Abstract
This study analyzed the association between individual and household factors and the incidence of trachoma among a population aged between 1 and 9 years in the state of Pernambuco. This was a population-based household study conducted using a population-based sample of residents from 96 census sectors of the 1778 sectors considered to be at social risk in the state. The estimated odds ratio of the univariate analysis presented a confidence interval of 95%. Weights and clusters were adjusted through the Generalized Linear and Latent Mixed Model (GLLAM) method. Trachoma cases were the dependent variable in the multivariate analysis. The independent variables were selected through the stepwise forward method, with an input criterion of 20% (p < 0.20) and an output criterion of 10% (p < 0.10). The prevalence was 6.65%. Trachoma was associated with a female sex, age of 5–9 years, either the absence of use or infrequent use of soap to wash the hands and face, the presence of nasal secretion, a lack of piped water from a public supply system, a greater number of rooms used for sleeping, a greater number of people living in the same household, and a family income of up to one minimum monthly wage. The prevalence of follicular trachoma in Pernambuco was higher than what is recommended by the World Health Organization (WHO).
1. Introduction
Associations of socioenvironmental factors within the field of healthcare have gained attention from the scientific community and international organizations [1]. Areas with inadequate sewage collection and treatment, precarious access to healthcare services, poor housing conditions, and low educational levels favor occurrences of neglected diseases [2], such as trachoma [3].
Trachoma is still considered to be the most important cause of avoidable blindness in the world [4]. The only source of transmission is humans with active trachoma [5]. Chlamydia trachomatis can be transmitted either from person to person or indirectly, by means of sharing contaminated objects, in addition to mechanical transmission through vectors, such as insects like the housefly [6]. The World Health Organization (WHO) has stated that the endemic disease will have been brought under control when the prevalence of active trachoma is <5% [7].
Poor and rural areas of 41 countries in Africa, Central and South America, Asia, Australia, and the Middle East are hyperendemic [7]. Since 2002, studies among schoolchildren have revealed a trachoma prevalence of >5% in several Brazilian states [8,9,10].
To reach the global elimination of trachoma as a cause of blindness by 2020, WHO established a goal of carrying out population-based surveys among samples to verify the epidemiological situation [7,11,12] and implementing the SAFE strategy to control trachoma (S—surgery in cases of trachomatous trichiasis; A—antibiotic therapy in cases of active trachoma; F—facial hygiene; and E—environmental improvements) [13]. Use of the SAFE strategy has been shown to be a tool for reducing the prevalence of active forms of the disease [14].
In Brazil, which is a signatory to resolution 51/2011 [15], pilot surveys have been conducted among populations that are considered to present a social risk in two states: Pernambuco and Tocantins. Based on a household survey conducted in Pernambuco, the objective of the present study was to ascertain individual and household risk factors for trachoma among children aged 1 to 9 years.
