Abstract
Pharmaceuticals (PhACs) are chemical substances that, after their use, can reach wastewater treatment plants, but the resulting treated wastewater (TWW) can still contain these contaminants. If TWWs are used for irrigation, PhACs can contaminate crops and also hinder their growth. The aim of this work was to evaluate the effects of 12 PhACs and their mixture at different doses on basil germination and early growth and on its photosynthetic pigment content. The germination percentage was not affected by PhACs even when applied at the highest doses. The results showed that the germination speed cannot be considered as an index of vigor of future seedlings as not all seeds that germinated first developed the best. PhACs between 25 and 100 ppb did not show negative effects on early growth and photosynthetic pigments of basil; in fact, in some cases the seeds even benefitted from their application as if it were a chemical treatment developed for the seeds. The highest assessed dose of PhACs always caused a reduction in growth parameters and the photosynthetic pigment content of basil, especially with climbazole, naproxen, triclosan, and the mixture of PhACs. In general, basil can be considered a species tolerant to PhACs after taking into account their average content in wastewater; however, more studies are needed to evaluate the long-term effects of PhACs and their translocation to edible parts.
1. Introduction
Pharmaceuticals (PhACs) are different chemical substances that are used in both human and veterinary medicine. Some of these have been recognized as contaminants of emerging concern (CECs) as they are increasingly widespread in the environment and have potential adverse effects on non-target organisms and humans [1]. Furthermore, the use of PhACs is increasing worldwide and will continue to increase in the future [2]. After their use, PhACs and/or their metabolites reach wastewater treatment plants through feces and urine where they undergo physical, chemical, and biological processes. The resulting treated wastewater (TWW) can still contain these contaminants [3,4]. Therefore, the use of TWW for irrigation, an agricultural practice already widespread in several regions affected by drought, can expose the soil environment to PhACs contamination [5].
Plants have the ability to absorb and accumulate PhACs in their tissues and is confirmed by several studies [6,7,8,9]. The effects vary in relation to plant species and the chemical and physical properties of the molecules [10,11]. Few studies have investigated the toxic effects of PhACs on seed germination, including reduced shoot growth and metabolic disorders [12,13,14]. In several experiments, hydrophobicity was considered the crucial factor that most affects the phytotoxicity of these compounds toward seeds [15]. Pino et al. [13] investigated the ecotoxicity of 15 PhACs in Lactuca sativa L. seeds and Chlamydomonas reinhardtii. They found that propranolol was the most toxic drug for root and hypocotyl elongation, followed by nonsteroidal anti-inflammatory drugs (NSAIDs), gemfibrozil, and tetracycline. However, the highest concentrations tested did not affect seed germination. Similarly, Bellino et al. [12] reported that chloramphenicol, spiramycin, spectinomycin, and vancomycin, belonging to four different chemical groups, adversely affected tomato root development but not seed germination. In contrast, Rude et al. [14] showed that other PhACs, such as paracetamol, ibuprofen, and amoxicillin, did not induce toxicity in the early growth of lettuce seeds. Therefore, the potential effects of PhACs on plant growth and development need to be fully assessed. Such studies would be of paramount importance considering the possible effects of PhACs on food chains, ecosystems, and human health.
Seed germination and root elongation tests are simple, sensitive, and inexpensive environmental bioassays [16,17], which are commonly used to evaluate the phytotoxicity of organic and/or inorganic compounds on plants [18]. Evaluation of the biological responses of crops to PhACs is important because plants could be increasingly irrigated with reclaimed wastewater. This hypothesis could represent a sustainable alternative to face the water shortage in drought areas, such as the Mediterranean basin.
Sweet basil (Ocimum basilicum L.) is an annual herb belonging to the Lamiaceae family, and the Ocimum genus represents the most widely used medicinal and aromatic plants [19]. In fact, basil is distributed throughout the world and comprises more than 60 cultivars [20]. Aromatic plants, such as sweet basil, can absorb and accumulate PhACs [21], which could alter their growth and yield. Although the toxic effects of a single pharmaceutical have been described at different trophic levels, current knowledge of the effects of a mixture of pharmaceuticals is still scarce. Therefore, the objective of this study was to evaluate the effects of 12 widely consumed pharmaceuticals from different therapeutic groups, alone or in combination, on the germination, early growth, and photosynthetic pigment content of Ocimum basilicum L. The authors wanted to simulate, at the laboratory level, the contact between basil seeds and TWW containing PhACs as TWW can be used for basil irrigation and, therefore, could be a threat that needs further evaluation.
