The Brilliance of Borrelia: Mechanisms of Host Immune Evasion by Lyme Disease-Causing Spirochetes
Abstract
1. Introduction
2. Innate Response
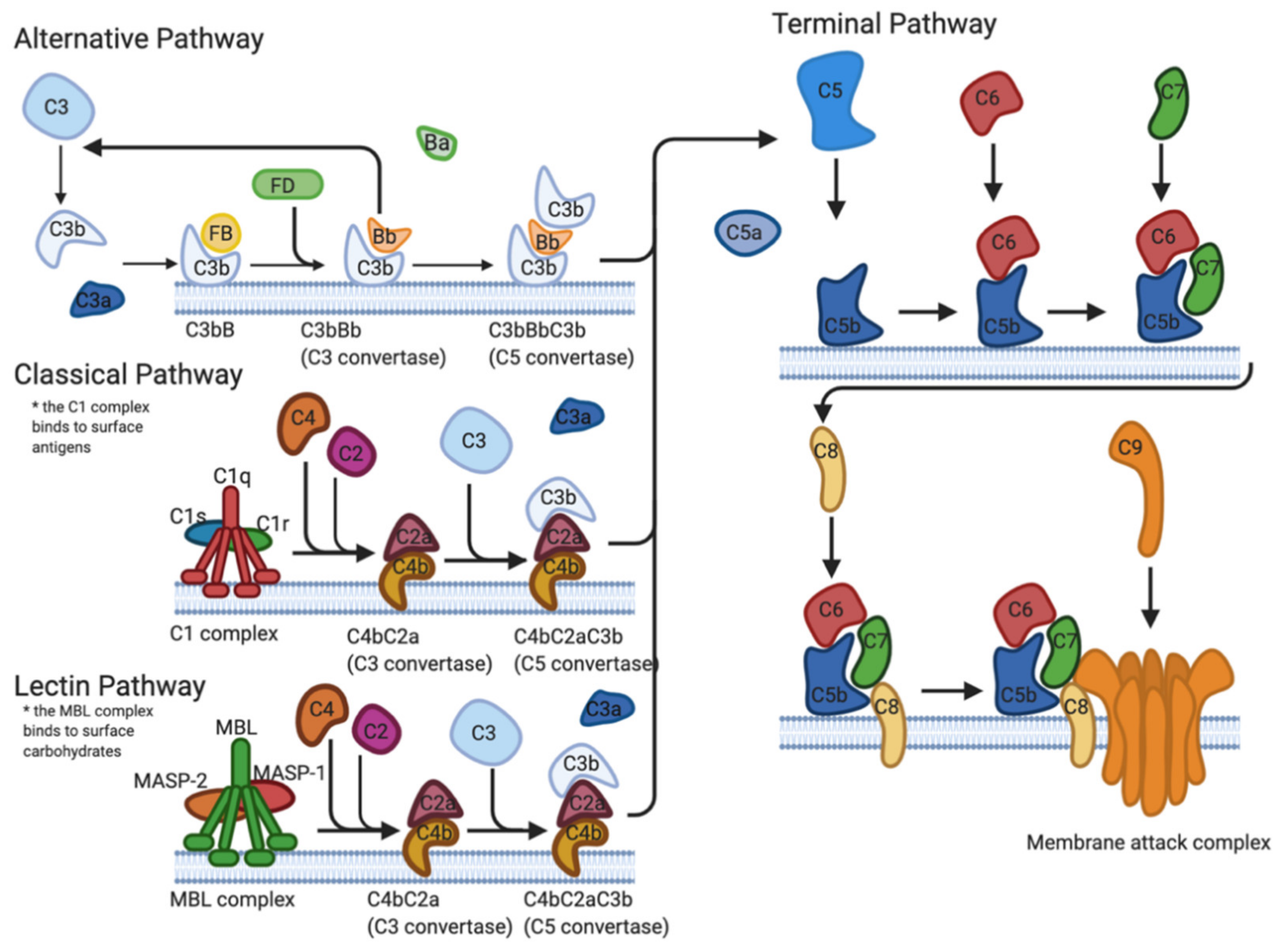
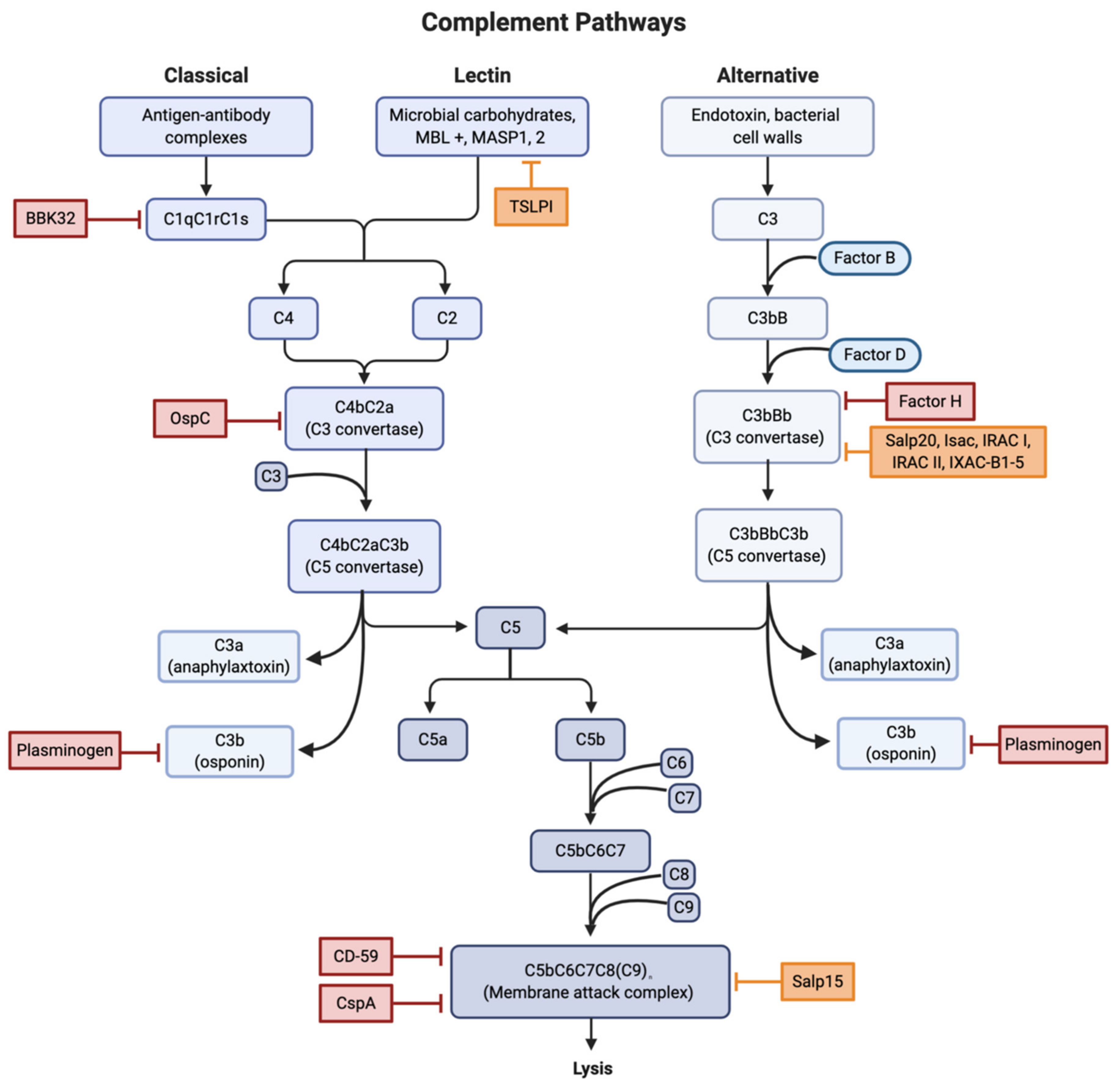
2.1. Complement Cascade
2.1.1. Direct Interference
2.1.2. Binding of Regulators
2.1.3. Tick Salivary Proteins
2.2. Antimicrobial Protein and Peptide Resistance
2.3. Macrophage Interference
2.4. Disabling of Chemokines and Alarmin Molecules
2.5. Neutralize Reactive Oxygen Species
2.6. Pleomorphic Forms
2.7. Intracellular Localization
3. Adaptive Response
3.1. The Humoral Response
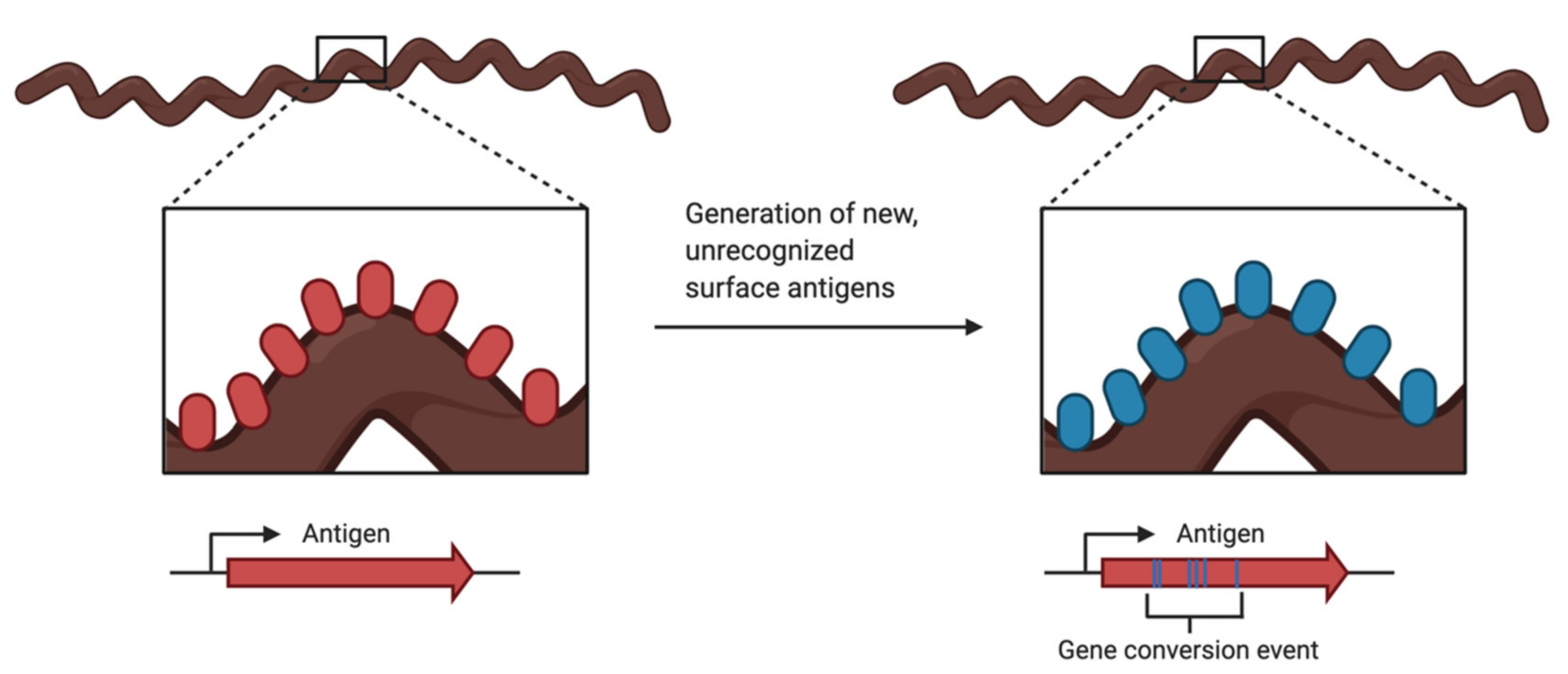
3.2. Antigenic Variation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steere, A.C.; Malawista, S.E.; Snydman, D.R.; Shope, R.E.; Andiman, W.A.; Ross, M.R.; Steele, F.M. An epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum. 1977, 20, 7–17. [Google Scholar] [CrossRef]
- Elbaum-Garfinkle, S. Close to home: A history of yale and lyme disease. Yale J. Biol. Med. 2011, 84, 103–108. [Google Scholar] [PubMed]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.R.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Prim. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Cerar, T.; Strle, F.; Stupica, D.; Ruzic-Sabljic, E.; McHugh, G.; Steere, A.C.; Strle, K. Differences in genotype, clinical features, and inflammatory potential of borrelia burgdorferi sensu stricto strains from Europe and the United States. Emerg. Infect. Dis. 2016, 22, 818–827. [Google Scholar] [CrossRef]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Sarma, J.V.; Ward, P.A. The complement system. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.W. Complement evasion in Borrelia spirochetes: Mechanisms and opportunities for intervention. Antibiotics 2019, 8, 80. [Google Scholar] [CrossRef]
- Ebady, R.; Niddam, A.F.; Boczula, A.E.; Kim, Y.R.; Gupta, N.; Tang, T.T.; Odisho, T.; Zhi, H.; Simmons, C.A.; Skare, J.T.; et al. Biomechanics of Borrelia burgdorferi Vascular Interactions. Cell Rep. 2016, 16, 2593–2604. [Google Scholar] [CrossRef]
- Fischer, J.R.; LeBlanc, K.T.; Leong, J.M. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect. Immun. 2006, 74, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.L.; Zhi, H.; Wager, B.; Höök, M.; Skare, J.T. Borrelia burgdorferi BBK32 Inhibits the Classical Pathway by Blocking Activation of the C1 Complement Complex. PLoS Pathog. 2016, 12, 1–28. [Google Scholar] [CrossRef]
- Barthel, D.; Schindler, S.; Zipfel, P.F. Plasminogen is a complement inhibitor. J. Biol. Chem. 2012, 287, 18831–18842. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.; Wallich, R.; Simon, M.M.; Kramer, M.D. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 12594–12598. [Google Scholar] [CrossRef]
- Önder, Ö.; Humphrey, P.T.; McOmber, B.; Korobova, F.; Francella, N.; Greenbaum, D.C.; Brisson, D. OspC is potent plasminogen receptor on surface of borrelia burgdorferi. J. Biol. Chem. 2012, 287, 16860–16868. [Google Scholar] [CrossRef] [PubMed]
- Caine, J.A.; Lin, Y.P.; Kessler, J.R.; Sato, H.; Leong, J.M.; Coburn, J. Borrelia burgdorferi outer surface protein C (OspC) binds complement component C4b and confers bloodstream survival. Cell. Microbiol. 2017, 19, 1–14. [Google Scholar] [CrossRef]
- Koenigs, A.; Hammerschmidt, C.; Jutras, B.L.; Pogoryelov, D.; Barthel, D.; Skerka, C.; Kugelstadt, D.; Wallich, R.; Stevenson, B.; Zipfel, P.F.; et al. BBA70 of borrelia burgdorferi is a novel plasminogen-binding protein. J. Biol. Chem. 2013, 288, 25229–25243. [Google Scholar] [CrossRef] [PubMed]
