Simple Summary
The post-weaning period in piglet production is associated with bacterial disorders and challenges such as diarrhea. Bioactive plant compounds are promising alternatives to traditional antimicrobial growth promoters, although translating lab studies to field conditions remains challenging. This study investigates the antimicrobial properties of bioactive plant compounds to tackle these issues. Carvacrol, eugenol, garlic oil, star anise oil, and tea tree oil were screened for their antimicrobial activities, effects on biofilm formation, bacterial communication, and adhesion to piglet intestinal mucus. Based on the results, two prototypes were created. Prototype two, containing carvacrol, eugenol, and star anise oil, showed stronger antimicrobial activity and better inhibition of biofilm formation and bacterial communication than prototype one, which contained garlic oil and tea tree oil. In part two of the study, 1000 post-weaning piglets were divided into four groups: the control group and three treatment groups receiving diets with prototype one, prototype two, or zinc oxide. Prototype two and zinc oxide improved body weight, daily gain, feed efficiency, and fecal scores compared to the control. The results of this study suggest that the compounds in prototype two can support piglets by likely influencing intestinal bacteria and demonstrate the potential of combining lab tests to develop effective feed additives.
Abstract
Finding effective alternatives to antibiotics and zinc oxide in livestock feed remains challenging, but phytogenic compounds show promising potential. In the first part of the present study, the in vitro antimicrobial activities of carvacrol, eugenol, garlic oil, star anise oil, and tea tree oil as well as their effects on the biofilm formation of two Escherichia coli field isolates, quorum sensing of Chromobacterium violaceum, and the adhesion of an E. coli field isolate to piglets’ small intestinal mucus were determined. Based on these results, two prototypes were formulated. Phytogenic feed additive (PFA) Core 2, containing carvacrol, eugenol, and star anise oil, showed stronger in vitro antimicrobial activity, inhibition of biofilm formation, and quorum sensing than PFA Core 1, which was mainly composed of garlic oil and tea tree oil. In the second part of the present study, 1000 post-weaning piglets were divided into four groups receiving a control or diets with either PFA Core 1, PFA Core 2, or zinc oxide. Only PFA Core 2 and zinc oxide significantly improved body weight, daily gain, feed efficiency, and fecal scores compared with the control, while PFA Core 1 increased the feed efficiency and fecal scores. The results show that feed additives based on carvacrol and eugenol can improve the growth performance of post-weaning piglets and reduce the incidence of diarrhea, possibly by influencing detrimental bacteria. Furthermore, the present study demonstrates the potential of combinations of in vitro assays to support the development of effective feed additives.
1. Introduction
For decades, antibiotics have been used by the livestock industry as in-feed antimicrobial growth promoters (AGPs). But rising concerns regarding the development of bacterial resistance to clinically important antibiotics have intensified the efforts to reduce their routine application in recent years. In this context, several countries have already banned the use of antibiotics as antimicrobial growth promoters [1], including the European Union in 2006 [2]. Because of this ban, zinc oxide (ZnO) was used as a replacement throughout Europe, especially in pig feed, to improve the animal’s growth performance and reduce the incidence of post-weaning diarrhea (PWD). As a growth promotor, ZnO is applied in pharmacological doses of 2000 to 4000 ppm, resulting in a correspondingly high accumulation in manure and the environment [3]. Therefore, the EU has restricted the application of ZnO beginning in 2022 [4]. These regulatory provisions, combined with an increasing pressure from consumers demanding more “natural” solutions, necessitates the search for alternatives to traditional AGPs in order to maintain efficient farming in the future. Especially promising in this regard are plant-derived bioactive compounds (hereinafter referred to as phytogenics).
Phytogenics are widely used in swine diets to influence palatability. Phytogenic flavors such as anise and garlic have been shown to positively impact feed intake in piglets [5]. Many phytogenics are also known to have bactericidal effects [6]. Furthermore, specific phytogenics have been shown to affect bacterial behavior and pathogenicity at concentrations below the minimum inhibitory concentration (sub-MICs) [7,8,9]. Several of these effects are attributed to the ability of specific phytogenics to interfere with the bacterial communication system quorum sensing [9]. Quorum sensing is based on the detection of secreted signal molecules, which accumulate in the environment in a cell density-dependent manner and are involved in the regulation of a wide variety of bacterial behaviors including bioluminescence, motility, virulence factor expression, biofilm formation, and pigment production [10,11,12,13,14]. Phytogenics that have been shown to influence quorum sensing include single substances like carvacrol [8], eugenol [15], or trans-anethole [16], and essential oils such as garlic oil [17] or tea tree oil [7]. Besides their various effects on pure bacterial cultures, challenge studies on the model organism Caenorhabditis elegans [18,19] and human cell lines [20] have demonstrated that phytogenics can also interfere with the quorum sensing of pathogenic bacteria in these models, leading to attenuated colonization and increased survival. Therefore, it is assumed that the application of phytogenics as feed additives in livestock could reduce the expression of bacterial virulence factors by interfering with quorum sensing signaling. This would likely prevent or reduce the burden of bacterial infections on the animal host while minimizing the selective pressure to develop resistance against the applied compound or treatment [21,22]. Livestock animals could benefit from these effects particularly during critical phases of their development, such as piglets in the post-weaning period [3].
The development of phytogenics-based feed additives for livestock animals, however, faces major challenges since severe limitations exist in terms of in vivo trial capacities as it is practically not feasible to test compounds in studies covering detailed dose–response relationships and possible combinations of different phytogenics. The sheer number of animals such trials would require contradicts the ethical responsibility and the “Replacement, Reduction, and Refinement” (3Rs) framework. The 3Rs aim to minimize the reliance on animal trials while maintaining robust scientific rigor [23]. Addressing these challenges requires alternative approaches to livestock feeding trials, such as leveraging advanced in vitro models and computational simulations.
Taking the above considerations into account and recognizing the limitations, the present study investigated the impact of carvacrol, eugenol, garlic oil, star anise oil, and tea tree oil as individual substances and as main constituents of two phytogenic blends as well as ZnO as a reference compound on three different bacterial models for quorum sensing-related traits. Subsequently, the two phytogenic blends were used as feed additives and studied in a feeding trial to evaluate their in vivo potential to reduce the incidence of PWD and improve the growth performance of piglets during the critical post-weaning phase in comparison to ZnO. Therefore, the hypothesis in the present study is that these two prototypes, developed based on the consistent effects of phytogenic compounds observed in a series of in vitro assays, will demonstrate distinct and corresponding responses in the piglet model. This will help enhance the understanding of how these models can predict in vivo effects.
2. Materials and Methods
2.1. Bacterial Strains and Test Compounds
Chromobacterium violaceum DSM 30191 was purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany) and cultured in trypto-casein soy broth (Biokar Diagnostics, Allone, France; TSB) at 30 °C under aerobic conditions.
Escherichia coli field isolates O88:H8 and O143:H4 (both isolated from swine) were provided by the veterinary pathology department of AGES GmbH Linz (Linz, Austria) and serotyped by the national reference laboratory for E. coli of AGES GmbH Graz (Graz, Austria). Both strains were classified as pathogenic due to their high-level growth in pure culture in various organs and their mucoid or hemolytic properties. For the mucus adherence assay, a F4-fimbriated E. coli strain isolated in Finland was obtained as a field isolate by Alimetrics Research Ltd. (Espoo, Finland). The E. coli field isolates were cultured under aerobic conditions at 37 °C in TSB that was supplemented with 2 g/L glucose (Carl Roth GmbH + Co. KG, Karlsruhe, Germany; TSB+) for biofilm assays.
