Abstract
Organophosphorus (OP) pesticides are a class of chemicals that are extensively used worldwide. The exposure to and use of organophosphates can be assessed by analyzing their metabolites and degradation products, such as dialkyl phosphate (DAP), dialkyl thiophosphate (DATP), and dialkyl dithiophosphate (DADTP). However, since these metabolites/hydrolysis products can result from the metabolism or breakdown of several organophosphorus pesticide families, they serve as nonspecific biomarkers and do not indicate the specific pesticide involved in exposure. In an earlier study, chemical derivatization using N-(2-(bromomethyl)benzyl)-N,N-diethylethanaminium bromide (CAX-B) was described to improve the signal intensity of numerous organophosphorus (OP) acids in liquid chromatography tandem mass spectrometry (LC-ESI-MS/MS) analysis. In the present study, CAX-B was employed to derivatize a set of seven phenolic compounds corresponding to the complementary portion of OP pesticides. The derivatization process using CAX-B was performed in acetonitrile with potassium carbonate at 50 °C for 30 min. LC-Orbitrap-ESI-MS/MS was used to analyze the resulting phenol derivatives and their fragmentation patterns were studied. Notably, the derivatized phenols were markedly more sensitive than the underivatized phenols when LC-ESI-MS/MS was used in MRM technique, without being affected by the sample matrix (soil or plant extracts). This derivatization technique aids in identifying OP pesticides, offers insights into their subfamily, and pinpoints a specific compound through the analysis of corresponding phenol derivative.
Keywords:
organophosphorus pesticides; phenols; derivatization; CAX-B; LC-MS/MS; soil; plants; sensitivity enhancement 1. Introduction
Organophosphorus (OP) pesticides are widely used in agriculture to control insect pests by inhibiting pest nervous system function. While OP pesticides are effective at increasing crop yields, they pose health risks to humans and animals because of their toxicity. Exposure, especially among agricultural workers, can cause neurological and respiratory issues, and OP residues harm nontarget species such as bees and aquatic life, disrupting ecosystems. Owing to these risks, the regulations related to OP pesticides are becoming more stringent, with efforts focused on finding safer alternatives and improving OP residue management to minimize environmental impact [,].
OP pesticides are categorized as nonpersistent pesticides exhibiting short lifetimes, e.g., the half-lives of dicrotophos and profenfos are several days in the soil [,,]. Their urinary metabolites and environmental decomposition products are commonly employed for the assessment of OP pesticide exposure and use []. At present, dozens of OP pesticides have been approved for use in agriculture, many of which contain O,O-dialkyl-substituted phosphate cores and reactive phenol groups. The most common metabolites and degradation products of OP pesticides are OP acids including dialkyl phosphate (DAP), dialkyl thiophosphate (DATP), and dialkyl-dithiophosphate (DADTP). The detection of these metabolites/hydrolysis products clearly demonstrates the incorporation of an OP pesticide but does not allow for the identification of the phenolic leaving group. Hence, identifying specific pesticides from these decomposition products or metabolites is unfeasible since they offer information solely about the OP pesticide class and not on a unique compound [,]. The determination of a specific phenol, e.g., 4-nitro phenol (PNP), is a more selective approach than the determination of dialkylphosphate derivative, as PNP is released after exposure to only a few OP pesticides (ethyl parathion, methyl parathion, and EPN), and the determination of a specific phenol, e.g., trichloropyridinol, is released from chlorpyrifos and chlorpyrifos-oxon [,]. Notably, although these phenols can be generated via the hydrolysis of some pesticides used in agriculture, they may have different potential sources in the environment. For example, NPs have been found in the environment in increasing quantities because of wastes from different industrial, agricultural, and medical activities []. However, identifying the two complementary degradation products, the OP acid and the phenolic leaving group, can provide insight into the specific pesticide involved. The analysis of phenolic compounds is usually performed by LC with UV detection [,,,,], LC with MS detection [,,,,], or various GC-MS detection methods [,,,,,,]. All these approaches require extensive sample preparation, involving the formation of volatile derivatives, cleanup, and sample concentration. When GC-MS is used, the cleanup requirements are slightly simpler, but the pre-derivatization steps are still necessary, and the detection sensitivities are relatively low. Francis et al. published an overview of the determination of p-nitrophenol (p-NP) and the remediation of media contaminated with this compound []. More recently, a review on the determination of organophosphorus metabolites in urine via chromatography-mass spectrometry-based methods was published [], with the references therein demonstrating the requirement for extensive sample preparation. The need to identify both complementary degradation products, the OP acid and the phenolic leaving group, which together offer insight into the specific pesticide used, has traditionally required two separate sample preparation approaches, as no single derivatizing agent has been capable of effectively targeting both phenols and organophosphorus acids using LC-MS/MS. This two separate steps add complexity to the analytical process and can introduce potential sources of errors.
Liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) is often the most suitable and effective analytical method. It is excellent in the detection of phenolic compounds that are challenging to analyze owing to their low molecular weight, limited fragmentation in MS/MS, and poor ionization efficiency. This technique can result in high sensitivity with minimal sample preparation, particularly when combined with appropriate derivatization strategies. Recently, we developed a simple analytical method to increase the identification sensitivity of specific environmentally relevant OP acids related to pesticides, utilizing chemical derivatization with CAX-B followed by LC-ESI-MS/MS [].
The aim of this study was to simplify and greatly increase the identification sensitivity and specificity of targeted environmentally relevant phenols related to pesticides by LC-ESI-MS/MS analysis in the positive mode by utilizing chemical derivatization with CAX-B. Although dansyl chloride, a well-known LC-MS/MS-compatible derivatizing agent has been employed for the analysis of alkyl phenols (notably endocrine disruptors) [], it is not suitable for the derivatization of organophosphorus (OP) acids. To date, no single universal derivatizing agent was utilized for both phenols and OP acids, which represent major metabolites and degradation products of organophosphorus pesticides. The method developed in this study is the first to enable the determination of both components of OP pesticide exposure using a single derivatizing agent. The CAX-B approach combines the most sensitive technique for phenol determination with the ability to provide detailed information about the exact phenol structure.