- Hallström, T.; Haupt, K.; Kraiczy, P.; Hortschansky, P.; Wallich, R.; Skerka, C.; Zipfel, P.F. Complement Regulator–Acquiring Surface Protein 1 of Borrelia burgdorferi Binds to Human Bone Morphogenic Protein 2, Several Extracellular Matrix Proteins, and Plasminogen. J. Infect. Dis. 2010, 202, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Kraiczy, P.; Hellwage, J.; Skerka, C.; Becker, H.; Kirschfink, M.; Simon, M.M.; Brade, V.; Zipfel, P.F.; Wallich, R. Complement Resistance of Borrelia burgdorferi Correlates with the Expression of BbCRASP-1, a Novel Linear Plasmid-encoded Surface Protein That Interacts with Human Factor H and FHL-1 and Is Unrelated to Erp Proteins. J. Biol. Chem. 2004, 279, 2421–2429. [Google Scholar] [CrossRef]
- Kraiczy, P.; Skerka, C.; Kirschfink, M.; Brade, V. Further Characterization of Complement Regulator-Acquiring Surface Proteins of. Society 2001, 69, 7800–7809. [Google Scholar] [CrossRef]
- Hallström, T.; Siegel, C.; Mörgelin, M.; Kraiczy, P.; Skerka, C.; Zipfel, P.F. CspA from Borrelia burgdorferi inhibits the terminal complement pathway. MBio 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Hart, T.; Nguyen, N.T.T.; Nowak, N.A.; Zhang, F.; Linhardt, R.J.; Diuk-Wasser, M.; Ram, S.; Kraiczy, P.; Lin, Y.P. Polymorphic Factor H-Binding Activity of CspA Protects Lyme Borreliae from the Host Complement in Feeding Ticks to Facilitate Tick-to-Host Transmission. PLoS Pathog. 2018, 14, 21007106. [Google Scholar] [CrossRef]
- Kenedy, M.R.; Vuppala, S.R.; Siegel, C.; Kraiczy, P.; Akins, D.R. CspA-mediated binding of human factor H inhibits complement deposition and confers serum resistance in Borrelia burgdorferi. Infect. Immun. 2009, 77, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Brissette, C.A.; Haupt, K.; Barthel, D.; Cooley, A.E.; Bowman, A.; Skerka, C.; Wallich, R.; Zipfel, P.F.; Kraiczy, P.; Stevenson, B. Borrelia burgdorferi infection-associated surface proteins ErpP, ErpA, and ErpC bind human plasminogen. Infect. Immun. 2009, 77, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, C.; Klevenhaus, Y.; Koenigs, A.; Hallström, T.; Fingerle, V.; Skerka, C.; Pos, K.M.; Zipfel, P.F.; Wallich, R.; Kraiczy, P. BGA66 and BGA71 facilitate complement resistance of Borrelia bavariensis by inhibiting assembly of the membrane attack complex. Mol. Microbiol. 2016, 99, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Pausa, M.; Pellis, V.; Cinco, M.; Giulianini, P.G.; Presani, G.; Perticarari, S.; Murgia, R.; Tedesco, F. Serum-Resistant Strains of Borrelia burgdorferi Evade Complement-Mediated Killing by Expressing a CD59-Like Complement Inhibitory Molecule. J. Immunol. 2003, 170, 3214–3222. [Google Scholar] [CrossRef]
- Józsi, M.; Tortajada, A.; Uzonyi, B.; Goicoechea de Jorge, E.; Rodríguez de Córdoba, S. Factor H-related proteins determine complement-activating surfaces. Trends Immunol. 2015, 36, 374–384. [Google Scholar] [CrossRef]
- Kraiczy, P.; Stevenson, B. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: Structure, function and regulation of gene expression. Ticks Tick. Borne Dis. 2013, 4, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Coleman, A.S.; Yang, X.; Kumar, M.; Zhang, X.; Promnares, K.; Shroder, D.; Kenedy, M.R.; Anderson, J.F.; Akins, D.R.; Pal, U. Borrelia burgdorferi Complement Regulator-Acquiring Surface Protein 2 does not contribute to complement resistance or host infectivity. PLoS ONE 2008, 3, 1–9. [Google Scholar] [CrossRef]
- Bykowski, T.; Woodman, M.E.; Cooley, A.E.; Brissette, C.A.; Brade, V.; Wallich, R.; Kraiczy, P.; Stevenson, B. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the lyme disease spirochete’s mammal-tick infection cycle. Infect. Immun. 2007, 75, 4227–4236. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Frye, A.M.; Nowak, T.A.; Kraiczy, P. New Insights Into CRASP-Mediated Complement Evasion in the Lyme Disease Enzootic Cycle. Front. Cell. Infect. Microbiol. 2020, 10, 1. [Google Scholar] [CrossRef]
- Hefty, P.S.; Jolliff, S.E.; Caimano, M.J.; Wikel, S.K.; Radolf, J.D.; Akins, D.R. Regulation of OspE-related, OspF-related, and Elp lipoproteins of Borrelia burgdorferi strain 297 by mammalian host-specific signals. Infect. Immun. 2001, 69, 3618–3627. [Google Scholar] [CrossRef]