Zinc oxide for the bacterial assays was purchased by Auhof Apotheke (Linz, Austria). Carvacrol, eugenol, garlic oil, star anise oil, and tea tree oil as well as “PFA Core 1” and “PFA Core 2” were provided by Delacon Biotechnik GmbH (Engerwitzdorf, Austria). “PFA Core 1” contained 65.8% tea tree oil, 32.8% garlic oil, and 1.4% citrus oil. “PFA Core 2” was composed of 40% carvacrol, 40% eugenol, and 20% star anise oil. Both essential oil cores were mixed with a carrier matrix. For individual essential oils, the main compounds are trans-anethole for star anise oil, terpinene-4-ol and γ-terpinene for tea tree oil, diallyl disulfide and diallyl trisulfide for garlic oil, and D-limonene for citrus oil.
2.2. Determination of Minimum Inhibitory Concentrations
The minimum inhibitory concentration (MIC) in each assay was optically determined as the lowest concentration of the test compound that completely inhibits visible growth (no obvious cell pellet or turbidity) after the incubation period (18 h at 30 °C for C. violaceum, 18 h at 37 °C for E. coli O88:H8 and O143:H4, and 24 h at 37 °C for the F4-fimbriated E. coli strain). The median value of all replicates was defined as the MIC value of the respective test compound.
The F4-fimbriated E. coli strain was only tested with carvacrol and tea tree oil. For MIC evaluation, both substances were diluted in a two-fold series in TSB containing 2% Tween 80 (Sigma-Aldrich, St. Louis, MO, USA).
2.3. Quorum Sensing Inhibition Assay (Chromobacterium violaceum)
The influence of the phytogenic test compounds on C. violaceum was assessed in concentrations ranging from 10,000 ppm to 5 ppm. Violacein production was quantified based on the protocol of Skogman et al. [24]. Serial dilutions ranging from 20,000 ppm to 10 ppm were prepared from stock solutions (200,000 ppm) of the phytogenic test compounds in ≥99.8% ethanol (VWR International GmbH, Vienna, Austria). Four technical replicates of each concentration (50 µL in each well) were arranged vertically in Nunc® polystyrene 96-well microtiter plates (Thermo Scientific, Waltham, MA, USA). An overnight culture of C. violaceum in 10 mL TSB was diluted to approximately 1–2 × 106 CFU/mL in fresh TSB, and aliquots of 50 µL were added to each test well and four wells containing only medium, serving as positive growth control without test compounds. An additional four wells containing only TSB were used as negative (sterility) controls. The starting bacterial concentration was verified using the colony count method. The microtiter plates as well as the colony count plates were incubated for 18 h at 30 °C. After the incubation period, MIC values were determined before the cells and violacein pigment were pelleted by centrifugation at 3000 rpm for 10 min. The supernatant was removed from the wells by pipetting and 200 µL ≥ 99.8% ethanol (VWR International GmbH, Vienna, Austria) was added to each well. The plates were sealed with Microseal ‘B’ PCR Plate Sealing Film (Bio-Rad, Hercules, CA, USA) and incubated overnight in the dark to allow the pelleted violacein pigment to dissolve. The next day, the plates were centrifuged at 3000 rpm for 10 min to sediment the decolored cells. Subsequently, 100 µL of the violacein-stained supernatant was transferred to a new microtiter plate and the absorbance was measured at 570 nm using a PHOmo microplate reader (Anthos Mikrosysteme GmbH, Friesoythe, Germany).
2.4. Biofilm Inhibition Assay
The effect of the phytogenic test compounds on the biofilm formation of E. coli O88:H8 and O143:H4 was assessed in a broth micro-dilution assay followed by crystal violet staining according to Axmann et al. [25], with minor modifications. Briefly, phytogenic test compounds were serially diluted in TSB+ and arranged vertically in 96-well microtiter plates, as described in Section 2.3. The outer columns of the microtiter plates were filled with sterile TSB+ to prevent evaporation from the central wells. Overnight cultures of the respective E. coli in TSB+ were adjusted to approximately 2 × 106 CFU/mL in fresh TSB+ and 50 µL of this dilution was placed in each test well. Four wells containing either only bacterial culture without phytogenic test compounds or sterile TSB+ medium served as positive or negative control, respectively. The plates were incubated under aerobic conditions for 18 h at 37 °C.
The antimicrobial activity of the phytogenic test compounds was evaluated as described in Section 2.2. After removal of the culture medium, the microtiter plates were washed twice with deionized H2O (diH2O) to remove the unattached cells and media components and air-dried for 30 min. The attached biomass was stained with 130 µL of a 0.1% (w/v) crystal violet solution (Alfa Aesar by Thermo Fisher GmbH, Kandel, Germany) in diH2O for 20 min at room temperature. The plates were then washed three times with diH2O and left to air-dry completely. The adherent dye was solubilized with 130 µL 30% (v/v) acetic acid (Merck KgaA, Darmstadt, Germany) and transferred to a new microtiter plate. Absorbance was measured at 570 nm with a PHOmo microplate reader (Anthos Mikrosysteme GmbH, Friesoythe, Germany). The mean of the four replicates was calculated followed by subtraction of the negative control measurements, and the results were expressed as a percentage biofilm relative to the positive control. Each test compound was assayed three to four times. In this study, the entirety of adherent cell mass that was stained by the crystal violet solution is referred to as biofilm without distinction of the maturation stage.
2.5. In Vitro Mucus Adherence Assay
In preparation of the mucus adhesion assay, an overnight culture of F4-fimbriated E. coli was grown at 37 °C in TSB without test compounds. From this seed culture, two successive overnight cultivations were performed for each treatment using 10% inoculum. This assay was only performed with carvacrol and tea tree oil in concentrations of 1/8th of the MIC (320 ppm for tea tree oil and 20 ppm for carvacrol). In addition, a second concentration of tea tree oil at 1/80th of the MIC was assayed to achieve a concentration level comparable to the tested carvacrol concentration. All treatments were cultured at 37 °C in three replicate vials. The turbidity of the cultures was followed by measuring the absorbance at 600 nm to ensure that the cell densities were close to those of the control culture without a test product. On the third day, each culture was further diluted tenfold in growth medium containing the respective test product at its specific concentration. Tritium-labeled thymidine was introduced in each vial to radioactively label the E. coli cells. For two hours, the culture was incubated at 37 °C. During incubation, the bacteria were taking up the radioactive nucleotide and incorporated it in their DNA. The radioactive-labeled bacteria were harvested by gentle centrifugation (3000× g for 5 min) and re-suspended in an equal volume of HEPES-Hank’s buffer (Nordic Biosite, Täby, Sweden). The suspension was used immediately for the adherence study.
For the in vitro adherence study, authentic mucus samples were collected from five piglets, pooled, and aliquoted. The aliquots were stored at −80 °C until further use in the study. To prepare mucus plates, the mucus samples were diluted with a coating buffer and cleared off all insoluble particles such as epithelial cells and bacteria by high-speed centrifugation. Mucus preparations were diluted to the final protein concentration of 0.1 mg/mL. These preparations were then used to coat microtiter wells that bind mucus irreversibly. The coated microtiter plates were washed twice with 300 mL of HEPES-Hank’s buffer to wash off the unbound mucus components before labeled E. coli cells were introduced (nine replicate wells per treatment) and incubated for 1 h at 37 °C. The reaction liquid was discarded and the wells were washed twice with 300 μL of HEPES-Hank’s buffer to remove unbound radioactive bacteria. Then, 250 μL of scintillation liquid was added and the radioactivity in each reaction well was measured. Additionally, the total radioactivity of each labeled E. coli culture used in the adherence study was measured to be able to determine the proportion of adhered cells. Hence, the remaining radioactivity in the wells was proportional to the number of adhered pathogenic bacteria.