2. Materials and Methods
2.1. Study Area
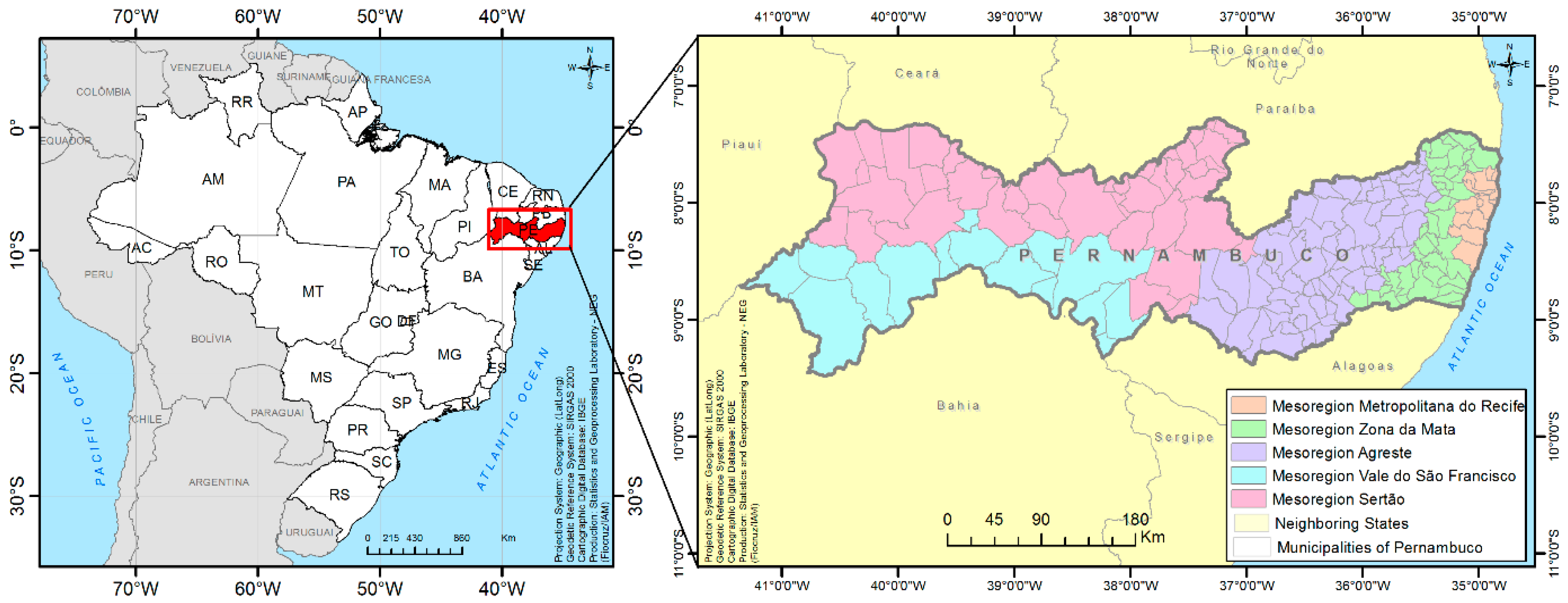
The present study was conducted in the state of Pernambuco, which is located in the northeastern region of Brazil. This state covers an area of 98,076,001 km2 and is divided into 184 municipalities, distributed into five mesoregions: Metropolitan Region of Recife, Zona da Mata, Agreste, Sertão do São Francisco, and Sertão Pernambucano (Figure 1) [16]. The estimated population in 2014 was 9,277,727 inhabitants [17].
Figure 1.
Geographical location of the state of Pernambuco and its division into mesoregions.
2.2. Study Design
This was a population-based study completed using data from the national household survey on trachoma that was conducted between 2014 and 2015.
2.3. Population Characterization
The sample selection parameters were the prevalence of active trachoma of 5%, confidence level of 95%, maximum margin of error of 0.02, and correction factor for a finite population with an effect of 4 [18]. The target population comprised 1778 census sectors that met the following social risk criteria: at least 50% of households with per capita income of up to ¼ of the minimum monthly wage and percentage of households connected to the general water supply network below 95% [17]. Of the 1778 eligible sectors, a randomized sample of 96 census sectors in which children between the ages of 1 and 9 years were living was selected. All residents were examined.
2.4. Sample Collection
A questionnaire was applied in the eligible households. It comprised individual questions (on sex, age group, use of bath and face towels, use of soap to wash the face and hands, whether the person slept alone, and presence of nasal secretion) and household questions (on type of home, water from the public network, any intermittence of water supply, type of sewage system, destination of sewage, destination of solid waste, flies in the household, number of rooms used for sleeping, educational level of the head of the family, family income, and number of people living in the household).
Cases of trachoma were diagnosed through an external eye examination, using a magnifying glass (2.5×) and either natural or artificial light, and were conducted by trained and standardized examiners [5].
The cases of trachoma among children and members of their families were classified as described by Thylefors et al. (1987) [19]. All cases were treated in accordance with the recommendations of the Ministry of Health [5].
2.5. Data Management and Analysis
The data were analyzed using STATA (version 12), with the exclusion of missing values. The data were adjusted by applying a correction factor [20] to account for random effects and cluster sample sizes [21,22].
The frequencies of all variables relating to the state of Pernambuco and its five mesoregions were defined and an association analysis of independent variables in relation to the dependent variable was then conducted through univariate analysis. Odds ratios were estimated with a 95% confidence interval. Weights and clusters were adjusted through the Generalized Linear and Latent Mixed Model (GLLAM) method [22].