- Miller, J.C.; Von Lackum, K.; Babb, K.; McAlister, J.D.; Stevenson, B. Temporal Analysis of Borrelia burgdorferi Erp Protein Expression throughout the Mammal-Tick Infectious Cycle. Infect. Immun. 2003, 71, 6943–6952. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.C.; Narayan, K.; Stevenson, B.; Pachner, A.R. Expression of Borrelia burgdorferi erp genes during infection of non-human primates. Microb. Pathog. 2005, 39, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Hellwage, J.; Meri, T.; Heikkilä, T.; Alitalo, A.; Panelius, J.; Lahdenne, P.; Seppälä, I.J.T.; Meri, S. The Complement Regulator Factor H Binds to the Surface Protein OspE of Borrelia burgdorferi. J. Biol. Chem. 2001, 276, 8427–8435. [Google Scholar] [CrossRef]
- Zipfel, P.F.; Skerka, C.; Hellwage, J.; Jokiranta, S.T.; Meri, S.; Brade, V.; Kraiczy, P.; Noris, M.; Remuzzi, G. Structure-function studies of the complement system. Biochem. Soc. Trans. 2002, 30, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.; Hallström, T.; Skerka, C.; Eberhardt, H.; Uzonyi, B.; Beckhaus, T.; Karas, M.; Wallich, R.; Stevenson, B.; Zipfel, P.F.; et al. Complement factor H-related proteins CFHR2 and CFHR5 represent novel ligands for the infection-associated CRASP proteins of Borrelia burgdorferi. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Nuttall, P.A. Tick saliva and its role in pathogen transmission. Wien. Klin. Wochenschr. 2019. [Google Scholar] [CrossRef]
- Schwan, T.G.; Piesman, J.; Golde, W.T.; Dolan, M.C.; Rosa, P.A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 1995, 92, 2909–2913. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, N.; Narasimhan, S.; Pal, U.; Bao, F.; Yang, X.F.; Fish, D.; Anguita, J.; Norgard, M.V.; Kantor, F.S.; Anderson, J.F.; et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 2005, 436, 573–577. [Google Scholar] [CrossRef]
- Pal, U.; Yang, X.; Chen, M.; Bockenstedt, L.K.; Anderson, J.F.; Flavell, R.A.; Norgard, M.V.; Fikrig, E. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Investig. 2004, 113, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Schuijt, T.J.; Hovius, J.W.R.; Van Burgel, N.D.; Ramamoorthi, N.; Fikrig, E.; Van Dam, A.P. The tick salivary protein Salp15 inhibits the killing of serum-sensitive Borrelia burgdorferi sensu lato isolates. Infect. Immun. 2008, 76, 2888–2894. [Google Scholar] [CrossRef]
- Wen, S.; Wang, F.; Ji, Z.; Pan, Y.Y.; Jian, M.; Bi, Y.F.; Zhou, G.; Luo, L.; Chen, T.; Li, L.; et al. Salp15, a Multifunctional Protein From Tick Saliva With Potential Pharmaceutical Effects. Front. Immunol. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Tyson, K.R.; Elkins, C.; de Silva, A.M. A Novel Mechanism of Complement Inhibition Unmasked by a Tick Salivary Protein That Binds to Properdin. J. Immunol. 2008, 180, 3964–3968. [Google Scholar] [CrossRef]
- Tyson, K.; Elkins, C.; Patterson, H.; Fikrig, E.; De Silva, A. Biochemical and functional characterization of Salp20, an Ixodes scapularis tick salivary protein that inhibits the complement pathway. Insect Mol. Biol. 2007, 16, 469–479. [Google Scholar] [CrossRef]
- Hourcade, D.E.; Akk, A.M.; Mitchell, L.M.; Zhou, H.; Hauhart, R.; Pham, C.T.N. Anti-complement activity of the Ixodes scapularis salivary protein Salp20. Mol. Immunol. 2016, 69, 62–69. [Google Scholar] [CrossRef]
- Mans, B.J. Chemical equilibrium at the tick-host feeding interface: A critical examination of biological relevance in hematophagous behavior. Front Physiol. 2019, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, B.; Beaufays, J.; Charon, C.; Lahaye, K.; Gensale, F.; Denis, V.; Charloteaux, B.; Decrem, Y.; Prévôt, P.P.; Brossard, M.; et al. Variability and action mechanism of a family of anticomplement proteins in ixodes ricinus. PLoS ONE 2008, 3. [Google Scholar] [CrossRef]
- Medicus, R.G.; Gotze, O.; Muller-Eberhard, H.J. Alternative pathway of complement: Recruitment of precursor properdin by the labile C3/ C5 convertsade and the potentiation of the pathway. J. Exp. Med. 1976, 144, 1076–1093. [Google Scholar] [CrossRef]
