Abstract
Ozone has the potential to improve the shelf life of fruits and vegetables by its antimicrobial properties, but the influence on the aroma properties has not been studied comprehensively at the molecular level. Therefore, the impact of ozone on odour-active compounds, total titratable acidity, ascorbic acid, weight loss, and fungal growth during storage is investigated in oranges and bananas. The fruits were stored at room temperature for 11 days either with or without ozone treatment. Interestingly, no significant impact of the ozone treatment on the contents of the selected odour-active compounds in bananas and oranges was observable. Furthermore, no impact on titratable acidity, weight loss, and ascorbic acid content were observable as a consequence of the ozone treatment at the respective ozone concentrations. In addition, the inhibitive effects of the growth and sporulation of moulds were demonstrated in this study. Finally, the data from this research show that ozonation has the potential of improving the shelf life of fruits without compromising their quality.
1. Introduction
Food waste is a recent and global problem. It is estimated that one third of food is lost along the value chain. In Switzerland, food waste amounts to around 2.8 million tons in one year [1]. High percentages of food waste are caused by the households, with fruits and vegetables representing an important group [1,2]. This group is highly susceptible to microbial decay and other forms of alteration, such as loss of texture, taste, and aroma [3,4].
The food waste of fruits and vegetables can be reduced in different ways. One aspect is to prolong their shelf life. This can be achieved by means of storage under controlled temperature and atmosphere [5]. A further possibility is an edible, protective coating on the fruits that reduces external influences and fruit transpiration [6]. A third possibility is the reduction in microbial load in the beginning of the storage to delay alteration through microbial decay. This can be achieved by methods such as radiation or the washing of the fruits [6,7,8]. The fruits are often washed with water that contains additives, such as chlorine, peracetic acid, and hydrogen peroxide [6,7,8].
Alternatively, the fruits can be exposed to ozone [8]. Ozone is an oxidizing agent with well-known antimicrobial effects that has been used for water treatments for a long time [7,9,10]. It can be generated from oxygen and decomposes again to oxygen very rapidly after application [7,10,11]. One major advantage is that no residues are left on the fruits, which makes it a harmless and non-toxic method in comparison to other methods, such as, for example, washing with chlorine [7,10,11]. Different forms of treatment exist: the food can be exposed either to a gaseous ozone atmosphere or it can be washed with ozonated water [7,9]. The exposition to gaseous ozone can be applied once at the beginning of storage or either regularly or continuously during storage [8].
The effect of ozone on different foods has been the focus of many studies. In addition to effectively reducing bacterial and fungal count, ozone is also able to degrade mycotoxins, pesticides, and chemical residues [9,10]. Many studies further investigated the influence of ozone on other quality parameters, such as sugars, acids, colour, firmness, weight loss, vitamins, phenolic compounds, and other important food constituents [7,9,12,13,14,15,16]. Most studies reported an improved shelf life and only slight effects on other quality parameters.
The influence of ozone on the aroma has only been studied partly in the form of sensory evaluations [13,14,17,18]. Different results were reported ranging from only minor changes to an improvement in the aroma by the ozone treatment during subsequent storage. However, the impact of ozone on the aroma properties of fruits and vegetables has not yet been studied at a molecular level. Although different studies analysed some volatile compounds [15,17,19], mostly semi-quantitative methods were applied and the impact on the aroma properties was not clearly demonstrated in all studies.
For this reason, this research investigates the impact of ozone on key aroma compounds in oranges and bananas at a molecular level. The effect of ozone on selected aroma active compounds in oranges and bananas has, to the researchers’ knowledge, never been reported before. The odorants were quantified using HS-SPME-GC-MS and isotopically substituted odorants as internal standards [20]. To investigate the impact of ozone on other important chemical constituents, ascorbic acid was quantified using HPLC-UV and the amount of titratable acids was determined. Additionally, the effect of ozone treatment on the growth and sporulation of artificially inoculated mould species was tested. The reason for performing the previously mentioned experiments was to demonstrate whether ozone had a significant effect on the aroma properties, further parameters relevant for the quality, and on the shelf life of the investigated bananas and oranges. Based on the results, the next goal was then to objectivate whether direct ozone treatment exhibited the potential to extend the shelf life of bananas and oranges, without compromising their quality, under storage settings similar to those in households. In this way, this research aims to answer the central question, which is whether ozone has the potential to be used as a tool to combat food waste at home.
2. Materials and Methods
2.1. Fruits
Organic oranges and bananas were purchased from a local supermarket (Zürich, Switzerland) on the first day of the experiments.
2.2. Design of Experiments
The experiments were conducted analogously for oranges and bananas. One part of the fruits was directly analysed after purchasing. The other fruits were prepared for storage. The fruits were separated into seven metallic boxes with a volume of 8.5 L (27.5 × 20.5 × 15 cm3). All fruits were stored at room temperature (20 ± 2 °C). Four boxes were treated with ozone during storage and three boxes were stored without additional treatment (control). The experiments started on Monday (day 0) and ended on Friday in the next week (day 11). The total storage time was 11 days. The ozone treatment was performed every day except for the weekend (day 5 and day 6) and the last storage day (day 11).
In a first experiment, the ozone treatment was applied only once per day. In a second experiment, the ozone treatment was applied three times per day except for day 0, when it was applied only two times. Thus, the ozone exposition was tripled in the second experiment. The experimental settings (storage time and temperature) were chosen to simulate the storage conditions of bananas and oranges in a household.
