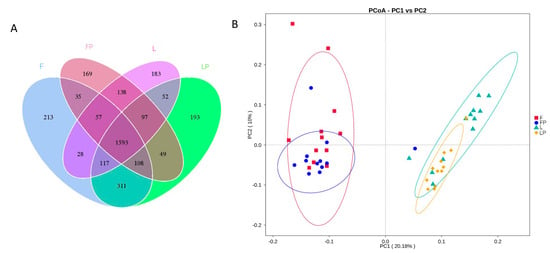
Figure 1.
Effects of different dietary treatments on the rumen bacteria flora in dairy calves: (A) Venn diagram of the OTUs; (B) Principal Coordinates Analysis (PCoA). (F, oat hay fed as free-choice; FP, oat hay fed as free-choice, with 0.15% CMP added to pelleted starter; L, fed with a diet of 16.7% oat hay; LP, fed with a diet of 16.7% oat hay, with 0.15% CMP added to pelleted starter).
According to Table 6, compared with the calves fed oat hay as free-choice, feeding 16.7% oat hay significantly decreased the ACE, Chao1, and Shannon indices (p < 0.01). Adding probiotics to the pelleted starter significantly increased the ACE, Chao, and Shannon indices (p < 0.05). There was an interaction between the oat hay feeding method and CMP on the Simpson and Shannon indices (p < 0.05).

Table 6.
Effects of dietary treatments on the community diversity of rumen bacteria in dairy calves.
At the phylum level, as shown in Table 7 and Figure 2A, feeding 16.7% oat hay reduced the relative abundance of Firmicutes, Actinobacteria, Unidentified-Bacteria, and Actinobacteriota (p < 0.05). Adding CMP to pelleted starter tended to increase the relative abundance of Fibrobacterota (p = 0.08) and decrease the relative abundance of Desulfobacterora (p < 0.05). The oat hay feeding method and CMP had interaction on the relative abundance of Unidentified-Bacteria (p = 0.06).

Table 7.
Effects of dietary treatments on the relative abundance of rumen bacteria flora in dairy calves (phylum level; %).
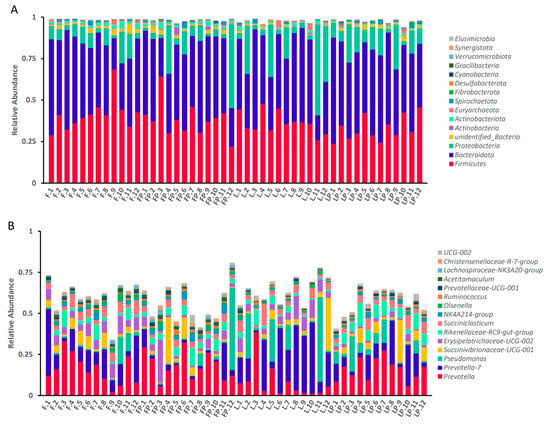
Figure 2.
Effect of dietary treatments on an accumulation map of rumen bacteria flora in dairy calves: (A) at the phylum level; (B) at the genus level. (F, oat hay fed as free-choice; FP, oat hay fed as free-choice, with 0.15% CMP added to pelleted starter; L, fed with a diet of 16.7% oat hay; LP, fed with a diet of 16.7% oat hay, with 0.15% CMP added to pelleted starter).
At the genus level, as shown in Table 8 and Figure 2B, feeding 16.7% oat hay increased the relative abundance of Prevotella_7 (p < 0.05), and decreased the relative abundance of Prevotella, NK4A214_group and Olsenella (p < 0.05). Adding CMP to pelleted starter increased the relative abundance of Prevotella, Rikenellaceae-RC9-gut-group, Ruminococus, NK4A214-group and Ruminococus (p < 0.05), and decreased the relative abundance of Prevotella-7 and Erysipelothiaceae-UCG-002 (p < 0.05). There was no interaction between oat hay and CMP at the genus level.