- Farries, T.C.; Lachmann, P.J.; Harrison, R.A. Analysis of the interactions between properdin, the third component of complement (C3), and its physiological activation products. Biochem. J. 1988, 252, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Alcorlo, M.; Tortajada, A.; De Córdoba, S.R.; Llorca, O. Structural basis for the stabilization of the complement alternative pathway C3 convertase by properdin. Proc. Natl. Acad. Sci. USA 2013, 110, 13504–13509. [Google Scholar] [CrossRef] [PubMed]
- Wagemakers, A.; Coumou, J.; Schuijt, T.J.; Oei, A.; Nijhof, A.M.; Van ’T Veer, C.; Van Der Poll, T.; Bins, A.D.; Hovius, J.W.R. An Ixodes ricinus Tick Salivary Lectin Pathway Inhibitor Protects Borrelia burgdorferi sensu lato from Human Complement. Vector-Borne Zoonotic Dis. 2016, 16, 223–228. [Google Scholar] [CrossRef]
- Schuijt, T.J.; Coumou, J.; Narasimhan, S.; Dai, J.; Deponte, K.; Wouters, D.; Brouwer, M.; Oei, A.; Roelofs, J.J.T.H.; Van Dam, A.P.; et al. A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the Lyme disease agent. Cell Host Microbe 2011, 10, 136–146. [Google Scholar] [CrossRef]
- Lusitani, D.; Malawista, S.E.; Montgomery, R.R. Borrelia burgdorferi Are Susceptible to Killing by a Variety of Human Polymorphonuclear Leukocyte Components. J. Infect. Dis. 2002, 185, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Tilly, K.; Stewart, P.; Bestor, A.; Battisti, J.M.; Rosa, P.A. Borrelia burgdorferi resistance to a major skin antimicrobial peptide is independent of outer surface lipoprotein content. Antimicrob. Agents Chemother. 2009, 53, 4490–4494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Troxell, B.; Xu, H.; Yang, X.F. Borrelia burgdorferi, a pathogen that lacks iron, encodes manganese-dependent superoxide dismutase essential for resistance to streptonigrin. J. Biol. Chem. 2012, 287, 19284–19293. [Google Scholar] [CrossRef] [PubMed]
- Sambri, V.; Marangoni, A.; Giacani, L.; Gennaro, R.; Murgia, R.; Cevenini, R.; Cinco, M. Comparative in vitro activity of five cathelicidin-derived sythetic peptides against Leptospira, Borrelia and Treponema pallidum. J. Antimicrob. Chemother. 2002, 50, 895–902. [Google Scholar] [CrossRef]
- Marchal, C.; Schramm, F.; Kern, A.; Luft, B.J.; Yang, X.; Schuijt, T.; Hovius, J.; Jaulhac, B.; Boulanger, N. Antialarmin effect of tick saliva during the transmission of lyme disease. Infect. Immun. 2011, 79, 774–785. [Google Scholar] [CrossRef]
- Bernard, Q.; Smith, A.A.; Yang, X.; Koci, J.; Foor, S.D.; Cramer, S.D.; Zhuang, X.; Dwyer, J.E.; Lin, Y.P.; Mongodin, E.F.; et al. Plasticity in early immune evasion strategies of a bacterial pathogen. Proc. Natl. Acad. Sci. USA 2018, 115, E3788–E3797. [Google Scholar] [CrossRef]
- Chung, Y.; Zhang, N.; Wooten, R.M. Borrelia burgdorferi elicited-IL-10 suppresses the production of inflammatory mediators, phagocytosis, and expression of co-stimulatory receptors by murine macrophages and/or dendritic cells. PLoS ONE 2013, 8, 1–13. [Google Scholar] [CrossRef]
- Lazarus, J.J.; Meadows, M.J.; Lintner, R.E.; Wooten, R.M. IL-10 Deficiency Promotes Increased Borrelia burgdorferi Clearance Predominantly through Enhanced Innate Immune Responses. J. Immunol. 2006, 177, 7076–7085. [Google Scholar] [CrossRef]
- Brown, J.P.; Zachary, J.F.; Teuscher, C.; Weis, J.J.; Wooten, R.M. Dual role of interleukin-10 in murine lyme disease: Regulation of arthritis severity and host defense. Infect. Immun. 1999, 67, 5142–5150. [Google Scholar] [CrossRef]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef]
- Hume, D.A. Macrophages as APC and the Dendritic Cell Myth. J. Immunol. 2008, 181, 5829–5835. [Google Scholar] [CrossRef]
- Hayward, J.; Sanchez, J.; Perry, A.; Huang, C.; Rodriguez Valle, M.; Canals, M.; Payne, R.J.; Stone, M.J. Ticks from diverse genera encode chemokine-inhibitory evasin proteins. J. Biol. Chem. 2017, 292, 15670–15680. [Google Scholar] [CrossRef]
