Abstract
Persistent organohalogen pollutants—including halogenated nitrophenols (HNCs), trichloroethylene (TCE), and per- and polyfluoroalkyl substances (PFAS)—pose serious environmental and health risks due to their stability, toxicity, and bioaccumulation potential. This review critically assesses current remediation technologies including advanced oxidation processes (AOPs), adsorption, membrane filtration, and thermal treatments. While these methods can be effective, they are often limited by high costs, energy demands, toxic byproduct formation, and sustainability concerns. Emerging biological approaches offer promising alternatives. Among these, fungal-based degradation methods (mycodegradation) remain significantly underrepresented in the literature, despite fungi demonstrating a high tolerance to contaminants and the ability to degrade structurally complex compounds. Key findings reveal that white-rot fungi such as Phanerochaete chrysosporium and Trametes versicolor possess enzymatic systems capable of breaking down persistent organohalogens under conditions that inhibit bacterial activity. This review also identifies critical research gaps, including the need for direct comparative studies between fungal and bacterial systems. The findings suggest that integrating mycodegradation into broader treatment frameworks could enhance the environmental performance and reduce the long-term remediation costs. Overall, this review highlights the importance of diversifying remediation strategies to include scalable, low-impact biological methods for addressing the global challenge of organohalogen contamination.
Keywords:
advanced oxidation processes (AOPs); adsorption technologies; environmental sustainability; halogenated nitrophenols (HNCs); membrane filtration; mycodegradation/biodegradation; organohalogen pollutants; per- and polyfluoroalkyl substances (PFAS); remediation technologies; trichloroethylene (TCE) 1. Introduction
Organohalogen compounds are persistent environmental pollutants, including halogenated nitrophenols (HNCs), trichloroethylene (TCE), and per- and polyfluoroalkyl substances (PFAS). HNCs are primarily used as intermediates in the production of industrial chemicals, dyes, polymers, pesticides, insecticides, fungicides, and explosives. TCE has been widely used as an industrial solvent, and PFAS are commonly found in firefighting foams, non-stick cookware, stain-resistant materials, and cosmetics [1,2,3]. Specific global production data for HNCs are limited, but the combined market value of chlorinated and fluorinated compounds has been estimated at USD 24.4 billion [4,5]. In 1995, TCE emissions alone exceeded 11,600 metric tonnes. Despite mounting regulations, PFAS production continues to grow rapidly, with the annual PFAS output now surpassing 230,000 tonnes and cumulative emissions of perfluoroalkyl acids estimated at over 46,000 tonnes [6,7,8,9,10]. Their extreme stability and bioaccumulative nature make these compounds exceptionally difficult to remediate. Although natural sources account for some of the ~8000 organohalogens found in the environment, industrial activities have significantly amplified their presence, posing serious ecological and public health risks [11,12]. Regulatory bodies now classify TCE as a probable human carcinogen, and PFAS are widely recognized for their long-term toxicity and global distribution [13,14].
A variety of treatment methods have been developed to address organohalogen contamination, including advanced oxidation processes (AOPs), adsorption systems, membrane filtration, and thermal treatment [15]. While these methods can achieve high removal efficiencies, they also face significant drawbacks: for example, AOPs can produce toxic intermediates, adsorption systems require frequent regeneration, membrane technologies pose brine disposal challenges, and thermal treatments are energy-intensive and may release harmful emissions. The widespread reliance on chemical inputs and energy-intensive processes raises concerns about the long-term sustainability of these technologies [15].
Existing literature reviews have extensively documented the performance and limitations of these conventional technologies [3,15,16]. However, they often neglect environmental cost assessments and rarely consider biological strategies within an integrated framework. Biodegradation has largely focused on bacterial pathways, despite emerging research highlighting the promising role of fungi. White-rot fungi such as Phanerochaete chrysosporium and Trametes versicolor produce extracellular enzymes—lignin peroxidase, manganese peroxidase, cytochrome P450, and laccase—that have shown potential for degrading TCE and PFAS under conditions where bacteria are less effective [17,18,19]. Fungi also demonstrate greater tolerance to contaminant concentrations and can colonize complex substrates via expansive mycelial networks [17,20]. However, comparative studies between fungal and bacterial remediation systems remain scarce, and fungal degradation mechanisms—particularly those involved in dechlorination and defluorination—are poorly understood, representing a significant research gap.
Given the growing regulatory pressure and the urgent need for scalable, low-impact alternatives, this review is both timely and necessary. It presents a balanced evaluation of current remediation strategies for organohalogen pollutants, while positioning fungal-based degradation (mycodegradation) within the broader landscape of chemical and physical treatment technologies. The novelty of this work lies in its integrative approach: synthesizing conventional and emerging biological methods through the lens of environmental sustainability, technical limitations, and future applicability. By identifying current challenges, comparative advantages, and research priorities, this review contributes to the development of more effective and environmentally responsible remediation solutions.
2. Overview of Halogenated Compounds and Their Applications
The selection of HNCs, TCE, and PFAS as the focal compounds in this review is based on their environmental relevance, structural diversity, and representation of distinct organohalogen subclasses. HNCs (halogenated nitrophenols) are commonly used as intermediates in the synthesis of dyes, pesticides, and polymers. While not always the final products released into the environment, they frequently appear as residues or byproducts due to improper disposal, runoff, or degradation of parent compounds. TCE (trichloroethylene) is a volatile chlorinated solvent with a long industrial history and is among the most prevalent groundwater contaminants worldwide. PFAS (per- and polyfluoroalkyl substances) constitute a large, chemically resilient class of fluorinated surfactants now receiving global regulatory attention due to their extreme persistence and widespread detection.
While other classes of organohalogens—such as PCBs, dioxins, and PBDEs—are also highly persistent and toxic, they fall outside the scope of this review, which focuses primarily on three representative chemical classifications. These compounds have been extensively reviewed elsewhere and are not included here; they are briefly mentioned in Section 4 to provide context.
Table 1 represents a summary of the selected organohalogen compounds, highlighting their structures, industrial applications, and carcinogenic classifications by the International Agency for Research on Cancer (IARC).

Table 1.
Chemical structures of organohalogens, applications, and toxicity classification.
2.1. Halogenated Nitrophenols (HNCs)
Chlorinated and fluorinated nitrophenols (Table 1, Figure 1A,B) are widely utilized as intermediates in the manufacture of industrial chemicals, including dyes, polymers, agricultural chemicals, and explosives [1]. Their extensive use has led to environmental contamination, with evidence of their harmful effects on both ecosystems and human health. For instance, chlorinated nitrophenols are key precursors in the production of neonicotinoids, namely, acetamiprid and imidacloprid, which are widely used in the agricultural sector due to their selective toxicity to insects [30]. Neonicotinoids account for 27% of the global insecticide market, valued at USD 5.1 billion, with an expected 5.4% compounding annual growth rate (CAGR) from 2024 to 2032 [4,31]; their persistence in the environment raises concerns about their long-term ecological impacts. Studies have shown that up to 75% of neonicotinoids applied to soil remain available for transport through surface and groundwater, leading to widespread contamination in the U.S. and Europe [32]. The environmental concentration of acetamiprid has been reported at 44.1 µg/L in lake water in Austin, TX, USA, and imidacloprid applications range from 1.7 to 312 g applied per hectare.
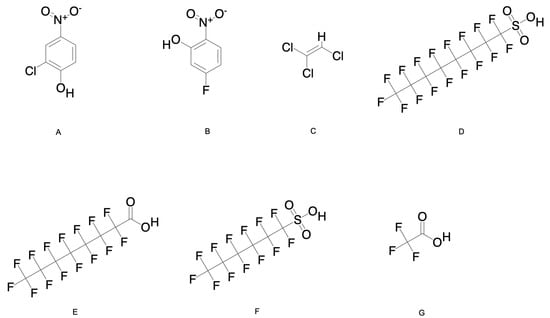
Figure 1.
(A–G) Chemical structures of HNCs, TCE, and PFAS chemicals. Chemical structures created using chem draw, SDF files downloaded from PubChem, access date 5 March 2025.
In contrast, fluorinated nitrophenols have not been classified as toxic by regulatory agencies. However, research by Gershon suggests that 5-halo-2-nitrophenols exhibit significant toxicity to fungi, with their reactivity following the sequence F > I > B > Cl [33]. Given their persistence and potential bioaccumulation, the environmental risks associated with HNCs necessitate the development of effective remediation strategies.
2.2. Trichloroethylene (TCE)
Trichloroethylene (TCE) (Table 1 and Figure 1C) is a widely used industrial solvent, primarily employed in vapor degreasing and cold cleaning of fabricated metal parts. Historically, it has also been utilized as a carrier solvent in insecticides, fungicides, and in the extraction of caffeine from coffee, although these applications have since been discontinued. Industries comprising metalworking, electronics, and transport manufacturing continue to rely on TCE for degreasing and as a chemical intermediate [2]. Despite regulatory efforts to limit emissions, TCE remains a prevalent environmental contaminant due to its extensive use and improper disposal. In 1995, the U.S. Toxic Chemical Release Inventory reported TCE emissions exceeding 11,600 metric tonnes, with the majority released into the atmosphere by metalworking facilities [7,34]. TCE is commonly detected in ambient air, surface water, and groundwater, with air concentrations ranging from 0.1 to 3.9 µg/m3 and drinking water levels reported between trace amounts and 50 µg/L. Exposure to the general population mainly occurs through inhalation and water ingestion, with estimated daily intakes of 11–33 µg from air and 2–20 µg from water [6].
Exposure to TCE occurs through inhalation, the ingestion of contaminated water, and occupational contact. The general U.S. population has measurable levels of TCE in their blood, with concentrations averaging 0.015 µg/L [7,13]. Occupational exposure was particularly significant in the 1980s, with over 400,000 workers in the U.S. potentially exposed to TCE [7,13]. Regulatory agencies, namely, the U.S. Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA), have set strict exposure limits, and the World Health Organization (WHO) has issued drinking water guidelines for TCE [13]. Despite these measures, TCE remains a significant environmental and public health concern due to its persistence and carcinogenic potential.
2.3. Per- and Polyfluoroalkyl Substances (PFAS)
Per- and polyfluoroalkyl substances (PFAS), including perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA), perfluorohexanesulfonic acid (PFHxS), and trifluoroacetic acid (TFA) (Table 1 and Figure 1D–G), are commonly referred to as “forever chemicals” due to their extreme environmental persistence. These compounds have been widely used since the 1940s in non-stick cookware, water-resistant fabrics, industrial coatings, and firefighting foams [3]. The U.S. EPA has identified nearly 15,000 PFAS variants, highlighting the complexity of regulating and remediating these pollutants [35]. Between 1992 and 2002, the annual PFOS and PFOA production was 3500 and 2500 tonnes, respectively, with the majority used in industrial applications [8,9]. Global production data for PFHxS remain limited; however, in 1997, the U.S. produced 227 tonnes of perfluorohexane sulfonyl fluoride (PFHxSF), a key precursor to PFHxS [36]. U.S. production ceased between 2000 and 2002, but manufacturing continues in China and Italy [36]. In contrast, the global TFA production was estimated at 21,000 tonnes in 2012, increasing to 26,000 tonnes in 2017, with projections reaching 34,000 tonnes by 2023 [37].
The persistence and widespread contamination of PFAS have led to growing concerns regarding their health effects. In December 2023, the International Agency for Research on Cancer (IARC) classified PFOA as a Group 1 carcinogen, indicating sufficient evidence of carcinogenicity in humans, and PFOS was placed in Group 2B, denoting possible carcinogenicity [14]. The highest recorded environmental concentrations of PFOS were found in tap water samples from Guangzhou and Shenzhen, China, at 297.5 ng/L; PFOA concentrations reached 62.3 µg/L in the Xiaoqing River in northern China [38,39]. These findings highlight the urgent need for effective PFAS treatment technologies to mitigate their impact on public health and ecosystems.
Trifluoroacetic acid (TFA), an ultrashort-chain PFAS, has emerged as a dominant contaminant in water sources, accounting for over 90% of the total PFAS mass in German drinking water [40]. Due to its high mobility and resistance to degradation, TFA has been detected in air, precipitation, and plant tissues, with concentrations exceeding 9 µg/L in some European groundwater samples [40,41,42]. The long-term effects of TFA exposure remain largely unknown, necessitating further research into its environmental fate and potential health risks.
Given the widespread detection of PFAS in water, soil, air, and biota, their remediation is an urgent priority to safeguard human health and ecological integrity. The bioaccumulative nature of PFAS leads to their persistence in food, with documented impacts on endocrine function, immune response, and developmental processes in humans and wildlife. Ecosystem-level effects include disruptions to aquatic life, with PFAS exposure linked to reproductive toxicity, metabolic disturbances, and bioaccumulation in fish and amphibians [43]. As global regulatory agencies tighten restrictions on PFAS usage, innovative remediation strategies are critical to preventing further contamination [14]. Addressing this issue is imperative to mitigate long-term environmental damage, protect drinking water sources, and reduce the burden of PFAS-related diseases on future generations.
