Simple Summary
Feline infectious peritonitis (FIP) is a fatal disease in cats and can present in various manifestations. Myocarditis is a, so far, rarely described pathology within the clinical spectrum of FIP. In the present study, suspicion of myocarditis was raised in 4/40 cats that presented with different cardiac manifestations. These four cats completely recovered during treatment with GS-441524 in combination with cardiovascular therapy and remained stable during a one-year-follow-up. Myocarditis can be an important feature in cats with FIP and should therefore be closely monitored in these cats.
Abstract
Feline infectious peritonitis (FIP) caused by feline coronavirus (FCoV) is a fatal disease in cats characterized by variable manifestations. Myocarditis represents a less commonly described pathology within the clinical spectrum of FIP. No research has described the successful treatment of FIP-induced myocarditis. In this study, 40 cats were included and treated with 15 mg/kg of GS-441524 every 24 h orally. All cats were diagnosed with FIP by reverse transcription quantitative PCR in effusion in combination with typical clinical and laboratory changes. Echocardiography was performed in all cats, and myocarditis was suspected in 4/40 cats. Equivocal wall thickness was diagnosed in 2/4 cats, while systolic dysfunction with biatrial dilation was diagnosed in the other 2/4 cats. One cat also presented with ventricular ectopy. A severe increase in cardiac troponin I was seen in all four cats (median 1.82 ng/mL (1.20–5.84 ng/mL)). Cardiac dimensions and electrocardiographic abnormalities completely normalized in all four cats during treatment with GS-441524 and remained stable after treatment discontinuation during a one-year follow-up period. Myocarditis can be a clinical feature of FIP and present with different cardiologic manifestations. FIP-induced myocarditis can be cured with GS-441524 in combination with symptomatic cardiovascular treatment including pimobendan, clopidogrel, furosemide, or atenolol, depending on the clinical presentation.
1. Introduction
Feline infectious peritonitis (FIP) caused by feline coronavirus (FCoV) is one of the most common infectious diseases in cats with fatal consequences if untreated [1]. The antiviral nucleoside analog GS-441524 has demonstrated high efficacy against FIP [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Cats with FIP can develop body cavity effusions, pyogranulomatous lesions in different organs including brain and eyes [19], as well as less common signs, such as dermatological manifestations [20,21,22]. Recently, cases of myocardial lesions [23,24] or myocarditis have also been described [25,26,27,28,29].
Myocarditis is characterized by the infiltration of inflammatory cells into the myocardium, often accompanied by degenerative alterations of cardiomyocytes. Myocarditis can present with different clinical signs; observed changes can include systolic and/or diastolic dysfunction, ventricular wall thickening and abnormal wall motion, pericardial effusion, and electrocardiographic changes [30].
Inflammation of the myocardium can arise from both infectious and non-infectious causes. Examples of infectious agents in cats include bacteria, such as Streptococcus suis [31], Bartonella henselae [32,33] causing (pyo-) granulomatous myocarditis, or Salmonella typhimurium [34] resulting in bacteremia-associated myocarditis. Myocarditis can also be caused by parasites, such as Hepatozoon silvestris [35], leading to lymphoplasmacytic and histiocytic myocarditis, as well as Sarcocystis felis [36] and Toxoplasma gondii [37,38]. Viruses other than FCoV have rarely been associated with myocarditis in cats. One study identified feline immunodeficiency virus (FIV) antigens in inflammatory cells, e.g., T-lymphocytes and macrophages, in the heart of cats with myocarditis and hypertrophic cardiomyopathy (HCM) phenotype. However, a definitive association between FIV and the development of myocarditis was not confirmed [39]. One case report described an association of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection with heart failure in a cat [40]. However, a myocardial biopsy was not performed, and it remains uncertain whether the virus directly induced myocarditis or whether it was secondary to a multisystem inflammatory syndrome, as described in humans [41]. In addition, FCoV has been detected by immunohistochemistry (IHC) in the myocardium in cats with FIP in necropsy [25,26]. In two cats that presented with a dilated cardiomyopathy (DCM) phenotype, necropsy revealed diffuse granulomatous inflammation in the heart, which led to the retrospective diagnosis of FIP [27].
Myocarditis in cats with FIP has not yet been prospectively described in living cats. Additionally, nothing is known about the development of myocarditis during the antiviral treatment of FIP. Therefore, the aims of this study were to describe the manifestation of FIP-associated myocarditis in a clinical setting and evaluate the outcome in cats with FIP-associated myocarditis treated with GS-441524 in combination with cardiovascular treatment.
2. Materials and Methods
2.1. Study Population and Protocol
Forty cats were originally included in a previously published prospective study by Zuzzi-Krebitz and colleagues (2024) [5]. In four cats, the suspicion of myocarditis was raised. Inclusion criteria for study participation in the previously published study were the presence of puncturable effusion, a negative result for FIV antibodies and feline leukemia virus (FeLV) antigens, and a diagnosis of FIP. The diagnosis of FIP was made based on a combination of clinical and laboratory parameters typically altered in cats with FIP, along with a positive FCoV reverse transcription quantitative PCR (RT-qPCR) from effusion. Clinical signs typical for FIP included lethargy, fever, hyporexia, and pleural/abdominal effusion. Laboratory findings included aregenerative anemia, lymphopenia, hyperbilirubinemia, hypalbuminemia, decreased albumin/globulin ratio (A/G), and elevated acute phase proteins [19,42,43] (Table 1).

Table 1.
Signalment, clinical signs, and laboratory parameters of the four cats with FIP-associated myocarditis before treatment (day 1), on day 42 of treatment, and at the end of treatment (day 84) with GS-441524.
All cats were treated with GS-441524 (15 mg/kg, every 24 h (q24h), per os (PO)). They were hospitalized in the clinic for seven days and underwent a detailed physical examination as well as laboratory analyses and abdominal ultrasonography. In addition, cardiac evaluations including electrocardiography (ECG) and echocardiography were performed in all cats before the initiation of GS-441524 treatment. After discharge from the hospital (LMU small animal clinic, Munich, Germany) on day 7, cats were treated at home and were not allowed to leave the house during the antiviral treatment period. All cats received a commercial diet. They were monitored on days 14, 28, 42, 56, 84, 168, 252, and 365 after treatment initiation. At these timepoints, all cats underwent a physical examination, abdominal ultrasonography and cardiac assessment (days 42, 84, 168, and 365). Laboratory analyses, including complete blood cell count (CBC) and serum biochemistry, were performed. Viral loads in blood, effusion, and feces were determined by RT-qPCR. Details on the evaluation of laboratory parameters and FCoV RT-qPCR are described in the original study [5].
This study complied with the German guidelines for prospective studies and was approved by the Government of Upper Bavaria (reference number 55.2-2532.Vet_02-20-52) and by the ethical committee of the Centre for Clinical Veterinary Medicine of LMU Munich (reference number 261-19-03-2021). Owners gave their written informed consent for their cats to participate in the study.