- Crijns, H.; Vanheule, V.; Proost, P. Targeting Chemokine—Glycosaminoglycan Interactions to Inhibit Inflammation. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Bhusal, R.P.; Eaton, J.R.O.; Chowdhury, S.T.; Power, C.A.; Proudfoot, A.E.I.; Stone, M.J.; Bhattacharya, S. Evasins: Tick Salivary Proteins that Inhibit Mammalian Chemokines. Trends Biochem. Sci. 2020, 45, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, J.J.; Tewary, P.; de la Rosa, G.; Yang, D. Alarmins initiate host defense. Adv. Exp. Med. Biol. 2007, 601, 185–194. [Google Scholar]
- Guerau-De-Arellano, M.; Huber, B.T. Chemokines and Toll-like receptors in Lyme disease pathogenesis. Trends Mol. Med. 2005, 11, 114–120. [Google Scholar] [CrossRef]
- Hirschfeld, M.; Kirschning, C.J.; Schwandner, R.; Wesche, H.; Weis, J.H.; Wooten, R.M.; Weis, J.J. Cutting edge: Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor. J. Immunol. 1999, 163, 2382–2386. [Google Scholar]
- Thannickal, V.J.; Fanburg, B.L. Reactive Oxygen Species in Cell Walls. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, L1005–L1028. [Google Scholar] [CrossRef]
- Boylan, J.A.; Lawrence, K.A.; Downey, J.S.; Gherardini, F.C. Borrelia burgdorferi membranes are the primary targets of reactive oxygen species. Mol. Microbiol. 2008, 68, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Gassent, M.D.; Elliot, N.L.; Seshu, J. sodA is essential for virulence of Borrelia burgdorferi in the murine model of Lyme disease. Mol. Microbiol. 2009, 71, 594–612. [Google Scholar] [CrossRef]
- Aguirre, J.D.; Clark, H.M.; McIlvin, M.; Vazquez, C.; Palmere, S.L.; Grab, D.J.; Seshu, J.; Hart, P.J.; Saito, M.; Culotta, V.C. A manganese-rich environment supports superoxide dismutase activity in a lyme disease pathogen, borrelia burgdorferi. J. Biol. Chem. 2013, 288, 8468–8478. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; He, M.; Oman, T.; Yang, X.F.; Norgard, M.V. A manganese transporter, BB0219 (BmtA), is required for virulence by the Lyme disease spirochete, Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 2009, 106, 3449–3454. [Google Scholar] [CrossRef]
- Meriläinen, L.; Herranen, A.; Schwarzbach, A.; Gilbert, L. Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms. Microbiology 2015, 161, 516–527. [Google Scholar] [CrossRef]
- Aberer, E.; Kersten, A.; Klade, H.; Poitschek, C.; Jurecka, W. Heterogeneity of Borrelia burgdorferi in the skin. Am. J. Dermatopathol. 1996, 18, 571–579. [Google Scholar] [CrossRef]
- Miklossy, J.; Kasas, S.; Zurn, A.D.; McCall, S.; Yu, S.; McGeer, P.L. Persisting atypical and cystic forms of Borrelia burgdorferi and local inflammation in Lyme neuroborreliosis. J. Neuroinflamm. 2008, 5, 1–18. [Google Scholar] [CrossRef]
- Murgia, R.; Cinco, M. Induction of cystic forms by different stress conditions in Borrelia burgdorferi. Apmis 2004, 112, 57–62. [Google Scholar] [CrossRef]
- Al-Robaiy, S.; Dihazi, H.; Kacza, J.; Seeger, J.; Schiller, J.; Huster, D.; Knauer, J.; Straubinger, R.K. Metamorphosis of Borrelia burgdorferi organisms—RNA, lipid and protein composition in context with the spirochetes’ shape. J. Basic Microbiol. 2010, 50, 5–17. [Google Scholar] [CrossRef]
- Brorson, S.H. Transformation of cystic forms of Borrelia burgdorferi to normal, mobile spirochetes. Infection 1997, 25, 240–246. [Google Scholar] [CrossRef]
- Smith, A.J.; Oertle, J.; Prato, D. Chronic Lyme Disease: Persistent Clinical Symptoms Related to Immune Evasion, Antibiotic Resistance and Various Defense Mechanisms of Borrelia burgdorferi. Open J. Med. Microbiol. 2014, 04, 252–260. [Google Scholar] [CrossRef]
- Pothineni, V.R.; Potula, H.S.K.; Ambati, A.; Mallajosyula, V.V.A.; Sridharan, B.; Inayathullah, M.; Ahmed, M.S.; Rajadas, J. Azlocillin can be the potential drug candidate against drug-tolerant Borrelia burgdorferi sensu stricto JLB31. Sci. Rep. 2020, 10, 3798. [Google Scholar] [CrossRef]
