Extremophile-Derived Bioactives in Cosmeceuticals: Bridging Nutraceuticals and Skincare for Holistic Wellness
Abstract
1. Introduction
2. Extremophiles as a Source of Bioactive Molecules
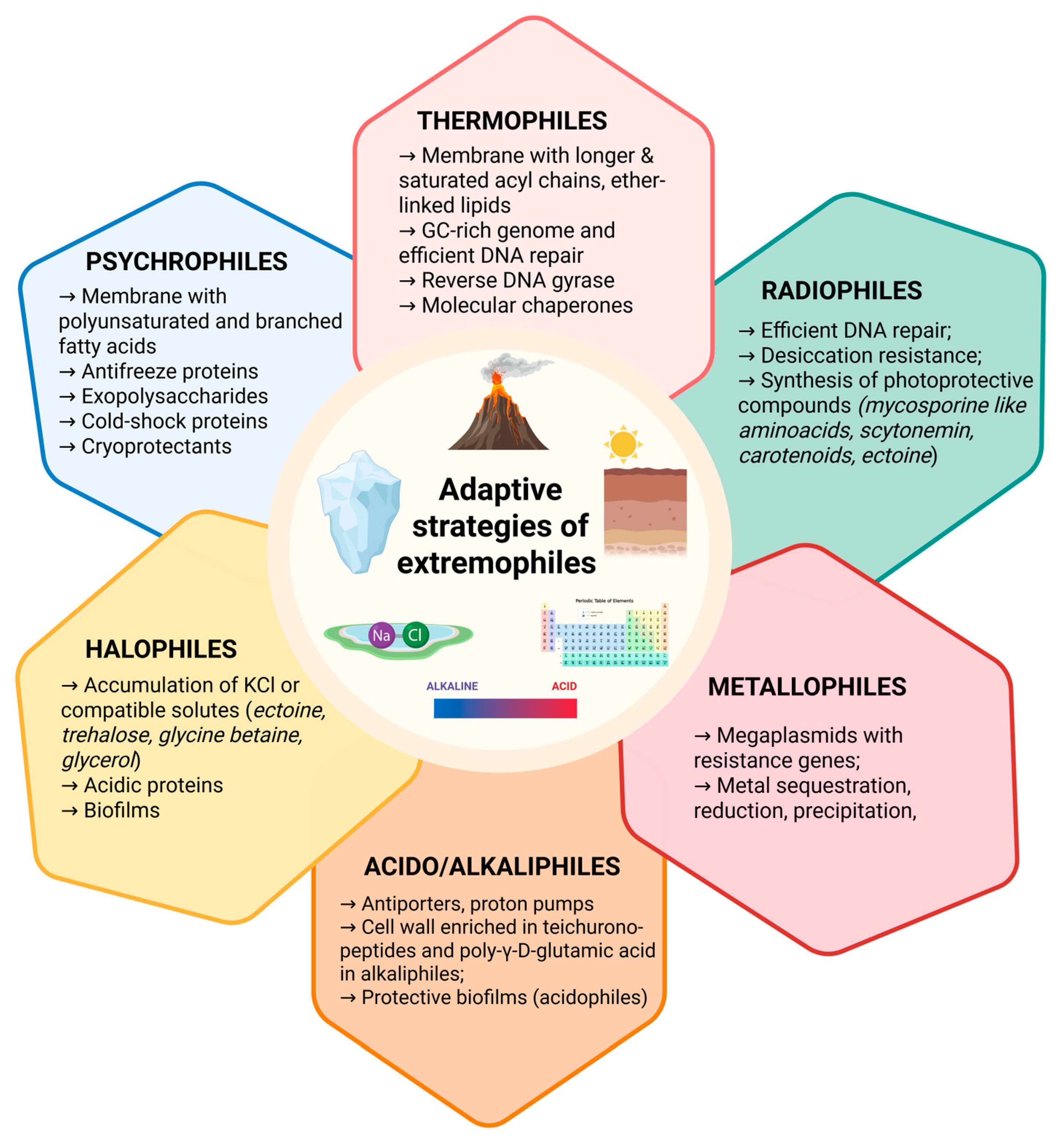
2.1. Adaptation Mechanisms to Extreme Temperatures and pH
2.1.1. Cold Adapted
2.1.2. Heat Adapted
2.1.3. pH Adapted
2.2. Adaptation Strategies to Salinity, Metals, Pressure, and Radiation
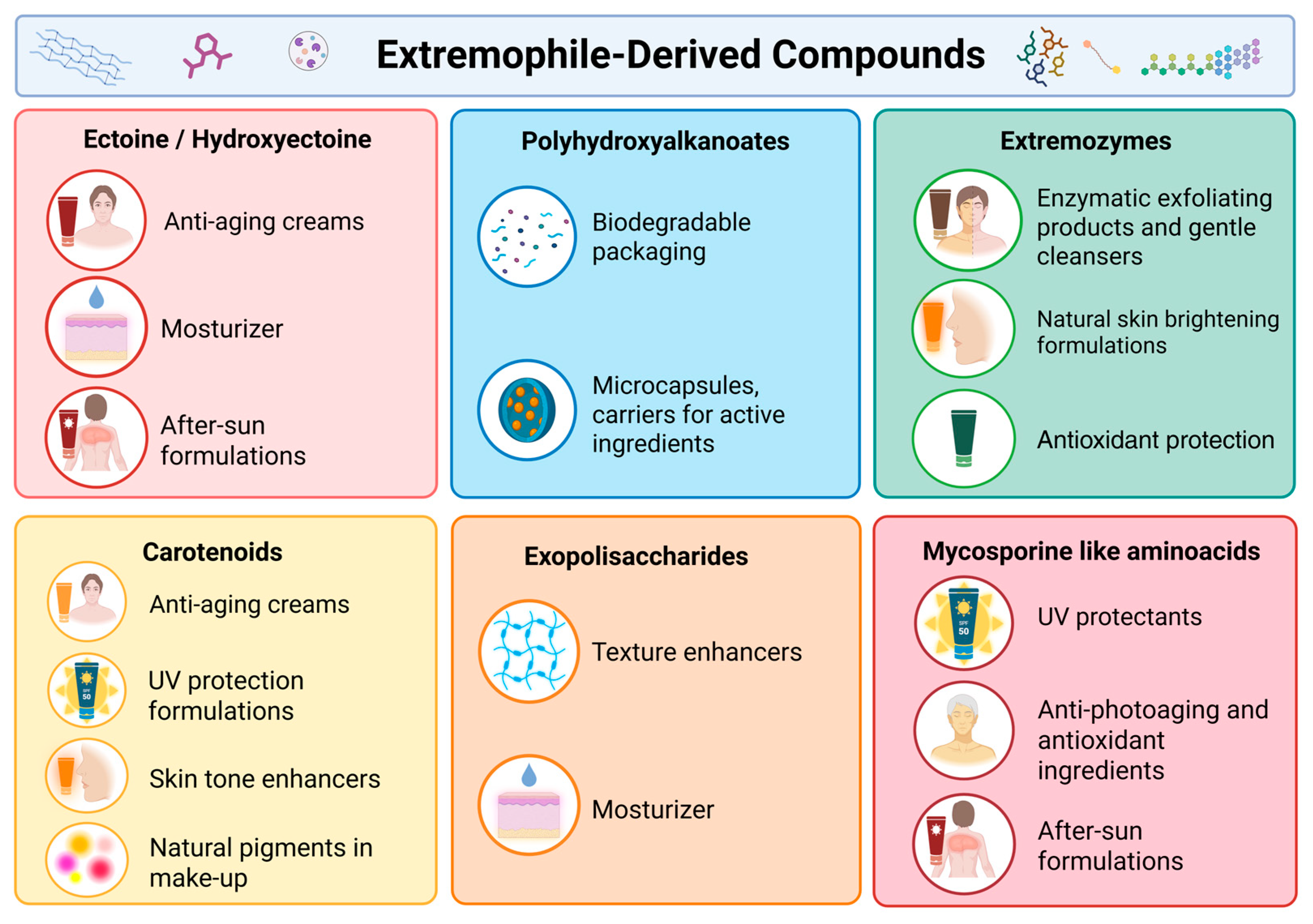
3. Cosmetic Applications of Extremophile-Derived Compounds
3.1. Extremozymes
3.1.1. Proteases
3.1.2. Lipases
3.1.3. Amylases
3.1.4. Chitinases
3.2. Ectoine
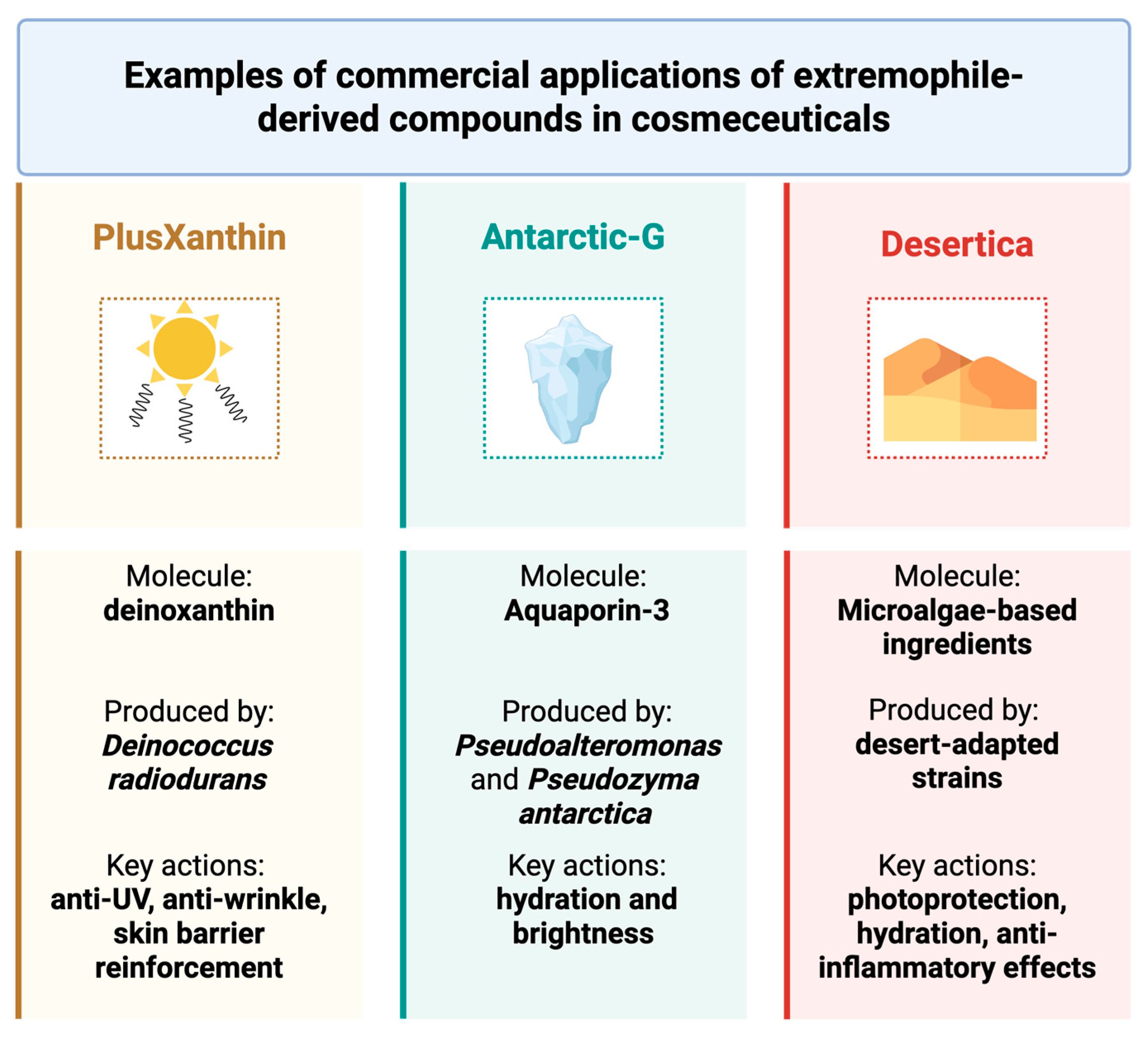
3.3. Carotenoids
3.4. Mycosporine-like Amino Acids
3.5. Exopolysaccharides and Polyhydroxyalkanoates
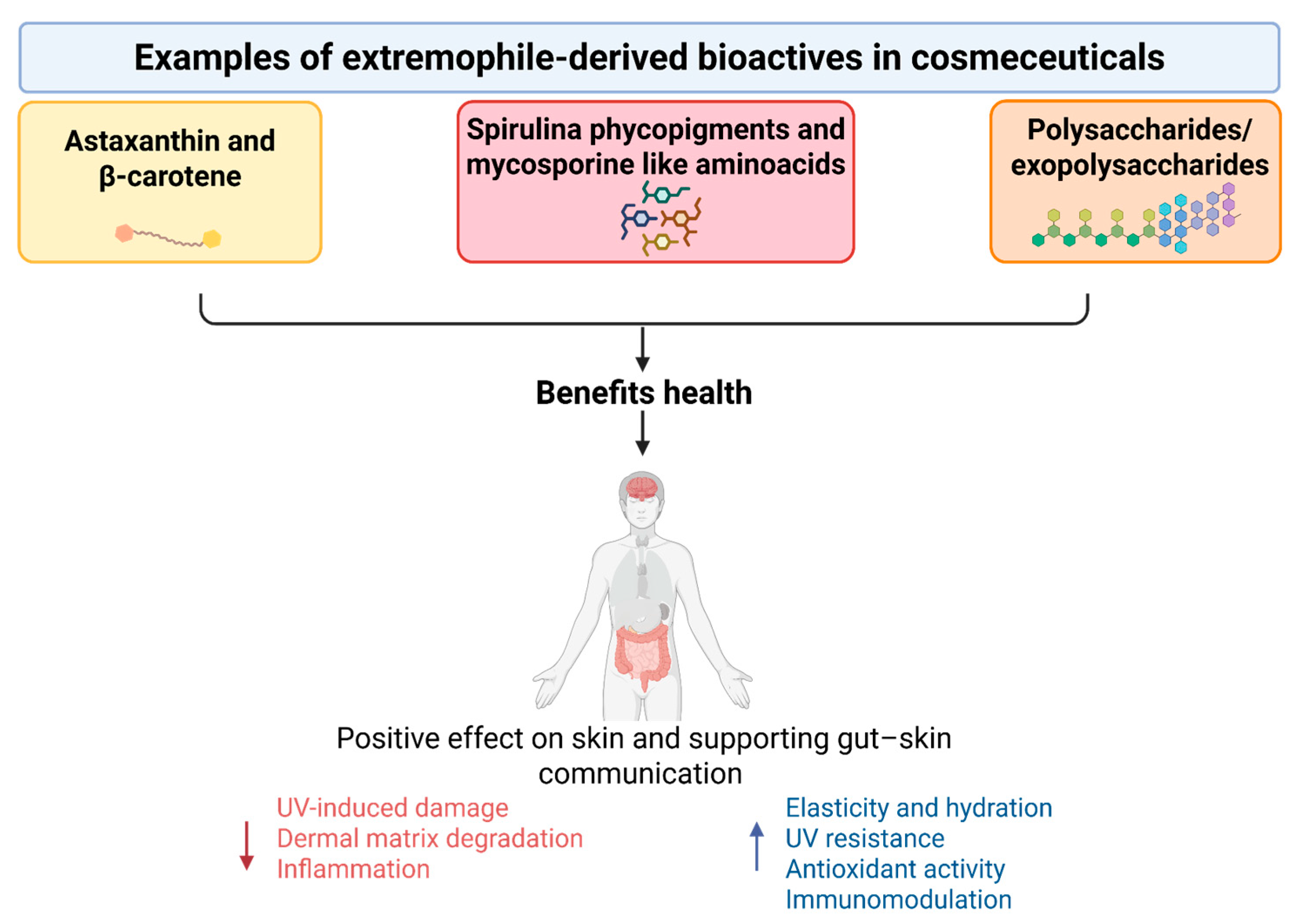
4. Nutraceutical Potential of Extremophile-Derived Compounds
5. Cosmeceutical Integration and Sustainability
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fusco, S.; Aulitto, M.; Bartolucci, S.; Contursi, P. A standardized protocol for the UV induction of Sulfolobus spindle-shaped virus 1. Extremophiles 2015, 19, 539–546. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aulitto, M.; Tom, L.M.; Ceja-Navarro, J.A.; Simmons, B.A.; Singer, S.W. Whole-Genome Sequence of Brevibacillus borstelensis SDM, Isolated from a Sorghum-Adapted Microbial Community. Microbiol. Resour. Announc. 2020, 9, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Aulitto, M.; Fusco, S.; Franzén, C.J.; Strazzulli, A.; Moracci, M.; Bartolucci, S.; Contursi, P. Draft Genome Sequence of Bacillus coagulans MA-13, a Thermophilic Lactic Acid Producer from Lignocellulose. Microbiol. Resour. Announc. 2019, 8, e00341-19. [Google Scholar] [CrossRef]
- Rampelotto, P.H. Extremophiles and extreme environments. Life 2013, 3, 482–485. [Google Scholar] [CrossRef]
- Giani, M.; Pire, C.; Martinez-Espinosa, R.M. Bacterioruberin: Biosynthesis, Antioxidant Activity, and Therapeutic Applications in Cancer and Immune Pathologies. Mar. Drugs 2024, 22, 167. [Google Scholar] [CrossRef]
- Ma, B.; Bu, Y.; Huang, J.; Liu, Y.; Guo, Z.; Yu, H.; Liang, T.; Wang, D. Bioactive compounds from deep-sea extremophiles: Emerging potential in cosmeceuticals and nutraceuticals. FEMS Microbiol. Lett. 2025, 372, fnaf102. [Google Scholar] [CrossRef]
- Irato, P.; Santovito, G. Enzymatic and Non-Enzymatic Molecules with Antioxidant Function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef]
- Forterre, P. Archaea: A Goldmine for Molecular Biologists and Evolutionists. Methods Mol. Biol. 2022, 2522, 1–21. [Google Scholar] [CrossRef]
- Gallo, G.; Aulitto, M. Advances in Extremophile Research: Biotechnological Applications through Isolation and Identification Techniques. Life 2024, 14, 1205. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Mougiakos, I.; Bianco, M.; Carbonaro, M.; Carpentieri, A.; Illiano, A.; Pucci, P.; Bartolucci, S.; Oost, J.v.d.; Fiorentino, G. A Hyperthermoactive-Cas9 Editing Tool Reveals the Role of a Unique Arsenite Methyltransferase in the Arsenic Resistance System of Thermus thermophilus HB27. mBio 2021, 12, e02813–e02821. [Google Scholar] [CrossRef]
- Gallo, G.; Antonucci, I.; Pirone, L.; Amoresano, A.; Contursi, P.; Limauro, D.; Pedone, E.; Bartolucci, S.; Fiorentino, G. A physicochemical investigation on the metal binding properties of TtSmtB, a thermophilic member of the ArsR/SmtB transcription factor family. Int. J. Biol. Macromol. 2019, 138, 1056–1063. [Google Scholar] [CrossRef]
- Bialkowski, K. Proteolytic activity of cosmetic enzyme peel products. Acta Biochim. Pol. 2022, 69, 895–899. [Google Scholar] [CrossRef]
- Białkowska, A.; Majewska, E.; Olczak, A.; Twarda-Clapa, A. Ice Binding Proteins: Diverse Biological Roles and Applications in Different Types of Industry. Biomolecules 2020, 10, 274. [Google Scholar] [CrossRef]
- Guerreiro, B.M.; Lima, J.C.; Silva, J.C.; Freitas, F. Polysaccharides in cryopreservation: Multidimensional systematic review of extremophilic traits and the role of selective pressure in structure-function relationships. Carbohydr. Polym. 2026, 371, 124462. [Google Scholar] [CrossRef] [PubMed]
- Feller, G. Psychrophilic enzymes: From folding to function and biotechnology. Scientifica 2013, 2013, 512840. [Google Scholar] [CrossRef]
- Alblooshi, A.S.; Nasar, M.I.; Rehman, S.S.U.; Alam, M.T. Genomic and metabolic network properties in thermophiles and psychrophiles compared to mesophiles. Sci. Rep. 2025, 15, 19757. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, N.; Ma, J.; Zhou, Y.; Wei, Q.; Tian, C.; Fang, Y.; Zhong, R.; Chen, G.; Zhang, S. Advances in cold-adapted enzymes derived from microorganisms. Front. Microbiol. 2023, 14, 1152847. [Google Scholar] [CrossRef] [PubMed]