2.2. Cardiac Examination
All 40 originally included cats [5] received a detailed cardiac examination, including the precise auscultation of the heart, ECG, and echocardiography. In cats with suspicion of myocarditis, cardiac troponin I (cTnI) was measured in fresh and cooled serum (reference interval (RI): <0.06 ng/mL) by chemiluminescence using Siemens® ADVIA Centaur XP (IDEXX Laboratories, Kornwestheim, Germany).
A 5 min 6-lead ECG (EKG 2000, Eickemeyer Medizintechnik, Tuttlingen, Germany) was recorded in right lateral recumbency without sedation. The underlying rhythm was determined, and the presence, number, and coupling interval of ventricular (VPCs) and atrial premature contractions (APCs) were documented. Additionally, signs of complexity (couplets and triplets) and malignancy (R-on-T phenomenon and ventricular tachycardia (VT)) or the presence of conduction abnormalities like atrioventricular blocks or bundle branch blocks were recorded.
Echocardiography (12–4 MHz transducer, Epiq 7, Philips Healthcare, Hamburg, Germany) was performed in right and left lateral recumbency without sedation, according to the published recommendations [44]. Interventricular septal (IVSd) and left ventricular posterior wall (LVPWd) thickness in end-diastole was measured in 2D using the leading edge-to-leading edge method. Left ventricular (LV) wall thickness ≥5 mm and <6 mm was considered equivocal, and hypertrophy was defined as IVSd and/or LVPWd ≥6 mm [45,46]. In cats >6 kg, weight-dependent reference intervals were used [47]. The left atrial-to-aortic root ratio (LA/Ao) was measured in right parasternal short-axis at end-systole as previously described [48]. The left atrial diameter (LAD) was measured in right parasternal long-axis optimized for the left atrium one frame before the mitral valve opening, as recommended [49]. Left atrial enlargement was defined as LA/Ao ≥1.6 and LAD ≥16 mm [49,50]. The right atrial diameter (RAD) was measured similarly to the LAD, and right atrial enlargement was diagnosed with RAD ≥12 mm [51]. Reduced systolic function was diagnosed with increased LV dimensions, especially in end-systole (LVIDs), and subsequently in end-diastole (LVIDd) according to breed-specific reference intervals [47]. In cats with suspected systolic dysfunction, the left ventricular global longitudinal strain (GLS) was measured using speckle-tracking echocardiography on an external work station (TOMTEC Imaging Systems GmbH, Unterschleißheim, Germany), as previously described [52]. According to previously published reference intervals for cats, reduced systolic function was defined as a GLS less negative than −21.18% [53].
Congestive heart failure (CHF) was confirmed when there was radiographic evidence of pulmonary edema, ultrasonographic detection of pleural and/or pericardial effusion, and associated clinical signs which resolved after the administration of diuretics [54].
2.3. Diagnosis of FIP-Induced Myocarditis
The modified Duke criteria proposed for the diagnosis of infectious endocarditis in humans [55], dogs [56], and cats [57] that have been adjusted for the diagnosis of myocarditis in dogs [58] were applied. According to these classifications, major and minor criteria have been proposed. The major criteria included cTnI >1.0 ng/mL and/or the detection of an infectious organism using a culture of blood/body fluid or PCR/RT-PCR. The minor criteria included a fever, a new or worsening heart murmur, ventricular arrhythmias, decreased left ventricular systolic function, heteroechogenicity of the left ventricular myocardium, pericardial effusion, and laboratory changes such as inflammatory leukogram, anemia, thrombocytopenia, and hypoalbuminemia. A high suspicion of myocarditis was raised if either two major criteria or one major and three minor criteria were fulfilled [58].
3. Results
Four cats were diagnosed with myocarditis (4/40; 10%) based on the fulfillment of two major criteria (cTnI >1.0 ng/mL and diagnosis of FIP) and additionally between one and two minor criteria for the diagnosis of myocarditis, as previously described [58]. All four cats were treated with GS-441524 at a dosage of 15 mg/kg q24h PO for 84 days. Additional treatment can be found in Table 2.

Table 2.
Additional symptomatic and cardiovascular treatment and other unrelated diseases developing during treatment with GS-441524.
3.1. Cardiac Findings
Cardiac auscultation was unremarkable in two cats (cats 38 and 39), one cat presented with gallop rhythm (cat 24), and one cat was arrhythmic with pulse deficits (cat 40). A severe increase in cTnI levels was seen in all four cats, with a median of 1.82 ng/mL (1.20–5.84 ng/mL) (RI < 0.06 ng/mL).
Two cats (cats 38 and 40) presented with equivocal cardiac wall thickness and heteroechogenicity of the left ventricular myocardium. The left atrial size was normal in both cats (Table 3). Cat 38 presented with equivocal cardiac dimensions and heterogenous echogenicity of the LV walls, which is considered abnormal in a young cat. In combination with severely elevated cTnI (1.37 ng/mL), acute myocardial damage was suspected and the suspicion of myocarditis was raised.

Table 3.
Echocardiographic measurements of the two equivocal cats (cats 38 and 40) before treatment (day 1), on day 42 of treatment, and at the end of treatment (day 84) with GS-441524.
Cat 40 also had 50 single VPCs in five minutes with a maximum coupling interval of 333 bpm, 28 single APCs with a maximum coupling interval of 320 bpm, and 7 fusion beats (Figure 1). Based on these findings, atenolol (1.5 mg/kg, q12h, PO) was started in cat 40. No further cardiac treatment was otherwise indicated in those two cats.
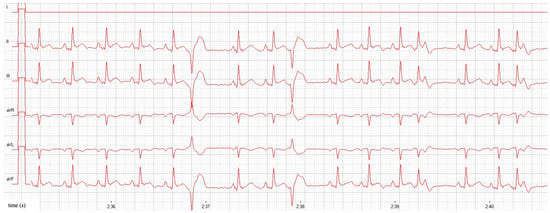
Figure 1.
Atrial and ventricular premature contractions in a cat with equivocal cardiac dimensions (cat 40). Before treatment, sinus rhythm with a heart rate of 166 bpm, single VPCs with a coupling interval of 333 bpm, and single APCs with a coupling interval of 320 bpm can be seen. Amplitude 10 mm/mV, paper speed 50 mm/s. APC = atrial premature contraction; VPC = ventricular premature contraction.