2. Materials and Methods
2.1. Pharmaceuticals
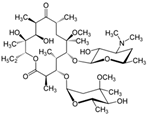
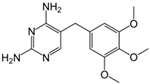
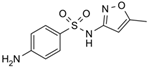
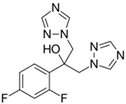
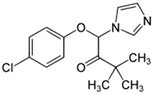
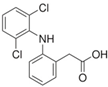
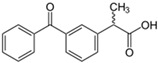
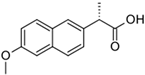
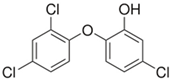
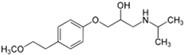
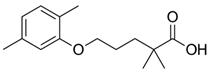
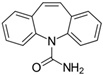
The PhACs used in the present study were carbamazepine (CBZ), clarithromycin (CLR), climbazole (CBZ), diclofenac (DCF), fluconazole (FCZ), gemfibrozil (GFZ), ketoprofen (KET), metoprolol (MTP), naproxen (NPX), sulfamethoxazole (SMX), triclosan (TCS), and trimethoprim (TMP). CLR, TMP, and SMX are antibiotics, CBZ and FCZ are antifungal drugs, DCF, KET, and NPX are anti-inflammatory drugs, and finally, TCS, MTP, GFZ, and CBZ are antibacterial, beta blocker, antilipemic, and antidepressant drugs, respectively. PhACs were selected considering their common presence in European wastewaters [22,23]. The chemical structures and properties of the selected PhACs are listed in Table 1.

Table 1.
Physicochemical properties (Molecular Weight; Chemical Structure; Chemical Class Water Solubility; KOW, Octanol/Water Coefficient; and pKa, Acid Ionization Constant) of selected CECs.
Solutions of each pharmaceutical were prepared at five different concentrations: 25, 50, 100, 200, and 600 ppb. To study a more realistic scenario, Ocimum basilicum seeds were also exposed to PhACs mixture solutions (MIX) at same concentrations.
Analytical standards (purity > 99%), used to prepare all solutions, were provided by Lab Instruments (Italy). Fresh stock solutions of pharmaceuticals were prepared in high purity water.
2.2. Experimental Procedure
Ten basil seeds were placed on Petri plates on Filter-Lab paper (85 mm) and moistened with 10 mL of each solution. Five replicates were tested for every solution, including controls without PhACs. The experiment was carried out in a growth chamber with Petri dishes randomly arranged. The first two days, the seeds remained in the dark to induce germination, then they were subjected to a photoperiod of 16:08 h light:dark with a light intensity of 10,000 l× at 24 °C and 60 % humidity. The germination parameters were recorded daily for the first 9 days, while the length of the roots and shoots, the fresh and dry weight, and the photosynthetic pigment content were determined on the 17th day of the experiment.
2.3. Germination Parameters
The parameters used to compare the germination data for representation and accuracy were as follows:
- Germination Energy (GE)
- Final Germination Percentage (FGP)
- Mean Germination Time (MGT)
- Seed Vigor Index I (SVI-I)
- Seed Vigor Index-II (SVI-II)
Details, measurement units, and calculation methods of each parameter are shown in Table 2.

Table 2.
Description of various parameters used to study seed germination.
2.4. Morphological Parameters and Photosynthetic Pigment Analysis
After 17 days of growth, the length of seedlings was measured using a ruler. Then, they were separated into roots and shoots to determine the fresh weight of the two seedling portions. Afterwards, roots and shoots were placed in paper bags, dried in an oven at 80 °C for 24 h, and weighed as described by Bibi et al. [28]. Seed Vigor Index I (SDI-I) and Seed Vigor Index II (SDI-II) were obtained by multiplying seedling length and seedling dry weight, respectively, using FPG [27].
For the determination of chlorophyll a (CHA) and b (CHB) and total carotenoids (TCA), 25 mg of dried plant material and 25 mg of MgO (to prevent phaeophytin formation) were placed in 15 mL tubes. Subsequently, 5 mL of methanol was added and the whole mixture was homogenized with stirring for 2 h. The samples were then centrifuged for 5 min at 4000 rpm. The supernatant was transferred in a 1 cm path length cuvette. The absorbance was measured using methanol solvent as a blank in a UV-Vis spectrophotometer at three different wavelengths: 666, 653, and 470 nm. According to the method described by Lichtenthaler and Wellburn [29], photosynthetic pigments were calculated as follows:
where OD represents the absorbance at a given wavelength.
CHA = 15.65 OD666 − 7.340 OD653
CHB = 27.05 OD653 − 11.21 OD666
TCA = (1000 OD470 − 2.860 CHA − 129.2 CHB)/245
2.5. Statistical Analysis
All experimental data were tested against the normal distribution of variables (Shapiro–Wilk test) and the homogeneity of variance (Bartlett test) using R studio software, version 4.1.3. The variables were then subjected to a suitable ANOVA and post-hoc test. The difference was significant when the p value ≤ 0.05.
