Abstract
The snack market is shifting toward healthier options, leading to a growing interest in organic snacks. Dried fruits are particularly popular due to their long shelf life and convenience. Freeze-drying helps preserve both the taste and nutrients of these fruits. Among them, peaches are noteworthy for their antioxidant and anti-inflammatory properties. The research assessed the impact of lactic fermentation using Lactiplantibacillus plantarum (P_LP) and Fructilactobacillus fructivorans (P_FF), followed by freeze-drying, on the physicochemical, structural, and sensory properties of peach slices. Fermentation increased acidity (>22 mg/kg), decreased sugars (up to 43.5%), and raised salt content (to ~0.5%), effectively altering the fruit’s chemical profile. Dry matter content decreased by 6.0% (P_LP) and 7.2% (P_FF), while water activity remained low (0.13–0.15). Color parameters changed notably: L* values decreased, and a* values increased, with total color differences (ΔE) exceeding 15. Structural changes included higher porosity (to 71.4% in P_LP and 72.8% in P_FF) and reduced hardness from 50.1 N (control) to 35.7 N (P_LP) and 28.2 N (P_FF), which may benefit processing. Water sorption isotherms suggested improved stability under elevated humidity. However, sensory analysis showed lower consumer acceptance of the fermented samples due to reduced sweetness, crunchiness, and overall palatability, along with undesirable flavors from F. fructivorans. While lactic fermentation holds the potential for creating fruit snacks with better functional value, further optimization is needed to enhance sensory appeal and market potential.
1. Introduction
The peach (Prunus persica) belongs to the genus Rosaceae. It is a drupe, inviting with its sweet taste and juicy flesh []. The country of origin of this fruit is China, where it is a symbol of longevity and prosperity due to its shape and sweetness. Considering economic and nutritional aspects, peaches are the second most important leafy fruit crop in the world []. The European Union is the second largest producer of this fruit after China. In 2017, the European Union’s production of peaches and related fruits totaled 3.9 million tons, of which 760,000 tons were for processed products, which in the case of peaches are very popular and include canned peach products, pureed peaches, and peach juice []. In addition to their taste, peach fruits are valued for their nutritional properties. They are a rich source of essential nutrients, such as vitamin C, flavonoids, carotenoids, anthocyanins, and retinol []. Moreover, peach phenolic compounds, such as quercetin, catechins, and cyanidin derivatives, have antioxidant, antimicrobial, and anti-inflammatory effects [].
The food market is very diverse, especially for the snacks segment, which continues to gain popularity among consumers []. The global snacking industry is steadily growing, and for many consumers, snacks have become a vital part of the daily diet []. Unfortunately, many commercially available snacks are low in nutritional value and contribute to health issues such as obesity and hypertension []. The current trend in the snack market is for manufacturers to emphasize their reduced fat and salt content in the formulation and increase protein, fiber, starch, and phytochemicals []. The market’s demand for foods with greater nutritional value poses a challenge for food producers, especially when producing dried fruits with desirable sensory qualities. Consumer expectations for snacks are evolving; they now seek not only good taste but also health benefits and convenience. Snacks made with organic and natural ingredients are becoming increasingly desirable. Additionally, visual appeal, particularly color, plays a key role in consumer acceptance [,].
The introduction of dried probiotic products has sparked rapid growth in this market niche []. Fermentation is a common food processing technology that allows food preservation. It not only extends shelf life but also enhances microbial safety by reducing the pH and inhibiting the growth of spoilage organisms. Lactic acid bacteria are commonly used in this context due to their effectiveness and functional properties [].
As the demand for healthier food options increases and fresh fruits encounter challenges related to perishability and seasonality, the transformation of fresh produce into stable, value-added products has emerged as a notable trend. Within this landscape, dried fruits have gained considerable popularity. Various drying methods effectively lower water content to safe levels, with freeze-drying recognized as one of the most efficient and widely adopted techniques []. In addition to increasing stability and extending expiration, dried products are more economical due to their reduced weight and volume, thus reducing packaging, transportation, and storage costs [].
The significance of this study can be attributed to its contribution to the development of innovative functional snacks that satisfy contemporary consumer demands for both health and taste. This research explores an innovative approach to creating shelf-stable, nutritious peach-based snacks enriched with probiotic benefits. The approach combines lactic acid fermentation with freeze-drying. In contradistinction to preceding studies, which predominantly concentrated on either fermentation or drying techniques in isolation, this study employed a distinctive approach by integrating both processes, thereby optimizing sensory and nutritional properties in a simultaneous manner. This dual-processing approach has been demonstrated to enhance the functional value of the product, while also preserving the desirable organoleptic characteristics that are essential for consumer acceptance. The objective of this study was to evaluate the technological suitability of freeze-dried peach snacks that have undergone fermentation using selected bacterial strains. The study applies a lactic fermentation process followed by freeze-drying of the resulting samples, with a focus on assessing both the functional and sensory properties of the final product.
2. Materials and Methods
2.1. Materials
The research material was peaches (Prunus persica L.) purchased from a market located in Warsaw, Poland. The fruits were selected based on two criteria to ensure they were at a similar level of ripeness: skin color and softness. We chose peaches that exhibited similar ripeness stages. Softness was assessed by gently pressing on the fruit, while changes in skin color served as an additional indicator of ripeness. Each fruit was manually cut into six uniform segments using a sterile stainless steel knife. Fermentation was carried out using the material immediately after purchase. The biological material Lactiplantibacillus plantarum ATCC 4080 (LP) and Fructilactobacillus fructivorans DSM 20203 (FF) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures GmbH (DSMZ, Braunschweig, Germany).
The chemical reagents used for analysis were of analytical grade.
2.2. Technological Treatment
2.2.1. Fermentation
Due to our previous pre-tests, the fermentation process was carried out for six days at a constant temperature of 25 °C using an incubator (BD-S115, Binder, Tuttlingen, Germany). The fermented samples P_LP and P_FF were prepared using the same method. First, cut peach samples were placed into glass jars. Next, a solution of water with 0.5% NaCl was added. Following that, a 10% v/v inoculum was added into the jars with peaches. This amount of inoculum was linked to the cultures of selected strains at ~1 × 108 CFU/mL. Inoculum was prepared in 0.85% NaCl solution using densimat (BioMerieux, Craponne, France). These conditions ensured consistent inoculum distribution and facilitated the effective progression of lactic acid fermentation. Finally, the jars were sealed to prevent air from entering.
2.2.2. Freeze-Drying
The plant material was frozen to −40 °C using a shock freezer (HCM 51.20, Irinox, Treviso, Italy). After 10 h of freezing, the samples were transferred to a freeze dryer (ALPHA 1–4, Christ, Osterode, Germany) and subjected to lyophilization. The drying process was carried out under the following conditions: shelf temperature of 25 °C, chamber pressure of 63 Pa, condenser temperature of −50 °C, and a total drying time of 48 h. The dried material was stored at 24 °C in polyethylene bags (PET12/Al8/PE100), providing a barrier to light and moisture until further analysis.