3. Overview of Current Treatment Methods and Research Gaps
A variety of treatment methods have been developed and are currently used to address the persistence of HNCs, TCE, and PFAS in the environment. Table 2 shows treatment methods for xenobiotic organohalogens along with advantages, disadvantages, and cost estimates. To provide a deeper understanding of these limitations and their implications, the following section will explore these technologies in greater detail, focusing on their applications, challenges, manufacturing processes, and associated environmental concerns.

Table 2.
Summary of treatment methods, advantages, disadvantages, cost considerations, and removal or degradation efficiency.
3.1. Treatment Methods
The remediation of persistent organohalogen pollutants requires a multifaceted approach due to the chemical diversity and environmental persistence of compounds like HNCs, TCE, and PFAS. This section provides a comparative overview of current treatment methods, including chemical (e.g., advanced oxidation processes and zero-valent iron systems), thermal, physical (e.g., membrane filtration and adsorption), and biological methods. These methods vary in their removal efficiency, operational costs, scalability, and environmental impact. Table 2 summarizes the key treatment methods alongside their respective advantages, limitations, cost considerations, and removal efficiencies. Building on this overview, the following discussion shifts focus on the unique potential of fungi as a sustainable and underutilized alternative for biodegradation.
3.2. Chemical Methods
Chemical treatment methods are widely employed for the degradation or transformation of persistent organic pollutants in water and soil [15]. These approaches involve the use of oxidants, catalysis, or reductants to break down contaminates into less harmful byproducts. While effective at targeting a broad range of pollutants, chemical treatments can also produce secondary waste, require precise operational control, and vary in cost-effectiveness depending on the pollutant treatment scale [90]. Among these, advanced oxidation processes (AOPs) have gained prominence due to their ability to generate highly reactive species capable of degrading recalcitrant compounds [91].
3.2.1. Advanced Oxidation Processes (Halogenated Nitrophenol, Trichloroethylene, and per- and Polyfluoroalkyl Substances)
AOPs (Table 2) encompass a suite of technologies designed to degrade organic compounds in effluent through the generation of highly reactive species, primarily hydroxy radicals (•OH). These radicals attack organic pollutants via hydrogen abstraction, electrophilic addition, electron transfer, or radical–radical reactions, resulting in mineralization to CO2, H2O, and inorganic byproducts [91,92].
The efficiency of AOPs can be hindered by competing reactions. For example, excess hydrogen peroxide (H2O2) and Fe3+ levels can cause H2O2 to scavenge •OH [93]. Optimizing reagent ratios is therefore critical [90].
The photo-Fenton process enhances •OH generation by combining UV-Vis radiation with the Fenton reaction. Of the UV wavelengths, UV-A (λ = 315–400 nm) is particularly effective in decomposing H2O2 [94,95]. Compared to the standard Fenton process, photo-Fenton systems offer superior degradation under optimized pH, temperature, and reagent conditions [96]. Optimal performance occurs at acidic pH (2.5–3.0), which prevents iron precipitation and supports the formation of photoactive [Fe(OH)]2+ complexes [97]. However, excess ferrous ions or H2O2 can reduce efficiency by promoting coagulation or self-reaction, respectively. Continuous reagent dosing can mitigate these effects [98].
AOPs, especially the photo-Fenton reaction, are attractive due to their comparative system design, ability to mineralize diverse pollutants, and suitability for treating mixed contaminant streams [15]. Nonetheless, they pose challenges including high reagent costs, energy demands, and environmental concerns. These systems may disrupt microbial communities [44], generate toxic intermediates requiring further treatment [45], and suffer from catalyst degradation over time [46].
Cost-effectiveness remains a critical barrier (Table 2). In photo-Fenton systems, reagents contribute over 60% of the total costs, while energy accounts for 11–14% [97]. The industrial-scale treatment of oil industry effluent using a photo-Fenton system was estimated at USD 2.62/m3, with H2O2 comprising the bulk of the chemical expenses [97]. Similarly, treating phenol-laden wastewater using solar photo-Fenton costs ~USD 2.87/m3 [98]. For emerging contaminants like PFAS, AOP costs vary widely: photocatalysis may reach USD 295/m3, Ti4O7 electrode technology costs ~USD 0.36/m2, and boron-doped diamond electrodes can exceed USD 7000/m2 [16]. These costs make AOPs less feasible for low-load or decentralized applications. Future research should focus on optimizing chemical utilization and exploring alternative cost-efficient treatment enhancements. The AOP performance varies based on the pollutant type and system optimization. PFAS degradation ranges from 15% to 100%, with photocatalysis typically achieving ~89% efficiency [15]. Enhancing catalyst durability, reagent efficiency, and energy use is key to improving sustainability [90].
The Fenton process is commonly used for degrading recalcitrant pollutants in water and soil. It relies on the reaction of H2O2 with ferrous iron to generate •OH radicals [99]. In one study, Fenton/UV treatment degraded 90% of 4-chloro-2-nitrophenol at pH 3.0–4.0, outperforming UV/TiO2 (80%) and UV/H2O2 (70%) at neutral pH [45]. The treatment costs ranged from USD 5.80 to USD 250.50 per kilogram (2007 values), with H2O2 being the most economical option [45].
Fenton-based systems are also effective against chlorinated organics like TCE, breaking C–Cl bonds to form less toxic compounds. However, they generate iron sludge and may require additional treatment to handle byproducts [90]. In situ chemical oxidation (ISCO) often applies Fenton chemistry by injecting H2O2 and iron into contaminated media, while above-ground systems use reactors for the degradation of TCE in water and industrial waste [100].
3.2.2. Photocatalysis (TiO2/UV ZnO/UV, UV/O3 and UV/H2O2)
Photocatalysis uses UV light to activate semiconductors like TiO2 or ZnO, generating electron–hole pairs that trigger redox reactions (Table 2). Conduction-band electrons reduce dissolved oxygen to superoxide radicals (O2•−), which degrade pollutants. While effective, photocatalysis is slower than other AOPs and often leads to incomplete mineralization, producing harmful intermediates [101]. Additionally, large-scale TiO2 and ZnO production has environmental impacts due to intensive mining and waste generation. For example, the sulfate process for TiO2 yields iron sulfate waste, while ZnO production relies on high-purity zinc, contributing to resource strain [102,103].
Catalytic hydrogenation reduces unsaturated compounds using metal catalysts like Pd, Pt, or Ni (Table 2). It is widely used to saturate alkenes, alkynes, and carbonyls, but presents sustainability concerns. These include greenhouse gas emissions, energy-intensive catalyst extraction, and heavy metal pollution [104]. Hydrogen gas use further adds risk due to its explosiveness [105].
Zero-valent iron (ZVI) is used for the reductive degradation of chlorinated solvents, VOCs, and heavy metals through electron transfer (Table 2). Bimetallic ZVI systems, enhanced with Pd, Pt, or Ni, increase reactivity by improving electron flow and reducing surface passivation. However, ZVI can generate toxic intermediates (e.g., vinyl chloride during TCE degradation) and alter redox conditions in groundwater, increasing metal mobility and contamination risks [106,107,108,109].
AOPs are efficient; however, their use raises environmental concerns. Intermediate compounds, iron sludge from Fenton processes, and acidification risks necessitate careful post-treatment [45,47,90,100]. The environmental footprint from secondary waste, energy use, and mined materials underscores the need for improved catalyst durability, optimized reagent use, and the development of sustainable materials.
3.2.3. Electrochemical Oxidation (EO) for PFAS Remediation
Electrochemical oxidation (EO) is an emerging method for degrading PFAS in water via direct electrode oxidation or reactive oxygen species (ROS) generation [49] (Table 2). EO enables PFAS mineralization without added chemicals but faces limitations in efficiency, cost, and scalability [110].
EO typically involves two steps: foam fractionation to concentrate PFAS, followed by anodic oxidation to degrade them. The reported degradation rates reach up to 86% for long-chain and 31% for short-chain PFAS, averaging 50% overall [50]. The electrode material is critical; boron-doped diamond (BDD) electrodes are preferred for their stability and performance, while newer materials like boron-doped graphene sponges show promise [111]. However, electrode wear increases costs and waste.
EO effectively treats diverse contaminants and has a simple, additive-free setup [48,49]. Yet, its high energy use raises carbon emissions, and BDD degradation adds to the operational costs [48]. Secondary pollutants may form, especially in chloride- or bromide-rich waters. Environmental concerns include incomplete mineralization, toxic byproducts, fluoride release, and impacts on aquatic ecosystems [50]. High energy demands and specialized electrodes limit scalability and sustainability, particularly in complex matrices with co-contaminants [50].
3.3. Adsorption Methods
Adsorption is widely used in water treatment to remove HNCs, TCE, and PFAS (Table 2). Pollutants are captured on the surface of materials like ion exchange resins, activated carbon, and biochar, each with varying performance and environmental profiles.
3.3.1. Ion Exchange
Ion exchange uses resins with sulfonic acid or amine groups to remove PFAS through electrostatic interactions [112]. These resins show high capacities (15–2415 mg/g) depending on the type used [15] (Table 2). Some can be regenerated, while many PFAS-specific resins are single-use, resulting in high disposal costs. Regeneration requires harsh chemicals, generating secondary waste and environmental risks. Pretreatment is often necessary to remove competing ions, increasing costs and complexity [113,114].
3.3.2. Activated Carbon and Biochar
Activated carbon and biochar remove organic pollutants via hydrophobic interactions and surface chemistry. Granular activated carbon (GAC) is common in drinking water treatment and can remove PFAS at ng/L levels, outperforming UV- and ozone-based methods [16,58,115]. However, GAC is less effective for short-chain PFAS [16].
Biochar, made from pyrolyzed biomass, is a lower-cost option with reduced CO2 emissions [15,116]. Its adsorption performance depends on feedstock and pyrolysis conditions. Modified biochar (e.g., magnetic or metal oxide-enriched) improves the PFAS uptake [117,118]. Red mud–sawdust biochar (RMSDN600) achieved 194.6 mg/g for PFOS, outperforming sawdust biochar (178.6 mg/g) [118]. Regeneration remains an issue; the efficiency drops from 90% to 60% after five cycles, and short-chain PFAS removal remains low (10–50%) [119].
GAC costs USD 0.10–1.00 per 3800 L treated water [108], but single-use anion exchange resins (AERs) are more cost-effective in the long term. AERs last over 160,000 bed volumes and use less media (4.2 g/m3), with a treatment cost of USD 0.28/m3 versus USD 0.45/m3 for GAC [56]. Though GAC is cheaper per kg, its higher usage increases the total cost. Regenerable AERs exist but involve chemical-intensive processes that generate PFAS-laden waste [56].
Environmental concerns also vary. Ion exchange resins are made from styrene and divinylbenzene; styrene is a Group 2 carcinogen and divinylbenzene resists degradation [60]. Their synthesis involves chloromethyl ethers (Group 1 carcinogens) and catalysts like AlCl3 and FeCl3, which harm soil systems [114,120,121,122,123]. These chemicals can also form secondary organic aerosols or raise toxicity concerns when reacting with trimethylamine or dimethylethanolamine [112]. GAC production and thermal regeneration emit greenhouse gases. Single-use GAC systems show the highest environmental impacts, including global warming, acidification, and fossil fuel depletion [59]. In contrast, single-use AERs have a lower impact due to less media consumption and no regeneration [27,56].
While adsorption technologies are effective, the performance varies by contaminant and application. Ion exchange resins offer high selectivity but require careful waste handling. GAC and biochar provide sustainable options for long-chain PFAS, though regeneration and short-chain removal remain key challenges. Improved material efficiency and disposal practices are essential for scaling up these technologies.
3.4. Physical Methods
Physical treatment methods rely on mechanical or phase-based separation to isolate or remove contaminants without chemically altering the tier structure. These approaches are particularly effective for persistent organic pollutants that resist degradation, such as HNCs, TCE, and PFAS. Common techniques include membrane filtration and soil vapor extraction (SVE), which are widely applied in water and soil remediation, respectively [63,64,71,72]. While these methods often achieve high contaminant removal efficiencies, they are associated with operation limitations such as fouling, high energy demands, brine or vapor management, and environmental burdens related to material disposal and system emissions [66,73].
3.4.1. Membrane Technologies: Reverse Osmosis and Nanofiltration
Membrane technologies, particularly reverse osmosis (RO) and nanofiltration (NF), are widely used to removed HNCs and PFAS (Table 2). These systems rely on semi-permeable membranes to reject contaminants via size exclusion, electrostatic repulsion, and hydrophobicity differences, but face operational, economic, and environmental challenges [63,124].