3. Results and Discussion
3.1. Germination Parameters
Figure 1 shows the percentage of basil germination as a function of each PhAC at different doses and of MIX solutions at same concentrations. The statistically lowest percentages of germination were observed for CLZ and MIX only at the highest dose, followed by NPX and TCS, which showed slightly lower percentages of germination relative to other treatments. The remaining theses did not show statistical difference. Similarly, Pino et al. [13] tested 15 PhACs on Lactuca sativa L. germination and found that even at the highest concentration tested (3000 mg L−1), seed germination was not affected. Hillis et al. [30] tested 10 antibiotics in carrots, lettuce, and alfa alfa and found that none of the antibiotics caused a significant decrease in seed germination. This result can be attributed to the seed coat, which is a protective barrier for the plant embryo against environmental contaminants [30,31,32].
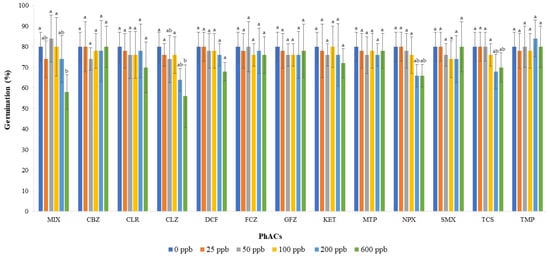
Figure 1.
Effect of mixture and individual pharmaceuticals on seed germination in basil. Each bar represents the mean value of five independent replicates, and the error bar shows the standard deviation. Different letters indicate significant differences between treatments (p < 0.05).
Table 3 shows the values of the germination indexes. GE was statistically the highest for 100 and 200 ppb solutions and the lowest for the control. With respect to the influence of PhACs, MIX and CBZ showed higher values of GE compared to those of other contaminants, whose GEs were quite comparable. On the FGP, this parameter was inversely proportional to the concentration of the solution applied, and the control showed the highest values. On the contrary, no significant differences were recorded when PhACs were considered individually or in combination. Therefore, in the first 4 days of the trial, the presence of PhACs was more helpful for the germination of the seeds, especially the PhACs mixture, possibly because of a short-term effect of chemical treatment on seeds. This was confirmed by the MGT values that resembled the GE trend. On the other hand, over the entire period of the trial, the PhACs solutions resulted in a lower inhibition of seed germination than the control. As the germinability varies in a seed population, it could be possible that seeds with the lowest intrinsic germinability, i.e., the ones that germinated later, were most affected by the addition of PhACs.

Table 3.
Effects of pharmaceuticals and different concentrations on germination indexes.
3.2. Early Growth Parameters
Figure 2 shows the length of the roots (A) and shoots (B) of the basil seedlings affected by each pharmaceutical or their mixture, added at different doses, with respect to the control. CLR, CLZ, DCF, and GFZ showed better values than the control when applied at 25 and 50 ppb doses, while FCZ and KET showed better values up to 200 ppb dose. Anyway, previous PhACs showed the hormesis phenomenon, that is, a biphasic response, favorable and inhibitory effect at low and high doses, respectively [33]. Similarly, other authors reported a greater growth of roots in some plants with the application of low doses of pharmaceuticals and a growth inhibition with the application of high doses of pharmaceuticals [34,35,36,37]. Finally, CBZ, SMX, and TMP did not cause modification in root length at any concentration of applied dose, while MTP, NPX, TCS, and MIX induced a significant reduction in root length only when higher doses were applied.
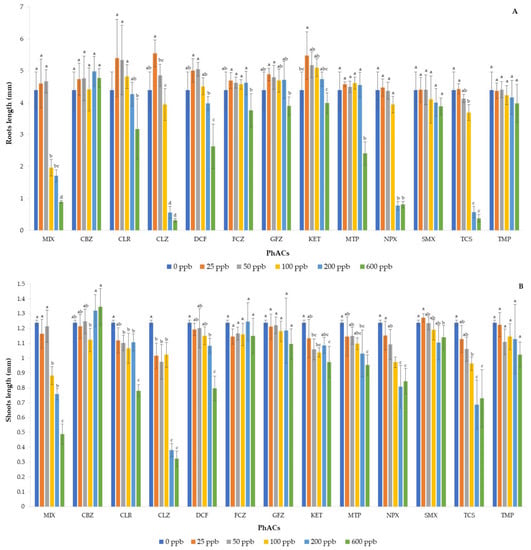
Figure 2.
Effect of the mixture and individual pharmaceuticals on the length of the root (A) and shoot (B) in basil seedlings. Each bar represents the mean value of five independent replicates, and the error bar shows the standard deviation. Different letters indicate significant differences between treatments (p < 0.05).
No PhACs studied showed hormesis, and none resulted in longer shoots than the control, except CBZ, which showed slightly higher values when applied at the highest doses. FCZ and GFZ did not cause any significant variation in the length of the shoot, while the remaining PhACs showed a similar trend to one observed for the corresponding root length.
