Molecular Characterization of Carbapenem Resistant Klebsiella pneumoniae in Malaysia Hospital
Abstract
1. Introduction
2. Results
2.1. Overview of Carbapenem Resistant Klebsiella pneumoniae Cases
2.2. Determination of Carbapenemases and Porin-Associated Genes
2.3. Genetic Relatedness between CRKP Strains
2.4. Statistical Analyses
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Data Collection and Bacterial Strains
4.3. PCR Detection of Carbapenemase Genes and Porin-Associated Genes
4.4. Pulsed-Field Gel Electrophoresis (PFGE)
4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munoz Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef]
- Nordmann, P.; Cuzon, G.; Naas, T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 2009, 9, 228–236. [Google Scholar] [CrossRef]
- Yigit, H.; Queenan, A.M.; Anderson, G.J.; Domenech-Sanchez, A.; Biddle, J.W.; Steward, C.D.; Alberti, S.; Bush, K.; Tenover, F.C. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Naas, T.; Poirel, L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791. [Google Scholar] [CrossRef]
- Gröbner, S.; Linke, D.; Schütz, W.; Fladerer, C.; Madlung, J.; Autenrieth, I.B.; Witte, W.; Pfeifer, Y. Emergence of carbapenem-non-susceptible extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolates at the university hospital of Tübingen, Germany. J. Med. Microbiol. 2009, 58, 912–922. [Google Scholar] [CrossRef]
- Aubert, D.; Naas, T.; Héritier, C.; Poirel, L.; Nordmann, P. Functional characterization of IS1999, an IS4 family element involved in mobilization and expression of β-lactam resistance genes. J. Bacteriol. 2006, 188, 6506–6514. [Google Scholar] [CrossRef]
- Tzouvelekis, L.; Markogiannakis, A.; Psichogiou, M.; Tassios, P.; Daikos, G. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin. Microbiol. Rev. 2012, 25, 682–707. [Google Scholar] [CrossRef]
- Palasubramaniam, S.; Karunakaran, R.; Gin, G.G.; Muniandy, S.; Parasakthi, N. Imipenem-resistance in Klebsiella pneumoniae in Malaysia due to loss of OmpK36 outer membrane protein coupled with AmpC hyperproduction. Int. J. Infect. Dis. 2007, 11, 472–474. [Google Scholar] [CrossRef]
- Abidin, N.Z.Z.; Sulong, A.; Alfizah, H.; Muttaqillah, N.A.S.; Ding, C.H. Molecular detection of the New Delhi metallo-β-lactamase-1 gene in Enterobacteriaceae isolates in a tertiary medical centre. Malays. J. Pathol. 2015, 37, 227–232. [Google Scholar]
- Hamzan, N.I.; Chan, Y.Y.; Abdul Rahman, R.; Hasan, H.; Abdul Rahman, Z. Detection of blaIMP4 and blaNDM1 harboring Klebsiella pneumoniae isolates in a university hospital in Malaysia. Emerg. Health Threat. J. 2015, 8, 26011. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Hashim, R.; Shukor, S.; Khalid, K.N.M.; Shamsudin, F.; Hussin, H. Characterization of the first isolate of Klebsiella pneumoniae carrying New Delhi metallo-β-lactamase and other extended spectrum β-lactamase genes from Malaysia. J. Med. Microbiol. 2013, 62, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Al-Marzooq, F.; Ngeow, Y.F.; Tay, S.T. Emergence of Klebsiella pneumoniae producing dual carbapenemases (NDM-1 and OXA-232) and 16S rRNA methylase (armA) isolated from a Malaysian patient returning from India. Int. J. Antimicrob. Agents 2015, 4, 445–446. [Google Scholar] [CrossRef]
- Zaidah, A.R.; Mohammad, N.I.; Suraiya, S.; Harun, A. High burden of Carbapenem-resistant Enterobacteriaceae (CRE) fecal carriage at a teaching hospital: Cost-effectiveness of screening in low-resource setting. Antimicrob. Resist. Infect. Control 2017, 6, 42. [Google Scholar] [CrossRef]
