Abstract
The nucleation and growth of crystals in igneous rocks is usually thought to occur under thermodynamic equilibrium conditions. However, recent studies on igneous textures and mineral compositions have shown that these processes probably occur under thermodynamic disequilibrium conditions. Titanomagnetite with variable crystal sizes can be observed in Hannuoba alkaline basalt, indicating disequilibrium crystallization processes (different cooling rates). The ratio of the maximum particle size to the area abundance of titanomagnetite, as determined by an analysis of previous studies on the texture of minerals, was negatively correlated with the apparent cooling rate. We analyzed the chemical composition and crystal size distribution of titanomagnetite in ten Hannuoba alkaline basalt samples to determine the connection between the apparent cooling rate and titanomagnetite composition. In Hannuoba samples, the cooling rate was found to affect cationic substitution in the titanomagnetite solid solution, and an increase in cooling rate led to a decrease in Ti4+ and an increase in Fe3+. The partition coefficient of Ti between titanomagnetite and the melt (DTi) is negatively correlated with the apparent cooling rate. These findings are consistent with those in experimental petrology and help us propose a better, more general geospeedometer. The cooling rate also impacted Mg2+ and Al3+, but they were more impacted by the melt composition and crystallinity of the coexisting melt. Therefore, a new geospeedometer was calibrated by considering the titanomagnetite composition, melt composition and the content of the clinopyroxene.The cooling rates of the Hannuoba basalt samples measured using the new geospeedometer calibrated in this study range from 0.7 to 7.0 (±0.5) °C/min. It cannot accurately predict the cooling rate from titanomagnetite in intermediate rock, felsic rock or Fe-rich basaltic melts. The new titanomagnetite geospeedometer can better measure the cooling rate of alkaline basalt and may help identify the effects of kinetically controlled crystallization on isotope fractionation, evaluate mineral thermobarometers and better recognize thermal remanence magnetization and ancient magnetic fields.
1. Introduction
The study of the cooling rate in igneous rocks is an effective supplement to the dynamics of igneous rocks and can further improve and constrain the dynamics of the magmatic process. The chemical compositional characteristics of rock-forming minerals have long been used to estimate temperature and pressure in igneous systems under thermodynamic equilibrium conditions [1].
However, recent studies suggest that major and minor element diversities in many rock-forming minerals, such as olivine [2,3], titanomagnetite [4,5,6,7], plagioclase [8,9,10] and clinopyroxene [11,12,13,14], could be significantly affected by cooling rate variations, which is a challenge for traditional geothermobarometers. In addition, if the cooling rate of magnetite is ignored, extending to geomagnetism can affect the intensity of the thermal remanence magnetization (T.R.M.) acquired by rocks during cooling to ambient temperature, which may lead to an underestimation or overestimation of the intensity of ancient magnetic fields [7,15,16]. Therefore, this encourages us to develop a mineral geospeedometer that records the cooling rate as magma rises [5,6,17,18,19].
In many igneous rocks, titanomagnetite is a common accessory mineral [20,21,22]. It can incorporate many major and trace elements, and cation redistribution in a titanomagnetite solid solution frequently occurs [4,6,23]. Chemical changes in titanomagnetite can reflect the variations in coexisting melt composition, temperature, pressure, oxygen fugacity, sulfur fugacity, undercooling, cooling rate and fluid compositions during the magma solidification process [6,18,24,25,26,27]. Chemical changes in titanomagnetite make it a useful petrogenetic indicator and geological processes chronicler mineral for mineralogy, petrology and geochemical studies [6,7,21,23,28,29,30,31,32,33,34,35]. Studying the distribution of cations in titanomagnetite can help us understand the magmatic environment in which minerals are formed.
Previous studies have found that pillows of mid-ocean ridge basalt (MORB) show a substantial change in textural and titanomagnetite mineralogical features as a function of cooling rate. These variations are of great importance to the characteristics of the MORB’s magnetization [7,15,36,37]. Recent experimental studies on trachybasalts, basalts and basaltic trachyandesites have shown that the cooling rate significantly affects the texture and chemical composition of titanomagnetite [5,6,7,38,39,40,41]. In these studies, when the cooling rate becomes faster, the particle size of titanomagnetite gradually decreases, and some titanomagnetites show dendritic morphology [5,6,7,40]. Compatible elements gradually replace other elements in the crystal lattice if the cooling rate is slow. The results of experimental petrology studies [6,34,42] show that, in the case of increasing cooling rates, titanomagnetite in basalt is rich in Mg2+, Al3+ and Fe3+ but poor in Ti4+ and Fe2+, and its ulvöspinel (Usp) end member is greatly reduced during the kinetically controlled crystallization process.
One titanomagnetite geospeedometer has been developed using cation redistribution from experiments conducted under suitable fO2 conditions to estimate the cooling rate of a dike outcropping on Mt Etna volcano [6]. The cooling rate from the innermost to the outermost part of the dike is from 0.02 to 1.13 °C/min [5,6]. However, the geospeedometer above does not take into account the composition of the melt (in Hannuoba basalt, Mg2+ and Al3+ in titanomagnetite are affected by melt composition) or other possible factors [6]. Melt composition may have an influence on titanomagnetite composition, so the geospeedometer should consider its influence on titanomagnetite. We need to find a geospeedometer suitable for experimental petrology and most field samples.
Zhou et al. [7] explored whether the Ti content in titanomagnetite is directly proportional to the titanomagnetite particle size in MORB. The results show that the rapid cooling rate leads to small particle sizes and a decrease in the Ti content in titanomagnetite. Crystal size may be an indicator of apparent cooling rate: when the cooling rate is slow, the nucleation rate is slow (relative to the crystal growth rate), and large crystals are easily formed. In contrast, when the cooling rate is fast, the nucleation rate is fast, and tiny crystals are easily formed. These phenomena are observed in field samples and products of high-temperature experiments, showing that the cooling rate is generally inversely proportional to the size of the crystals [5,6,7,13,43,44]. However, the crystal size is determined by various factors, such as the crystallization time, nucleation rate, cooling rate, magma mixing, and overgrowth [34,45,46,47,48,49]. Previous experimental studies explored the relationship between the cooling process and mineral quantitative textural parameters (e.g., [34,43]), indicating that the crystal size may not accurately reflect the cooling rate. So, more theoretical and case studies on the relationship between cooling rate, titanomagnetite texture and chemical composition are needed.
Hannuoba basalt is representative of Cenozoic basalt in eastern China [50,51,52,53,54,55,56,57,58]. Titanomagnetite with variable crystal sizes and texture can be observed in Hannuoba alkaline basalt, indicating kinetically controlled crystallization processes (different cooling rates).
In this study, we determine better parameters to describe the apparent cooling rate using the crystal size distribution of titanomagnetite in Hannuoba basalt. We found that the ratio of the maximum particle size to the area abundance of titanomagnetite could represent an apparent cooling rate in Hannuoba alkaline basalt. Furthermore, this study attempts to determine how cooling rate affects cation redistribution in titanomagnetite by comparing the apparent cooling rate of natural samples and the chemical composition of titanomagnetite with experimental petrology. The study shows that, although rock content and mineral content can influence cation redistribution, the cooling rate still plays a critical role in the composition of titanomagnetite despite other geological factors. The new geospeedometer obtained in this study is suitable for mafic rocks, especially alkaline basalt.
2. Geological Setting
The Hannuoba basalt is one of many Cenozoic basalts in eastern China (~200 km NW of Beijing) and includes alkaline and subalkaline basalts [50,51,52,53,54,55,56,57,58] (Figure 1a). Overall, Hannuoba basalt is a fissure overflow basalt, a vast lava slab that has unconformity above the Archean, Jurassic, Cretaceous, and Yanshanian igneous rocks and earlier intrusive rocks. Hannuoba basalt is a multicycle product with at least two large magma cycles at approximately 26 Ma in the early–middle Miocene. Its area is up to 1700 km2, where alkaline and subalkaline basalts are interlayered, forming as much as 90% of the outcrop [54]. Mantle xenoliths are widely found in Hannuoba alkaline basalt. Previous studies suggested that Hannuoba xenoliths bearing alkaline basalt derived from isotopically homogeneous sources and crustal contamination are negligible [53,54], although the nature of the source rocks remains under debate [53,54,59,60]. High-pressure eclogite fractionation may cause compositional diversity in basalts [54].
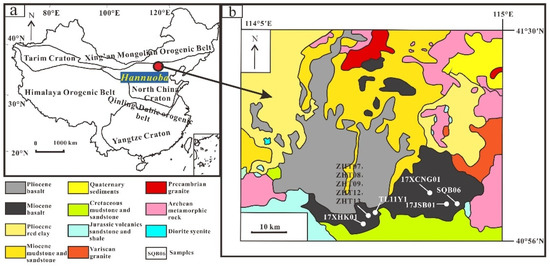
Figure 1.
The location of Hannuoba basalt in China (a) and the geologic map of Hannuoba (b). The geologic map of Hannuoba was modified by Zhi et al. [54].
3. Methods
3.1. Quantitative Mineral Texture Analysis
In recent years, quantitative rock structure analysis has gradually become an important research focus in igneous rock [34,43,44,61,62,63]. By quantifying a series of structural parameters, such as mineral or porosity content, three-dimensional morphology, degree of self-shape, grain size distribution, spatial distribution, degree of orientation and dihedral angle [42,45,47,63,64,65,66,67,68], we can quantitatively understand the diversity of igneous rock structure characteristics and the dynamic process of magma crystallization [4,42,43,44,45,46,47,66,69,70].
Marsh [45] introduced crystal size distribution (CSD) theory into the study of magmatic systems. In recent years, this theory has been widely used to discuss the crystallization kinetics of igneous rock systems (e.g., [4,43,44,46,61,64,69,70,71,72,73,74,75,76,77,78,79,80,81,82]). In simple terms, CSD theory refers to the representation of a mineral in a rock in the covariant diagram of ln(n) (the natural logarithm of population density) and L (crystal size). Equation (1) clearly shows that if the crystal growth rate is independent of the crystal size and the nucleation rate exponentially increases with time, a closed system produces a semi-logarithmic linear relationship between crystal density and crystal length [45,47,62]:
In Equation (1), G is the crystal growth rate, and τ is the crystallization time. The intercept is (the natural logarithm of the final nucleation density of the crystal), and the slope is .