The VI-I index confirmed that doses 25 and 50 ppb resulted in the best vigor, while the same index decreased with increasing concentration of solutions (Table 2). In addition, the PhACs studied were segregated by the VI-I index in two groups: one composed of MIX, CLZ, NPX, and TCS, characterized by the lowest vigor, and the other one, represented by the remaining PhACs, showing the best vigor.
Figure 3 shows the dry weight of the roots (A) and the shoots (B). CBZ, CLZ, DCF, NPX, and TCS showed hormesis, that is, a significant increase in the dry weight of the root when applied at 100 ppb concentration (200 ppb for CBZ) compared to the control, while they decreased the same parameter at higher solution concentrations. KET and MIX did not influence the dry weight of the root at any concentration, while the remaining PhACs resulted in a weight reduction with the highest doses. Regarding shoot weight, all PhACs showed the same trend as that for the corresponding root dry weight, except for CBZ and NPX that did not show hormesis as observed for the roots. It was evident that doses of 200 and especially 600 ppb drastically reduced the dry weights of the seedlings, highlighting the toxicity of the various compounds with respect to the applied dose. In general, the effects of PhACs on plants depend on the class of pharmaceuticals and its concentration and on the plant species [38]. Therefore, it is possible that some of the PhACs studied can stimulate basil growth in low doses due to possible hormone-like effects and/or seed protection against seedling damping.
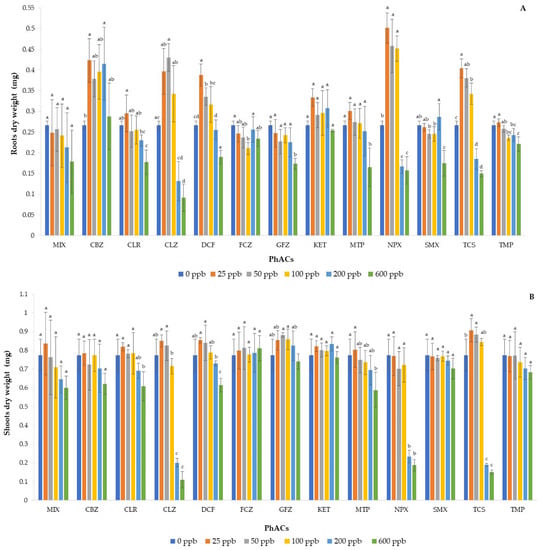
Figure 3.
Effect of the mixture and individual pharmaceuticals on the dry weight of the root (A) and shoot (B) in basil seedlings. Each bar represents the mean value of five independent replicates, and the error bar shows the standard deviation. Different letters indicate significant differences between treatments (p < 0.05).
The VI-II index confirmed that doses of 25 to 100 ppb resulted in better vigor compared to control and doses of higher concentrations (Table 2). Among the PhACs studied, CLZ and NPX showed the highest VI-II index due to their larger dry weight, especially at 25 to 100 ppb; however, significant differences in FGP values for each PhAC were not observed. In general, CLZ, NPX, and TCS showed the worst results for the aforementioned parameters at the highest dose. Regarding CLZ, Richter et al. [39] found that this compound negatively influenced seedling emergence, shoot length, and biomass of Brassica napus L. and Avena sativa L. In general, demethylase inhibitor fungicides, such as climbazole, can act as plant growth inhibitors that interact with sterol biosynthesis and inhibit gibberellin biosynthesis. Climbazole is an imidazole and has a higher toxicity than triazoles, such as FCZ, and isoxazoles, such as SMX [40]. In fact, in our study, FCZ and SMX showed slight toxicity only at the highest applied dose for some parameters. In particular, FCZ did not affect basil growth as its behavior is similar to that of other triazoles used as plant fungicides and growth promoters [41,42]. However, García-Valcárcel et al. [43] tested different azoles on lamb lettuce and found higher weights for plants grown in the presence of azoles compared to the control in the initial days of the experiment, and the same was observed in the present study for CLZ applied at lower doses.
The worst results of TCS at higher doses were possibly due to blockage of fatty acid synthesis in plants by inhibiting a reductase implicated in various metabolic pathways [44]. As a result, plants exposed to high concentrations of triclosan soon died. Regarding NPX, Pawłowska et al. [34] conducted a study on the effects of NPX and DCF on growth parameters of spring barley and found that NPX had the greatest negative effect on the plant. High doses of NPX can induce the formation of reactive oxygen species (ROS), lipid peroxidation, reduction of membrane integrity, and change in the antioxidant and redox system that, finally, negatively influence the root apparatus in plants [36].
With respect to MIX, the length of roots and shoots was significantly lower than the control at a dose of 100 ppb, showing greater toxicity of this dose than that of a single contaminant. However, at doses of 200 and 600 ppb, the toxicity of the mixture was in any case lower than that of CLZ, NPX, and TCS considered individually. Furthermore, the dry weight of the roots and shoots did not show significant differences compared to the control, even at the highest dose applied. These results could be derived from interactions among the different contaminants that have mitigated the effect of individual PhACs, lowering their toxic effect.