- Low, Y.M.; Yap, P.S.X.; Abdul Jabar, K.; Ponnampalavanar, S.; Karunakaran, R.; Velayuthan, R.; Chong, C.W.; Abu Bakar, S.; Md Yusof, M.Y.; Teh, C.S.J. The emergence of carbapenem resistant Klebsiella pneumoniae in Malaysia: Correlation between microbiological trends with host characteristics and clinical factors. Antimicrob. Resist. Infect. Control 2017, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Kashiwa, M.; Arai, K.; Nagano, N.; Saito, R. Comparison of the Modified-Hodge test, Carba NP test, and carbapenem inactivation method as screening methods for carbapenemase-producing Enterobacteriaceae. J. Microbiol. Methods 2016, 128, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Girlich, D.; Poirel, L.; Nordmann, P. Value of the modified Hodge test for detection of emerging carbapenemases in Enterobacteriaceae. J. Clin. Microbiol. 2012, 50, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Carvalhaes, C.G.; Picao, R.C.; Nicoletti, A.G.; Xavier, D.E.; Gales, A.C. Cloverleaf test (modified Hodge test) for detecting carbapenemase production in Klebsiella pneumoniae: Be aware of false positive results. J. Antimicrob. Chemother. 2010, 65, 249–251. [Google Scholar] [CrossRef]
- Tamma, P.D.; Simner, P.J. Phenotypic Detection of Carbapenemase-Producing Organisms from Clinical Isolates. J. Clin. Microbiol. 2018, 56, e01140-18. [Google Scholar] [CrossRef]
- Nordmann, P.; Poirel, L. The difficult to control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin. Microbiol. Infect. 2014, 20, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile β-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Oueslati, S.; Nordmann, P.; Poirel, L. Heterogeneous hydrolytic features for OXA-48-like β-lactamases. J. Antimicrob. Chemother. 2015, 70, 1059–1063. [Google Scholar] [CrossRef]
- Walther-Rasmussen, J.; Høiby, N. OXA-type carbapenemases. J. Antimicrob. Chemother. 2006, 57, 373–383. [Google Scholar] [CrossRef]
- Goodman, K.; Simner, P.; Tamma, P.; Milstone, A. Infection control implications of heterogeneous resistance mechanisms in carbapenem-resistant Enterobacteriaceae (CRE). Expert Rev. Anti-Infect. Ther. 2016, 14, 95–108. [Google Scholar] [CrossRef]
- Pereira, P.S.; Borghi, M.; de Araújo, C.F.M.; Aires, C.A.M.; Oliveira, J.C.R.; Asensi, M.D.; Carvalho-Assef, A.P.D.A. Clonal dissemination of OXA-370-producing Klebsiella pneumoniae in Rio de Janeiro, Brazil. Antimicrob. Agents Chemother. 2015, 59, 4453–4456. [Google Scholar] [CrossRef]
- Potron, A.; Poirel, L.; Nordmann, P. Derepressed transfer properties leading to the efficient spread of the plasmid encoding carbapenemase OXA-48. Antimicrob. Agents Chemother. 2014, 58, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef]
- Johnson, A.P.; Woodford, N. Global spread of antibiotic resistance: The example of New Delhi metallo-β-lactamase (NDM)-mediated carbapenem resistance. J. Med. Microbiol. 2013, 62, 499–513. [Google Scholar] [CrossRef]
- Hsu, L.-Y.; Apisarnthanarak, A.; Khan, E.; Suwantarat, N.; Ghafur, A.; Tambyah, P.A. Carbapenem-resistant Acinetobacter baumannii and Enterobacteriaceae in south and southeast Asia. Clin. Microbiol. Rev. 2017, 30, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Weeks, J.; Livermore, D.M.; Toleman, M.A. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: An environmental point prevalence study. Lancet Infect. Dis. 2011, 11, 355–362. [Google Scholar] [CrossRef]
