Scalable Advanced Dual-Engineered Superhydrophobic Aluminum Surfaces for Industrial-Grade Corrosion Protection
Abstract
1. Introduction
2. Materials and Methods
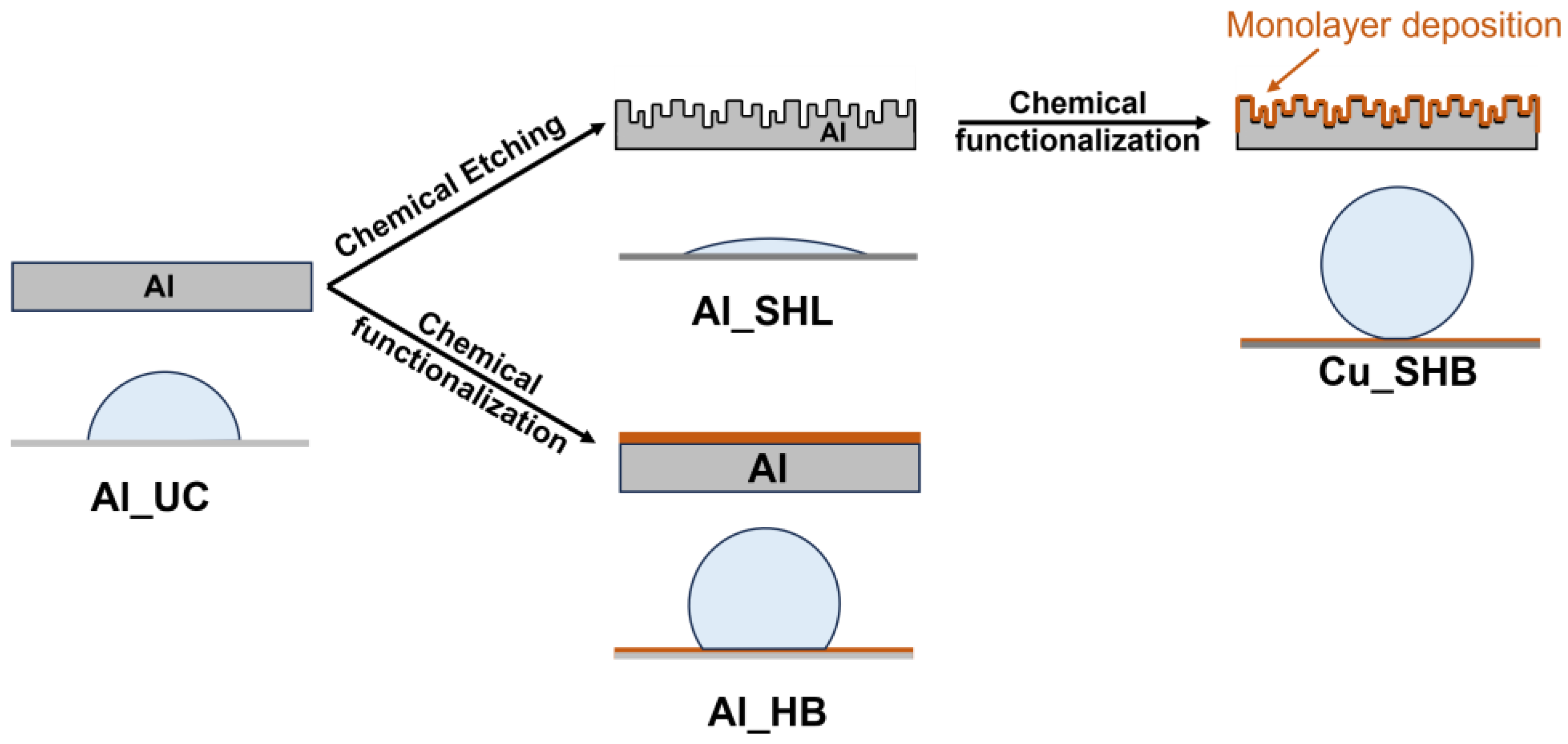
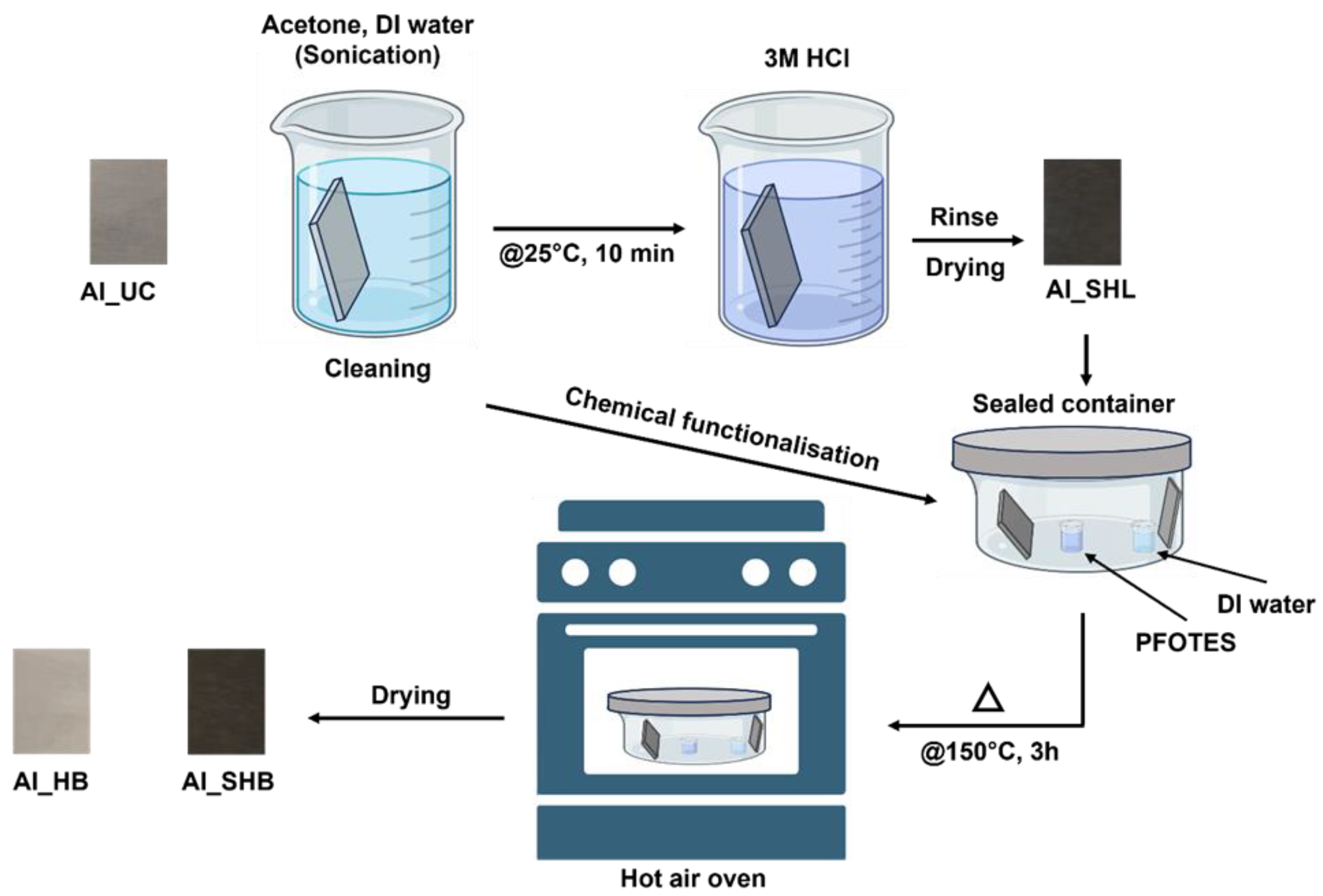
2.1. Substrate Preparation and Cleaning
2.2. Chemical Etching for Surface Nanostructuring
2.3. Chemical Functionalization
2.4. Surface Characterization
2.5. Electrochemical Measurements
2.6. Salt Spray Test
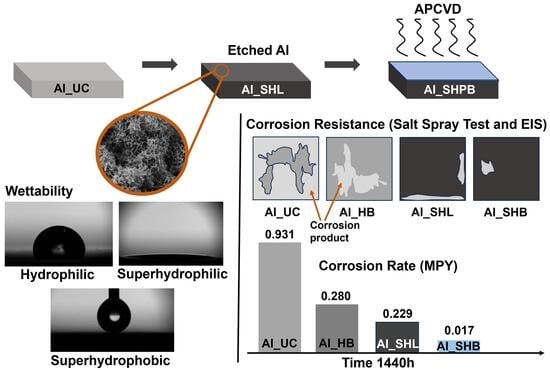
3. Results and Discussion
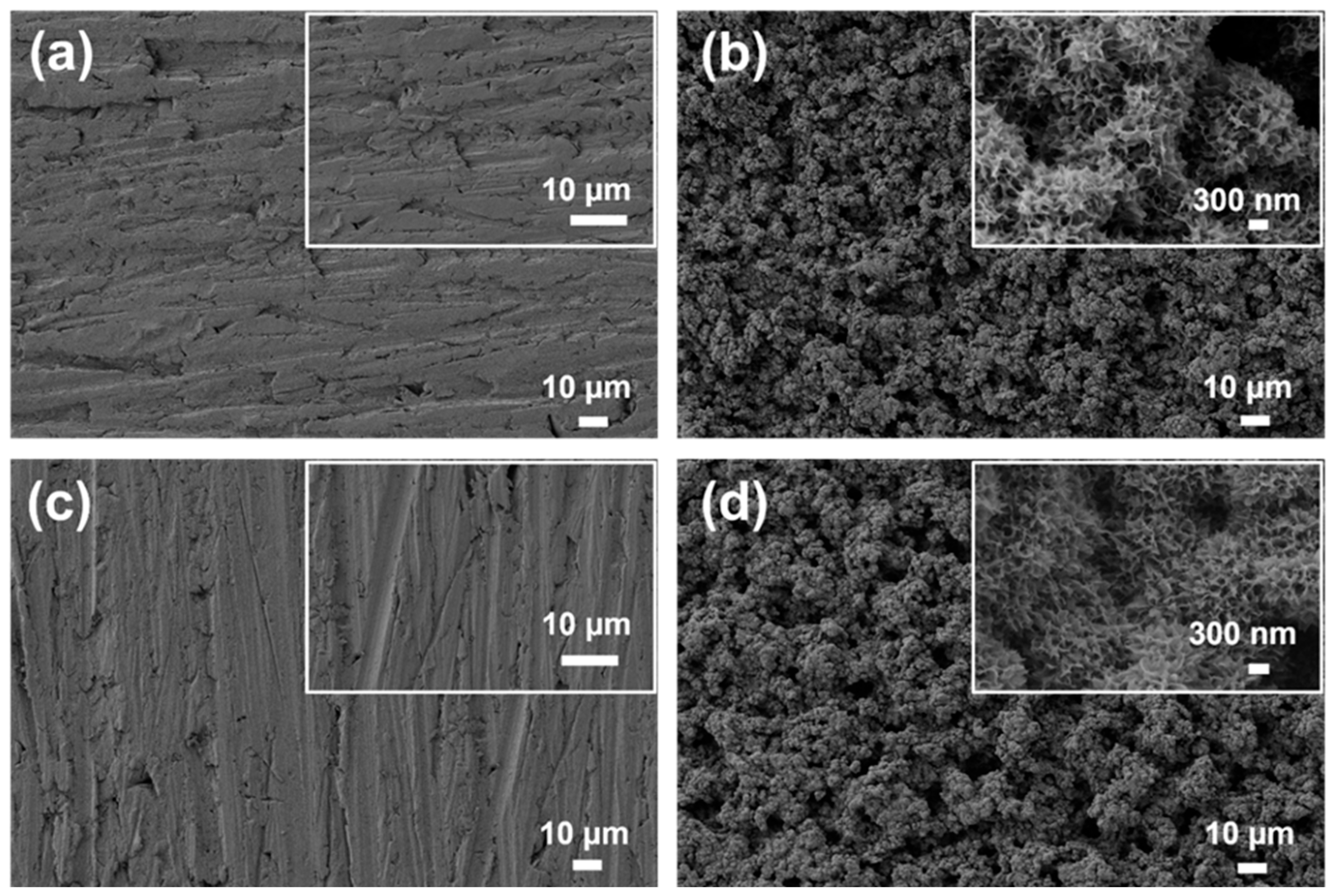
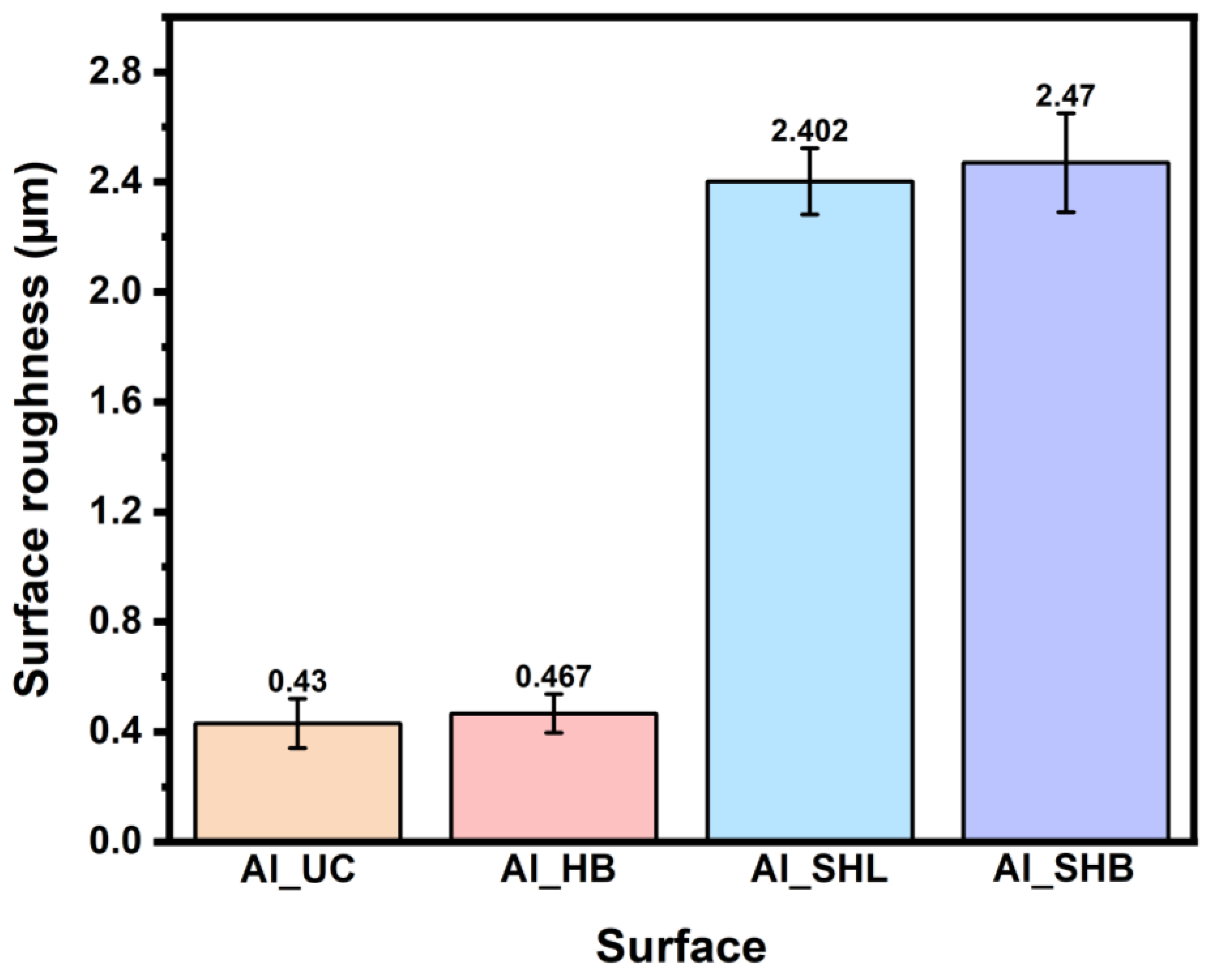
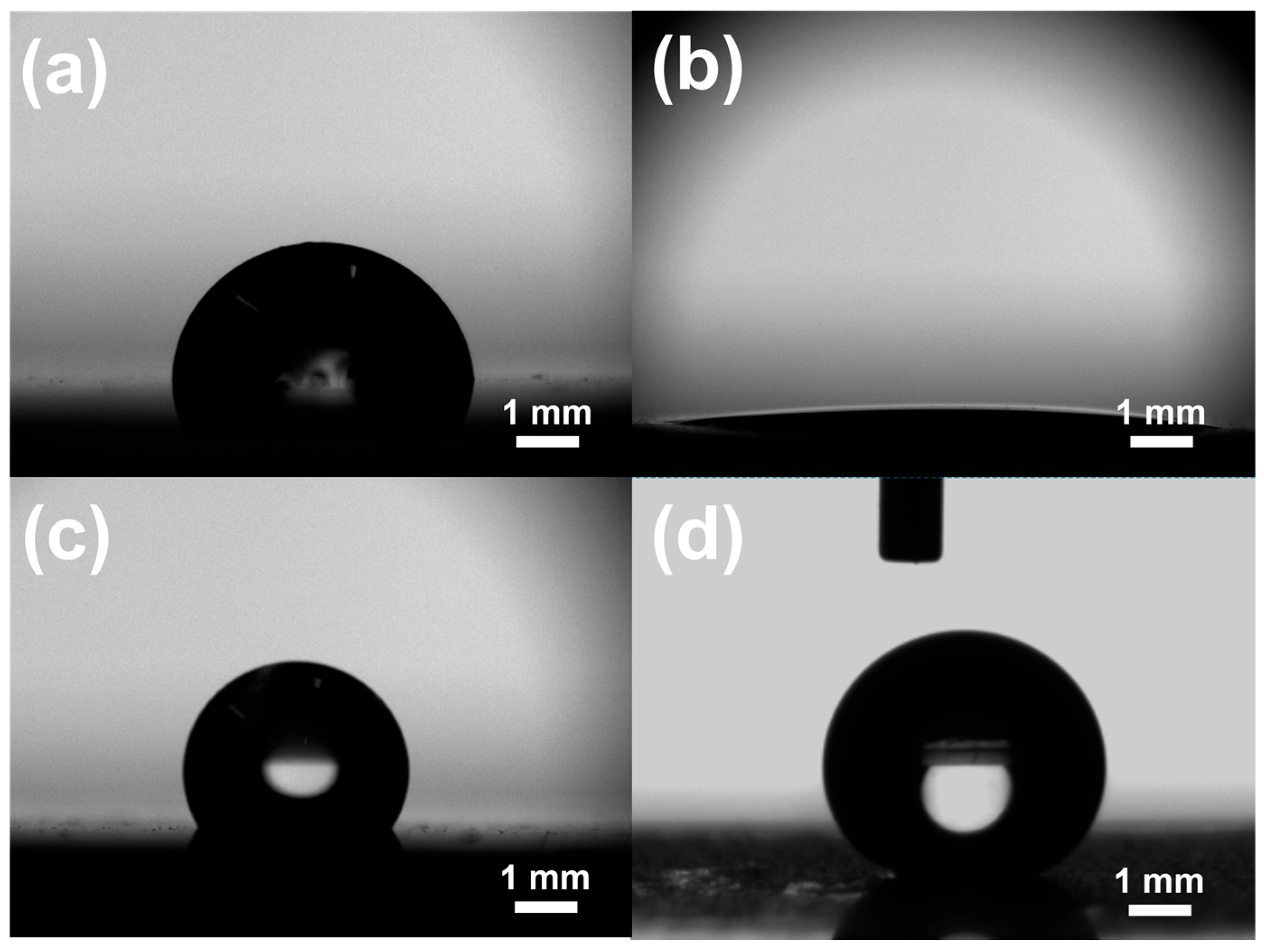
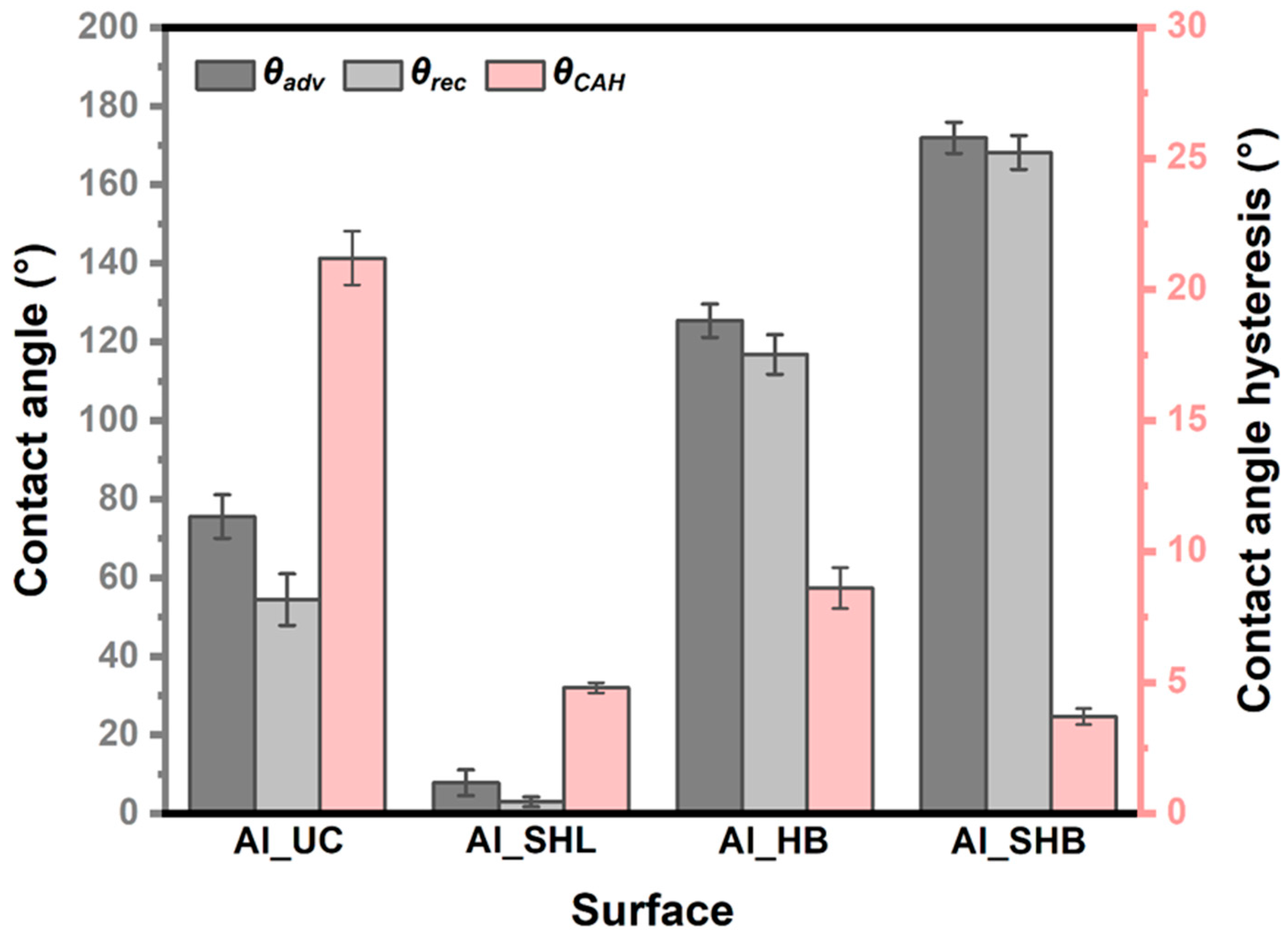
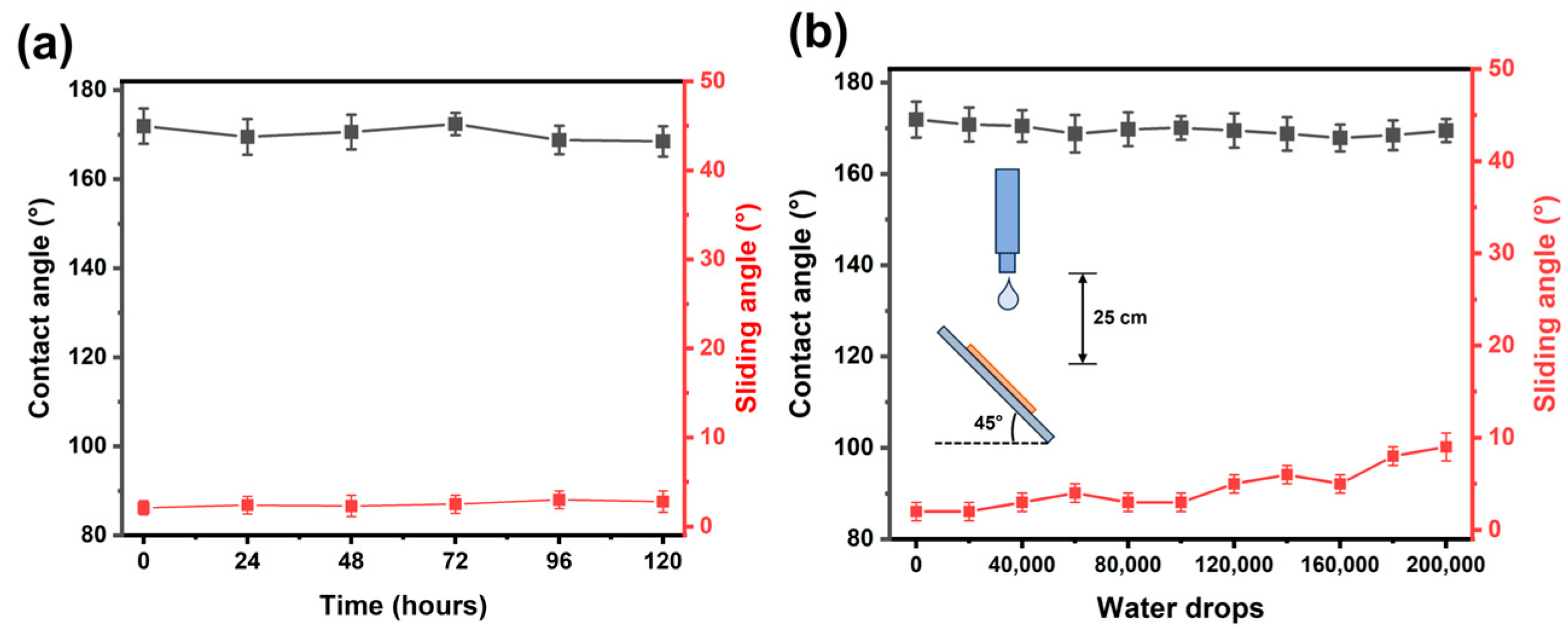
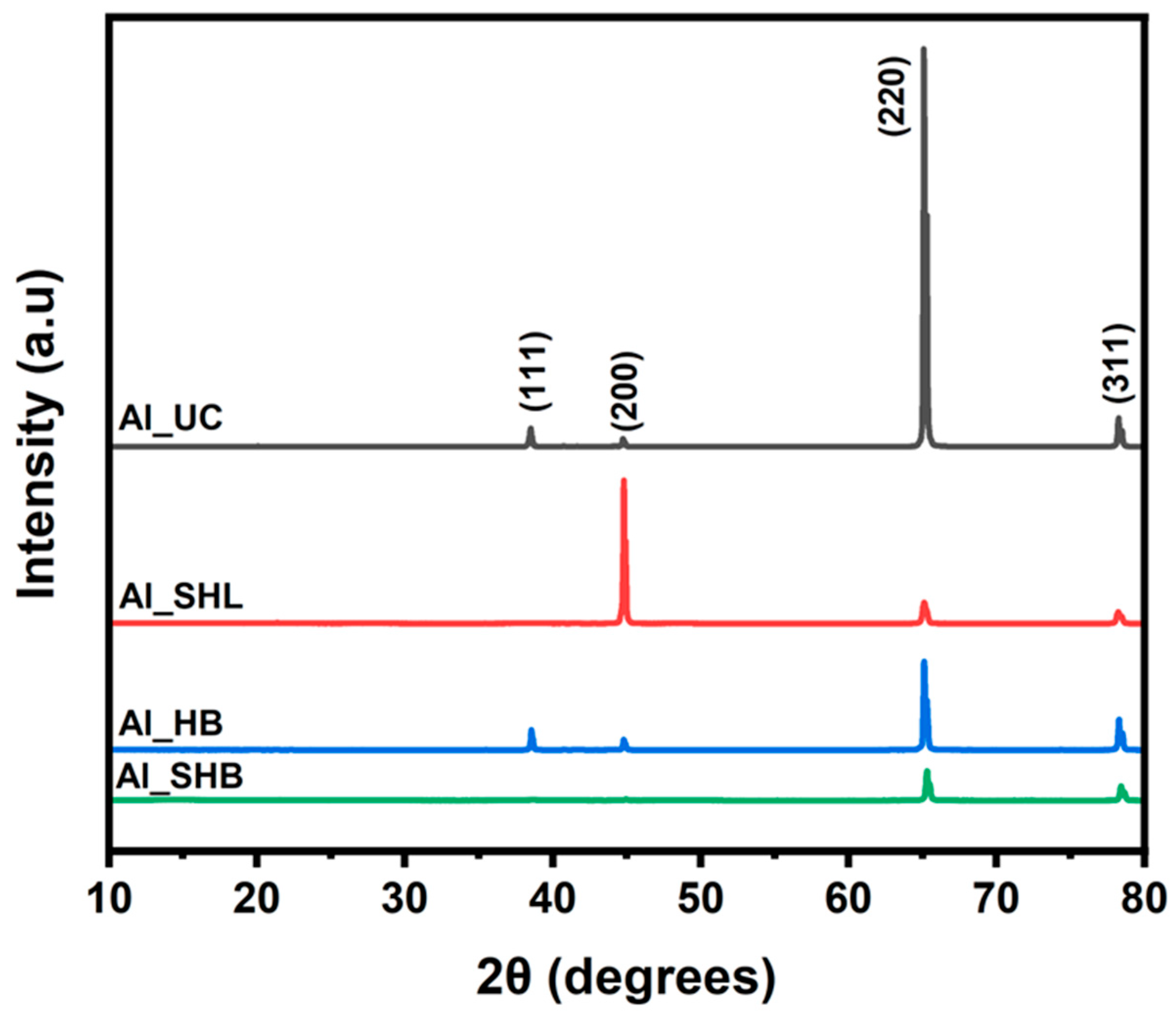
3.1. Surface Characterization
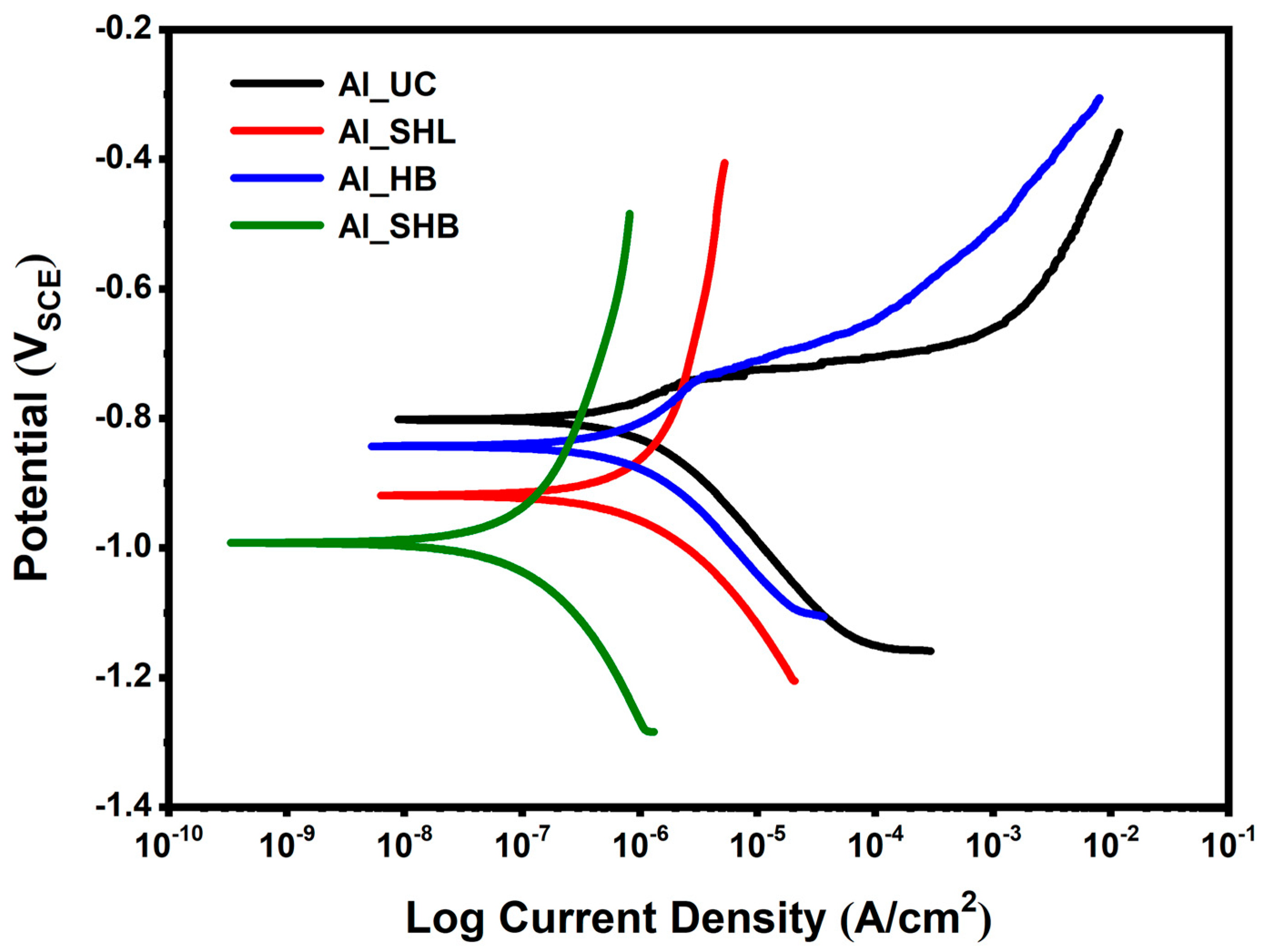
3.2. Electrochemical Measurements
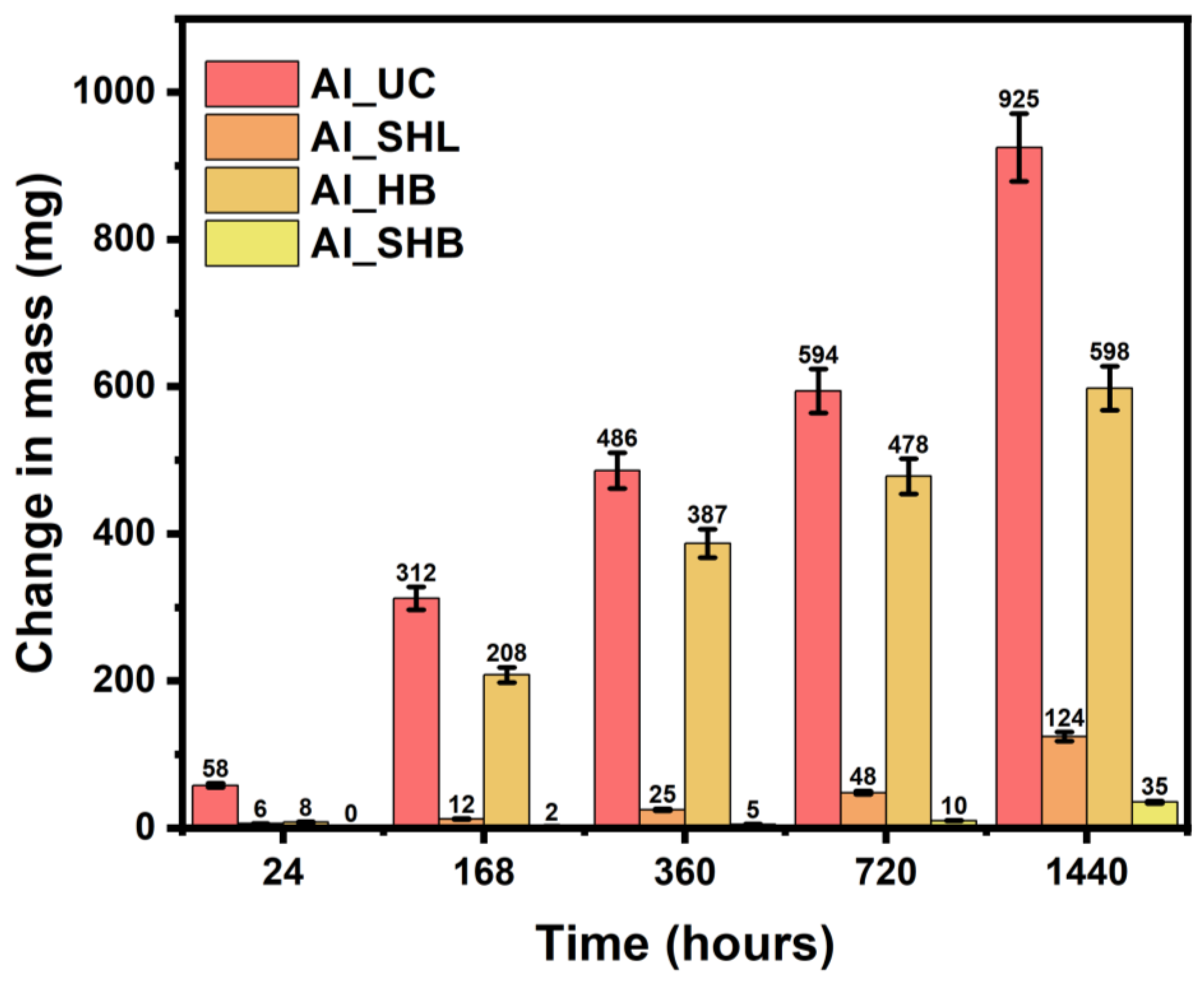
3.3. Corrosion Morphology over Time