3.3. Photosynthetic Pigments
Figure 4 shows the concentration of CHA (A), CHB (B), and TCA (C) from basil seedlings as a function of each PhAC at different doses and of MIX at same doses. The trend of CHA content was quite similar among the studied PhACs, and from 100 ppb to the highest dose, its content decreased in all treatments, although to a different extent, with CLZ, NPX, TCS, and MIX resulting in the largest reduction in CHA concentration. Finally, CBZ, KET, and MTP showed slightly higher content at the single dose of 25 ppb. Instead, all PhACs resulted in the highest CHB concentration when applied at a dose of 25 ppb, while its concentration decreased from 100 ppb with increasing concentration of the dose.
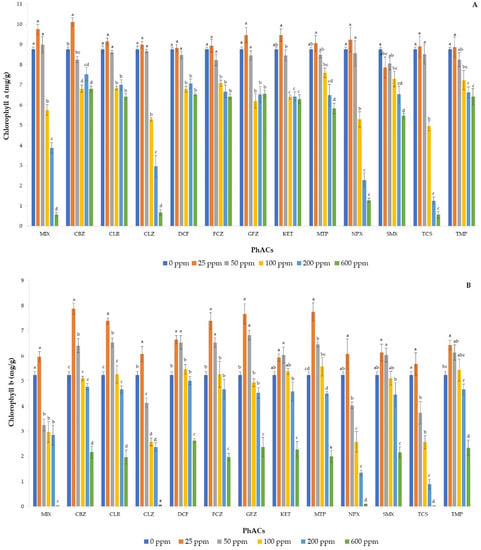
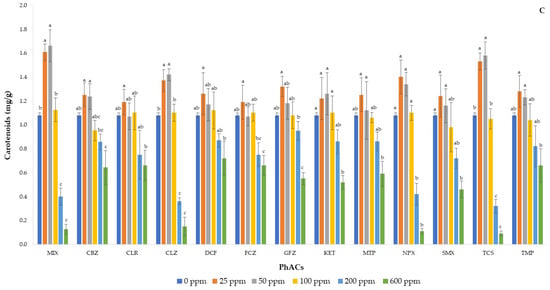
Figure 4.
Concentrations of chlorophyll a (A), chlorophyll b (B), and total carotenoids (C) in basil plants after exposure to mixture and individual pharmaceuticals. Each bar represents the mean of five independent replicates, and the error bar shows the standard deviation. Different letters indicate significant differences between treatments (p < 0.05).
The worst results were recorded with the application of 600 ppb of MIX, CLZ, NPX, and TCS, which resulted in an almost zero concentration of both chlorophylls. In fact, the latter are pigments involved in photosynthesis and, therefore, responsible for plant growth, with CHA being more abundant because it is the principal pigment involved in photosynthesis, while CHB is an accessory pigment [45]. Changes in their concentration are indicative of the cumulative impact of environmental stress [46] and mainly attributed to metabolic disorders or ROS accumulation in chloroplasts [47]. Therefore, all contaminants tested, at doses higher than 50 ppb, induced a stress condition in basil seedlings as the reduction in chlorophyll content is a typical stress response [48]. Similarly, Opriș et al. [49] tested the effects of non-steroidal anti-inflammatory drugs (NSAIDs) on green leafy vegetables and found a reduction in chlorophylls, carotenoids, polyphenols, and flavonoids as a function of the dose applied. Wang et al. [50] also confirmed the toxicity of NSAIDs in green alga with a reduction in the concentration of photosynthetic pigments.
A significant reduction in the content of TCA was observed mainly with the application of the highest dose of contaminants, that is, 600 ppb and, only in some cases, 200 ppb. Therefore, carotenoids appeared to be less sensitive than chlorophylls to the addition of different contaminants in the basil growth medium. Carotenoids are secondary metabolites that help absorb light and protect elements against photodamage and ROS-induced oxidative stress [51,52]. Therefore, the reduction in carotenoids occurred only in the case of severe stress [49] and, in general, their trend resembled those of chlorophylls, confirming the highest toxic effect of CLZ, NPX, TCS, and MIX on basil seedlings.
4. Conclusions
The results of this study suggest that PhACs doses between 25 and 50 ppb do not interfere with basil germination and early growth and, rather, some PhACs show positive effects on these parameters as if they were a chemical treatment developed for seeds. Therefore, considering the doses of contaminants normally present in the soil or added with wastewater, it can be stated that basil does not suffer from the current environmental contamination of the PhACs studied. However, if PhACs somehow reach doses of 200 to 600 ppb, the effects on germination and early growth are hazardous, especially when basil is treated with CLZ, NPX, TCS, and MIX.