- Al-Marzooq, F.; Yusof, M.Y.M.; Tay, S.T. Molecular analysis of antibiotic resistance determinants and plasmids in Malaysian isolates of multidrug resistant Klebsiella pneumoniae. PLoS ONE 2015, 10, e0133654. [Google Scholar] [CrossRef]
- Yan, J.; Pu, S.; Jia, X.; Xu, X.; Yang, S.; Shi, J.; Sun, S.; Zhang, L. Multidrug resistance mechanisms of carbapenem resistant Klebsiella pneumoniae strains isolated in Chongqing, China. Ann. Lab. Med. 2017, 37, 398–407. [Google Scholar] [CrossRef][Green Version]
- Liew, S.M.; Rajasekaram, G.; Puthucheary, S.D.; Chua, K.H. Detection of VIM-2-, IMP-1-and NDM-1-producing multidrug-resistant Pseudomonas aeruginosa in Malaysia. J. Glob. Antimicrob. Resist. 2018, 13, 271–273. [Google Scholar] [CrossRef]
- Lai, K.; Ma, Y.; Guo, L.; An, J.; Ye, L.; Yang, J. Molecular characterization of clinical IMP-producing Klebsiella pneumoniae isolates from a Chinese Tertiary Hospital. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 42. [Google Scholar] [CrossRef]
- Yan, J.J.; Ko, W.C.; Tsai, S.H.; Wu, H.M.; Wu, J.J. Outbreak of infection with multidrug-resistant Klebsiella pneumoniae carrying bla IMP-8 in a university medical center in Taiwan. J. Clin. Microbiol. 2001, 39, 4433–4439. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, L.; Pascual, A.; Hernández-Allés, S.; Alvarez-Díaz, D.; Suárez, A.I.; Tran, J.; Benedí, V.J.; Jacoby, G.A. Roles of β-lactamases and porins in activities of carbapenems and cephalosporins against Klebsiella pneumoniae. Antimicrob. Agents Chemother. 1999, 43, 1669–1673. [Google Scholar] [CrossRef] [PubMed]
- Doumith, M.; Ellington, M.J.; Livermore, D.M.; Woodford, N. Molecular mechanisms disrupting porin expression in ertapenem-resistant Klebsiella and Enterobacter spp. clinical isolates from the UK. J. Antimicrob. Chemother. 2009, 63, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Neoh, H.-m.; Tan, X.-E.; Sapri, H.F.; Tan, T.L. Pulsed-field gel electrophoresis (PFGE): A review of the “gold standard” for bacteria typing and current alternatives. Infect. Genet. Evol. 2019, 74, 103935. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, Y.; Du, M.; Bai, Y.; Liu, B.; Gong, M.; Song, H.; Tong, Y.; Liu, Y. Prospective investigation of carbapenem-resistant Klebsiella pneumonia transmission among the staff, environment and patients in five major intensive care units, Beijing. J. Hosp. Infect. 2019, 101, 150–157. [Google Scholar] [CrossRef]
- Snitkin, E.S.; Zelazny, A.M.; Thomas, P.J.; Stock, F.; Henderson, D.K.; Palmore, T.N.; Segre, J.A.; Program, N.C.S. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci. Transl. Med. 2012, 4, ra116–ra148. [Google Scholar] [CrossRef]
- Swaminathan, M.; Sharma, S.; Blash, S.P.; Patel, G.; Banach, D.B.; Phillips, M.; LaBombardi, V.; Anderson, K.F.; Kitchel, B.; Srinivasan, A. Prevalence and risk factors for acquisition of carbapenem-resistant Enterobacteriaceae in the setting of endemicity. Infect. Control Hosp. Epidemiol. 2013, 34, 809–817. [Google Scholar] [CrossRef]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.P.; Lin, Y.T.; Lin, J.C.; Chen, T.L.; Yeh, K.M.; Chang, F.Y.; Chuang, H.C.; Wu, H.S.; Tseng, C.P.; Siu, L.K. Klebsiella pneumoniae in gastrointestinal tract and pyogenic liver abscess. Emerg. Infect. Dis. 2012, 18, 1322. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Bachman, M. Colonization, Infection, and the Accessory Genome of Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Correa, L.; Martino, M.D.V.; Siqueira, I.; Pasternak, J.; Gales, A.C.; Silva, C.V.; Camargo, T.Z.S.; Scherer, P.F.; Marra, A.R. A hospital-based matched case–control study to identify clinical outcome and risk factors associated with carbapenem-resistant Klebsiella pneumoniae infection. BMC Infect. Dis. 2013, 13, 80. [Google Scholar] [CrossRef]