2.3. Ozone Treatment
The ozone treatment consisted of the following steps. First, the metallic boxes were closed tightly. The boxes were then vacuumized with a vacuum pump that was connected to the boxes through a valve in the lid. Vacuumization was conducted for either 30 s (first experiment) or 10 s (second experiment). The boxes were fully vacuumized in both experiments, the different times to apply the vacuum were due to the use of different vacuum pumps. The valve was closed and the ozone generator was subsequently connected to the valve. The ozone generator (LR-Z128, unknown supplier from China) was commercially purchased and produced an ozone output of 350 mg/h as determined by an APOA-370 ozone-measuring device (HORIBA, Ltd., Oberursel, Germany). The ozone treatment was performed for 15 s. The ozone concentration in one box was 0.17 mg/L (81 ppm). The valve was closed and the fruits in the boxes were exposed to the ozone for 20 min in the closed boxes. After this ozone exposition period, the boxes were opened.
2.4. Sample Preparation
One sample consisting of two fruits was drawn from each box on storage days 2, 4, 7, 9, and 11. Five samples consisting of two fruits each were analysed before storage (day 0). The samples were prepared as described in what follows.
Two oranges were pressed for juice by an electric juice maker. In the first experiment, the complete juice was mixed with the same amount of a saturated calcium chloride solution (50% (w/w) of anhydrous calcium chloride in distilled water) and stored at −18 °C until analysis. In the second experiment, one part of the juice was mixed with the calcium chloride solution for odorant analysis. Another part of the juice was used directly for ascorbic acid quantification and a third part was stored as it was at −18 °C for titratable acidity analysis.
Two bananas were peeled and cut into pieces. One part was directly mixed with the same amount of calcium chloride solution in a smoothie mixer. The homogenized slurry was stored at −18 °C until the analyses of odorants and total titratable acids. Another part of the sliced bananas was directly used for ascorbic acid quantification.
As one sample was drawn from each box, four ozone-treated samples and three control samples were obtained for analysis for each measuring day.
2.5. Quantification of Odorants
The selected odour-active compounds were quantified with headspace-solid phase micro-extraction-gas chromatography-mass spectrometry (HS-SPME-GC-MS). Two grams of the sample were filled into a brown SPME glass vial and internal standards were subsequently added. The internal standards, which were mostly isotopically substituted odorants, were added in the amount as expected in the sample (0.014 –27.563 µg).
The closed vial was incubated for 10 min at 40 °C during shaking. The extraction with the CAR/PDMS fibre (Merck KGaA, Darmstadt, Germany) was then performed for 10 min. The fibre was injected into a TRACE GC Ultra (Thermo Fisher Scientific, Reinach, Switzerland) operating in the split/splitless mode. The volatiles were desorbed at 250 °C before being separated on a DB-FFAP column (30 m length, 0.25 mm inner diameter, and 0.25 μm film thickness; Agilent Technologies, Basel, Switzerland). The helium carrier flow was constant at 1.2 mL/min. The split flow was 10 mL/min and the splitless time was 0.2 min at the beginning of the run. The oven was set to 40 °C at the beginning and, after 4 min, the temperature was increased by 7 °C every minute until 240 °C was reached. The temperature of 240 °C was held for 30 min.
The coupled TSQ Quantum mass spectrometer (Thermo Fisher Scientific) was run in the EI mode with an electron ionisation energy of 70 eV. The ion source was operated at 200 °C and the transfer line was heated to 280 °C. The selected odorants were quantified in the SIM mode. The concentrations of the analytes were calculated by the areas of analytes and standards, amount of standard added, sample weight, and individual calibrations for each odorant. The calibration curves and quantifier ions of the analytes and standards are listed in Table S1 in the Supplementary Materials.
2.6. Quantification of Ascorbic Acid
The pH of 10 mL of orange juice was adjusted to 2.5 with o-phosphoric acid (85% solution). The mixture was centrifuged for 5 min at 4000 rpm, and the supernatant was filtered at 0.2 µm (regenerated cellulose, with a diameter of 15 mm) and used for direct analysis with HPLC or stored immediately at −18 °C for a maximum of ten days.
For the bananas, the extraction medium consisted of 3% meta-phosphoric acid, 0.05% EDTA disodium salt, and 8.4% glacial acetic acid, in distilled water. Of the peeled banana pieces, 15 g were mixed with 25 mL extraction medium for 30–60 s using a Polytron (PT 10–35 GT, Kinematica AG; Luzern, Switzerland) while being cooled with ice. The mixture was shaken in a bench shaker for 5 min at 800 rpm under light protection, using a pre-cooled block before being centrifuged for 3 min at 4000 rpm. The supernatant was stored in the dark on ice, while the residue was extracted for a second time with 10 mL of extraction medium (bench shaker, 800 rpm, 5 min). After centrifugation for 5 min at 4000 rpm, the two supernatants were combined, filtered at 0.2 µm (syringe filter, regenerated cellulose, with a diameter of 15 mm), and analysed directly by HPLC or stored immediately at −18 °C for a maximum of ten days.
Ascorbic acid quantification was conducted with an Agilent 1260 Infinity Binary LC system coupled with a 1260 DAD detector. The separation of the analytes was performed using an Agilent Poroshell 120 EC-C18 (2.1 × 5 mm, 2.7µm) column. The flow rate was set at 1.5 mL/min and the column temperature at 20 °C. The mobile phase A consisted of 20 mM monobasic potassium phosphate buffer (pH 2.5) and the mobile phase B was a mixture of 60% methanol and 40% acetonitrile. The gradient elution was as follows: 0–0.5 min, 5% B; 0.6–3 min, 25% B; 3.1–3.9 min, 70% B; and 4–5 min, 5% B. The re-equilibration time was two minutes. The injection volume was 4 µL. UV detection was performed at 243.5 nm (band width of 4 nm).
For the quantification, a reference standard of ascorbic acid was used (L-Ascorbic acid, Sigma Aldrich, Buchs, Switzerland). The calibration curve was built using the peak area at the corresponding wavelength under the chromatographic conditions described above. The calibration was analysed for each batch of analysis. The following linear regressions were used for quantification:
Linear range: 5–750 µg/mL.