2.6. Animals, Diets, and In Vivo Trial Design
A total of one thousand healthy post-weaning piglets (500 ♂; 500 ♀; DanBred × Duroc), with an age at weaning of 25 ± 2 days, were allocated to four treatments and 100 pens with solid partitions and slotted floor in total (5 ♂ & 5 ♀ per pen, 25 pens per treatment). The average housing temperature was kept at about 30 °C during the first week after weaning and was gradually reduced by 1.8 °C per week down to 22 °C from day 53 of age onwards. Post-weaning piglets had ad libitum access to feed provided in mash form and water supplied by drinking bowls throughout the experiment. Prior to weaning, piglets had access to creep feed formulated without added antibiotics, organic acids, polysaccharides, enzymes, yeast/egg products, porcine plasma, or zootechnical feed additives. The concentrations of zinc and copper were maintained at adequate (nutritional) levels, but not in excess, to avoid the potentially confounding effects of these additives.
The four dietary treatments included a negative control group receiving an unsupplemented basal diet (negative control; NC), a positive control group whose diet was supplemented with ZnO at a pharmacological level (3 kg/t of feed; ZnO), and the treatment groups PFA 1 and PFA 2 that were fed basal diets supplemented with PFA 1 or PFA 2, respectively, at 1 kg/t of feed each.
Phytogenic prototype PFA 1 consisted of wheat bran, limestone, and a blend of turmeric and fenugreek seed powder, with the addition of PFA Core 1. Phytogenic prototype PFA 2 was composed of limestone, a blend of turmeric and fenugreek seed powder, and PFA Core 2. Both PFA cores were added at the same inclusion level of 50 g/kg to the respective prototype.
The 42-day observation period was divided into two feeding periods: a starter period of two weeks (from 25 to 38 days of age) and a subsequent grower period of four weeks (from 39 to 66 days of age). The basal diets for each period were formulated as recommended by the Society of Nutrition Physiology [26], with the exception of zinc, and are presented in Table 1. Delacon Biotechnik GmbH (Austria) supplied the phytogenic prototypes. Zinc oxide (Spezialfutter Neuruppin GmbH & Co. KG, Neuruppin, Germany) was supplemented at the expense of Tixosil (>97% silicon dioxide), while PFA 1 and PFA 2 were included at the expense of wheat bran. This substitution was made to leverage the prebiotic potential of wheat bran and the carrier components of the phytogenics, in contrast to the nutritionally inert nature of Tixosil and ZnO.

Table 1.
Piglet feed composition and calculated nutrients of starter and grower diets.
2.7. Piglet Growth Performance and Determination of Diarrhea Score
All piglets were observed twice daily for any abnormalities, abnormal behavior, and clinical signs of sickness throughout the 42 d experimental period.
Pen body weights (BWs) were recorded weekly, as was the amount of feed supplied to each pen during the preceding week. Piglet BW gain was calculated by dividing the mean BW per pen at the end of each period by the mean BW per pen at the start of each period and number of piglets per pen. Feed consumption per piglet was estimated as the total feed supplied per pen and period, corrected for dispersed/leftover feed and the number of piglets per pen. The feed-to-gain ratio (FCR) was calculated from the relationship of the weekly corrected feed intake and BW gain per piglet for this period. The diarrhea scores were determined on a pen-basis using a scale from 0 to 3 (0 = normal; 1 = soft feces; 2 = mild diarrhea; 3 = severe diarrhea).
2.8. Statistical Analysis
Chromobacterium violaceum violacein production and E. coli biofilm formation was analyzed using the Glimmix procedure of SAS (SAS 9.4, SAS Institute Inc., Cary, NC, USA), with substance concentration as a fixed effect and the day of measurement (biological replicate) as a random effect. Data from the mucus adherence assay were analyzed with the Student’s t-test. A comprehensive overview of the C. violaceum and E. coli results is given in Figure 1, with a detailed presentation of the results supplied in Supplementary Figures S1–S8.
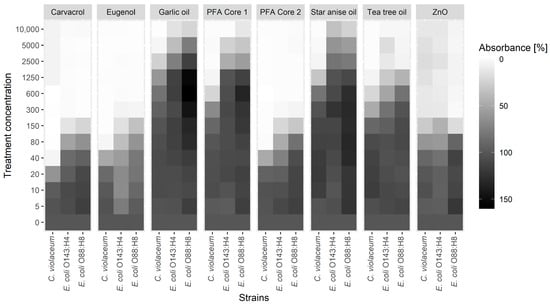
Figure 1.
Impact of carvacrol, eugenol, garlic oil, star anise oil, tea tree oil, phytogenic feed additive (PFA) Core 1, PFA Core 2, and ZnO on violacein production of C. violaceum and biofilm formation of E. coli strains O143:H4 and O88:H8. Absorbance values for each concentration were normalized to the respective positive control (containing no phytogenic compound).
The in vivo study design was a random complete block design, with weaning batch as the blocking factor and pen as the experimental unit for statistical purposes for recoded parameters. Growth performance was analyzed using the Glimmix procedure of SAS. Treatment was used as a fixed effect and the results were presented as the least square means with standard error of the mean. Body weight day 1 was used as the co-factor for analysis of BW at day 15, BW at day 42, average daily gain (ADG), and average daily feed intake (ADFI).
Fecal consistency was assessed using a generalized linear mixed model (GLMM) with binomial distribution, fitted via the glmer function in R (v4.1.2). The original 4-point fecal score (0 = normal; 1 = soft feces; 2 = mild diarrhea; 3 = severe diarrhea) was recorded into a binary outcome: 0 = normal feces, 1 = altered feces. The model included the treatment, period, as well as their interaction as fixed effects, and weaning batch as a random effect. Type III tests with Kenward–Roger approximation were performed using the car package.
3. Results
3.1. Effect of Selected Phytogenics on Bacterial Growth, Violacein Production, and Biofilm Formation
In the present study, the influence of ZnO, two phytogenic prototype essential oil cores (PFA Core 1 and PFA Core 2), and their respective main compounds garlic oil and tea tree oil, as well as carvacrol, eugenol, and star anise oil, was initially investigated on bacterial susceptibility, biofilm formation of E. coli strains O88:H8 and O143:H4, and violacein production of C. violaceum. Treatment with each substance exerted a similar effect on the growth of all bacterial strains (Table 2). In particular, carvacrol and eugenol showed very strong bactericidal effects (MIC of 150–600 ppm), whereas tea tree oil exhibited substantial lower antimicrobial activities against all three E. coli strains (MIC of 2560–10,000 ppm) and C. violaceum (MIC of 2500 ppm). Similarly, garlic oil and star anise oil only inhibited the growth of C. violaceum at 10,000 and 5000 ppm, respectively, while the growth of both tested E. coli strains was not inhibited. Zinc oxide did not inhibit the growth of the E. coli and C. violaceum strains up to the highest tested concentration of 10,000 ppm. The MIC values of substance combinations PFA Core 1 and PFA Core 2 were similar to those of the respective individual substances.

Table 2.
Minimum inhibitory concentrations of tested substances [ppm] against three E. coli strains and C. violaceum.