2. Materials and Methods
2.1. Chemicals and Reagents
All chemicals were purchased from commercial suppliers: 4-bromo-2-chlorophenol (1), 2-methyl-4-(methylthio)phenol (2), 2-methyl-4-nitrophenol (3), 3,5,6 trichloro-pyridine-2-ol (4), 4-nitrophenol (5), 4-(methylthio)phenol (6), N)4-hydroxyphenyl)acetamide (7), and potassium carbonate were attained from Sigma-Aldrich (St. Louis, MO, USA). N-(2-(Bromomethyl)benzyl)-N,N-diethylethanaminium bromide (CAX-B) was synthesized in house as described by Wang et al. []. Formic acid (MS grade), acetonitrile (MS grade), methanol (MS grade), and water (MS grade) were purchased from Biolab (Jerusalem, Israel). Leaves were gathered from lemon and orange trees, and soil was collected from the Negev (south Israel).
2.2. Sample Preparation
A stock solution (1 mg/mL) of phenols 1–7 (Figure 1) was prepared in acetonitrile (ACN). This solution was further diluted in acetonitrile/acetonitrile extracts of soil and plants before undergoing derivatization to concentrations of 0.02–10 ng/mL.
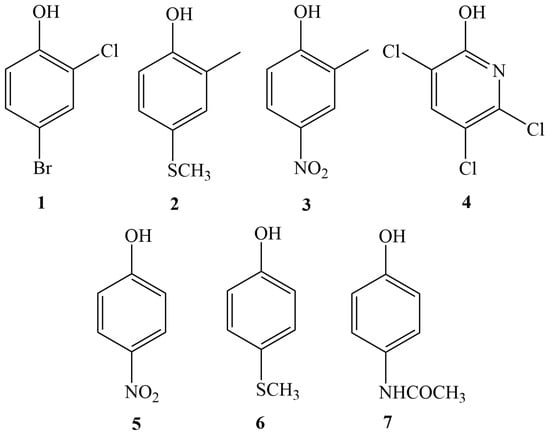
Figure 1.
The structures of the investigated phenols derived from the hydrolysis of organophosphorus (OP) pesticides.
2.2.1. Synthesis of N-(2-(Bromomethyl)benzyl)-N,N-diethylethanaminium Bromide (CAX-B) Reagent
CAX-B was synthesized from α,α’-Dibromo-o-xylene and Et3N according to the procedure published by Wang et al. [] with a 52% yield. CAX-B was characterized by high-resolution mass spectrometry (HRMS) using LC-MS Orbitrap instrumentation. The observed M+ ion was detected at m/z 284.1005, in excellent agreement with the calculated theoretical value of m/z 284.1008 (mass error < 2 ppm). The high mass accuracy supports the assignment of the molecular formula. Furthermore, the experimentally determined isotopic distribution closely matched the theoretical pattern, with mass deviations of less than 2 ppm and abundance deviations below 1.5%.
2.2.2. Derivatization Procedure
To a sample tube containing 500 µL of an acetonitrile/acetonitrile extract with analyte concentrations ranging from 10 pg to 5 ng, 1 mg of K2CO3 and 1 mg of CAX-B was added. The mixture was stirred at 50 °C and 900 rpm for 30 min using an Eppendorf thermomixer. Next, the solution was diluted in a ratio of 1:2 with water and transferred to an LC vial for LC-ESI-MS/MS analysis.
2.2.3. Spiking of Environmental Matrix Extracts
Plant leaves (10.5 g) and soil (10.2 g) were each mixed with 20 mL of acetonitrile in a 50 mL polypropylene tube, vortexed for 2 min, and left undisturbed at room temperature in a fume hood for 1 h. The acetonitrile extracts obtained were filtered through a 0.22 µm Teflon filter into clean glass bottles and stored at 4 °C. A 500 µL aliquot of each extract was fortified with the analytes and promptly processed using the previously described derivatization procedure.
2.3. Instrumentation
2.3.1. LC-ESI-MS/MS (MRM)
The analytes were separated using an Agilent 1290 Infinity II UHPLC system (Waldbronn, Germany). EPI (enhanced product ion) and MRM experiments were carried out with an SCIEX 6500+ QTRAP mass spectrometer (Framingham, MA, USA) controlled by Analyst software (version 1.7.2) and equipped with a Turbo V ion source operated in positive and negative ESI mode. Gradient elution was performed using a reversed-phase Gemini C18 separation column (3.0 μm, 150 mm × 2.1 mm ID; Phenomenex, Switzerland) at a flow rate of 0.3 mL/min, with the column temperature consistently maintained at 40 °C.
For separating intact phenols, the mobile phase comprised of water with 1 mM ammonium formate as phase A and methanol with 1 mM ammonium formate as phase B. For the separation of CAX-phenol derivatization products, phase A was water with 0.1% formic acid, and phase B was methanol with 0.1% formic acid. The separation gradient began with 100% mobile phase A and ramped to 95% B over 6 min. Then, the flow was held at 95% B for 3.5 min, switched back to 100% A, and held for 5.5 additional minutes to equilibrate the column for the next sample. The total LC cycle time was 15 min and the injection volume was 10 μL.
The ESI inlet conditions were as follows: gas 1, air (60 psi, 2.7 bar); gas 2, air (30 psi, 4.0 bar); ion spray voltage, 5500 V; ion source temperature, 500 °C; and curtain gas, nitrogen (35 psi, 2.4 bar). For the enhanced product ion (EPI) (MS/MS) experiments, the collision gas was set to “medium” and the collision energy was set between 10 and 50 V.