Equations:
2.7. Quantification of the Total Titratable Acids
In the first experiment, the total titratable acids were analysed in the samples with calcium chloride. In the second experiment, the analyses were conducted with the pure juice for oranges and the mixture with calcium chloride for bananas. Pre-experiments confirmed no differences in the results between the samples with and without calcium chloride for oranges. Of either juice or the mixture with calcium chloride, 10 mL were titrated automatically with a 785 DMP Titrino (Metrohm Schweiz AG, Zofingen, Switzerland) using 0.1 N sodium hydroxide (Roth AG, Arlesheim, Switzerland) until a pH of 8.1 was reached. The content of the total titratable acids was calculated by Titrino as malic acid equivalents for bananas and citric acid equivalents for oranges.
2.8. Weight Loss
The weight of the fruits was documented in order to analyse the weight loss of the fruits over time. All fruits were weighed on day 0 before either being stored or directly analysed. In the first experiment, the samples were weighed only on the day they were analysed. In the second experiment, all samples were weighed until they were analysed on storage days 2, 4, 7, 9, and 11.
2.9. Artificial Contamination of the Fruits with Moulds
Oranges and bananas were washed for 2 min in bleach and then rinsed two times using deionised water. The fruits were dried in a biosafety cabinet with laminar air flow. Once dried, they were glued to a plastic Petri dish to keep the fruits in the same position during the course of the experiment and set inside the metallic box. Spores of Aspergillus niger 8AS9, Penicillium digitatum DSM 2750, and Penicillium expansum DSM 62841 were pricked on the fruits (1000 spores/infection site). Ozonisation was performed as indicated above. The growth and sporulation of each mould was visually inspected.
2.10. Statistics
Tukey’s test (α = 0.05) for analysing the significance of differences and data visualization were performed using Python 3.10.
3. Results
3.1. Impact of One Ozone Treatment per Day on Odour-Active Compounds, Total Titratable Acidity, Weight Loss, and Mould Growth
The impact of one ozone treatment with 81 ppm ozone for 20 min per day on different quality parameters during the storage of oranges and bananas was tested in a first experiment. The quantified odorants were selected based on the existing literature on key odorants in oranges, freshly pressed orange juice [21,22,23], and bananas [24,25]. The odorant concentrations in the bananas during storage without and with ozone treatment are displayed in Figure 1 and Table S3.
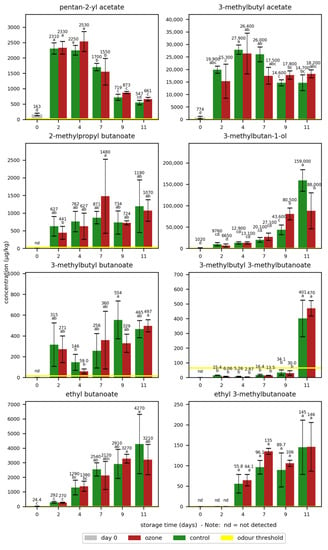
Figure 1.
Mean concentrations of the selected odorants with standard deviations in bananas during storage without (control) and with ozone treatment once a day. Different letters after the value indicate a significant difference between the samples for the compound.
The analyses included eight important banana odorants, with mainly acetates and butanoates as important fruity-smelling esters in bananas [24]. With concentrations surpassing their odour threshold at the latest on day 2, this research confirms the importance of most of the quantified odorants for the banana odour. Especially high concentrations were found for 3-methylbutyl acetate and 3-methylbutan-1-ol. Only 3-methylbutyl 3-methylbutanoate showed concentrations below its odour threshold until day 9, and ethyl 3-methylbutanoate was only detectable from day 4 onwards.
In the summary of all odorants, no significant differences were found between the samples treated with ozone and the control samples for most odorants, with the exception of 3-methylbutan-1-ol. Its concentration on day 9 was significantly higher in the ozone-treated sample, whereas its concentration after 11 days was significantly higher in the control sample. Even though the differences were significant, no clear trend was observed. Small and non-significant differences between the two groups in the concentrations of the other odorants did not indicate a trend regarding a possible influence of the ozone treatment. 2-Methylpropyl butanoate showed mostly higher concentrations in the control samples compared to the ozone-treated samples, except for day 7. In contrast, the ethyl 3-methylbutanote and pentan-2-yl acetate concentrations were slightly higher in the ozone-treated samples on most days.
Generally, high standard deviations were observed for most samples, independent of the storage type and day. The broad range of odorant concentrations in the samples indicated high natural variations in the bananas. As demonstrated by the non-significance of most differences, the natural variations had a greater influence on the odorant concentrations than the storage type.
In addition to the natural variations in the fruits, the storage time had a higher impact on the odorant concentrations than the storage type. Most odorants showed low concentrations at the beginning of the storage and an increase over time. A very steep increase from day 0 to day 2 was observed for pentan-2-yl acetate, 3-methylbutyl acetate, 2-methylpropyl butanoate, and 3-methylbutyl butanoate. 3-Methylbutyl 3-methylbutanoate showed a steep increase from day 9 to day 11 and reached a concentration above its odour threshold only on day 11. A slower increase rate was observed for 3-methylbutan-1-ol and ethyl butanoate. The formation of odorants during storage is part of the ripening process of the bananas, which continues after harvesting [4]. Especially, esters are known to increase during banana ripening [26,27,28,29], and 3-methylbutan-1-ol was reported to increase during ripening until a certain ethanol level is reached [29]. Liu et al. [29] observed a decrease in esters at a certain time of storage, which was highly dependent on the storage temperature. This study observed a decreasing effect at the end of storage for 3-methylbutyl acetate and pentan-2-yl acetate.