In this study, we used a two-dimensional thin section method to obtain quantitative data on the crystal sizes of igneous rocks [45,47,62]. Appropriate microscope magnification was selected to obtain reflection photomicrographs according to the crystal size of titanomagnetite. Ensuring that each sample had at least 200 crystals was conducive to obtaining the three-dimensional shape parameters of the crystal, avoiding interruptions in the crystal size interval and reducing errors [83]. Due to observation accuracy and image resolution limitation, titanomagnetite with a particle size less than 0.01 mm should be omitted. Photoshop (Adobe Photoshop, Adobe Systems Incorporated, San Jose, CA, USA) and CorelDRAW (CorelDRAW Graphics Suite, Corel, Ottawa, ON, Canada) were used to distinguish the mineral crystals and manually depict the mineral boundaries. The images were output at the original size in TIFF format and imported into ImageJ software (Image J, National Institutes of Health, Bethesda, MD, USA); the measuring scale and statistical parameters were set, and the area of titanomagnetite, the aspect ratio of the fitting ellipse and roundness were then analyzed. To obtain 3D texture characteristics of titanomagnetite, a more convenient, fast and accurate conversion method was realized by 2D length (l) and width (w), which is widely used at present [66]. Furthermore, based on the formula above, the cut-section effect and intersection–probability effect were also considered. We then imported the abovementioned data into CSD corrections 1.38 ((Higgins, 2000), Canada) [62]. In this software, the textural characteristics, morphological characteristics, roundness and other parameters of titanomagnetite were set and calculated, and the characteristic size distribution curve of titanomagnetite was obtained.
3.2. Sampling and Mineral Identification
We selected ten representative samples based on extensive petrographic observations, whose matrix minerals have different grain size characteristics. They are all Miocene basalts. The location of each sample is shown in Figure 1b.
BSE images of ten samples were obtained by a TESCAN MIRA3 high-performance field emission scanning electron microscope in Resources Exploration Experiment and Training Center–Research Center of Genetic Mineralogy, China University of Geosciences, Beijing. Representative images are shown in Figure 2. The accelerating voltage was 20 kV. The current was about 260 pA, and the beam size was 85.0 nm.
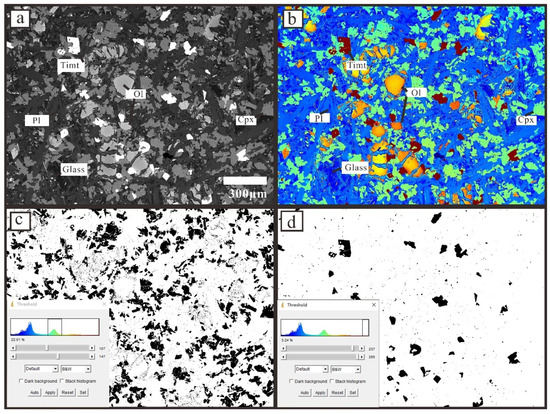
Figure 2.
An example (17XHK01) of distinguishing all minerals and obtaining their contents. (a): BSE photograph of 17XHK01; (b): Jet color is given to the BSE grayscale photograph to make it easier to use ImageJ Threshold to distinguish different minerals; (c): The outlines of clinopyroxene using ImageJ Threshold; (d): The outlines of titanomagnetite using ImageJ Threshold.Pl: Plagioclase, Cpx: Clinopyroxene, Timt: Titanomagnetite, Ol: Olivine. The mineral symbols are referred to as Warr [84].
Different minerals (glass, olivine, clinopyroxene, titanomagnetite, plagioclase) and their respective contents can be distinguished by the gray range of the BSE photographs (Figure 2a,b). Clinopyroxene (Figure 2c), titanomagnetite (Figure 2d) and other minerals can be distinguished by grayscale, and ImageJ Threshold tools can measure their contents. This method can quickly distinguish minerals and estimate the mineral content, but the mineral outline image obtained by this method has a little more noise, and the accuracy of mineral morphology is not very high, so it cannot be used for CSD analysis.
3.3. Rock Major Element Analysis
Analyses of the major elements of whole rocks were carried out in the Laboratory of Geochemical Micro area of the State Key Laboratory of Geological Processes and Mineral Resources, China University of Geoscience, Beijing. The instrument model was a Shimadzu XRF-1800 wavelength dispersive X-ray fluorescence spectrometer. Samples were prepared by a vitreous melting method. In a platinum crucible, analytical samples (0.7 g) with a crystal size of at least 200 mesh, a flux (lithium metaborate and lithium tetraborate mixed flux, 7 g) and a release agent (lithium bromide) were weighed at a ratio of 1:10. After thorough mixing, the crucible was placed in an HMS-II-MXZ oven and melted for 30 min at 950~1100 °C. Then, the fused beads were loaded into the XRF instrument for determination for 30 min. The prepared fuses were placed in an X-ray fluorescence spectrometer for measurement, and the calibration curve method was used to quantitatively analyze the major elements of the rock. The produced measurement and data quality were monitored by repeat analyses of international basalt standards BCR-2 and BHVO-2. The analytical precision (RSD, relative standard deviation) and accuracy (RE, relative error between measured and recommended values) are better than 5% for major elements, with many elements agreeing to within 2% of the reference values.
3.4. Mineral Major Element Analysis
Electron microprobe analysis was performed to determine the major elements in titanomagnetite from the ten samples. In each sample, 15 particles of different sizes were randomly selected to determine the overall chemical characteristics of titanomagnetite. Two independent tests were conducted to obtain two sets of data, with 30 points in total. The aim was to test the reliability of statistical rules. All experiments were carried out in the Electron Probe Laboratory of the Beijing Research Institute of Uranium Geology. A JEOL-JXA-8100 electron probe microanalyzer (EPMA) was used, and the following standards were adopted for the various chemical elements: jadeite (Si), corundum (Al), forsterite (Mg), andradite (Fe), chromite (Cr), calcium vanadate (V), nickel oxide (Ni), rutile (Ti), labradorite (Ca), and spessartine (Mn). The accelerating voltage was 20 kV, beam current was 10-8 A, electron probe beam size was 2 μm, and exit angle was 40°. Peak and background counting times were 10 and 5 s for Mg, Al, Fe and Ti, 20 and 10 s for V, Cr, Si, Ca, Mn and Ni. The standards used were SPI standard minerals and were analyzed as internal standards to monitor data quality. Analyses are accurate up to 1%~2% for major elements (>10%) and 2%~10% relative for minor elements (0.5%~10%).
4. Results
4.1. Petrology
We studied Miocene Hannuoba xenolith bearing alkaline basalt. The basalt sample has a porphyritic texture overall. The phenocrysts are olivine and clinopyroxene. The fine-grained groundmass contains olivine, plagioclase, clinopyroxene, titanomagnetite and fresh glass (Figure 3). In the reflected light (Figure 4a), we identified the opaque mineral as titanomagnetite. SEM allows us to easily segment different minerals and glasses to obtain the crystalline volume content of different minerals. We investigated ten alkaline basalt samples with significant differences in the crystal size of the groundmass titanomagnetite for analysis. The fine- and coarse-grained structures are relative concepts in this article, the coarse-grained titanomagnetite denotes grains between 0.1 mm and 0.2 mm, and the fine-grained titanomagnetite denotes grains between 0.03 mm and 0.1 mm. Titanomagnetite crystals (Figure 3) are primarily granular, and skeleton crystals are visible in the fine-grained samples. There are a small number of euhedral and subhedral titanomagnetite grains. There is no composition zoning in titanomagnetite. We assume that each titanomagnetite grain is just one crystal to ensure the analysis accuracy. The modal abundance of titanomagnetite ranges from approximately 3% to 5%. The crystal size ranges from 10 μm to 100 μm. Titanomagnetite in the samples accords with the description of the characteristics of titanomagnetite in Hannuoba alkaline basalt in previous research [54].
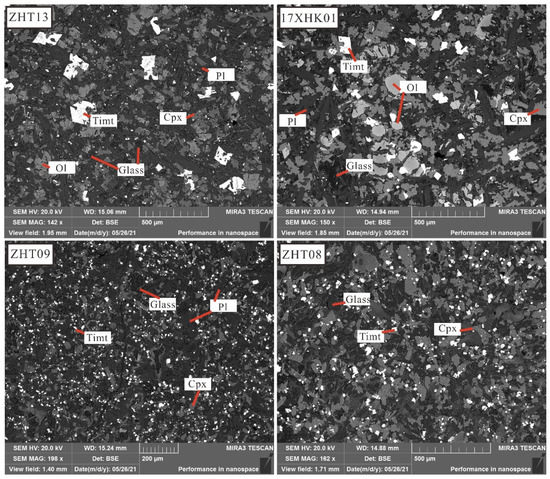
Figure 3.
Representative BSE photographs of Hannuoba alkaline basalt with different crystal sizes. Samples with coarse-grained phenocrysts, such as 17XHK01 and ZHT13, have relatively coarse-grained groundmass crystals, while samples without phenocrysts, such as ZHT09 and ZHT08, have relatively fine-grained groundmass crystals. The crystal sizes significantly differ, implying different cooling rates during melting solidification. Pl: Plagioclase, Cpx: Clinopyroxene, Timt: Titanomagnetite, Ol: Olivine. The mineral symbols are referred to as Warr [84].
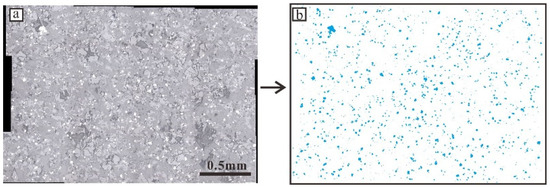
Figure 4.
An example (17JSB01) of titanomagnetite particle outlines is extracted from a stitched photomicrograph taken via a reflection microscope. (a): The reflection microscope photograph of 17JSB01. The white mineral particles are titanomagnetite; (b): Image of titanomagnetite particles outlined manually used for CSD analysis.
4.2. Quantitative Textural Parameters
In these samples, clinopyroxene and plagioclase are typical mineral phases, and some samples have a small amount of olivine and apatite. Titanomagnetite is about 4.5%, clinopyroxene is from 18.8 to 28.7%, plagioclase is from 28.0 to 49.4%. The phase abundance of other major minerals is presented in Table 1.

Table 1.
Phase abundances of major minerals (%).
Outlined images of the titanomagnetite crystals for all the samples are provided in Appendix A Figure A1. Photoshop and CorelDRAW were used to distinguish the mineral crystals and manually depict the mineral boundaries. One example is shown in Figure 4. The textural parameters of all the titanomagnetite samples by the images are presented in Table 2. The CSD curves of the ten samples by the parameters are plotted in Figure 5 with negative slopes, as shown on a semilogarithmic CSD diagram. The CSD curves of all the samples have negative slopes, and three types of CSD curves (Higgins, 2006a) are shown in Figure 5: S-CSD (semilogarithmic CSDs) lines (17JSB01, ZHT07), L-CSD (lognormal CSDs) lines (17XCNG01, SQB06, ZHT08, ZHT09, ZHT12) and F-CSD (fractal CSDs) lines (17XHK01, TL11Y1, ZHT13).