3.4. Quantitative Mass Change and Corrosion Rates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiang, L.; Tao, J.; Xia, X.; Zhao, Z.; Chen, Q.; Su, Y.; Chai, S.; Zheng, Z.; Sun, J. Impact of marine atmospheric corrosion on the microstructure and tensile properties of 7075 high-strength aluminum alloy. Materials 2023, 16, 2396. [Google Scholar] [CrossRef]
- Huang, G.Q. Corrosion behavior of aluminum alloys in seawater. Corros. Sci. Technol. 2002, 31, 215. [Google Scholar]
- Knudsen, O.Ø.; Bertram, J. Corrosion of Aluminium in Marine Environments. 2023. Available online: https://www.sintef.no/contentassets/adf31392734e496c805e9cbfad888c2f/report---corrosion-of-al-in-marine-environments.pdf (accessed on 20 August 2025).
- Olkowicz, K.; Buczko, Z.; Nasiłowska, B.; Kowalczyk, K.; Czwartos, J. Superhydrophobic coating based on porous aluminum oxide modified by polydimethylsiloxane (PDMS). Materials 2022, 15, 1042. [Google Scholar] [CrossRef] [PubMed]
- Rahul, N.; Kumar, N.; Sett, S. Delayed Condensation on Nanoparticle Coated Transparent Lubricant-Infused Surfaces. In Proceedings of the 27th National and 5th International ISHMT-ASTFE Heat and Mass Transfer Conference, 2023, IIT Patna, Patna-801106, Bihar, India, 14–17 December 2024; Begel House Inc.: Danbury, CT, USA, 2024. [Google Scholar]