Cat 24 presented with systolic dysfunction based on increased LVIDs above breed-specific reference intervals [47] and reduced LV GLS (−10.19%) (Table 4). The left and right ventricle were affected, and the cat showed biatrial enlargement. Due to the presence of pulmonary edema, the cat was considered to be in CHF and treatment with furosemide (1.25 mg/kg, q12h, PO), pimobendan (0.3 mg/kg, q12h, PO), and rivaroxaban (0.6 mg/kg, q24h, PO) was started (Table 2). Prior to the start of the study, the cat was additionally diagnosed with uveitis and chorioretinitis caused by FIP and was therefore treated with prednisolone acetate eye drops. These eye drops were substituted with ketorolac-trometamol eye drops on day 12. This change in eye drop formulation was necessary due to supply shortages, which made the originally administered product temporarily unavailable. On the same day, the general condition of this cat deteriorated and the cat showed symptoms of fever, vomiting, and erythema cutis. At this time, sinus tachycardia with an instantaneous rate of 220 bpm, which was interrupted by a single episode of VT with an instantaneous rate of 340 bpm, was noted on monitor observation. The VT converted into sinus rhythm after approximately three seconds without medical intervention. The ECG of the cat was closely monitored for the next days; the VT never recurred, and only rare isolated VPCs that did not require any treatment could be detected. Rivaroxaban had to be discontinued on the same day due to petechiae and pronounced thrombocytopenia (68.00 ×109/L; RI: 180–550) of unknown origin but was restarted once the platelet count returned to the reference interval on day 19. As these abnormalities improved within a few days after the discontinuation of the ketorolac-trometamol eye drops, drug reaction was suspected.

Table 4.
Echocardiographic measurements of two cats with systolic dysfunction (cats 24 and 39) before treatment (day 1), on day 42 of treatment and at the end of treatment (day 84) with GS-441524.
Cat 39 also presented with systolic dysfunction based on increased LVIDs above breed-specific reference intervals [47] and reduced LV GLS (−16.22%) (Table 4). The left and right ventricle were affected, and the cat showed biatrial enlargement. The cat also showed mild pericardial effusion, which was thought to be secondary to FIP and not a sign of congestion in this cat. The cat was started on pimobendan (0.24 mg/kg, q12h, PO) and clopidogrel (18.75 mg/cat, q24h, PO). Diuretics were not considered necessary (Table 2). The echocardiographic data of all four cats can be seen in Table 3 and Table 4.
3.2. Outcome
All four cats had already presented with cardiac changes prior to treatment, but in three cats, the cardiac situation deteriorated within the first days after treatment initiation. However, during the treatment with GS-441524, all four cats recovered completely from their suspected myocarditis based on the normalization of the cardiac dimensions. Except for cat 24, abnormal cardiac parameters normalized in all cats by day 42, and all cardiac medications could be stopped in those three cats on the same day. Cat 24 still had signs of systolic dysfunction on day 42, and cardiovascular treatment (pimobendan, clopidogrel, and furosemide) was continued until day 84 when the cardiac dimensions normalized and the cardiovascular treatment could also be discontinued. The arrhythmias in cat 40 had already resolved by day 14 (based on 10 min ECG recording). Echocardiography and ECG were unremarkable in all cats during the follow-up examinations on days 168, 252, and 365 after treatment initiation. Echocardiographic follow-ups are demonstrated in Figure 2 and Figure 3. All clinical and laboratory signs typical of FIP improved during antiviral treatment [5]; in most cats, the improvement of other clinical and laboratory parameters (normalization by day 28–42 at the latest), such as the Karnofsky’s score, hematocrit, bilirubin concentration, and albumin concentration, occurred more rapidly than the recovery from FIP-induced myocarditis (Table 1).
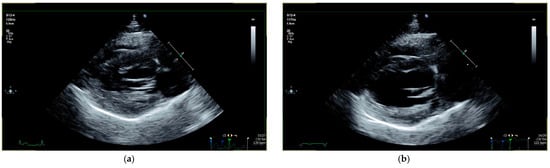
Figure 2.
Echocardiographic follow-up in a cat with equivocal cardiac dimensions (cat 40): (a) before treatment, cat 40 presented with equivocal left ventricular wall thickness (IVSd 5.1 mm and LVPWd 5.6 mm) and VPCs at the initial examination. (b) Day 42 after treatment initiation, cardiac dimensions completely normalized (IVSd 4.9 mm and LVPWd 4.8 mm). IVSd = interventricular septum thickness at end-diastole; LVPWd = left ventricular posterior wall thickness at end-diastole; VPC = ventricular premature contraction.
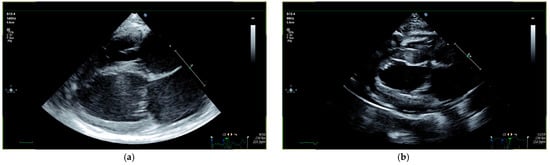
Figure 3.
Echocardiographic follow-up in a cat with systolic dysfunction (cat 24): (a) before treatment, cat 24 presented with systolic dysfunction (LVIDs 13.5 mm and GLS −10.19%) and biatrial dilation (LA/Ao 2.43, LAD 17 mm, RAD 13.1 mm) at the initial examination; (b) day 84 after treatment initiation, systolic function (LVIDs 10.6 mm and GLS −28.29%) and atrial dimensions (LA/Ao 1.44, LAD 14 mm, RAD 10.9 mm) completely normalized. GLS = global longitudinal strain; LA/Ao = left atrial-to-aortic root ratio; LAD = left atrial diameter; LVIDs = left ventricular internal diameter at end-systole; RAD = right atrial diameter.
4. Discussion
The results of this study demonstrate that myocarditis can be an important feature of FIP and can be effectively treated with an effective antiviral compound when combined with symptomatic treatment with cardiovascular drugs. The variable manifestation of myocarditis and a lack of validated diagnostic criteria complicates the diagnosis, but cardiac changes successfully resolve during treatment.
Myocarditis is a rare problem in cats, but a few reports about FIP-induced myocarditis exist [25,26,27,28,29]. The low number of case reports about FIP-associated myocarditis likely depends on their relatively low importance compared with other lesions caused by FIP. So far, diagnosis in those previously described cases was determined postmortem by histopathological examination and not by detailed cardiologic assessments of living cats. Two previous histopathology reports [25,26] identified pyogranulomatous infiltration in the myocardium, and IHC confirmed the presence of FCoV-positive macrophages. Multifocal areas of pyogranulomatous vasculitis were observed in these cats, were characterized by macrophages, neutrophils, and occasional lymphocytes, and were frequently centered around cores of lytic necrosis. The observed thickening of the myocardium was attributed to edema and the presence of inflammatory infiltrates, including lymphocytes, plasma cells, and FCoV-positive macrophages [25,26]. However, these cases only described changes in necropsy, and, so far, no detailed descriptions of FIP-associated myocarditis in cats in vivo and during their follow-up exist. There are also no reports available on the clinical or therapeutic management of cardiomyopathy in cats with FIP, likely because, until a few years ago, FIP was considered invariably fatal. Therefore, recent research focused primarily on the antivirals available for treatment rather than providing recommendations or guidelines for the supportive care of associated diseases such as cardiomyopathies. With the availability of efficient antiviral drugs, there is now an opportunity and necessity to investigate these additional treatments.