A multivariate analysis was conducted using trachoma cases as the dependent variable. The independent variables were selected through the stepwise forward method, with an input criterion of 20% (p < 0.2) and an output criterion of 10% (p < 0.10).
An agglomeration indicator (AI) was built based on the following formula:
where
x: No. of people residing in the household;
y: Mean no. of rooms used for sleeping = No. of rooms/No. of households.
2.6. Ethics Statement
This research project was approved by the Ethics Committee of the Aggeu Magalhães Institute, Fiocruz, Pernambuco (CAEE 21192013.0.0000.5190).
3. Results
A total of 4238 households were evaluated, which presented 446 cases of trachoma among the 7423 children examined. The prevalence of trachoma in the state was 6.65% (CI 5.39–8.17).
The variables for the final logistic regression model (in bold) were selected in the univariate analysis, presented in Table 1 and Table 2: five individual variables (sex, age group, use of a bath and face towel, use of soap to wash the face and hands, and presence of nasal secretion) and eight household variables (water from the public network, intermittence of water supply, type of sewage, destination of sewage, destination of solid waste, flies in the household, number of rooms used for sleeping, and number of people living in the household).

Table 1.
Univariate analysis on variables relating to individual characteristics of cases of trachoma in the population between 1 and 9 years of age investigated in Pernambuco and mesoregions, 2014–2015.

Table 2.
Univariate analysis on variables relating to household characteristics of cases of trachoma in the population between 1 and 9 years of age investigated in Pernambuco and mesoregions, 2014–2015.
Table 3 presents the final adjusted model, in which trachoma is associated with the following individual variables: being a girl between the ages of 5 and 9 years and not using or only sometimes using soap to wash the face and hands. In the state of Pernambuco, children who presented nasal secretion had a 94% higher chance of having trachoma.

Table 3.
Final adjusted model for associations of individual and household characteristics of cases with trachoma in the population between 1 and 9 years of age investigated in Pernambuco and mesoregions, 2014–2015.
The following household characteristics were associated with trachoma: not having a piped water supply from the public network, a greater number of rooms used for sleeping, and a greater number of people in the household. These factors increased the chances of occurrence of the disease (Table 3). Most homes had two rooms for sleeping, with an agglomeration indicator of 2.2 people per room.
4. Discussion
An analysis on the risk factors for trachoma provides important information for planning and implementing actions in disease control programs [5,14,15,23]. The present study revealed factors that were associated with occurrences of the disease, using households as the database.
Among the factors investigated, it was observed that girls had greater chances of contracting trachoma in Pernambuco and in the mesoregion of Zona da Mata. This association has also been reported in population-based surveys in Senegal [24], Ethiopia [25], Gambia, and Tanzania [13]. This is probably because girls more frequently help in tending to younger siblings and because affective behavior is closer among girls [13,25]. However, another explanation could be a higher susceptibility to infection by C. trachomatis among females, as reported by Ngondi et al. (2008) [25].
In the present population-based study, as in studies conducted in Africa [18,26,27], children of school and preschool ages were assessed. However, unlike studies from the African continent, the results from the present investigation demonstrated that children of a school age had greater chances of presenting the disease than preschool children. This is possibly explained by reinfections that occur with an increasing age [18].
Studies conducted in Brazil have shown that school-age children (5–9 years) have greater chances of presenting trachoma [10,28,29], thus corroborating the results of the present study. Last et al. (2014) [18] showed that cases occurred predominantly among children between 0 and 5 years of age, in a household survey conducted in Guinea-Bissau. This was similar to what was observed in a school survey conducted in Brazil between 2002 and 2008 [8,9]. Schools are frequently the environment for conducting evaluations and interventions in relation to diseases because children are easily accessible and available in this setting. In the specific case of Brazil, school surveys are unable to characterize trachoma among children between the ages of 1 and 4 years [30], because most individuals of this age group are not enrolled in public schools.
Facial cleaning is among the recommendations of the SAFE strategy [31,32,33,34,35,36]. A greater risk of disease transmission persists when hygiene behaviors are not translated into routine attitudes [37]. The prevalence of trachoma is reduced through a higher frequency of facial cleaning [10,29,35,38].