The same experiment was analogously performed with oranges. The concentrations of the quantified odorants in oranges are displayed in Figure 2 and Table S4. With concentrations above their odour thresholds, this research confirms the importance of the analysed odorants for the orange odour. Especially, limonene [21,22,23] showed concentrations drastically above its odour threshold. The α-terpineol concentration was below its odour threshold throughout the whole storage period and might therefore not contribute to the sensory properties of oranges.
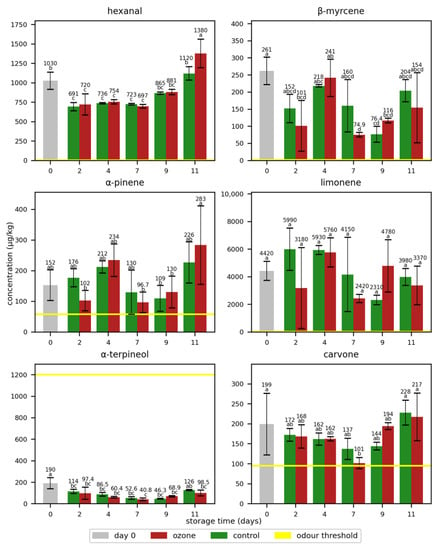
Figure 2.
Mean concentrations of the selected odorants with standard deviations in oranges during storage without (control) and with ozone treatment once a day. Different letters after the value indicate a significant difference between the samples for the compound.
In contrast to the bananas, the storage time did not significantly affect the concentrations and no ripening effect was observed. This is in line with the non-climacteric properties of the oranges [4]. Instead, the concentrations varied over time and, as in the case of the banana odorants, showed high standard deviations that corresponded to the natural variations of the odorant concentrations in the oranges. Interestingly, the hexanal concentrations increased at the end of the storage period.
Concerning a possible effect of ozone on the odorant concentrations, no significant differences were observed between the samples treated with ozone and the control samples, with the exception of hexanal, whose concentration on day 11 was significantly higher in the ozone-treated sample than that of the control sample. As in the case of the odorants in the bananas, no clear trend was observed in the oranges between the samples with and without ozone treatment. Thus, it was concluded that the applied ozone exposition had no impact on the odour properties of the oranges.
In addition to the selected key odorants, the total titratable acidity was measured (Figure 3, Table S5). The total acid concentration determined as malic acid decreased during storage in the bananas, which is in line with the findings of Youryon and Supapvanich [30]. In contrast to the bananas, the total acid concentration determined as citric acid remained relatively stable in the oranges. The total titratable acidity was not significantly affected by the ozone treatment in both bananas and oranges, and only slight differences were observed between the control samples and those treated with ozone.
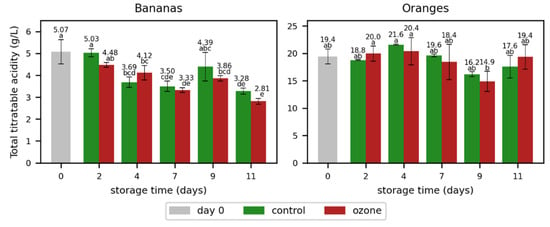
Figure 3.
Total titratable acidity determined as malic acid in bananas and citric acid in oranges during storage without (control) and with ozone treatment once a day. Different letters after the value indicate a significant difference between the samples for the total titratable acidity.
The weight loss was higher for bananas than for oranges (Figure 4). The bananas had only around 80% of their initial weight at the end of the storage, while the oranges maintained over 93% of their weight at the same point in time. However, no significant differences were observed in bananas with respect to weight loss between the control samples and those treated with ozone. The weight loss in oranges was by trend higher for the samples treated with ozone, but the differences were only significant on day 7. This could be an indication of an impact of ozone on the weight loss in oranges, which has to be verified with more intense ozone treatments.
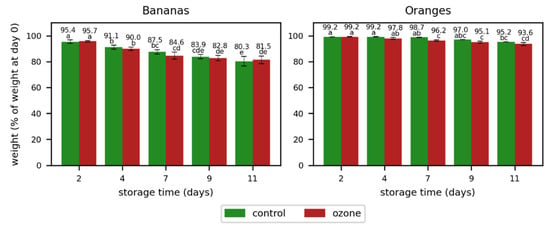
Figure 4.
Weight loss in bananas and oranges during storage without (control) and with ozone treatment once a day. Different letters after the value indicate a significant difference between the samples for the weight (% of weight at day 0).
These data show that the applied ozone treatment did not affect important quality parameters, such as key odorants, total acid concentration, and weight loss, neither in oranges nor in bananas. In addition, it was investigated whether the ozone treatment had a positive impact on the shelf life by inhibiting mould growth on the fruits. On bananas, only the growth of P. expansum was slightly reduced. The growth of the other moulds applied was not affected. On oranges, ozone did not affect the growth of any mould tested.
3.2. Impact of Three Ozone Treatments per Day on Odour-Active Compounds, Total Titratable Acidity, Ascorbic Acid, Weight Loss, and Mould Growth
In a second experiment, a higher ozone exposition per day was tested. In this case, the same settings as during the first experiment were applied, with the exception of three ozone treatments per day instead of one. Consequently, the ozone exposition was tripled. In addition to the previously analysed parameters, ascorbic acid was analysed as a further quality parameter regarding the impact of ozone on vitamins.