Table 2.
Titanomagnetite textural parameters of the Hannuoba alkaline basalt samples.
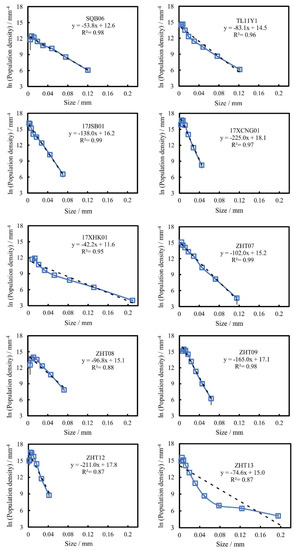
Figure 5.
Titanomagnetite crystal size distribution for all the studied Hannuoba alkaline basalt samples. The black dashed lines are the regressions of all data. A shallower slope represents a larger size range and a coarser-grained sample.
4.3. Rock Major Element
The whole-rock data for the major elements in each sample are presented in Table 3. For whole-rock major elements, the ranges of SiO2, Al2O3, FeO, CaO, and MgO are 46.16~50.50 wt%, 13.40~16.38 wt%, 11.27~13.20 wt%, 7.20~9.78 wt% and 5.33~9.38 wt%, respectively. The samples are all alkaline basalt, and most of the samples are trachybasalt or very close to trachybasalt in this study (Figure 6).

Table 3.
Whole-rock major element data for the Hannuoba alkaline basalt (wt%).
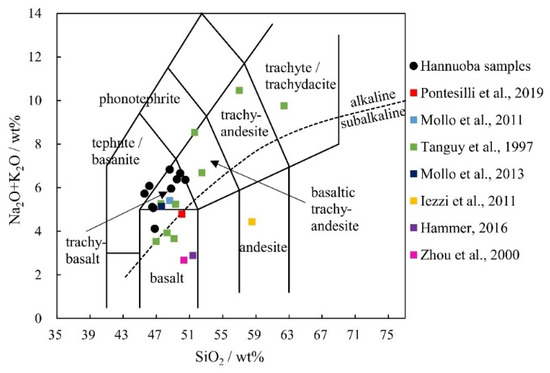
Figure 6.
Whole-rock TAS diagram [85] of natural rocks and synthesized mixtures used in experimental petrology. The alkaline line is cited by Miyashiro [86]. Other samples come from nature [5,7,87] and synthetic materials in experimental petrology [4,6,34,42].
4.4. Mineral Major Element
The major chemical titanomagnetite composition of all particles is reported in Supplementary Materials Table S1. Two independent tests were conducted to obtain two sets of data (15 particles for each test), marked as ‘sample-a’ and ‘sample-b’, respectively. The average major chemical data of each test in each sample are shown in Table 4. In these samples, there is no statistical difference in the average composition of major elements between the two sets of data. The two sets of data represent the average composition characteristics of major elements of titanomagnetite, and these average data are consistent with the statistical rules. For titanomagnetite mineral major elements, Al2O3 are 0.28~2.77 wt%, FeO are 43.74~51.33 wt%, Fe2O3 are 13.18~28.32 wt%, MgO are 0.71~3.61 wt%, and TiO2 are 21.03~27.26 wt%. In the FeO-Fe2O3-TiO2 ternary diagram, the main oxide was near the titanomagnetite series, as Mollo et al. [6] reported (Figure 7). Compared with data in Mollo et al. [6], titanomagnetite in Hannuoba samples is more closed to Usp unit.

Table 4.
Major elements in titanomagnetite from the Hannuoba alkaline basalt (wt%) and the number of cations based on four oxygen atoms.
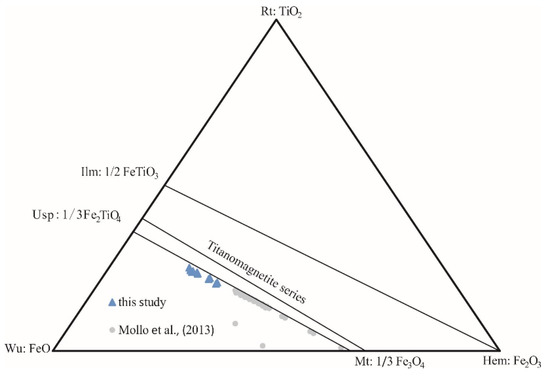
Figure 7.
Titanomagnetite compositions from Hannuoba samples are plotted in the FeO–Fe2O3–TiO ternary diagram. The error bars are within the symbols [6].
5. Discussion
5.1. Magma Cooling Process
It is vital to understand the magmatic ascent and flow process in Hannuoba, especially because the cooling rate may change during this process. CSD data are appropriate for exploring the fundamental aspects of crystal nucleation and growth during the progressive solidification of Hannuoba alkaline basalt.
Based on covariant relations among CSD variables, we can further discuss the characteristics of magma systems and various ascent and flow dynamic processes and reveal the significance of dynamics. The slope and intercept of the CSD regression line are negatively correlated (Figure 8a). The correlation between slope and intercept is well-known and is related to the closure of the system [66]. The slope of the CSD regression line and the maximum particle size determine the survival time of an open or closed system. At the same time, the giant crystal often more easily enters or leaves the system and is usually independent of CSD changes, so the relationship between the maximum particle size and the slope and intercept can reveal many crucial magmatic consolidation dynamics. The slope and maximum particle size are positively correlated (Figure 8b), showing that the nucleation rate and growth rate are stable and that titanomagnetites are formed under the stable and open system. The relationship between the intercept and maximum particle size is shown as negative correlations (Figure 8c), representing the characteristics of a closed system [45,62,63,66]. Overall, the titanomagnetite in these samples probably nucleates and grows in a stable open or closed system and is fractionally influenced by the environment.
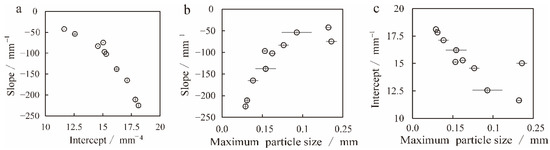
Figure 8.
Covariant relation among CSD variables and dynamical significance. (a): The relationship between the slope and intercept of the CSD regression line. (b): The relationship between slope and maximum particle size. (c): Relationship between intercept and maximum particle size.
Most titanomagnetite in most Hannuoba samples is represented by a straight line in the classic CSD diagram of ln (population density) versus size (S-CSD) (Figure 5), indicating a simple nucleation and growth process [47]. These simple straight CSD lines imply a relatively stable cooling rate, which was experimentally confirmed [43].
S-CSD lines represent the process of crystal nucleation and growth in a stable system without magma mixing [45]. This implies that titanomagnetite crystallizes in a simple and stable environment during the consolidation of Hannuoba alkaline basalt.
L-CSD lines show concave downward CSDs as tiny crystals, which may be due to a decrease in nucleation density during the final stage of groundmass crystallization or the possible Ostwald ripening process [48,49,62,72,74,77,88,89]. Another possible explanation is that the minor fine-grained crystals that are smaller than 0.01 mm are omitted. Some of them are too difficult to quantify during CSD analysis, resulting in the loss of minor fine-grained crystals. Under such conditions, these L-CSD lines can be seen as approximate S-CSD lines. In experiments (e.g., [34]), regardless of how the dwelling time changes (the cooling process ends; the temperature drops to the target temperature and keeps constant for some time), the chemical composition of titanomagnetite remains stable under a fixed cooling rate. However, textural information can be reflected in the CSD diagram: a longer dwelling time results in a larger slope of the CSD lines and a smaller intercept, resulting in the loss of small crystals and the formation of L-CSD. Titanomagnetites with different sizes in the L-CSD samples (for ZHT09 and 17XCNG01 as examples in Figure 9) have homogeneous chemical characteristics, suggesting that the titanomagnetites in these samples undergo a fixed cooling process with some dwelling time.
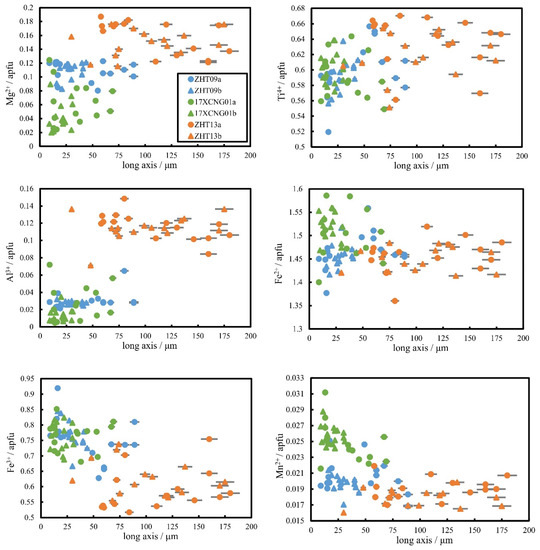
Figure 9.
Relationship between major element concentrations in titanomagnetite reported by atom per formula unit (apfu) and the 2D-shape long axis of titanomagnetite in L-CSD (ZHT09 and 17XCNG01) and F-CSD (ZHT13) that was tested. The 2D-shape axis of titanomagnetite is measured by BSE images through distance measurement tools. The vast majority of titanomagnetite is round and granular, and the long axis of the section can approximately represent the particle size. The error is less than 5%. Two independent tests were conducted to obtain two sets of data (15 particles for each test), marked as ‘sample-a’ and ‘sample-b’, respectively. Some error bars smaller than the symbols are not shown.
F-CSD lines might result from two-stage crystallization, crystal aggregation or magma mixing [47,62,63]. If the situation above exists, there will be two different crystal groups of titanomagnetite in the F-CSD sample (for ZHT13 as an example in Figure 9), and the chemical composition of titanomagnetite in different crystal groups should be different because physical and chemical conditions must change in the two crystallization processes, which will influence the cation substitution of titanomagnetite. However, the chemical composition in ZHT13 is almost fixed, and it seems that there are not two titanomagnetite crystal groups (Figure 9). Hannuoba samples are xenolith-bearing alkaline basalts without crustal magma chamber processes [53,54], so magma mixing is also less likely. Another assumption is that the CSD curve may also be fractal at a fixed cooling rate. It has been found in experimental petrology [4,43]. It means that titanomagnetite might undergo one fixed cooling rate. So, the F-CSD curves may not reflect two-stage crystallization or magma mixing.
Combined with CSD theory, experimental petrology and the geological background of Hannuoba alkaline basalt, these titanomagnetites most likely experienced only one cooling process, and magma mixing and aggregation are unlikely.