- Patel, G.; Huprikar, S.; Factor, S.H.; Jenkins, S.G.; Calfee, D.P. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect. Control Hosp. Epidemiol. 2008, 29, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Basukala, P.; Basukala, O.; Parajuli, K.; Pokhrel, B.M.; Rijal, B.P. Detection of biofilm production and antibiotic resistance pattern in clinical isolates from indwelling medical devices. Curr. Microbiol. 2015, 70, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Pitout, J.D.; Nordmann, P.; Poirel, L. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob. Agents Chemother. 2015, 59, 5873–5884. [Google Scholar] [CrossRef]
- Xu, Y.; Gu, B.; Huang, M.; Liu, H.; Xu, T.; Xia, W.; Wang, T. Epidemiology of carbapenem resistant Enterobacteriaceae (CRE) during 2000–2012 in Asia. J. Thorac. Dis. 2015, 7, 376. [Google Scholar]
- Balm, M.N.; Ngan, G.; Jureen, R.; Lin, R.T.; Teo, J.W. OXA-181-producing Klebsiella pneumoniae establishing in Singapore. BMC Infect. Dis. 2013, 13, 58. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Dani, A. Colonization and infection. Cent. Eur. J. Urol. 2014, 67, 86. [Google Scholar]
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388. [Google Scholar] [CrossRef]
- Friedman, N.D.; Kaye, K.S.; Stout, J.E.; McGarry, S.A.; Trivette, S.L.; Briggs, J.P.; Lamm, W.; Clark, C.; MacFarquhar, J.; Walton, A.L. Health care–associated bloodstream infections in adults: A reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 2002, 137, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General principles of antimicrobial therapy. In Mayo Clinic Proceedings. Elsevier 2011, 86, 156–167. [Google Scholar]
- McGregor, J.C.; Rich, S.E.; Harris, A.D.; Perencevich, E.N.; Osih, R.; Lodise, T.P., Jr.; Miller, R.R.; Furuno, J.P. A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin. Infect. Dis. 2007, 45, 329–337. [Google Scholar] [CrossRef]
- Naas, T.; Cuzon, G.; Villegas, M.-V.; Lartigue, M.-F.; Quinn, J.P.; Nordmann, P. Genetic structures at the origin of acquisition of the β-lactamase blaKPC gene. Antimicrob. Agents Chemother. 2008, 52, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Héritier, C.; Tolün, V.; Nordmann, P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2004, 48, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, F.M.; Dib-Hajj, F.; Shang, W.; Gootz, T.D. High-level carbapenem resistance in a Klebsiella pneumoniae clinical isolate is due to the combination of blaACT-1 β-lactamase production, porin OmpK35/36 insertional inactivation, and down-regulation of the phosphate transport porin PhoE. Antimicrob. Agents Chemother. 2006, 50, 3396–3406. [Google Scholar] [CrossRef]
- CDC. Standard operating procedure for PulseNet PFGE of Escherichia coli O157: H7, Escherichia coli non-O157 (STEC), Salmonella serotypes, Shigella sonnei and Shigella flexneri. Available online: https://www.cdc.gov/pulsenet/pdf/ecoli-shigella-salmonella-pfge-protocol-508c.pdf (accessed on 18 May 2018).