The odorant concentrations in the bananas during storage are summarized in Figure 5 and Table S3. As in the first experiment, high natural variations of odorant concentrations were found in the bananas and an increase in odorant concentrations as result of the ripening process was observed. Interestingly, the concentrations of 3-methylbutyl acetate, pentan-2-yl acetate, and ethyl butanoate decreased on day 11. The statistical data evaluation revealed no significant effect of the higher ozone exposition on the concentrations of the analysed odorants. Although the differences were mostly small and not significant, slight trends were observed for certain odorants. The 2-methylpropyl butanoate and 3-methylbutan-1-ol concentrations were higher in the samples treated with ozone than in the control samples. The ethyl butanoate concentrations were lower in the samples treated with ozone, except for day 11.
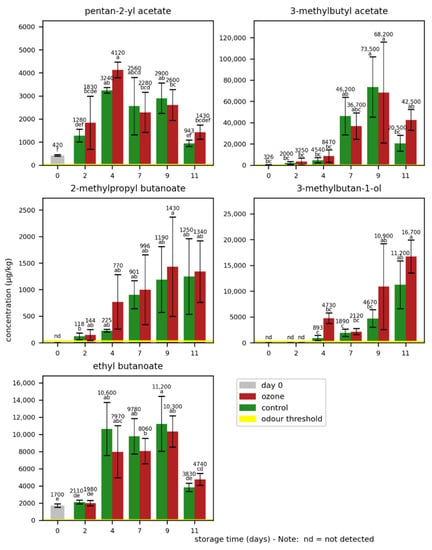
Figure 5.
Mean concentrations of the selected odorants with standard deviations in bananas during storage without (control) and with ozone treatment three times a day. Different letters after the value indicate a significant difference between the samples for the compound.
A similar observation was established for the odorants in oranges when three ozone treatments per day were applied (Figure 6 and Table S4). Interestingly, mostly higher concentrations were found in the control samples, but the differences were only significant for day 7 and day 9 in carvone and for day 2 and day 4 in α-terpineol. Consequently, the higher ozone exposition of the fruits did not clearly affect the concentrations of key odorants in oranges. However, the observed trend suggests that higher ozone expositions than the ones applied in this study might influence the concentrations of key odorants in oranges.
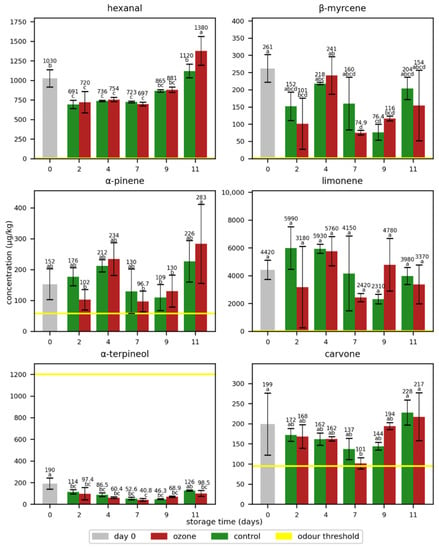
Figure 6.
Mean concentrations of the selected odorants with standard deviations in oranges during storage without (control) and with ozone treatment three times a day. Different letters after the value indicate a significant difference between the samples for the compound.
The higher ozone exposition in the second experiment did not significantly affect the total acid concentration (Figure 7 and Table S5) and the weight loss (Figure 8). Except for day 11, the weight loss in the oranges showed a trend of a higher weight loss in the samples treated with ozone; however, this weight loss was less pronounced than in the first experiment. As the weight loss in the ozone sample was significantly lower on day 11, the ozone treatment showed no clear impact on the weight loss in oranges.
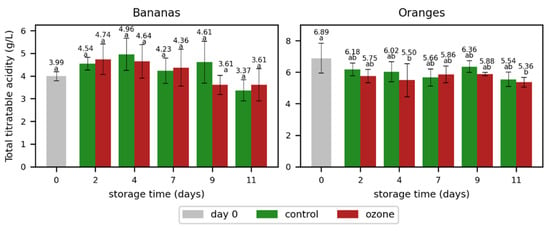
Figure 7.
Total titratable acidity determined as malic acid in bananas and citric acid in oranges during storage without (control) and with ozone treatment three times a day. Different letters after the value indicate a significant difference between the samples for the total titratable acidity.
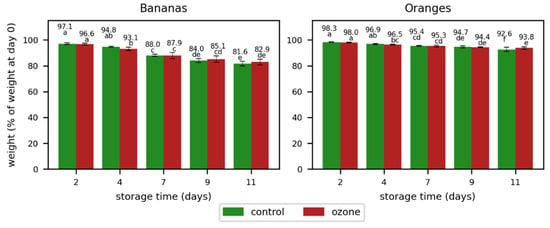
Figure 8.
Weight loss in bananas and oranges during storage without (control) or with ozone treatment three times a day. Different letters after the value indicate a significant difference between the samples for the weight (% of weight at day 0).
As a further quality parameter, the ascorbic acid concentration was measured (Figure 9 and Table S6). It was relatively stable over the whole storage time in oranges but decreased during the storage of bananas. The decrease started rapidly, but the concentration stayed relatively stable from day 7 onwards. Interestingly, the ascorbic acid concentrations in bananas were mostly higher in the samples treated with ozone, compared to the untreated ones. The effect was the opposite in oranges with lower concentrations in the samples treated with ozone. However, the differences were not significant in both fruits. Consequently, the applied ozone treatment did not negatively affect the ascorbic acid concentration in both fruits.
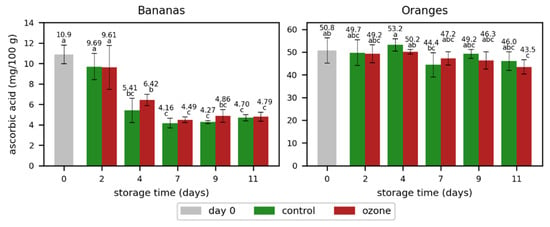
Figure 9.