Table 8.
Effects of dietary treatments on the relative abundance of rumen bacteria flora in dairy calves (genus level; %).
LEfSe analysis showed that the 46 different bacterial strains in the four groups are (Figure 3). Twenty-three species are in the F group, 16 species in the FP group, four species in the L group, and three species in the LP group. At the class level, the Bacilli in the F group are highly enriched. At the order level, the FP group is highly enriched with the order of Oscillospirales.
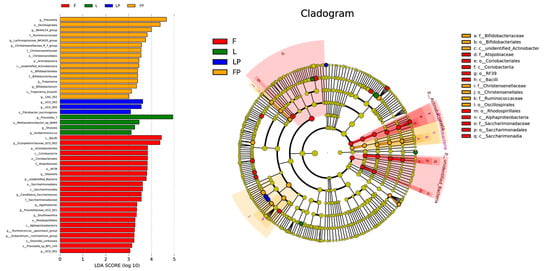
Figure 3.
LEfSe analysis of rumen bacterial flora in dairy calves. (F, oat hay fed as free-choice; FP, oat hay fed as free-choice, with 0.15% CMP added to pelleted starter; L, fed with a diet of 16.7% oat hay; LP, fed with a diet of 16.7% oat hay, with 0.15% CMP added to pelleted starter).
Pearson correlation analysis was carried out on the growth performance, fecal index, and ruminal fermentation parameters and the top 15 bacteria. As shown in Figure 4, Prevotella-7 was positively correlated with ADG at 1–84 d in calves (p < 0.05). Prevotella-7 and Prevotella-UCG-001 were positively correlated with pH (p < 0.05), while Pseudomonas in Proteobacteria was negatively correlated with pH (p < 0.05). Prevotella and NK4A214-group were positively correlated with the concentration of acetic acid (p < 0.05), while Prevotella-7 and Prevotellaceae-UCG-001 were negatively correlated with the concentration of acetic acid (p < 0.05). Erysipolitrichaceae-UCG-002 was negatively correlated with the concentration of butyric acid (p < 0.05). Prevotellaceae-UCG-001 in Bacteroidota was negatively correlated with the concentration of butyric acid and isobutyric acid (p < 0.05).
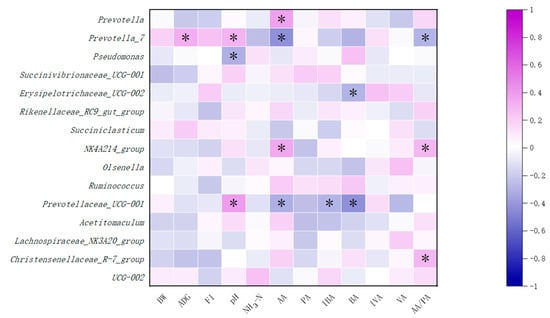
Figure 4.
Correlation analysis between ruminal microorganisms and fermentation parameters. Numbers in the figure represent Pearson’s correlation coefficients, “*” stands for significant correlation at p < 0.05. BW, body weight; ADG, average daily gain at 1–84 d; FI, fecal index; NH3-N, ammonia nitrogen; AA, acetic acid; PA, propionic acid; BA, butyric acid; IVA, isobutyric acid; VA, valeric acid; IVA, isovaleric acid.