- Greco, L.; Ullo, S.; Rigano, L.; Fontana, M.; Berardesca, E.; Cameli, N. Evaluation of the Filming and Protective Properties of a New Trehalose and Ceramides Based Ingredient. Cosmetics 2019, 6, 62. [Google Scholar] [CrossRef]
- Cleland, D.; Krader, P.; McCree, C.; Tang, J.; Emerson, D. Glycine betaine as a cryoprotectant for prokaryotes. J. Microbiol. Methods 2004, 58, 31–38. [Google Scholar] [CrossRef]
- Buenger, J.; Driller, H. Ectoin: An effective natural substance to prevent UVA-induced premature photoaging. Ski. Pharmacol. Physiol. 2004, 17, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Dey, P. Bacterial exopolysaccharides as emerging bioactive macromolecules: From fundamentals to applications. Res. Microbiol. 2023, 174, 104024. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cornejo, S.; Otero, M.C.; Banerjee, A.; Gordillo-Fuenzalida, F. Biological properties of exopolysaccharides produced by Bacillus spp. Microbiol. Res. 2023, 268, 127276. [Google Scholar] [CrossRef]
- Driessen, A.J.M.; Albers, S.-V. Membrane Adaptations of (Hyper)Thermophiles to High Temperatures. In Physiology and Biochemistry of Extremophiles; Wiley Online Library: Hoboken, NJ, USA, 2007; pp. 104–116. [Google Scholar]
- Vieille, C.; Zeikus, G.J. Hyperthermophilic Enzymes: Sources, Uses, and Molecular Mechanisms for Thermostability. Microbiol. Mol. Biol. Rev. 2001, 65, 1–43. [Google Scholar] [CrossRef]
- Liao, Z.; Gopalasingam, C.C.; Kameya, M.; Gerle, C.; Shigematsu, H.; Ishii, M.; Arakawa, T.; Fushinobu, S. Structural insights into thermophilic chaperonin complexes. Structure 2024, 32, 679–689. [Google Scholar] [CrossRef]
- Somayaji, A.; Dhanjal, C.R.; Lingamsetty, R.; Vinayagam, R.; Selvaraj, R.; Varadavenkatesan, T.; Govarthanan, M. An insight into the mechanisms of homeostasis in extremophiles. Microbiol. Res. 2022, 263, 127115. [Google Scholar] [CrossRef]
- Kochhar, N.; Shrivastava, S.; Ghosh, A.; Rawat, V.S.; Sodhi, K.K.; Kumar, M. Perspectives on the microorganism of extreme environments and their applications. Curr. Res. Microb. Sci. 2022, 3, 100134. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Dopson, M. Life in acid: pH homeostasis in acidophiles. Trends Microbiol. 2007, 15, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mirete, S.; Morgante, V.; González-Pastor, J.E. Acidophiles: Diversity and Mechanisms of Adaptation to Acidic Environments. In Adaption of Microbial Life to Environmental Extremes: Novel Research Results and Application; Stan-Lotter, H., Fendrihan, S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 227–251. [Google Scholar]
- Ianutsevich, E.A.; Danilova, O.A.; Grum-Grzhimaylo, O.A.; Tereshina, V.M. The Role of Osmolytes and Membrane Lipids in the Adaptation of Acidophilic Fungi. Microorganisms 2023, 11, 1733. [Google Scholar] [CrossRef]
- Luo, W.; Li, Y.; Chen, S.; Liang, Y.; Liu, X. Roles and Regulation of Quorum Sensing of Acidophiles in Bioleaching: A Review. Microorganisms 2024, 12, 422. [Google Scholar] [CrossRef]
- Ferrer, A.; Orellana, O.; Levican, G. Oxidative Stress and Metal Tolerance in Extreme Acidophiles. Acidophiles Life Extrem. Acidic Environ. 2016, 1, 63–76. [Google Scholar]
- Oren, A. Life at high salt concentrations, intracellular KCl concentrations, and acidic proteomes. Front. Microbiol. 2013, 4, 315. [Google Scholar] [CrossRef]
- Buommino, E.; Schiraldi, C.; Baroni, A.; Paoletti, I.; Lamberti, M.; De Rosa, M.; Tufano, M.A. Ectoine from halophilic microorganisms induces the expression of hsp70 and hsp70B’ in human keratinocytes modulating the proinflammatory response. Cell Stress. Chaperones 2005, 10, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.T.; Lindsay, Y.E.; Caumette, P.; Herbert, R.A.; Hannan, j. Identification of trehalose and glycine betaine as compatible solutes in the moderately halophilic sulfate reducing bacterium, Desulfovibrio halophilus. FEMS Microbiol. Lett. 1996, 140, 203–207. [Google Scholar] [CrossRef]
- Sharma, N.; Farooqi, M.S.; Chaturvedi, K.K.; Lal, S.B.; Grover, M.; Rai, A.; Pandey, P. The Halophile Protein Database. Database 2014, 2014, bau114. [Google Scholar] [CrossRef]
- Ng, H.S.; Wan, P.-K.; Kondo, A.; Chang, J.-S.; Lan, J.C.-W. Production and Recovery of Ectoine: A Review of Current State and Future Prospects. Processes 2023, 11, 339. [Google Scholar] [CrossRef]
- Kanekar, P.P.; Kanekar, S.P. Metallophilic, Metal-Resistant, and Metal-Tolerant Microorganisms. In Diversity and Biotechnology of Extremophilic Microorganisms from India; Springer Nature Singapore: Singapore, 2022; pp. 187–213. [Google Scholar]
- Sprocati, A.R.; Alisi, C.; Segre, L.; Tasso, F.; Galletti, M.; Cremisini, C. Investigating heavy metal resistance, bioaccumulation and metabolic profile of a metallophile microbial consortium native to an abandoned mine. Sci. Total Environ. 2006, 366, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, L.; Bazylinski, D.A. Deep-sea piezosphere and piezophiles: Geomicrobiology and biogeochemistry. Trends Microbiol. 2010, 18, 413–422. [Google Scholar] [CrossRef]