- Zheng, S.; Li, C.; Fu, Q.; Xiang, T.; Hu, W.; Wang, J.; Ding, S.; Liu, P.; Chen, Z. Fabrication of a micro-nanostructured superhydrophobic aluminum surface with excellent corrosion resistance and anti-icing performance. Rsc Adv. 2016, 6, 79389–79400. [Google Scholar] [CrossRef]
- Orejon, D.; Oh, J.; Preston, D.J.; Yan, X.; Sett, S.; Takata, Y.; Miljkovic, N.; Sefiane, K. Ambient-mediated wetting on smooth surfaces. Adv. Colloid Interface Sci. 2024, 324, 103075. [Google Scholar] [CrossRef] [PubMed]
- Rahul, N.; Park, B.; Pradhan, S.K.; Sung, H.E.; Jeong, I.H.; Yun, Y.S.; Oh, M.S. Scalable Engineering of Superhydrophobic Copper Surfaces with Enhanced Corrosion Resistance by Combined Nanostructuring and Chemical Vapor Deposition. Materials 2025, 18, 3981. [Google Scholar] [CrossRef]
- Thomas, T.M.; Mahapatra, P.S. Fabrication of hierarchically textured aluminum-based superhydrophobic surfaces for anti-frosting application. Mater. Today Proc. 2022, 56, 1267–1273. [Google Scholar] [CrossRef]