4. Discussion
4.1. Dry Matter Intake and Growth Performance
Weaning is a critical period for calves, and growth in this period directly affects future production performance. Karami et al. [28] fed calves aged 70–120 days with diets containing different concentrate-to-forage ratios (50:50, 65:35, and 80:20), and found that decreasing the forage level increased the daily gain of calves linearly. Similarly, we also found that feeding 16.7% oat hay increased the ADG from calves fed oat hay as free-choice. The positive effect of forage on calf growth may be related to the improvement of the rumen environment and the enhancement of rumen muscle development [29], which in turn contributes to an increase in the solid feed intake in the late weaning period [30]. Meanwhile, feeding 16.7% oat hay increased the intake of protein and starch, which is also an important reason for the increased ADG of calves in our research. The feeding effect varies depending on the animal growth stage, environment, dosage of probiotics, strains, etc. Similar to the results of this experiment, the addition of CMP did not have significant effect on body weight and daily gain. Maamouri et al. [31] fed calves with 28 g/d of live yeast Saccharomyces cerevisiae, and found that the experimental group calves had a higher body weight and feed conversion rate, whereas Zhang et al. [32] supplemented with 1 × 108 cfu/d Lactobacillus plantarum and Bacillus subtilis for calves, and no significant difference was observed regarding DMI and ADG. In our study, the results indicate that the addition of CMP has no significant effect on the body weight and daily gain of calves. The possible reason could be that the beneficial effects of probiotics would be observed only when the animals are not in good health.
4.2. Diarrhea Incidence
Pre-weaning and post-weaning are two critical stages for calves, in which they are particularly susceptible to intestinal infectious diseases [33]. The mortality rate of calves due to diarrhea and gastrointestinal disease is as high as 56.5%, causing significant economic losses for global dairy farms [34]. Karamzadeh-Dehaghani et al. [35] found that feeding calves with 3 g/d compound probiotics can effectively reduce the incidence of diarrhea and fecal score of calves. Stefańska et al. [36] fed calves with 250 mg/d multi-strain probiotics composed of Lactobacillus casei, Lactobacillus salivarius, and Lactobacillus sake, and found that the fecal score was lower than that of the control group, which is consistent with our results. We found that adding CMP can reduce the fecal index of calves, and the reason may be that probiotics can produce antibacterial compounds in vivo, such as hydrogen peroxide, organic acids, and bacteriocin [37]. These compounds can eliminate pathogenic bacteria, improve mucosal immunity, and enhance intestinal health by establishing beneficial intestinal flora.
4.3. Serum Antioxidant and Immune Indicators
The concentration levels of blood biochemicals can reflect the health and nutritional level of animals [38]. MDA is the product of lipid peroxidation of cell membranes, and its content indirectly reflects the production of free radicals and the degree of lipid peroxidation of cells [39]. Guo et al. [40] fed calves with different doses of multi-strain probiotics, and found that the content of MDA in calf serum was higher than that without probiotics, which is in agreement with our results. The MDA level in calf serum was decreased in the group with CMP, and the CAT activity was significantly higher than in the other groups. CAT is an antioxidant enzyme that removes hydrogen peroxide from the body and protects the mitochondrial membrane from destruction. The increase of CAT activity indicates that the body’s defense performance is stronger [41]. The IgA, IgG, and IgM contents were significantly higher in the group adding CMP than in the group without CMP, which was similar to the research of Wu et al. [42], suggesting that the CMP can improve the immunity of calves in this study. Moreover, feeding 16.7% oat hay increased the content of IgA, IgG, and IgM significantly in serum, which may be due to the increase in the intake of pelleted starter. The higher content of protein, vitamins, and trace elements in pelleted starter can improve the immune function of calves.