- Ichiye, T. Enzymes from piezophiles. Semin. Cell Dev. Biol. 2018, 84, 138–146. [Google Scholar] [CrossRef]
- Gabani, P.; Singh, O.V. Radiation-resistant extremophiles and their potential in biotechnology and therapeutics. Appl. Microbiol. Biotechnol. 2013, 97, 993–1004. [Google Scholar] [CrossRef]
- Coppola, D.; Verde, C.; Giordano, D. Isolation of UV-Resistant Marine Bacteria by UV-C Assays. Methods Mol. Biol. 2022, 2498, 293–305. [Google Scholar] [CrossRef]
- Singh, D.K.; Pathak, J.; Pandey, A.; Singh, V.; Ahmed, H.; Rajneesh; Kumar, D.; Sinha, R.P. Chapter 15—Ultraviolet-screening compound mycosporine-like amino acids in cyanobacteria: Biosynthesis, functions, and applications. In Advances in Cyanobacterial Biology; Singh, P.K., Kumar, A., Singh, V.K., Shrivastava, A.K., Eds.; Academic Press: New York, NY, USA, 2020; pp. 219–233. [Google Scholar]
- Urrea-Victoria, V.; Hernández, A.R.; Castellanos, L.; Alves, I.A.; Novoa, D.M.A. The role of mycosporine-like amino acids in skin care formulations: A patent review (2014–2024). Photochem. Photobiol. Sci. 2025, 24, 847–861. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Long, P.F.; Young, A.R. Mycosporine-Like Amino Acids for Skin Photoprotection. Curr. Med. Chem. 2018, 25, 5512–5527. [Google Scholar] [CrossRef] [PubMed]
- Venetikidou, M.; Lykartsi, E.; Adamantidi, T.; Prokopiou, V.; Ofrydopoulou, A.; Letsiou, S.; Tsoupras, A. Proteolytic Enzyme Activities of Bromelain, Ficin, and Papain from Fruit By-Products and Potential Applications in Sustainable and Functional Cosmetics for Skincare. Appl. Sci. 2025, 15, 2637. [Google Scholar] [CrossRef]
- Lentzen, G.; Schwarz, T. Extremolytes: Natural compounds from extremophiles for versatile applications. Appl. Microbiol. Biotechnol. 2006, 72, 623–634. [Google Scholar] [CrossRef]
- Upadhyaya, C.; Patel, H.; Patel, I.; Upadhyaya, T. Extremophilic Exopolysaccharides: Bioprocess and Novel Applications in 21st Century. Fermentation 2025, 11, 16. [Google Scholar] [CrossRef]
- Mesbah, N.M. Industrial Biotechnology Based on Enzymes From Extreme Environments. Front. Bioeng. Biotechnol. 2022, 10, 870083. [Google Scholar] [CrossRef]
- Stege, H.; Roza, L.; Vink, A.A.; Grewe, M.; Ruzicka, T.; Grether-Beck, S.; Krutmann, J. Enzyme plus light therapy to repair DNA damage in ultraviolet-B-irradiated human skin. Proc. Natl. Acad. Sci. USA 2000, 97, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Luze, H.; Nischwitz, S.P.; Zalaudek, I.; Mullegger, R.; Kamolz, L.P. DNA repair enzymes in sunscreens and their impact on photoageing-A systematic review. Photodermatol. Photoimmunol. Photomed. 2020, 36, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Stamatas, G.N. Protein degradation in the stratum corneum. Int. J. Cosmet. Sci. 2024, 46, 590–597. [Google Scholar] [CrossRef]
- Trevisol, T.C.; Henriques, R.O.; Souza, A.J.A.; Furigo, A., Jr. An overview of the use of proteolytic enzymes as exfoliating agents. J. Cosmet. Dermatol. 2022, 21, 3300–3307. [Google Scholar] [CrossRef]
- Bhatt, H.B.; Sani, R.K.; Amoozegar, M.A.; Singh, S.P. Editorial: Extremozymes: Characteristics, structure, protein engineering and applications. Front. Microbiol. 2024, 15, 1423463. [Google Scholar] [CrossRef]
- Lourenco, C.B.; Ataide, J.A.; Cefali, L.C.; Novaes, L.C.; Moriel, P.; Silveira, E.; Tambourgi, E.B.; Mazzola, P.G. Evaluation of the enzymatic activity and stability of commercial bromelain incorporated in topical formulations. Int. J. Cosmet. Sci. 2016, 38, 535–540. [Google Scholar] [CrossRef]
- Balachandran, C.; Vishali, A.; Nagendran, N.A.; Baskar, K.; Hashem, A.; Abd Allah, E.F. Optimization of protease production from Bacillus halodurans under solid state fermentation using agrowastes. Saudi J. Biol. Sci. 2021, 28, 4263–4269. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Verma, V.; Dubey, V.K.; Srivastava, A.; Garg, S.K.; Singh, V.P.; Arora, P.K. Industrial applications of fungal lipases: A review. Front. Microbiol. 2023, 14, 1142536. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jin, T.; Nian, B.; Cheng, W. Solvent Tolerance Improvement of Lipases Enhanced Their Applications: State of the Art. Molecules 2024, 29, 2444. [Google Scholar] [CrossRef]
- Higaki, S. Lipase inhibitors for the treatment of acne. J. Mol. Catal. B Enzym. 2003, 22, 377–384. [Google Scholar] [CrossRef]
- Nakase, K.; Momose, M.; Yukawa, T.; Nakaminami, H. Development of skin sebum medium and inhibition of lipase activity in Cutibacterium acnes by oleic acid. Access Microbiol. 2022, 4, acmi000397. [Google Scholar] [CrossRef]