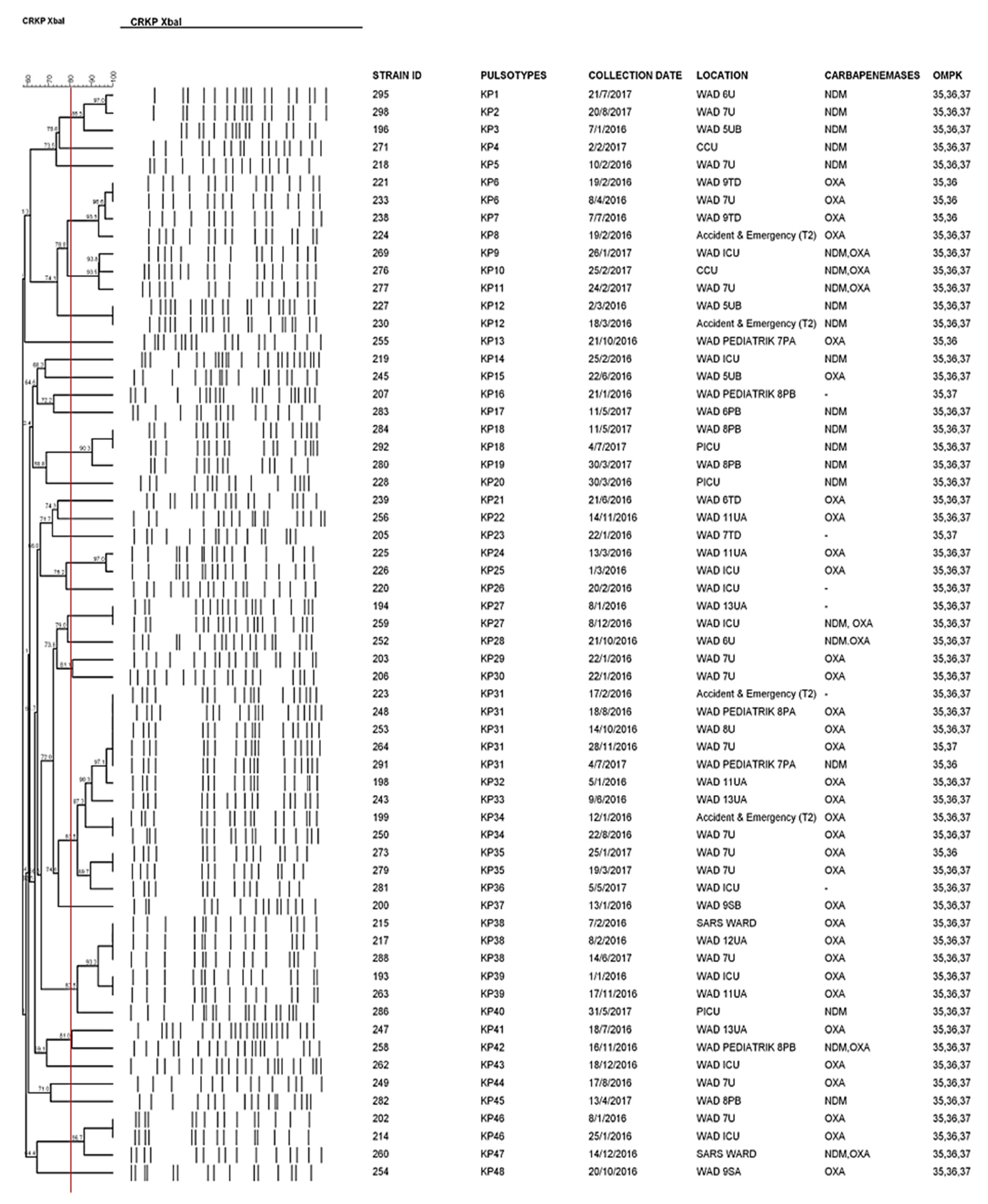
| In Hospital Mortality | p Value | OR (95% CI) | |||
|---|---|---|---|---|---|
| Yes | No | ||||
| Gender | Female | 8 (34.8%) | 15 (65.2%) | 0.292 | |
| Male | 18 (48.6%) | 19 (51.4%) | |||
| Ethnic | Malay | 6 (33.3%) | 12 (66.7%) | 0.246 | |
| Chinese | 13 (50%) | 13 (50%) | |||
| Indian | 5 (35.7%) | 9 (64.3%) | |||
| Others | 2 (100%) | 0 | |||
| Carbapenemase gene | No carbapenemase | 3 (60.0%) | 2 (40.0%) | 0.118 | |
| OXA | 17 (51.5%) | 16 (48.5%) | |||
| NDM | 3 (20.0%) | 12 (80.0%) | 0.081 (0.0067–0.43) | ||
| OXA & NDM | 3 (42.9%) | 4 (57.1%) | |||
| porin loss | No loss | 22 (43.1%) | 29 (56.9%) | 0.163 | |
| Loss of OMPK 36 | 0 | 3 (100%) | |||
| Loss of OMPK 37 | 4 (66.7%) | 2 (33.3%) | |||
| Infection model | CA | 2 (50.0%) | 2 (50.0%) | 0.003 | |
| HA | 18 (60.0%) | 12 (40.0%) | 0.26 (0.17–1.66) | ||
| HC | 2 (100%) | 0 | |||
| Unsure | 1 (100%) | 0 | |||
| Colonized | 3 (13.0%) | 20 (87.0%) | 0.02 (0.00053–0.17) | ||
| ICU admission | No | 11 (44.0%) | 14 (56.0%) | 0.93 | |
| Yes | 15 (42.9%) | 20 (57.1%) | |||
| Co-morbidity | CKD | 5 (62.5%) | 3 (37.5%) | 0.24 | |
| DM | 9 (47.4%) | 10 (52.6%) | 0.668 | ||
| Malignancy | 1 (50.0%) | 1 (50.0%) | 0.847 | ||