The observed values for biofilm formation and violacein production at each treatment concentration were compared to an untreated positive control to identify potential sub-MIC effects. Both parameters decreased in a dose-dependent manner at sub-MIC levels in response to each tested substance (Figure 1 and Figures S1–S8). Eugenol and carvacrol proved to be the most potent of the substances tested in reducing biofilm formation of the E. coli strains. Interestingly, although carvacrol showed identical effects on the biofilm formation of both E. coli strains, eugenol elicited a different response in the two strains, with a concentration of 10 ppm disturbing biofilm formation in E. coli O143:H4, while 80 ppm was required to do so in E. coli O88:H8. The biofilm-reducing effect of the carvacrol-eugenol-based PFA Core 2 was in the same range as that of the two individual substances with 80 ppm for E. coli O88:H8 and 40 ppm for E. coli O143:H4. In contrast, the third component of PFA Core 2, star anise oil, showed comparatively weak effects and required a minimum of 5000 ppm to achieve a significant response. Garlic oil did not show any significant effects below 5000 ppm on the biofilm formation of either strain. Tea tree oil significantly reduced the biofilm formation of both E. coli strains in three to four sub-MICs by −96.6% to −48.5%. Likewise, PFA Core 1 significantly decreased (−87.7% to −44%) the biofilm formation of E. coli O88:H8 and O143:H4 in two and three sub-MICs (down to 2500 ppm), respectively. Although ZnO did not inhibit the growth of either E. coli strain, the substance did show a strong impact on their biofilm formation capabilities, with the lowest effective concentration ranging from 150 ppm (−81.4%) for E. coli O88:H8 to 80 ppm (−41.7%) for E. coli O143:H4.
Violacein production of C. violaceum was most efficiently reduced with carvacrol, eugenol, and PFA Core 2, with the lowest effective concentration being 20 ppm. While tea tree oil and PFA Core 1 showed similar potentials to inhibit violacein production in the first three sub-MICs (−96.5% to −32.4% at 1250–300 ppm), garlic and star anise oil proved to be less effective, with a violacein-inhibiting concentration of 600 ppm and 300 ppm, respectively. Interestingly, low concentrations of tea tree oil (10 ppm) and star anise oil (10–150 ppm) slightly increased the violacein production of C. violaceum. Comparable to the observations on biofilm formation, ZnO decreased the violacein signal down to a concentration of 40 ppm, despite having no effect on the growth of C. violaceum.
3.2. Effect of Carvacrol and Tea Tree Oil on Adhesion of F4-Fimbriated E. coli to Piglets’ Small Intestinal Mucus In Vitro
The effect of carvacrol and tea tree oil on the mucus-binding properties of an F4-fimbriated E. coli strain was evaluated in an in vitro mucus adherence assay. Close to 20% of the radioactive-labeled F4-fimbriated E. coli introduced into the microtiter wells adhered to the mucus coating (Figure 2). This suggests that all receptor sites in the mucus became occupied by the introduced bacterial cells grown in the absence of the test products. Treatment with tea tree oil and carvacrol showed opposite effects on the adhesion of F4-fimbriated E. coli cells; exposure to tea tree oil reduced the proportion of adhered cells in both tested concentrations (−52.6% at 320 ppm and −15.8% at 32 ppm), while carvacrol enhanced bacterial adhesion by +24.9% at 20 ppm.
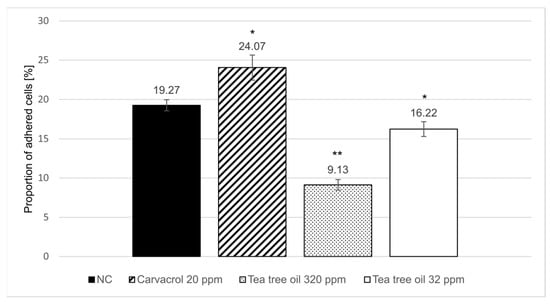
Figure 2.
Mucus-bound F4-fimbriated E. coli presented as percentage of introduced labeled bacteria. The error bars indicate standard error of nine replicate wells and asterisks indicate the statistical difference in comparison to the negative control (NC) analyzed with a t-test (*~p < 0.05, **~p < 0.001).
3.3. Effect of Phytogenic Prototypes on Piglet Growth Performance and Health Parameters
The in vivo study was run without any adverse technical events. Growth performance data are shown in Table 3. Piglets receiving a pharmacological dosage of 3 kg ZnO per ton of feed showed the best growth performance with a 12.56% improved ADG during the 42-day trial period, resulting in a 9.19% difference in BW at the end of the trial compared to the NC. The two phytogenic prototypes did not differ significantly in their effect on the piglets. However, in comparison to the NC, PFA 2 significantly improved ADG (7.05% from day 1 to day 42) and FCR (−4.4% from day 1 to day 42), which led to a 5.25% increased final BW at the end of the study. Additionally, dietary supplementation with PFA 2 caused ADFI to increase significantly during the first two weeks of the study compared to the NC. Supplementation with PFA 1, on the other hand, only improved FCR to a significant extent.

Table 3.
Effects of ZnO and phytogenic prototypes PFA 1 and PFA 2 on growth performance parameters of post-weaning piglets during the 42-day feeding trial.
The mortality observed throughout the whole study period of 42 days was NC: 1.6%, ZnO: 0.4%, PFA 1: 0.8%, and PFA 2: 0.4% (Table S1). The medication rate amounted to NC: 6.4%, ZnO 5.6%, PFA 1: 7.2%, and PFA 2: 5.5% (Table S2). Clinical signs of PWD were found in 2.4% (6 animals) of piglets fed the unsupplemented basal diet (NC) and 1.2% (3 animals) or 0.8% (2 animals) of piglets fed the diet supplemented with PFA 1 or PFA 2, respectively. No piglets receiving ZnO were affected by clinical signs of PWD. Remaining piglets showed normal activity and alertness, a normal coat and eyes, as well as normal feces and urine.
The fecal scores were recorded based on a scale from 0 to 3 (0 = normal; 1 = soft feces; 2 = mild diarrhea; 3 = severe diarrhea) and are depicted in Table 4. Since no signs of severe diarrhea (scale 3) were observed during the in vivo study, fecal scores were binarized as 0 = normal and 1 = altered (soft to severe diarrhea). During the starter feeding period (day 1 to 14), zinc oxide supplementation (ZnO, treatment 2) resulted in the highest probability of normal feces (0.821), followed by PFA 2 (treatment 4; 0.681), PFA 1 (treatment 3; 0.579), and the negative control (NC, treatment 1; 0.422). In the grower feeding period (day 15 to 42), all supplemented treatments showed high probabilities of normal feces, with ZnO again being the highest (0.983), followed by PFA 2 (0.898), PFA 1 (0.855), and NC (0.870).

Table 4.
Effects of ZnO and phytogenic prototypes PFA 1 and PFA 2 on fecal scores of post-weaning piglets during the 42-day feeding trial.
4. Discussion
Many challenges in livestock production are associated with bacterial infections. This is especially the case for critical phases early in the life of an animal. In this regard, piglets are among the most susceptible livestock species, particularly during the weaning period, with massive environmental, dietary, and social changes for these young animals. Stress and an increased susceptibility to health-issues such as diarrhea are the consequence. One of the most common strategies to manage PWD is the application of ZnO at pharmacological concentrations [3]. However, with increasing restrictions on the use of traditional AGPs like antibiotics and ZnO, new strategies are required to promote animal welfare and livestock production efficacy. Therefore, the present study assessed the potential of selected phytogenics as alternatives to ZnO, the interactions of these substances with different bacterial models, and their performance in a piglet feeding trial.
The microbroth dilution assay of the individual substances revealed a substantially stronger antibacterial efficacy of carvacrol and eugenol compared to tea tree oil, garlic oil, and star anise oil in all tested strains. This is well in line with available references in which carvacrol [25,27,28] and eugenol [28] proved to be potent substances to inhibit bacterial growth, while tea tree oil [7] and garlic oil [29,30] showed only weak antibacterial efficacy. The application of lethal concentrations of a substance could, however, be a strong inducer of resistance development. Consequently, it is of great interest to explore the potential of phytogenics to prevent the expression of pathogenic traits without exerting selective pressure towards resistance development or compromising the “healthy” functions of the microbiome. In this regard, the influence of phytogenics on the cell density-dependent bacterial communication system quorum sensing has gained considerable interest as many virulence-related genes are regulated by quorum sensing [21]. Therefore, several bacterial models related to quorum sensing were applied in this study to investigate the inhibitory potential of phytogenics.