- Spalletta, A.; Joly, N.; Martin, P. Latest Trends in Lipase-Catalyzed Synthesis of Ester Carbohydrate Surfactants: From Key Parameters to Opportunities and Future Development. Int. J. Mol. Sci. 2024, 25, 3727. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; WeiZhuo, X.; Wei, X. A review on lipase-catalyzed synthesis of geranyl esters as flavor additives for food, pharmaceutical and cosmetic applications. Food Chem. Adv. 2022, 1, 100052. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Wang, H.-M.D.; Shieh, C.-J. Enzymes in Biomedical, Cosmetic and Food Application. Catalysts 2024, 14, 162. [Google Scholar] [CrossRef]
- Kettelarij, J.; Proquin, H.; Beetstra, R.; Roesink, C.; ter Burg, W.; Verhoeven, J.; Wezenbeek, J.; Woutersen, M.; Brand, W. Enzymes in Consumer Products. An Inventory of Non-Food Products, Regulatory Frameworks, Hazards and Considerations for Risk Assessment; RIVM: Bilthoven, The Netherlands, 2023. [Google Scholar]
- Acer, Ö.; Bekler, F.M.; Pirinççioğlu, H.; Güven, R.G.; Güven, K. Purification and Characterization of Thermostable and Detergent-Stable α-Amylase from Anoxybacillus sp. AH1. Food Technol. Biotechnol. 2016, 54, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Bandal, J.N.; Tile, V.A.; Sayyed, R.Z.; Jadhav, H.P.; Azelee, N.I.W.; Danish, S.; Datta, R. Statistical Based Bioprocess Design for Improved Production of Amylase from Halophilic Bacillus sp. H7 Isolated from Marine Water. Molecules 2021, 26, 2833. [Google Scholar] [CrossRef]
- Abo-Kamer, A.M.; Abd-El-salam, I.S.; Mostafa, F.A.; Mustafa, A.-E.-R.A.; Al-Madboly, L.A. A promising microbial α-amylase production, and purification from Bacillus cereus and its assessment as antibiofilm agent against Pseudomonas aeruginosa pathogen. Microb. Cell Factories 2023, 22, 141. [Google Scholar] [CrossRef]
- Salamanca-Córdoba, M.A.; Zambrano-Pérez, C.A.; Mejía-Arbeláez, C.; Motta, A.; Jiménez, P.; Restrepo-Restrepo, S.; Celis-Ramírez, A.M. Seborrheic dermatitis and its relationship with Malassezia spp. Infectio 2021, 25, 120–129. [Google Scholar] [CrossRef]
- Ekundayo, F.O.; Folorunsho, A.E.; Ibisanmi, T.A.; Olabanji, O.B. Antifungal activity of chitinase produced by Streptomyces species isolated from grassland soils in Futa Area, Akure. Bull. Natl. Res. Cent. 2022, 46, 95. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Odeniyi, O.A.; Majengbasan, O.S.; Igwaran, A.; Moloantoa, K.M.; Khetsha, Z.P.; Iwarere, S.A.; Daramola, M.O. Chitinases: Expanding the boundaries of knowledge beyond routinized chitin degradation. Environ. Sci. Pollut. Res. 2024, 31, 38045–38060. [Google Scholar] [CrossRef]
- Saunte, D.M.L.; Gaitanis, G.; Hay, R.J. Malassezia-Associated Skin Diseases, the Use of Diagnostics and Treatment. Front. Cell Infect. Microbiol. 2020, 10, 112. [Google Scholar] [CrossRef]
- de Andrade, R.; de Araujo, N.K.; Torres-Rego, M.; Furtado, A.A.; Daniele-Silva, A.; de Souza Paiva, W.; de Medeiros Dantas, J.M.; da Silva, N.S.; da Silva-Junior, A.A.; Ururahy, M.A.G.; et al. Production and Characterization of Chitooligosaccharides: Evaluation of Acute Toxicity, Healing, and Anti-Inflammatory Actions. Int. J. Mol. Sci. 2021, 22, 10631. [Google Scholar] [CrossRef] [PubMed]
- Choi Seong, S.; Yim Jun, H.; Abdalla Kozhir, K.; Jeon Young, J. Ectoine Production: Genetic, Biochemical, and Biotechnological Perspectives. J. Life Sci. 2025, 35, 635–645. [Google Scholar] [CrossRef]
- Liu, M.; Liu, H.; Shi, M.; Jiang, M.; Li, L.; Zheng, Y. Microbial production of ectoine and hydroxyectoine as high-value chemicals. Microb. Cell Fact. 2021, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Orhan, F.; Akıncıoğlu, A.; Ceyran, E. Ectoine production from a novel bacterial strain and high-purity purification with a cost-effective and single-step method. J. Biotechnol. 2024, 388, 24–34. [Google Scholar] [CrossRef]
- Bilstein, A.; Heinrich, A.; Rybachuk, A.; Mosges, R. Ectoine in the Treatment of Irritations and Inflammations of the Eye Surface. Biomed. Res. Int. 2021, 2021, 8885032. [Google Scholar] [CrossRef]
- del Olmo, J.A.; Melero, A.; Pino, A.; Martínez de Cestafe, N.; Gartziandia, O.; Ucelay López de Heredia, M.; Torrecilla, J.; Gómez, L.; Benito Cid, S.; Alonso, J.M.; et al. Enhanced Ocular Retention and Anti-Allergic Efficacy of a Novel HA–Ectoine–CMC Eye Drop for Dry Eye Disease Management. J. Pharm. BioTech Ind. 2025, 2, 16. [Google Scholar] [CrossRef]
- Cheng, W.; An, Q.; Zhang, J.; Shi, X.; Wang, C.; Li, M.; Zhao, D. Protective Effect of Ectoin on UVA/H2O2-Induced Oxidative Damage in Human Skin Fibroblast Cells. Appl. Sci. 2022, 12, 8531. [Google Scholar] [CrossRef]