5.2. A New Parameter: Estimation for Apparent Cooling Rate
The cooling rate can be determined when the degree of undercooling and the growth time of a mineral are already known. However, the degree of undercooling and the growth time are difficult to independently determine for natural samples and can only be determined via experimental petrology [4,6,43]. To explore the cooling rate of natural samples, we can determine the apparent cooling rate using the CSD curve of titanomagnetite [4,43,45,46,47,66,83]. For a series of comagmatic samples, the shallower the slope is, the slower the cooling rate and the larger the crystal size, as indicated by CSD theory [46,47] and observed in experimental petrology [4,43]. However, the potential coarsening process will influence the maximum particle size, CSD slope, intercept and other texture parameters, and these influences will affect the estimation of the cooling rate in natural samples. We need to identify a new parameter to represent the apparent cooling rate in natural samples.
Magnetite and spinel are common spinel minerals in basic and ultrabasic igneous rocks. Their crystallization habits are similar, they are an equiaxed crystal system, symmetric m3m, and often octahedral {111}. Therefore, in the experimental petrology of Giuliani et al. [43], the cooling rate experiment of spinel can be compared with the crystallization law of titanomagnetite, which has certain reference value. As is shown in Figure 10a–d, in the experimental study performed by Giuliani et al. [43], the cooling rate was negatively correlated with the spinel CSD slope, maximum particle size and the ratio of the maximum particle size to the area abundance and positively correlated with the CSD intercept. These parameters can all describe the apparent cooling rate in natural samples to some extent. However, in a constant cooling rate (80 °C/min) experiment [34], the dwelling time was negatively correlated with the titanomagnetite CSD intercept and positively correlated with the maximum particle size and CSD slope. The ratio of maximum particle size to area abundance in titanomagnetite remains stable at a fixed cooling rate, regardless of how the dwelling time changes (Figure 10e–h). The possible Ostwald ripening process greatly influences the maximum particle size. Continuous crystal growth will happen when the dwelling time lasts, and the titanomagnetite content also changes.
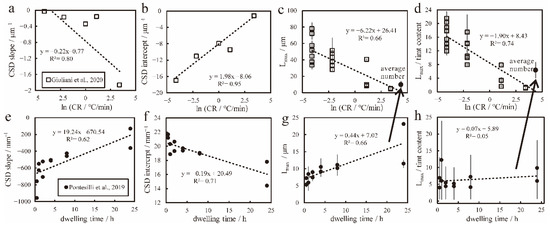
Figure 10.
The relationship between quantitative textural parameters and cooling rate [43] and dwelling time [34]. (a): The relationship between ln(cooling rate (CR)) and CSD slope; (b): the relationship between ln(CR) and CSD intercept; (c): the relationship between ln(CR) and Lmax (maximum particle size); (d): the relationship between ln(CR) and Lmax/timt content (ratio of maximum particle size to area abundance); (e): the relationship between dwelling time and CSD slope; (f): the relationship between dwelling time and CSD intercept; (g): the relationship between dwelling time and Lmax; (h): the relationship between dwelling time and the Lmax/timt content. Lmax is the average of the ten largest particles [34]; Lmax is the average of the four largest particles [43]. The fitting line is for all data.
The CSD slope, intercept and maximum particle size (Lmax) exhibit a strong trend with dwelling time and cooling rate. However, the ratio of maximum particle size to area abundance seems to not be affected by dwelling time but only changes with cooling rate. From 80 °C/min to 0.01 °C/min, the ratio of the maximum particle size to the titanomagnetite area abundance (Lmax/timt content) ranges from 1.02 to 20.38, including the average number under a fixed cooling rate in Pontesilli et al. [34]. Due to the different physical and chemical conditions (e.g., quenching temperature, starting temperature, pressure, oxygen fugacity, and starting melt composition), the average number under a fixed cooling rate in the experiment in Pontesilli et al. [34] is not very appropriate but also near to the fitting line (black solid point in Figure 10c,d). However, in the same experiment, the ratio of the maximum particle size to the area abundance can describe the cooling rate (black hollow point in Figure 10c,d), and this parament also makes sense in Hannuoba samples. In Hannuoba alkaline basalt samples, the Lmax/timt content ranges from 9.22 to 48.98, implying various apparent cooling rates.
5.3. Cation Redistribution Behavior with the Apparent Cooling Rate in Titanomagnetite in Hannuoba Samples
The cooling rate is one factor that influences the redistribution of cations in the titanomagnetite solid solution. Melt composition is also a key factor that influences the redistribution of cations in the titanomagnetite solid solution. Under equilibrium conditions, the main factor that changes crystal composition is the coexisting melt composition. When the melt cools and deviates from equilibrium conditions, the composition of the melt near the titanomagnetite surface changes, resulting in a change in the chemical components of the titanomagnetite; this is reflected in the partition coefficients of key major elements. Therefore, it is important to consider the partition coefficient of major elements in titanomagnetite.
For the Hannuoba basalt, as the Lmax/timt content decreases (increasing the apparent cooling rate), titanomagnetite crystals become progressively enriched in Fe3+ and depleted in Mg2+, Al3+ and Ti4+. Fe2+ shows a large fluctuation range, which is relatively unrelated to the cooling rate. The contents of other cations, such as Cr3+ and Ca2+, are low and have little relationship with the cooling rate. Thus, only Fe3+ and Ti4+ variations are consistent with the experimental petrology results [6,7]. Here, we use the partition coefficient to describe the proportion of incompatible major elements between titanomagnetite and the melt. The partition coefficients of Mg, Al and Ti are strongly and negatively related to the Lmax/timt content and are better than ion (apfu). Similarly, only DTi (the partition coefficient of Ti between titanomagnetite and the melt) is consistent with the experimental results and is more related to the apparent cooling rate. Therefore, in the natural samples, the contents of Ti4+, DTi and Fe3+ in titanomagnetite have strong relationships with the apparent cooling rate (Figure 11).
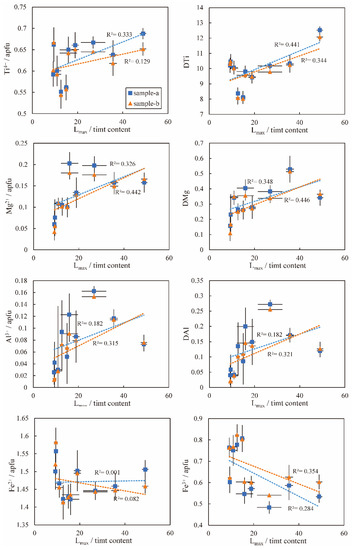
Figure 11.
Relationship between titanomagnetite major element contents (reported by atom per formula unit (apfu)) and Mg, Al, Ti partition coefficient (DMg, DAl and DTi: MgO, Al2O3 and TiO2 content in titanomagnetite and rock are reported by weight percent (wt%)) in Hannuoba samples and Lmax/timt content. Two independent tests were conducted to obtain two sets of data (15 particles for each test), marked as ‘sample-a’ and ‘sample-b’, respectively. Some error bars smaller than the symbols are not shown.
The main end members of titanomagnetite (Fe2+(1+x)Fe3+(2−2x)Ti4+xO4) (0 < x < 1) are ulvöspinel (Fe2TiO4) and magnetite (FeFe2O4) [20,33]. In the case of an increasing cooling rate, Fe2+ and Ti4+, from the ulvöspinel end member, gradually decreases [6,7]. Fe3+ replaces Ti4+, so Fe3+ and Fe2+, which are derived from the magnetite endmember, increase. These replacements explain why Fe2+ exhibits a large fluctuation but is irrelevant to the cooling rate.
The composition of the residual melt dynamically changes during the solidification process as the minerals successively crystallize. The variations in the crystallinity of the melt affect the residual melt composition and the chemical composition of crystals in the groundmass, including titanomagnetite. In this study, the crystallinity of melt is reflected in the degree of crystallization of clinopyroxene, plagioclase and other minerals. In Hannuoba basalt, clinopyroxene and plagioclase are mainly groundmass crystals. When the apparent cooling rate increases, the content of groundmass clinopyroxene increases, and most Mg2+ enters it, resulting in decreased Mg content in titanomagnetite (Figure 11 and Figure 12). Small titanomagnetite mostly crystallizes later than large titanomagnetite crystals. Although several small-crystal sections may be large crystals because of the cross-section effect (the plane randomly cut through the crystal does not always pass through the center of the crystal, and the size of the major axis of the section can vary arbitrarily from zero to the longest axis of its triad) [90,91] and intersection probability problem (the cross-section is always easier to cut through larger crystals, and smaller ones are less likely to be cut through) [91]. The slight positive correlations between Mg2+, Al3+ and the long axis of titanomagnetite for sample ZHT09 (Figure 9) suggest that small titanomagnetite (<50 μm) compositions are significantly affected by the near solidus residual melt composition. Therefore, the different crystallinities affect cationic substitution in the titanomagnetite solid solution.
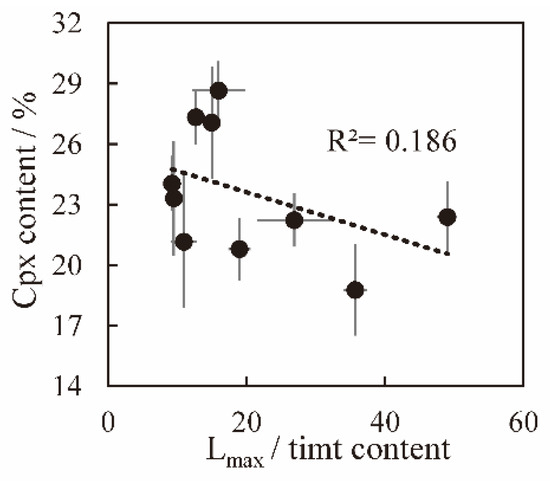
Figure 12.
Relationship between volume content of Cpx (clinopyroxene) in Hannuoba samples and Lmax/timt content. Some error bars smaller than the symbols are not shown.
For other cations, the correlations between the contents of Ca2+, Ni2+, and V3+ and the cooling rate are minimal.
Therefore, the cooling rate does control the partition coefficient in titanomagnetite, but there are significant differences in crystallinity during the crystallization process under kinetically controlled conditions. This phenomenon implies that the cooling rate influences the partition coefficients of elements under kinetically controlled conditions to some extent.