The relationship between a clean face and reduced chances of trachoma is one of the strongest associations found in the literature [29,38,39,40]. In Pernambuco, a lack of the use or only occasional use of soap to wash the hands and face increases the risk of transmitting trachoma by more than 60%, in comparison with the regular use of soap. In some mesoregions of this state, this risk was tripled or even higher. In Brazil, the school health program, a partnership between healthcare and educational bodies, can conduct combined actions to direct children to clean their faces and hands as a way to decrease transmission of this disease, since most cases have been observed among school-age children.
C. trachomatis can be found in nasal secretion [18,41], which can increase the chance of transmission among children living in endemic areas. The presence of nasal secretion among children in Pernambuco demonstrates that this factor was associated with occurrences of trachoma.
Among the household factors, a lack of water supply from the public network in Pernambuco was shown to increase the chances of having trachoma. In this state, intermittence of the water supply has led to people storing water in barrels, buckets, or water tanks to ensure that they have a supply for domestic consumption. When there is no piped public water network, water is drawn from wells, mines, or cacimbas. The results found may be related to the amount and/or quality of water to which the population has access, since intermittence of the water supply leads to an inadequate storage or use of water of an uncontrolled quality [27,38,42,43,44]. Moreover, with little water available, hygiene actions are less frequent. Studies in Gambia [45] and Ethiopia [46] demonstrated that there was a reduction in the transmission of C. trachomatis infection with improved sanitation and water access.
In Pernambuco and in the mesoregions of the Metropolitan Region of Recife and Sertão do São Francisco, the more rooms that there were in a household, the greater the risk of trachoma was. This result goes against the results found in other surveys conducted in Brazil, which indicated that a smaller number of rooms presented a higher risk of trachoma [10,29].
An agglomeration indicator was built with the aim of understanding this phenomenon. It was found that larger households concentrated more residents, which increased the chance of transmission, even though there were more rooms that could be used for sleeping. According to Favacho et al. (2018) [29], the greater the number of people in a household was, the higher the risk of the disease was. Hence, larger families contributed more towards the incidence of infection [13]. Moreover, with more people, there was a greater chance of sharing beds. In the mesoregions of Agreste and Sertão Pernambucano, most cases identified were from those who shared their bed with other residents. This could explain the maintenance of the transmission cycle of the disease within households with greater numbers of people that was found in the present study.
The target population of the present investigation was selected using social risk criteria. Therefore, it was expected that there would be no significant differences between individuals earning up to one minimum monthly wage and more than one minimum monthly wage. In the final model, it was seen that in the mesoregion of Sertão Pernambucano, people earning up to one minimum monthly wage were more likely to contract the disease. This association, in addition to the social criteria established, related to a population that resided in an area of the state with a low development index [47].
Population-based surveys imply operational difficulties and high costs. Trachoma is a disease that is known to be related to poverty [3]. Therefore, the team that conducted the national survey opted to work with the social risk criteria, which may have led to a limitation in the present study since this may represent a bias in sample selection. These options were implemented to fill the gap of knowledge about this disease in silent areas that nevertheless form part of populations living in areas of extreme poverty.
5. Conclusions
The prevalence of follicular trachoma in Pernambuco was higher than what is recommended by the WHO. The factors that were associated with maintaining the transmission chain of trachoma were girls of a school age, those who did not have the habit or had an infrequent habit of cleaning their hands and face, individuals who did not have access to water from the public network, individuals who were part of larger families, and individuals whose family income was up to one minimum monthly wage.
The present study demonstrates the need for monitoring and surveillance measures aimed towards trachoma. These need to be implemented together with intersectoral actions to promote health and improve socioeconomic, environmental, and educational conditions, in order to reduce the transmission of trachoma.