At first, the biosensor strain C. violaceum was used as a model to determine the QS-controlled violacein production. The treatment of C. violaceum with all individual substances at sub-MIC concentrations inhibited or reduced the production of violacein (Figure 1). These results suggest that the tested substances may interfere with quorum sensing of this model strain, although at different concentrations. Similar results were obtained by Burt et al. [8] for carvacrol, Alvarez et al. [7] for tea tree oil, Noumi et al. [31] for star anise oil, and Zhou et al. [15] for eugenol, which reported an inhibition of violacein production in C. violaceum at concentrations comparable to those in the present study.
The literature references for the effect of garlic oil on C. violaceum are not available to the authors’ knowledge. However, Bodini et al. [32] found that aqueous garlic extracts inhibited the violacein production of two C. violaceum strains at similar concentrations as the garlic essential oil used in the present studies. Most studies investigating the effects of ZnO are using nanoparticles in antibacterial assays. For example, Al-Shabib et al. [33] and Khan et al. [34] used different types of ZnO nanoparticles and observed violacein reduction in C. violaceum biosensor strains beginning at 50 ppm and 25 ppm, respectively.
Quorum sensing has also been shown to be involved in the formation of biofilms [11,13]. The protective environment of biofilms plays an important role in bacterial infections by conferring resistance towards antibiotics and host defense systems [12,35]. In this study, carvacrol, eugenol, tea tree oil, and ZnO showed a significant impact on the biofilm formation of two E. coli field isolates at sub-MIC concentrations, although carvacrol, eugenol, and ZnO exhibited these effects at much lower concentrations than tea tree oil (Figure 1). Star anise oil did not have an MIC within the tested concentration range and only showed an effect on biofilm formation at concentrations of 5000 ppm or higher. Garlic oil, on the other hand, seemed to have weak biofilm-promoting properties on E. coli strain O88 at sub-MIC concentrations between 300 and 1250 ppm. The disturbance of E. coli biofilm production by carvacrol [25], eugenol [36], ZnO [33], and tea tree oil [37] was also found by previous studies in a similar concentration range, causing the effects in the present study. Despite the slightly stronger antimicrobial effects against C. violaceum compared with the two pathogenic E. coli field isolates, the phytogenic compounds decreased the violacein production, and hence the quorum sensing, of C. violaceum in a similar sub-MIC range than the biofilm formation of the E. coli field isolates. Therefore, it is hypothesized that the reduction of biofilm formation induced by carvacrol, eugenol, tea tree oil, and ZnO could be related to their disturbing influence on quorum sensing. Likewise, Burt et al. [8] found that carvacrol suppressed violacein production at concentrations consistent with carvacrol’s inhibiting effect on the biofilm formation of C. violaceum, Salmonella enterica subsp. Typhimurium, and Staphylococcus aureus. They concluded that carvacrol’s activity to inhibit biofilm formation may be related to the disruption of quorum sensing.
The third model applied in this study evaluated the impact of tea tree oil and carvacrol on the ability of an F4-fimbriated E. coli strain to adhere to the small intestinal mucus of piglets. This is especially relevant in the livestock industry, as F4-fimbriated E. coli are one of the major pathogens associated with PWD, and the production of F4 fimbriae is regulated by quorum sensing [14]. Previous studies demonstrated the potential of cranberry extract to inhibit the adherence of F4- and F18-fimbriated E. coli to intestinal villi in vitro and intestinal epithelium in vivo [38]. A subsequent challenge experiment revealed that cranberry supplementation of feed and drinking water reduced diarrhea as well as the extent and duration of F18-fimbriated E. coli excretion [38]. Thus, it was speculated that tea tree oil, which reduced the adhesion of F4-fimbriated E. coli in the present study, might also increase the resilience of piglets against PWD when supplemented in feed. Treatment with carvacrol, on the other hand, resulted in a higher number of adherent cells, which may indicate increased expression of adhesion factors. However, it is more likely that the chemical stress induced by carvacrol led to the occurrence of autoaggregation events. This type of bacterial behavior serves to protect the involved bacteria from external stresses [39]. However, further studies are necessary to determine the exact nature and cause of this observation.
Based on the results of the investigation of the individual phytogenic compounds, two prototypes were formulated, PFA Core 1 and PFA Core 2. PFA Core 1 was based on garlic oil and tea tree oil, while the second prototype, PFA Core 2, was based on carvacrol, eugenol, and star anise oil. The prototypes were formulated based on three different considerations: Due to their antibacterial efficacy and sub-MIC effects, carvacrol and eugenol were selected as the most potent substances from the present in vitro tests and used as the basis for one of the prototypes. The convincing results of tea tree oil in the mucus adherence assay led to the assumption that this substance could be promising in terms of increasing the resilience of piglets to PWD. Therefore, the second prototype was based on tea tree oil. In addition, both garlic oil and star anise oil were added to the prototypes due to their potential feed intake-stimulating properties, although the present results suggest the little relevance of both substances in terms of their influence on PWD caused by pathogenic E. coli. The subsequent study of the bactericidal activity as well as quorum sensing and the biofilm inhibitory properties of the two prototypes showed that the effects of both PFA cores correlated with those of their respective individual main components (Figure 1 and Table 2).
The in vitro test of ZnO, which served as a positive control in the in vivo feeding trial, revealed no bactericidal activity in the applied concentration range. Evidence from the literature suggests that particle size has a major influence on the antibacterial activity of ZnO [40]. ZnO nanoparticles especially showed stronger antibacterial effects than larger commercial particles, which were used in this study to mimic the application in livestock. Consequently, the high average particle size in combination with low water solubility might have been responsible for the lack of bactericidal activity of the pharmaceutical-grade ZnO applied in the present study. Despite this, a strong inhibition of E. coli biofilm formation and C. violaceum violacein production was observed in a wide range of tested ZnO concentrations. These findings indicate that ZnO may not only function through bactericidal effects but also inhibit bacterial traits in the sub-MIC range.
Side effects like the onset of toxic effects during prolonged administration and the accumulation of the heavy metal zinc in the environment prompted the European Union to ban the use of medicinal doses of ZnO, starting from June 2022 [3]. At the same time, the 3Rs principle is already firmly established in the EU legislation and requires scientists to carefully consider the 3Rs principle in their search for alternative feed additives [23]. Due to this background and the encouraging results of the in vitro assays, where the prototypes showed similar effects as the individual substances, only the prototypes were included in the subsequent in vivo trials. Therefore, the second part of the present study evaluated the in vivo potential of the formulated phytogenic feed additive prototypes as ZnO alternatives in piglets during the nursery period. While feed additives containing carvacrol have already been employed in previous studies, the use of tea tree oil in weaning piglets is much less common.
The supplementation of feed with ZnO resulted in the expected improvement in the growth performance of the animals along with a significant reduction in fecal scores. Similar benefits of ZnO on piglet growth parameters and fecal scores were reported by other studies. For instance, Molist et al. [41] observed a reduced incidence of diarrhea and an 8.2% increase in body weight on day 12 post-weaning in piglets receiving 3000 ppm ZnO compared to an unsupplemented control, which correlates with the 9.1% increase on day 15 post-weaning in the present study. Not only ZnO but also PFA 1 and PFA 2 supplementation improved the fecal scores of piglets between day 1 and 14 of the trial, with PFA 2 showing a greater effect than PFA 1. However, these observations were made under conditions with a low incidence of pathogens, as the overall mortality and medication rates as well as growth performance suggest a good health status of the whole group of animals observed in this study. The incidence of diarrhea, with a total of only 1.1% of all animals requiring medication due to PWD, was lower than the stated averages in the literature. The reported PWD rates in piglets receiving no ZnO or antibiotic supplementation are in the range of 34% to 51.1% for individual groups in Denmark [42,43] and between 3.6% and 14.3% in organized Indian farms [44]. The variations in the incidence of diarrhea and PWD might be the result of differing housing conditions, since, for example, low hygiene standards can lead to the increased susceptibility of piglets to stressors and diarrhea [3]. Due to the low overall morbidity of the piglets in this study, significant differences in health parameters like fecal scores are difficult to achieve, and the clinical relevance of the observed changes is up for discussion. Nevertheless, both ZnO- and carvacrol-containing feed additives have been demonstrated to improve piglet fecal scores in previous studies [41,45,46]. Therefore, it is assumed that also the differences found in the feeding trial are of clinical relevance.