5.4. Calibration of a New Titanomagnetite Geospeedometer
The equation for the geospeedometer established by Mollo et al. [6] should be re-examined because, in this study, the content changes in Mg and Al in titanomagnetite are mainly controlled not by the cooling rate but by the possible melt composition and crystallinity of the melt. Therefore, the geospeedometer that applies to MgO and Al2O3 might be unsuitable in this study. It is possible that Equation (2) is not applicable because of different systems, but as described below, Mollo et al.’s [6] equation is indeed affected by the melt composition and degree of crystallization. The cooling rate equation
is used to calculate the cooling rates of the Hannuoba samples, ranging from 0.0001 °C/min to 0.06 °C/min. In other words, the cooling rates calculated by Equation (2) are close to 0, and within the SEE of Equation (2) (0.507 °C/min), which is not applicable and accurate in this study because the Lmax/timt content greatly differs (9.22 to 48.98), implying variations in the cooling rate. The results should have a range of variation and should not be close to 0. Importantly, the negative correlation between TiO2/(Al2O3 + MgO) and Lmax/timt content (Figure 13b) is inconsistent with the kinetically controlled crystallization trend. This means that when the cooling rate decreases, the Lmax/timt content and TiO2/(Al2O3 + MgO) should both increase. However, this is not shown in Figure 13b. Using the titanomagnetite data (Figure 13d) and experiments of this study (Figure 13c) [6], the ratio of the partition coefficient of Ti between liquid and titanomagnetite to Fe2+ (DTi/Fe2+: and DTi is Ti4+ in titanomagnetite to TiO2 in the melt) in titanomagnetite is consistent with the trend of equilibrium (Figure 13d). This means that when the cooling rate decreases, the Lmax/timt content increases. The DTi/Fe2+ should also increase, as shown in Figure 14d, indicating that DTi/Fe2+ is an important parameter of titanomagnetite composition reflecting cooling rate. For titanomagnetite in Hannuoba alkaline basalt, the contents of Mg2+ and Al3+ cannot sensitively reflect the cooling rate because of changes in the composition and crystallinity of the coexisting melt. In natural dike samples [5], the changes in melt composition were not clear, so Mg2+ and Al3+ were slightly influenced by the coexisting melt. Therefore, using this geospeedometer [6] to calculate the cooling rate may ignore the influence of melt composition. DTi/Fe2+ is expected to be consistent with natural and experimental samples to measure the cooling rate. Under kinetically controlled conditions, the crystallization of clinopyroxene, plagioclase and other minerals results in variations in the residual melt composition, which is reflected in the crystallinity of the melt. Different pressure conditions, quenching temperatures and cooling rates lead to different crystallinities in the melt. The temperature and pressure of Hannuoba alkaline basalt are 1070–1146 ± 28 °C and −1–6.7 ± 1.4 kbar, which is close to experiment petrology (see Appendix A for a detailed overview).
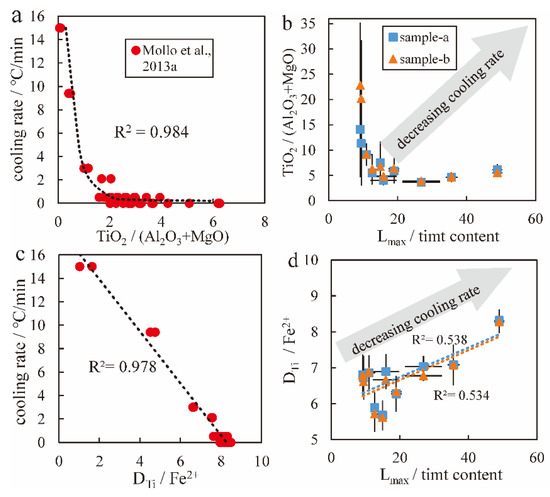
Figure 13.
Relationship between the (a): cooling rate and TiO2/(Al2O3 + MgO) in titanomagnetite; (b): Lmax/timt content and TiO2/(Al2O3 + MgO) in titanomagnetite; (c): cooling rate and DTi/Fe2+; (d): Lmax/timt content and DTi/ Fe2+. Two independent tests were conducted to obtain two sets of data (15 particles for each test), marked as ‘sample-a’ and ‘sample-b’, respectively. The error bar is the standard deviation of fifteen electron probe data points for each sample. TiO2, Al2O3 and MgO are wt%, and timt Fe2+ is apfu.
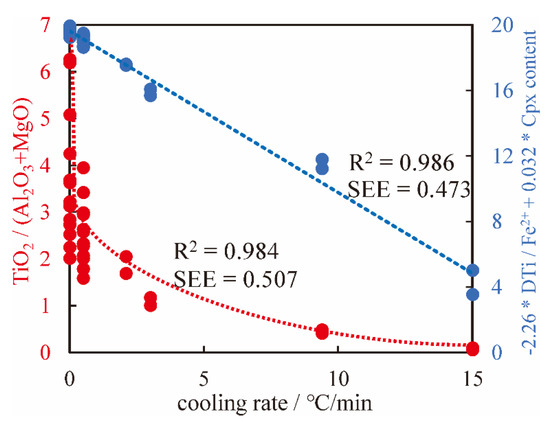
Figure 14.
Comparison between Equations (2) [6] and (3). Equation (3) yields the blue line, and Equation (2) yields the red curve. Equation (3) has a better fitting degree and smaller error ranges. Although the SEE in Equation (3) is slightly lower than that in Equation (2) [6], and the errors in Equation (3) mainly arise from a higher cooling rate, while the errors in Equation (2) [6] mainly arise from a lower cooling rate. Equation (3) may better predict the low cooling rate, which is more common in natural samples [5,87].
The experiment [6] provides texture and chemical compositional data that can help us to build a calibration dataset. The dataset is provided in Supplementary Materials Table S2. The regression equation is obtained by multiple linear regression using parameters consistent with the equilibrium trend in both natural samples and experimental petrology, as well as other possible parameters (such as initial temperature; final temperature; pressure; clinopyroxene volume content; glass volume content; Ti4+, Al3+, Fe3+, Fe2+, Mn2+, Mg2+, and Mn2+ in titanomagnetite; DTi, DAl, DMg, DMn, and DTi/Fe2+, etc. Finally, the regression is as follows:
We compared Equation (2) with Equation (3), which shows that the accuracy of the equation has been improved, especially at a slow cooling rate. At a slow cooling rate, the points are more gathered in Equation (3) than in Equation (2) (Figure 14).
Pressure has little effect on the geospeedometer, according to Equation (3). Using the geospeedometer developed in this study, we can more accurately estimate the cooling rate of melt solidification.
Equation (2) in Mollo et al., [6] and Equation (3) in this study are used to estimate the cooling rate of alkaline basalt from Hannuoba (Figure 15). The cooling rates of the Hannuoba alkaline basalt range from 0.7 to 7.0 (±0.5) °C/min, as determined using the new geospeedometer (Equation (3)) proposed in this study. For Equation (2), the Hannuoba samples mostly nucleated and grew in a very low and constant cooling rate environment with a change in Lmax/timt content. For Equation (3), a low cooling rate with a Lmax/timt content represents equilibrium conditions, and rapid cooling results in a small Lmax/timt content, showing kinetically controlled conditions. As seen when comparing Equations (2) and (3), the calculated cooling rate is more accurate when the cooling rate is slow (<3 °C/min) (Figure 14), and the influence of crystallinity on Equation (3) is eliminated to a greater extent, demonstrating that the geospeedometer can reflect the real conditions in nature. Our samples show that rapid cooling is rare in continental basalt, suggesting that prediction accuracy is quite important under a slower cooling rate (<10 °C/min) and that the geospeedometer in this study is more suitable for a lower cooling rate.
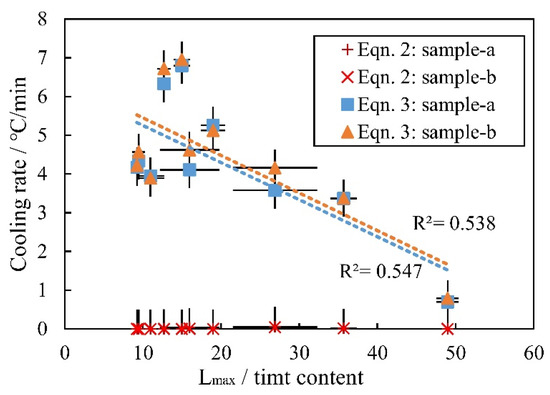
Figure 15.
Comparison between the results calculated from Equations (2) and (3). Two independent tests were conducted to obtain two sets of data (15 particles each test), marked as ‘sample-a’ and ‘sample-b’, respectively. Some error bars smaller than the symbols are not shown.
Equation (3) is used to predict other samples, and the results are listed in Supplementary Materials Table S3. This geospeedometer is found to have a large error under some conditions. It cannot accurately predict the cooling rate from titanomagnetite when the rock is solidified, except when under natural conditions in the laboratory [34]. It cannot be used to predict the cooling rate in intermediate rock, acidic rock or Fe-rich basaltic melts [4,42,87]. Ilmenite and chromite cannot be used in this geospeedometer [42]. If the melt condition, oxygen fugacity and pressure are relatively unchanged, Equation (2) can accurately predict the cooling rate of natural samples [5,6,7].
6. Conclusions
In a quantitative textural analysis of igneous rocks, we found that the ratio of the maximum particle size to the area abundance of titanomagnetite was utilized to obtain information about the apparent cooling rate in Hannuoba alkaline basalt in this study. The disequilibrium crystallization process is reflected in the solid solution substitution of alkaline basalt groundmass titanomagnetite. DTi/Fe2+ (the ratio of the partition coefficient of Ti between liquid and titanomagnetite to Fe2+ in titianomagnetite) and Cpx volume content have strong correlations with the cooling rate in this study. The composition and crystallinity of the coexisting melt compensate for the effect of the cooling rate on Mg2+ and Al3+. Taking the partition coefficient of Ti between the liquid and titanomagnetite, Fe2+ in titanomagnetite and crystallinity into account, a new titanomagnetite geospeedometer is calibrated. The new geospeedometer can better predict slow cooling rates [5,6,87], which are common in natural samples. The cooling rates of the Hannuoba alkaline basalt are estimated to range from 0.7 to 7.0 (±0.5) °C/min, which might also cover the ranges of cooling rates for many other continental alkaline basalts.
Our study suggests that the relationship between crystal size distribution and mineral chemistry is a promising tool for exploring the effects of disequilibrium crystallization on cation redistribution in rock-forming minerals. In addition, researchers prefer using common rock-making minerals in igneous rock, such as clinopyroxene, olivine and plagioclase, to calculate temperature, pressure, water content, oxygen fugacity and other information [1,24,92,93,94,95,96,97] to explore the source lithology and solidification history of igneous rocks. However, kinetically controlled crystallization processes increase the uncertainties of such calculations since equilibrium conditions are assumed in these models. Therefore, the influence of the cooling rate should be fully considered when calculating this information. The new titanomagnetite geospeedometer can better measure the cooling rate of basalt and may help evaluate mineral thermobarometers [8,9,10,11,12,13,14,15,16,17,17,18,18,19,19,20,21,22,23,24,25,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100] and better recognize thermal remanence magnetization and ancient magnetic fields [7,15,16].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min12111412/s1, Table S1: The original data of major elements in titanomagnetite from the Hannuoba alkaline basalts (wt%); Table S2: Database for Equation (3) regression; Table S3: Specific results which using Equations (2) and (3) to predict other samples; Table S4: The original data of major elements in clinopyroxene from the Hannuoba alkaline basalts (wt%).