This study is the first describing the echocardiographic features of in vivo FIP-associated myocardial changes and follow-up examinations during antiviral treatment. In the described cats, either equivocal wall thickness or systolic dysfunction with biatrial enlargement were the most important findings, and both manifestations completely resolved with treatment with GS-441524. In severe cases, it should be considered that additionally supportive cardiovascular treatment is necessary.
Biatrial enlargement or hypertrophic phenotype have been previously described as “comorbidities” associated with FIP [3,13,26]. These findings suggest cardiac involvement in some cats with FIP. The presence of cardiac manifestations in 10% of the originally included cats suggests that FIP-induced myocarditis is probably more common than previously thought. Thus, it is important to consider myocarditis in cats with FIP, measure cTnI for the evaluation of cardiomyocyte damage, and potentially refer the cats for a detailed cardiac examination. Pleural effusion is not only a consequence of vasculitis caused by FIP but can also be a sign of heart failure due to myocarditis.
Endomyocardial biopsy is considered the gold standard for the in vivo diagnosis of myocarditis in humans [59]. However, since this procedure is highly invasive in cats [60], a suspected diagnosis often relies on historical and clinical data in combination with laboratory findings such as cTnI [61], ECG, and echocardiography [58,62]. The criteria proposed by Lakhdhir and colleagues (2020) for dogs [58] can be used as diagnostic tools to confirm the suspicion of myocarditis. In the present study, all four cats fulfilled two major criteria (cTnI > 1.0 ng/mL and diagnosis of FIP), as well as between one and two minor criteria, including decreased left ventricular systolic function (cats 24 and 39), pericardial effusion (cat 39), ventricular arrhythmias (cat 40), and the heteroechogenicity of the myocardium (cats 38 and 40). Certain laboratory changes, such as the elevation of acute phase proteins and fever can be related to FIP itself [19,42,43] and are therefore not indicative of myocarditis in cats with FIP.
In this study, NTproBNP levels were not measured, as cTnI is generally considered a more sensitive marker for myocardial damage, and it can be severely elevated in cases with myocarditis [63]. On the other hand, NTproBNP primarily reflects myocardial stretch or volume overload and has a strong correlation with the left atrial size. It is therefore suited to diagnose moderate to severe echocardiographic changes, but equivocal or mild disease might not be detected [64,65]. The in-house SNAP test provides a qualitative result (normal vs. abnormal) and might only be helpful in severe cases to distinguish cardiac-related respiratory distress from non-cardiac related disease [66]. Normal SNAP test results do not guarantee the absence of cardiomyopathy, especially in cases of mild heart disease [67]. It is therefore recommended to measure cTnI as a more appropriate biomarker for myocardial damage in combination with echocardiographic examination.
SARS-CoV-2 can cause myocarditis in humans [68] and is associated with an inflammatory reaction and cytokine storm [69]. Cardiac complications of coronavirus disease 2019 (COVID-19) include acute myocardial injury with increased cTnI levels, decreased ejection fraction, arrhythmias, thromboembolism, and pericarditis [70]. COVID-19-associated myocarditis is caused by direct cell injury and T-lymphocyte-mediated cytotoxicity, possibly being augmented by cytokine storm syndrome, especially by interleukin 6 (IL-6) [71,72]. Interestingly, SARS-CoV-2 has also been reported to cause myocarditis in cats [40,73,74], and further studies are needed to determine if FCoV infects the myocardium in a similar manner. In one study, it could be shown that cats with FIP display the increased transcription of inflammatory cytokines in the heart muscle and liver when compared to cats without FIP, although the increase was lower in the heart compared to the liver. This suggests a later and more reactive involvement of the myocardium in the disease process [75]. In the present study, cTnI was higher in the two cats with systolic dysfunction (2.27 and 5.84 ng/mL) compared to the two equivocal cats (1.37 and 1.20 ng/mL), and more pronounced cardiac damage as a result of the cytokine reaction may be a possible explanation for the different disease manifestations.
The use of the modified Duke’s criteria to diagnose myocarditis might be controversial in a multisystemic disease such as FIP. Without the use of endomyocardial biopsies, it remains unclear whether the virus itself and the associated inflammatory reaction or the massive cytokine response were responsible for myocarditis in the affected cats. However, in the present cases with a definitive diagnosis of FIP and the remission of myocarditis after treatment initiation, it is highly likely that the changes in the myocardium were caused by FIP.
All of the affected cats in this study exhibited cardiac changes prior to the initiation of treatment, but in three cats, the cardiologic situation deteriorated within the first days after treatment initiation. However, since the echocardiographic measurements and the general condition of the cats overall improved with GS-441524 treatment, it is strongly presumed that myocarditis is a consequence of FIP and not an adverse reaction to the drug. Thus, GS-441524 can be considered an effective and safe treatment for FIP-induced myocarditis. However, it is also important to mention that severe myocardial damage might not always be reversible with GS-441524 and supportive cardiovascular treatment. Furthermore, myocarditis should be recognized as a serious comorbidity in cats with FIP.
All cats received a uniform dosage of 15 mg/kg q24h PO, regardless of the presence of additional neurologic or ocular signs. In a previous publication by Krentz and colleagues (2021) [15], cats were treated with 5 mg/kg q24h PO GS-441524, increasing the dose to 10 mg/kg in cats with neurological or ocular involvement. However, the analysis of the multicomponent drug (Xraphconn®) used in this study revealed that the actual active GS-441524 content was more than twice the amount stated by the manufacturer (personal communication, J. Horak). Based on these findings, a dosage of 15 mg/kg was selected for all FIP manifestations. The four cats in this case series were treated for 84 days. They were randomly assigned to the long treatment group [5], and this decision was not based on the presence of cardiac manifestations.
Both myocarditis manifestations completely resolved with treatment with GS-441524. However, it could be argued that myocarditis resolved on its own or without causal treatment with GS-441524, as seen in stress-induced transient myocardial thickening (TMT) in cats [76]. Nevertheless, without GS-441524, the cats with FIP would have died from the disease itself and myocarditis would have had no chance to resolve on its own.
A limitation of this study was that cTnI was only measured in those of the 40 included cats in which myocarditis was suspected based on echocardiography, and cats with mild cardiac damage could have been missed. CTnI was not reassessed in cats with suspected myocarditis, and the reassessment relied solely on the improvement observed in cardiac dimensions. Further prospective studies with larger sample sizes should be performed in the future. As another limitation, only a high suspicion of myocarditis was raised and no definitive diagnosis via biopsy was made. Other reasons for myocarditis in cats, such as Toxoplasma gondii [37,38] or Bartonella henselae [32,33], were not excluded. However, due to the improvement of the general condition and cardiac changes during treatment with GS-441524, it was considered to be proven that myocarditis was FIP-induced.