4.4. Rumen Fermentation Parameters
Rumen is the main digestive organ of ruminants and plays a key role in the normal growth of calves. Rumen pH, NH3-N, and VFA are critical indexes to assess rumen health. Llamas-Lamas et al. [43] found that when the proportion of alfalfa hay in the diet increased from 56% to 86%, the rumen pH value of dairy cows increased significantly. This is similar to the results of the present study, where there was a downward trend in the rumen pH values of calves in the 16.7% oat hay group compared to those in the oat hay free-choice feeding group. The reason may be that insufficient dietary fiber or lack of fiber effectiveness reduced the chewing time of ruminants, which would lead to decreased saliva secretion and decreased rumen pH [44]. Moreover, the higher intake of starch can easily ferment in the rumen to produce a large amount of VFA, leading to a decrease in rumen pH. Jiang et al. [45] fed calves with 1 × 1010 cfu Lactobacillus plantarum daily, and found that the concentration of butyric acid and microbial protein were increased. However, in our study, the effect of CMP on rumen fermentation parameters was not found, which may be due to the joint action of multiple strains or the difference of dairy calves. Different concentrate-to-forage ratios in ruminant diets affect the intake of energy and nonstructural carbohydrates, thus affecting rumen fermentation [46]. In this study, feeding 16.7% oat hay reduced the acetic acid concentration and the acetic acid to propionic acid ratio, and increased the propionic acid and valeric acid concentration. Consistent with the results of this study, Olijhoek et al. [47] fed dairy cows with dietary concentrate to forage ratios of 49:51, 70:30, and 91:9. The results showed that, with the increase of concentrate in the diets, the ruminal concentration of propionic acid of increased, while the concentration of acetic acid decreased. Therefore, when the ratio of concentrate to forage in the diet increased, the rumen fermentation pattern of dairy cows would change accordingly.
4.5. Rumen Bacteria Community
An increase of rumen microbial diversity promotes the stability of bacterial communities in the rumen ecosystem [48]. In this study, the ACE, Chao1, and Shannon indices of the CMP group increased significantly. The results showed that feeding CMP increased the richness and diversity of rumen bacteria in calves, which helped to maintain the balance and stability of the gastrointestinal bacteria. At the phylum level, the dominant microflora in rumen of weaned calves are Firmicutes, Bacteroides, and Proteobacteria, which is consistent with the previous research of Mao et al. [49]. Firmicutes participate in the degradation of cellulose, hemicellulose, starch, and oligosaccharides, which is closely related to energy conversion and harvest [50]. With an increasing proportion of forage in the diet, the relative abundance of Firmicutes also increased [51], which was consistent with the results of this experiment. The relative abundance of Actinobacteria is negatively correlated with a high-fat diet and positively correlated with fiber intake [52]. In this study, feeding 16.7% oat hay reduced the contents of Actinobacterta and Actinobacteriota, which may be related to the difference in dietary structure.
Fibrobacterota are the main bacteria for rumen fiber degradation, and can digest and ferment low-quality forage to produce VFA [53]. In this study, the addition of CMP showed a trend to increase the relative abundance of Fibrobacterota, suggesting that CMP could affect rumen fermentation. Prevotella has been proved to be involved in the production of VFAs, which are further used as energy by the host [54]. Rikenellaceae-RC9-gut-group may reduce the methane production by participating in VFA production and hydrogen scavenging [55]. Ruminococcus is ubiquitous in the human intestine and ruminant rumen microorganisms, and plays an important role in the fermentation of cellulose-rich feed and resistant starch [56,57]. Feeding CMP increased the relative abundance of Prevotella, Rikenellaceae-RC9-gut-group, NK4A214-group, and Ruminococcus. Fermentation of these known bacteria is helpful to stabilize rumen pH, reduce ammonia concentration, and improve fiber digestibility [58,59,60]. It has been reported that Erysipolorichaceae-UCG-002 is related to VFA synthesis [61], but its specific function still needs to be explored. Prevotella-7 often participates in the inflammatory reaction by producing redox proteins or increasing resistance to the host [62]. Olsenella is a beneficial bacterium among actinomycetes, and its abundance is limited by a high-fat diet [63]. In this experiment, feeding 16.7% oat hay reduced the abundance of Prevotella, NK4A214-group, and Olsenella, which may be related to higher intake of pelleted starter.