Author Contributions
Conceptualization, C.M.G.d.B., Z.M.d.M., and G.C.G.; methodology, C.M.G.d.B., Z.M.d.M., C.C.B., S.M.C.d.A., A.L.S.d.O., U.R.M., C.F., M.d.T.V., M.d.F.C.L., and G.C.G.; software, C.M.G.d.B., U.R.M., C.F., M.d.T.V., and G.C.G.; validation, C.M.G.d.B., Z.M.d.M., C.C.B., A.L.S.d.O., U.R.M., C.F., M.d.T.V., and G.C.G.; formal analysis, C.M.G.d.B., Z.M.d.M., C.C.B., S.M.C.d.A., A.L.S.d.O., U.R.M., C.F., M.d.T.V., and G.C.G.; investigation, C.M.G.d.B., Z.M.d.M., C.C.B., S.M.C.d.A., and G.C.G.; resources, C.M.G.d.B., Z.M.d.M., C.C.B., S.M.C.d.A., A.L.S.d.O., U.R.M., C.F., M.d.T.V., and G.C.G.; data curation, C.M.G.d.B., Z.M.d.M., C.C.B., S.M.C.d.A., A.L.S.d.O., U.R.M., C.F., M.d.T.V., M.d.F.C.L., and G.C.G.; writing—original draft preparation, C.M.G.d.B., Z.M.d.M., C.C.B., S.M.C.d.A., A.L.S.d.O., U.R.M., C.F., M.d.T.V., and G.C.G.; writing—review and editing, C.M.G.d.B.; visualization, C.M.G.B; supervision, C.M.G.d.B., Z.M.d.M., and G.C.G.; project administration, C.M.G.d.B., Z.M.d.M., and G.C.G.; funding acquisition C.M.G.d.B., Z.M.d.M., and G.C.G.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001 and by the National Health Fund, through agreement TC 210/2011 between FIOCRUZ and the Department of Epidemiological Surveillance of the Ministry of Health (SVS/MS).
Acknowledgments
Fiocruz, the Department of Epidemiological Surveillance of the Ministry of Health (SVS/MS), the State Secretariat of Health of Pernambuco, the managers and advisors of the Sanar Program of Pernambuco, to all technicians and the population that participated in the National Household Survey Trachoma, 2014 and 2015.
Conflicts of Interest
The authors declare that there were no conflicts of interest in conducting this study. The authors alone were responsible for the content and writing of this article.
Declaration of Non-Publication
The authors declare that this submission has not been previously published and is not considered simultaneously for any other publication.
Ethical Approval
The project was approved by the research ethics committee of the Oswaldo Cruz Foundation, in Pernambuco (CAEE 21192013.0.0000.5190).
References
- Szwarcwald, C.L.; Bastos, F.I.; Andrade, C.L.T. Medidas de desigualdad em salud: La discusión de alguns aspectos metodológicos com uma aplicación para la mortalidad neonatal em el Município de Rio de Janeiro, 2000. Cad. Saúde Pública 2002, 18, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Michael, E.; Madon, S. Socio-ecological dynamics and challenges to the governance of Neglected Tropical Disease control. Infect. Dis. Poverty 2017, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Brandão, E.; Romero, S.; Silva, M.A.L.; Santos, F.L.N. Neglected tropical diseases in Brazilian children and adolescents: Data analysis from 2009 to 2013. Infect. Dis. Poverty 2017, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, S.P.; Pascolini, D.; Rose-Nussbaumer, J. Trachoma: Global magnitude of a preventable cause of blindness. Br. J. Ophthalmol. 2009, 93, 563–568. [Google Scholar] [CrossRef]
- Brazil, Ministry of Health, Health Surveillance Secretariat. Tracking Manual Tracking and Its Removal as a Cause of Blindness; Brazil Ministry of Health: Brasilia, Brazil, 2014. [Google Scholar]
- King, J.D.; Ngondi, J.; Kasten, J.; Diallo, M.O.; Zhu, H.; Cromwell, E.A.; Emerson, P.M. Randomised trial of face-washing to develop a standard definition of clean face for monitoring trachoma control programmes. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 7–16. [Google Scholar] [CrossRef]
- World Health Organization. Trachoma; Fact Sheet Nº 382. Available online: http://www.who.int/mediacentre/factsheets/fs382/en/ (accessed on 20 August 2017).