- Wang, K.; Cui, B.; Wang, Y.; Luo, W. Microbial Production of Ectoine: A Review. ACS Synth. Biol. 2025, 14, 332–342. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, S.; Lv, P.; Sun, S.; Ma, C.; Xu, P.; Su, H.; Yang, C. High ectoine production by an engineered Halomonas hydrothermalis Y2 in a reduced salinity medium. Microb. Cell Fact. 2019, 18, 184. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wu, J.; Lao, C.; Wang, J.; Xu, Y.; Li, H.; Yuan, L.; Chen, X.; Yao, J. Multistep Metabolic Engineering of Escherichia coli for High-Level Ectoine Production. ACS Synth. Biol. 2025, 14, 1230–1239. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, B.; Liao, Q.; Huang, X.; Mi, M.; Huang, M.; Wu, Y.; Wu, S.; Wang, X.; Hu, X. Multi-gene metabolic engineering of Pichia pastoris to synthesize ectoine. J. Biosci. Bioeng. 2025, 139, 347–353. [Google Scholar] [CrossRef]
- Lu, B.; Zhao, S.; Zhang, J.; Zhan, J.; Zhang, J.; Liu, Z.; Zhang, J. Anti-inflammatory and antioxidant effects on skin based on supramolecular hyaluronic acid-ectoin. J. Mater. Chem. B 2024, 12, 8408–8419. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Silva, T.d.C.D.; da Silva Andrade, R.F.; Ootani, M.A.; Mendes, P.V.D.; da Silva, M.R.F.; Souza, K.S.; dos Santos Correia, M.T.; da Silva, M.V.; de Oliveira, M.B.M. Biotechnological potential of carotenoids produced by extremophilic microorganisms and application prospects for the cosmetics industry. Adv. Microbiol. 2020, 10, 397. [Google Scholar] [CrossRef]
- Kuzucu, M. Extremophilic Solutions: The Role of Deinoxanthin in Counteracting UV-Induced Skin Harm. Curr. Issues Mol. Biol. 2023, 45, 8372–8394. [Google Scholar] [CrossRef] [PubMed]
- Paredes Contreras, B.V.; Vermelho, A.B.; Casanova, L.; de Alencar Santos Lage, C.; Spindola Vilela, C.L.; da Silva Cardoso, V.; Pacheco Arge, L.W.; Cardoso-Rurr, J.S.; Correa, S.S.; Passos De Mansoldo, F.R.; et al. Enhanced UV-B photoprotection activity of carotenoids from the novel Arthrobacter sp. strain LAPM80 isolated from King George Island, Antarctica. Heliyon 2025, 11, e41400. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Kim, T.; Byeon, H.; Rayamajhi, V.; Lee, J.; Jung, S.; Shin, H. Improved Production of Astaxanthin from Haematococcus pluvialis Using a Hybrid Open–Closed Cultivation System. Appl. Sci. 2024, 14, 1104. [Google Scholar] [CrossRef]
- Jeong, S.W.; Kim, J.H.; Kim, J.W.; Kim, C.Y.; Kim, S.Y.; Choi, Y.J. Metabolic Engineering of Extremophilic Bacterium Deinococcus radiodurans for the Production of the Novel Carotenoid Deinoxanthin. Microorganisms 2020, 9, 44. [Google Scholar] [CrossRef]
- Shah, M.M.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-Like Amino Acids from Red Macroalgae: UV-Photoprotectors with Potential Cosmeceutical Applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Singh, V.K.; Jha, S.; Rana, P.; Gupta, A.; Singh, A.P.; Kumari, N.; Mishra, S.; Singh, P.R.; Jaiswal, J.; Sinha, R.P. Application of Synthetic Biology Approaches to High-Yield Production of Mycosporine-like Amino Acids. Fermentation 2023, 9, 669. [Google Scholar] [CrossRef]
- Pedrosa, V.M.; Sanches, A.G.; da Silva, M.B.; Gratao, P.L.; Isaac, V.L.; Gindri, M.; Teixeira, G.H. Production of mycosporine-like amino acid (MAA)-loaded emulsions as chemical barriers to control sunscald in fruits and vegetables. J. Sci. Food Agric. 2022, 102, 801–812. [Google Scholar] [CrossRef]
- Katoch, M.; Mazmouz, R.; Chau, R.; Pearson, L.A.; Pickford, R.; Neilan, B.A. Heterologous Production of Cyanobacterial Mycosporine-like Amino Acids Mycosporine-Ornithine and Mycosporine-Lysine in Escherichia coli. Appl. Environ. Microbiol. 2016, 82, 6167–6173. [Google Scholar] [CrossRef]
- Ying, R.; Zhang, Z.; Zhu, H.; Li, B.; Hou, H. The Protective Effect of Mycosporine-Like Amino Acids (MAAs) from Porphyra yezoensis in a Mouse Model of UV Irradiation-Induced Photoaging. Mar. Drugs 2019, 17, 470. [Google Scholar] [CrossRef]
- Kim, S.; Park, B.G.; Jin, H.; Lee, D.; Teoh, J.Y.; Kim, Y.J.; Lee, S.; Kim, S.J.; Moh, S.H.; Yoo, D.; et al. Efficient production of natural sunscreens shinorine, porphyra-334, and mycosporine-2-glycine in Saccharomyces cerevisiae. Metab. Eng. 2023, 78, 137–147. [Google Scholar] [CrossRef]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kogej, T.; Gostincar, C.; Volkmann, M.; Gorbushina, A.A.; Gunde-Cimerman, N. Mycosporines in Extremophilic Fungi—Novel Complementary Osmolytes? Environ. Chem. 2006, 3, 105–110. [Google Scholar] [CrossRef]
- Singh, A.; Cizkova, M.; Bisova, K.; Vitova, M. Exploring Mycosporine-Like Amino Acids (MAAs) as Safe and Natural Protective Agents against UV-Induced Skin Damage. Antioxidants 2021, 10, 683. [Google Scholar] [CrossRef]
- Gloster, T.M.; Toksoy Oner, E. Microbial exopolysaccharide production by polyextremophiles in the adaptation to multiple extremes. FEBS Lett. 2025. [Google Scholar] [CrossRef]