Author Contributions
Conceptualization, Z.-F.Y.; methodology, Z.-F.Y. and Z.-H.X.; software, Z.-F.Y.; validation, Z.-F.Y., Z.-H.X., R.X., X.-H.A. and J.-N.Q.; formal analysis, Z.-F.Y. and Z.-H.X.; investigation, Z.-F.Y. and Z.-H.X.; resources, Z.-F.Y.; data curation, Z.-H.X., R.X., X.-H.A. and J.-N.Q.; writing—original draft preparation, Z.-H.X.; writing—review and editing, Z.-F.Y. and Z.-H.X.; visualization, Z.-H.X.; supervision, Z.-F.Y.; project administration, Z.-F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Fundamental Research Funds for the Central Universities (2652019049), the 111 Project (B18048), National Natural Science Foundation of China (92162213) and National Natural Science Foundation of China (42272037). This is CUGB petro-geochemical contribution No. PGC-2015-0093.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the reviewers for their constructive and thorough reviews. We also thank them for their comments and editorial work. This paper has benefited from discussions with Qing-Bao Jiang, Jie Li, Ying-Ying Li, Lin-Yu Qu, Ye Yuan, Xiao-Jie Jiang, Li-Jun Zhou, Hui-Zhong Peng, Tong Rao, Sheng-Yue He, Yu Yang, Ben Ma and Kai-Xuan Jia. The authors are grateful to Silvio Mollo and Keith Putirka for their useful review about crystallization kinetics. We thank Pei-Pei Li for providing information and a discussion of the temperature and pressure estimation and calculation of alkaline basalt. We are also grateful to one anonymous reviewer for their constructive suggestions on CSD theory. This work is supported by the Fundamental Research Funds for the Central Universities (2652019049), the 111 Project (B18048), National Natural Science Foundation of China (92162213) and National Natural Science Foundation of China (42272037). This is CUGB petro-geochemical contribution No. PGC-2015-0093.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Additional Discussion: Estimation of Temperature and Pressure of Hannuoba Alkaline Basalt
The composition of clinopyroxene is sensitive to temperature and pressure. The clinopyroxene single-mineral thermobarometers and mineral–melt equilibrium thermobarometers can estimate the depth of the magma chamber, temperature and pressure during crystallization [1,95,101,102,103,104]. The application degree is higher than olivine and plagioclase thermobarometers, and the clinopyroxene thermobarometers are more accurate [103].
In this study, electron microprobe analysis was performed to determine the major elements in clinopyroxene from ten samples. In each sample, 15 particles of different sizes were selected randomly to determine the overall chemical characteristics of clinopyroxene. All experiments were carried out in the Electron Probe Laboratory of the Beijing Research Institute of Uranium Geology. A JEOL-JXA-8100 electron probe microanalyzer (EPMA) was used, and the following standards were adopted for the various chemical elements: jadeite (Si), corundum (Al), forsterite (Mg), andradite (Fe), rutile (Ti), labradorite (Ca), potassium niobate (K), jadeite (Na), and spessartine (Mn). The accelerating voltage was 20 kV, the beam current was 10−8 A, the electron probe beam size was 2 μm, and the exit angle was 40°. Wavelength dispersive spectrometry (WDS) analysis was used for analysis, and ZAF was used for correction. The major chemical clinopyroxene composition of all particles is reported in Supplementary Materials Table S4.
The clinopyroxene thermobarometers are useful for determining the depth of magma storage and for reconstructing the magmatic processes of previous eruptions. In addition, it is necessary to check whether chemical equilibrium exists between minerals and the melt before estimating temperature and pressure. Some mineral–melt equilibrium tests were carried out to show whether clinopyroxene and the melt were in chemical equilibrium. Fe-Mg exchange coefficients (KD(Fe-Mg)cpx-liq = 0.28 ± 0.08) are an important index to measure the equilibrium between clinopyroxene and the melt [1]. However, it has been proven for a long time that the KD(Fe-Mg)cpx-liq value is not a reliable indicator [13,14]. Furthermore, crystallization kinetics experiments have shown that the composition of clinopyroxene is always affected by a degree of undercooling [13,14,102,105]. KD(Fe-Mg)cpx-liq value increases with the degree of undercooling [105]. Therefore, it is not reliable to only use KD(Fe-Mg)cpx-liq as an indicator to measure the equilibrium between the clinopyroxene and the melt. However, Mollo et al. [14] indicated that the clinopyroxene end-member components (EnFs (Mg2Si2O6 + Fe2Si2O6), DiHd (CaMgSi2O6 + CaFeSi2O6), CaTs (CaAl2SiO6), Jd (NaAlSi2O6), etc.) are a series of reliable indicators. Mineral–melt equilibrium is considered when the difference between the predicted and calculated end-member components is within 1 SEE (∆DiHd = ±0.06, ∆EnFs = ±0.05, ∆CaTs = ±0.03, ∆Jd= ±0.02). Considering the calibration error of the model, it can be considered to be in equilibrium when ∆DiHd = ±0.1 [106] (Figure A1).
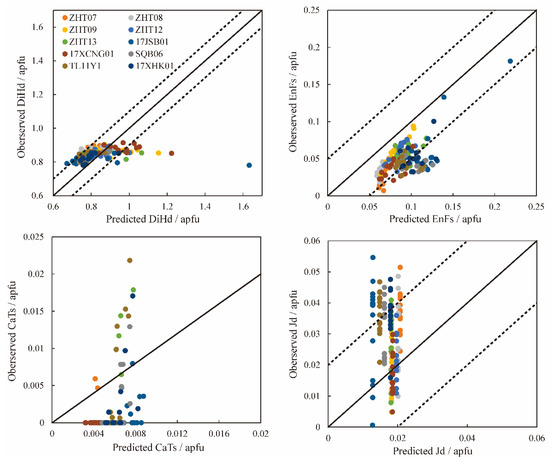
Figure A1.
Clinopyroxene–melt equilibrium tests comparing measured and predicted DiHd, EnFs, CaTs and Jd. [14]. Dashed lines encompass the calibration errors of the models. The calibration error of DiHd is ±0.1 [107]. The solid line is the 1:1 line. The points outside the dashed line do not calculate the temperature and pressure.
We estimate the pressure of the Hannuoba alkaline basalt using a water-independent melt–clinopyroxene equilibrium barometer Equation (A1) (±1.4 kbar, [101]):
and a water-independent clinopyroxene barometer Equation (A2) (±3.1 kbar, [1]):
We estimate the temperature of the Hannuoba alkaline basalt using a P-H2O-independent melt–clinopyroxene equilibrium thermometer Equation (A3) (±28 °C, [108]):
This thermometer is considered to be more suitable for the temperature estimation of mafic alkaline magmas [108].
The pressure and temperature (Figure A2) are 0.1–6.7 ± 1.4 kbar (Equation (A1)) and −1.0–3.7 ± 3.1 kbar (Equation (A2)) and 1070.4–1146.5 ± 28 °C (Equation (A3)). This accords with the temperature and pressure characteristics of alkaline basalt. Hannuoba alkaline magma may continue to crystallize in the middle and upper crust. This process allows magma to cool at different rates.
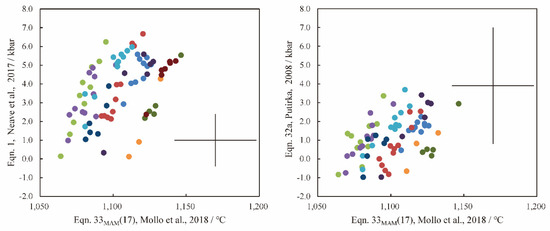
Figure A2.
The calculated pressure and temperature. The calculated pressure by Equation (A1) (±1.4 kbar, [101]) and Equation (A2) (±3.1 kbar, [1]). The calculated temperature by Equation (A3) (±28 °C, [108]). The points are all in clinopyroxene–melt equilibrium (Figure A1). The legend is the same as in Figure A1.