RO membranes, with pore sizes of 0.1–1 nm, under high pressure, force water through, rejecting salts, nitrophenol, and PFAS. Their dense polyamide structure promotes dielectric and electrostatic exclusion, achieving near-complete removal of both long- and short-chain PFAS, with up to 90% recovery [15,63,124,125,126,127]. Carboxylate groups enhance rejection, though fouling, especially at high PFAS concentrations, increases operational costs. Interestingly, fouled membranes may exhibit improved PFAS rejection [15,125,126].
NF membranes (e.g., DK, DL, NF270, NF90, and ENSA-K1) are effective for long-chain PFAS, achieving >95% rejection in most cases [128]. DK membranes reject 96.5% of PFOS at 10 ppm; DL achieves 98% POFS and 95% PFOA rejection; NF279 maintains 95–98% rejection at low concentrations (1 µg/L). However, NF is less effective for short-chain PFAS. For example, NF270 achieves only 92% PFBS rejection in Ca2+ rich water due to weakened electrostatic repulsion, with performance dropping below 70% in adverse conditions [129,130,131]. The feed chemistry impacts performance. ESNA-K1 achieves 97% PFAS rejection with FeCl3 or CaCl2, but only 91.7% with NaCl [132]. NF performs best under neutral pH and low ionic strength. Compared to RO (99–100% rejection), NF is cost-effective against RO but less consistent for small molecules [133].
Innovations aim to enhance rejection, reduce fouling, and improve sustainability. Biodegradable membranes (e.g., PLA and PHAs) reduce environmental impact but struggle with cost and durability [134]. Novel materials include amyloid fibril membranes, which achieve 99% removal of long- and medium-chain PFAS and over 96% for short-chain PFAS; graphene oxide–polyethyleneimine membranes, which provide 96.5% PFAS rejection with antifouling properties; metal–organic framework membranes, which achieve 98.4% rejection; and functionalized MXene hollow fibres, which remove 96% of PFOS through size exclusion and electrostatic interactions [16]. RO and NF remain among the most effective treatment options. PFAS rejection ranges from 69 to 99%, and for HNCs, up to ~99% [16,63]. Energy demands have decreased (2.5 kWh/m3 vs. 20 kWh/m3 in the 1970s), but capital costs and system complexity persist [65,135].
Brine management remains a key environmental issue (Table 2). Disposal methods (e.g., deep-well injection and evaporation ponds) carry risks such as groundwater contamination, high costs, and marine impacts [64]. By 2015, membrane waste reached ~12,000 tonnes/year, mostly incinerated or landfilled due to limited recycling [66].
Membrane production also poses hazards. RO/NF membranes use thin-film composites with polyamide layers synthesized from trimesoyl chloride (TMC), m-phenylenediamine (MPD), and piperazine (PIP). TMC is carcinogenic; MPD is toxic and binds sediments; PIP degradation varies from 15 to 98%, raising persistence concerns [67,68,69,70].
3.4.2. Soil Vapor Extraction (SVE) for TCE Remediation
Soil vapor extraction (SVE) is a widely used in situ method for removing volatile organic compounds (VOCs) like TCE from the unsaturated vadose zone (Table 2). It applies a vacuum to extraction wells to volatilize contaminants, which are then treated using carbon adsorption, thermal oxidation, or biofiltration [136]. SVE is cost-effective, adaptable, and efficient, with field studies reporting initial TCE removal rates of 1.5 kg/day, declining by 73% in a week and 90% within a month. Over two years, ~30 kg of TCE was extracted, mainly from deeper layers [136], reflecting SVE’s typical pattern of rapid early removal followed by slower rates. The performance depends on the soil type, contaminant volatility, and system design. Coarse soils promote airflow and vapor transport, while fine-grained soils reduce efficiency. Thermal enhancement improves vaporization, enabling up to 90% TCE removal by increasing volatility and reducing adsorption [137].
SVE’s strengths include rapid VOC removal, low site disruption, and adaptability [72,73]. However, it is ineffective for semi-volatile or non-volatile compounds [71]. Low-permeability zones can trap contaminants, requiring longer operation or additional treatment. Extracted vapors must be treated to prevent air pollution [73]. Thermal treatment is effective for volatile compounds but requires adequate soil permeability. It can produce hazardous byproducts needing further treatment, and incomplete remediation may leave residual contamination [73].
Costs vary by site. At the Verona Well Field (MI), 20,415 m3 of soil was treated for USD 2.18 million (USD 81/m3). At the Sacramento Army Depot (CA), 189,514 m3 was treated for USD 3.53/m3, with 62.6 kg of VOCs removed for USD 10,709/kg [75].
Environmental concerns include fossil fuel energy use, CO2 emissions from thermal oxidation, and potential vapor migration into buildings or groundwater [72,73]. Careful monitoring and integrated methods are needed to mitigate risks.
3.5. Thermal Treatment for Contaminant Removal
Thermal treatment technologies are widely used for remediating soil and groundwater contaminated with chlorinated organics like TCE and PFAS (Table 2). These methods elevate subsurface temperatures to volatilize or degrade pollutants, often requiring >1000 °C for complete mineralization. While highly effective, thermal treatments also present significant environmental, economic, and operational challenges [138]. In situ thermal treatment enhances contaminant mobility through heat, often using steam injection to extract chlorinated organics such as TCE in vapor or liquid forms [138]. TCE volatilizes between 100 and 600 °C, but complete destruction via incineration requires temperatures above 1000 °C [139].
PFAS, due to their strong C–F bonds, are more resistant to thermal degradation. High-temperature incineration and plasma arc technologies (>1000 °C) can achieve >90% PFAS removal, though the efficiency depends on the compound type and treatment conditions [140]. PFSAs are more thermally stable than PFCAs; heating at 400 °C for 30 min yields only 60–71% PFSA degradation [54,55]. Byproducts such as hydrogen fluoride necessitate advanced emission controls [55]. Removal efficiencies vary: TCE removal ranges from 36% to 78%, and for PFAS, from 60% to 90%, depending on the soil properties and temperature [53,54,55]. Although plasma and incineration technologies offer high performance, ensuring complete mineralization and avoiding hazardous emissions remain critical concerns.
Thermal treatments require no chemical additives, reducing the risks of secondary contamination, and are effective in complex pollution scenarios [15]. They are largely unaffected by co-contaminants [15]. However, drawbacks include high energy consumption, increased operational costs, and environmental impacts from fuel use and emissions [15]. Subsurface heterogeneity complicates the heat distribution, causing uneven volatilization and incomplete treatment. Effective remediation requires extensive site characterization and monitoring [138]. Additionally, unknown contaminant distributions and temperature variations can reduce efficiency [138].
3.6. Biodegradation of Halogenated Nitrophenol, Trichloroethylene, and per- and Polyfluoroalkyl Substances
Biodegradation is a natural and sustainable remediation strategy that uses microorganisms to transform or mineralize environmental pollutants in soil and water (Table 2). Bacteria and fungi leverage metabolic pathways to convert contaminants into less harmful byproducts. This approach has been investigated for halogenated nitro compounds (HNCs), trichloroethylene (TCE), and emerging contaminants like PFAS. However, its practical application at scale is constrained by variable efficiency, environmental dependencies, and the risk of producing toxic intermediates.
Bacteria play a pivotal role due to their versatile metabolic systems. They utilize mechanisms such as biosorption, bioaccumulation, and enzymatic degradation to process contaminants. Some strains can reduce toxic heavy metals like hexavalent chromium to less harmful forms through enzymatic reduction. The formation of bacterial biofilms improves degradation efficiency by creating microenvironments that enhance enzyme activity and provide protection against environmental stressors [141,142].
Bacterial degradation of halogenated hydrocarbons, particularly TCE, is well documented. Anaerobic bacteria such as Dehalococcoides species can achieve complete reductive dechlorination of TCE to ethene, a non-toxic end product. However, incomplete degradation may produce hazardous intermediates like vinyl chloride, which is more toxic than TCE itself. The success of bacterial remediation depends heavily on site-specific factors, including the pH, redox potential, oxygen availability, and presence of electron donors [142].
Fungi, particularly members of the Basidiomycota and Ascomycota phyla, complement bacterial activity through the secretion of powerful extracellular enzymes. Basidiomycetes, such as white-rot fungi, are well-known for degrading persistent organic pollutants using enzymes like laccases, lignin peroxidases, and manganese peroxidases. These enzymes enable the breakdown of recalcitrant compounds including polycyclic aromatic hydrocarbons (PAHs), pesticides, and halogenated organics that resist bacterial degradation [143]. Ascomycetes also contribute via oxidative metabolism and enzyme production. Their extensive mycelial networks increase contact with contaminants and improve pollutant mobility in soil. Fungal laccases exhibit higher redox potentials than bacterial analogues, enabling them to oxidize a wider range of persistent substrates [143].
Mycodegradation—the fungal-based approach—shows promise for PFAS treatment. Though still in the early stages, some fungal species have demonstrated the ability to disrupt the carbon–fluorine bond through oxidative mechanisms [144].
4. Mycodegradation Potential
The remediation of persistent organohalogen pollutants requires a multifaceted approach due to the chemical diversity and environmental persistence of compounds like HNCs, TCE, and PFAS. Section 3 outlined a range of conventional methods—chemical, thermal, physical, and bacterial—with varying degrees of success, cost, and sustainability. Table 2 summarizes these treatment methods and their key characteristics. Building on this context, this section shifts focus to mycodegradation—fungal-based biodegradation—as a promising and underutilized alternative.
Fungi represent a vast and diverse kingdom with an estimated 11.7–13.2 million species across seven major phyla, yet they remain underrepresented in biodegradation research [143]. Amongst them, filamentous fungi offer strong potential due to their extensive mycelial networks, which allow penetration into soils and substrates. This structural advantage facilities pollutant interaction and immobilization through mechanisms such as biosorption, metal chelation by melanin-like polymers, and the formation of insoluble oxalates [143]. To understand the relevance of these fungal traits in practice, it is important to assess how mycodegradation compares to established physical, chemical, and bacterial approaches.
4.1. Comparative Evaluation of Mycodegradation and Conventional Approaches
Building on the advantages discussed in Section 3.1, Section 3.2, Section 3.3, Section 3.4, Section 3.5 and Section 3.6, this section positions mycodegradation within the broader context of established remediation methods to support its potential development and application. Table 3 provides a side-by-side comparison of fungal biodegradation with conventional physical, chemical, and biological methods. These technologies vary widely in terms of mechanism, scalability, cost, environmental impact, and effectiveness for specific pollutant classes such as HNCs, TCE, and PFAS [16,50,54,82]. Biodegradation continues to gain attention as a low-impact alternative to conventional remediation technologies. As summarized in Table 3, biological strategies—particularly mycodegradation—offer promising advantages, including low energy requirements, low toxicity, and the ability to degrade a broad range of organic pollutants with minimal site disturbance [77]. However, the field still lacks robust, scalable systems that move beyond lab-scale demonstrations.

Table 3.
Comparison of mycodegradation and conventional remediation approaches.
Fungal-based biodegradation systems benefit from unique extracellular enzymatic pathways—including laccase, manganese peroxidase, lignin peroxidase, and chloroperoxidase—that enable them to degrade complex, halogenated pollutants that may persist under bacterial treatment [82,83,84]. Additionally, fungi exhibit resilience in extreme environmental conditions such as salinity, pH variation, and pollutant toxicity, where bacterial systems often underperform [83,84].
Despite these advantages, mycodegradation remains underutilized relative to both bacterial systems and conventional chemical or physical approaches. Most published studies are confined to highly controlled laboratory environments, often using sterilized media or model compounds. These conditions fail to replicate the complexity of real-world matrices, where nutrient competition, co-contaminants, and environmental fluctuations pose significant barriers. There is a pressing need for field trials that assess fungal colonization, enzyme persistence, and pollutant breakdown under dynamic environmental conditions [85,86,87].
Conventional technologies such as AOPs, thermal desorption, and membrane filtration offer high removal rates and are more frequently deployed at scale. However, they carry significant trade-offs. AOPs can generate intermediate byproducts of uncertain toxicity and require high reagent input [15,90,93]. Membrane systems like reverse osmosis demand high energy and result in concentrated brines that require further disposal, creating an additional waste stream [16,55,63]. Thermal treatment is often cost-prohibitive and energy-intensive, producing airborne emissions that must be tightly controlled [50,53].