Ascorbic acid mean concentrations with standard deviations in bananas and oranges without (control) or with ozone treatment three times a day. Different letters after the value indicate a significant difference between the samples for the compound.
For bananas, the application of three ozone treatments per day revealed the growth inhibition of A. niger for three days, compared to the control sample. The growth or sporulation of P. digitatum and P. expansum, respectively, was delayed by one day. On oranges, the effect was far less pronounced. The three moulds did not grow well on oranges. However, while the growth of A. niger and P. digitatum was not affected, P. expansum was slightly inhibited (Table S2).
Therefore, the higher ozone exposition of the fruits receiving three treatments per day delayed fungal growth without significantly affecting key odorants, total titratable acidity, weight loss, and ascorbic acid content in bananas and oranges.
4. Discussion
These results show that the exposition of ozone to oranges and bananas with 81 ppm for 20 min either once or three times a day did not negatively affect the concentration of key odorants, total titratable acidity, ascorbic acid content, and weight loss. In addition, the ozone treatment during storage led to the concentration-dependent delay of mould growth, depending on the fruit and mould used.
Consequently, the tested ozone treatment slightly improved the shelf life of the fruits without significantly affecting their quality in terms of aroma and health aspects.
The effect of ozone on odorants in bananas and oranges has not yet been investigated. However, the influence of ozone on volatiles in orange juice has been studied [19]. Juice was exposed for a period ranging from 1 to 6 min to gaseous ozone. A clear impact of ozone on the volatiles in orange juice was observed, especially after longer exposition times [19]. The relative amounts of limonene decreased with higher exposition times, while other compounds, such as β-myrcene and α-terpineol, increased. The researchers suggested oxidation and hydrolysis of and reduction in terpenes to be responsible for the changes in orange volatiles at longer ozone exposition times. Tiwari et al. [16] reported a degradative effect of ozone on the ascorbic acid content in orange juice. However, the total titratable acidity was not affected by the ozone treatment [16].
These aforementioned observations were mostly contrary to the results discussed in this study. One reason for this might be the difference in the application of ozone on whole oranges in contrast to orange juice. When applying ozone on whole oranges, the ozone molecules might only react with the peel constituents and not influence the inner part of the fruit, which was analysed in this case. The peel might have a protective function for valuable orange constituents, such as odorants, acids, and vitamins. The same effect might have occurred in bananas and could explain why key odorants, total titratable acids, and ascorbic acid were not affected by ozone in this study. On the contrary, a slight effect was observed on fungal growth, which occurs on the fruit peels. Further studies will be necessary to clarify a possible protective function of orange and banana peels and how different parts of the fruits are impacted by the application of a gaseous ozone atmosphere.
Furthermore, the effect of ozone on the shelf life and quality parameters has been studied in other fruits than oranges and bananas before.
Ozone treatments ranging from 0.5 to 1.5 mg/L for 60 min before the refrigerated storage of apricots reduced fungal decay significantly during storage time [14]. However, the researchers observed a significantly higher weight loss in the ozone-treated fruits. Interestingly, the ozonated apricots kept their sensory properties for a longer storage time than the not ozonated ones.
Ozone application on tomatoes (25 or 45 mg/m3 for 2 h/day for 16 days) showed no significant influence on the titratable acidity [31]. The ozone treatment improved the weight loss during storage. Until 13 days of storage, the ascorbic acid content was not significantly affected by the ozone treatment. Lower ascorbic acid concentrations in the ozone-treated samples were observed after 16 days of storage. The authors explained this by the oxidizing effect of ozone that leads to a loss in antioxidant compounds. They explained the low effect of ozone on the ascorbic acid content by the action of the tomato pericarp as a physical barrier for the ozone. Ascorbic acid is concentrated in the aqueous bulk of the tomatoes and, therefore, not affected by the treatment [31]. This is in line with the underlying assumption of the protective function of the orange peel. The ozone treatment further lowered the percentage of damaged and spoiled tomatoes during storage in their study [31].
Nadas et al. [13] investigated the effect of ozone (1.5 µL/L) on strawberries during three days at 2 °C before the fruits were exposed to room temperature for storage. The ozone treatment delayed Botrytis cinerea growth and reduced the natural decay incidence. They observed a lower weight loss of the ozone-treated samples directly after cold storage, but this effect was not observed again during the following storage at ambient temperature. The titratable acidity was lower in the ozone-treated sample. They reported a loss of odour directly after cold storage in the ozone-treated strawberries, but this effect diminished after two days at room temperature. Pérez et al. [15] similarly investigated the effects of a 3-day ozone treatment on cold-stored strawberries before being stored for 5 days at room temperature. They observed an increase in the ascorbic acid concentration in the ozone-treated fruits. They explained the higher content by the formation of antioxidative compounds of the fruits as a reaction to the oxidative stress caused by ozone and other radicals formed by the ozone treatment. Similar to the results of this study, they found no significant differences in the organic acid concentrations of malic acid and citric acid between the ozone-treated fruits and the control group. After five days of storage at room temperature, low concentrations of esters were found in the ozone-treated strawberries. However, the impact on the odour was not studied and the natural variations of volatile compounds in the strawberries were not discussed. The aqueous ozone treatment of strawberries (up to 3.5 mg/L for 5 min) did not affect the titratable acidity and sensory characteristics of the strawberries during cold storage [12]. In addition, the treatment delayed and reduced the decay caused by B. cinerea.
In regard of the obtained results, it can be concluded that the direct ozonation of fruits might be a good alternative to other solutions, such as coatings and edible films [32,33], which are also effective in prolonging the storage time of fruits and vegetables. In this way, it can be postulated that the use of ozone devices in households, which increase the shelf life of fruits, might be helpful to decrease food waste in the future.