5. Conclusions
This study demonstrates that myocarditis can be an associated feature of FIP and therefore should be considered as a potential complication. A thorough physical examination and cTnI testing are recommended in cats with FIP, and in cases of suspected myocarditis and/or clinical deterioration, a comprehensive cardiological assessment should be performed. FIP-associated myocarditis can effectively be cured with GS-441524 in combination with individually adapted cardiovascular treatment. This study also demonstrates that it is extremely important that FIP and all associated features are properly diagnosed and managed by veterinarians, ideally in veterinary hospitals with specialists who are able to manage complicated and critical cases.
Author Contributions
Conceptualization, K.H. and R.H.-L.; methodology, K.B., J.F., A.-M.Z.-K., J.S., J.E. and A.M.S.; software, G.W., validation, K.B., J.F., A.-M.Z.-K. and K.Z.; formal analysis, K.B., J.F., A.-M.Z.-K. and K.Z.; investigation, K.B., J.F., A.-M.Z.-K., K.Z., J.S., J.E. and A.M.S.; resources, K.H. and G.W.; writing—original draft preparation, K.B., J.F. and K.H.; writing—review and editing, K.H., R.H.-L., G.W. and A.M.S.; visualization, K.B. and J.F.; supervision, K.H. and G.W.; project administration, K.H.; funding acquisition, K.H. and R.H.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the UZH Global Strategy and Partnerships Funding Scheme, «A bi-center interdisciplinary study investigating the first successful oral treatment of fatal coronavirus infection in cats» (K-53420-02 01).
Institutional Review Board Statement
The animal study protocol was approved by the Government of Upper Bavaria, reference number 55.2-2532.Vet_02-20-52 and by the ethical committee (reference number 345-19-12-2022) of the Centre for Clinical Veterinary Medicine of the LMU Munich. The prospective study in cats fulfilled the German guidelines for prospective studies with written informed owner consent.
Informed Consent Statement
The owners of the cats in the prospective study gave written informed consent to participate.
Data Availability Statement
The authors confirm that the datasets analyzed during the study are available from the corresponding author upon reasonable request.
Acknowledgments
The GS-441524 tablets were provided by BOVA Specials, London, UK. The authors thank the cardiology team of the LMU Small Animal Clinic.
Conflicts of Interest
The authors declare that they have no conflict of interest. The GS-441524 tablets were provided by BOVA Specials, London, UK, but BOVA played no role in the interpretation of study data or the decision to submit the manuscript for publication. No commercial conflict of interest exists as the information is solely for scientific dissemination.
Abbreviations
The following abbreviations are used in this manuscript:
| LMU | Ludwig Maximilians University |
| FIP | Feline infectious peritonitis |
| FCoV | Feline coronavirus |
| cTnI | Cardiac Troponin I |
| ECG | Electrocardiography |
| FIV | Feline immunodeficiency virus |
| HCM | Hypertrophic cardiomyopathy |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| IHC | Immunohistochemistry |
| DCM | Dilated cardiomyopathy |
| FeLV | Feline leukemia virus |
| RT-qPCR | Reverse transcription quantitative polymerase chain reaction |
| A/G | Albumin/globulin ratio |
| q24h | Every 24 h |
| PO | Per os |
| CBC | Complete blood cell count |
| RI | Reference interval |
| VPCs | Ventricular premature contractions |
| APCs | Atrial premature contractions |
| VT | Ventricular tachycardia |
| IVSd | Interventricular septal thickness in end-diastole |
| LVPWd | Left ventricular posterior wall thickness in end-diastole |
| LV | Left ventricular |
| LA/Ao | Left atrial-to-aortic root ratio |
| LAD | Left atrial diameter |
| RAD | Right atrial diameter |
| LVIDs | Left ventricular internal diameter in end-systole |
| LVIDd | Left ventricular internal diameter in end-systole |
| GLS | Global longitudinal strain |
| CHF | Congestive heart failure |
| q8h | Every 8 h |
| q12h | Every 12 h |
| q6h | Every 6 h |
| COVID-19 | Coronavirus disease 2019 |
| TMT | Transient myocardial thickening |
| UK | United Kingdom |
References
- Sparkes, A.H.; Gruffydd-Jones, T.J.; Harbour, D.A. Feline infectious peritonitis: A review of clinicopathological changes in 65 cases, and a critical assessment of their diagnostic value. Vet. Rec. 1991, 129, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Novicoff, W.; Nadeau, J.; Evans, S. Unlicensed GS-441524-Like Antiviral Therapy Can Be Effective for at-Home Treatment of Feline Infectious Peritonitis. Animals 2021, 11, 2257. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Syme, H.; Tayler, S. Thirty-two cats with effusive or non-effusive feline infectious peritonitis treated with a combination of remdesivir and GS-441524. J. Vet. Intern. Med. 2023, 37, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Katayama, M.; Uemura, Y. Therapeutic Effects of Mutian® Xraphconn on 141 Client-Owned Cats with Feline Infectious Peritonitis Predicted by Total Bilirubin Levels. Vet. Sci. 2021, 8, 328. [Google Scholar] [CrossRef]
- Zuzzi-Krebitz, A.-M.; Buchta, K.; Bergmann, M.; Krentz, D.; Zwicklbauer, K.; Dorsch, R.; Wess, G.; Fischer, A.; Matiasek, K.; Hönl, A.; et al. Short Treatment of 42 Days with Oral GS-441524 Results in Equal Efficacy as the Recommended 84-Day Treatment in Cats Suffering from Feline Infectious Peritonitis with Effusion—A Prospective Randomized Controlled Study. Viruses 2024, 16, 1144. [Google Scholar] [CrossRef]