2.3. Sensory Evaluation
The sensory profiling and overall acceptance evaluation were conducted by 30 trained panelists. In the sensory evaluation room, the panelists were asked to assess the selected samples encoded with three-digit numbers []. Participants assessed the following attributes: color, intensity of odor, overall tastiness, hardness, crispness, acidity, sweetness, saltiness, and overall product quality. A 5-point hedonic scale was used, allowing scores with one decimal precision, where 1 represented the lowest and 5 represented the highest level of acceptability. The participants were informed of the purpose of the survey and that the responses would be anonymous. No formal relationship was used to recruit participants to the study. The mean, variance, and standard deviation were calculated for each attribute of all samples and sessions individually. This part was conducted at the University of Life Sciences in Poznan.
2.4. Physical Evaluation
2.4.1. Dry Matter and Water Activity
The gravimetric method was used for dry matter determination. Approximately 1 g of material was weighed into prepared vessels and dried in an oven at 70 °C for 24 h.
The water activity was determined using an Aqualab water activity meter (Decagon Devices Inc., Pullman, WA, USA) with an accuracy of ±0.001. All measurements were conducted in triplicate.
2.4.2. Color of Samples
The color coordinates of the samples were determined using the CR-5 spectrometer (Konica Minolta Sensing Inc., Osaka, Japan) in the CIE L*a*b* system. The measurement parameters were Illuminant D65, angle 2°, and calibration with white and black standards. All measurements were made in ten repetitions. The total color difference (ΔE) and chroma (C*) were also calculated [].
2.4.3. Structure
- Light Microscopy (LM)
Digital imaging of the 3D constructs was performed using a VHX-950F microscope (Keyence, Neu-Isenburg, Germany) under magnifications of 5×.
- Scanning Microscope (SEM)
To obtain information on the internal structure of the dried material, a 0.5 cm thick fragment was cut and mounted on an aluminum sample holder using carbon tape. To improve surface conductivity, the material was coated with a 5 nm gold layer using a sputter coater (Leica EM ACE200; Leica Mikrosysteme GmbH, Vienna, Austria). Observations were performed using a scanning electron microscope (Phenom XL, Phenom-World, Eindhoven, The Netherlands) operated at an accelerating voltage of 10 kV and under a pressure of 60 Pa. Images were acquired at 150× magnification.
- Microtomography (µCT)
A sample with dimensions of 2 × 3 cm (width × height) was cut from the dried material using a razor blade and vertically mounted on a metal stage. Scans were performed using an X-ray source operating at 50 kV and 200 μA. The analysis was carried out with a rotation step of 0.3° and a spatial resolution of 13.4 µm. The total scanning time for each sample was 1 h and 25 min. The obtained images were reconstructed based on 100 projections using NRecon software version 1.6.10 (Bruker, Billerica, MA, USA). Cross-sectional views in the X, Y, and Z directions were obtained using DataViewer version 1.5.2.6 (Bruker). The reconstructed datasets were imported into CTAn software (Bruker, Kontich, Belgium) and binarized using a threshold range of 20–220. A three-dimensional analysis was performed to determine the total porosity, pore size distribution, and Euler number [].
2.4.4. Texture Measurement
The texture of dried samples was assessed using a TA.XT2i Texture Analyser (Stable Micro Systems, Godalming, UK). Textural parameters were determined via a compression test. Samples (size 1 × 1 cm) were placed on the instrument platform and compressed at randomly selected points using a 20 mm diameter aluminum platen probe, operating at a crosshead speed of 1 mm/s. The test was terminated when the sample was compressed to 50% of its original height. The maximum force required to deform the sample was recorded from the resulting force–distance curves []. Each measurement was performed in ten replicates per sample.
2.4.5. Hygroscopicity and Sorption Isotherm
Hygroscopicity was assessed by measuring the water vapor uptake of the samples under controlled conditions at a water activity level of 0.75. For this purpose, freeze-dried peach samples were cut into pieces of similar weight, accurately weighed, and placed in a desiccator containing a saturated sodium chloride solution to maintain the desired humidity []. The samples were removed at predetermined intervals, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 12, 24, 48, and 72 h, and their mass was recorded after each time point.
Hygroscopicity (H) was calculated using the following equation:
where
H = (uτ − u0)/d.m.
uτ—moisture content of the sample at a given time τ during the hygroscopicity test (kg H2O),
u0—initial moisture content of the sample (kg H2O),
d.m.—dry matter content of the sample (kg/kg).
Sorption isotherms were determined using an Aquadyne DVS analyzer (Quantachrome, Boynton Beach, FL, USA), which automatically controlled the relative humidity (RH) and continuously recorded changes in sample mass over time []. Approximately 25 mg of each sample was placed on quartz weighing pans. The analysis was conducted at 25 °C, across a relative humidity range from 0.3% to 75.3%, divided into 10 steps of varying RH increments. Before the measurement, the samples were pre-dried in a vacuum oven (30 °C, 10 mPa) for 48 h. This was followed by an additional drying step in the DVS analyzer for 180 min. During the sorption experiment, the RH was increased stepwise, with each new humidity level applied only when the change in sample mass was less than 0.001% per minute and not earlier than 180 min. For RH levels above 70%, this stabilization period was extended to at least 210 min per step.
2.5. Chemical
2.5.1. Total Acidity
The total acidity (TA) was determined using a titration meter and a pH meter (SevenCompact S210, Mettler–Toledo GmbH, Greifensee, Switzerland) []. Approximately 5 g of the sample (m) was weighed and diluted with distilled water to a total volume of 150 mL, followed by boiling. The hot solution was filtered through filter paper, and the filtrate was brought to a final volume of 50 mL with distilled water. Next, 25 mL of the filtrate was titrated using a 0.1 M sodium hydroxide (NaOH) solution until the pH of the sample reached 8.1. The total acidity was then calculated and expressed as a percentage of lactic acid according to the following equation:
where
Total acidity = (VNaOH · MNaOH · 90.08 · 100 · K)/m
VNaOH—volume of NaOH solution used (mL),
MNaOH—molarity of NaOH solution (mol/L),
90.08—molar mass of lactic acid (g/mol),
m—mass or volume of the analyzed sample (g or mL).
K—lactic acid coefficient = 0.09.
2.5.2. Salt Content
The salt content was determined using the Mohr method []. The preparation of the filtrate for determination was identical to that used in the total acidity analysis. Additionally, the solution was neutralized with 0.1 M NaOH. Subsequently, 1 mL of 5% concentrated potassium chromate solution (K2CrO4) was added, and the sample was titrated with 0.1 M silver nitrate (AgNO3) solution until a stable brick-red coloration appeared. The results were calculated using the following equation:
where
NaCl content (%) = (V ∙ M ∙ 0.05845/m) ∙ 100
V—volume of AgNO3 solution used for titration (mL),
M—molarity of AgNO3 solution (mol/L),
m—mass of the product in the titrated solution (g).