The promising ability of tea tree oil to reduce the adherence of F4-fimbriated E. coli cells to piglets’ small intestinal mucus appeared to have only minor, if any, effects on fecal scores and the incidence of diarrhea in the presented feeding trial. However, since the reasons for the occurrence of PWD were not further explored, it cannot be specified whether the observed diarrhea events were caused by the F4-fimbriated E. coli strains in the present study. Notably, a subsequent study on PFA 2 was recently published [47]. In this study, the incorporation of PFA 2 in the diets of weaned piglets challenged with F4-Enterotoxigenic E. coli appeared to offer significant benefits in terms of reducing pathogen shedding and improving intestinal histomorphometry. This additional evidence strengthens the hypothesis on the mechanism through which PFA 2 interacts with pathogenic E. coli to support piglets during the post-weaning period and underscores the importance of continued research in this area.
Beneficial effects on growth parameters were observed for those substances that also led to improved fecal scores: The addition of PFA 2 to the feed resulted in a significant improvement of BW, ADG, and FCR, and thus performed close to ZnO. In contrast, supplementation with PFA 1 only improved FCR in a significant manner. These results reflect the in vitro observations, with PFA Core 2 showing effects at much lower concentrations compared to PFA Core 1 in the E. coli biofilm inhibition assay and the C. violaceum quorum sensing inhibition assay.
Blends containing carvacrol as one of their main constituents have previously been demonstrated to improve nutrient digestibility and the immunological as well as morphological gut health parameters of weaning piglets [45,46,48,49]. For example, dietary supplementation with a mixture of carvacrol and thymol reduced inflammatory responses by decreasing the expression of tumor necrosis factor α [45,48]. A similar mixture has also been shown to promote nutrient digestibility, enhance trypsin and chymotrypsin activity, and improve intestinal morphology via an increased villus height to crypt depth ratio [49]. The combination of all these effects may reduce the adverse effects of stress caused by weaning and bacterial infections, leading to the improved growth parameters observed in the present and other studies [45,46,49].
Differences in the application dosage may in turn explain the deviations between the present results and one of the few available studies on the use of tea tree oil as a feed additive, conducted by Wang et al. [50]. In contrast to the present study, these authors observed positive effects on the growth performance of weaned piglets at a tea tree oil dosage almost 15 times higher than the concentration used in our piglet study. This high amount of tea tree oil applied by Wang et al. is consistent with the concentrations that caused strong effects in the in vitro assays of the present study. This is a further indication that the substance concentrations that were necessary to achieve effects in our in vitro assays correlate with the substance doses required to improve the health status and growth performance of weaning piglets.
Similar to the present work, Grilli et al. [51] and Kelly et al. [52] successfully transferred results from in vitro assays to an in vivo context while investigating feed additives that reduce Campylobacter jejuni infectivity. However, it is important to note that most in vitro assays only investigate single aspects. These models usually lack the complexity of whole biological systems, such as the animal interphase or the microbiome, and thus simplify the in vivo situation. Nevertheless, a combination of various in vitro assays may serve as a useful toolbox for enhancing the understanding of the effects of phytogenics and predicting their effectiveness in vivo. Such an approach would increase the chances of success in formulating phytogenic feed additives to develop alternatives for traditional antimicrobial growth promoters, while minimizing the need for extensive animal trials.
5. Conclusions
The results of this study show that the dietary inclusion of a carvacrol–eugenol-star anise oil-based phytogenic feed additive improves growth parameters and fecal scores in weaned piglets compared to an unsupplemented control. While animal responses to feed additives can greatly vary, the growth performance improvement in this study was similar to, but less effective than, the addition of ZnO. The applied in vitro assays suggest that this effect could be associated with but not limited to the ability of carvacrol and eugenol to influence bacterial behavior and communication at the dosages included in the piglet study. Considering the in vitro results with tea tree oil, a single assay may not be sufficient to make predictions for these types of compounds. Taken together, this study has demonstrated that the applied in vitro models have the potential to support the development of phytogenic feed additives in order to find alternatives to traditional antimicrobial growth promoters in livestock feed while respecting the 3Rs principle. Particularly the use of a set of different in vitro assays with consistent results for prototype candidates can help to achieve this goal.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani15111661/s1, Figure S1: Effect of carvacrol on biofilm formation of E. coli strains O143:H4 and O88:H8 and violacein production of C. violaceum; Figure S2: Effect of eugenol on biofilm formation of E. coli strains O143:H4 and O88:H8 and violacein production of C. violaceum; Figure S3: Effect of garlic oil on biofilm formation of E. coli strains O143:H4 and O88:H8 and violacein production of C. violaceum; Figure S4: Effect of PFA Core 1 on biofilm formation of E. coli strains O143:H4 and O88:H8 and violacein production of C. violaceum; Figure S5: Effect of PFA Core 2 on biofilm formation of E. coli strains O143:H4 and O88:H8 and violacein production of C. violaceum; Figure S6: Effect of star anise oil on biofilm formation of E. coli strains O143:H4 and O88:H8 and violacein production of C. violaceum; Figure S7: Effect of tea tree oil on biofilm formation of E. coli strains O143:H4 and O88:H8 and violacein production of C. violaceum; Figure S8: Effect of ZnO on biofilm formation of E. coli strains O143:H4 and O88:H8 and violacein production of C. violaceum; Table S1: Mortality and causes of death from day 1 to day 42 on trial (day 25 to day 66 of age); Table S2: Clinical signs and medications from d 1 to d 42 on trial (d 25 to d 66 of age).
Author Contributions
Conceptualization, supervision, project administration, funding acquisition, T.A. and S.A.; methodology, A.W., T.A., K.M. and T.R.; validation, A.W. and S.A.; formal analysis, T.A.; investigation, A.W., K.M. and T.R.; resources, A.W., T.A., K.M. and T.R.; data curation, T.A.; writing—original draft preparation, writing—review and editing, A.W. and T.A.; visualization, A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was created within a research project of the Austrian Competence Centre for Feed and Food Quality, Safety and Innovation (FFoQSI; FFG project number 881882). The COMET-K1 competence center FFoQSI is funded by the Austrian federal ministries BMK, BMDW, and the Austrian provinces Lower Austria, Upper Austria and Vienna within the scope of COMET—Competence Centers for Excellent Technologies. The program COMET is handled by the Austrian Research Promotion Agency FFG.
Institutional Review Board Statement
The animals used in the study were raised and treated according to European Union Directive 2010/63/EU covering the protection of animals used for experimental or other purposes and according to the recommendation of Commission 2007/526/CE covering the accommodation and care of animals used for experimental and other scientific purposes. During the study, appropriate animal health and welfare inspections were carried out. The study is approved by the Ethics Committee of Landesamt für Gesundheit und Soziales Berlin (protocol code A-0439/17 and date of approval at 19 December 2018).
Informed Consent Statement
The study animals were owned by the commercial farm “Asmussen Agro GmbH, Germany” (H.-P. Asmussen, Stolzenhainer Straße 5, D-06917 Jessen).