- Benhadda, F.; Zykwinska, A.; Colliec-Jouault, S.; Sinquin, C.; Thollas, B.; Courtois, A.; Fuzzati, N.; Toribio, A.; Delbarre-Ladrat, C. Marine versus Non-Marine Bacterial Exopolysaccharides and Their Skincare Applications. Mar. Drugs 2023, 21, 582. [Google Scholar] [CrossRef]
- Sabroso, E.; Martínez, J.M.; Sánchez-León, E.; Rodríguez, N.; Amils, R.; Abrusci, C. Production and Characterisation of an Exopolysaccharide by Vreelandella titanicae Zn11_249 Isolated from Salar de Uyuni (Bolivia). Polymers 2025, 17, 2362. [Google Scholar] [CrossRef]
- Aytar, M.; Uygun, D.A.; Başbülbül, G. Production and biological activities of exopolysaccharides synthesized by thermophilic bacilli isolated from hot springs in Türkiye. Int. Microbiol. 2025, 28, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, H.; Zhao, Y.; Cong, B.; Zhang, P. Structural characteristics and immunomodulatory activity of an exopolysaccharide from Antarctic Pseudoalteromonas sp. LP6-12-2. Extremophiles 2025, 29, 29. [Google Scholar] [CrossRef]
- Yang, F.; Wang, F.; Gan, L.; Wang, C.; Dong, C.; Zou, X. Production and Partial Characterization of Exopolysaccharide from a Newly Isolated Halophilic Strain Halomonas sp. DT-Z4; Research Square: Durham, NC, USA, 2025. [Google Scholar]
- Naykodi, A.; Doriya, K.; Thorat, B.N. Optimization and Characterization of Exopolysaccharide Production from Alkali-tolerant Alkalihalobacillus sp. using Response Surface Methodology for Cr (VI) Biosorption. Biocatal. Agric. Biotechnol. 2025, 64, 103522. [Google Scholar] [CrossRef]
- Murugesan, H.; Shobanan, S.; Tamilmani, K.K.; Sushmitha, T.J.; Balachandran, K.R.S.; Peter, D.M.; Rangamaran, V.R.; Gopal, D. Exopolysaccharide Production by Bacillus sp. NIOTSM16 from Arabian Sea Seamounts: Characterization and Optimization. Microbiology 2025, 94, 433–441. [Google Scholar] [CrossRef]
- Oztekin, S.; Dikmetas, D.N.; Karbancıoglu-Guler, F. A novel exopolysaccharide from cold-adapted yeast Rhodotorula glutinis, along with structural, rheological, antioxidant, and antibiofilm properties. Biomass Convers. Biorefinery 2025, 15, 1507–1523. [Google Scholar] [CrossRef]
- Karsavran, K.; Görünmek, M.; Çakmak, Z.E.; Çakmak, T. Exopolysaccharides as novel cryoprotectants released by Antarctic Chlamydomonas sp. ASYA25. Int. J. Biol. Macromol. 2025, 321, 146454. [Google Scholar] [CrossRef]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Guo, Q.; Xin, Y.; Liu, Y. Comprehensive review in moisture retention mechanism of polysaccharides from algae, plants, bacteria and fungus. Arab. J. Chem. 2022, 15, 104163. [Google Scholar] [CrossRef]
- Han, S.-I.; Heo, Y.M.; Jeon, M.S.; Kyung, S.; Kang, S.; Kwon, S.-J.; Ryu, J.H.; Kim, J.H.; Ahn, J.-W. The effect of exopolysaccharides from EMS-induced Porphyridium cruentum mutant on human epidermal and dermal layers. Front. Mar. Sci. 2024, 11, 1365311. [Google Scholar] [CrossRef]
- Li, F.; Hu, X.; Qin, L.; Li, H.; Yang, Y.; Zhang, X.; Lu, J.; Li, Y.; Bao, M. Characterization and protective effect against ultraviolet radiation of a novel exopolysaccharide from Bacillus marcorestinctum QDR3-1. Int. J. Biol. Macromol. 2022, 221, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Rotter, A.; Varamogianni-Mamatsi, D.; Zvonar Pobirk, A.; Gosenca Matjaz, M.; Cueto, M.; Diaz-Marrero, A.R.; Jonsdottir, R.; Sveinsdottir, K.; Catala, T.S.; Romano, G.; et al. Marine cosmetics and the blue bioeconomy: From sourcing to success stories. iScience 2024, 27, 111339. [Google Scholar] [CrossRef]
- Banerjee, A.; Sarkar, S.; Govil, T.; Gonzalez-Faune, P.; Cabrera-Barjas, G.; Bandopadhyay, R.; Salem, D.R.; Sani, R.K. Extremophilic Exopolysaccharides: Biotechnologies and Wastewater Remediation. Front. Microbiol. 2021, 12, 721365. [Google Scholar] [CrossRef]
- Thakur, A.; Musiol, M.; Duale, K.; Kowalczuk, M. Exploring the Future of Polyhydroxyalkanoate Composites with Organic Fillers: A Review of Challenges and Opportunities. Polymers 2024, 16, 1768. [Google Scholar] [CrossRef] [PubMed]
- Getino, L.; Martin, J.L.; Chamizo-Ampudia, A. A Review of Polyhydroxyalkanoates: Characterization, Production, and Application from Waste. Microorganisms 2024, 12, 2028. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Ray, S.; Sankhyan, S. Recent Challenges and Trends of Polyhydroxyalkanoate Production by Extremophilic Bacteria Using Renewable Feedstocks. Polymers 2023, 15, 4385. [Google Scholar] [CrossRef]
- Obulisamy, P.K.; Mehariya, S. Polyhydroxyalkanoates from extremophiles: A review. Bioresour. Technol. 2021, 325, 124653. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Panariello, L.; Morganti, P.; Danti, S.; Baroni, A.; Lazzeri, A.; Fusco, A.; Donnarumma, G. Skin-Compatible Biobased Beauty Masks Prepared by Extrusion. J. Funct. Biomater. 2020, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, N.; Gan, H.; Ilangovan, M.; Kimura, S.; Kasuya, K.I.; Isobe, N.; Iwata, T. Coastal and deep-sea biodegradation of polyhydroxyalkanoate microbeads. Sci. Rep. 2024, 14, 10302. [Google Scholar] [CrossRef]