- Yin, Y.; Li, T.; Wang, C.; Liu, X.; Ouyang, H.; Ji, W.; Liu, J.; Liao, X.; Li, J.; Hu, C. A retrospective study of clinical and laboratory features and treatment on cats highly suspected of feline infectious peritonitis in Wuhan, China. Sci. Rep. 2021, 11, 5208. [Google Scholar] [CrossRef]
- Taylor, S.S.; Coggins, S.; Barker, E.N.; Gunn-Moore, D.; Jeevaratnam, K.; Norris, J.M.; Hughes, D.; Stacey, E.; MacFarlane, L.; O’brien, C.; et al. Retrospective study and outcome of 307 cats with feline infectious peritonitis treated with legally sourced veterinary compounded preparations of remdesivir and GS-441524 (2020–2022). J. Feline Med. Surg. 2023, 25, 1098612X231194460. [Google Scholar] [CrossRef]
- Addie, D.D.; Covell-Ritchie, J.; Jarrett, O.; Fosbery, M. Rapid Resolution of Non-Effusive Feline Infectious Peritonitis Uveitis with an Oral Adenosine Nucleoside Analogue and Feline Interferon Omega. Viruses 2020, 12, 1216. [Google Scholar] [CrossRef]
- Coggins, S.J.; Norris, J.M.; Malik, R.; Govendir, M.; Hall, E.J.; Kimble, B.; Thompson, M.F. Outcomes of treatment of cats with feline infectious peritonitis using parenterally administered remdesivir, with or without transition to orally administered Gs-441524. J. Vet. Intern. Med. 2023, 37, 1772–1783. [Google Scholar] [CrossRef]
- Murphy, B.; Perron, M.; Murakami, E.; Bauer, K.; Park, Y.; Eckstrand, C.; Liepnieks, M.; Pedersen, N. The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies. Vet. Microbiol. 2018, 219, 226–233. [Google Scholar] [CrossRef]
- Zwicklbauer, K.; Krentz, D.; Bergmann, M.; Felten, S.; Dorsch, R.; Fischer, A.; Hofmann-Lehmann, R.; Meli, M.L.; Spiri, A.M.; Alberer, M.; et al. Long-term follow-up of cats in complete remission after treatment of feline infectious peritonitis with oral GS-441524. J. Feline Med. Surg. 2023, 25, 1098612X231183250. [Google Scholar] [CrossRef] [PubMed]
- Cosaro, E.; Pires, J.; Castillo, D.; Murphy, B.G.; Reagan, K.L. Efficacy of Oral Remdesivir Compared to GS-441524 for Treatment of Cats with Naturally Occurring Effusive Feline Infectious Peritonitis: A Blinded, Non-Inferiority Study. Viruses 2023, 15, 1680. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C.; Perron, M.; Bannasch, M.; Montgomery, E.; Murakami, E.; Liepnieks, M.; Liu, H. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. J. Feline Med. Surg. 2019, 21, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Bai, Y.; Wang, Y.; Yang, L.; Jin, Y.; Dong, J. Effect of GS-441524 in combination with the 3C-like protease inhibitor GC376 on the treatment of naturally transmitted feline infectious peritonitis. Front. Vet. Sci. 2022, 9, 1002488. [Google Scholar] [CrossRef]
- Krentz, D.; Zenger, K.; Alberer, M.; Felten, S.; Bergmann, M.; Dorsch, R.; Matiasek, K.; Kolberg, L.; Hofmann-Lehmann, R.; Meli, M.L.; et al. Curing Cats with Feline Infectious Peritonitis with an Oral Multi-Component Drug Containing GS-441524. Viruses 2021, 13, 2228. [Google Scholar] [CrossRef]
- Krentz, D.; Zwicklbauer, K.; Felten, S.; Bergmann, M.; Dorsch, R.; Hofmann-Lehmann, R.; Meli, M.L.; Spiri, A.M.; von Both, U.; Alberer, M.; et al. Clinical Follow-Up and Postmortem Findings in a Cat That Was Cured of Feline Infectious Peritonitis with an Oral Antiviral Drug Containing GS-441524. Viruses 2022, 14, 2040. [Google Scholar] [CrossRef]
- Dickinson, P.J.; Bannasch, M.; Thomasy, S.M.; Murthy, V.D.; Vernau, K.M.; Liepnieks, M.; Montgomery, E.; Knickelbein, K.E.; Murphy, B.; Pedersen, N.C. Antiviral treatment using the adenosine nucleoside analogue GS-441524 in cats with clinically diagnosed neurological feline infectious peritonitis. J. Vet. Intern. Med. 2020, 34, 1587–1593. [Google Scholar] [CrossRef]
- Addie, D.D.; Silveira, C.; Aston, C.; Brauckmann, P.; Covell-Ritchie, J.; Felstead, C.; Fosbery, M.; Gibbins, C.; Macaulay, K.; McMurrough, J.; et al. Alpha-1 Acid Glycoprotein Reduction Differentiated Recovery from Remission in a Small Cohort of Cats Treated for Feline Infectious Peritonitis. Viruses 2022, 14, 744. [Google Scholar] [CrossRef]
- Tasker, S.; Addie, D.D.; Egberink, H.; Hofmann-Lehmann, R.; Hosie, M.J.; Truyen, U.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Feline Infectious Peritonitis: European Advisory Board on Cat Diseases Guidelines. Viruses 2023, 15, 1847. [Google Scholar] [CrossRef]
- Bauer, B.S.; Kerr, M.E.; Sandmeyer, L.S.; Grahn, B.H. Positive immunostaining for feline infectious peritonitis (FIP) in a Sphinx cat with cutaneous lesions and bilateral panuveitis. Vet. Ophthalmol. 2013, 16 (Suppl. 1), 160–163. [Google Scholar] [CrossRef]
- Cannon, M.J.; Silkstone, M.A.; Kipar, A.M. Cutaneous lesions associated with coronavirus-induced vasculitis in a cat with feline infectious peritonitis and concurrent feline immunodeficiency virus infection. J. Feline Med. Surg. 2005, 7, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Trotman, T.K.; Mauldin, E.; Hoffmann, V.; Del Piero, F.; Hess, R.S. Skin fragility syndrome in a cat with feline infectious peritonitis and hepatic lipidosis. Vet. Dermatol. 2007, 18, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Montali, R.J.; Strandberg, J.D. Extraperitoneal Lesions in Feline Infectious Peritonitis. Vet. Pathol. 1972, 9, 109–121. [Google Scholar] [CrossRef]
- Kotsyumbas, G.I.; Khalaniia, M.R. Pathomorphology of cats with myocardial infectious peritonitis. Sci. Messenger LNU Vet. Med. Biotechnol. Ser. Vet. Sci. 2019, 21, 177–184. [Google Scholar] [CrossRef]
- Ernandes, M.A.; Cantoni, A.M.; Armando, F.; Corradi, A.; Ressel, L.; Tamborini, A. Feline coronavirus-associated myocarditis in a domestic longhair cat. J. Feline Med. Surg. Open Rep. 2019, 5, 2055116919879256. [Google Scholar] [CrossRef]