References
- Putirka, K. Thermometers and Barometers for Volcanic Systems. Rev. Mineral. Geochem. 2008, 69, 61–120. [Google Scholar] [CrossRef]
- Faure, F.; Trolliard, G.; Christian, N.; Montel, J.-M. A developmental model of olivine morphology as a function of the cooling rate and the degree of undercooling. Contrib. Mineral. Petrol. 2003, 145, 251–263. [Google Scholar] [CrossRef]
- Sossi, P.A.; O’Neill, H.S.C. Liquidus temperatures of komatiites and the effect of cooling rate on element partitioning between olivine and komatiitic melt. Contrib. Mineral. Petrol. 2016, 171, 49. [Google Scholar] [CrossRef]
- Iezzi, G.; Mollo, S.; Torresi, G.; Guido, V.; Cavallo, A.; Scarlato, P. Experimental solidification of an andesitic melt by cooling. Chem. Geol. 2011, 283, 261–273. [Google Scholar] [CrossRef]
- Mollo, S.; Lanzafame, G.; Masotta, M.; Iezzi, G.; Ferlito, C.; Scarlato, P. Cooling history of a dike as revealed by mineral chemistry: A case study from Mt. Etna volcano. Chem. Geol. 2011, 288, 39–52. [Google Scholar] [CrossRef]
- Mollo, S.; Putirka, K.; Iezzi, G.; Scarlato, P. The control of cooling rate on titanomagnetite composition: Implications for a geospeedometry model applicable to alkaline rocks from Mt. Etna volcano. Contrib. Mineral. Petrol. 2013, 165, 457–475. [Google Scholar] [CrossRef]
- Zhou, W.; Der Voo, R.V.; Peacor, D.R.; Zhang, Y. Variable Ti-content and grain size of titanomagnetite as a function of cooling rate in very young MORB. Earth Planet. Sci. Lett. 2000, 179, 9–20. [Google Scholar] [CrossRef]
- Cashman, K.V. Relationship between plagioclase crystallization and cooling rate in basaltic melts. Contrib. Mineral. Petrol. 1993, 113, 126–142. [Google Scholar] [CrossRef]
- Iezzi, G.; Mollo, S.; Shahini, E.; Cavallo, A.; Scarlato, P. The cooling kinetics of plagioclase feldspar as revealed by electron-microprobe mapping. Am. Mineral. 2014, 99, 898–907. [Google Scholar] [CrossRef]
- Mollo, S.; Putirka, K.; Iezzi, G.; Del Gaudio, P.; Scarlato, P. Plagioclase–melt (dis)equilibrium due to cooling dynamics: Implications for thermometry, barometry and hygrometry. Lithos 2011, 125, 221–235. [Google Scholar] [CrossRef]
- Lofgren, G.; Huss, G.; Wasserburg, G. An experimental study of trace-element partitioning between Ti-Al-clinopyroxene and melt: Equilibrium and kinetic effects including sector zoning. Am. Mineral. 2006, 91, 1596–1606. [Google Scholar] [CrossRef]
- Masotta, M.; Pontesilli, A.; Mollo, S.; Armienti, P.; Ubide, T.; Nazzari, M.; Scarlato, P. The role of undercooling during clinopyroxene growth in trachybasaltic magmas: Insights on magma decompression and cooling at Mt. Etna volcano. Geochim. Cosmochim. Acta 2020, 268, 258–276. [Google Scholar] [CrossRef]
- Mollo, S.; Del Gaudio, P.; Ventura, G.; Iezzi, G.; Scarlato, P. Dependence of clinopyroxene composition on cooling rate in basaltic magmas: Implications for thermobarometry. Lithos 2010, 118, 302–312. [Google Scholar] [CrossRef]
- Mollo, S.; Putirka, K.; Misiti, V.; Soligo, M.; Scarlato, P. A new test for equilibrium based on clinopyroxene–melt pairs: Clues on the solidification temperatures of Etnean alkaline melts at post-eruptive conditions. Chem. Geol. 2013, 352, 92–100. [Google Scholar] [CrossRef]
- Ferk, A.; Leonhardt, R.; Hess, K.-U.; Koch, S.; Egli, R.; Krása, D.; Dingwell, D.B. Influence of cooling rate on thermoremanence of magnetite grains: Identifying the role of different magnetic domain states. J. Geophys. Res. Solid Earth 2014, 119, 1599–1606. [Google Scholar] [CrossRef]
- Koch, S.; Ferk, A.; Hess, K.-U.; Leonhardt, R. Cooling rate dependence of synthetic SD, PSD, MD magnetite. In Proceedings of the American Geophysical Union, Fall Meeting 2010, San Francisco, CA, USA, 1 January 2010. [Google Scholar]
- Canil, D.; Lacourse, T. Geothermometry using minor and trace elements in igneous and hydrothermal magnetite. Chem. Geol. 2020, 541, 119576. [Google Scholar] [CrossRef]
- Ghiorso, M.S.; Sack, O. Fe-Ti oxide geothermometry: Thermodynamic formulation and the estimation of intensive variables in silicic magmas. Contrib. Mineral. Petrol. 1991, 108, 485–510. [Google Scholar] [CrossRef]
- Jolles, J.S.R.; Lange, R.A. High-resolution Fe–Ti oxide thermometry applied to single-clast pumices from the Bishop Tuff: A re-examination of compositional variations in phenocryst phases with temperature. Contrib. Mineral. Petrol. 2019, 174, 70. [Google Scholar] [CrossRef]
- Bosi, F.; Hålenius, U.; Skogby, H. Crystal chemistry of the magnetite-ulvöspinel series. Am. Mineral. 2009, 94, 181–189. [Google Scholar] [CrossRef]
- Dupuis, C.; Beaudoin, G. Discriminant diagrams for iron oxide trace element fingerprinting of mineral deposit types. Miner. Depos. 2011, 46, 319–335. [Google Scholar] [CrossRef]
- Jang, Y.D.; Naslund, H.R.; McBirney, A.R. The differentiation trend of the Skaergaard intrusion and the timing of magnetite crystallization: Iron enrichment revisited. Earth Planet. Sci. Lett. 2001, 189, 189–196. [Google Scholar] [CrossRef]
- Nadoll, P.; Angerer, T.; Mauk, J.L.; French, D.; Walshe, J. The chemistry of hydrothermal magnetite: A review. Ore Geol. Rev. 2014, 61, 1–32. [Google Scholar] [CrossRef]
- Frost, B.R. Introduction to oxygen fugacity and its petrologic importance. Rev. Mineral. Geochem. 1991, 25, 1–9. [Google Scholar]
- Hou, T.; Botcharnikov, R.; Moulas, E.; Just, T.; Berndt, J.; Koepke, J.; Wang, M.; Yang, Z.; Holtz, F. Kinetics of Fe–Ti Oxide Re-equilibration in Magmatic Systems: Implications for Thermo-oxybarometry. J. Petrol. 2021, 61, egaa116. [Google Scholar] [CrossRef]
- Toplis, M.; Carroll, M. An Experimental Study of the Influence of Oxygen Fugacity on Fe-Ti Oxide Stability, Phase Relations, and Mineral–Melt Equilibria in Ferro-Basaltic Systems. J. Petrol. 1995, 36, 1137–1170. [Google Scholar] [CrossRef]
- Whalen, J.; Chappell, B. Opaque mineralogy and mafic mineral chemistry of I- and S-type granites of the Lachlan Fold Belt, southeast Australia. Am. Mineral. 1988, 73, 281–296. [Google Scholar]
- Dare, S.; Barnes, S.-J.; Beaudoin, G.; Méric, J.; Boutroy, E.; Potvin-Doucet, C. Trace elements in magnetite as petrogenetic indicators. Miner. Depos. 2014, 49, 785–796. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.-J.; Beaudoin, G. Variation in trace element content of magnetite crystallized from a fractionating sulfide liquid, Sudbury, Canada: Implications for provenance discrimination. Geochim. Cosmochim. Acta 2012, 88, 27–50. [Google Scholar] [CrossRef]
- Huang, X.-W.; Zhou, M.-F.; Qiu, Y.-Z.; Qi, L. In-situ LA-ICP-MS trace elemental analyses of magnetite: The Bayan Obo Fe-REE-Nb deposit, North China. Ore Geol. Rev. 2015, 65, 884–899. [Google Scholar] [CrossRef]
- Nadoll, P.; Mauk, J.L.; Hayes, T.S.; Koenig, A.E.; Box, S.E. Geochemistry of Magnetite from Hydrothermal Ore Deposits and Host Rocks of the Mesoproterozoic Belt Supergroup, United States. Econ. Geol. 2012, 107, 1275–1292. [Google Scholar] [CrossRef]
- Nielsen, R.; Forsythe, L.; Gallahan, W.; Fisk, M. Major and trace-element magnetite-melt equilibria. Chem. Geol. 1994, 117, 167–191. [Google Scholar] [CrossRef]
- Pearce, C.I.; Henderson, C.M.B.; Telling, N.D.; Pattrick, R.A.D.; Charnock, J.M.; Coker, V.S.; Arenholz, E.; Tuna, F.; van der Laan, G. Fe site occupancy in magnetite-ulvöspinel solid solutions: A new approach using X-ray magnetic circular dichroism. Am. Mineral. 2010, 95, 425–439. [Google Scholar] [CrossRef]
- Pontesilli, A.; Masotta, M.; Nazzari, M.; Mollo, S.; Armienti, P.; Scarlato, P.; Brenna, M. Crystallization kinetics of clinopyroxene and titanomagnetite growing from a trachybasaltic melt: New insights from isothermal time-series experiments. Chem. Geol. 2019, 510, 113–129. [Google Scholar] [CrossRef]
- She, H.-D.; Fan, H.-R.; Yang, K.-F.; Li, X.-C.; Wang, Q.-W.; Zhang, L.-F.; Liu, S.; Li, X.-H.; Dai, Z.-H. In situ trace elements of magnetite in the Bayan Obo REE-Nb-Fe deposit: Implications for the genesis of mesoproterozoic iron mineralization. Ore Geol. Rev. 2021, 139, 104574. [Google Scholar] [CrossRef]
- Gee, J. Calibration of magnetic granulometric trends in oceanic basalts. Earth Planet. Sci. Lett. 1999, 170, 377–390. [Google Scholar] [CrossRef]
- Marshall, M.; Cox, A. Magnetism of Pillow Basalts and Their Petrology. Geol. Soc. Am. Bull. 1971, 82, 537–552. [Google Scholar] [CrossRef]
- Isobe, H.; Gondo, T. Dendritic magnetite crystals in rapid quenched fine spherules produced by falling experiments through the high temperature furnace with controlled gas flow. J. Mineral. Petrol. Sci. 2013, 108, 227–237. [Google Scholar] [CrossRef]
- Mollo, S.; Giacomoni, P.P.; Andronico, D.; Scarlato, P. Clinopyroxene and titanomagnetite cation redistributions at Mt. Etna volcano (Sicily, Italy): Footprints of the final solidification history of lava fountains and lava flows. Chem. Geol. 2015, 406, 45–54. [Google Scholar] [CrossRef]
- Szramek, L.; Gardner, J.E.; Hort, M. Cooling-induced crystallization of microlite crystals in two basaltic pumice clasts. Am. Mineral. 2010, 95, 503–509. [Google Scholar] [CrossRef]
- Turner, M.; Cronin, S.J.; Stewart, R.B.; Bebbington, M.; Smith, I.E.M. Using titanomagnetite textures to elucidate volcanic eruption histories. Geology 2008, 36, 31–34. [Google Scholar] [CrossRef]
- Hammer, J. Influence of fO2 and cooling rate on the kinetics and energetics of Fe-rich basalt crystallization. Earth Planet. Sci. Lett. 2006, 248, 618–637. [Google Scholar] [CrossRef]
- Giuliani, L.; Iezzi, G.; Vetere, F.; Behrens, H.; Mollo, S.; Cauti, F.; Ventura, G.; Scarlato, P. Evolution of textures, crystal size distributions and growth rates of plagioclase, clinopyroxene and spinel crystallized at variable cooling rates from a mid-ocean ridge basaltic melt. Earth-Sci. Rev. 2020, 204, 103165. [Google Scholar] [CrossRef]