While these technologies provide speed and control, their environmental and economic costs remain substantial. In contrast, fungal systems are self-replicating, non-invasive, and potentially cost-effective over long treatment durations. However, a key limitation persists: laboratory studies have demonstrated fungi’s potential, but these results have not yet translated into reliable, field-scale applications. The absence of standardized protocols for fungal inoculation, reactor design, and performance monitoring continue to hinder broader adoption and commercial integration.
A defining strength of fungi lies in their extracellular oxidative enzymes, particularly lignin peroxidases, manganese peroxidase, and laccase, which are capable of degrading complex and persistent organic compounds [146]. A leading example is Phanerochaete chrysosporium, a white-rot fungus extensively studied for its ligninolytic activity. It not only mineralizes lignin into simpler compounds that benefit surrounding microbial communities but also degrades a wide range of hazardous substances including dioxins, polychlorinated biphenyls, chlorobenzenes, DDT, chlorophenols, phenoxy herbicides, and trichloroethylene (TCE) [18,146].
Despite these capabilities, mycodegradation remains significantly underexplored compared to bacterial approaches. This trend is evident in the distribution of biodegradation literature, where bacterial studies comprise 74% of the total (Figure 2). The dominance of bacterial research is even more produced in the remediation of specific organohalogen pollutants: bacterial-focused studies account for 89% of the literature on halogenated nitrophenol (HNCs), 97% on TCE, and 83% on PFAS (Figure 2). However, fungi have demonstrated the ability to tolerate and degrade higher concentrations of these persistent pollutants, often outperforming bacteria under similar conditions [17].
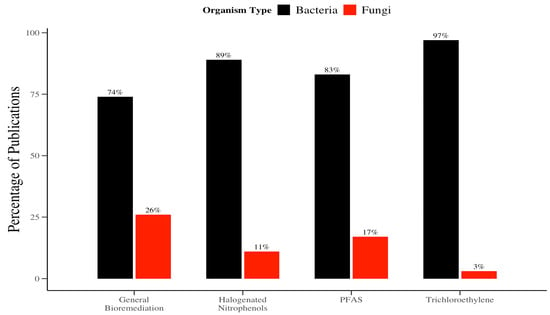
Figure 2.
Proportion of research on bacterial versus fungal remediation.
Given fungi’s enzymatic versatility, structural reach, and resilience in toxic environments, they represent a largely untapped resource for biodegradation, particularly for challenging compounds such as HNCs, TCE, and PFAS. There is a clear need for increased research that systematically evaluates fungal species and pathways capable of degrading these pollutants, especially as environmental contamination becomes more complex and persistent.
Figure 2 was developed by conducting a structured keyword search on the Web of Science and PubChem databases to compare the volume of published literature on bacterial versus fungal biodegradation of organohalogen compounds. Searches were performed using combinations of terms including “bioremediation/biodegradation and bacteria,” “bioremediation/biodegradation and fungi,” “TCE and bacteria and bioremediation/biodegradation,” “TCE and fungi and mycodegradation,” and similar phrases for halogenated nitrophenols and PFAS. Searches were limited to peer-reviewed articles and review papers published in English. The search included publications from 1913 to June 2024 to capture both historical and contemporary research trends. The total number of publications retrieved for each search term was manually recorded and used to generate the comparative bar chart. This approach was intended to illustrate the relative research emphasis on bacterial versus fungal remediation pathways across compound classes.
Given the promising attributes of fungi for pollutant degradation, it is important to assess their performance against specific classes of organohalogen compounds. The following subsections provide examples of available studies on the fungal degradation of halogenated nitrophenols, trichloroethylene, and PFAS; three persistent pollutant groups with high environmental relevance. These examples highlight both the emerging potential and current limitations of mycodegradation research.
4.2. Mycodegradation of Organohalogen Compounds
Fungi have emerged as promising candidates for the degradation of persistent organohalogen compounds due to their diverse enzymatic systems and adaptability to harsh environments. The following subjections explore specific classes of organohalogens that have been targeted in mycodegradation research. These include halogenated nitrophenols, chlorinated solvents such as trichloroethylene (TCE), and poly- and perfluoroalkyl substances (PFAS). Each case highlights the capacity of select fungal species to transform or mineralize these compounds under laboratory or environmental conditions and identifies current limitations and knowledge gaps [82,84,86,147,148].
4.2.1. Mycodegradation of Halogenated Nitrophenol
Literature on the fungal degradation of halogenated nitrophenols remains limited. In a study by Čapek, the concentration of the parent compound 2C4NP (Table 1) was significantly reduced, with over 90% degradation observed within 72 h when exposed to resistant strains of Trichophyton mentagrophytes, Microsporum gypseum, and Aspergillus niger. Thin layer chromatography confirmed the transformation of 2C4NP into four distinct, though chemically uncharacterized, metabolites (Figure 2). The study reported that the metabolites were less toxic than the original compound, suggesting that the transformation can be regarded as defence-detoxification. Only resistant strains were able to degrade 2C4NP, suggesting that enzymatic reactivity was induced as part of an adaptive response [82]. This study highlights the potential for mycodegradation of halogenated nitrophenols. To the best of the author’s knowledge, there are no published papers on the fungal degradation of fluorinated nitrophenols.
4.2.2. Mycodegradation of Trichloroethylene
A range of white-rot fungi have been investigated for their ability to degrade trichloroethylene (TCE) (Table 1 and Figure 1C) under aerobic conditions. In a 12-day screening study, Trametes versicolor, Irpex lacteus, Ganoderma lucidum, and Phanerochaete chrysosporium were assessed for TCE degradation. T. versicolor demonstrated the highest efficiency, achieving approximately 45% removal in both defined and malt extract media under static conditions (Figure 3). To enhance its degradation performance, further optimization using mycelial pellets, shaking incubation (135 rpm), varying glucose concentrations (2, 8, and 19 g/L), and reoxygenation with pure O2 on day four resulted in TCE removal rates ranging from 42% to 80%. The highest theoretical chloride release (78.7%) was observed under conditions of 8 g/L glucose and reoxygenation, indicating substantial mineralization. Mechanistic evidence suggested cytochrome P450 enzymes as the primary drivers of TCE degradation in T. versicolor, rather than the classical ligninolytic enzymes consisting of manganese peroxidase, lignin peroxidase, or laccases. The proposed mechanism involves oxidative dechlorination, leading to the release of chloride ions and carbon dioxide. P. chrysosporium exhibited moderate activity; its reliance on LiP/MnP appeared insufficient for effective TCE breakdown. I. lacteus and G. lucidum showed minimal to moderate degradation, highlighting the need for further investigation and optimization of culture conditions [84,147].
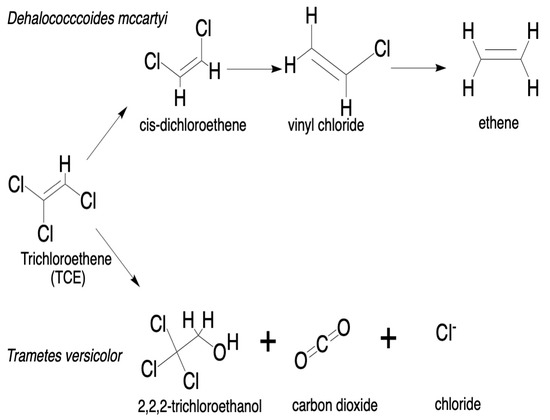
Figure 3.
Comparative degradation pathways of trichloroethylene (TCE) by Dehalococcoides mccartyi and Trametes versicolor. In the upper pathway, anaerobic bacterial degradation of TCE occurs via stepwise reductive dechlorination to ethene [145]. The lower pathway represents the proposed fungal degradation route, in which T. versicolor oxidatively transforms TCE into 2,2,2-trichloroethanol, carbon dioxide, and chloride ions. Redrawn from [145,147], using Chem Draw (version 23.1.2.7); file downloads from PubChem accessed 23 May 2025.
The fungal mechanism, though less defined, is believed to be mediated by cytochrome P450 enzymes [149]. This suggests partial mineralization of the compound, including ethene. The bacterial pathway is included for comparison only; this section focuses on the emerging mycodegradation potential of white-rot fungi. More recently, research has expanded to include other fungal species, namely, Pleurotus ostreatus and Pleurotus eryngii. In liquid cultures, both fungi tolerated TCE concentrations of 14 mg/L and 140 mg/L over 14 days without any observable morphological changes. In soil microcosm experiments with 140 mg/kg of TCE contamination, P. eryngii achieved 8% greater removal than P. ostreatus and 32% more than natural attenuation. After four weeks, both species achieved the complete degradation of TCE, outperforming natural attenuation by 44%. These findings support the growing interest in exploring diverse fungal species for the development of effective mycodegradation strategies targeting chlorinated solvents, namely, TCE [18].
Although Trametes versicolor is primarily studied in liquid cultures for TCE degradation, field studies using this fungus for related pollutants demonstrate its practical potential. In a bio-pile trial targeting pentachlorophenol (PCP), T. versicolor was applied in engineered soil cells that required no irrigation, tolerated natural temperature fluctuations, and showed consistent contaminant reduction over 2.5 years. The low-maintenance system used a sawdust-based inoculum and passive aeration, highlighting the feasibility of outdoor applications [150]. While field-scale use for TCE is yet to be demonstrated, these findings support the fungus’s promise for real-world remediation strategies.
4.2.3. Mycodegradation of PFAS
The biological degradation of PFAS remains poorly documented, with only a limited number of studies exploring the mycodegradation of PFAS, particularly PFOS (Table 1 and Figure 1D), and fluorotelomer alcohols (FTOHs) (Table 1). Phanerochaete chrysosporium and Aspergillus niger have been investigated for their potential to transform these compounds. Initial experiments assessed fungal transformation activities against 6:2 and 8:2 FTOHs at concentrations of 3 mg/L and 8 mg/L [19,86], respectively, providing early insight into the mycodegradation potential of PFAS-contaminated environments. P. chrysosporium successfully transformed 50% of 6:2 FTOH and 70% of 8:2 FTOH over 28 days, producing metabolites which include 5:3 polyfluorinated acid (40%), 5:2 FTOH (10%), and PFHxA (4%) (Figure 4). However, A. niger exhibited no measurable transformation of 6:2 FTOH after 35 days. Environmental fungal isolates (Envi 5 and Envi 7) displayed limited PFOS and PFOA degradation, with up to 20% transformation within 14–28 days, though no intermediate metabolites were detected. The study suggests that fungi can contribute to PFAS degradation, but complete mineralization remains a challenge [19,86]. These findings highlight a significant gap in the literature, particularly regarding the mechanisms, consistency, and scalability of fungal PFAS degradation under environmentally relevant conditions.
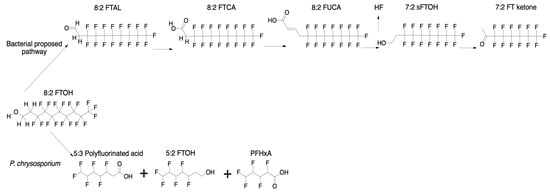
Figure 4.
Proposed aerobic degradation pathways of 8:2 FTOH by bacterial and fungal systems. The upper pathway reflects the most widely studied bacterial transformation route, progressing through intermediates such as 8:2 FTAL, 8:2 FTCA, and 7:2 FT ketone. While specific bacteria are not consistently identified at each step, studies frequently implicate Pseudomonas and Acidimicrobium spp. [148]. The lower pathway shows limited fungal transformation by Phanerochaete chrysosporium, yielding 5:3 polyfluorinated acid, 5:2 FTOH, and PFHxA. The figure highlights the current research bias toward bacterial systems and the need for greater exploration of fungal degradation mechanisms. Redrawn from [19,148] using ChemDraw (version 23.1.2.7_and PubChem files accessed on 23 May 2025.
Table 4 highlights fungi from seven classes studied for mycodegradation and unique enzyme production, showcasing their versatility in degrading complex organohalogen compounds.

Table 4.
Fungi identified as being capable of the degradation of organohalogens and halogenated substrates.
4.3. Mycodegradation of Halogenated Organic Pollutants and Halogenated Substrates
In addition to well-studied halogenated pollutants such as HNCs, TCE, and PFAS, fungi have demonstrated the capacity to degrade a broader array of halogenated organics, including reactive dyes, pesticides, and pharmaceuticals. These compounds often co-occur in industrial wastewater, agricultural runoff, and contaminated soils, yet have received less attention in fungal biodegradation studies. Some studies have also explored the fungal utilization of halogen-containing compounds like potassium fluoride, though these are not considered environmental pollutants. This section consolidates the evidence for fungal degradation across these pollutant groups, emphasizing removal efficiency, mechanistic gaps, and considerations for real-world applications.