- Hodzic, E. Lyme borreliosis: Is there a preexisting (natural) variation in antimicrobial susceptibility among Borrelia burgdorferi strains? Bosn. J. Basic Med. Sci. 2015, 15, 1–13. [Google Scholar] [CrossRef][Green Version]
- Hodzic, E.; Imai, D.M.; Escobar, E. Generality of post-antimicrobial treatment persistence of Borrelia burgdorferi strains N40 and B31 in genetically susceptible and resistant mouse strains. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Bugrysheva, J.V.; Newman, S.A. Sleeper cells: The stringent response and persistence in the Borreliella (Borrelia) burgdorferi enzootic cycle. Environ. Microbiol. 2017, 19, 3846–3862. [Google Scholar] [CrossRef]
- Costerton, A.J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Sapi, E.; Bastian, S.L.; Mpoy, C.M.; Scott, S.; Rattelle, A.; Pabbati, N.; Poruri, A.; Burugu, D.; Theophilus, P.A.S.; Pham, T.V.; et al. Characterization of Biofilm Formation by Borrelia burgdorferi In Vitro. PLoS ONE 2012, 7, 1–11. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Barbour, A.G. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 1984, 57, 521–525. [Google Scholar]
- Srivastava, S.Y.; De Silva, A.M. Characterization of borrelia burgdorferi aggregates. Vector-Borne Zoonotic Dis. 2009, 9, 323–329. [Google Scholar] [CrossRef]
- Sapi, E.; Balasubramanian, K.; Poruri, A.; Maghsoudlou, J.S.; Socarras, K.M.; Timmaraju, A.V.; Filush, K.R.; Gupta, K.; Shaikh, S.; Theophilus, P.A.S.; et al. Evidence of in vivo existence of Borrelia biofilm in borrelial lymphocytomas. Eur. J. Microbiol. Immunol. 2016, 6, 9–24. [Google Scholar] [CrossRef]
- Grab, D.J.; Lanners, H.N.; Martin, L.N.; Chesney, J.; Cai, C.; Adkisson, H.D.; Bucala, R. Interaction of Borrelia burgdorferi with peripheral blood fibrocytes, antigen-presenting cells with the potential for connective tissue targeting. Mol. Med. 1999, 5, 46–54. [Google Scholar] [CrossRef]
- Montgomery, R.R.; Nathanson, M.H.; Malawista, S.E. The fate of Borrelia burgdorferi, the agent for Lyme disease, in mouse macrophages: Destruction, survival, recovery. J. Immunol. 1993, 150, 909–915. [Google Scholar]
- Ma, Y.; Sturrock, A.; Weis, J.J. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect. Immun. 1991, 59, 671–678. [Google Scholar] [CrossRef]
- Wu, J.; Weening, E.H.; Faske, J.B.; Höök, M.; Skare, J.T. Invasion of eukaryotic cells by Borrelia burgdorferi requires β1 integrins and Src kinase activity. Infect. Immun. 2011, 79, 1338–1348. [Google Scholar] [CrossRef]
- Janeway, C.J.; Travers, P.; Walport, M.; Shlomchik, M.J. The Humoral Immune Response. In Immunobiology: The Immune System in Healt and Disease; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Elsner, R.A.; Hastey, C.J.; Olsen, K.J.; Baumgarth, N. Suppression of Long-Lived Humoral Immunity Following Borrelia burgdorferi Infection. PLoS Pathog. 2015, 11, 1–19. [Google Scholar] [CrossRef]
- Hastey, C.J.; Elsner, R.A.; Barthold, S.W.; Baumgarth, N. Delays and diversions mark the development of B cell responses to Borrelia burgdoferi infection. J. Immunol. 2012, 188, 5612–5622. [Google Scholar] [CrossRef]
- Tunev, S.S.; Hastey, C.J.; Hodzic, E.; Feng, S.; Barthold, S.W.; Baumgarth, N. Lymphoadenopathy during lyme borreliosis is caused by spirochete migration-induced specific B cell activation. PLoS Pathog. 2011, 7, 20–24. [Google Scholar] [CrossRef]
- McKisic, M.D.; Barthold, S.W. T-cell-independent responses to Borrelia burgdorferi are critical for protective immunity and resolution of lyme disease. Infect. Immun. 2000, 68, 5190–5197. [Google Scholar] [CrossRef]
- Elsner, R.A.; Hastey, C.J.; Baumgarth, N. CD4+ T cells promote antibody production but not sustained affinity maturation during Borrelia burgdorferi infection. Infect. Immun. 2015, 83, 48–56. [Google Scholar] [CrossRef]
- Hastey, C.J.; Ochoa, J.; Olsen, K.J.; Barthold, S.W.; Baumgarth, N. MyD88- and TRIF-independent induction of Type I interferon drives naive B cell accumulation but not loss of lymph node architecture in lyme disease. Infect. Immun. 2014, 82, 1548–1558. [Google Scholar] [CrossRef]