LC-ESI-MS/MS (MRM) Analysis of the Phenols Prior to Derivatization
The phenols related to organophosphorus pesticides (1–7) were analyzed without modification to evaluate their responses via LC-ESI-MS/MS prior to derivatization. Except for phenol 7, which yielded a protonated molecule [M+H]+, the other phenols (1–6) predominantly yielded deprotonated molecules [M-H]− with the most abundant MRM transitions listed in Table 1.

Table 1.
LC-MS/MS (MRM) data and parameters for phenols 1–7 acquired via LC-QTRAP.
LC-ESI-MS/MS (MRM) Analysis of CAX-Phenol Derivatives
The detection and identification of all phenols after the derivatization reaction with CAX-B was carried out in positive ion MRM mode using the most abundant MRM transitions listed in Table 2.

Table 2.
LC-MS/MS (MRM) data and parameters for CAX-phenols 1–7 acquired via LC-QTRAP.
2.3.2. LC-ESI-HRMS/MS (Orbitrap Mass Spectrometry)
The analytes were separated using an Agilent 1290 high-performance LC system (Palo Alto, CA, USA). The MS and MS/MS experiments were conducted with a Thermo Scientific Q Exactive Plus Orbitrap MS (Thermo Fisher Scientific, Bremen, Germany) equipped with a heated-ESI (HESI) source operating in positive and negative ion modes.
The parameters for the Orbitrap HESI were identical to those used in our previous study [] and are as follows: electrospray voltage of 1.25 kV, auxiliary gas flow rate of 10 (arbitrary units), sheath gas flow rate of 45 (arbitrary units), auxiliary gas heater temperature of 400 °C, sweep gas flow rate of 2 (arbitrary units), and capillary temperature of 275 °C. The instrument was calibrated using positive and negative ESI calibration solutions prepared according to the operating manual. All samples were analyzed in two alternating experimental modes: full-scan mode from m/z 60–500 with a resolving power of 70,000, an automatic gain control (AGC) target of 1 × 106, and a data-independent acquisition (DIA) experiment with an inclusion list, a resolving power of 35,000, and an AGC target of 5 × 105. The collision energies were varied between 10 and 50 eV, and the normalized collision energy (NCE) was 35 eV.
2.4. Comparison of the Sensitivity of the Phenols Before and After Derivatization with CAX-B
At first, we employed the Orbitrap using the same parameters as in our previous study [] to analyze the generated fragments, which helped confirm the structures, especially those of the phenols after derivatization. In contrast, the MS/MS parameters for the QTRAP, including collision energies and declustering potential (DP), were specifically optimized to maximize the intensity of the MS/MS (MRM) signals. Subsequently, we compared the signal-to-noise ratios of the most prominent MRM transitions for the phenolic compounds before and after derivatization. This study involved analyzing solutions of underivatized phenols and their derivatives at concentrations ranging from 0.02 to 10 ng/mL. For this analysis, the most intense product ions were first selected. The underivatized phenols were then examined in negative ion mode, with the exception of phenol 7, which was analyzed in positive ion mode. The phenols were subsequently derivatized using CAX-B and analyzed in positive ion mode. Finally, the signal-to-noise (S/N) ratios of the multiple reaction monitoring (MRM) transition signals were compared between the underivatized and derivatized analytes.
3. Results and Discussion
The identification strategy, including the general formula of the OP pesticides, their degradation products (the OP acid and the complementary phenol residual) and reaction schemes, is illustrated in Figure 2.
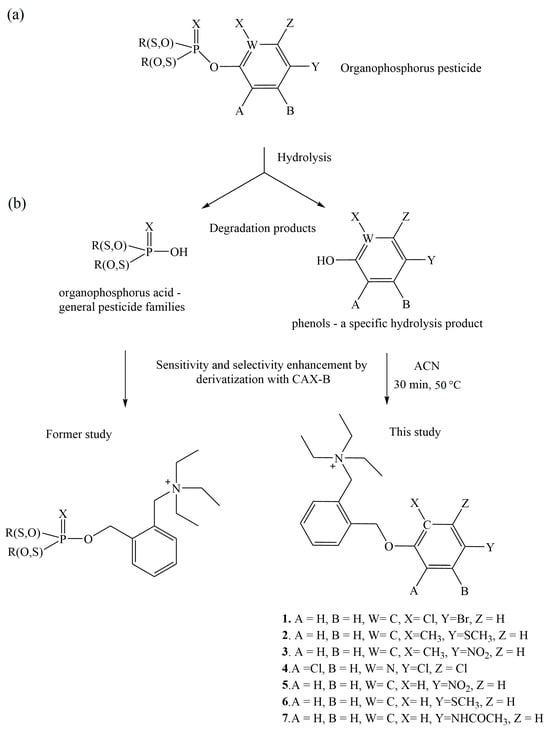
Figure 2.
The identification strategy: The general structure of organophosphorus (OP) pesticides (a); the derived hydrolysis products/metabolites (the OP acid and the phenol) (b); the reaction scheme of the OP acid (former study []).