4.3.1. Mycodegradation of Reactive Halogenated Dyes and Colorants
Synthetic dyes are structurally complex and resistant to conventional treatment. Fungi such as Peniophora sp., Scedosporium apiospermum, Penicillium roquefortii, Aspergillus fumigatus, and Polyporus brumalis have demonstrated a capacity to degrade halogenated dyes like Rhodamine B, Poly R-478, and reactive black [155,156,159,163,168]. Despite promising results under saline and pH-variable conditions, most studies report decolorization as a proxy for degradation without confirming mineralization or assessing the toxicity of transformation products. Experimental designs often overlook operational constraints found in wastewater systems, such as surfactant interference and co-contaminant complexity. Field validation and long-term studies on enzyme stability and pollutant rebound are lacking. Further research is needed to establish reliable metrics such as TOC removal or ecotoxicity reduction.
4.3.2. Mycodegradation of Halogenated Pesticides and Herbicides
Fungi including Mucor racemosus, Mucor genevensis, Eurotium sp., Hexagonia sp., and Penicillium species have demonstrated degradation of organochlorine and organophosphorus pesticides like aldrin, dieldrin, chlorpyrifos, and 2,4-D [152,154,160,161,162,163,164]. Candida species have shown dual capabilities in degrading hydrocarbons and halogenated pesticides [151,152]. While promising, most of this work is based on single-compound degradation in nutrient-rich laboratory media, with little information on real-world performance in complex matrices. Mechanisms remain poorly resolved for many compounds, and few studies evaluate fungal efficacy under competitive microbial conditions. Application-focused research should prioritize metabolite tracking and the performance under variable moisture.
4.3.3. Mycodegradation of Halogenated Pharmaceuticals
Pharmaceuticals including chloramphenicol, diclofenac, and fluoroquinolones [156,160,165,166,167] have been targeted by fungi such as Trametes versicolor, Trichoderma sp., Trichosporon asahii, Mucor hiemalis, Aspergillus niger, and Penicillium roquefortii. These compounds are often resistant to conventional treatment and persist in aquatic environments. While fungal transformation has been demonstrated, most studies are limited to short-term liquid cultures, frequently omitting degradation kinetics, metabolite profiling, and ecotoxicological impacts. Comparative assessments against bacterial systems are rare, and field-based trials are virtually absent. Future work should focus on identifying transformation pathways, evaluating the longevity and adaptability of fungal systems, and simulating performance in mixed-pollutant wastewater scenarios.
4.3.4. Fungal Chloroperoxidase Producers and Potassium Fluoride Utilization
In addition to pollutant-specific degradation, some fungi produce broad-spectrum oxidative enzymes that may facilitate the breakdown of a wide array of halogenated compounds. Notably, Caldariomyces fumago and Curvularia inaequalis are known producers of chloroperoxidase (CPO), an enzyme capable of catalyzing halogenation and oxidative cleavage reactions, including those involving stable carbon–halogen bonds such as the C–F bond.
C. fumago has been shown to transform monofluorophenol [144], suggesting its CPO may participate in reactions relevant to fluorinated pollutant degradation. C. inaequalis, by contrast, produces a vanadate-dependent chloroperoxidase [169], further expanding the mechanistic diversity available for fungal remediation strategies.
An additional finding is the observed 43% increase in the biomass of Cordyceps militaris in the presence of potassium fluoride (KF) [153]. This suggests a tolerance of stimulatory interaction; it is unclear whether fluoride is actively utilized or merely tolerated. This observation raises questions about the role of fluorine in fungal metabolism and whether selective or transformation processes are involved. Most studies involving CPO have been conducted in vitro using purified enzymes, with a notable lack of research on intact fungal systems or CPO expression in environmental matrices. It remains unclear whether the enzymatic activity observed under laboratory conditions translates to effective pollutant breakdown in situ. To advance application, future work should prioritize confirming CPO-mediated degradation under realistic conditions, investigating gene regulation in fungi exposed to halogenated compounds, and exploring the interplay between fluoride exposure and fungal metabolic pathways. Without such studies, the role of CPO and fluoride-adapted fungi in field-scale biodegradation remains largely theoretical.
4.4. Limitations of Mycodegradation Approaches
Fungal-based remediation currently faces several challenges that limit its practical deployment. Degradation rates can be variable and are influenced by environmental factors such as pH, moisture, and co-contaminants [86,87]. Most research remains confined to controlled laboratory settings, with few studies demonstrating effectiveness in complex field environments. Mechanistic understanding of fungal pathways, particularly for defluorination and dechlorination, are still limited [144]. Additionally, some effective fungal strains may raise biosafety concerns due to their opportunistic pathogenicity [158].
5. Conclusions
Advancing mycodegradation will require a deliberate shift in research focus from optimizing fungal strains in isolation to validating their performance in complex, real-world environments. Laboratory studies have provided evidence of fungi’s potential, but these successes must now be translated into pilot-scale systems that account for site-specific challenges such as co-contaminant interference, environmental variability, and competitive microbial dynamics. Comparative trials with conventional technologies under matched conditions are essential to determine where fungi can outperform, complement, or offer unique advantages over chemical, thermal, or bacterial methods.
As global attention intensifies around the persistent threat of organohalogen pollutants, the limitations of conventional remediation approaches are becoming increasingly evident. While technologies such as advanced oxidation, thermal treatment, adsorption, and membrane filtration are effective, they are also energy-intensive, chemically demanding, and often generate secondary waste streams. In contrast, mycodegradation offers a lower-impact, biologically driven alternative that harnesses the metabolic and enzymatic capacity of fungi to degrade structurally complex and otherwise recalcitrant contaminants. Examples include Phanerochaete chrysosporium and Trametes versicolor, both of which have demonstrated the ability to degrade pollutants such as trichloroethylene and PFAS under conditions where bacterial remediation is often less effective. Caldariomyces fumago, which produces chloroperoxidase, has shown potential for cleaving the exceptionally strong carbon–fluoride bond found in monofluorophenols—illustrating the specificity and catalytic power fungi can offer in tackling fluorinated compounds. However, species selection must be approached with care to avoid introducing pathogenic fungi, which may pose a risk to human and ecological health.
Rather than positioning mycodegradation as a niche or supplemental tool, this review argues for its integration as a core component of sustainable remediation strategies. By leveraging the unique biochemical capabilities and ecological adaptability of fungi, environmental management can move toward more circular, regenerative approaches. Future work should prioritize elucidating fungal degradation pathways, optimizing growth and inoculation conditions for field applications, and establishing scalable treatment models across a range of contaminated media. Incorporating fungi into the remediation toolkit is not only a technical refinement—it represents a paradigm shift in how we approach long-term, ecologically grounded pollution control.
Author Contributions
Conceptualization, G.A.J. and A.S.B.; methodology, G.A.J. and A.S.B.; software, G.A.J.; validation, G.A.J. and A.S.B.; formal analysis, G.A.J.; investigation, G.A.J.; resources, G.A.J. and A.S.B.; data curation, G.A.J.; writing—original draft preparation, G.A.J.; writing—review and editing, G.A.J., A.T. and A.S.B.; visualization, G.A.J., J.A.B. and S.K.B.; supervision, A.S.B., L.K. and A.T.; project administration, G.A.J.; funding acquisition, G.A.J. and A.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the RMIT Research Scholarship (RRSS-SC) from RMIT University.
Data Availability Statement
The data presented in this review are available on request from the corresponding author.
Acknowledgments
The authors acknowledge RMIT University and the ARC Training Centre for the Transformation of Australia’s Biosolids Resource for making this research possible.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hardman, D.J. Biotransformation of halogenated compounds. Crit. Rev. Biotechnol. 1991, 11, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.B. Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; JSTOR: Lyon, France, 1996; Volume 63, Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Dry-Cleaning-Some-Chlorinated-Solvents-And-Other-Industrial-Chemicals-1995 (accessed on 23 May 2025).
- Wanninayake, D.M. Comparison of currently available PFAS remediation technologies in water: A review. J. Environ. Manag. 2021, 283, 111977. [Google Scholar] [CrossRef] [PubMed]
- Neonicotinoid Pesticide Market—By Type (Imidacloprid, Thiamethoxam, Clothianidin, Dinotefuran, Acetamiprid) By Crop Type (Cereals, Oilseed, Fruits, Vegetables, Pulses), By Application Method & Forecast, 2024–2032. Available online: https://www.gminsights.com/industry-analysis/neonicotinoid-pesticide-market#:~:text=Neonicotinoid%20Pesticide%20Market%20was%20valued,owing%20to%20its%20versatile%20applications (accessed on 31 August 2024).
- Global Fluorinated Compounds Market Growth 2024–2030. Available online: https://www.researchreportsworld.com/global-fluorinated-compounds-market-26325246 (accessed on 31 August 2024).
- E.P.A, U.S. Sources, Emission and Exposure to Trichloroethylene (TCE) and Related Chemicals. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=21006 (accessed on 6 March 2025).
- Wu, C.; Schaum, J. Exposure assessment of trichloroethylene. Environ. Health Perspect. 2000, 108 (Suppl. 2), 359–363. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.; Roccaro, P. Removal of poly-and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef]
- Dixit, F.; Barbeau, B.; Mostafavi, S.G.; Mohseni, M. Removal of legacy PFAS and other fluorotelomers: Optimized regeneration strategies in DOM-rich waters. Water Res. 2020, 183, 116098. [Google Scholar] [CrossRef]
- Evich, M.G.; Davis, M.J.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J. Per-and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef]
- Gribble, G.W. Naturally Occurring Organohalogen Compounds—A Comprehensive Review. In Naturally Occurring Organohalogen Compounds; Kinghorn, A.D., Falk, H., Gibbons, S., Asakawa, Y., Liu, J.-K., Dirsch, V.M., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 1–546. [Google Scholar] [CrossRef]
- Gribble, G.W. The diversity of naturally produced organohalogens. Chemosphere 2003, 52, 289–297. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. N.T.P. Report on Carcinogens Background Document for Trichloroethylene. Available online: https://ntp.niehs.nih.gov/sites/default/files/ntp/newhomeroc/roc10/tce.pdf (accessed on 21 December 2024).
- Zahm, S.; Bonde, J.P.; Chiu, W.A.; Hoppin, J.; Kanno, J.; Abdallah, M.; Blystone, C.R.; Calkins, M.M.; Dong, G.-H.; Dorman, D.C.; et al. Carcinogenicity of perfluorooctanoic acid and perfluorooctanesulfonic acid. Lancet Oncol. 2024, 25, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Ibrar, I.; Al-Juboori, R.A.; Singh, L.; Ganbat, N.; Kazwini, T.; Karbassiyazdi, E.; Samal, A.K.; Subbiah, S.; Altaee, A. Updated review on emerging technologies for PFAS contaminated water treatment. Chem. Eng. Res. Des. 2022, 182, 667–700. [Google Scholar] [CrossRef]
- Das, S.; Ronen, A. A Review on Removal and Destruction of Per- and Polyfluoroalkyl Substances (PFAS) by Novel Membranes. Membranes 2022, 12, 662. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of fungi in bioremediation of emerging pollutants. Front. Mar. Sci. 2023, 10, 1070905. [Google Scholar] [CrossRef]
- Mayans, B.; Camacho-Arévalo, R.; García-Delgado, C.; Alcántara, C.; Nägele, N.; Antón-Herrero, R.; Escolástico, C.; Eymar, E. Mycoremediation of Soils Polluted with Trichloroethylene: First Evidence of Pleurotus Genus Effectiveness. Appl. Sci. 2021, 11, 1354. [Google Scholar] [CrossRef]
- Tseng, N.; Wang, N.; Szostek, B.; Mahendra, S. Biotransformation of 6:2 Fluorotelomer Alcohol (6:2 FTOH) by a Wood-Rotting Fungus. Environ. Sci. Technol. 2014, 48, 4012–4020. [Google Scholar] [CrossRef]
- Linde, D.; Ayuso-Fernández, I.; Laloux, M.; Aguiar-Cervera, J.E.; de Lacey, A.L.; Ruiz-Dueñas, F.J.; Martínez, A.T. Comparing Ligninolytic Capabilities of Bacterial and Fungal Dye-Decolorizing Peroxidases and Class-II Peroxidase-Catalases. Int. J. Mol. Sci. 2021, 22, 2629. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Zhang, J.-J.; Zhou, N.-Y. The gene cluster for para-nitrophenol catabolism is responsible for 2-chloro-4-nitrophenol degradation in Burkholderia sp. strain SJ98. Appl. Environ. Microbiol. 2014, 80, 6212–6222. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.K.; Srivastava, A.; Garg, S.K.; Singh, V.P. Recent advances in degradation of chloronitrophenols. Bioresour. Technol. 2018, 250, 902–909. [Google Scholar] [CrossRef]
- Arora, P.K.; Sasikala, C.; Ramana, C.V. Degradation of chlorinated nitroaromatic compounds. Appl. Microbiol. Biotechnol. 2012, 93, 2265–2277. [Google Scholar] [CrossRef]
- Arora, P.K.; Srivastava, A.; Singh, V.P. Bacterial degradation of nitrophenols and their derivatives. J. Hazard. Mater. 2014, 266, 42–59. [Google Scholar] [CrossRef]
- Huang, M.-z.; Huang, K.-l.; Ren, Y.-g.; Lei, M.-x.; Huang, L.; Hou, Z.-k.; Liu, A.-p.; Ou, X.-m. Synthesis and Herbicidal Activity of 2-(7-Fluoro-3-oxo-3, 4-dihydro-2 H-benzo [b][1, 4] oxazin-6-yl) isoindoline-1, 3-diones. J. Agric. Food Chem. 2005, 53, 7908–7914. [Google Scholar] [CrossRef]
- Ilaš, J.; Anderluh, P.Š.; Dolenc, M.S.; Kikelj, D. Recent advances in the synthesis of 2H-1, 4-benzoxazin-3-(4H)-ones and 3, 4-dihydro-2H-1, 4-benzoxazines. Tetrahedron 2005, 61, 7325–7348. [Google Scholar] [CrossRef]
- Ganton, M.D.; Kerr, M.A. Aryl Amidation Routes to Dihydropyrrolo [3, 2-e] indoles and Pyrrolo [3, 2-f] tetrahydroquinolines: Total Synthesis of the (±)-CC-1065 CPI Subunit. J. Org. Chem. 2007, 72, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Brunn, H.; Arnold, G.; Körner, W.; Rippen, G.; Steinhäuser, K.G.; Valentin, I. PFAS: Forever chemicals—Persistent, bioaccumulative and mobile. Reviewing the status and the need for their phase out and remediation of contaminated sites. Environ. Sci. Eur. 2023, 35, 20. [Google Scholar] [CrossRef]
- López, S.E.; Salazar, J. Trifluoroacetic acid: Uses and recent applications in organic synthesis. J. Fluor. Chem. 2013, 156, 73–100. [Google Scholar] [CrossRef]
- Pesticides & Bee Toxicity. Available online: https://www.mda.state.mn.us/protecting/bmps/pollinators/beetoxicity (accessed on 21 August 2024).