2.5.3. Total Sugar Content
The determination of the sugar content (TSC) was performed using liquid chromatography []. The analytical system was equipped with a quaternary pump (Waters 515, Milford, MA, USA), an autosampler (Waters 717), a column oven, and a refractive index detector (Waters 2414). For the extraction, 0.3 g of the sample was mixed with Milli-Q water (Direct-Q™ 5 Ultrapure, Water System from Millipore; Bedford, MA, USA) preheated to 80 °C. The mixture was incubated in a circular-vibrating shaker for 4 h to facilitate sugar extraction. The solution was centrifuged for 5 min at 4350 rpm and then filtered using a 0.22 µm hydrophobic PTFE syringe filter (Millex-FG, Millipore, Milford, MA, USA). A 1 µL aliquot of the filtrate was injected into the chromatographic column. Chromatographic separation was carried out on a Waters Sugar-Pak I column (300 × 6.5 mm). The mobile phase consisted of Milli-Q redistilled water and was delivered at a constant flow rate of 0.6 mL/min. The column oven was maintained at 90 °C, while the detector operated at 50 °C. The total sugar content was calculated as the sum of sucrose, fructose, and glucose. All measurements were conducted in duplicate.
2.6. Statistical Treatment
All data were analyzed using MS Excel 2019. Data are presented in graphs and in tables as mean ± standard deviation. The obtained results were subjected to a statistical analysis using Statistica 13 software (StatSoft, Warsaw, Poland). A one-way analysis of variance (ANOVA) followed by Tukey’s HSD post hoc test was performed to assess significant differences among all sample groups at a significance level of α = 0.05.
3. Results and Discussion
3.1. Basic and Color Attributes of Freeze-Dried Peach Samples
The freeze-dried peach sample (P) had the highest dry matter content at 96.9%. The fermented samples showed lower dry matter, about 6% (freeze-dried peach sample fermented with L. plantarum—P_LP) or 7% (freeze-dried peach sample fermented with I. fructivorans—P_FF) compared with the control (Table 1). This decrease suggested that the fermentation process in brine made the fruit tissue retain more water, which remained even after freeze-drying. During fermentation, water may be absorbed into the fruit tissue, while at the same time, water-soluble compounds can diffuse into the brine. The higher the salt content in brine, the faster this process was. In the presented study, the amount of salt was relatively small compared with those presented in the literature, usually from 2 to 10%.

Table 1.
Physical properties of dried samples.
Additionally, the reduction in sugar content was likely due to its consumption by lactic acid bacteria (LAB) during growth and lactic acid production. It was demonstrated that a minimal addition of sodium chloride (0.5%) to the brine could exert a favorable influence on the proliferation and viability of lactic acid bacteria. This was achieved by instigating the osmotic stress, to which the bacteria could adapt through a range of physiological and metabolic alterations [,]. Moreover, the loss of soluble substances, such as sugars and polyphenols, into the brine may further contribute to decreased dry matter content [,]. In a study comparing methods regardless of pretreatment, freeze-drying carrot slices resulted in higher dry matter content (93.6–95.8%) than other methods, such as convection, microwave convection, and microwave vacuum drying [].
The water activity (aw) was low and did not change much among the samples, ranging from 0.13 to 0.15 (Table 1). This meant that even though the fermented samples had more moisture, their resistance to microbial spoilage was similar to that of the control sample []. However, a low aw, below 0.3, meant that dried samples were considered microbiologically and chemically safe [].
When analyzing the samples as potential snack products, it was essential to consider their sensory attributes, such as texture, color, and taste, as these elements must be appealing to consumers to ensure product acceptance and market success. Among them, color is one of the most significant parameters that define food quality as it significantly influences consumer preferences and purchasing decisions [].
In the present study, color was used as one of the quality indicators to evaluate the quality of peach-based fermented snack products. The color parameters of the CIE L*a*b* system were analyzed to observe the impact of the fermentation process and the strain used on the product’s color. The results showed that the color parameters were dependent on the fermentation process. The control sample was also characterized by a lighter color as evidenced by the highest values of the L* parameter, the lowest intensity of the red color (the lowest value of a* parameter), and a higher yellow color (the highest value of b* parameter) (Table 1). Chroma (C*), representing color intensity, was the highest in the control and lower in the fermented samples, confirming that fermentation resulted in less vivid coloration. The parameter that could give us the final information that could influence the consumer choice was the total color difference (ΔE), calculated compared with the control. Its value was high in both fermented samples (18.2 for P_LP and 15.6 for P_FF), indicating a substantial visual change in color due to fermentation.
Fermentation with both strains (P_LP and P_FF) significantly influenced the color parameters of the freeze-dried peach samples. A decrease in lightness (L*) and yellow-blue components was observed; in contrast, the red-green coefficient was increased after fermentation (Table 1).
Hashemi et al. [] suggest that fermentation contributes to pigment degradation or enzymatic modifications affecting brightness. The increase in the red-green component (a*) indicates a shift toward a more intense red hue, possibly due to enhanced anthocyanin stability or Maillard reaction products formed during fermentation. This can be confirmed by the previous study of the fermentation of peach juice with Lactobacillus strains; authors have noted that it inhibits the Maillard reaction by converting reducing sugars into non-reducing forms, thereby inhibiting browning processes [].
The significant decrease in the yellow-blue component (b*) suggested that the breakdown/leakage from peach tissue of carotenoids was causing a loss of yellow pigments (which was confirmed by the carotenoid test, not presented here). These changes showed how fermentation strains could affect the color of the fruit, which may influence how consumers see and feel about the product []. Kao, Wang, Tsai and Lin [] have observed changes during the fermentation of cucumbers. After fermentation, the visual appearance differs significantly from the fresh product due to changes in chlorophyll. Moreover, differences in color parameters between the presented variants may be due to changes in the chemical composition of the matrix that occurred during fermentation, changes in their components, and interactions during processing.
3.2. Microstructure and Texture of Freeze-Dried Peaches
According to Nowak and Jakubczyk [], consumers prioritize color and structure-texture properties when evaluating foods. Structure-texture porosity is an important and characteristic feature of freeze-dried food, closely related to its physicochemical changes [].
A comprehensive evaluation of the internal structure and texture of freeze-dried peach samples was carried out using light microscopy (LM), scanning electron microscopy (SEM), and X-ray microtomography (μCT). The imaging techniques allowed visualization of the structural changes induced by lactic acid fermentation and the subsequent freeze-drying process.
As shown in Figure 1 (light microscope—LM), the peach freeze-dried sample (P), which had not undergone fermentation, showed a relatively compact and homogeneous structure on both light microscopy and SEM. The tissue appeared dense, with minimal damage to the crumb cells and a limited number of pores. In contrast, samples fermented with Lactiplantibacillus plantarum (P_LP) and Fructilactobacillus fructivorans (P_FF) showed significant structural loosening. SEM images revealed intense cellular damage and the presence of larger, irregular voids. μCT scanning further confirmed these changes, showing increased porosity and more heterogeneous internal structure in the fermented samples. In a study by Han et al. [] and fermented chili peppers, it was noted that after fermentation, the tissue of the peppers loosened significantly, and the texture softened.