3.1. Derivatization Strategy and Optimization of the Derivatization Reaction Between Phenols and CAX-B
The CAX-B derivatizing agent has been shown to be highly effective for derivatizing analytes containing organophosphorus acids [], aromatic hydroxyl groups [], cyanide [], and azide [] via LC-ESI-MS/MS. In this study, we aimed to develop a sensitive and selective method for identifying phenolic compounds derived from pesticide dissociation via LC-ESI-MS/MS. We selected the CAX-B derivatizing agent, which features a cationic tag to generate precursor ions and informative product ions that exhibit high responsiveness in ESI(+)-MS/MS. CAX-B has previously been shown to be an effective derivatizing agent for phosphonic acid derivatives, providing insights into the sub-family of organophosphorus pesticides (as demonstrated in a prior study). In the current study, CAX-B was applied to detect phenols. By analyzing both components of organophosphorus pesticides—the organophosphorus acid, which reveals the pesticide sub-family, and the phenol, which identifies the specific pesticide used, we were able to gather comprehensive information on the pesticide composition. Furthermore, compared to alkylating agents like pentafluorobenzyl bromide, CAX-B provides both greater sensitivity and improved solubility, thanks to its cationic nature, which enables it to react even in aqueous environments. Unlike dansyl chloride and other acylating agents, CAX-B does not degrade in water. Acetonitrile, a non-protic polar solvent suitable for extracting pesticides and their degradation products, was selected as the solvent (0.5 mL), and potassium carbonate (1 mg/mL) was chosen as the base, following a previously developed protocol for derivatizing OP acids with CAX-B []. The derivatization reaction parameters were tuned, with a focus on the influence of reaction time (15–60 min) and temperature (50–70 °C) on the conversion yields of the phenolic compounds. As the derivatization of OP acids was accomplished within 0.5 h at 70 °C containing 1 mg/mL potassium carbonate and CAX-B, the same conditions were initially applied for the derivatization of phenols. However, phenols are more reactive, and milder reaction conditions are sufficient for the completion of the reaction. The final reaction conditions were as follows: 50 °C for 0.5 h in acetonitrile, with the same concentration of potassium carbonate and CAX-B (1 mg each). The reaction mixture was diluted 1:2 with water before LC-ESI-MS/MS analysis. Under these conditions, the conversion yields exceeded 95%, as evidenced by the disappearance of intact phenols detected via LC-ESI-MS/MS, and the signal intensities of the derivatization products reached a plateau. Notably, an attempt was made to use water as a reaction solvent as an alternative to ACN. This derivatization (alkylation) of phenols could be accomplished in water, unlike the alkylation of OP acids, owing to the greater nucleophilicity of phenolate ions than water. The reaction did occur in water; however, the signal intensities of the derivatization products were several-fold lower. Therefore, ACN was chosen as the best solvent candidate.
3.2. High-Resolution MS/MS (Orbitrap) and MRM Analysis (QTRAP) of Targeted Phenols
Initially, all seven phenolic compounds (1–7) were analyzed in their native forms using Orbitrap-ESI-MS/MS experiments. Phenolic compounds 1–6 yielded deprotonated molecules [M-H]−, whereas phenol 7 yielded a protonated molecule [M+H]+. The Orbitrap-ESI-MS/MS spectra of phenolic compounds 1–7 at a normalized collision energy (NCE) of 35 eV and collision energies of 10 to 60 eV were interpreted, revealing poor MS/MS information, with a few to only one single dominant product ion (Figure S1). The ESI(-)-MS/MS spectra of phenol 1 (M-H− 204.9062) revealed two product ions at m/z 168.9295 (loss of HCl) and m/z 78.9189 (Br−), phenol 2 (M-H− 153.0385) with only one product ion at m/z 138.0145 (loss of radical methyl), phenol 3 (M-H− 152.0354) with product ions at m/z 122.0374 (loss of NO), m/z 121.0296 (loss of HNO), m/z 106.0425, and m/z 93.0347, phenol 4 (M-H− 195.9127) with a product ion at m/z 159.9362 (loss of HCl, negligible abundance), phenol 5 (M-H− 138.0197) with dominant product ions at m/z 108.0218 (loss of NO) and m/z 92.0268 (loss of NO2), phenol 6 (M-H− 139.0227) with a product ion at m/z 123.9989 (loss of radical methyl) and phenol 7 (M+H+ 152.0704) with product ions at m/z 134.0599 (loss of water), m/z 110.0599 (loss of ethenone), m/z 93.0334, and m/z 65.0387. In the second step, QTRAP in MS/MS (MRM mode) was utilized to determine the response of the phenols via standard LC-ESI-MS/MS prior to derivatization. We developed an optimized MRM method for determining the phenols 1–6 in negative ion mode and for phenol 7 in positive ion mode. On the basis of these findings, the most intense MRM transition was used for the determination of the intact phenols. A summary of all MRM transitions of the intact phenols (1–7) is presented in Table 1.
3.3. High-Resolution MS/MS (Orbitrap) and MRM Analysis (QTRAP) of Targeted Phenols and Structural Insights Gained After Derivatization