- Ren, S.; Guo, S.; Yang, L.; Wang, C. Effect of composite biodegradable biomaterials on wound healing in diabetes. Front. Bioeng. Biotechnol. 2022, 10, 1060026. [Google Scholar] [CrossRef]
- Cubas, A.L.V.; Bianchet, R.T.; Reis, I.; Gouveia, I.C. Plastics and Microplastic in the Cosmetic Industry: Aggregating Sustainable Actions Aimed at Alignment and Interaction with UN Sustainable Development Goals. Polymers 2022, 14, 4576. [Google Scholar] [CrossRef]
- Bikiaris, N.; Nikolaidis, N.F.; Barmpalexis, P. Microplastics (MPs) in Cosmetics: A Review on Their Presence in Personal-Care, Cosmetic, and Cleaning Products (PCCPs) and Sustainable Alternatives from Biobased and Biodegradable Polymers. Cosmetics 2024, 11, 145. [Google Scholar] [CrossRef]
- Rodriguez-Cendal, A.I.; Gomez-Seoane, I.; de Toro-Santos, F.J.; Fuentes-Boquete, I.M.; Senaris-Rodriguez, J.; Diaz-Prado, S.M. Biomedical Applications of the Biopolymer Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Drug Encapsulation and Scaffold Fabrication. Int. J. Mol. Sci. 2023, 24, 11674. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.M.; Wnek, G.E. Poly(hydroxyalkanoates): Emerging Biopolymers in Biomedical Fields and Packaging Industries for a Circular Economy. Biomed. Mater. Devices 2025, 3, 19–44. [Google Scholar] [CrossRef]
- Park, H.; He, H.; Yan, X.; Liu, X.; Scrutton, N.S.; Chen, G.Q. PHA is not just a bioplastic! Biotechnol. Adv. 2024, 71, 108320. [Google Scholar] [CrossRef]
- Peng, J.; Guo, F.; Liu, S.; Fang, H.; Xu, Z.; Wang, T. Recent Advances and Future Prospects of Mycosporine-like Amino Acids. Molecules 2023, 28, 5588. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, Q.; Orfila, C.; Zhao, J.; Zhang, L. Systematic Review and Meta-Analysis on the Effects of Astaxanthin on Human Skin Ageing. Nutrients 2021, 13, 2917. [Google Scholar] [CrossRef]
- Bjorklund, G.; Gasmi, A.; Lenchyk, L.; Shanaida, M.; Zafar, S.; Mujawdiya, P.K.; Lysiuk, R.; Antonyak, H.; Noor, S.; Akram, M.; et al. The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions. Molecules 2022, 27, 7167. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Heinrich, U.; Jungmann, H.; Sies, H.; Tronnier, H. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am. J. Clin. Nutr. 2000, 71, 795–798. [Google Scholar] [CrossRef]
- Heinrich, U.; Gärtner, C.; Wiebusch, M.; Eichler, O.; Sies, H.; Tronnier, H.; Stahl, W. Supplementation with beta-carotene or a similar amount of mixed carotenoids protects humans from UV-induced erythema. J. Nutr. 2003, 133, 98–101. [Google Scholar] [CrossRef]
- Havas, F.; Krispin, S.; Cohen, M.; Loing, E.; Farge, M.; Suere, T.; Attia-Vigneau, J. A Dunaliella salina Extract Counteracts Skin Aging under Intense Solar Irradiation Thanks to Its Antiglycation and Anti-Inflammatory Properties. Mar. Drugs 2022, 20, 104. [Google Scholar] [CrossRef]
- Caimi, A.T.; Yasynska, O.; Rivas Rojas, P.C.; Romero, E.L.; Morilla, M.J. Improved stability and biological activity of bacterioruberin in nanovesicles. J. Drug Deliv. Sci. Technol. 2022, 77, 103896. [Google Scholar] [CrossRef]
- Bax, C.E.; Diaz, D.; Li, Y.; Vazquez, T.; Patel, J.; Grinnell, M.; Ravishankar, A.; Maddukuri, S.; Keyes, E.; Yan, D.; et al. Herbal supplement Spirulina stimulates inflammatory cytokine production in patients with dermatomyositis in vitro. iScience 2023, 26, 108355. [Google Scholar] [CrossRef]
- Chwil, M.; Mihelic, R.; Matraszek-Gawron, R.; Terlecka, P.; Skoczylas, M.M.; Terlecki, K. Comprehensive Review of the Latest Investigations of the Health-Enhancing Effects of Selected Properties of Arthrospira and Spirulina Microalgae on Skin. Pharmaceuticals 2024, 17, 1321. [Google Scholar] [CrossRef]
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in clinical practice: Evidence-based human applications. Evid. Based Complement. Altern. Med. 2011, 2011, 531053. [Google Scholar] [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Ou, J.; Wang, Z.; Liu, X.; Song, B.; Chen, J.; Li, R.; Jia, X.; Huang, R.; Xiang, W.; Zhong, S. Regulatory effects of marine polysaccharides on gut microbiota dysbiosis: A review. Food Chem. X 2022, 15, 100444. [Google Scholar] [CrossRef]
- Gao, T.; Wang, X.; Li, Y.; Ren, F. The Role of Probiotics in Skin Health and Related Gut-Skin Axis: A Review. Nutrients 2023, 15, 3123. [Google Scholar] [CrossRef]
- Nagar, S.; Antony, R.; Thamban, M. Extracellular polymeric substances in Antarctic environments: A review of their ecological roles and impact on glacier biogeochemical cycles. Polar Sci. 2021, 30, 100686. [Google Scholar] [CrossRef]