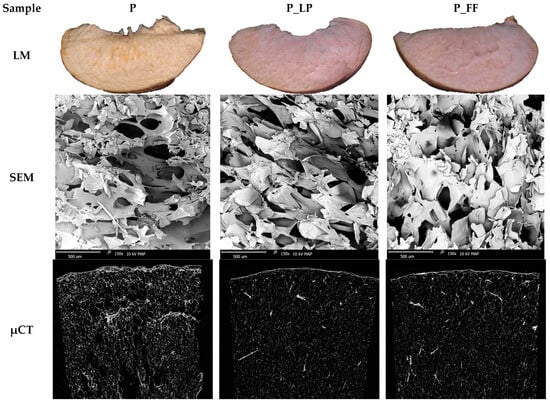
Figure 1.
Internal morphology; LM—light microscope; SEM—scanning electron microscope (magnification ×150); µCT—micro-tomography; P—freeze-dried peach sample; P_LP—fermented with Lactiplantibacillus plantarum and freeze-dried peach samples; P_FF—fermented with Fructilactobacillus fructivorans and freeze-dried peach sample.
The quantitative data from the microtomographic analysis confirmed a notable increase in porosity and a significant reduction in Euler number following fermentation (Table 2). Compared with the control sample (67.7%), porosity increased by approximately 5.3% in the P_LP sample and by 7.4% in the P_FF sample. At the same time, the Euler number decreased markedly, indicating a more complex and interconnected pore structure. Specifically, the Euler number dropped by 51.7% in P_LP (from −411,032 to −623,697) and by 102.5% in P_FF (to −832,126), demonstrating the strong impact of fermentation on internal microstructural connectivity.

Table 2.
Structural and mechanical properties of peach samples.
Liu et al. [] described changes occurring in the cell wall morphology and mechanical properties of puffing dried peach slices. They showed that the process of treating the fruit caused uneven distribution of pores in the cells, which simultaneously led to changes in the mechanical properties of the products. The process carried out at high temperatures also caused cell wall destruction, formation of new pores, and cellulose degradation.
Wang et al. [] obtained increased porosity of apples after blanching and freeze-drying processes. On the other hand, in a study by Kayacan et al. [], the microstructure of dried pear slices showed that it was the products obtained by freeze-drying that had the highest pore diameter and spongy texture.
Plant products’ texture strongly correlates with the integration of the cell wall of the raw tissue []. The pore size distribution graph (Figure 2) shows a shift toward larger pore diameters in the fermented variants, further confirming the structural degradation of the fruit tissues due to microbial activity. These changes directly affected the mechanical properties—the control sample showed the highest hardness (50.1 N; Table 2), while the values for P_LP and P_FF significantly decreased to 35.72 N for P_LP and 28.2 N for P_FF.
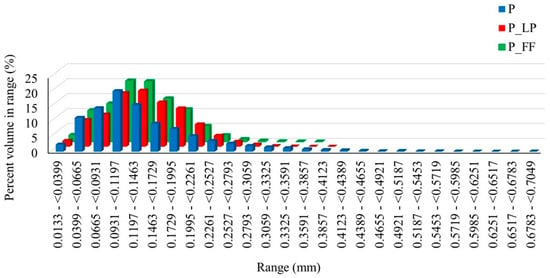
Figure 2.
Percent in volume range in tested samples. P—freeze-dried peach sample; P_LP—fermented with Lactiplantibacillus plantarum and freeze-dried peach samples; P_FF—fermented with Fructilactobacillus fructivorans and freeze-dried peach sample.
In a study by Ge et al. [] on the quality of Paocai, a popular fermented vegetable in Asian countries, the hardness values of samples after fermentation decreased significantly. The tissue was weakened, and it was observed that cell walls became loose and wrinkled with pores scattered in the tissue. Shang et al. [] investigated the physicochemical properties of pickled chayote during natural fermentation on an industrial scale. The original hardness value of the chayote was 153.64 N. This value decreased significantly during fermentation. After 21 days of fermentation, the hardness values gradually stabilized at 51.27 N. The authors concluded that the decrease in hardness values of pickled chayote may be due to the loss of cell wall strength and adhesion due to pH changes during fermentation. This observation can also be applied to the fermented freeze-dried peach samples studied in this work.
3.3. Chemical Changes Induced by Lactic Acid Fermentation
Fermentation using Lactiplantibacillus plantarum (P_LP) and Fructilactobacillus fructivorans (P_FF) caused observed changes in the chemical composition of freeze-dried peach samples (Table 3). These changes occurred due to the dynamic interactions of microbial activity, enzyme processes, and diffusion in the brine during fermentation [,,].

Table 3.
Chemical composition of peach samples.
The salt content in the fermented peach samples increased significantly compared with the control sample. Specifically, the salt levels measured approximately 0.50% for both fermented samples and only 0.16% for the control. This increase was likely due to the peaches absorbing salt from the brine, which enhanced flavor. The elevated salt content could influence the overall sensory experience of the fermented product, which was also shown in sensory analysis. The salt content in fermented samples showed the maximum intake level, while the salt addition was 0.5 m/v in brine. The diffusion of salt into the fruit tissue and the movement of soluble compounds into the brine suggested an osmotic balance shift, affecting the composition of the fruit []. The salt content measured in the control samples was due to its presence in the peach samples. Moreover, the addition of NaCl could create osmotic stress, to which LAB must adapt. For example, Waśko et al. [] examined Lactobacillus rhamnosus, which exhibited increased cell viability and biomass concentration when exposed to 0.5 M NaCl during exponential growth. This suggested that even a slight increase in salt concentration could improve the resilience of LAB.
Additionally, the total acidity in the fermented samples increased after fermentation. It reached 22.5 mg/kg in P_LP and 21.7 mg/kg in P_FF, in contrast to just 3.8 mg/kg in the control sample. This substantial rise in acidity was linked to the metabolic processes of the bacteria, which generated organic acids, mainly lactic acid, as they broke down sugars [,]. The resultant acidic environment symbolized lactic acid fermentation, promoting preservation, lowering pH levels, and imparting a distinctive sour taste to the final product.
Fermentation significantly reduced the total sugar content, with a 43.5% decrease in P_LP and a 31.2% decrease in P_FF compared with the control peach sample. This reduction could be attributed to the consumption of sugars (mainly glucose, fructose, and sucrose) by lactic acid bacteria (LAB) as a carbon source for growth and acid production []. The amount of sugar reduction may depend on the specific needs of each type of bacteria, the composition of sugars in the fruit juice, the time of fermentation, and how sucrose is broken down into simpler sugars []. Fructobacillus ferments glucose; however, fructose is fermented faster than glucose [].
The ratios of total sugar to total acidity and salt content illustrated the impact of fermentation on the biochemical balance of the peach samples. In the control sample (P), the sugar-to-acidity ratio was approximately 11.13, while it decreased drastically in the fermented samples to 1.06 for P_LP and 1.34 for P_FF. Similarly, the sugar-to-salt ratio dropped from 264.38 in the control to 47.80 in P_LP and 60.63 in P_FF. These changes reflected the intense microbial metabolism of sugars into organic acids during fermentation and the associated increase in salt concentration due to processing.
Analyzing both TA (total acidity) and TSC (total sugar content) as indicators of the lactic acid fermentation process can be based on the information that LAB used sugars to generate energy through glycolysis, leading to the production of lactic acid and other organic acids, which explained the increase in acidity and decrease in sugar content [].