| Other diseases | 14 (48.3%) | 15 (51.7%) | 0.455 | ||
| Invasive devices | Indwelling catheter | 20 (48.8%) | 21 (51.2%) | 0.211 | |
| Mechanical ventilator | 17 (44.7%) | 21 (55.3%) | 0.773 | ||
| Tracheostomy | 3 (37.5%) | 5 (62.5%) | 0.721 | ||
| Central venous catheter | 23 (51.1%) | 22 (48.9%) | 0.035 | 12.90 (2.23–210.37) | |
| Art line | 13 (48.1%) | 14 (51.9%) | 0.496 | ||
| Peripheral line | 17 (40.5%) | 25 (59.5%) | 0.495 | ||
| Nasogastric tubes | 19 (45.2%) | 23 (54.8%) | 0.649 | ||
| Stoma | 5 (71.4%) | 2 (28.6%) | 0.11 | ||
| Other | 9 (50.0%) | 9 (50.0%) | 0.495 | ||
| Empiric treatment | Yes | 17 (47.2%) | 19 (52.8%) | 0.457 | |
| No | 9 (37.5%) | 15 (62.5%) | |||
| Anti CRKP treatment | Yes | 15 (57.7%) | 11 (42.3%) | 0.05 | |
| No | 11 (32.4%) | 23 (67.6%) | |||
| Monoinfection | Yes | 6 (54.5%) | 5 (45.5%) | 0.406 | |
| No | 20 (40.8%) | 29 (59.2%) | |||
| Total | 26 (43.3%) | 34 (56.7%) | |||
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio (HR) | 95% CI for HR | p Value | Odd Ratio | 95% CI for HR | p Value | |
| Ethnic | 0.78 | 0.53–1.2 | 0.22 | |||
| Carbapenemase genes | ||||||
| OXA | 2.5 | 1.1–5.6 | 0.029 | |||
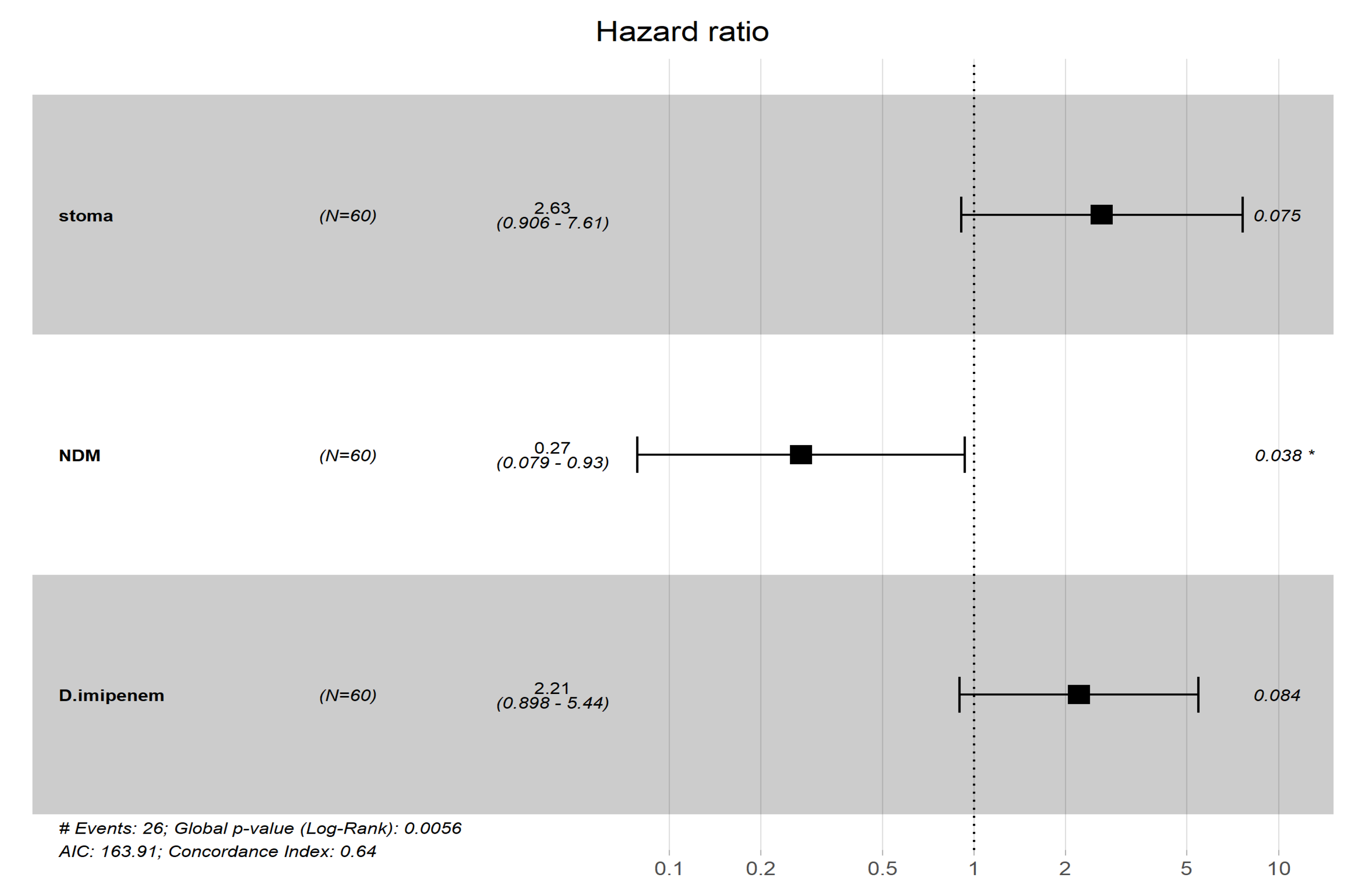