- Eom, H.; Lee, J.; Kim, J.; Hong, M.; Kim, K.; Jang, H.; Lee, D.; Lim, H.; Jiong, S.; Choi, W. Scalable and tunable micro/nanostructuring of aluminum surfaces via chemical etching for enhanced heat dissipation. Appl. Therm. Eng. 2025, 274, 126729. [Google Scholar] [CrossRef]
- Fang, C.; Pu, M.; Zhou, X.; Lei, W.; Pei, L.; Wang, C. Facile preparation of hydrophobic aluminum oxide film via sol-gel method. Front. Chem. 2018, 6, 308. [Google Scholar] [CrossRef]
- da Silva, R.G.; Malta, M.I.; da Silva, J.J.; da Silva Filho, W.L.; Cirino, J.A.; de Oliveira, S.H.; Vinhas, G.M.; Vieira, M.R. Study of a fluorine-free silane-based film on an aluminum alloy via drop-coating method with the purpose of providing hydrophobic and corrosion protection properties. Mater. Chem. Phys. 2025, 329, 130099. [Google Scholar] [CrossRef]
- Barthwal, S.; Barthwal, S. Engineering a robust, multifunctional superhydrophobic/oleophobic microporous aluminum surface via a two-step chemical etching process. Surf. Interfaces 2024, 46, 103933. [Google Scholar] [CrossRef]