- Guarnieri, C.; Bertola, L.; Ferrari, L.; Quintavalla, C.; Corradi, A.; Di Lecce, R. Myocarditis in an FIP-Diseased Cat with FCoV M1058L Mutation: Clinical and Pathological Changes. Animals 2024, 14, 1673. [Google Scholar] [CrossRef]
- Yoshida, T.; Ichikawa, N.; Koike, M.; Hirokawa, T.; Sasaoka, K.; Machida, N.; Taguchi, M. Two feline cases of dilated cardiomyopathy-like disease caused by feline infectious peritonitis virus. J. Anim. Clin. Med. 2016, 25, 148–152. [Google Scholar]
- Murphy, B.G.; Castillo, D.; Neely, N.E.; Kol, A.; Brostoff, T.; Grant, C.K.; Reagan, K.L. Serologic, Virologic and Pathologic Features of Cats with Naturally Occurring Feline Infectious Peritonitis Enrolled in Antiviral Clinical Trials. Viruses 2024, 16, 462. [Google Scholar] [CrossRef] [PubMed]
- Repyak, K.; Atiee, G.; Cook, A.; Bryan, L.; Gremillion, C. Thoracic radiographic findings in cats with feline infectious peritonitis. J. Feline Med. Surg. 2025, 27, 1098612X241309823. [Google Scholar] [CrossRef]
- Lampejo, T.; Durkin, S.M.; Bhatt, N.; Guttmann, O. Acute myocarditis: Aetiology, diagnosis and management. Clin. Med. 2021, 21, e505–e510. [Google Scholar] [CrossRef]
- Wood, J.; Reagan, K.L.; Gunther-Harrington, C.; Sykes, J.E. Identification of Streptococcus suis in a cat with endomyocarditis. J. Feline Med. Surg. Open Rep. 2021, 7, 20551169211012346. [Google Scholar] [CrossRef]
- Joseph, J.; Oxford, E.; Santilli, R. Transient myocardial thickening in a Bartonella henselae–positive cat. J. Vet. Cardiol. 2018, 20, 198–203. [Google Scholar] [CrossRef]
- Varanat, M.; Broadhurst, J.; Linder, K.E.; Maggi, R.G.; Breitschwerdt, E.B. Identification of Bartonella henselae in 2 Cats with Pyogranulomatous Myocarditis and Diaphragmatic Myositis. Vet. Pathol. 2012, 49, 608–611. [Google Scholar] [CrossRef]
- Vercelli, A.; Cicero, E.L.; Pazzini, L. Salmonella typhimurium Endocarditis and Myocarditis in a Cat. Case Rep. Vet. Med. 2019, 2019, 7390530. [Google Scholar] [CrossRef]
- Kegler, K.; Nufer, U.; Alic, A.; Posthaus, H.; Olias, P.; Basso, W. Fatal infection with emerging apicomplexan parasite Hepatozoon silvestris in a domestic cat. Parasites Vectors 2018, 11, 428. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Kennedy, F.A.; Murphy, A.J.; Soliman, M.; Mansfield, L.S. Sarcocystosis of Sarcocystis felis in cats. J. Egypt Soc. Parasitol. 2006, 36, 1071–1085. [Google Scholar]
- Romito, G.; Fracassi, F.; Cipone, M. Transient myocardial thickening associated with acute myocardial injury and congestive heart failure in two Toxoplasma gondii-positive cats. J. Feline Med. Surg. Open Rep. 2022, 8, 20551169221131266. [Google Scholar] [CrossRef]
- Simpson, K.E.; Devine, B.C.; Gunn-Moore, D. Suspected toxoplasma—Associated myocarditis in a cat. J. Feline Med. Surg. 2005, 7, 203–208. [Google Scholar] [CrossRef]
- Rolim, V.M.; Casagrande, R.A.; Wouters, A.T.B.; Driemeier, D.; Pavarini, S.P. Myocarditis caused by Feline Immunodeficiency Virus in Five Cats with Hypertrophic Cardiomyopathy. J. Comp. Pathol. 2016, 154, 3–8. [Google Scholar] [CrossRef]
- Chetboul, V.; Foulex, P.; Kartout, K.; Klein, A.M.; Sailleau, C.; Dumarest, M.; Delaplace, M.; Gouilh, M.A.; Mortier, J.; Le Poder, S. Myocarditis and Subclinical-Like Infection Associated with SARS-CoV-2 in Two Cats Living in the Same Household in France: A Case Report with Literature Review. Front. Veter. Sci. 2021, 8, 748869. [Google Scholar] [CrossRef]
- Morris, S.B.; Schwartz, N.G.; Patel, P.; Abbo, L.; Beauchamps, L.; Balan, S.; Lee, E.H.; Paneth-Pollak, R.; Geevarughese, A.; Lash, M.K.; et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection–United Kingdom and United States, March–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1450–1456. [Google Scholar] [CrossRef]
- Thayer, V.; Gogolski, S.; Felten, S.; Hartmann, K.; Kennedy, M.; A Olah, G. 2022 AAFP/EveryCat Feline Infectious Peritonitis Diagnosis Guidelines. J. Feline Med. Surg. 2022, 24, 905–933. [Google Scholar] [CrossRef]
- Hartmann, K. Feline infectious peritonitis. Vet. Clin. Small Anim. Pract. 2005, 35, 39–79. [Google Scholar] [CrossRef]
- Thomas, W.P.; Gaber, C.E.; Jacobs, G.J.; Kaplan, P.M.; Lombard, C.W.; Vet, M.; Moise, N.S.; Moses, B.L. Recommendations for Standards in Transthoracic Two-Dimensional Echocardiography in the Dog and Cat. J. Vet. Intern. Med. 1993, 7, 247–252. [Google Scholar] [CrossRef]
- Häggström, J.; Fuentes, V.L.; Wess, G. Screening for hypertrophic cardiomyopathy in cats. J. Vet. Cardiol. 2015, 17, S134–S149. [Google Scholar] [CrossRef]
- Luis Fuentes, V.; Abbott, J.; Chetboul, V.; Côté, E.; Fox, P.R.; Häggström, J.; Kittleson, M.D.; Schober, K.; Stern, J.A. Acvim consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J. Vet. Intern. Med. 2020, 34, 1062–1077. [Google Scholar] [CrossRef]
- Häggström, J.; Andersson, A.O.; Falk, T.; Nilsfors, L.; OIsson, U.; Kresken, J.G.; Höglund, K.; Rishniw, M.; Tidholm, A.; Ljungvall, I. Effect of body weight on echocardiographic measurements in 19,866 pure-bred cats with or without heart disease. J. Vet. Intern. Med. 2016, 30, 1601–1611. [Google Scholar] [CrossRef]
- Rishniw, M.; Erb, H.N. Evaluation of four 2-dimensional echocardiographic methods of assessing left atrial size in dogs. J. Vet. Intern. Med. 2000, 14, 429–435. [Google Scholar]
- Duler, L.; Scollan, K.F.; LeBlanc, N.L. Left atrial size and volume in cats with primary cardiomyopathy with and without congestive heart failure. J. Vet. Cardiol. 2019, 24, 36–47. [Google Scholar] [CrossRef]
- Schober, K.E.; Chetboul, V. Echocardiographic evaluation of left ventricular diastolic function in cats: Hemodynamic determinants and pattern recognition. J. Vet. Cardiol. 2015, 17, S102–S133. [Google Scholar] [CrossRef]