Among the germination parameters considered in the present study, the percentage of germination is the parameter least sensitive to contaminants, while a higher germination speed is not necessarily indicative of better development of future seedlings. In fact, MIX caused faster germination compared to other PhACs but later showed higher toxicity, resulting in less vigorous seedlings. More studies are needed to evaluate the long-term growth of basil and the possible translocation of PhACs to its edible parts.
Author Contributions
Conceptualization, G.B., A.T., F.D.M. and C.C. (Claudio Cocozza); methodology, G.B., A.T., F.D.M. and C.C. (Claudio Cocozza); software, A.T., C.C. (Claudio Cacace) and F.D.M.; validation, G.B. and C.C. (Claudio Cocozza); formal analysis, A.T., C.C. (Claudio Cacace) and F.D.M.; investigation, G.B., A.T., F.D.M. and C.C. (Claudio Cocozza); resources, G.B.; data curation, A.T. and F.D.M.; writing—original draft preparation, A.T., C.C. (Claudio Cacace) and F.D.M.; writing—review and editing, A.T., F.D.M., C.C. (Claudio Cocozza) and G.B.; visualization, C.C. (Claudio Cocozza) and G.B.; supervision, C.C. (Claudio Cocozza) and G.B.; project administration, G.B.; funding acquisition, G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministero Dell’istruzione Dell’università E Della Ricerca, Italy, grant number: 2017C5CLFB.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Madikizela, L.; Ncube, S.; Chimuka, L. Uptake of pharmaceuticals by plants grown under hydroponic conditions and natural occurring plant species: A review. Sci. Total Environ. 2018, 636, 477–486. [Google Scholar] [CrossRef] [PubMed]
- González Peña, O.I.; López Zavala, M.Á.; Cabral Ruelas, H. Pharmaceuticals Market, Consumption Trends and Disease Incidence Are Not Driving the Pharmaceutical Research on Water and Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 2532. [Google Scholar] [CrossRef] [PubMed]
- Khasawneh, O.F.S.; Palaniandy, P. Occurrence and removal of pharmaceuticals in wastewater treatment plants. Process Saf. Environ. Prot. 2021, 150, 532–556. [Google Scholar] [CrossRef]
- Shaheen, J.F.; Sizirici, B.; Yildiz, I. Fate, transport, and risk assessment of widely prescribed pharmaceuticals in terrestrial and aquatic systems: A review. Emerg. Contam. 2022, 8, 216–228. [Google Scholar] [CrossRef]
- Al-Farsi, R.S.; Ahmed, M.; Al-Busaidi, A.; Choudri, B.S. Translocation of pharmaceuticals and personal care products (PPCPs) into plant tissues: A review. Emerg. Contam. 2017, 3, 132–137. [Google Scholar] [CrossRef]
- Bártíková, H.; Podlipná, R.; Skálová, L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere 2016, 144, 2290–2301. [Google Scholar] [CrossRef]
- De Mastro, F.; Brunetti, G.; De Mastro, G.; Ruta, C.; Stea, D.; Murgolo, S.; De Ceglie, C.; Mascolo, G.; Sannino, F.; Cocozza, C.; et al. Uptake of different pharmaceuticals in soil and mycorrhizal artichokes from wastewater. Environ. Sci. Pollut. Res. 2022, 30, 33349–33362. [Google Scholar] [CrossRef]
- Stando, K.; Korzeniewska, E.; Felis, E.; Harnisz, M.; Bajkacz, S. Uptake of Pharmaceutical Pollutants and Their Metabolites from Soil Fertilized with Manure to Parsley Tissues. Molecules 2022, 27, 4378. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, Y.; Zhang, H.; Wang, J.; Lian, K.; Ai, L. Uptake and Transport of Different Concentrations of PPCPs by Vegetables. Int. J. Environ. Res. Public Health 2022, 19, 15840. [Google Scholar] [CrossRef]
- Corbel, S.; Mougin, C.; Martin-Laurent, F.; Crouzet, O.; Bru, D.; Nélieu, S.; Bouaïcha, N. Evaluation of phytotoxicity and ecotoxicity potentials of a cyanobacterial extract containing microcystins under realistic environmental concentrations and in a soil-plant system. Chemosphere 2015, 128, 332–340. [Google Scholar] [CrossRef]
- Venkata, L.; Pullagurala, R.; Rawat, S.; Adisa, I.O.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Plant uptake and translocation of contaminants of emerging concern in soil. Sci. Total Environ. 2018, 636, 1585–1596. [Google Scholar] [CrossRef]