- Leong, H.J.-Y.; Teoh, M.-L.; Beardall, J.; Convey, P. Green beauty unveiled: Exploring the potential of microalgae for skin whitening, photoprotection and anti-aging applications in cosmetics. J. Appl. Phycol. 2024, 36, 3315–3328. [Google Scholar] [CrossRef]
- Ali Abdelrahim, N.; Noshy Saad, M.; Ashraf Zaghloul, M.; Samueal Neaz, O. Upcycled cosmetic ingredients: Extraction process optimization via green solvents by the means of I-optimal and Box–Behnken designs. Discov. Appl. Sci. 2024, 6, 672. [Google Scholar] [CrossRef]
- Stubbs, S.; Yousaf, S.; Khan, I. A review on the synthesis of bio-based surfactants using green chemistry principles. DARU J. Pharm. Sci. 2022, 30, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N. Unveiling the Anti-Aging Potential of Marine Natural Bioproducts. Mar. Drugs 2025, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.M.; Corona, B.; ten Klooster, R.; Worrell, E. Sustainability of reusable packaging–Current situation and trends. Resour. Conserv. Recycl. X 2020, 6, 100037. [Google Scholar] [CrossRef]
| Stress Factor | Bioactive Compound(s) | Function in Cosmetics |
|---|---|---|
| Radiation (UV, ionizing) | Mycosporine-like amino acids (MAAs), Scytonemin, Carotenoids (e.g., deinoxanthin), Ectoine | UV protection, antioxidant, anti-photoaging |
| High Salinity | Ectoine, Hydroxyectoine, Glycine betaine, Trehalose | Hydration, anti-inflammatory, barrier support |
| Cold (psychrophilic) | Antifreeze proteins (AFPs), Exopolysaccharides (EPSs), Trehalose | Moisturization, cryoprotection, anti-pollution |
| Heat (thermophilic) | Extremozymes (proteases, lipases, amylases), Chaperones | Enzymatic exfoliation, stability in formulations |
| Acidic pH | Organic antioxidants (e.g., glutathione), Peroxidases | Anti-oxidative, anti-inflammatory |
| Desiccation | Ectoine, Hydroxyectoine, EPSs | Moisturization, barrier protection |
| High Pressure | Pressure-stable extremozymes | Robust processing, formulation stability |
| Metal-rich environments | EPS with chelating properties | Anti-pollution, detoxifying claims |
| Microorganism | EPS Name/Type | Key Properties |
|---|---|---|
| Vreelandella titanicae Zn11_249 | Branched heteropolysaccharide | Antioxidant, non-cytotoxic, cosmetic potential |
| Geobacillus, Parageobacillus, Aeribacillus, Anoxybacillus | Thermophilic EPS | Antioxidant, antibacterial, prebiotic |
| Pseudoalteromonas sp. LP6-12-2 | Mannose-rich EPS | Immunomodulatory, anti-aging potential |
| Halomonas sp. DT-Z4 | Fructose-dominated EPS | High thermal stability, oil retention |
| Alkalihalobacillus sp. | EPS under alkaline stress | Biosorption, rheology modifier |
| Bacillus sp. NIOTSM16 | EPS with fatty acids | Moisturizing, anti-pollution |
| Rhodotorula glutinis (cold-adapted yeast) | β-D-glucan EPS | Antioxidant, antibiofilm, cryoprotectant |
| Chlamydomonas sp. ASYA25 | Microalgal EPS | Cryoprotectant, membrane integrity |
| Compound Class | Limitations and Risks | References |
|---|---|---|
| Ectoine/Hydroxyectoine | Limited large-scale production due to cost; regulatory constraints in food use; potential allergenicity not fully evaluated. | [36,80,83] |
| Extremozymes | Expensive production and purification; possible immunogenicity if used in topical or ingestible formulations; few toxicity studies in humans. | [8,9] |
| Carotenoids | Instability under light and oxygen; bioavailability issues; production yield still limited for some extremophilic sources. | [85] |
| EPS | Complex purification and structure variability; high production cost. | [101] |
| PHA | High production costs of PHA compared with other (bio)polymers | [118] |
| MAAs | Low accumulation of MAAs in organisms, limited green extraction process, difficult identification, and high cost | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maresca, E.; Carbone, M.; Gallo, G.; Fusco, S.; Aulitto, M. Extremophile-Derived Bioactives in Cosmeceuticals: Bridging Nutraceuticals and Skincare for Holistic Wellness. Life 2025, 15, 1787. https://doi.org/10.3390/life15121787
Maresca E, Carbone M, Gallo G, Fusco S, Aulitto M. Extremophile-Derived Bioactives in Cosmeceuticals: Bridging Nutraceuticals and Skincare for Holistic Wellness. Life. 2025; 15(12):1787. https://doi.org/10.3390/life15121787
Chicago/Turabian StyleMaresca, Emanuela, Micaela Carbone, Giovanni Gallo, Salvatore Fusco, and Martina Aulitto. 2025. "Extremophile-Derived Bioactives in Cosmeceuticals: Bridging Nutraceuticals and Skincare for Holistic Wellness" Life 15, no. 12: 1787. https://doi.org/10.3390/life15121787
APA StyleMaresca, E., Carbone, M., Gallo, G., Fusco, S., & Aulitto, M. (2025). Extremophile-Derived Bioactives in Cosmeceuticals: Bridging Nutraceuticals and Skincare for Holistic Wellness. Life, 15(12), 1787. https://doi.org/10.3390/life15121787