- Visser, L.; Sloan, C.; Stern, J. Echocardiographic Assessment of Right Ventricular Size and Function in Cats with Hypertrophic Cardiomyopathy. J. Vet. Intern. Med. 2017, 31, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Hertzsch, S.; Wess, G. Two-dimensional speckle tracking-derived global longitudinal strain in healthy doberman pinschers: Method evaluation, variability, and reference values. J. Vet. Cardiol. 2023, 45, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Schoebel, J.; Friederich, J.; Eberhard, J.; Feldhuetter, E.; Wess, G. Reference intervals of two-dimensional speckle tracking–derived endocardial global longitudinal strain analysis in 132 healthy cats. J. Vet. Cardiol. 2024, 57, 39–46. [Google Scholar] [CrossRef]
- Côté, E. Feline congestive heart failure: Current diagnosis and management. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 1055–1064. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K. Infective Endocarditis in Dogs: Diagnosis and Therapy. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 665–684. [Google Scholar] [CrossRef]
- Palerme, J.S.; Jones, A.E.; Ward, J.L.; Balakrishnan, N.; Linder, K.E.; Breitschwerdt, E.B.; Keene, B.W. Infective endocarditis in 13 cats. J. Vet. Cardiol. 2016, 18, 213–225. [Google Scholar] [CrossRef]
- Lakhdhir, S.; Viall, A.; Alloway, E.; Keene, B.; Baumgartner, K.; Ward, J. Clinical presentation, cardiovascular findings, etiology, and outcome of myocarditis in dogs: 64 cases with presumptive antemortem diagnosis (26 confirmed postmortem) and 137 cases with postmortem diagnosis only (2004–2017). J. Vet. Cardiol. 2020, 30, 44–56. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Rush, J.E.; Keene, B.W.; Eicker, S.W. Endomyocardial biopsy in cats. Am. J. Vet. Res. 1990, 51, 1765–1768. [Google Scholar] [CrossRef]
- Langhorn, R.; Willesen, J. Cardiac Troponins in Dogs and Cats. J. Vet. Intern. Med. 2015, 30, 36–50. [Google Scholar] [CrossRef]
- Szaluś-Jordanow, O.; Stabińska-Smolarz, M.; Czopowicz, M.; Moroz, A.; Mickiewicz, M.; Łobaczewski, A.; Chrobak-Chmiel, D.; Kizerwetter-Świda, M.; Rzewuska, M.; Sapierzyński, R.; et al. Focused Cardiac Ultrasound Examination as a Tool for Diagnosis of Infective Endocarditis and Myocarditis in Dogs and Cats. Animals 2021, 11, 3162. [Google Scholar] [CrossRef]
- Oyama, M.A. Using Cardiac Biomarkers in Veterinary Practice. Clin. Lab. Med. 2015, 35, 555–566. [Google Scholar] [CrossRef]
- Fox, P.R.; Oyama, M.A.; Reynolds, C.; Rush, J.E.; DeFrancesco, T.C.; Keene, B.W.; Atkins, C.E.; MacDonald, K.A.; Schober, K.E.; Bonagura, J.D.; et al. Utility of plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) to distinguish between congestive heart failure and non-cardiac causes of acute dyspnea in cats. J. Vet. Cardiol. 2009, 11 (Suppl. 1), S51–S61. [Google Scholar] [CrossRef]
- Wess, G.; Daisenberger, P.; Mahling, M.; Hirschberger, J.; Hartmann, K. Utility of measuring plasma N-terminal pro-brain natriuretic peptide in detecting hypertrophic cardiomyopathy and differentiating grades of severity in cats. Vet. Clin. Pathol. 2011, 40, 237–244. [Google Scholar] [CrossRef]
- Hezzell, M.J.; Rush, J.E.; Humm, K.; Rozanski, E.A.; Sargent, J.; Connolly, D.J.; Boswood, A.; Oyama, M.A. Differentiation of Cardiac from Noncardiac Pleural Effusions in Cats Using Second-Generation Quantitative and Point-of-Care Nt-Probnp Measurements. J. Vet. Intern. Med. 2016, 30, 536–542. [Google Scholar] [CrossRef]
- Lu, T.L.; Côté, E.; Kuo, Y.W.; Wu, H.H.; Wang, W.Y.; Hung, Y.W. Point-of-Care N-Terminal Pro B-Type Natriuretic Peptide Assay to Screen Apparently Healthy Cats for Cardiac Disease in General Practice. J. Vet. Intern. Med. 2021, 35, 1663–1672. [Google Scholar] [CrossRef]
- Shu, H.; Zhao, C.; Wang, D.W. Understanding COVID-19-related myocarditis: Pathophysiology, diagnosis, and treatment strategies. Cardiol. Plus 2023, 8, 72–81. [Google Scholar] [CrossRef]
- Bavishi, C.; Bonow, R.O.; Trivedi, V.; Abbott, J.D.; Messerli, F.H.; Bhatt, D.L. Special Article–Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog. Cardiovasc. Dis. 2020, 63, 682–689. [Google Scholar] [CrossRef]
- Dmytrenko, O.; Lavine, K.J. Cardiovascular Tropism and Sequelae of SARS-CoV-2 Infection. Viruses 2022, 14, 1137. [Google Scholar] [CrossRef]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T., Jr.; Chahal, C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020, 17, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Esfandiarei, M.; McManus, B.M. Molecular Biology and Pathogenesis of Viral Myocarditis. Annu. Rev. Pathol. Mech. Dis. 2007. [Google Scholar] [CrossRef] [PubMed]
- Carvallo, F.R.; Martins, M.; Joshi, L.R.; Caserta, L.C.; Mitchell, P.K.; Cecere, T.; Hancock, S.; Goodrich, E.L.; Murphy, J.; Diel, D.G. Severe SARS-CoV-2 Infection in a Cat with Hypertrophic Cardiomyopathy. Viruses 2021, 13, 1510. [Google Scholar] [CrossRef] [PubMed]
- Ferasin, L.; Fritz, M.; Ferasin, H.; Becquart, P.; Corbet, S.; Gouilh, M.A.; Legros, V.; Leroy, E.M. Infection with SARS-CoV-2 variant B.1.1.7 detected in a group of dogs and cats with suspected myocarditis. Vet. Rec. 2021, 189, e944. [Google Scholar] [CrossRef]
- Malbon, A.J.; Fonfara, S.; Meli, M.L.; Hahn, S.; Egberink, H.; Kipar, A. Feline Infectious Peritonitis as a Systemic Inflammatory Disease: Contribution of Liver and Heart to the Pathogenesis. Viruses 2019, 11, 1144. [Google Scholar] [CrossRef]
- Matos, J.N.; Pereira, N.; Glaus, T.; Wilkie, L.; Borgeat, K.; Loureiro, J.; Silva, J.; Law, V.; Kranjc, A.; Connolly, D.; et al. Transient Myocardial Thickening in Cats Associated with Heart Failure. J. Vet. Intern. Med. 2017, 32, 48–56. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).