We hypothesized that using CAX-B to form a consistently charged molecule and analyzing the phenolic compounds after derivatization using LC-ESI(+)MS2 would enhance sensitivity. This is because cations typically form gaseous ions more efficiently than anions in the electrospray ionization sources of mass spectrometers []. To test this hypothesis, derivatization with CAX-B was used, followed by LC-ESI(+)MS2 analyses. First, all seven phenolic compounds (1–7) were derivatized with CAX-B and analyzed using Orbitrap-ESI-MS2, revealing more information-rich mass spectra than those of the intact phenols. The Orbitrap-ESI-MS2 spectra of phenols 1–3 and phenols 4–7 after derivatization with CAX-B are shown in Figure 3 and Figure S2, respectively. In this study, we analyzed the dissociation processes using a comprehensive empirical set of ESI-MS/MS fragmentation rules developed in our laboratory []. Figure S3 illustrates the proposed structures of all seven CAX-phenol (1–7) major fragment ions that were observed in the ESI-MS2 spectra of the cationized molecules M+. Orbitrap-ESI-MS2 spectra of all CAX-phenols 1–7 revealed ions representing the quaternary amines derived from the CAX-B derivatizing agent; the ion at m/z 100.1119 is attributed to the N-ethyl-N-ethylideneethanaminium and the ion at m/z 86.0963 (N-ethyl-N-methyleneethanaminium). The Orbitrap-ESI-MS/MS spectrum of CAX-phenol 1 (M+ 410.0871) revealed several additional product ions: an ion at m/z 308.9664 (loss of neutral triethylamine from the precursor ion), an ion at m/z 272.9906 (loss of trimethylamine and HCl) from the precursor ion, an ion at m/z 230.0490 (loss of trimethylamine and a Br radical), an ion at m/z 195.0803 (loss of trimethylamine, Br radical and Cl radical), and an ion at m/z 165.0698 (C13H9+). The Orbitrap-ESI-MS/MS spectrum of CAX-phenol 2 (M+ 358.2192) revealed a product ion at m/z 257.0994 (elimination of triethylamine from the precursor ion), an ion at m/z 209.0959 (elimination of trimethylamine and methanethiol) from the precursor ion, an ion at m/z 181.1010 (C14H13+), and an ion at m/z 141.0368 (protonated 4-mercapto, 2-methyl, 1-phenol). The ESI-MS/MS spectrum of CAX-phenol 3 (M+ 357.2166) revealed product ions at m/z 256.0963 (loss of neutral triethylamine from the precursor ion) at low abundance, m/z 210.1037 (loss of neutral triethylamine and loss of NO2 radical) from the precursor ion, and an m/z 195.0805 (loss of trimethylamine, NO2, and methyl radical from the precursor ion). The ESI-MS/MS spectrum of CAX-phenol 4 (M+ 401.0946) revealed product ions at m/z 299.9738 (loss of neutral triethylamine from the precursor ion), m/z 265.0052 (loss of triethyl amine and Cl radical from the precursor ion), m/z 229.0286 (loss of trimethylamine, HCl and Cl radical from the precursor ion), m/z 201.0336 (C15H15O+), and m/z 137.0151 (C8H6Cl+). The ESI-MS/MS spectrum of CAX-phenol 5 (M+ 343.2010) revealed product ions at m/z 242.0807 (loss of triethylamine from the precursor ion), m/z 196.0883 (loss of triethyl amine and NO2 radical from the precursor ion), and m/z 179.0854 (C14H11+). The Orbitrap-ESI-MS/MS spectrum of CAX-phenol 6 (M+ 344.2043) revealed product ions at m/z 243.0835 (loss of neutral triethylamine from the precursor ion), m/z 195.0803 (loss of trimethylamine and methanethiol) from the precursor ion, m/z 167.0854 (C13H11+), and m/z 127.0211 (protonated 4-mercapto 1-phenol). The ESI-MS/MS spectrum of CAX-phenol 7 (M+ 355.2372) revealed product ions at m/z 254.1173 (loss of triethylamine from the precursor ion), m/z 212.1068 (loss of triethyl amine and NO2 radical from the precursor ion), m/z 184.1119 (C13H14N+), and m/z 167.0853 (C13H11+).
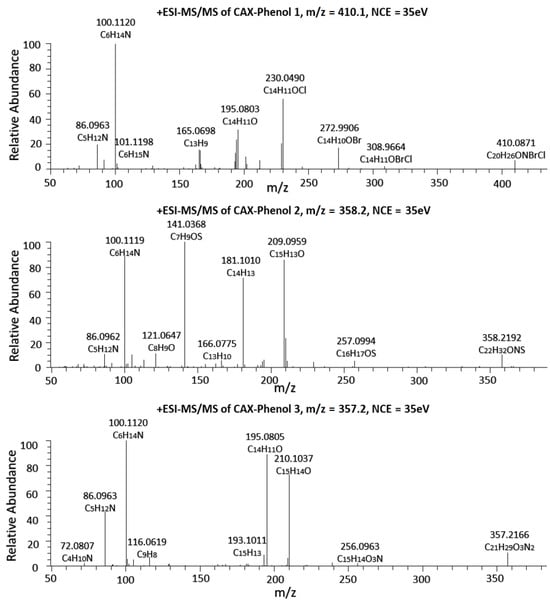
Figure 3.
Orbitrap ESI-MS/MS spectra of 10 ng/mL solutions of 4-bromo-2-chlorophenol (phenol 1), 2-methyl-4-(methylthio)phenol (phenol 2), and 2-methyl-4-nitrophenol (phenol 3) after derivatization with CAX-B at a normalized collision energy (NCE) of 35 eV (<3 ppm error).
EPI (QTRAP-MS/MS) experiments were carried out for all derivatization products. MS/MS of the cationized molecules (M+) was performed by varying the collision energy (10 eV–60 eV). The most intense product ions were used for MRM transitions (detection and identification). In addition to determining the MRM transitions, their signal intensity ratios were defined and retention times were noted. A summary of all the MRM transitions of CAX-phenols (1–7) is presented in Table 2.
3.4. Method Evaluation
Method evaluation was conducted in ACN to assess performance by analyzing calibration curve linearity, limits of quantitation (LOQs), limits of identification (LOIs), and CAX-phenol derivative stability. The reactions for all phenols were performed in ACN at concentrations of 0.02, 0.05, 0.1, 1, 0.5, 1, and 10 ng/mL. All calibration curves for the CAX-phenol derivatives exhibited linearity within the range of 0.05–10 ng/mL, with a correlation coefficient (R2) greater than 0.99, as shown in Figure S4 (blue line). To assess the method’s repeatability, three separate derivatizations were performed at each concentration point in the calibration curve. The relative standard deviations (RSDs) of the most intense MRM signal for each analyte were then measured and were found to be lower than 20%.
All CAX-phenols (1–7) exhibited more than two MRM signals with signal-to-noise ratios above 3 at a spike concentration of 0.05 ng/mL in acetonitrile. This allowed for the determination of the identification limit at the low parts-per-trillion (ppt) level. Additionally, the CAX-phenols (1–7) demonstrated stability, showing no noticeable dissociation after 24 h when left on the LC-MS tray at 20 °C. Typical MRM chromatograms of phenols 1–7 after derivatization are shown in Figure 4.
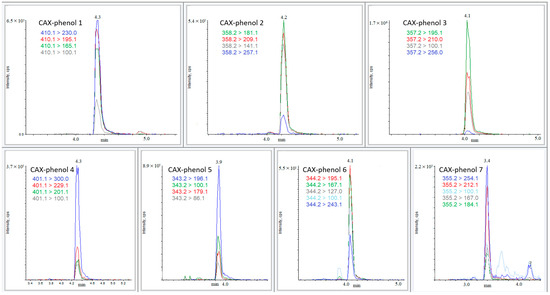
Figure 4.