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental Risks and Challenges Associated with Neonicotinoid Insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef]
- Borsuah, J.F.; Messer, T.L.; Snow, D.D.; Comfort, S.D.; Mittelstet, A.R. Literature Review: Global Neonicotinoid Insecticide Occurrence in Aquatic Environments. Water 2020, 12, 3388. [Google Scholar] [CrossRef]
- Gershon, H.; Clarke, D.; Gershon, M. Antifungal activity of halophenols and halonitrophenols. Monatshefte Chem. 1995, 126, 1161. [Google Scholar] [CrossRef]
- Agency, E.P. TRI Basic Data Files: Calendar Years 1987-Present. Available online: https://www.epa.gov/toxics-release-inventory-tri-program/tri-basic-data-files-calendar-years-1987-present (accessed on 28 December 2024).
- Xu, B.; Liu, S.; Zhou, J.L.; Zheng, C.; Weifeng, J.; Chen, B.; Zhang, T.; Qiu, W. PFAS and their substitutes in groundwater: Occurrence, transformation and remediation. J. Hazard. Mater. 2021, 412, 125159. [Google Scholar] [CrossRef]
- Ministry of Economy, Trade and Industry. The 238th Chemical Substances Council—Review Subcommittee: Document 6 [in Japanese]. Available online: https://www.meti.go.jp/shingikai/kagakubusshitsu/shinsa/pdf/238_s06_00.pdf (accessed on 9 March 2025).
- Lindley, A.A. An Inventory of Fluorspar Production, Industrial Use, and Emissions of Trifluoroacetic Acid (TFA) in the Period 1930 to 1999. J. Geosci. Environ. Prot. 2023, 11, 1–16. [Google Scholar] [CrossRef]
- Jin, Y.H.; Liu, W.; Sato, I.; Nakayama, S.F.; Sasaki, K.; Saito, N.; Tsuda, S. PFOS and PFOA in environmental and tap water in China. Chemosphere 2009, 77, 605–611. [Google Scholar] [CrossRef]
- Wang, C.C.; Li, Q.F.; Lu, Y.L.; Wang, T.Y.; Khan, K.; Wang, P.; Meng, J.; Zhou, Y.G.; Yvette, B.; Suriyanarayanan, S. Simulating transport, flux, and ecological risk of perfluorooctanoate in a river affected by a major fluorochemical manufacturer in northern China. Sci. Total Environ. 2019, 657, 792–803. [Google Scholar] [CrossRef]
- Arp, H.P.H.; Gredelj, A.; Glüge, J.; Scheringer, M.; Cousins, I.T. The Global Threat from the Irreversible Accumulation of Trifluoroacetic Acid (TFA). Environ. Sci. Technol. 2024, 58, 19925–19935. [Google Scholar] [CrossRef] [PubMed]
- Neuwald, I.J.; Hübner, D.; Wiegand, H.L.; Valkov, V.; Borchers, U.; Nödler, K.; Scheurer, M.; Hale, S.E.; Arp, H.P.H.; Zahn, D. Ultra-short-chain PFASs in the sources of German drinking water: Prevalent, overlooked, difficult to remove, and unregulated. Environ. Sci. Technol. 2022, 56, 6380–6390. [Google Scholar] [CrossRef]
- Tian, Y.; Yao, Y.; Chang, S.; Zhao, Z.; Zhao, Y.; Yuan, X.; Wu, F.; Sun, H. Occurrence and phase distribution of neutral and ionizable per-and polyfluoroalkyl substances (PFASs) in the atmosphere and plant leaves around landfills: A case study in Tianjin, China. Environ. Sci. Technol. 2018, 52, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, K.; Park, K.; Seong, C.; Yu, S.D.; Kim, P. Adverse effects of perfluoroalkyl acids on fish and other aquatic organisms: A review. Sci. Total Environ. 2020, 707, 135334. [Google Scholar] [CrossRef]
- Alexander, J.; Karaolia, P.; Fatta-Kassinos, D.; Schwartz, T. Impacts of Advanced Oxidation Processes on Microbiomes During Wastewater Treatment. In Advanced Treatment Technologies for Urban Wastewater Reuse; Fatta-Kassinos, D., Dionysiou, D.D., Kümmerer, K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 129–144. [Google Scholar] [CrossRef]
- Saritha, P.; Aparna, C.; Himabindu, V.; Anjaneyulu, Y. Comparison of various advanced oxidation processes for the degradation of 4-chloro-2 nitrophenol. J. Hazard. Mater. 2007, 149, 609–614. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, M.; Zhang, S.; Qu, Y. Electron-Enriched Pd Nanoparticles for Selective Hydrogenation of Halonitrobenzenes to Haloanilines. Catalysts 2021, 11, 543. [Google Scholar] [CrossRef]
- Tang, H.Q.; Xiang, Q.Q.; Lei, M.; Yan, J.C.; Zhu, L.H.; Zou, J. Efficient degradation of perfluorooctanoic acid by UV-Fenton process. Chem. Eng. J. 2012, 184, 156–162. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.; Wang, X. A review of electrochemical oxidation technology for advanced treatment of medical wastewater. Front. Chem. 2022, 10, 1002038. [Google Scholar] [CrossRef]
- Najafinejad, M.S.; Chianese, S.; Fenti, A.; Iovino, P.; Musmarra, D. Application of Electrochemical Oxidation for Water and Wastewater Treatment: An Overview. Molecules 2023, 28, 4208. [Google Scholar] [CrossRef]
- Smith, S.J.; Lauria, M.; Ahrens, L.; McCleaf, P.; Hollman, P.; Bjälkefur Seroka, S.; Hamers, T.; Arp, H.P.H.; Wiberg, K. Electrochemical Oxidation for Treatment of PFAS in Contaminated Water and Fractionated Foam—A Pilot-Scale Study. ACS EST Water 2023, 3, 1201–1211. [Google Scholar] [CrossRef]
- Stroo, H.F.; Unger, M.; Ward, C.H.; Kavanaugh, M.C.; Vogel, C.; Leeson, A.; Marqusee, J.A.; Smith, B.P. Peer reviewed: Remediating chlorinated solvent source zones. Environ. Sci. Technol. 2003, 37, 224A–230A. [Google Scholar] [CrossRef]
- Friis, A.K.; Edwards, E.A.; Albrechtsen, H.J.; Udell, K.S.; Duhamel, M.; Bjerg, P.L. Dechlorination after thermal treatment of a TCE-contaminated aquifer: Laboratory experiments. Chemosphere 2007, 67, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Costanza, J.; Davis, E.L.; Mulholland, J.A.; Pennell, K.D. Abiotic degradation of trichloroethylene under thermal remediation conditions. Environ. Sci. Technol. 2005, 39, 6825–6830. [Google Scholar] [CrossRef]
- Chalivendra, S. Mechanisms of PFAS degradation in thermal destruction processes. J. Res. Appl. Sci. Biotechnol. 2023, 2, 317–323. [Google Scholar] [CrossRef]
- Alinezhad, A.; Challa Sasi, P.; Zhang, P.; Yao, B.; Kubátová, A.; Golovko, S.A.; Golovko, M.Y.; Xiao, F. An Investigation of Thermal Air Degradation and Pyrolysis of Per- and Polyfluoroalkyl Substances and Aqueous Film-Forming Foams in Soil. ACS EST Eng. 2022, 2, 198–209. [Google Scholar] [CrossRef]
- Ellis, A.C.; Boyer, T.H.; Fang, Y.; Liu, C.J.; Strathmann, T.J. Life cycle assessment and life cycle cost analysis of anion exchange and granular activated carbon systems for remediation of groundwater contaminated by per-and polyfluoroalkyl substances (PFASs). Water Res. 2023, 243, 120324. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Technical and Cost Considerations for Treatment Technologies to Remove PFAS from Drinking Water. Available online: https://www.epa.gov/system/files/documents/2024-04/2024-pfas-tech-cost_final-508.pdf (accessed on 9 March 2025).
- Belkouteb, N.; Franke, V.; McCleaf, P.; Köhler, S.; Ahrens, L. Removal of per-and polyfluoroalkyl substances (PFASs) in a full-scale drinking water treatment plant: Long-term performance of granular activated carbon (GAC) and influence of flow-rate. Water Res. 2020, 182, 115913. [Google Scholar] [CrossRef] [PubMed]
- Stoiber, T.; Evans, S.; Temkin, A.M.; Andrews, D.Q.; Naidenko, O.V. PFAS in Drinking Water: An Emergent Water Quality Threat. Available online: https://www.ewg.org/sites/default/files/u352/Stoiber_Evans_WaterSolutions_2020.pdf (accessed on 9 March 2025).
- (IARC), International Agency for Research on Cancer. Styrene (Group 2B). Available online: https://inchem.org/documents/iarc/vol82/82-07.html?utm (accessed on 7 January 2025).
- Taylor, J.S.; Jacobs, E.P. Reverse Osmosis and Nanofiltration; American Water Works Association: Denver, CO, USA, 1996; pp. 9.1–9.70. ISBN 0070015597/9780070015593. [Google Scholar]
- Elazhar, F.; Touir, J.; Elazhar, M.; Belhamidi, S.; El Harrak, N.; Zdeg, A.; Hafsi, M.; Amor, Z.; Taky, M.; Elmidaoui, A. Techno-economic comparison of reverse osmosis and nanofiltration in desalination of a Moroccan brackish groundwater. Desalination Water Treat. 2015, 55, 2471–2477. [Google Scholar] [CrossRef]
- Yahya, A.A.; Rashid, K.T.; Ghadhban, M.Y.; Mousa, N.E.; Majdi, H.S.; Salih, I.K.; Alsalhy, Q.F. Removal of 4-Nitrophenol from Aqueous Solution by Using Polyphenylsulfone-Based Blend Membranes: Characterization and Performance. Membranes 2021, 11, 171. [Google Scholar] [CrossRef]
- Valdés, H.; Saavedra, A.; Flores, M.; Vera-Puerto, I.; Aviña, H.; Belmonte, M. Reverse Osmosis Concentrate: Physicochemical Characteristics, Environmental Impact, and Technologies. Membranes 2021, 11, 753. [Google Scholar] [CrossRef]
- Shenvi, S.S.; Isloor, A.M.; Ismail, A. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Lawler, W.; Bradford-Hartke, Z.; Cran, M.J.; Duke, M.; Leslie, G.; Ladewig, B.P.; Le-Clech, P. Towards new opportunities for reuse, recycling and disposal of used reverse osmosis membranes. Desalination 2012, 299, 103–112. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mahmoud, S.A.; Mohamed, A.A. Nanomaterials-modified reverse osmosis membranes: A comprehensive review. RSC Adv. 2024, 14, 18879–18906. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 78138, 1,3,5-Benzenetricarbonyl Trichloride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/78138 (accessed on 4 January 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 7935, m-Phenylenediamine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/m-Phenylenediamine (accessed on 4 January 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 4837, Piperazine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Piperazine (accessed on 4 January 2025).