- Luna, E.J.; Lopes, M.F.; Medina, N.H.; Favacho, J.; Cardoso, M.R. Prevalence of Trachoma in Schoolchildren in Brazil. Ophthalmic Epidemiol. 2016, 23, 360–365. [Google Scholar] [CrossRef]
- Lopes, M.F.C.; Luna, E.J.A.; Medina, N.H.; Cardoso, M.R.; Freitas, H.S.; Koizumi, I.K.; Bernardes, N.A.F.A.; Guimarães, J.A. Prevalence of trachoma in Brazilian schoolchildren. Rev. Saude Publica 2013, 47, 451–459. [Google Scholar] [CrossRef]
- Lucena, A.R.; Cruz, A.A.V.; Akaishi, P. Epidemiology of trachoma in the village of Araripe plateau—Ceará State. Arq. Bras. Oftalmol. 2010, 73, 271–275. [Google Scholar] [CrossRef]
- Solomon, A.W.; Pavluck, A.L.; Courtright, P.; Aboe, A.; Adamu, L.; Alemayehu, W.; Alemu, M.; Alexander, N.D.; Kello, A.B.; Bero, B.; et al. The Global Trachoma Mapping Project: Methodology of a 34-Country Population-Based Study. Ophthalmic Epidemiol. 2015, 22, 214–225. [Google Scholar] [CrossRef]
- Solomon, A.W.; Kurylo, E. The global trachoma mapping project. Community Eye Health 2014, 27, 18. [Google Scholar]
- Blake, I.M.; Burton, M.J.; Bailey, R.L.; Solomon, A.W.; West, S.; Muñoz, B.; Holland, M.J.; Mabey, D.C.; Gambhir, M.; Basáñez, M.G.; et al. Estimating household and community transmission of ocular Chlamydia trachomatis. PLoS Negl. Trop. Dis. 2009, 3, e401. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, P.; Einterz, E. The Limits of Medical Interventions for the Elimination of Preventable Blindness. Trop. Med. Health 2014, 42, 43–52. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHA51.11 Global Elimination of Blinding Trachoma. 1998. Available online: http://www.who.int/blindness/causes/WHA51.11/en/ (accessed on 20 August 2017).
- Aggeu Magalhães Institute, Statistics and Geoprocessing Center. Map of the State of Pernambuco by Mesoregion; Aggeu Magalhães Institute, Statistics and Geoprocessing Center: Recife, Brazil, 2018. [Google Scholar]
- Brazilian Institute of Geography and Statistics. Available online: https://www.ibge.gov.br/estatisticas-novoportal/sociais/populacao/9662-censo-demografico-2010.html?&t=downloads (accessed on 20 February 2018).
- Last, A.R.; Burr, S.E.; Weiss, H.A.; Harding-Esch, E.M.; Cassama, E.; Nabicassa, M.; Mabey, D.C.; Holland, M.J.; Bailey, R.L. Risk factors for active trachoma and ocular Chlamydia trachomatis infection in treatment-naïve trachoma-hyperendemic communities of the Bijagós Archipelago, Guinea Bissau. PLoS Negl. Trop. Dis. 2014, 8, e2900. [Google Scholar] [CrossRef] [PubMed]
- Thylefors, B.; Dawson, C.R.; Jones, B.R.; West, S.K.; Taylor, H.R. A simple system for the assessment of trachoma and its complications. Bull. World Health Organ. 1987, 65, 477–483. [Google Scholar]
- Cochran, W.G. Samping Techniques, 3rd ed.; John Wiley: Hoboken, NJ, USA, 1977. [Google Scholar]
- Anderson, R.M.; May, R.M. Age-related changes in the rate of disease transmission: Implications for the design of vaccination programmes. J. Hyg. 1985, 94, 365–436. [Google Scholar] [CrossRef]
- Ximenes, R.A.A.; Martelli, C.M.; Merchán-Hamann, E.; Montarroyos, U.R.; Braga, M.C.; Lima, M.L.; Turchi, M.D.; Costa, M.A.; Alencar, L.C.; Moreira, R.C.; et al. Multilevel analysis of hepatitis A infection in children and adolescents: A household survey in the Northeast and Central-west regions of Brazil. Int. J. Epidemiol. 2008, 37, 852–861. [Google Scholar] [CrossRef]
- World Health Organization. Working to overcome the global impact of neglected tropical diseases—Summary. Wkly. Epidemiol. Rec. 2011, 86, 113–120. Available online: https://www.ncbi.nlm.nih.gov/pubmed/21438440 (accessed on 11 October 2019).