3.4. Hygroscopicity and Water Sorption Isotherms
The water absorption properties of dried products from their surroundings were a very important characteristic. It affected their physical, chemical, and microbiological stability [,]. The ability of freeze-dried peach samples to absorb water from the environment was evaluated using hygroscopicity measurements and water sorption isotherm analysis. These tests provided significant information on the behavior of products during storage, especially under conditions of increased relative humidity. Moisture sorption isotherms describe the sorption behavior of material stored in an environment of varying humidity [,]. As illustrated in Figure 3, the data analysis revealed that all examined samples exhibited a characteristic type II sorption isotherm, commonly found in plant materials rich in sugars and fiber. An increase in relative humidity correlated with a notable rise in moisture content across all samples. However, clear distinctions emerged between the control sample (P)—freeze-dried peach without fermentation—and the fermented samples (P_LP and P_FF), which underwent lactic acid fermentation prior to freeze-drying. Specifically, the fermented samples exhibited a significantly lower moisture content, particularly at relative humidity levels above 50%. This reduction in water vapor sorption capacity suggested that lactic acid fermentation altered the sorption properties of peach tissue. These changes were likely due to structural and chemical modifications induced by the fermentation process, including osmotic effects. The use of a low-salt brine during fermentation may have led to salt migrating into the fruit tissue while soluble compounds such as sugars and polyphenols were released into the surrounding medium. These alterations may diminish the availability of hydrophilic binding sites or modify the architecture of the cell wall, thereby restricting moisture uptake at elevated humidity levels [].
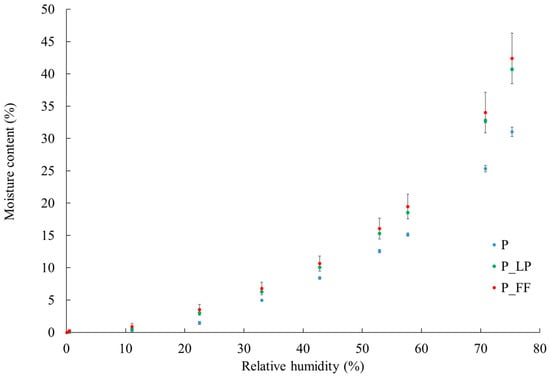
Figure 3.
Water vapor adsorption isotherms in freeze-dried and fermented peaches. P—freeze-dried peach sample; P_LP—fermented with Lactiplantibacillus plantarum and freeze-dried peach samples; P_FF—fermented with Fructilactobacillus fructivorans and freeze-dried peach sample.
This phenomenon can enhance the stability of freeze-dried fermented products in high humidity, reducing hygroscopicity and lowering the risk of moisture-related degradation. This helps extend shelf life and maintain product quality.
The significant differences between the variants indicated that the fermentation process significantly affected the sorption properties. The fermented samples (P_LP and P_FF) showed higher water sorption capacity than the control sample (P). Specifically, the P_FF sample absorbed the highest amounts of water, which may be related to its more porous structure (Table 2) and higher pore connectivity, which facilitated water penetration and accumulation. This was also confirmed by the results of pore size distribution (Figure 2), which showed a higher proportion of large pores in fermented samples.
The results of the hygroscopicity test (Figure 4) also showed significant differences between the samples. After 72 h of exposure in an atmosphere with constant relative humidity (RH = 75), all samples showed an increase in water content, with the dynamics of this process being highest in the first hours (1–8 h) and then slowing down, reaching a plateau after about 24 h. The highest moisture gain was recorded for the P_FF sample, slightly lower for P_LP, while the control sample absorbed the least water. This may be due to the compact structure and lower porosity of the control sample (67.74%).
The increased hygroscopicity of fermented samples may be directly related to transformations occurring during fermentation, such as the breakdown of pectins and hemicelluloses or the synthesis of hydrophilic compounds (e.g., organic acids) that increase water-binding capacity []. These observations are consistent with reports in the literature—including a paper by Mubeen et al. [], where fermentation of sea buckthorn beverages led to a loosening of the particle structure and increased water absorption.
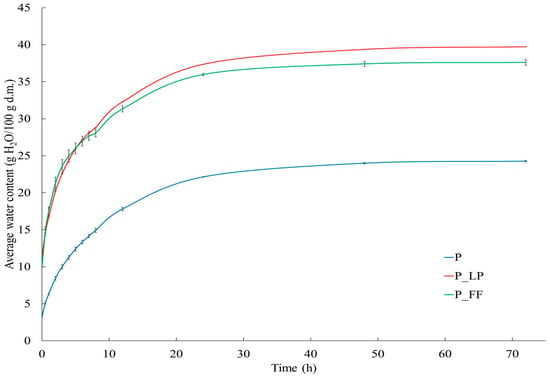
Figure 4.
Hygroscopic properties of tested peach samples. P—freeze-dried peach sample; P_LP—fermented with Lactiplantibacillus plantarum and freeze-dried peach samples; P_FF—fermented with Fructilactobacillus fructivorans and freeze-dried peach sample.
These findings have important practical implications; increased sorption and hygroscopicity can lead to reduced product storage life, increased stickiness, or loss of crispness, as confirmed in sensory evaluation tests. Therefore, although fermentation improves functional and sensory qualities, proper packaging and storage conditions are necessary to prevent the adverse effects of excessive moisture absorption.
3.5. Sensory Evaluation
Consumer acceptance is influenced by various factors, including the product’s sensory characteristics []. The study revealed no significant variation in color and type of odor between the samples (Figure 5). Such characteristics are typical for freeze-dried snacks as the process preserves the properties of the fruit and bioactive compounds []. The color change of the product may be due to microbiological interactions causing the release of pigments such as carotenoids and anthocyanins into the brine and resulting in decoloring of the samples []. Lactic fermentation also allows for flavor enhancement as a result of the enzymatic activity of microorganisms [].
The results demonstrated that the fermented peaches exhibited significantly diminished palatability, crunchiness, and sweetness compared with the control sample (Figure 5). Conversely, the acidity levels of the fermented peaches were higher, a phenomenon that may be attributed to the lactic fermentation process in brine. Increasing acidity after fermentation can improve the sensory experience by masking undesirable flavors (TA, see Table 2). Fermentation by increasing acidity changes the sugar/acid ratio and sugar/salt ratio in the product, allowing for an increase in the overall flavor profile. Those ratios were decreased in fermented samples, as was described in Section 3.4. Lactic fermentation can, therefore, contribute to a more balanced “overall assessment” of the product evaluation, revealing that the non-fermented sample received the highest rating [].
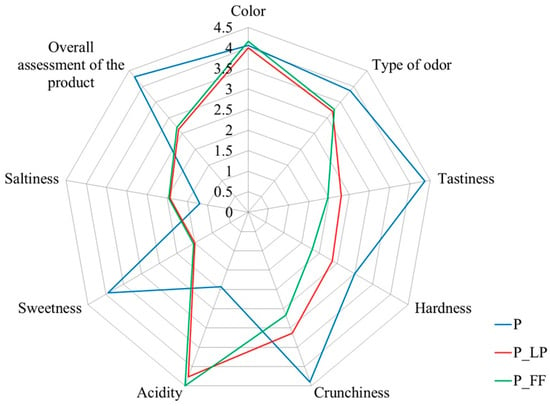
Figure 5.