- Bellino, A.; Lofrano, G.; Carotenuto, M.; Libralato, G.; Baldantoni, D. Antibiotic effects on seed germination and root development of tomato (Solanum lycopersicum L.). Ecotoxicol. Environ. Saf. 2018, 148, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.; Muñiz, S.; Val, J.; Navarro, E. Phytotoxicity of 15 common pharmaceuticals on the germination of Lactuca sativa and photosynthesis of Chlamydomonas reinhardtii. Environ. Sci. Pollut. Res. 2016, 23, 22530–22541. [Google Scholar] [CrossRef]
- Rede, D.; Santos, L.H.M.L.M.; Ramos, S.; Oliva-Teles, F.; Antão, C.; Sousa, S.R.; Delerue-Matos, C. Individual and mixture toxicity evaluation of three pharmaceuticals to the germination and growth of Lactuca sativa seeds. Sci. Total Environ. 2019, 673, 102–109. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L. Phytotoxicity of veterinary antibiotics to seed germination and root elongation of crops. Ecotoxicol. Environ. Saf. 2016, 126, 228–237. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Priac, A.; Badot, P.M.; Crini, G. Treated wastewater phytotoxicity assessment using Lactuca sativa: Focus on germination and root elongation test parameters. Comptes Rendus Biol. 2017, 340, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Di Salvatore, M.; Carafa, A.M.; Carratù, G. Assessment of heavy metals phytotoxicity using seed germination and root elongation tests: A comparison of two growth substrates. Chemosphere 2008, 73, 1461–1464. [Google Scholar] [CrossRef]
- Calderón Bravo, H.; Vera Céspedes, N.; Zura-Bravo, L.; Muñoz, L.A. Basil Seeds as a Novel Food, Source of Nutrients and Functional Ingredients with Beneficial Properties: A Review. Foods 2021, 10, 1467. [Google Scholar] [CrossRef]
- Sipos, L.; Balázs, L.; Székely, G.; Jung, A.; Sárosi, S.; Radácsi, P.; Csambalik, L. Optimization of Basil (Ocimum basilicum L.) Production in LED Light Environments—A Review. Sci. Hortic. 2021, 289, 110486. [Google Scholar] [CrossRef]
- Kowalska, G. Pesticide Residues in Some Polish Herbs. Agriculture 2020, 10, 154. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Xue, J.; Zhao, Y.; Taylor, A.A.; Zenobio, J.E.; Sun, Y.; Han, Z.; Salawu, O.A.; Zhu, Y. Abundance, fate, and effects of pharmaceuticals and personal care products in aquatic environments. J. Hazard. Mater. 2022, 424, 127284. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Varela Della Giustina, S.; Llorca, M.; Barcelo, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef] [PubMed]
- ISTA. ISTA Handbook on Seedling Evaluation; International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2006. [Google Scholar]
- Scott, S.; Jones, R.; Williams, W. Review of data analysis methods for seed germination. Crop Sci. 1984, 24, 1192–1199. [Google Scholar] [CrossRef]
- Orchard, T. Estimating the parameters of plant seedling emergence. Seed Sci. Technol. 1977, 5, 61–69. [Google Scholar]
- Abdul- Baki, A.A.; Anderson, J.D. Physiological and biochemical deterioration of seeds. In Seed Biology; Kozlowski, T.T., Ed.; Academic Press: New York, NY, USA, 1972; Volume 2, pp. 283–315. [Google Scholar]
- Bibi, A.; Sadaqat, H.A.; Tahir, M.H.N.; Akram, H.M. Screening of sorghum (Sorghum bicolor var Moench) for drought tolerance at seedling stage in polyethylene glycol. J. Anim. Plant Sci. 2012, 22, 671–678. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Hillis, D.G.; Fletcher, J.; Solomon, K.R.; Sibley, P.K. Effects of ten antibiotics on seed germination and root elongation in three plant species. Arch. Environ. Contam. Toxicol. 2011, 60, 220–232. [Google Scholar] [CrossRef]
- An, J.; Zhou, Q.; Sun, F.; Zhang, L. Ecotoxicological effects of paracetamol on seedgermination and seedling development of wheat (Triticum aestivum L.). J. Hazard. Mater. 2009, 169, 751–757. [Google Scholar] [CrossRef]
- Vazquez-Roig, P.; Andreu, V.; Blasco, C.; Picó, Y. Risk assessment on the presence of pharmaceuticals in sediments, soils and waters of the Pego–OlivaMarshlands (Valencia, eastern Spain). Sci. Total Environ. 2012, 440, 24–32. [Google Scholar] [CrossRef]
- Novak, P.J.; Arnold, W.A.; Blazer, V.S.; Halden, R.U.; Klaper, R.D.; Kolpin, D.W.; Kriebel, D.; Love, N.G.; Martinović-Weigelt, D.; Patisaul, H.B.; et al. On the Need for a National (U.S.) Research Program to Elucidate the Potential Risks to Human Health and the Environment Posed by Contaminants of Emerging Concern. Environ. Sci. Technol. 2011, 45, 3829–3830. [Google Scholar] [CrossRef]