- Harding-Esch, E.M.; Kadimpeul, J.; Sarr, B.; Sane, A.; Badji, S.; Laye, M.; Sillah, A.; Burr, S.E.; MacLeod, D.; Last, A.R.; et al. Population-based prevalence survey of follicular trachoma and trachomatous trichiasis in the Casamance region of Senegal. BMC Public Health 2017, 18, 62. [Google Scholar] [CrossRef]
- Ngondi, J.; Gebre, T.; Shargie, E.B.; Graves, P.M.; Ejigsemahu, Y.; Teferi, T.; Genet, A.; Mosher, A.W.; Endeshaw, T.; Zerihun, M.; et al. Risk factors for active trachoma in children and trichiasis in adults: A household survey in Amhara Regional State, Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 432–438. [Google Scholar] [CrossRef]
- King, J.D.; Odermatt, P.; Utzinger, J.; Ngondi, J.; Bamani, S.; Kamissoko, Y.; Boubicar, K.; Hassan, A.S.; Nwobi, B.C.; Jip, N.; et al. Trachoma among children in community surveys from four African countries and implications of using school surveys for evaluating prevalence. Int. Health 2013, 5, 280–287. [Google Scholar] [CrossRef]
- Ferede, A.T.; Dadi, A.F.; Tariku, A.; Adane, A.A. Prevalence and determinants of active trachoma among preschool-aged children in Dembia District, Northwest Ethiopia. Infect. Dis. Poverty 2017, 6, 128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meneghim, R.L.F.D.S.; Padovani, C.R.; Schellini, S.A. Trachoma in schoolchildren of the city of Botucatu, Sao Paulo, Brazil: Detection and health promotion of a neglected disease. Rev. Bras. Oftalmol. 2016, 75, 360–364. [Google Scholar] [CrossRef][Green Version]
- Favacho, J.; Alves da Cunha, A.J.L.; Gomes, S.; Freitas, F.B.; Queiroz, M.A.F.; Vallinoto, A.C.R.; Ishak, R.; Ishak, M.O.G. Prevalence of trachoma in school children in the Marajó Archipelago, Brazilian Amazon, and the impact of the introduction of educational and preventive measures on the disease over eight years. PLoS Negl. Trop. Dis. 2018, 12, e0006282. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.A.P.; Souza, W.V.; Luna, C.F.; Gouveia, G.C. Analysis of interventions and socio environmental factors associated with the occurrence of trachoma in Pernambuco in two surveys on schoolchildren conducted in 2006 and 2012. Cad. Saude Coletiva 2016, 24, 435–442. [Google Scholar] [CrossRef][Green Version]
- Abashawl, A.; Macleod, C.; Riang, J.; Mossisa, F.; Dejene, M.; Willis, R.; Flueckiger, R.M.; Pavluck, A.L.; Tadesse, A.; Adera, T.H.; et al. Prevalence of Trachoma in Gambella Region, Ethiopia: Results of Three Population-Based Prevalence Surveys Conducted with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016, 23, 77–83. [Google Scholar] [CrossRef]
- Adera, T.H.; Macleod, C.; Endriyas, M.; Dejene, M.; Willis, R.; Chu, B.K.; Letamo, Y.; Misganaw, T.; Mesele, T.; Mekonnen, E.; et al. Prevalence of and Risk Factors for Trachoma in Southern Nations, Nationalities, and Peoples’ Region, Ethiopia: Results of 40 Population-Based Prevalence Surveys Carried Out with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016, 23, 84–93. [Google Scholar] [CrossRef]
- Adamu, M.D.; Mpyet, C.; Muhammad, N.; Umar, M.M.; Muazu, H.; Olamiju, F.; Isiyaku, S.; Onyebuchi, U.; Bosso, U.A.; William, A.; et al. Prevalence of Trachoma in Niger State, North Central Nigeria: Results of 25 Population-Based Prevalence Surveys Carried Out with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016, 23, 63–69. [Google Scholar] [CrossRef]
- Stocks, M.E.; Ogden, S.; Haddad, D.; Addiss, D.G.; Mcguire, C.; Freeman, M.C. Effect of Water, Sanitation, and Hygiene on the Prevention of Trachoma: A Systematic Review and Meta-Analysis. PLoS Med. 2014, 11, e1001605. [Google Scholar] [CrossRef]