5. Conclusions
This investigation of the effect of direct ozone application on bananas and oranges showed, at a molecular level, that the odour quality is not negatively affected in the applied setting. In addition, the ozone treatment delayed fungal growth and did not negatively affect the total acid content, weight loss, and ascorbic acid concentration during storage. Therefore, it can be concluded that the direct ozone treatment of fruits shows a potential in prolonging their shelf life without compromising quality, which could be a promising treatment in order to reduce food waste.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app131910885/s1. Table S1: Calibration lines of odorants; Table S2: Growth and sporulation of A. niger, P. digitatum, and P. expansum on artificially contaminated bananas and oranges; Table S3: Concentrations of the selected odorants in bananas during storage without and with ozone treatment; Table S4: Concentrations of the selected odorants in oranges during storage without and with ozone treatment; Table S5: Total acid concentrations in the bananas and oranges during storage without or with ozone treatment once and three times a day; Table S6: Ascorbic acid concentrations in the bananas and oranges with and without ozone treatment three times a day.
Author Contributions
Conceptualization, I.C. and L.U.; methodology, I.C., L.U., L.F. and A.A.; validation, formal analysis and investigation, L.U., E.G., A.A., S.P. and A.F.I.; data curation, I.C. and L.U.; writing—original draft preparation, L.U. and I.C.; writing—review and editing, I.C., L.U., E.G., A.A. and L.F.; visualization, L.U.; supervision, I.C.; project administration, I.C.; funding acquisition, I.C. and L.F. All authors have read and agreed to the published version of the manuscript.
Funding
Innosuisse Innovation Project Nr. 51390.1 IP-EE.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Katrin Jedrys, Giverny Ganz and Lucy Laila Tulinski-Withers for their great support during the project. Last but not least, we thank Daniel Bertschi (Novartis AG), who inspired us to investigate the impact of ozone on the quality and safety of fruits within the framework of the Innosuisse-funded project “SONEVA—the fresh keeping drawer against food waste”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beretta, C.; Hellweg, S. Potential Environmental Benefits from Food Waste Prevention in the Food Service Sector. Resour. Conserv. Recycl. 2019, 147, 169–178. [Google Scholar] [CrossRef]
- Beretta, C.; Stucki, M.; Hellweg, S. Environmental Impacts and Hotspots of Food Losses: Value Chain Analysis of Swiss Food Consumption. Environ. Sci. Technol. 2017, 51, 11165–11173. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Rodgrigo, D.; Zanini, S.F. Safety Issues on the Preservation of Fruits and Vegetables. In Fruit Preservation; Rosenthal, A., Deliza, R., Welti-Chanes, J., Barbosa-Canovas, G.V., Eds.; Food Engineering Series; Springer Science+Business Media: New York, NY, USA, 2018; pp. 21–43. [Google Scholar]
- Escobedo-Avellaneda, Z.; Guerrero-Beltran, J.A.; Tapia, M.S.; Barbosa-Canovas, G.V.; Welti-Chanes, J. Minimal Processing of Fruits. In Fruit Preservation; Rosenthal, A., Deliza, R., Welti-Chanes, J., Barbosa-Canovas, G.V., Eds.; Food Engineering Series; Springer Science+Business Media: New York, NY, USA, 2018; pp. 67–92. [Google Scholar]
- Bhat, N.R. Postharvest Storage Systems: Biology, Physical Factors, Storage, and Transport. In Handbook of Fruits and Fruit Processing; Sinha, N.K., Sidhu, J.S., Barta, J., Wu, J.S.B., Cano, M.P., Eds.; John Wiley & Sons: Chichester, UK, 2012; pp. 87–102. [Google Scholar]
- Balla, C.; Farkas, J.; Dalmadi, I. Developments in Minimal Processing of Fruits. In Handbook of Fruits and Fruit Processing; Sinha, N.K., Sidhu, J.S., Barta, J., Wu, J.S.B., Cano, M.P., Eds.; John Wiley & Sons: Chichester, UK, 2012; pp. 153–173. [Google Scholar]
- Sarron, E.; Gadonna-Widehem, P.; Aussenac, T. Ozone Treatments for Preserving Fresh Vegetables Quality: A Critical Review. Foods 2021, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Muthukumarappan, K. Ozone in Fruit and Vegetable Processing. In Ozone in Food Processing; O’Donnell, C., Tiwari, B.K., Cullen, P.J., Rice, R.G., Eds.; John Wiley & Sons: Chichester, UK, 2012; pp. 55–80. [Google Scholar]
- Horvitz, S.; Cantalejo, M.J. Application of Ozone for the Postharvest Treatment of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2014, 54, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Afsah-Hejri, L.; Hajeb, P.; Ehsani, R.J. Application of Ozone for Degradation of Mycotoxins in Food: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1777–1808. [Google Scholar] [CrossRef]
- O’Donnell, C.; Tiwari, B.K.; Cullen, P.J.; Rice, R.G. Status and Trends of Ozone in Food Processing. In Ozone in Food Processing; John Wiley & Sons: Chichester, UK, 2012; pp. 1–6. [Google Scholar]