5. Conclusions
This study revealed that, compared with calves fed oat hay as free-choice, feeding a diet of 16.7% chopped oat hay increased the pelleted starter intake and ADG of dairy calves. Adding CMP to the pelleted starter resulted in lower diarrhea incidence, higher serum immunity and antioxidant capacity, bacterial diversity and richness, relative abundance of beneficial bacteria, and lower abundance of harmful bacteria in the rumen. Therefore, based on the results of this study, it is recommended to moderately limit the feeding of chopped oat hay and add CMP to the pelleted starter for calves aged 3–5 months.
Author Contributions
Y.-Q.G. and Y.-R.H.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—review & editing. S.-R.L., M.W. and Z.-Y.X.: Investigation, Methodology, Project administration. D.-W.L., B.-L.S., Y.-K.L., G.-B.L., M.D. and W.-F.H.: Writing—review, Methodology. Q.-S.L.: Supervision, Writing review & editing, Methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Industry-University-Research Cooperation Project: The research and application of postbiotic preparations in dairy cows (H20210464), National Nature Science Foundation of China (31872382), Modern Agricultural Industrial Technology System of Guangdong Province (2022KJ127).
Institutional Review Board Statement
This study was conducted on the experimental dairy farm of the South China Agricultural University, Hezhou City, Guangxi, China. The animal study was reviewed and approved by the Ethical Committee of the South China Agricultural University (approval number SCAU#2013-10). All procedures involving dairy calf feeding, management, and animal welfare strictly followed the South China Agricultural University experimental guidelines during the experiment.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors gratefully thank all of the staff of the dairy farm for their assistance in feeding and care of the animals. We also acknowledge the members of the College of Animal Science of South China Agricultural University for their assistance with rumen and blood sampling.
Conflicts of Interest
The authors declare no conflict of interest.
References
- LeBlanc, S.J.; Lissemore, K.D.; Kelton, D.F.; Duffield, T.F.; Leslie, K.E. Major advances in disease prevention in dairy cattle. J. Dairy Sci. 2006, 89, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Kertz, A.F.; Hill, T.M.; Quigley Iii, J.D.; Heinrichs, A.J.; Linn, J.G.; Drackley, J.K. A 100-Year Review: Calf nutrition and management. J. Dairy Sci. 2017, 100, 10151–10172. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Alugongo, G.M.; Li, J.; Wang, Y.; Li, S.; Cao, Z. How forage feeding early in life influences the growth rate, ruminal environment, and the establishment of feeding behavior in pre-weaned calves. Animals 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Nagata, R.; Ohtani, N.; Ichijo, T.; Ikuta, K.; Sato, S. Effects of dietary forage and calf starter diet on ruminal pH and bacteria in Holstein calves during weaning transition. Front. Microbiol. 2016, 7, 1575. [Google Scholar] [CrossRef]
- Laarman, A.H.; Oba, M. Effect of calf starter on rumen pH of Holstein dairy calves at weaning. J. Dairy. Sci. 2011, 94, 5661–5664. [Google Scholar] [CrossRef]
- Diao, Q.; Zhang, R.; Fu, T. Review of strategies to promote rumen development in calves. Animals 2019, 9, 490. [Google Scholar] [CrossRef]
- Nemati, M.; Amanlou, H.; Khorvash, M.; Mirzaei, M.; Moshiri, B.; Ghaffari, M.H. Effect of different alfalfa hay levels on growth performance, rumen fermentation, and structural growth of Holstein dairy calves. J. Anim. Sci. 2016, 94, 1141–1148. [Google Scholar] [CrossRef]