Radar plots of sensory evaluation for peach samples. P—freeze-dried peach sample; P_LP—fermented with Lactiplantibacillus plantarum and freeze-dried peach samples; P_FF—fermented with Fructilactobacillus fructivorans and freeze-dried peach sample.
In the control sample, most of the sensory characteristics of the dried peach products were highly accepted. Consequently, enhancing the sweetness, crunchiness, and tastiness of fermented peaches was necessary to make the snack more acceptable to consumers. The advancement of fermentation technology was identified as an essential element in the development of valuable fruit snacks.
Combining these findings with those provided in our other article [], we can conclude that this fermented peach snack is rich in lactic acid bacteria (LAB), with levels of 8.38 log CFU/g for sample P_LP and 7.86 log CFU/g for sample P_FF. Additionally, the P_FF sample exhibited a higher level of flavonoids, while both samples maintained a comparable level of polyphenols when compared with the non-fermented peach sample. There was also evidence of reduced degradation of carotenoids and anthocyanins in this species. Given these characteristics, this snack can be considered a potentially valuable source of LAB and active compounds.
4. Conclusions
Freeze-dried peach snacks subjected to lactic fermentation with Lactiplantibacillus plantarum and Fructilactobacillus fructivorans strains exhibited significant physicochemical, structural, and sensory changes compared with the control sample. The fermentation process led to a decline of approximately 6–7% in dry matter content, primarily due to water absorption and the loss of soluble substances, such as sugars, while also contributing to an increase in acidity and a perceived increase in salinity due to the presence of added NaCl. Microscopic images and microtomographic analysis confirmed that fermentation induced a loosening of the cellular structure and increased porosity, particularly in samples fermented with F. fructivorans; these changes promoted better water vapor permeability, resulting in increased moisture sorption and higher hygroscopicity, as well as a faster water weight gain under elevated relative humidity conditions. Moreover, fermentation significantly altered the color of the samples by reducing brightness and intensity and modifying the shade, which may have implications for consumer perception of the product. Despite the technological advancements, sensory analysis indicated that consumer acceptance of the fermented samples was lower in terms of sweetness, crunchiness, and overall palatability compared with the unfermented product. Consequently, while lacto-fermentation holds promise as a method for developing fruit snacks with enhanced functional value, further optimization of the process, especially regarding the sensory profile, is essential to improve consumer acceptance and market viability. Adjusting fermentation parameters, potentially by incorporating monosaccharides into the brine, could promote the growth of lactic acid bacteria (LAB) and help maintain desirable sensory attributes such as sweetness and saltiness. Following freeze-drying, it may be tempting to utilize high-sugar solutions to boost consumer appeal; however, this approach could adversely affect the product’s texture due to increased porosity after freeze-drying.
Author Contributions
Conceptualization, E.J.-T., S.O., Z.D., K.P. and K.R.; methodology, E.J.-T., K.R. and K.P.; software, E.J.-T.; validation, E.J.-T., K.R. and K.P.; formal analysis, E.J.-T., K.R., K.P., S.O., Z.D., K.G. and J.S.; investigation, E.J.-T., S.O., Z.D., K.G., J.S., K.P. and K.R.; data curation, E.J.-T., S.O., Z.D., K.G., J.S., K.P. and K.R.; writing—original draft preparation, E.J.-T., S.O., Z.D., K.G., J.S., K.P. and K.R; writing—review and editing, E.J.-T., S.O., Z.D., K.G., J.S., K.P. and K.R.; visualization, E.J.-T., S.O. and Z.D.; supervision, E.J.-T., K.P. and K.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. However some research equipment used for this research was purchased as part of the “Food and Nutrition Centre—modernisation of the WULS campus to create a Food and Nutrition Research and Development Centre (CŻiŻ)” co-financed by the European Union from the European Regional Development Fund under the Regional Operational Programme of the Mazowieckie Voivodeship for 2014–2020 (Project No. RPMA.01.01.00-14-8276/17).
Institutional Review Board Statement
Studies were performed following the Code of Ethics of the World Medical Association (with consent of the PULS Rector’s Committee for the Ethics of Scientific Research Involving Humans No. 6/2025 from 21 February 2025.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. The participation was entirely voluntary.
Data Availability Statement
The authors confirm that all data supporting the findings of this study are available within the article.
Acknowledgments
The created graphical abstract shows the experimental workflow and key findings. For some figures, the abstract authors have used elements generated with DALL·E 3 from OpenAI, accessed through ChatGPT-4 (February 2025 version). All graphical components for the graphical abstract were verified for scientific accuracy, and microscopy-related visuals were conceptually modeled based on microtomographic data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Giménez-Campillo, C.; Arroyo-Manzanares, N.; Pastor-Belda, M.; Campillo, N.; Viñas, P. Discrimination of the geographical origin of peaches by the monitoring of volatile organic compounds by gas chromatography with mass spectrometry and chemometric tools. J. Food Compos. Anal. 2024, 129, 106125. [Google Scholar] [CrossRef]
- Lv, J.; Niu, L.; Xu, L.; Sun, X.; Wang, L.; Rong, H.; Zou, L. A visual identification method of the growth posture of young peach fruits in orchards. Sci. Hortic. 2024, 335, 113355. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Stoukogiorgos, G.; Limnaios, A.; Katsimichas, A.; Thanou, I.; Taoukis, P. Pulsed electric field pretreatment for energy efficient processing of industrial peach cultivars. Innov. Food Sci. Emerg. Technol. 2025, 100, 103931. [Google Scholar] [CrossRef]
- Li, Z.; Deng, X.; Zhao, Q.; Chen, J.; Zhu, Y.; Ye, X.; Chen, S.; Cheng, H. Assessing Frozen Flavor Variations and Process Adaptability in Peach (Prunus persica L. Batsch) Cultivars Based on Molecular Sensory Technology. Food Biosci. 2025, 65, 106047. [Google Scholar] [CrossRef]
- Bento, C.; Gonçalves, A.C.; Silva, B.; Silva, L.R. Peach (Prunus persica): Phytochemicals and health benefits. Food Rev. Int. 2022, 38, 1703–1734. [Google Scholar] [CrossRef]