| NDM | 0.25 | 0.073–0.83 | 0.024 | 0.27 | 0.079–0.93 | 0.038 |
| OXA & NDM | 0.74 | 0.22–2.5 | 0.62 | |||
| No carbapenemases | 1.6 | 0.46–5.3 | 0.47 | |||
| MHT | 1.3 | 0.51–3.2 | 0.6 | |||
| Porin-associated gene | ||||||
| No porin loss | 1.1 | 0.39–3.3 | 0.82 | |||
| OMPK 36 | 3.8 × 10−8 | 0-Inf | 1 | |||
| OMPK 37 | 1.2 | 0.39–3.4 | 0.79 | |||
| CRKP infection models | ||||||
| HA | 1.6 | 0.7–3.7 | 0.26 | |||
| CA | 2.6 | 0.59–12 | 0.21 | |||
| Colonizer | 0.23 | 0.069–0.76 | 0.016 | |||
| Invasive devices | ||||||
| Indwelling catheter | 1.8 | 0.72–4.6 | 0.2 | |||
| Central venous catheter | 2.2 | 0.65–7.3 | 0.21 | |||
| Stoma | 3.5 | 1.2–9.9 | 0.019 | 2.63 | 0.906–7.61 | 0.075 |
| Co-morbidity of chronic kidney diseases | 1.6 | 0.58–4.2 | 0.38 | |||
| Previous exposure of antibiotics | ||||||
| Piperacillin-tazobactam | 1.6 | 0.7–3.5 | 0.27 | |||
| Second-generation cephalosporin | 0.43 | 0.1–1.8 | 0.25 | |||
| Third-generation cephalosporin | 0.41 | 0.18–0.93 | 0.032 | |||
| Empirical antibiotic treatment | ||||||
| Amoxicillin-clavulanate | 1.9 | 0.57–6.6 | 0.29 | |||
| Meropenem/imipenem | 1.8 | 0.79–4 | 0.17 | |||
| Definitive antibiotic treatment | ||||||
| Meropenem | 1.1 | 0.42–2.7 | 0.88 | |||