- Imani, M.; Mirzaei, M.; Baghbanzadeh-Nobari, B.; Ghaffari, M.H. Effects of forage provision to dairy calves on growth performance and rumen fermentation: A meta-analysis and meta-regression. J. Dairy Sci. 2017, 100, 1136–1150. [Google Scholar] [CrossRef]
- O’Hara, E.; Neves, A.L.; Song, Y.; Guan, L.L. The role of the gut microbiome in cattle production and health: Driver or passenger? Annu. Rev. Anim. Biosci. 2020, 8, 199–220. [Google Scholar] [CrossRef]
- Tavoukjian, V. Faecal microbiota transplantation for the decolonization of antibiotic-resistant bacteria in the gut: A systematic review and meta-analysis. J. Hosp. Infect. 2019, 102, 174–188. [Google Scholar] [CrossRef]
- Andremont, A.; Cervesi, J.; Bandinelli, P.; Vitry, F.; de Gunzburg, J. Spare and repair the gut microbiota from antibiotic-induced dysbiosis: State-of-the-art. Drug Discov. Today 2021, 26, 2159–2163. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Vi, R.B.; McLeod, K.R.; Klotz, J.L.; Heitmann, R.N. Rumen development, intestinal growth and hepatic metabolism in the pre-and postweaning ruminant. J. Dairy Sci. 2004, 87, E55–E65. [Google Scholar]
- Cangiano, L.R.; Yohe, T.T.; Steele, M.A.; Renaud, D.L. Invited Review: Strategic use of microbial-based probiotics and prebiotics in dairy calf rearing. Appl. Anim. Sci. 2020, 36, 630–651. [Google Scholar] [CrossRef]
- Timmerman, H.M.; Koning, C.J.; Mulder, L.; Rombouts, F.M.; Beynen, A.C. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysiss, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.; Rose, C.L.; Omi, S.K.; Forry, K.R.; Durall, D.M.; Bigg, W.L. Starch determination by perchloric acid vs enzymes: Evaluating the accuracy and precision of six colorimetric methods. J. Agric. Food Chem. 1991, 39, 2–11. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Li, J.; Meng, Q.S.; Wu, D.L.; Xu, M. Effects of butyric acid supplementation of acidified milk on digestive function and weaning stress of cattle calves. Livest. Sci. 2019, 225, 78–84. [Google Scholar] [CrossRef]
- Marcondes, M.I.; Pereira, T.R.; Chagas, J.; Filgueiras, E.A.; Castro, M.; Costa, G.P.; Sguizzato, A.; Sainz, R.D. Performance and health of Holstein calves fed different levels of milk fortified with symbiotic complex containing pre-and probiotics. Trop. Anim. Health Prod. 2016, 48, 1555–1560. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Parente, E.; Zotta, T.; Ercolini, D. A comparison of bioinformatic approaches for 16S rRNA gene profiling of food bacterial microbiota. Int. J. Food Microbiol. 2018, 265, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Alikhani, M.; Khorvash, M.; Hashemzadeh, F.; Sadeghi-Sefidmazgi, A.; Rafiee, H.; Ferraretto, L.F. Effects of different forage to concentrate ratios on performance, plasma metabolites, and feeding behaviour of weaned dairy calves from 70 to 120 days of age. Ital. J. Anim. Sci. 2021, 20, 1317–1327. [Google Scholar] [CrossRef]
- Castells, L.; Bach, A.; Aris, A.; Terré, M. Effects of forage provision to young calves on rumen fermentation and development of the gastrointestinal tract. J. Dairy Sci. 2013, 96, 5226–5236. [Google Scholar] [CrossRef]
- Castells, L.; Bach, A.; Araujo, G.; Montoro, C.; Terré, M. Effect of different forage sources on performance and feeding behavior of Holstein calves. J. Dairy Sci. 2012, 95, 286–293. [Google Scholar] [CrossRef]