- Good-Jacobson, K.L.; Shlomchik, M.J. Plasticity and Heterogeneity in the Generation of Memory B Cells and Long-Lived Plasma Cells: The Influence of Germinal Center Interactions and Dynamics. J. Immunol. 2010, 185, 3117–3125. [Google Scholar] [CrossRef] [PubMed]
- Menten-Dedoyart, C.; Couvreur, B.; Thellin, O.; Drion, P.V.; Herry, M.; Jolois, O.; Heinen, E. Influence of the Ixodes ricinus tick blood-feeding on the antigen-specific antibody response in vivo. Vaccine 2008, 26, 6956–6964. [Google Scholar] [CrossRef]
- Menten-Dedoyart, C.; Couvreur, B.; Jolois, O.; Van Lerberghe, P.B.; Duwez, L.; Drion, P.; Heinen, E. Kinetic study of the antibody response during the blood meal of Ixodes ricinus: Implication on plasma cell maturation in vivo and for anti-Ixodes vaccination. Vaccine 2011, 29, 2044–2050. [Google Scholar] [CrossRef]
- Anguita, J.; Ramamoorthi, N.; Hovius, J.W.R.; Das, S.; Thomas, V.; Persinski, R.; Conze, D.; Askenase, P.W.; Rincón, M.; Kantor, F.S.; et al. Salp15, an Ixodes scapularis salivary protein, inhibits CD4+ T cell activation. Immunity 2002, 16, 849–859. [Google Scholar] [CrossRef]
- Tomás-Cortázar, J.; Martín-Ruiz, I.; Barriales, D.; Pascual-Itoiz, M.Á.; De Juan, V.G.; Caro-Maldonado, A.; Merino, N.; Marina, A.; Blanco, F.J.; Flores, J.M.; et al. The immunosuppressive effect of the tick protein, Salp15, is long-lasting and persists in a murine model of hematopoietic transplant. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Vinuesa, C.G.; Linterman, M.A.; Goodnow, C.C.; Randall, K.L. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol. Rev. 2010, 237, 72–89. [Google Scholar] [CrossRef]
- Kalish, R.A.; McHugh, G.; Granquist, J.; Shea, B.; Ruthazer, R.; Steere, A.C. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10-20 years after active Lyme disease. Clin. Infect. Dis. 2001, 33, 780–785. [Google Scholar] [CrossRef]
- Craft, J.E.; Grodzicki, R.L.; Shrestha, M.; Fischer, D.K.; García-Blanco, M.; Steere, A.C. The antibody response in Lyme disease. Yale J. Biol. Med. 1984, 57, 561–565. [Google Scholar]
- Hilton, E.; Tramontano, A.; DeVoti, J.; Sood, S.K. Temoral study of immunglobulin M seroreactivity to Borrelia burgdorferi in patients treated for Lyme borreliosis. J. Clin. Microbiol. 1997, 35, 774–776. [Google Scholar] [CrossRef]
- Kalish, R.A.; Kaplan, R.F.; Taylor, E.; Jones-Woodward, L.; Steere, A.C. Evaluation of study patients with Lyme disease, 10-20-year follow-up. J. Infect. Dis. 2001, 183, 453–460. [Google Scholar] [CrossRef]
- Henriksson, A.; Link, H.; Cruz, M.; Stiernstdedt, G. Immunoglobulin abnormalities in cerebrospinal fluid and blood over the course of lymphocytic meningoradiculitis (Bannwarth’s syndrome). Ann. Neurol. 1986, 20, 337–345. [Google Scholar] [CrossRef]
- Hammers-Berggren, S.; Lecech, A.M.; Karlsson, M.; Andersson, U.; Hansen, K.; Stiernstedt, G. Serological follow-up after treatment of Borrelia arthritis and acrodermatitis chronica atrophicans. Scand. J. Infect. Dis. 1994, 26, 339–347. [Google Scholar] [CrossRef]
- Chaconas, G.; Castellanos, M.; Verhey, T.B. Changing of the guard: How the Lyme disease spirochete subverts the host immune response. J. Biol. Chem. 2020, 295, 301–313. [Google Scholar] [CrossRef]
- Zhang, J.-R.; Hardham, J.M.; Barbour, A.G.; Norris, S.J. Antigenic VAriation in Lyme Disease Borreliae by Promiscuous Recombination of VMP-like Sequence Cassettes. Cell 1997, 89, 275–285. [Google Scholar] [CrossRef]
- Norris, S.J. vls Antigenic Variation Systems of Lyme Disease Borrelia: Eluding Host Immunity through both Random, Segmental Gene Conversion and Framework Heterogeneity. Microbiol. Spectr. 2015, 2, 1–29. [Google Scholar] [CrossRef]
- Purser, J.E.; Norris, S.J. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 2000, 97, 13865–13870. [Google Scholar] [CrossRef]