- Lestari, D.; Nugroho, F.S.; Pramitasari, R.; Canti, M.; Sustaningrum, R. Snack Bar Formulated from Moringa and Cashew can Prevent Anemia During Pregnancy. Appl. Food Res. 2025, 5, 100815. [Google Scholar] [CrossRef]
- Alefew, Y.D.; Tiruneh, A.T.; Yehuala, T.F. Optimization of extrusion conditions for development of high quality rice-lupin-pumpkin based extruded snack food. Heliyon 2024, 10, e40913. [Google Scholar] [CrossRef]
- Acharya, S.; Kalahal, S.P.; Prajapati, S.; Patria, D.G.; Lin, J. Utilization of flaxseed by-product to develop a healthy brown rice extruded snack and identification of its physicochemical properties. Future Foods 2025, 11, 100566. [Google Scholar] [CrossRef]
- Uzun, D.E.; Nemli, E.; Apak, R.; Bener, M.; Tomas, M.; Yağcı, S.; Capanoglu, E. Starch-based composite formulation of chickpea flour and black carrot (Daucus carota l.) pomace in extruded snacks: In vitro gastrointestinal behavior and stability of bioactive compounds. Int. J. Biol. Macromol. 2025, 293, 139075. [Google Scholar] [CrossRef]
- Pęksa, A.; Nemś, A.; Nadal, E.S.; Noguera-Artiaga, L.; Issa-Issa, H.; Tajner-Czopek, A.; Carbonell-Barrachina, Á.A.; Kita, A. Sensory profile and consumer acceptability of third generation snacks from colored flesh potatoes. LWT 2025, 217, 117460. [Google Scholar] [CrossRef]
- Botta-Arias, V.L.; Ramos-Escudero, F.; Muñoz, A.M.; Anticona, M. Nutritional composition, phenolic compounds, and sensory evaluation of osmosonicated orange peel snacks impregnated with plant extracts. Appl. Food Res. 2024, 4, 100486. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, C.; Su, S.; Sun, A.; Du, T.; Wang, J.; Liu, J.; Zhang, W. Metal-phenolic networks enhanced the protection of excipients for probiotics during freeze-drying. Food Res. Int. 2025, 206, 116097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lin, S.-J.; Qi, X.-Y.; Xu, B.-C.; Qin, L.; Huang, X.-H. The interaction of Lactiplantibacillus plantarum and Latilatilactobacillus sakei regulated the formation of characteristic quality attributes of low-salt fermented tilapia. Food Biosci. 2024, 62, 105167. [Google Scholar] [CrossRef]
- Gunes, R. In vitro gastrointestinal digestion of anthocyanins from marshmallows enriched with blackthorn fruit powders obtained by convective hot air and freeze drying treatments. Food Res. Int. 2025, 205, 116001. [Google Scholar] [CrossRef] [PubMed]
- Vidal, V.A.S.; Juel, S.S.; Mukhatov, K.; Jensen, I.-J.; Lerfall, J. Impact of processing conditions on the rehydration kinetics and texture profile of freeze-dried carbohydrates sources. J. Agric. Food Res. 2025, 19, 101693. [Google Scholar]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Zhang, M.; Zhou, C.; Ma, L.; Su, W.; Jiang, J.; Hu, X. Influence of ultrasound on the microbiological, physicochemical properties, and sensory quality of different varieties of pumpkin juice. Heliyon 2024, 10, e27927. [Google Scholar] [CrossRef]
- Lisiecka, K.; Wójtowicz, A.; Samborska, K.; Mitrus, M.; Oniszczuk, T.; Combrzyński, M.; Soja, J.; Lewko, P.; Kasprzak Drozd, K.; Oniszczuk, A. Structure and texture characteristics of novel snacks expanded by various methods. Materials 2023, 16, 1541. [Google Scholar] [CrossRef]
- Karwacka, M.; Galus, S.; Janowicz, M. The effect of apple pomace powder and calcium ions on selected physicochemical properties of freeze-dried carrot-orange-ginger snacks. J. Sci. Food Agric. 2024, 104, 1713–1722. [Google Scholar] [CrossRef]
- Matys, A.; Witrowa-Rajchert, D.; Parniakov, O.; Wiktor, A. Assessment of the effect of air humidity and temperature on convective drying of apple with pulsed electric field pretreatment. LWT 2023, 188, 115455. [Google Scholar] [CrossRef]
- Hassan, B.; Mustapha, A.T.; Al-Awaadh, A.M.; Ahmed, K.A. Physical and moisture sorption thermodynamic properties of Sukkari date (Phoenix dactylifera L.) powder. CyTA-J. Food 2020, 18, 264–273. [Google Scholar] [CrossRef]
- Wang, Z.; Tong, Y.; Tong, Q.; Liu, Y.; Xu, W. Effects of different lactic acid bacteria on phenolic profiles, antioxidant capacities, and volatile compounds in purple sweet potato juice. J. Food Sci. Technol. 2024, 61, 1800–1810. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Janiszewska-Turak, E. Influence of the Salt Addition during the Fermentation Process on the Physical and Chemical Properties of Dried Yellow Beetroot. Appl. Sci. 2024, 14, 524. [Google Scholar] [CrossRef]
- Yang, S.; Meng, Z.; Li, Y.; Chen, R.; Yang, Y.; Zhao, Z. Evaluation of physiological characteristics, soluble sugars, organic acids and volatile compounds in ‘Orin’apples (Malus domestica) at different ripening stages. Molecules 2021, 26, 807. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, A.; Fliss, I.; Filteau, M. High-throughput characterization of the effect of sodium chloride and potassium chloride on 31 lactic acid bacteria and their co-cultures. Front. Microbiol. 2024, 15, 1328416. [Google Scholar] [CrossRef] [PubMed]
- Glaasker, E.; Konings, W.N.; Poolman, B. Osmotic regulation of intracellular solute pools in Lactobacillus plantarum. J. Bacteriol. 1996, 178, 575–582. [Google Scholar] [CrossRef]
- Fan, S.; Breidt, F.; Price, R.; Pérez-Díaz, I. Survival and Growth of Probiotic Lactic Acid Bacteria in Refrigerated Pickle Products. J. Food Sci. 2017, 82, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Ignaczak, A.; Salamon, A.; Kowalska, J.; Marzec, A.; Kowalska, H. Influence of Pre-Treatment and Drying Methods on the Quality of Dried Carrot Properties as Snacks. Molecules 2023, 28, 6407. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- de Araújo, J.S.F.; de Souza, E.L.; Oliveira, J.R.; Gomes, A.C.A.; Kotzebue, L.R.V.; da Silva Agostini, D.L.; de Oliveira, D.L.V.; Mazzetto, S.E.; da Silva, A.L.; Cavalcanti, M.T. Microencapsulation of sweet orange essential oil (Citrus aurantium var. dulcis) by liophylization using maltodextrin and maltodextrin/gelatin mixtures: Preparation, characterization, antimicrobial and antioxidant activities. Int. J. Biol. Macromol. 2020, 143, 991–999. [Google Scholar]