QTRAP-MS/MS (MRM) chromatograms of 1 ng/mL solutions of phenols (1–7) after derivatization with CAX-B and dilution at a ratio of 1:2 with water prior to analysis.
3.5. Application in Real-World Matrices
After proving that the method could be used to transform phenols into their corresponding CAX-phenols, we turned our attention to evaluating its effectiveness in real-life samples. The ability of CAX-B to react with phenols 1–2, 4, and 6 in acetonitrile extracts of soil and of phenols 1–4 and 6 in acetonitrile extracts of plants, was tested. Notably, two phenols (3 and 5) were observed in the blank soil samples. According to the literature, such phenols are commonly found in soil, probably due to industrial pollution, pesticide degradation, and other sources [].
It is well established that environmental matrices impact both ionization efficiency (enhancement or suppression) and derivatization rates during LC-MS analysis. The influence of the matrices were determined by comparing phenol-spiked blank matrix acetonitrile extracts with pure acetonitrile, followed by derivatization with CAX-B before analysis. Plant and soil extracts in ACN were fortified with several phenols at concentrations ranging from 0.02 to 10 ng/mL, after which the derivatization procedure was applied. The linearity and reproducibility of the analysis under the optimized conditions were evaluated. The calibration curves for the derivatization products were linear in the range of 0.05–10 ng/mL, with correlation coefficients (R2) greater than 0.99 (Figure S4). No peak interferences were observed near retention times, with signal suppression or enhancement of less than 20%.
Figure 5 shows the extracted ion chromatograms (EICs) of the most intense MRM transitions of CAX-phenols 1 and 4 after spiking to a concentration of 50 pg/mL in acetonitrile, acetonitrile extract of plants, and acetonitrile extract of soil, followed by derivatization with CAX-B and LC-ESI-MS/MS analysis.
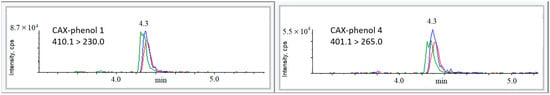
Figure 5.
The role of the matrix in affecting derivatization rate and ionization efficiency of the derivative product of phenols 1 and 4. Comparison of signal intensities of the dominant MRM transition between spiked acetonitrile extracts of plants (green), soil (red), and spiked ACN (blue). Phenols 1 and 4 were added to acetonitrile and acetonitrile extracts of blank matrices, followed by the immediate addition of 1 mg of CAX-B and 1 mg of K2CO3 and stirring for 0.5 h at 50 °C prior to LC-ESI-MS/MS analysis. Response ratios lower or higher than 100% indicate signal suppression or enhancement.
3.6. Sensitivity Enhancement After Derivatization with CAX-B Using a QqQ Instrument
For assessing identification sensitivity between the underivatized phenols and the phenols after derivatization with CAX-B, all phenols at concentrations of 1 ng/mL and above, according to their displayed LOQ level, were reacted with CAX-B to the corresponding phenol derivatives, and the mixtures were diluted 1:2 with water and analyzed by LC-ESI-MS/MS employing MRM mode. The most intense positive MRM transitions of the phenolic compound derivatives (1–6) were compared with the most intense negative ion MRM transitions of the intact compounds (except phenol 7) which yielded a protonated molecule [M+H]+ in its intact form. For the underivatized phenols, the highest intensity MRM transition exhibited a signal-to-noise ratio (S/N) of 10–30 at concentration between 1 ng/mL and 100 ng/mL, as a function of the sensitivity of each phenol. However, for CAX-phenol derivatives, at concentrations of 20–50 pg/mL (LOI level), a signal-to-noise ratio (S/N) of nearly 10 was detected. Therefore, it was demonstrated that CAX-phenols are more sensitive by several orders of magnitude than the underivatized phenols (Figure 6).
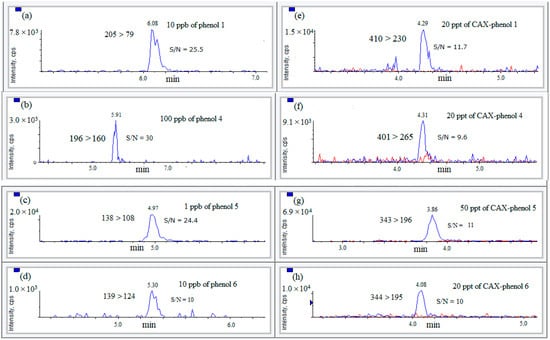
Figure 6.
Differences in the sensitivity of phenols 1, 4, 5, and 6 before and after reaction with CAX-B in absolute acetonitrile. Extracted ion chromatograms (EICs) of the main quantifying MRM transition (left column) of phenol 1 (a); phenol 4 (b); phenol 5 (c); phenol 6 (d) and the derivatization products (right column) of CAX-phenol 1 (e); CAX-phenol 4 (f); CAX-phenol 5 (g) and CAX-phenol 6 (h) along with the blank samples (in red) were prepared by the same procedure. The concentrations of the aforementioned phenols at the LOQ level are between 1 and 100 ppb, whereas the LOQ concentrations of their corresponding derivatives are between 20 and 50 ppt. The X-axis represents the elution time, whereas the y-axis represents the intensity.