- Maamouri, O.; Ben Salem, M. The effect of live yeast Saccharomyces cerevisiae as probiotic supply on growth performance, feed intake, ruminal pH and fermentation in fattening calves. Vet. Med. Sci. 2022, 8, 398–404. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, M.; Tu, Y.; Zhang, N.F.; Deng, K.D.; Ma, T.; Diao, Q.Y. Effect of oral administration of probiotics on growth performance, apparent nutrient digestibility and stress-related indicators in Holstein calves. J. Anim. Physiol. Anim. Nutr. 2016, 100, 33–38. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Gao, Z.; Li, Q.; Qiu, X.; Wu, F.; Guan, T.; Cao, B.; Su, H. Effects of compound probiotics on growth performance, rumen fermentation, blood parameters, and health status of neonatal Holstein calves. J. Dairy Sci. 2022, 105, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Galarza, C.; LeBlanc, S.J.; Jones-Bitton, A.; DeVries, T.J.; Rushen, J.; de Passillé, A.M.; Endres, M.I.; Haley, D.B. Associations between management practices and within-pen prevalence of calf diarrhea and respiratory disease on dairy farms using automated milk feeders. J. Dairy Sci. 2018, 101, 2293–2308. [Google Scholar] [CrossRef] [PubMed]
- Karamzadeh-Dehaghani, A.; Towhidi, A.; Zhandi, M.; Mojgani, N.; Fouladi-Nashta, A. Combined effect of probiotics and specific immunoglobulin Y directed against Escherichia coli on growth performance, diarrhea incidence, and immune system in calves. Animal 2021, 15, 100124. [Google Scholar] [CrossRef] [PubMed]
- Stefańska, B.; Sroka, J.; Katzer, F.; Goliński, P.; Nowak, W. The effect of probiotics, phytobiotics and their combination as feed additives in the diet of dairy calves on performance, rumen fermentation and blood metabolites during the preweaning period. Anim. Feed. Sci. Technol. 2021, 272, 114738. [Google Scholar] [CrossRef]
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.T.; Hörmannsperger, G.; Huys, G. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef]
- Salisu, I.B.; Shahid, A.A.; Ali, Q.; Rao, A.Q.; Husnain, T. Nutritional assessment of dietary Bt and CP4EPSPS proteins on the serum biochemical changes of rabbits at different developmental stages. Front. Nutr. 2018, 5, 49. [Google Scholar] [CrossRef]
- Jia, F.; Dou, W.; Hu, F.; Wang, J. Effects of thermal stress on lipid peroxidation and antioxidant enzyme activities of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Fla. Entomol. 2011, 94, 956–963. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Z.; Deng, M.; Li, Y.; Liu, G.; Liu, D.; Liu, Q.; Liu, Q.; Sun, B. Effects of a multi-strain probiotic on growth, health, and fecal bacterial flora of neonatal dairy calves. Anim. Biosci. 2022, 35, 204. [Google Scholar] [CrossRef]
- Jiang, Z.; Lin, Y.; Zhou, G.; Luo, L.; Jiang, S.; Chen, F. Effects of dietary selenomethionine supplementation on growth performance, meat quality and antioxidant property in yellow broilers. J. Agric. Food Chem. 2009, 57, 9769–9772. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Luo, R.; Chen, H.; Nie, C.; Niu, J.; Chen, C.; Xu, Y.; Li, X.; Zhang, W. Effect of a multispecies probiotic mixture on the growth and incidence of diarrhea, immune function, and fecal microbiota of pre-weaning dairy calves. Front. Microbiol. 2021, 12, 681014. [Google Scholar] [CrossRef] [PubMed]
- Llamas-Lamas, G.; Combs, D.K. Effect of forage to concentrate ratio and intake level on utilization of early vegetative alfalfa silage by dairy cows. J. Dairy Sci. 1991, 74, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Erdman, R.A. Dietary buffering requirements of the lactating dairy cow: A review. J. Dairy Sci. 1988, 71, 3246–3266. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, H.J.; Cui, Z.Q.; Zhang, Y.G. Effects of supplementation with Lactobacillus plantarum 299v on the performance, blood metabolites, rumen fermentation and bacterial communities of preweaning calves. Livest. Sci. 2020, 239, 104120. [Google Scholar] [CrossRef]