- Contigiani, E.V.; Kronberg, M.F.; Jaramillo Sánchez, G.; Gómez, P.L.; García-Loredo, A.B.; Munarriz, E.; Alzamora, S.M. Ozone Washing Decreases Strawberry Susceptibility to Botrytis Cinerea While Maintaining Antioxidant, Optical and Sensory Quality. Heliyon 2020, 6, e05416. [Google Scholar] [CrossRef]
- Nadas, A.; Olmo, M.; García, J.m. Growth of Botrytis Cinerea and Strawberry Quality in Ozone-Enriched Atmospheres. J. Food Sci. 2003, 68, 1798–1802. [Google Scholar] [CrossRef]
- Panou, A.A.; Karabagias, I.K.; Riganakos, K.A. The Effect of Different Gaseous Ozone Treatments on Physicochemical Characteristics and Shelf Life of Apricots Stored under Refrigeration. J. Food Process. Preserv. 2018, 42, e13614. [Google Scholar] [CrossRef]
- Pérez, A.G.; Sanz, C.; Ríos, J.J.; Olías, R.; Olías, J.M. Effects of Ozone Treatment on Postharvest Strawberry Quality. J. Agric. Food Chem. 1999, 47, 1652–1656. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Muthukumarappan, K.; O’Donnell, C.P.; Cullen, P.J. Kinetics of Freshly Squeezed Orange Juice Quality Changes during Ozone Processing. J. Agric. Food Chem. 2008, 56, 6416–6422. [Google Scholar] [CrossRef]
- De Santis, D.; Garzoli, S.; Vettraino, A.M. Effect of Gaseous Ozone Treatment on the Aroma and Clove Rot by Fusarium Proliferatum during Garlic Postharvest Storage. Heliyon 2021, 7, e06634. [Google Scholar] [CrossRef]
- Karakosta, E.S.; Karabagias, I.K.; Riganakos, K.A. Shelf Life Extension of Greenhouse Tomatoes Using Ozonation in Combination with Packaging under Refrigeration. Ozone Sci. Eng. 2019, 41, 389–397. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Rodrigues, T.H.S.; Fernandes, F.A.N.; de Brito, E.S.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Cavalcante, R.S.; Almeida, F.D.L.; Rodrigues, S. An Untargeted Chemometric Evaluation of Plasma and Ozone Processing Effect on Volatile Compounds in Orange Juice. Innov. Food Sci. Emerg. Technol. 2019, 53, 63–69. [Google Scholar] [CrossRef]
- Schieberle, P.; Grosch, W. Quantitative Analysis of Aroma Compounds in Wheat and Rye Bread Crusts Using a Stable Isotope Dilution Assay. J. Agric. Food Chem. 1987, 35, 252–257. [Google Scholar] [CrossRef]
- Sellami, I.; Mall, V.; Schieberle, P. Changes in the Key Odorants and Aroma Profiles of Hamlin and Valencia Orange Juices Not from Concentrate (NFC) during Chilled Storage. J. Agric. Food Chem. 2018, 66, 7428–7440. [Google Scholar] [CrossRef] [PubMed]
- Buettner, A.; Schieberle, P. Evaluation of Aroma Differences between Hand-Squeezed Juices from Valencia Late and Navel Oranges by Quantitation of Key Odorants and Flavor Reconstitution Experiments. J. Agric. Food Chem. 2001, 49, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Seideneck, R.; Schieberle, P. Comparison of the Key Aroma Compounds in Hand-Squeezed and Unpasteurised, Commercial NFC Juices Prepared from Brazilian Pera Rio Oranges. Eur. Food Res. Technol. 2011, 232, 995–1005. [Google Scholar] [CrossRef]
- Xiao, Z.; Chen, H.; Niu, Y.; Zhu, J. Characterization of the Aroma-Active Compounds in Banana (Musa AAA Red Green) and Their Contributions to the Enhancement of Sweetness Perception. J. Agric. Food Chem. 2021, 69, 15301–15313. [Google Scholar] [CrossRef]
- Pino, J.A.; Febles, Y. Odour-Active Compounds in Banana Fruit Cv. Giant Cavendish. Food Chem. 2013, 141, 795–801. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Li, J.; Luo, J.; Chen, W.; Li, X. Comparative Study of Volatile Compounds in the Fruit of Two Banana Cultivars at Different Ripening Stages. Molecules 2018, 23, 2456. [Google Scholar] [CrossRef]
- Vermeir, S.; Hertog, M.L.A.T.M.; Vankerschaver, K.; Swennen, R.; Nicolaï, B.M.; Lammertyn, J. Instrumental Based Flavour Characterisation of Banana Fruit. LWT—Food Sci. Technol. 2009, 42, 1647–1653. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.P.; Yuan, R.C.; Chen, Y.F.; Chen, W.X. Changes in Volatile Compounds and Associated Relationships with Other Ripening Events in Banana Fruit. J. Hortic. Sci. Biotechnol. 2010, 85, 283–288. [Google Scholar] [CrossRef]
- Liu, T.-T.; Yang, T.-S. Optimization of Solid-Phase Microextraction Analysis for Studying Change of Headspace Flavor Compounds of Banana during Ripening. J. Agric. Food Chem. 2002, 50, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Youryon, P.; Supapvanich, S. Physicochemical Quality and Antioxidant Changes in ‘Leb Mue Nang’ Banana Fruit during Ripening. J. Agric. Nat. Resour. 2017, 51, 47–52. [Google Scholar] [CrossRef]
- Venta, M.B.; Broche, S.S.C.; Torres, I.F.; Pérez, M.G.; Lorenzo, E.V.; Rodriguez, Y.R.; Cepero, S.M. Ozone Application for Postharvest Disinfection of Tomatoes. Ozone Sci. Eng. 2010, 32, 361–371. [Google Scholar] [CrossRef]
- Suseno, N.; Savitri, E.; Sapei, L.; Padmawijaya, K.S. Improving Shelf-life of Cavendish Banana Using Chitosan Edible Coating. Procedia Chem. 2014, 9, 113–120. [Google Scholar] [CrossRef]
- Kolgesiz, S.; Tas, C.E.; Koken, D.; Genc, M.H.; Yalcin, I.; Kalender, K.; Unal, S.; Unal, H. Extending the Shelf Life of Bananas with Cinnamaldehyde-Impregnated Halloysite/Polypropylene Nanocomposite Films. ACS Food Sci. Technol. 2023, 3, 340–349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).