4. Conclusions
CAX-B was established as an effective derivatizing agent that enhances the sensitivity and selectivity of the entire set of analyzed phenols using MS/MS (MRM) in positive ion mode, even in complex matrices such as soil and plant extracts. These phenols, which serve as the leaving groups released by organophosphorus (OP) pesticides, can act as unique markers of pesticide exposure. Derivatizing pesticide-related phenols into their corresponding derivatives significantly improved detection sensitivity—by up to several orders of magnitude—compared with underivatized phenols in LC-ESI-MS/MS analysis, enabling their determination in the low ppt range. This derivatization strategy with CAX-B has been optimized for the simultaneous and rapid determination of alkyl phosphates (as shown in previous work) and their phenolic counterparts, facilitating the identification of the organophosphorus pesticide involved, as demonstrated in this study. Generally, CAX-B can be applied in various fields, including environmental monitoring, food analysis, and plant metabolomics, wherever precise and sensitive phenol detection is required. Its superior performance in terms of yield, speed, and sensitivity makes it a preferred choice over many traditional derivatization reagents for phenols like silylation, acylation, and alkylation agents. Notably, although in this study, CAX-B was shown to efficiently derivatize a range of phenols which can serve as markers for organophosphorus pesticides, including those bearing strongly electron-donating and strongly electron-withdrawing substituents, it may also be suitable for other classes of phenolic compounds, such as metabolites of phenoxy acid herbicides, endocrine-disrupting compounds, and others.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12060193/s1, Figure S1: Orbitrap ESI-MS/MS spectra of 100 ng/mL solutions of 4-Bromo-2-chlorophenol (phenol 1), 2-methyl-4-(methylthio)phenol (phenol 2), 2-methyl-4-nitrophenol (phenol 3), 3,5,6 trichloro pyridine-2-ol (phenol 4), 4-nitrophenol (phenol 5), 4-(methylthio)phenol (phenol 6), and N-(4-hydroxyphenyl)acetamide (phenol, 7), (<3 ppm error); Figure S2: Orbitrap ESI-MS/MS spectra of 10 ng/mL solutions of 3,5,6 trichloro pyridine-2-ol (phenol 4), 3,5,6 trichloro pyridine-2-ol (phenol 5), 4-(methylthio)phenol (phenol 6), and N-(4hydroxyphenyl)acetamide (phenol 7) after derivatization with CAX-B at a normalized collision energy (NCE) of 35 V, (<3 ppm error). Figure S3: Plausible structures of CAX-phenols (1–7) fragment ions observed in the ESI-MS2 spectra; Figure S4: Linear calibration curves of CAX-phenol derivatives (1–7) related to pesticides. The derivatization reaction was carried out in pure ACN (blue line), and in ACN extract of soil (orange) and plant (gray) at the concentration range of 0.02–10 ng/mL.
Author Contributions
Conceptualization, A.W.; methodology, A.W.; validation, M.M., A.T., A.W. and A.S.; formal analysis, A.S., A.T., A.W., M.M., T.S.Y. and M.B.; investigation, A.S. and A.T.; data curation, A.S. and A.T.; writing—original draft preparation, A.W., T.S.Y. and M.M.; writing—review and editing, A.W., T.S.Y. and M.M.; visualization, A.S., A.T., T.S.Y., M.M. and A.W.; supervision, A.W., M.M. and T.S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- USEPA. The Environmental Fate and Effect Division (EFED) Reregistration Eligibility Decision (RED) Chapter for Dicrotophos; Office of Pesticide Programs Special Docket for Pesticide Reregistration Risk Assessments, USEPA: Washington, DC, USA, 1999.
- USEPA. Reregistration Eligibility Decision (RED). DCPA; United States Environmental Protection Agency: Washington, DC, USA, 1998.
- USEPA. The Environmental Fate and Effect Division (EFED) Environmental Risk Assessment for Profenofos; Office of Pesticide Programs Special Docket for Pesticide Reregistration Risk Assessments, USEPA: Washington, DC, USA, 1998.
- Reemtsma, T.; Lingott, J.; Roegler, S. Determination of 14 monoalkyl phosphates, dialkyl phosphates and dialkyl thiophosphates by LC-MS/MS in human urinary samples. Sci. Total Environ. 2011, 409, 1990–1993. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, G.; Dar, M.A.; Nimesh, S.; López-Chuken, U.J.; Villarreal-Chiu, J.F. Microbial degradation of organophosphate pesticides: A review. Pedosphere 2018, 28, 190–208. [Google Scholar] [CrossRef]
- Hernández, F.; Sancho, J.V.; Pozo, O.J. An estimation of the exposure to organophosphorus pesticides through the simultaneous determination of their main metabolites in urine by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2004, 808, 229–239. [Google Scholar] [CrossRef]
- Arora, P.K.; Srivastava, A.; Singh, V.P. Bacterial degradation of nitrophenols and their derivatives. J. Hazard. Mater. 2014, 266, 42–59. [Google Scholar] [CrossRef]
- Sharma, N.; Jain, A.; Singh, V.K.; Verma, K.K. Solid-phase extraction combined with headspace single-drop microextraction of chlorophenols as their methyl ethers and analysis by high-performance liquid chromatography-diode array detection. Talanta 2011, 83, 994–999. [Google Scholar] [CrossRef]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Determination of phenols in waters by stir membrane liquid–liquid–liquid microextraction coupled to liquid chromatography with ultraviolet detection. J. Chromatogr. A 2011, 1218, 2176–2181. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, W.W.; Chen, B.; Wu, M.; Li, S.G. Determination of 13 phenolic compounds in rice wine by high-performance liquid chromatography. Food Anal. Methods 2015, 8, 825–832. [Google Scholar] [CrossRef]
- Puig, D.; Barceló, D. Comparative study of on-line solid phase extraction followed by UV and electrochemical detection in liquid chromatography for the determination of priority phenols in river water samples. Anal. Chim. Acta 1995, 311, 63–69. [Google Scholar] [CrossRef]