- Luo, Y.; Zeng, X.-B.; Hu, Y.-Y.; Li, D.-Y.; Liu, X.-Y.; Liu, Y.-X.; Zhou, D.-Y. Differences and mechanisms of color deterioration in three types of ready-to-eat shellfishes during storage. Food Chem. 2025, 469, 142459. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D.; Jouki, M. Improving bioactive properties of peach juice using Lactobacillus strains fermentation: Antagonistic and anti-adhesion effects, anti-inflammatory and antioxidant properties, and Maillard reaction inhibition. Food Chem. 2021, 365, 130501. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-C.; Wang, H.-M.; Tsai, S.-J.; Lin, J.-Y. Sensory and microbial analyses on naturally lacto-fermented cucumbers. Int. J. Gastron. Food Sci. 2023, 32, 100714. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The freeze-drying of foods—The characteristic of the process course and the effect of its parameters on the physical properties of food materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Oyinloye, T.M.; Yoon, W.B. Effect of Freeze-Drying on Quality and Grinding Process of Food Produce: A Review. Processes 2020, 8, 354. [Google Scholar] [CrossRef]
- Han, Q.; Wu, Y.; Jin, J.; Zhang, L.; Tong, S.; Li, C.; Luo, H. Effect of fresh pepper at different ripening stages on the physiochemical quality and flavor of fermented Chinese pepper. J. Food Compos. Anal. 2025, 137, 106892. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J.; Li, D.; Liu, C.; Zhang, Z.; Jiang, N.; Niu, L.; Zhang, M.; Cheng, J. Cell wall components, cell morphology, and mechanical properties of peach slices submitted to drying. Dry. Technol. 2020, 38, 1776–1789. [Google Scholar] [CrossRef]
- Wang, H.-O.; Fu, Q.-Q.; Chen, S.-J.; Hu, Z.-C.; Xie, H.-X. Effect of Hot-Water Blanching Pretreatment on Drying Characteristics and Product Qualities for the Novel Integrated Freeze-Drying of Apple Slices. J. Food Qual. 2018, 2018, 1347513. [Google Scholar] [CrossRef]
- Kayacan, S.; Sagdic, O.; Doymaz, I.; Karasu, S. The effect of different drying methods on total bioactive properties, individual phenolic compounds, rehydration ability, color, and microstructural characteristics of Asian pear. J. Food Process. Preserv. 2022, 46, e16682. [Google Scholar] [CrossRef]
- Ge, L.; Huang, Y.; Li, X.; Wang, N.; Liu, J.; Liu, M.; Mei, Y.; Yang, M.; Zhao, J.; Zhao, N. Temperature-driven divergence in molecular distribution and microbial invasion and the associated texture softening during dual-phase fermentation of Paocai. Food Chem. 2024, 457, 140171. [Google Scholar] [CrossRef]
- Shang, Z.; Ye, Z.; Li, M.; Ren, H.; Cai, S.; Hu, X.; Yi, J. Dynamics of microbial communities, flavor, and physicochemical properties of pickled chayote during an industrial-scale natural fermentation: Correlation between microorganisms and metabolites. Food Chem. 2022, 377, 132004. [Google Scholar] [CrossRef]
- Mousavi, Z.E.; Mousavi, S.M.; Razavi, S.H.; Hadinejad, M.; Emam-Djomeh, Z.; Mirzapour, M. Effect of fermentation of pomegranate juice by Lactobacillus plantarum and Lactobacillus acidophilus on the antioxidant activity and metabolism of sugars, organic acids and phenolic compounds. Food Biotechnol. 2013, 27, 1–13. [Google Scholar] [CrossRef]
- Özcan, M.M.; Uslu, N. The effect of fermentation with different additives on bioactive compounds, antioxidant activity, phenolic component, fatty acid composition and mineral substance contents of capers fruits. J. Food Meas. Charact. 2023, 17, 3896–3908. [Google Scholar] [CrossRef]
- Prieto-Santiago, V.; Aguiló-Aguayo, I.; Ortiz-Solà, J.; Anguera, M.; Abadias, M. Selection of a probiotic for its potential for developing a synbiotic peach and grape juice. Foods 2024, 13, 350. [Google Scholar] [CrossRef]
- Waśko, A.; Polak-Berecka, M.; Gustaw, W. Increased viability of probiotic Lactobacillus rhamnosus after osmotic stress. Acta Aliment. 2013, 42, 520–528. [Google Scholar] [CrossRef]
- Abouloifa, H.; Khodaei, N.; Rokni, Y.; Karboune, S.; Brasca, M.; D’Hallewin, G.; Salah, R.B.; Saalaoui, E.; Asehraou, A. The prebiotics (Fructo-oligosaccharides and Xylo-oligosaccharides) modulate the probiotic properties of Lactiplantibacillus and Levilactobacillus strains isolated from traditional fermented olive. World J. Microbiol. Biotechnol. 2020, 36, 185. [Google Scholar] [CrossRef] [PubMed]
- Van Durme, J.; Goiris, K.; De Winne, A.; De Cooman, L.; Muylaert, K. Evaluation of the volatile composition and sensory properties of five species of microalgae. J. Agric. Food Chem. 2013, 61, 10881–10890. [Google Scholar] [CrossRef] [PubMed]
- Verón, H.E.; Contreras, L.; Isla, M.I.; Torres, S. Assessment of technological and functional features of Lactiplantibacillus and Fructobacillus strains isolated from Opuntia ficus-indica fruits. NFS J. 2023, 31, 110–122. [Google Scholar] [CrossRef]
- Patil, M.; Jadhav, A.; Patil, U. Functional characterization and in vitro screening of Fructobacillus fructosus MCC 3996 isolated from Butea monosperma flower for probiotic potential. Lett. Appl. Microbiol. 2020, 70, 331–339. [Google Scholar] [CrossRef]
- Supplement. 3 XVIII Annual Scientific Meeting, Tucuman Biology Society. Tucuman—Argentina. BIOCELL 2002, 26 (Suppl. S), 153–203.
- Jakubczyk, E.; Ostrowska-Ligeza, E.; Gondek, E. Moisture sorption characteristics and glass transition temperature of apple puree powder. Int. J. Food Sci. Technol. 2010, 45, 2515–2523. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Jaskulska, A. The Effect of Freeze-Drying on the Properties of Polish Vegetable Soups. Appl. Sci. 2021, 11, 654. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, H.; Li, Y.; Tian, L.; Ye, B.; Yan, W.; Liu, G.; Yang, Y. Evaluating the dynamic effects of complex probiotics as cellulase replacements during fermentation of apple pomace. Int. J. Food Microbiol. 2024, 425, 110896. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, B.; Aregbe, A.Y.; Murtaza, S.; Yaqoob, S.; Xiong, Y.; Ma, Y. Investigating the impact of lactic acid bacteria fermentation on the phytochemical composition, antioxidant and functional properties, and sensory attributes of Monk Fruit Sweetened Sea Buckthorn Beverage. J. Food Meas. Charact. 2025, 19, 3188–3204. [Google Scholar] [CrossRef]
- Zang, Z.; Wan, F.; Ma, G.; Xu, Y.; Wang, T.; Wu, B.; Huang, X. Enhancing peach slices radio frequency vacuum drying by combining ultrasound and ultra-high pressure as pretreatments: Effect on drying characteristics, physicochemical quality, texture and sensory evaluation. Ultrason. Sonochem. 2024, 103, 106786. [Google Scholar] [CrossRef]
- Boasiako, T.A.; Boateng, I.D.; Ma, Y. Valorization of jujube puree using lactic acid bacteria and Acetobacter pasteurianus tri-cultured co-fermentation matrix: Insight into their nutritional, sensory, volatile and non-volatile metabolomics, and physicochemical properties. LWT 2025, 218, 117431. [Google Scholar] [CrossRef]
- Ossowski, S.; Rybak, K.; Pobiega, K.; Sękul, J.; Domżalska, Z.; Gregorek, K.; Gramza-Michałowska, A.; Janiszewska-Turak, E. Antioxidant Activity and Microbial Quality of Freeze-Dried, Lactic Acid Fermented Peach Products. Molecules 2025, 30, 2360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).