- Shi, Y.; Zhao, S.; Diao, Z.; Ye, Y.; Wang, Q.; Wang, Y. Modeling and Analysis of the Soil Vapor Extraction Equipment for Soil Remediation. Electronics 2023, 12, 151. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, L.; Miao, Y.; Qiu, L. Research progress in the enhancement technology of soil vapor extraction of volatile petroleum hydrocarbon pollutants. Environ. Sci. Process. Impacts 2021, 23, 1650–1662. [Google Scholar] [CrossRef]
- Soares, A.A.; Pinho, M.T.; Albergaria, J.T.; Domingues, V.; da Conceição, M.; Alvim-Ferraz, M.; De Marco, P.; Delerue-Matos, C. Sequential Application of Soil Vapor Extraction and Bioremediation Processes for the Remediation of Ethylbenzene-Contaminated Soils. Water Air Soil Pollut. 2012, 223, 2601–2609. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency Office of Solid Waste and Emergency Response Technology Innovation Office. Case Study: Verona Well Field Superfund Site Verona, Michigan. Available online: https://clu-in.org/download/remed/verona.pdf (accessed on 9 March 2025).
- EPA, U.S. Site Acceleration Approach Documents. Available online: https://www.clu-in.org/products/costperf/sve/saad2.htm (accessed on 5 March 2025).
- United States Environmental Protection Agency. Cost and Performance Report: Soil Vapor Extraction System at Intersil, Inc. Available online: https://www.frtr.gov/costperformance/pdf/Intersil.pdf (accessed on 5 March 2025).
- Sharma, I. Bioremediation techniques for polluted environment: Concept, advantages, limitations, and prospects. In Trace Metals in the Environment—New Approaches and Recent Advances; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Dar, A.; Naseer, A. Recent Applications of Bioremediation and Its Impact; Hazardous Waste Management; IntechOpen: London, UK, 2022; p. 49. ISBN 1803553782/9781803553788. [Google Scholar]
- Cervantes, P.A.M.; Ziarati, P.; de Frutos Madrazo, P. Bioremediation. In Encyclopedia of Sustainable Management; Idowu, S.O., Schmidpeter, R., Capaldi, N., Zu, L., Del Baldo, M., Abreu, R., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 298–305. [Google Scholar] [CrossRef]
- Lewis, T.; Clayton, J.; Skelton, N. Remediation of TCE-Impacted Groundwater via Enhanced Biodegradation in the Adelaide Plains. Available online: https://aclca.com.au/wp-content/uploads/2023/06/ACLCA-SA-Remediation-of-TCE-Impacted-Groundwater-via-Enhanced-Biodegrradation-in-the-Adelaide-Plains.pdf (accessed on 5 March 2025).
- Arora, P.K.; Jain, R.K. Metabolism of 2-chloro-4-nitrophenol in a Gram negative bacterium, Burkholderia sp. RKJ 800. PLoS ONE 2012, 7, e38676. [Google Scholar] [CrossRef]
- Čapek, A.; Šimek, A.; Leiner, J.; Weichet, J. Antimicrobial agents: VII. Microbial degradation of the antifungal agent 2-chloro-4-nitrophenol (Nitrofungin). Folia Microbiol. 1970, 15, 350–353. [Google Scholar] [CrossRef]
- Shukla, A.K.; Upadhyay, S.N.; Dubey, S.K. Current trends in trichloroethylene biodegradation: A review. Crit. Rev. Biotechnol. 2014, 34, 101–114. [Google Scholar] [CrossRef]
- Marco-Urrea, E.; Gabarrell, X.; Caminal, G.; Vicent, T.; Adinarayana Reddy, C. Aerobic degradation by white-rot fungi of trichloroethylene (TCE) and mixtures of TCE and perchloroethylene (PCE). J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2008, 83, 1190–1196. [Google Scholar] [CrossRef]
- Huang, S.; Jaffé, P.R. Defluorination of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) by Acidimicrobium sp. strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419. [Google Scholar] [CrossRef]
- Tseng, N.S.-l. Feasibility of Biodegradation of Polyfluoroalkyl and Perfluoroalkyl Substances. Master’s Thesis, UCLA, Los Angeles, CA, USA, 2012. [Google Scholar]
- Ayilara, M.S.; Babalola, O.O. Bioremediation of environmental wastes: The role of microorganisms. Front. Agron. 2023, 5, 1183691. [Google Scholar] [CrossRef]
- Service, N.N.O. What Is Hypoxia? Available online: https://oceanservice.noaa.gov/hazards/hypoxia/?utm (accessed on 5 February 2025).
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques-classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.; Teixeira, A.C.S.C.; Ruotolo, L.A.M. Critical review of Fenton and photo-Fenton wastewater treatment processes over the last two decades. Int. J. Environ. Sci. Technol. 2023, 20, 13995–14032. [Google Scholar] [CrossRef]
- Fujihira, M.; Satoh, Y.; Osa, T. Heterogeneous photocatalytic oxidation of aromatic compounds on semiconductor materials: The photo-fenton type reaction. Chem. Lett. 2006, 10, 1053–1056. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Pliego, G.; Zazo, J.A.; Garcia-Muñoz, P.; Munoz, M.; Casas, J.A.; Rodriguez, J.J. Trends in the Intensification of the Fenton Process for Wastewater Treatment: An Overview. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2611–2692. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Ameta, R.; Chohadia, A.K.; Jain, A.; Punjabi, P.B. Chapter 3—Fenton and Photo-Fenton Processes. In Advanced Oxidation Processes for Waste Water Treatment; Ameta, S.C., Ameta, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 49–87. [Google Scholar] [CrossRef]
- Amaral-Silva, N.; Martins, R.C.; Nunes, P.; Castro-Silva, S.; Quinta-Ferreira, R.M. From a lab test to industrial application: Scale-up of Fenton process for real olive mill wastewater treatment. J. Chem. Technol. Biotechnol. 2017, 92, 1336–1344. [Google Scholar] [CrossRef]
- Gar Alalm, M.; Tawfik, A.; Ookawara, S. Investigation of optimum conditions and costs estimation for degradation of phenol by solar photo-Fenton process. Appl. Water Sci. 2017, 7, 375–382. [Google Scholar] [CrossRef]
- Smara, M.; Khalladi, R.; Moulai-Mostefa, N.; Madi, K.; Mansour, D.; Lekmine, S.; Benslama, O.; Tahraoui, H.; Zhang, J.; Amrane, A. Efficiency of Hydrogen Peroxide and Fenton Reagent for Polycyclic Aromatic Hydrocarbon Degradation in Contaminated Soil: Insights from Experimental and Predictive Modeling. Processes 2024, 12, 621. [Google Scholar] [CrossRef]
- Baciocchi, R. Principles, Developments and Design Criteria of In Situ Chemical Oxidation. Water Air Soil Pollut. 2013, 224, 1717. [Google Scholar] [CrossRef]
- Emilio, C.A.; Jardim, W.F.; Litter, M.I.; Mansilla, H.D. EDTA destruction using the solar ferrioxalate advanced oxidation technology (AOT): Comparison with solar photo-Fenton treatment. J. Photochem. Photobiol. A Chem. 2002, 151, 121–127. [Google Scholar] [CrossRef]
- Gázquez, M.J.; Bolívar, J.P.; García-Tenorio García-Balmaseda, R.; Vaca, F. A review of the production cycle of titanium dioxide pigment. Mater. Sci. Appl. 2014, 5, 441–458. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide-From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Lai, C.; Zhang, Y.; Lin, X.; Ding, S. Recent advances in noble metal-based catalysts for CO oxidation. RSC Adv. 2024, 14, 30566–30581. [Google Scholar] [CrossRef]
- Chandra, T.; Zebrowski, J.P. Hazards associated with laboratory scale hydrogenations. J. Chem. Health Saf. 2016, 23, 16–25. [Google Scholar] [CrossRef]
- Namakka, M.; Rahman, M.R.; Said, K.A.B.M.; Muhammad, A. Insights into micro-and nano-zero valent iron materials: Synthesis methods and multifaceted applications. RSC Adv. 2024, 14, 30411–30439. [Google Scholar] [CrossRef]
- Mines, P.D.; Kaarsholm, K.M.; Droumpali, A.; Andersen, H.R.; Hwang, Y. Estimating dehalogenation reactivity of nanoscale zero-valent iron by simple colorimetric assay by way of 4-chlorophenol reduction. Environ. Eng. Res. 2020, 25, 197–204. [Google Scholar] [CrossRef]
- Jang, M.H.; Lim, M.; Hwang, Y.S. Potential environmental implications of nanoscale zero-valent iron particles for environmental remediation. Environ. Health Toxicol. 2014, 29, e2014022. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Zhou, P.; Liu, Y.; Cheng, F.; Liu, Y.; Cheng, X.; Zhang, Y.; Wang, Q. Removal of contaminants by activating peroxymonosulfate (PMS) using zero valent iron (ZVI)-based bimetallic particles (ZVI/Cu, ZVI/Co, ZVI/Ni, and ZVI/Ag). RSC Adv. 2020, 10, 28232–28242. [Google Scholar] [CrossRef] [PubMed]
- Aulenta, F.; Catervi, A.; Majone, M.; Panero, S.; Reale, P.; Rossetti, S. Electron transfer from a solid-state electrode assisted by methyl viologen sustains efficient microbial reductive dechlorination of TCE. Environ. Sci. Technol. 2007, 41, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Duinslaeger, N.; Radjenovic, J. Electrochemical degradation of per- and polyfluoroalkyl substances (PFAS) using low-cost graphene sponge electrodes. Water Res. 2022, 213, 118148. [Google Scholar] [CrossRef]
- Simon, G.P. Ion Exchange Training Manual; Springer: Berlin/Heidelberg, Germany, 1991; ISBN 0442006527. [Google Scholar]
- Dixit, F.; Dutta, R.; Barbeau, B.; Berube, P.; Mohseni, M. PFAS removal by ion exchange resins: A review. Chemosphere 2021, 272, 129777. [Google Scholar] [CrossRef]
- Maul, G.A.; Kim, Y.; Amini, A.; Zhang, Q.; Boyer, T.H. Efficiency and life cycle environmental impacts of ion-exchange regeneration using sodium, potassium, chloride, and bicarbonate salts. Chem. Eng. J. 2014, 254, 198–209. [Google Scholar] [CrossRef]
- Álvarez-Merino, M.; Fontecha-Cámara, M.; López-Ramón, M.; Moreno-Castilla, C. Temperature dependence of the point of zero charge of oxidized and non-oxidized activated carbons. Carbon 2008, 46, 778–787. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef]
- Niu, B.; Yang, S.; Li, Y.; Zang, K.; Sun, C.; Yu, M.; Zhou, L.; Zheng, Y. Regenerable magnetic carbonized Calotropis gigantea fiber for hydrophobic-driven fast removal of perfluoroalkyl pollutants. Cellulose 2020, 27, 5893–5905. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Du, J.; Qi, F. Adsorption of Perfluorooctane sulfonate (PFOS) onto metal oxides modified biochar. Environ. Technol. Innov. 2020, 19, 100816. [Google Scholar] [CrossRef]
- Dalahmeh, S.S.; Alziq, N.; Ahrens, L. Potential of biochar filters for onsite wastewater treatment: Effects of active and inactive biofilms on adsorption of per-and polyfluoroalkyl substances in laboratory column experiments. Environ. Pollut. 2019, 247, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, X.; Zhu, X.; Li, Y. Removal of Intermediate Aromatic Halogenated DBPs by Activated Carbon Adsorption: A New Approach to Controlling Halogenated DBPs in Chlorinated Drinking Water. Environ. Sci. Technol. 2017, 51, 3435–3444. [Google Scholar] [CrossRef] [PubMed]
- Lyon, F. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. World Health Organization, International Agency for Research on Cancer. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/08/14-002.pdf (accessed on 21 December 2024).
- Yu, F.; Luo, G. Modeling of gaseous methylamines in the global atmosphere: Impacts of oxidation and aerosol uptake. Atmos. Chem. Phys. 2014, 14, 12455–12464. [Google Scholar] [CrossRef]
- Haneke, K.E. Toxicological Summary for Dimethylethanolamine (DMAE) and Selected Salts and Esters. Available online: https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/dimethylethanolamine_508.pdf (accessed on 21 December 2024).