- Labandeira-Rey, M.; Skare, J.T. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 2001, 69, 446–455. [Google Scholar] [CrossRef]
- Bankhead, T.; Chaconas, G. The role of VlsE antigenic variation in the Lyme disease spirochete: Persistence through a mechanism that differs from other pathogens. Mol. Microbiol. 2007, 65, 1547–1558. [Google Scholar] [CrossRef]
- Rogovskyy, A.S.; Casselli, T.; Tourand, Y.; Jones, C.R.; Owen, J.P.; Mason, K.L.; Scoles, G.A.; Bankhead, T. Evaluation of the importance of VlsE antigenic variation for the enzootic cycle of Borrelia burgdorferi. PLoS ONE 2015, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, T. Role of the VlsE lipoprotein in immune avoidance by the lyme disease spirochete borrelia burgdorferi. For. Immunopathol. Dis. Ther. 2016, 7, 191–203. [Google Scholar] [CrossRef]
- Eicken, C.; Sharma, V.; Klabunde, T.; Lawrenz, M.B.; Hardham, J.M.; Norris, S.J.; Sacchettini, J.C. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J. Biol. Chem. 2002, 277, 21691–21696. [Google Scholar] [CrossRef] [PubMed]
- Hudson, C.R.; Frye, J.G.; Quinn, F.D.; Gherardini, F.C. Increased expression of Borrelia burgdorferi vlsE in response to human endothelial cell membranes. Mol. Microbiol. 2001, 41, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Brázda, V.; Coufal, J.; Liao, J.C.C.; Arrowsmith, C.H. Preferential binding of IFI16 protein to cruciform structure and superhelical DNA. Biochem. Biophys. Res. Commun. 2012, 422, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Bikard, D.; Loot, C.; Baharoglu, Z.; Mazel, D. Folded DNA in Action: Hairpin Formation and Biological Functions in Prokaryotes. Microbiol. Mol. Biol. Rev. 2010, 74, 570–588. [Google Scholar] [CrossRef] [PubMed]
- Brázda, V.; Laister, R.C.; Jagelská, E.B.; Arrowsmith, C. Cruciform structures are a common DNA feature important for regulating biological processes. BMC Mol. Biol. 2011, 12. [Google Scholar] [CrossRef]
- Walia, R.; Chaconas, G. Suggested Role for G4 DNA in Recombinational Switching at the Antigenic Variation Locus of the Lyme Disease Spirochete. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Dahan, D.; Tsirkas, I.; Dovrat, D.; Sparks, M.A.; Singh, S.P.; Galletto, R.; Aharoni, A. Pif1 is essential for efficient replisome progression through lagging strand G-quadruplex DNA secondary structures. Nucleic Acids Res. 2018, 46, 11847–11857. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Le Piazza, A.; Bermejo, R.; Kriegsman, B.; Colosio, A.; Teulade-Fichou, M.P.; Foiani, M.; Nicolas, A. G-quadruplex-induced instability during leading-strand replication. EMBO J. 2011, 30, 4033–4046. [Google Scholar] [CrossRef]
- Kolinjivadi, A.M.; Sannino, V.; de Antoni, A.; Técher, H.; Baldi, G.; Costanzo, V. Moonlighting at replication forks—A new life for homologous recombination proteins BRCA1, BRCA2 and RAD51. FEBS Lett. 2017, 591, 1083–1100. [Google Scholar] [CrossRef]
- Polleys, E.J.; House, N.C.M.; Freudenreich, C.H. Role of recombination and replication fork restart in repeat instability. DNA Repair (Amst.) 2017, 56, 156–165. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, C.; Brissette, C.A. The Brilliance of Borrelia: Mechanisms of Host Immune Evasion by Lyme Disease-Causing Spirochetes. Pathogens 2021, 10, 281. https://doi.org/10.3390/pathogens10030281
Anderson C, Brissette CA. The Brilliance of Borrelia: Mechanisms of Host Immune Evasion by Lyme Disease-Causing Spirochetes. Pathogens. 2021; 10(3):281. https://doi.org/10.3390/pathogens10030281
Chicago/Turabian StyleAnderson, Cassidy, and Catherine A. Brissette. 2021. "The Brilliance of Borrelia: Mechanisms of Host Immune Evasion by Lyme Disease-Causing Spirochetes" Pathogens 10, no. 3: 281. https://doi.org/10.3390/pathogens10030281
APA StyleAnderson, C., & Brissette, C. A. (2021). The Brilliance of Borrelia: Mechanisms of Host Immune Evasion by Lyme Disease-Causing Spirochetes. Pathogens, 10(3), 281. https://doi.org/10.3390/pathogens10030281