Data Availability Statement
None of the data were deposited in an official repository. Data can be provided upon request via e-mail by the corresponding author.
Conflicts of Interest
Tobias Aumiller is an employee of Delacon Biotechnik GmbH, a manufacturer for phytogenic feed additives, which is a wholly owned subsidiary of Cargill, Incorporated. Anika Weitmann and Sonja Axmann are employed in the Institute for Animal Nutrition and Feed, AGES GmbH—Austrian Agency for Health and Food Safety. Teemu Rinttilä is employed at Alimetrics Research Ltd. These authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADFI | Average daily feed intake |
| ADG | Average daily gain |
| AGP | Antimicrobial growth promoter |
| BW | Body weight |
| diH2O | Deionized H2O |
| FCR | Feed conversion ratio |
| ME | Metabolizable energy |
| MIC | Minimum inhibitory concentration |
| NC | Negative control |
| PWD | Post-weaning diarrhea |
| TSB | Trypto-casein soy broth |
| ZnO | Zinc oxide |
References
- Casewell, M.; Friis, C.; Marco, E.; McMullin, P.; Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003, 52, 159–161. [Google Scholar] [CrossRef] [PubMed]
- No 1831/2003; Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. L 268/229. Official Journal of the European Union—European Commission: Brussels, Belgium, 2003.
- Bonetti, A.; Tugnoli, B.; Piva, A.; Grilli, E. Towards Zero Zinc Oxide: Feeding Strategies to Manage Post-Weaning Diarrhea in Piglets. Animals 2021, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- 2001/82/EC; Comission Implementing Decision of 26.6.2017 Concerning, in the Framework of Article 35 of Directive 2001/82/EC of the European Parliament and of the Council, the Marketing Authorisations for Veterinary Medicinal Products Containing “Zinc Oxide” to Be Administered Orally to Food Producing Species. Official Journal of the European Union—European Commission: Brussels, Belgium, 2017.
- Langendijk, P.; Bolhuis, J.E.; Laurenssen, B.F.A. Effects of pre- and postnatal exposure to garlic and aniseed flavour on pre- and postweaning feed intake in pigs. Livest. Sci. 2007, 108, 284–287. [Google Scholar] [CrossRef]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties--an overview. Forsch. Komplementmed. 2009, 16, 79–90. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Moreira, M.R.; Ponce, A. Antiquorum Sensing and Antimicrobial Activity of Natural Agents with Potential Use in Food. J. Food Saf. 2012, 32, 379–387. [Google Scholar] [CrossRef]
- Burt, S.A.; Ojo-Fakunle, V.T.; Woertman, J.; Veldhuizen, E.J. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef]
- Reichling, J. Anti-biofilm and Virulence Factor-Reducing Activities of Essential Oils and Oil Components as a Possible Option for Bacterial Infection Control. Planta Med. 2020, 86, 520–537. [Google Scholar] [CrossRef]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143 Pt 12, 3703–3711. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Gonzalez Barrios, A.F.; Zuo, R.; Hashimoto, Y.; Yang, L.; Bentley, W.E.; Wood, T.K. Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (MqsR, B3022). J. Bacteriol. 2006, 188, 305–316. [Google Scholar] [CrossRef]
- Yang, K.; Meng, J.; Huang, Y.C.; Ye, L.H.; Li, G.J.; Huang, J.; Chen, H.M. The role of the QseC quorum-sensing sensor kinase in epinephrine-enhanced motility and biofilm formation by Escherichia coli. Cell Biochem. Biophys. 2014, 70, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Sturbelle, R.T.; de Avila, L.F.; Roos, T.B.; Borchardt, J.L.; da Conceicao Rde, C.; Dellagostin, O.A.; Leite, F.P. The role of quorum sensing in Escherichia coli (ETEC) virulence factors. Vet. Microbiol. 2015, 180, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zheng, H.; Tang, Y.; Yu, W.; Gong, Q. Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnol. Lett. 2013, 35, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Hançer Aydemir, D.; Çifci, G.; Aviyente, V.; Boşgelmez-Tinaz, G. Quorum-sensing inhibitor potential of trans-anethole aganist Pseudomonas aeruginosa. J. Appl. Microbiol. 2018, 125, 731–739. [Google Scholar] [CrossRef]
- Li, W.-R.; Ma, Y.-K.; Shi, Q.-S.; Xie, X.-B.; Sun, T.-L.; Peng, H.; Huang, X.-M. Diallyl disulfide from garlic oil inhibits Pseudomonas aeruginosa virulence factors by inactivating key quorum sensing genes. Appl. Microbiol. Biotechnol. 2018, 102, 7555–7564. [Google Scholar] [CrossRef]
- Ganesh, P.S.; Rai, R.V. Inhibition of quorum-sensing-controlled virulence factors of Pseudomonas aeruginosa by Murraya koenigii essential oil: A study in a Caenorhabditis elegans infectious model. J. Med. Microbiol. 2016, 65, 1528–1535. [Google Scholar] [CrossRef]
- Liu, W.; Lu, H.; Chu, X.; Lou, T.; Zhang, N.; Zhang, B.; Chu, W. Tea polyphenols inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances resistance to Klebsiella pneumoniae infection in Caenorhabditis elegans model. Microb. Pathog. 2020, 147, 104266. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.H.; Kim, J.A.; Neupane, G.P.; Cho, M.H.; Lee, C.S.; Lee, J. Low concentrations of honey reduce biofilm formation, quorum sensing, and virulence in Escherichia coli O157:H7. Biofouling 2011, 27, 1095–1104. [Google Scholar] [CrossRef]
- Vattem, D.A.; Mihalik, K.; Crixell, S.H.; McLean, R.J.C. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia 2007, 78, 302–310. [Google Scholar] [CrossRef]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef]
- MacArthur Clark, J. The 3Rs in research: A contemporary approach to replacement, reduction and refinement. Brit J. Nutr. 2018, 120, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Skogman, M.E.; Kanerva, S.; Manner, S.; Vuorela, P.M.; Fallarero, A. Flavones as Quorum Sensing Inhibitors Identified by a Newly Optimized Screening Platform Using Chromobacterium violaceum as Reporter Bacteria. Molecules 2016, 21, 1211. [Google Scholar] [CrossRef] [PubMed]
- Axmann, S.; Schorpp, A.; Strassgüttl, J.; Aumiller, T. Effects of phytogenic substances on growth and biofilm formation of and field isolates. Die Bodenkult. J. Land Manag. Food Environ. 2021, 72, 1–8. [Google Scholar] [CrossRef]
- Society of Nutrition Physiology. Recommendations for the Supply of Energy and Nutrients to Pigs; DLG-Verlags-GmbH, Frankfurt am Main: Frankfurt, Germany, 2006. [Google Scholar]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Liu, X.X.; Liu, R.; Zhao, R.T.; Wang, J.S.; Cheng, Y.Y.; Liu, Q.; Wang, Y.Y.; Yang, S.M. Synergistic Interaction Between Paired Combinations of Natural Antimicrobials Against Poultry-Borne Pathogens. Front. Microbiol. 2022, 13, 811784. [Google Scholar] [CrossRef]