- Pawłowska, B.; Telesiński, A.; Biczak, R. Effect of diclofenac and naproxen and their mixture on spring barley seedlings and Heterocypris incongruens. Environ. Toxicol. Pharmacol. 2021, 88, 103746. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.; Redshaw, C.H. Evaluation of biological endpoints in crop plants after exposure to non-steroidal anti-inflammatory drugs (NSAIDs): Implications for phytotoxicological assessment of novel contaminants. Ecotox. Environ. Saf. 2015, 112, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Svobodníková, L.; Kemerova, M.; Zezulka, S.; Babula, P.; Sendecká, K. Root response in Pisum sativum under naproxen stress: Morphoanatomical, cytological, and biochemical traits. Chemosphere 2020, 258, 127411. [Google Scholar] [CrossRef] [PubMed]
- Zezulka, S.; Kummerová, M.; Babula, P.; Hájková, M.; Oravec, M. Sensitivity of physiological and biochemical endpoints in early ontogenetic stages of crops under diclofenac and paracetamol treatments. Environ. Sci. Pollut. Res. 2019, 26, 3965–3979. [Google Scholar] [CrossRef]
- Bartrons, M.; Peñuelas, J. Pharmaceuticals and personal-care products in plants. Trends Plant Sci. 2017, 22, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.; Wick, A.; Ternes, T.A.; Coors, A. Ecotoxicity of climbazole, a fungicide contained in antidandruff shampoo. Environ. Toxicol. Chem. 2013, 32, 2816–2825. [Google Scholar] [CrossRef]
- Bhagat, J.; Singh, N.; Nishimura, N.; Shimada, Y. A comprehensive review on environmental toxicity of azole compounds to fish. Chemosphere 2021, 262, 128335. [Google Scholar] [CrossRef]
- Currey, C.J.; Erwin, J.E. Foliar applications of plant growth regulators affect stem elongation and branching of 11 Kalanchoe species. HortTechnology 2012, 22, 338–344. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, X.L.; Yong, T.W.; Yang, W.Y. Seed treatment with uniconazole powder induced drought tolerance of soybean in relation to changes in photosynthensis and chlorophyll fluorescence. Res. Crop 2012, 13, 147–154. [Google Scholar]
- García-Valcárcel, A.I.; Loureiro, I.; Escorial, C.; Molero, E.; Tadeo, J.L. Uptake of azoles by lamb’s lettuce (Valerianella locusta L.) grown in hydroponic conditions. Ecotoxicol. Environ. Saf. 2016, 124, 138–146. [Google Scholar] [CrossRef]
- McLeod, R.; Muench, S.P.; Rafferty, J.B.; Kyle, D.E.; Mui, E.J.; Kirisits, M.J.; Mack, D.G.; Roberts, C.W.; Samuel, B.U.; Lyons, R.E.; et al. Triclosan inhibits the growth of Plasmodium falciparum and Toxoplasma gondii by inhibition of Apicomplexan Fab I. Int. J. Parasitol. 2001, 31, 109–113. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Młodzińska, E. Survey of plant pigments: Molecular and environmental determinants of plant colors. Acta Biol. Cracov. Bot. 2009, 51, 7–16. [Google Scholar]
- Cao, X.; Cui, X.; Xie, M.; Zhao, R.; Xu, L.; Ni, S.; Cui, Z. Amendments and bioaugmentation enhanced phytoremediation and micro- ecology for PAHs and heavy metals co-contaminated soils. J. Hazard. Mater. 2022, 426, 128096. [Google Scholar] [CrossRef] [PubMed]
- Munneé-Bosch, S.; Alegre, L. Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta 2000, 210, 925–931. [Google Scholar] [CrossRef]
- Opriș, O.; Lung, I.; Soran, M.L.; Ciorîță, A.; Copolovici, L. Investigating the effects of non-steroidal anti-inflammatory drugs (NSAIDs) on the composition and ultrastructure of green leafy vegetables with important nutritional values. Plant Physiol. Biochem. 2020, 151, 342–351. [Google Scholar] [CrossRef]
- Wang, H.; Jin, M.; Mao, W.; Chen, C.; Fu, L.; Li, Z.; Du, S.; Liu, H. Photosynthetic toxicity of non-steroidal anti-inflammatory drugs (NSAIDs) on green algae Scenedesmus obliquus. Sci. Total Environ. 2020, 707, 136176. [Google Scholar] [CrossRef]
- Barizão, É.O.; Visentainer, J.V.; de Cinque Almeida, V.; Ribeiro, D.; Chisté, R.C.; Fernandes, E. Citharexylum Solanaceum Fruit Extracts: Profiles of Phenolic Compounds and Carotenoids and Their Relation with ROS and RNS Scavenging Capacities. Food Res. Int. 2016, 86, 24–33. [Google Scholar] [CrossRef]
- Othman, R.; Mohd Zaifuddin, F.A.; Hassan, N.M. Carotenoid biosynthesis regulatory mechanisms in plants. J. Oleo Sci. 2014, 63, 753–760. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).