- Luan, J.; Plaisier, A. Study on treatment of wastewater containing nitrophenol compounds by liquid membrane process. J. Membr. Sci. 2004, 229, 235–239. [Google Scholar] [CrossRef]
- Appleman, T.D.; Dickenson, E.R.; Bellona, C.; Higgins, C.P. Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids. J. Hazard. Mater. 2013, 260, 740–746. [Google Scholar] [CrossRef]
- Bellona, C.; Drewes, J.E.; Xu, P.; Amy, G. Factors affecting the rejection of organic solutes during NF/RO treatment—A literature review. Water Res. 2004, 38, 2795–2809. [Google Scholar] [CrossRef]
- Sawyer, L.; James, M.N.G. Carboxyl–carboxylate interactions in proteins. Nature 1982, 295, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Fu, Q.S.; Criddle, C.S.; Leckie, J.O. Effect of flux (transmembrane pressure) and membrane properties on fouling and rejection of reverse osmosis and nanofiltration membranes treating perfluorooctane sulfonate containing wastewater. Environ. Sci. Technol. 2007, 41, 2008–2014. [Google Scholar] [CrossRef]
- Liu, C.J.; Strathmann, T.J.; Bellona, C. Rejection of per-and polyfluoroalkyl substances (PFASs) in aqueous film-forming foam by high-pressure membranes. Water Res. 2021, 188, 116546. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Xu, C.; Zhi, R.; Miao, R.; Liang, T.; Yue, X.; Lv, Y.; Liu, T. Perfluorooctane sulfonate and perfluorobutane sulfonate removal from water by nanofiltration membrane: The roles of solute concentration, ionic strength, and macromolecular organic foulants. Chem. Eng. J. 2018, 332, 787–797. [Google Scholar] [CrossRef]
- Xiong, J.; Hou, Y.; Wang, J.; Liu, Z.; Qu, Y.; Li, Z.; Wang, X. The rejection of perfluoroalkyl substances by nanofiltration and reverse osmosis: Influencing factors and combination processes. Environ. Sci. Water Res. Technol. 2021, 7, 1928–1943. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Rosa, M.J.; Nyström, M. The role of membrane charge on nanofiltration performance. J. Membr. Sci. 2005, 265, 160–166. [Google Scholar] [CrossRef]
- Soriano, A.l.; Gorri, D.; Urtiaga, A. Selection of high flux membrane for the effective removal of short-chain perfluorocarboxylic acids. Ind. Eng. Chem. Res. 2019, 58, 3329–3338. [Google Scholar] [CrossRef]
- Fouad, K.; Elhenawy, Y.; El-Hadek, M.A.; Bassyouni, M. A Critical Review of Sustainable Biodegradable Polymeric Reverse Osmosis Membranes. In Engineering Solutions Toward Sustainable Development; Springer: Cham, Switzerland, 2024; pp. 175–194. [Google Scholar]
- Schunke, A.J.; Hernandez Herrera, G.A.; Padhye, L.; Berry, T.-A. Energy recovery in SWRO desalination: Current status and new possibilities. Front. Sustain. Cities 2020, 2, 9. [Google Scholar] [CrossRef]
- Stewart, L.; Lutes, C.; Truesdale, R.; Schumacher, B.; Zimmerman, J.H.; Connell, R. Field Study of Soil Vapor Extraction for Reducing Off-Site Vapor Intrusion. Ground Water Monit. Remediat. 2020, 40, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Qian, Y.; Yuan, G.-X.; Wang, C.-X. Research progress on the soil vapor extraction. J. Groundw. Sci. Eng. 2020, 8, 57–66. [Google Scholar] [CrossRef]
- Truex, M.J.; Gillie, J.M.; Powers, J.G.; Lynch, K.P. Assessment of In Situ Thermal Treatment for Chlorinated Organic Source Zones. Remediat.-J. Environ. Cleanup Costs Technol. Tech. 2009, 19, 7–17. [Google Scholar] [CrossRef]
- Wang, B.; Wu, A.; Li, X.; Ji, L.; Sun, C.; Shen, Z.; Chen, T.; Chi, Z. Progress in fundamental research on thermal desorption remediation of organic compound-contaminated soil. Waste Dispos. Sustain. Energy 2021, 3, 83–95. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Bezerra de Souza, B.; Casarini, M.M.; Kewalramani, J.A. A Review of PFAS Destruction Technologies. Int. J. Environ. Res. Public Health 2022, 19, 16397. [Google Scholar] [CrossRef]
- Syed, Z.; Sogani, M.; Rajvanshi, J.; Sonu, K. Microbial Biofilms for Environmental Bioremediation of Heavy Metals: A Review. Appl. Biochem. Biotechnol. 2023, 195, 5693–5711. [Google Scholar] [CrossRef]
- Liu, C.-J.; Deng, S.-G.; Hu, C.-Y.; Gao, P.; Khan, E.; Yu, C.-P.; Ma, L.Q. Applications of bioremediation and phytoremediation in contaminated soils and waters: CREST publications during 2018–2022. Crit. Rev. Environ. Sci. Technol. 2023, 53, 723–732. [Google Scholar] [CrossRef]
- Wu, B.; Hussain, M.; Zhang, W.; Stadler, M.; Liu, X.; Xiang, M. Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 2019, 10, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.D. Fluorophenol oxidation by a fungal chloroperoxidase. Biotechnol. Lett. 2007, 29, 45–49. [Google Scholar] [CrossRef]
- Liu, P.-W.G.; Wu, Y.-J.; Whang, L.-M.; Lin, T.-F.; Hung, W.-N.; Cho, K.-C. Molecular tools with statistical analysis on trichloroethylene remediation effectiveness. Int. Biodeterior. Biodegrad. 2020, 154, 105050. [Google Scholar] [CrossRef]
- Torres-Farradá, G.; Thijs, S.; Rineau, F.; Guerra, G.; Vangronsveld, J. White Rot Fungi as Tools for the Bioremediation of Xenobiotics: A Review. J. Fungi 2024, 10, 167. [Google Scholar] [CrossRef]
- Marco-Urrea, E.; Parella, T.; Gabarrell, X.; Caminal, G.; Vicent, T.; Adinarayana Reddy, C. Mechanistics of trichloroethylene mineralization by the white-rot fungus Trametes versicolor. Chemosphere 2008, 70, 404–410. [Google Scholar] [CrossRef]
- Shahsavari, E.; Schwarz, A.; Aburto-Medina, A.; Ball, A.S. Biological Degradation of Polycyclic Aromatic Compounds (PAHs) in Soil: A Current Perspective. Curr. Pollut. Rep. 2019, 5, 84–92. [Google Scholar] [CrossRef]
- Yadav, J.S.; Bethea, C.; Reddy, C.A. Mineralization of Trichloroethylene (TCE) by the White Rot Fungus Phanerochaete chrysosporium. Bull. Environ. Contam. Toxicol. 2000, 65, 28–34. [Google Scholar] [CrossRef]
- Walter, M.; Boyd-Wilson, K.; Boul, L.; Ford, C.; McFadden, D.; Chong, B.; Pinfold, J. Field-scale bioremediation of pentachlorophenol by Trametes versicolor. Int. Biodeterior. Biodegrad. 2005, 56, 51–57. [Google Scholar] [CrossRef]
- Shakoori, A.; Qureshi, A.; Saquib, Z. Endosulfan Degrading Yeast Isolated from Food Industry Effluents and Their Role in Environmental Cleanup; CAB International: Wallingford, UK, 2001; Volume 21, pp. 69–94. [Google Scholar]
- Bisht, J.; Harsh, N.S.K.; Palni, L.M.S.; Agnihotri, V.; Kumar, A. Bioaugmentation of endosulfan contaminated soil in artificial bed treatment using selected fungal species. Bioremediat. J. 2019, 23, 196–214. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Zhang, H.; Xiao, L.; Lei, Z.; Kaul, S.C.; Wadhwa, R.; Zhang, Z. Low Dose of Fluoride in the Culture Medium of Cordyceps militaris Promotes Its Growth and Enhances Bioactives with Antioxidant and Anticancer Properties. J. Fungi 2021, 7, 342. [Google Scholar] [CrossRef]
- Tigini, V.; Prigione, V.; Di Toro, S.; Fava, F.; Varese, G.C. Isolation and characterisation of polychlorinated biphenyl (PCB) degrading fungi from a historically contaminated soil. Microb. Cell Fact. 2009, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Rigas, F.; Dritsa, V.; Chatzidakis, J.; Marchant, R. Detoxification characteristics of selected Polyporus species for bioremediation applications. In Proceedings of the 8th International Conference on Environmental Science and Technology, Lemnos Island, Greece, 8–10 September 2003; pp. 739–746. [Google Scholar]
- Blanco-Orta, M.F.; Garcia-de la Cruz, R.F.; Paz-Maldonado, L.M.T.; Pedraza-Gonzalez, D.A.; Morales-Avila, M.M.; Balderas-Hernandez, V.E.; Gonzalez-Ortega, O.; Perez-Martinez, A.S. Assessing three industrially produced fungi for the bioremediation of diclofenac. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2023, 58, 661–670. [Google Scholar] [CrossRef]
- Cameron, M.D.; Timofeevski, S.; Aust, S.D. Enzymology of Phanerochaete chrysosporium with respect to the degradation of recalcitrant compounds and xenobiotics. Appl. Microbiol. Biotechnol. 2000, 54, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Rougeron, A.; Giraud, S.; Alastruey-Izquierdo, A.; Cano-Lira, J.; Rainer, J.; Mouhajir, A.; Le Gal, S.; Nevez, G.; Meyer, W.; Bouchara, J.P. Ecology of ScedosporiumSpecies: Present Knowledge and Future Research. Mycopathologia 2018, 183, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, V.; Bankole, P.O.; Jooju, B.; Sadasivam, S.K. Degradation and detoxification of reactive yellow dyes by Scedosporium apiospermum: A mycoremedial approach. Arch. Microbiol. 2022, 204, 324. [Google Scholar] [CrossRef]
- Esterhuizen-Londt, M.; Hendel, A.-L.; Pflugmacher, S. Mycoremediation of diclofenac using Mucor hiemalis. Toxicol. Environ. Chem. 2017, 99, 795–808. [Google Scholar] [CrossRef]
- Maya, K.; Upadhyay, S.N.; Singh, R.S.; Dubey, S.K. Degradation kinetics of chlorpyrifos and 3,5,6-trichloro-2-pyridinol (TCP) by fungal communities. Bioresour. Technol. 2012, 126, 216–223. [Google Scholar] [CrossRef]
- Henn, C.; Arakaki, R.M.; Monteiro, D.A.; Boscolo, M.; da Silva, R.; Gomes, E. Degradation of the Organochlorinated Herbicide Diuron by Rainforest Basidiomycetes. Biomed. Res. Int. 2020, 2020, 5324391. [Google Scholar] [CrossRef]
- Vithalani, P.; Bhatt, N.S. Mycoremediation of Rhodamine B through Aspergillus fumigatus P5 and evaluation of degradative pathway. Int. J. Environ. Sci. Technol. 2023, 20, 13209–13218. [Google Scholar] [CrossRef]
- Vroumsia, T.; Steiman, R.; Seigle-Murandi, F.; Benoit-Guyod, J.L.; Groupe pour l’Étude du Devenir des Xénobiotiques dans l’Environnement (GEDEXE). Fungal bioconversion of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4-dichlorophenol (2,4-DCP). Chemosphere 2005, 60, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Losantos, D.; Li, Y.; Sarra, M. Biotransformation of chloramphenicol by white-rot-fungi Trametes versicolor under cadmium stress. Bioresour. Technol. 2023, 369, 128508. [Google Scholar] [CrossRef] [PubMed]
- Nykiel-Szymanska, J.; Bernat, P.; Slaba, M. Biotransformation and detoxification of chloroacetanilide herbicides by Trichoderma spp. with plant growth-promoting activities. Pestic. Biochem. Physiol. 2020, 163, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Jałowiecki, Ł.; Płaza, G.; Ejhed, H.; Nawrotek, M. Aerobic biodegradation of norfloxacin and ofloxacin by a microbial consortium. Arch. Environ. Prot. 2023, 45, 40–47. [Google Scholar] [CrossRef]
- Fontes, B.D.; Kleingesinds, E.K.; Giovanella, P.; Pessoa, A.; Sette, L.D. Laccases produced by Peniophora from marine and terrestrial origin: A comparative study. Biocatal. Agric. Biotechnol. 2021, 35, 8. [Google Scholar] [CrossRef]
- Vanschijndel, J.; Vollenbroek, E.G.M.; Wever, R. The chloroperoxidase from the fungus curvularia-inaequalis—A novel vanadium enzyme. Biochim. Biophys. Acta 1993, 1161, 249–256. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).