- Ross, Z.M.; O’Gara, E.A.; Hill, D.J.; Sleightholme, H.V.; Maslin, D.J. Antimicrobial Properties of Garlic Oil against Human Enteric Bacteria: Evaluation of Methodologies and Comparisons with Garlic Oil Sulfides and Garlic Powder. Appl. Environ. Microbiol. 2001, 67, 475–480. [Google Scholar] [CrossRef]
- Dussault, D.; Vu, K.D.; Lacroix, M. In vitro evaluation of antimicrobial activities of various commercial essential oils, oleoresin and pure compounds against food pathogens and application in ham. Meat Sci. 2014, 96, 514–520. [Google Scholar] [CrossRef]
- Noumi, E.; Ahmad, I.; Adnan, M.; Patel, H.; Merghni, A.; Haddaji, N.; Bouali, N.; Alabbosh, K.F.; Kadri, A.; Caputo, L.; et al. Illicium verum L. (Star Anise) Essential Oil: GC/MS Profile, Molecular Docking Study, In Silico ADME Profiling, Quorum Sensing, and Biofilm-Inhibiting Effect on Foodborne Bacteria. Molecules 2023, 28, 7691. [Google Scholar] [CrossRef]
- Bodini, S.F.; Manfredini, S.; Epp, M.; Valentini, S.; Santori, F. Quorum sensing inhibition activity of garlic extract and p-coumaric acid. Lett. Appl. Microbiol. 2009, 49, 551–555. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Ahmed, F.; Khan, R.A.; Ahmad, I.; Alsharaeh, E.; Khan, M.S.; Hussain, A.; Rehman, M.T.; Yusuf, M.; et al. Biogenic synthesis of Zinc oxide nanostructures from Nigella sativa seed: Prospective role as food packaging material inhibiting broad-spectrum quorum sensing and biofilm. Sci. Rep.-Uk 2016, 6, 36761. [Google Scholar] [CrossRef]
- Khan, M.F.; Husain, F.M.; Zia, Q.; Ahmad, E.; Jamal, A.; Alaidarous, M.; Banawas, S.; Alam, M.M.; Alshehri, B.A.; Jameel, M.; et al. Anti-quorum Sensing and Anti-biofilm Activity of Zinc Oxide Nanospikes. ACS Omega 2020, 5, 32203–32215. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Lee, J.-H.; Gwon, G.; Kim, S.-I.; Park, J.G.; Lee, J. Essential Oils and Eugenols Inhibit Biofilm Formation and the Virulence of Escherichia coli O157:H7. Sci. Rep.-Uk 2016, 6, 36377. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, J.; Gong, X.; Wu, X.; Liu, L.; Chi, F. Rosemary and Tea Tree Essential Oils Exert Antibiofilm Activities In Vitro against Staphylococcus aureus and Escherichia coli. J. Food Prot. 2020, 83, 1261–1267. [Google Scholar] [CrossRef]
- Coddens, A.; Loos, M.; Vanrompay, D.; Remon, J.P.; Cox, E. Cranberry extract inhibits in vitro adhesion of F4 and F18(+) Escherichia coli to pig intestinal epithelium and reduces in vivo excretion of pigs orally challenged with F18(+) verotoxigenic E. coli. Vet. Microbiol. 2017, 202, 64–71. [Google Scholar] [CrossRef]
- Trunk, T.; Khalil, H.S.; Leo, J.C. Bacterial autoaggregation. Aims Microbiol. 2018, 4, 140–164. [Google Scholar] [CrossRef]
- Rutherford, D.; Jira, J.; Kolarova, K.; Matolinova, I.; Micova, J.; Remes, Z.; Rezek, B. Growth Inhibition of Gram-Positive and Gram-Negative Bacteria by Zinc Oxide Hedgehog Particles. Int. J. Nanomed. 2021, 16, 3541–3554. [Google Scholar] [CrossRef]
- Molist, F.; Hermes, R.G.; de Segura, A.G.; Martin-Orue, S.M.; Gasa, J.; Manzanilla, E.G.; Perez, J.F. Effect and interaction between wheat bran and zinc oxide on productive performance and intestinal health in post-weaning piglets. Brit J. Nutr. 2011, 105, 1592–1600. [Google Scholar] [CrossRef]
- Eriksen, E.O.; Kudirkiene, E.; Christensen, A.E.; Agerlin, M.V.; Weber, N.R.; Nodtvedt, A.; Nielsen, J.P.; Hartmann, K.T.; Skade, L.; Larsen, L.E.; et al. Post-weaning diarrhea in pigs weaned without medicinal zinc: Risk factors, pathogen dynamics, and association to growth rate. Porc. Health Manag. 2021, 7, 54. [Google Scholar] [CrossRef]
- Carstensen, L.; Ersboll, A.K.; Jensen, K.H.; Nielsen, J.P. Escherichia coli post-weaning diarrhoea occurrence in piglets with monitored exposure to creep feed. Vet. Microbiol. 2005, 110, 113–123. [Google Scholar] [CrossRef]
- VinodhKumar, O.R.; Singh, B.R.; Sinha, D.K.; Pruthvishree, B.S.; Tamta, S.; Dubal, Z.B.; Karthikeyan, R.; Rupner, R.N.; Malik, Y.S. Risk factor analysis, antimicrobial resistance and pathotyping of Escherichia coli associated with pre- and post-weaning piglet diarrhoea in organised farms, India. Epidemiol. Infect. 2019, 147, e174. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Song, M.H.; Lee, J.H.; Oh, H.J.; Kim, Y.J.; An, J.W.; Go, Y.B.; Song, D.C.; Cho, H.A.; Cho, S.Y.; et al. Phytogenic feed additives alleviate pathogenic Escherichia coli-induced intestinal damage through improving barrier integrity and inhibiting inflammation in weaned pigs. J. Anim. Sci. Biotechnol. 2022, 13, 107. [Google Scholar] [CrossRef]
- Tan, B.F.; Lim, T.; Boontiam, W. Effect of dietary supplementation with essential oils and a Bacillus probiotic on growth performance, diarrhoea and blood metabolites in weaned pigs. Anim. Prod. Sci. 2021, 61, 64–71. [Google Scholar] [CrossRef]
- Alberto, T.-P.; Keiner, A.; Le Gall, M.; Molist, F.; Guan, X.; Middelkoop, A.; Jiménez-Moreno, E.; Balfagón, A.; Mantovani, G.; Nofrarías, M.; et al. Impact of a Phytogenic Feed Additive on Diarrhea Incidence, Intestinal Histomorphology and Fecal Excretion of F4-Fimbriated Enterotoxigenic Escherichia coli in Post-Weaning Piglets. Stresses 2025, 5, 8. [Google Scholar] [CrossRef]
- Wei, H.K.; Xue, H.X.; Zhou, Z.X.; Peng, J. A carvacrol-thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal 2017, 11, 193–201. [Google Scholar] [CrossRef]
- Xu, Y.T.; Liu, L.; Long, S.F.; Pan, L.; Piao, X.S. Effect of organic acids and essential oils on performance, intestinal health and digestive enzyme activities of weaned pigs. Anim. Feed. Sci. Tech. 2018, 235, 110–119. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, Y.; Liu, L.; Huang, F.; Dong, B. Effects of Three-Layer Encapsulated Tea Tree Oil on Growth Performance, Antioxidant Capacity, and Intestinal Microbiota of Weaned Pigs. Front. Vet. Sci. 2021, 8, 789225. [Google Scholar] [CrossRef]
- Grilli, E.; Vitari, F.; Domeneghini, C.; Palmonari, A.; Tosi, G.; Fantinati, P.; Massi, P.; Piva, A. Development of a feed additive to reduce caecal Campylobacter jejuni in broilers at slaughter age: From in vitro to in vivo, a proof of concept. J. Appl. Microbiol. 2013, 114, 308–317. [Google Scholar] [CrossRef]
- Kelly, C.; Gundogdu, O.; Pircalabioru, G.; Cean, A.; Scates, P.; Linton, M.; Pinkerton, L.; Magowan, E.; Stef, L.; Simiz, E.; et al. The In Vitro and In Vivo Effect of Carvacrol in Preventing Campylobacter Infection, Colonization and in Improving Productivity of Chicken Broilers. Foodborne Pathog. Dis. 2017, 14, 341–349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).