- Adarraga, O.; Agustín-Sáenz, C.; Bustero, I.; Brusciotti, F. Superhydrophobic and oleophobic microtextured aluminum surface with long durability under corrosive environment. Sci. Rep. 2023, 13, 1737. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Dutta, M.; Nallana, R.; Sett, S. Sustainable Non-PFAS Hydrophobic Surfaces from Naturally Derived Sepiolite, Myristic Acid, and Ethyl Cellulose for Stable Dropwise Condensation. Small 2025, e05314. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Calabrese, L.; Proverbio, E. Anticorrosion superhydrophobic surfaces on AA6082 aluminum alloy by HF/HCl texturing and self-assembling of silane monolayer. Materials 2022, 15, 8549. [Google Scholar] [CrossRef]
- Cui, C.; Cao, Y.; Qi, B.; Wei, J.; Yuan, J.; Wang, Y. Convenient and large-scale fabrication of cost-effective superhydrophobic aluminum alloy surface with excellent reparability. Langmuir 2021, 37, 7810–7820. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Momen, G.; Jafari, R. The thermodynamic stability of the Cassie–Baxter regime determined by the geometric parameters of hierarchical superhydrophobic surfaces. Appl. Mater. Today 2023, 34, 101893. [Google Scholar] [CrossRef]
- Wang, X.; Fu, C.; Zhang, C.; Qiu, Z.; Wang, B. A comprehensive review of wetting transition mechanism on the surfaces of microstructures from theory and testing methods. Materials 2022, 15, 4747. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Nosonovsky, M.; Chae Jung, Y. Towards optimization of patterned superhydrophobic surfaces. J. R. Soc. Interface 2007, 4, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yin, Y.; Chen, S.; Chang, X.; Cheng, S. Super-hydrophobic surfaces improve corrosion resistance of copper in seawater. Electrochim. Acta 2007, 52, 3709–3713. [Google Scholar] [CrossRef]
- Yunying, X.; Wenxin, H.; Yi, C.; Guangxin, W.; Jieyu, Z. Effect of heat treatment process on microstructure and corrosion resistance of Al–10% Si–24% Zn coating. Surf. Coat. Technol. 2020, 401, 126305. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Z.; Ye, Y.; Wang, G.; He, X. Effects of various Mg/Si ratios on microstructure and performance property of Al-Mg-Si alloy cables. Mater. Charact. 2016, 119, 114–119. [Google Scholar] [CrossRef]
- ASTM B117-19; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2019.