- Peng, G.; Lu, Y.; He, Q.; Mmereki, D.; Zhou, G.; Chen, J.; Tang, X. Determination of 3,5,6-trichloro-2-pyridinol, phoxim and chlorpyrifos-methyl in water samples using a new pretreatment method coupled with high-performance liquid chromatography. J. Sep. Sci. 2015, 38, 4204–4210. [Google Scholar] [CrossRef]
- Hofmann, D.; Hartmann, F.; Herrmann, H. Analysis of nitrophenols in cloud water with a miniaturized light-phase rotary perforator and HPLC-MS. Anal. Bioanal. Chem. 2008, 391, 161–169. [Google Scholar] [CrossRef]
- Jáuregui, O.; Moyano, E.; Galceran, M.T. Liquid chromatography–atmospheric pressure chemical ionization mass spectrometry for chlorinated phenolic compounds: Application to the analysis of polluted soils. J. Chromatogr. A 1998, 823, 241–248. [Google Scholar] [CrossRef]
- Puig, D.; Barceló, D. Off-line and on-line solid-phase extraction followed by liquid chromatography for the determination of priority phenols in natural waters. Chromatographia 1995, 40, 435–444. [Google Scholar] [CrossRef]
- Cappiello, A.; Famiglini, G.; Palma, P.; Berloni, A.; Bruner, F. New approach for the analysis of acidic pesticides in water by LC/MS with a particle beam interface. Environ. Sci. Technol. 1995, 29, 2295–2300. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Liu, Y.; Wang, Y.; Li, S. Simultaneous determination of nine phenolic compounds in imitation wild Dendrobium officinale samples using ultrahigh-performance liquid chromatography–tandem mass spectrometry. J. Sep. Sci. 2020, 43, 3117–3125. [Google Scholar] [CrossRef]
- Saraji, M.; Bakhshi, M. Determination of phenols in water samples by single-drop microextraction followed by in-syringe derivatization and gas chromatography–mass spectrometric detection. J. Chromatogr. A 2005, 1098, 30–36. [Google Scholar] [CrossRef]
- Padilla-Sánchez, J.A.; Plaza-Bolaños, P.; Romero-González, R.; Barco-Bonilla, N.; Martínez-Vidal, J.L.; Garrido-Frenich, A. Simultaneous analysis of chlorophenols, alkylphenols, nitrophenols and cresols in wastewater effluents, using solid phase extraction and further determination by gas chromatography–tandem mass spectrometry. Talanta 2011, 85, 2397–2404. [Google Scholar] [CrossRef]
- Sobhi, H.R.; Esrafili, A.; Farahani, H.; Gholami, M.; Baneshi, M.M. Simultaneous derivatization and extraction of nitrophenols in soil and rain samples using modified hollow-fiber liquid-phase microextraction followed by gas chromatography–mass spectrometry. Environ. Monit. Assess. 2013, 185, 9055–9065. [Google Scholar] [CrossRef]
- Faludi, T.; Balogh, C.; Serfőző, Z.; Molnár-Perl, I. Analysis of phenolic compounds in the dissolved and suspended phases of Lake Balaton water by gas chromatography-tandem mass spectrometry. Environ. Sci. Pollut. Res. 2015, 22, 11966–11974. [Google Scholar] [CrossRef]
- Padilla-Sánchez, J.A.; Plaza-Bolaños, P.; Romero-González, R.; Garrido-Frenich, A.; Martínez Vidal, J.L. Application of a quick, easy, cheap, effective, rugged and safe-based method for the simultaneous extraction of chlorophenols, alkylphenols, nitrophenols and cresols in agricultural soils, analyzed by using gas chromatography–triple quadrupole-mass spectrometry/mass spectrometry. J. Chromatogr. A 2010, 1217, 5724–5731. [Google Scholar] [CrossRef]
- Sánchez-Brunete, C.; Miguel, E.; Tadeo, J.L. Determination of tetrabromobisphenol-A, tetrachlorobisphenol-A and bisphenol-A in soil by ultrasonic assisted extraction and gas chromatography–mass spectrometry. J. Chromatogr. A 2009, 1216, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- USEPA. SW-846 Test Method 8041A: Phenols by Gas Chromatography; US Environmental Protection Agency: Washington, DC, USA, 1995; pp. 1–28.
- Tchieno, F.M.M.; Tonle, I.K. p-Nitrophenol determination and remediation: An overview. Rev. Anal. Chem. 2018, 37, 20170019. [Google Scholar] [CrossRef]
- Birolli, W.G.; Lanças, F.M.; Dos Santos Neto, Á.J.; Silveira, H.C.S. Determination of pesticide residues in urine by chromatography-mass spectrometry: Methods and applications. Front. Public Health 2024, 12, 1336014. [Google Scholar] [CrossRef] [PubMed]
- Yamin, T.S.; Madmon, M.; Hindi, A.; Shifrovich, A.; Prihed, H.; Blanca, M.; Weissberg, A. Enhanced LC-ESI-MS/MS sensitivity by cationic derivatization of organophosphorus acids. Molecules 2023, 28, 6090. [Google Scholar] [CrossRef]
- Pernica, M.; Poloucká, P.; Seifertová, M.; Šimek, Z. Determination of alkylphenols in water samples using liquid chromatography–tandem mass spectrometry after pre-column derivatization with dansyl chloride. J. Chromatogr. A 2015, 1417, 49–56. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Q.; Yao, Y.; Giese, R.W. Cationic xylene tag for increasing sensitivity in mass spectrometry. J. Am. Soc. Mass Spectrom. 2015, 26, 1713–1721. [Google Scholar] [CrossRef]
- Madmon, M.; Shifrovich, A.; Tamar, S.Y.; Weissberg, A. Simple and fast determination of free cyanide in drinking water by liquid chromatography electrospray ionization tandem mass spectrometry following “in vial” derivatization. Int. J. Mass Spectrom. 2021, 463, 116553. [Google Scholar] [CrossRef]
- Prihed, H.; Shifrovitch, A.; Yamin, T.S.; Madmon, M.; Belay, C.; Blanca, M.; Weissberg, A. Rapid and simple identification of trace amounts of sodium azide in beverages and bodily fluids followed by derivatization and liquid chromatography–electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2023, 37, e9624. [Google Scholar] [CrossRef]
- Weissberg, A.; Dagan, S. Interpretation of ESI(+)-MS-MS Spectra—Towards the Identification of “Unknowns”. Int. J. Mass Spectrom. 2011, 299, 158–168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).