- Sutton, J.D. Altering milk composition by feeding. J. Dairy Sci. 1989, 72, 2801–2814. [Google Scholar] [CrossRef]
- Olijhoek, D.W.; Hellwing, A.; Noel, S.J.; Lund, P.; Larsen, M.; Weisbjerg, M.R.; Børsting, C.F. Feeding up to 91% concentrate to Holstein and Jersey dairy cows: Effects on enteric methane emission, rumen fermentation and bacterial community, digestibility, production, and feeding behavior. J. Dairy Sci. 2022, 105, 9523–9541. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, P.; Wang, L.; Zhao, Z.; Chen, Y.; Yang, Y. Bacterial community diversity associated with different levels of dietary nutrition in the rumen of sheep. Appl. Microbiol. Biotechnol. 2017, 101, 3717–3728. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Zhang, M.; Liu, J.; Zhu, W. Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: Membership and potential function. Sci. Rep. 2015, 5, 16116. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Parmar, N.R.; Solanki, J.V.; Patel, A.B.; Shah, T.M.; Patel, A.K.; Parnerkar, S.; Kumar JI, N.; Joshi, C.G. Metagenome of Mehsani buffalo rumen microbiota: An assessment of variation in feed-dependent phylogenetic and functional classification. J. Mol. Microb. Biotech. 2014, 24, 249–261. [Google Scholar] [CrossRef]
- Geurts, L.; Neyrinck, A.M.; Delzenne, N.M.; Knauf, C.; Cani, P.D. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: Novel insights into molecular targets and interventions using prebiotics. Benef. Microbes 2014, 5, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Wang, R.; Ma, Z.Y.; Zhang, X.M.; Jiao, J.Z.; Zhang, Z.G.; Ungerfeld, E.M.; Yi, K.L.; Zhang, B.Z.; Long, L. Dietary selection of metabolically distinct microorganisms drives hydrogen metabolism in ruminants. ISME J. 2022, 16, 2535–2546. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Hehemann, J.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Daghio, M.; Ciucci, F.; Buccioni, A.; Cappucci, A.; Casarosa, L.; Serra, A.; Conte, G.; Viti, C.; McAmmond, B.M.; Van Hamme, J.D. Correlation of breed, growth performance, and rumen microbiota in two rustic cattle breeds reared under different conditions. Front. Microbiol. 2021, 12, 652031. [Google Scholar] [CrossRef] [PubMed]
- Ze, X.; Duncan, S.H.; Louis, P.; Flint, H.J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012, 6, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Moraïs, S.; Mizrahi, I. The road not taken: The rumen microbiome, functional groups, and community states. Trends Microbiol. 2019, 27, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Nocek, J.E.; Kautz, W.P.; Leedle, J.; Allman, J.G. Ruminal supplementation of direct-fed microbials on diurnal pH variation and in situ digestion in dairy cattle. J. Dairy Sci. 2002, 85, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.H.; Shan, A.S.; Ma, N.; Ma, Q.Q.; Sun, Z.W. Effect of supplemental Bacillus cultures on rumen fermentation and milk yield in Chinese Holstein cows. J. Anim. Physiol. Anim. Nutr. 2010, 94, 429–436. [Google Scholar] [CrossRef]
- Jeyanathan, J.; Martin, C.; Eugène, M.; Ferlay, A.; Popova, M.; Morgavi, D.P. Bacterial direct-fed microbials fail to reduce methane emissions in primiparous lactating dairy cows. J. Anim. Sci. Biotechnol. 2019, 10, 41. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Yang, C.; Zhang, J.; Kalwar, Q.; Liang, Z.; Li, C.; Du, M.; Yan, P.; Long, R.; Han, J. Effects of dietary energy levels on rumen fermentation, microbial diversity, and feed efficiency of yaks (Bos grunniens). Front. Microbiol. 2020, 11, 625. [Google Scholar] [CrossRef]
- Hofer, U. Pro-inflammatory prevotella? Nat. Rev. Microbiol. 2014, 12, 5. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).