- Devra, R.S.; Gupta, R.; Rahul, N.; Sett, S.; Vadali, M. Transitioning Surface Wettability of Ti6Al4V via Laser Ablation and Post-processing Methods. J. Bio-Tribo-Corros. 2025, 11, 39. [Google Scholar] [CrossRef]
- Rahul, N.; Kalita, S.; Sen, P.; Shil, B.; Sen, D. Enhanced pool boiling heat transfer characteristics on microstructured copper surfaces coated with hybrid nanofluid. J. Therm. Anal. Calorim. 2024, 149, 6281–6293. [Google Scholar] [CrossRef]
- Lee, H.S.; Singh, J.K.; Ismail, M.A.; Bhattacharya, C.; Seikh, A.H.; Alharthi, N.; Hussain, R.R. Corrosion mechanism and kinetics of Al-Zn coating deposited by arc thermal spraying process in saline solution at prolong exposure periods. Sci. Rep. 2019, 9, 3399. [Google Scholar] [CrossRef] [PubMed]
- Sacilotto, D.G.; Kunst, S.R.; Soares, L.G.; Carone, C.L.P.; Arnold, D.C.M.; Oliveira, C.T.; Ferreira, J.Z. Corrosion resistance of 5052 aluminum alloy using hydrophobic silane coatings. Matéria 2025, 30, e20240919. [Google Scholar]
- Boinovich, L.B.; Emelyanenko, A.M.; Modestov, A.D.; Domantovsky, A.G.; Emelyanenko, K.A. Not simply repel water: The diversified nature of corrosion protection by superhydrophobic coatings. Mendeleev Commun. 2017, 27, 254–256. [Google Scholar] [CrossRef]
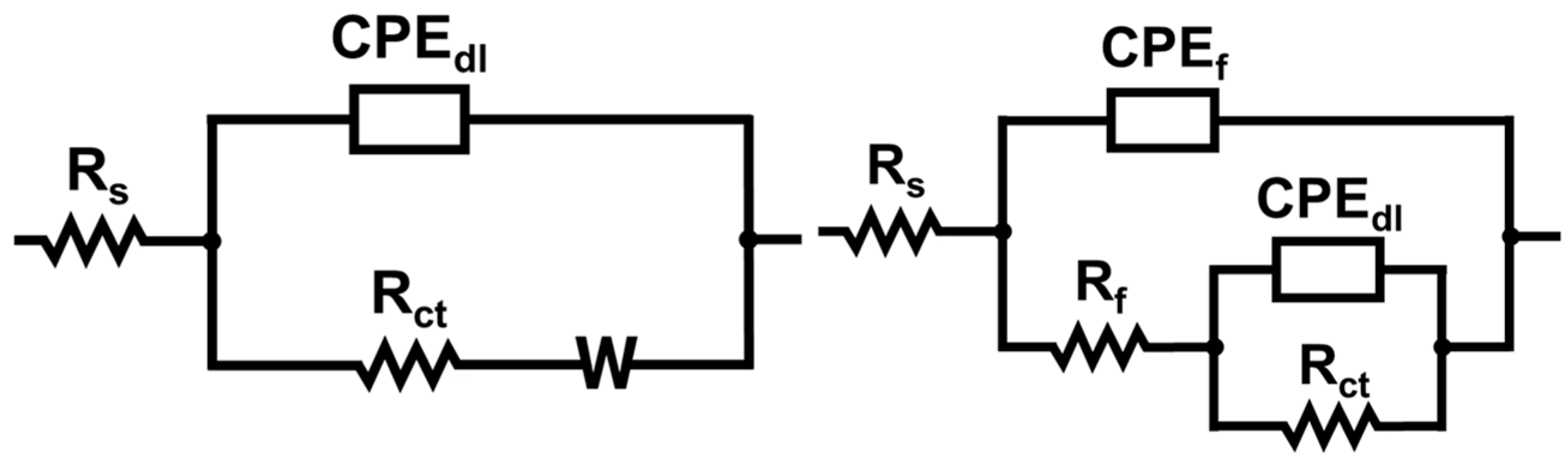
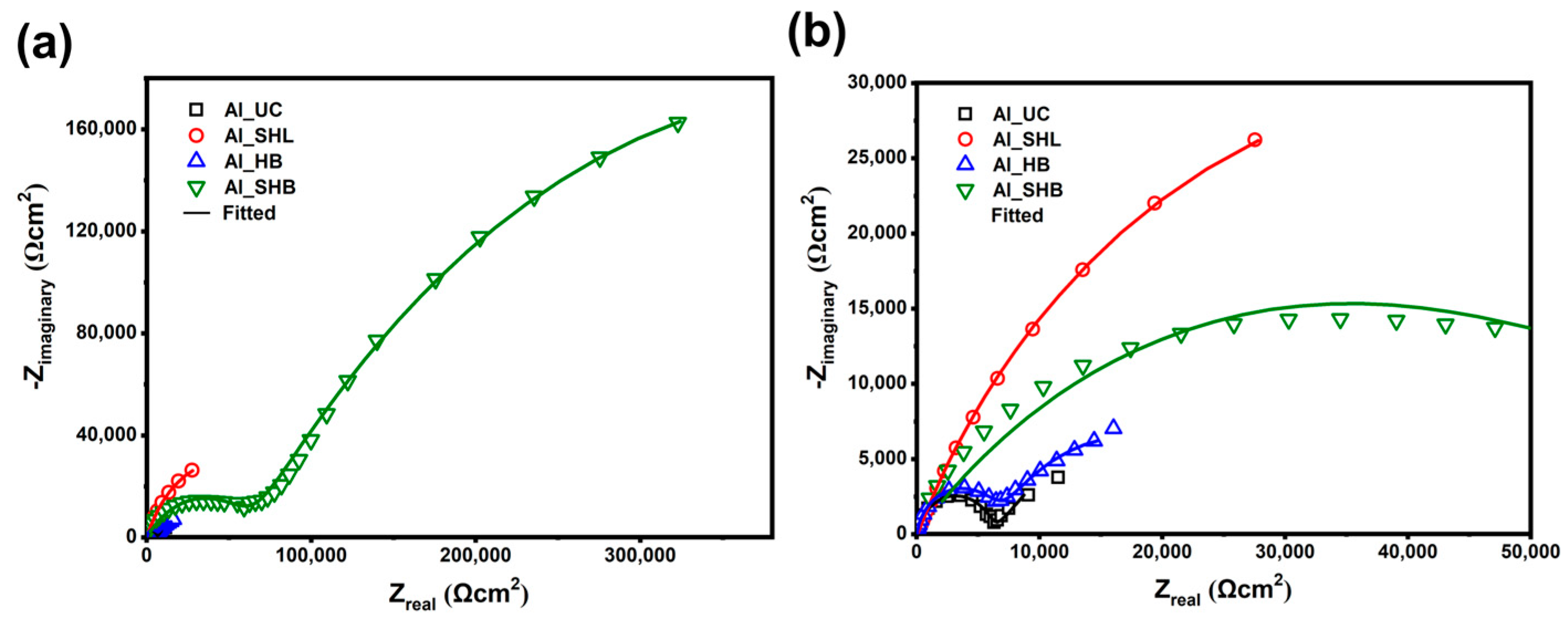
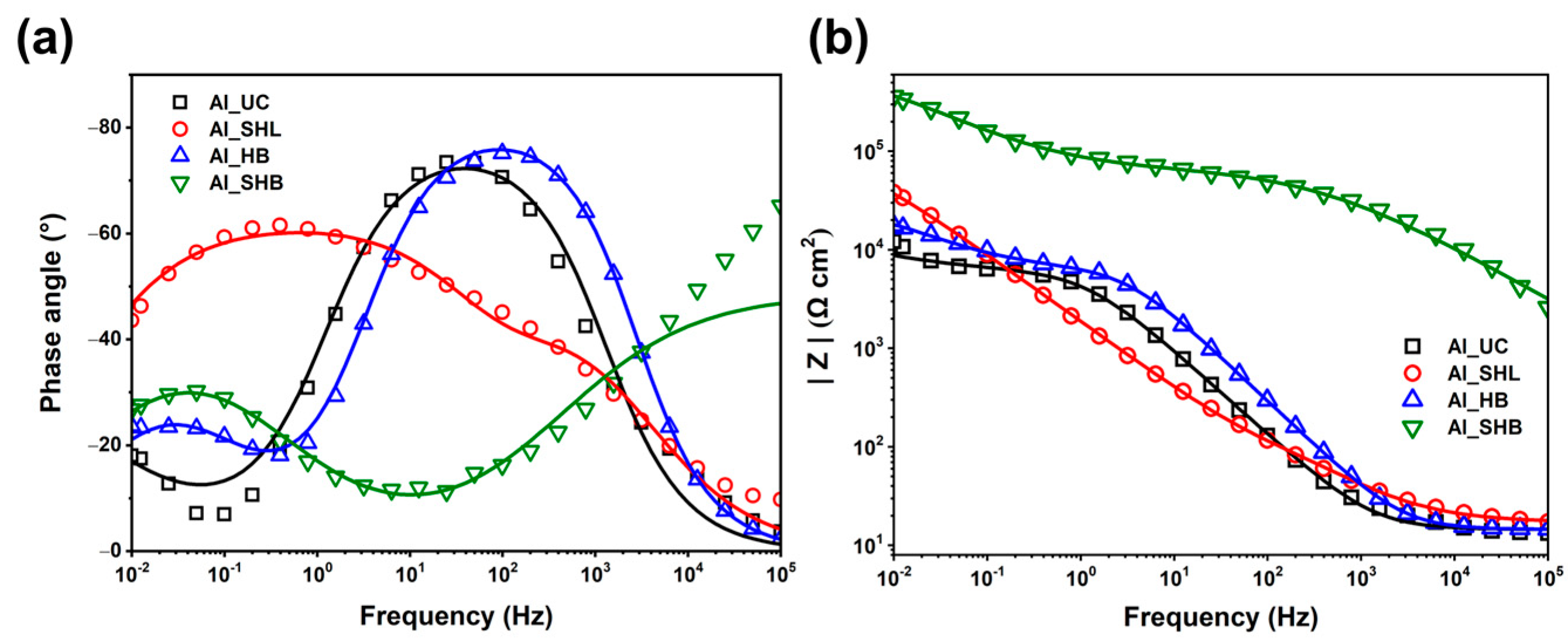

| Sample | Rs | Rf | Rct | CPEf | CPEdl | m | n | Rp (Ω cm2) | IE (%) |
|---|---|---|---|---|---|---|---|---|---|
| (Ω cm2) | (F/cm2) | ||||||||