| Imipenem | 2.2 | 0.9–5.4 | 0.084 | 2.21 | 0.898–5.44 | 0.084 |
| Colistin | 1.3 | 0.58–2.8 | 0.55 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, M.Y.; Teng, F.E.; Chua, K.H.; Ponnampalavanar, S.; Chong, C.W.; Abdul Jabar, K.; Teh, C.S.J. Molecular Characterization of Carbapenem Resistant Klebsiella pneumoniae in Malaysia Hospital. Pathogens 2021, 10, 279. https://doi.org/10.3390/pathogens10030279
Lau MY, Teng FE, Chua KH, Ponnampalavanar S, Chong CW, Abdul Jabar K, Teh CSJ. Molecular Characterization of Carbapenem Resistant Klebsiella pneumoniae in Malaysia Hospital. Pathogens. 2021; 10(3):279. https://doi.org/10.3390/pathogens10030279
Chicago/Turabian StyleLau, Min Yi, Fui Enn Teng, Kek Heng Chua, Sasheela Ponnampalavanar, Chun Wie Chong, Kartini Abdul Jabar, and Cindy Shuan Ju Teh. 2021. "Molecular Characterization of Carbapenem Resistant Klebsiella pneumoniae in Malaysia Hospital" Pathogens 10, no. 3: 279. https://doi.org/10.3390/pathogens10030279
APA StyleLau, M. Y., Teng, F. E., Chua, K. H., Ponnampalavanar, S., Chong, C. W., Abdul Jabar, K., & Teh, C. S. J. (2021). Molecular Characterization of Carbapenem Resistant Klebsiella pneumoniae in Malaysia Hospital. Pathogens, 10(3), 279. https://doi.org/10.3390/pathogens10030279