- Bailey, R.; Lietman, T. The SAFE strategy for the elimination of trachoma by 2020: Will it work? Bull. World Health Organ. 2001, 79, 233–236. [Google Scholar]
- Emerson, P.M.; Burton, M.; Solomon, A.W.; Bailey, R.; Mabey, D. The SAFE strategy for trachoma control: Using operational research for policy, planning and implementation. Bull. World Health Organ. 2006, 84, 613–619. [Google Scholar] [CrossRef]
- Freeman, M.C.; Stocks, M.E.; Cumming, O.; Jeandron, A.; Higgins, J.P.; Wolf, J.; PrüssUstün, A.; Bonjour, S.; Hunter, P.R.; Fewtrell, L.; et al. Systematic review: Hygiene and health: Systematic review of handwashing practices worldwide and update of health effects. Trop. Med. Int. Health 2014, 19, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Mpyet, C.; Muhammad, N.; Adamu, M.D.; Muazu, H.; Umar, M.M.; Goyol, M.; Yahaya, H.B.; Onyebuchi, U.; Ogoshi, C.; Hussaini, T.; et al. Prevalence of Trachoma in Kano State, Nigeria: Results of 44 Local Government Area-Level Surveys. Ophthalmic Epidemiol. 2017, 24, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Ketema, K.; Tiruneh, M.; Woldeyohannes, D.; Muluye, D. Active trachoma and associated risk factors among children in BasoLiben District of East Gojjam, Ethiopia. BMC Public Health 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Ndayishimiye, O.; Willems, J.; Manirakiza, E.; Smith, J.L.; Gashikanyi, R.; Kariyo, L.; Ndayishimiye, S.; Niyoniziziye, B.; Niyonkuru, A.; Nkunda, A.; et al. Population-based survey of active trachoma in 11 districts of Burundi. Ophthalmic Epidemiol. 2011, 18, 146–149. [Google Scholar] [CrossRef]
- Burr, S.E.; Hart, J.D.; Edwards, T.; Baldeh, I.; Bojang, E.; Harding-Esch, E.M.; Holland, M.J.; Lietman, T.M.; West, S.K.; Mabey, D.C.; et al. Association between ocular bacterial carriage and follicular trachoma following mass azithromycin distribution in The Gambia. PLoS Negl. Trop. Dis. 2013, 7. [Google Scholar] [CrossRef]
- Erlanger, T.E.; Keiser, J.; Caldas De Castro, M.; Bos, R.; Singer, B.H.; Tanner, M.; Utzinger, J. Effect of water resource development and management on Lymphatic filariasis, and estimates of populations at risk. Am. J. Trop. Med. Hyg. 2005, 73, 523–533. [Google Scholar] [CrossRef]
- Bero, B.; Macleod, C.; Alemayehu, W.; Gadisa, S.; Abajobir, A.; Adamu, Y.; Dejene, M.; Mekasha, A.; Habtamu Jemal, Z.; Yadeta, D.; et al. Prevalence of and Risk Factors for Trachoma in Oromia Regional State of Ethiopia: Results of 79 Population-Based Prevalence Surveys Conducted with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016, 23, 392–405. [Google Scholar] [CrossRef]
- Prüss Ustün, A.; Bartram, J.; Clasen, T.; Colford, J.M.; Cumming, O.; Curtis, V.; Bonjour, S.; Dangour, A.D.; De France, J.; Fewtrell, L.; et al. Burden of disease from inadequate water, sanitation and hygiene in low and middle-income settings: A retrospective analysis of data from 145 countries. Trop. Med. Int. Health 2014, 19, 894–905. [Google Scholar] [CrossRef]
- Migchelsen, S.J.; Sepulveda, N.; Martin, D.L.; Cooley, G.; Gwyn, S.; Pickering, H.; Joof, H.; Makalo, P.; Bailey, R.; Burr, S.E.; et al. Serology refects a decline in the prevalence of trachoma in two regions of The Gambia. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Tadesse, B.; Worku, A.; Kumie, A.; Yimer, S.A. Effect of water, sanitation and hygiene interventions on active trachoma in North and South Wollo zones of Amhara Region, Ethiopia: A Quasi-experimental study. PLoS Negl. Trop. Dis. 2017, 11. [Google Scholar] [CrossRef]
- Atlas do Desenvolvimento Humano no Brasil. IDHM 2013. Available online: http://www.atlasbrasil.org.br/2013/pt/consulta/ (accessed on 20 February 2018).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).