| Al_UC | 14.5 | - | 6143 | - | 5.05 × 10−6 | - | 0.87 | 6143 | - |
| Al_SHL | 17.36 | 341 | 93,570 | 9.72 × 10−5 | 4.94 × 10−5 | 0.71 | 0.75 | 93,911 | 93.4 |
| Al_HB | 14.51 | 6817 | 21,050 | 1.35 × 10−5 | 4.50 × 10−4 | 0.91 | 0.69 | 27,867 | 70.8 |
| Al_SHB | 12.9 | 66,540 | 738,581 | 2.48 × 10−7 | 1.04 × 10−5 | 0.54 | 0.56 | 805,121 | 99.2 |
| Sample | Ecorr (V) | icorr (µA/cm2) | Corrosion Rate (mpy) | IE (%) |
|---|---|---|---|---|
| Al_UC | −0.802 | 2.150 | 0.931 | - |
| Al_SHL | −0.919 | 0.529 | 0.229 | 75.4 |
| Al_HB | −0.843 | 0.647 | 0.280 | 69.9 |
| Al_SHB | −0.992 | 0.0395 | 0.017 | 98.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahul, N.; Sung, H.-E.; Lee, S.W.; Oh, M.-S. Scalable Advanced Dual-Engineered Superhydrophobic Aluminum Surfaces for Industrial-Grade Corrosion Protection. Metals 2025, 15, 1248. https://doi.org/10.3390/met15111248
Rahul N, Sung H-E, Lee SW, Oh M-S. Scalable Advanced Dual-Engineered Superhydrophobic Aluminum Surfaces for Industrial-Grade Corrosion Protection. Metals. 2025; 15(11):1248. https://doi.org/10.3390/met15111248
Chicago/Turabian StyleRahul, N., Ho-Eon Sung, Sang Won Lee, and Min-Suk Oh. 2025. "Scalable Advanced Dual-Engineered Superhydrophobic Aluminum Surfaces for Industrial-Grade Corrosion Protection" Metals 15, no. 11: 1248. https://doi.org/10.3390/met15111248
APA StyleRahul, N., Sung, H.-E., Lee, S. W., & Oh, M.-S. (2025). Scalable Advanced Dual-Engineered Superhydrophobic Aluminum Surfaces for Industrial-Grade Corrosion Protection. Metals, 15(11), 1248. https://doi.org/10.3390/met15111248