- Yang, Z.-F. Combining Quantitative Textural and Geochemical Studies to Understand the Solidification Processes of a Granite Porphyry: Shanggusi, East Qinling, China. J. Petrol. 2012, 53, 1807–1835. [Google Scholar] [CrossRef]
- Marsh, B. Crystal size distribution (CSD) in rocks and the kinetics and dynamics of crystallization—I. Theory. Contrib. Mineral. Petrol. 1988, 99, 277–291. [Google Scholar] [CrossRef]
- Marsh, B. Crystallization of Silicate Magmas Deciphered Using Crystal Size Distributions. J. Am. Ceram. Soc. 2007, 90, 746–757. [Google Scholar] [CrossRef]
- Marsh, B.D. On the Interpretation of Crystal Size Distributions in Magmatic Systems. J. Petrol. 1998, 39, 553–599. [Google Scholar] [CrossRef]
- Voorhees, P.W. The theory of Ostwald ripening. J. Stat. Phys. 1985, 38, 231–252. [Google Scholar] [CrossRef]
- Voorhees, P.W. Ostwald Ripening of Two-Phase Mixtures. Annu. Rev. Mater. Sci. 1992, 22, 197–215. [Google Scholar] [CrossRef]
- Xu, R.; Liu, Y.; Wang, X.; Zong, K.; Hu, Z.; Chen, H.; Zhou, L. Crust recycling induced compositional-temporal-spatial variations of Cenozoic basalts in the Trans-North China Orogen. Lithos 2017, 274–275, 383–396. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Graham, D.; Su, S.; Deng, J. Geochemistry of Cenozoic basalts and mantle xenoliths in Northeast China. Lithos 2007, 96, 108–126. [Google Scholar]
- Zhao, X.M.; Cao, H.H.; Mi, X.; Evans, N.J.; Qi, Y.H.; Huang, F.; Zhang, H.F. Combined iron and magnesium isotope geochemistry of pyroxenite xenoliths from Hannuoba, North China Craton: Implications for mantle metasomatism. Contrib. Mineral. Petrol. 2017, 172, 40. [Google Scholar] [CrossRef]
- Qian, S.P.; Ren, Z.Y.; Zhang, L.; Hong, L.B.; Liu, J.Q. Chemical and Pb isotope composition of olivine-hosted melt inclusions from the Hannuoba basalts, North China Craton: Implications for petrogenesis and mantle source. Chem. Geol. 2015, 401, 111–125. [Google Scholar] [CrossRef]
- Zhi, X.; Song, Y.; Frey, F.A.; Feng, J.; Zhai, M. Geochemistry of Hannuoba basalts, eastern China: Constraints on the origin of continental alkalic and tholeiitic basalt. Chem. Geol. 1990, 88, 1–33. [Google Scholar] [CrossRef]
- Zeng, G.; Huang, X.-W.; Zhou, M.-F.; Chen, L.-H.; Xu, X.-S. Using chalcophile elements to constrain crustal contamination and xenolith-magma interaction in Cenozoic basalts of eastern China. Lithos 2016, 258–259, 163–172. [Google Scholar] [CrossRef]
- Sun, P.; Niu, Y.; Guo, P.; Duan, M.; Wang, X.; Gong, H.; Xiao, Y. The Lithospheric Thickness Control on the Compositional Variation of Continental Intraplate Basalts: A Demonstration Using the Cenozoic Basalts and Clinopyroxene Megacrysts from Eastern China. J. Geophys. Res. Solid Earth 2020, 125, e2019JB019315. [Google Scholar] [CrossRef]
- Zhou, X.; Armstrong, R.L. Cenozoic volcanic rocks of eastern China—Secular and geographic trends in chemistry and strontium isotopic composition. Earth Planet. Sci. Lett. 1982, 58, 301–329. [Google Scholar] [CrossRef]
- Zou, H.; Zindler, A.; Xu, X.; Qi, Q. Major, trace element, and Nd, Sr and Pb isotope studies of Cenozoic basalts in SE China: Mantle sources, regional variations, and tectonic significance. Chem. Geol. 2000, 171, 33–47. [Google Scholar] [CrossRef]
- Yang, Z.-F.; Li, J.; Liang, W.; Luo, Z.-H. On the chemical markers of pyroxenite contributions in continental basalts in Eastern China: Implications for source lithology and the origin of basalts. Earth-Sci. Rev. 2016, 157, 18–31. [Google Scholar] [CrossRef]
- Yang, Z.-F.; Zhou, J.-H. Can we identify source lithology of basalt? Sci. Rep. 2013, 3, 1856. [Google Scholar] [CrossRef]
- Higgins, M.; Debecq, A.; Auwera, J.V.; Nomikou, P. Chemical and textural diversity of Kameni (Greece) dacites: Role of vesiculation in juvenile and mature basal crystal masses. Contrib. Mineral. Petrol. 2021, 176, 13. [Google Scholar] [CrossRef]
- Higgins, M.D. Quantitative Textural Measurements in Igneous and Metamorphic Petrology; Cambridge University Press: New York, NY, USA, 2006; pp. 1–228. [Google Scholar]
- Higgins, M.D. Verification of ideal semi-logarithmic, lognormal or fractal crystal size distributions from 2D datasets. J. Volcanol. Geotherm. Res. 2006, 154, 8–16. [Google Scholar] [CrossRef]
- Boorman, S.; Boudreau, A.; Kruger, F. The Lower Zone-Critical Zone Transition of the Bushveld Complex: A Quantitative Textural Study. J. Petrol. 2004, 45, 1209–1235. [Google Scholar] [CrossRef]
- Harvey, P.; Laxton, R.R. The estimation of finite strain from the orientation distribution of passively deformed linear markers: Eigenvalue relationships. Tectonophysics 1980, 70, 285–307. [Google Scholar] [CrossRef]
- Higgins, M.D. Measurement of crystal size distributions. Am. Mineral. 2000, 85, 1105–1116. [Google Scholar] [CrossRef]
- Holness, M.; Cheadle, M.; McKenzie, D.A.N. On the Use of Changes in Dihedral Angle to Decode Late-stage Textural Evolution in Cumulates. J. Petrol. 2005, 46, 1565–1583. [Google Scholar] [CrossRef]
- Morgan, D.J.; Jerram, D.A. On estimating crystal shape for crystal size distribution analysis. J. Volcanol. Geotherm. Res. 2006, 154, 1–7. [Google Scholar] [CrossRef]
- Arzilli, F.; Piochi, M.; Mormone, A.; Agostini, C.; Carroll, M.R. Constraining pre-eruptive magma conditions and unrest timescales during the Monte Nuovo eruption (1538 ad; Campi Flegrei, Southern Italy): Integrating textural and CSD results from experimental and natural trachy-phonolites. Bull. Volcanol. 2016, 78, 72. [Google Scholar] [CrossRef]
- Cashman, K.V. Crystal Size Distribution (CSD) Analysis of Volcanic Samples: Advances and Challenges. Front. Earth Sci. 2020, 8, 291. [Google Scholar] [CrossRef]
- Fornaciai, A.; Perinelli, C.; Armienti, P.; Favalli, M. Crystal size distributions of plagioclase in lavas from the July–August 2001 Mount Etna eruption. Bull. Volcanol. 2015, 77, 70. [Google Scholar] [CrossRef]
- Higgins, M. Origin of megacrysts in granitoids by textural coarsening: A crystal size distribution (CSD) study of microcline in the Cathedral Peak Granodiorite, Sierra Nevada, California. Geol. Soc. Lond. Spec. Publ. 1999, 168, 207–219. [Google Scholar] [CrossRef]
- Higgins, M.; Chandrasekharam, D. Nature of Sub-volcanic Magma Chambers, Deccan Province, India: Evidence from Quantitative Textural Analysis of Plagioclase Megacrysts in the Giant Plagioclase Basalts. J. Petrol. 2007, 48, 885–900. [Google Scholar] [CrossRef]
- Higgins, M.; Roberge, J. Crystal Size Distribution of Plagioclase and Amphibole from Soufriere Hills Volcano, Montserrat: Evidence for Dynamic Crystallization-Textural Coarsening Cycles. J. Petrol. 2003, 44, 1401–1411. [Google Scholar] [CrossRef]
- Higgins, M.D. Magma dynamics beneath Kameni volcano, Thera, Greece, as revealed by crystal size and shape measurements. J. Volcanol. Geotherm. Res. 1996, 70, 37–48. [Google Scholar] [CrossRef]
- Higgins, M.D. Origin of Anorthosite by Textural Coarsening: Quantitative Measurements of a Natural Sequence of Textural Development. J. Petrol. 1998, 39, 1307–1323. [Google Scholar] [CrossRef]
- Higgins, M.D. A crystal size-distribution study of the Kiglapait layered mafic intrusion, Labrador, Canada: Evidence for textural coarsening. Contrib. Mineral. Petrol. 2002, 144, 314–330. [Google Scholar] [CrossRef]
- Moss, S.; Russell, J.; Smith, B.; Brett, R. Olivine crystal size distributions in kimberlite. Am. Mineral. 2010, 95, 527–536. [Google Scholar] [CrossRef]
- Ngonge, E.; Archanjo, C.; Hollanda, M. Plagioclase crystal size distribution in some tholeiitic mafic dykes in Cabo Frio-Buzios, Rio de Janeiro, Brazil. J. Volcanol. Geotherm. Res. 2013, 255, 26–42. [Google Scholar] [CrossRef]
- O’Driscoll, B.; Donaldson, C.H.; Troll, V.R.; Jerram, D.A.; Emeleus, C.H. An Origin for Harrisitic and Granular Olivine in the Rum Layered Suite, NW Scotland: A Crystal Size Distribution Study. J. Petrol. 2007, 48, 253–270. [Google Scholar] [CrossRef]
- Vinet, N.; Higgins, M. Magma Solidification Processes beneath Kilauea Volcano, Hawaii: A Quantitative Textural and Geochemical Study of the 1969–1974 Mauna Ulu Lavas. J. Petrol. 2010, 51, 1297–1332. [Google Scholar] [CrossRef]
- Vinet, N.; Higgins, M. What can crystal size distributions and olivine compositions tell us about magma solidification processes inside Kilauea Iki lava lake, Hawaii? J. Volcanol. Geotherm. Res. 2011, 208, 136–162. [Google Scholar] [CrossRef]
- Mock, A.; Jerram, D.A. Crystal Size Distributions (CSD) in Three Dimensions: Insights from the 3D Reconstruction of a Highly Porphyritic Rhyolite. J. Petrol. 2005, 46, 1525–1541. [Google Scholar] [CrossRef]
- Warr, L.N. IMA–CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Maitre, R.W.L.; Streckeisen, A.; Zanettin, B. A Chemical Classification of Volcanic Rocks Based on the Total Alkali-Silica Diagram. J. Petrol. 1986, 27, 745–750. [Google Scholar] [CrossRef]
- Miyashiro, A. Nature of alkalic volcanic rock series. Contrib. Mineral. Petrol. 1978, 66, 91–104. [Google Scholar] [CrossRef]
- Tanguy, J.-C.; Condomines, M.; Kieffer, G. Evolution of the Mount Etna magma: Constraints on the present feeding system and eruptive mechanism. J. Volcanol. Geotherm. Res. 1997, 75, 221–250. [Google Scholar] [CrossRef]
- Cashman, K.V.; Ferry, J.M. Crystal size distribution (CSD) in rocks and the kinetics and dynamics of crystallization—III. Metamorphic crystallization. Contrib. Mineral. Petrol. 1988, 99, 401–415. [Google Scholar] [CrossRef]
- Hunter, R.H. Texture development in cumulate rocks. In Developments in Petrology; Cawthorn, R.G., Ed.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 15, pp. 77–101. [Google Scholar]
- Royet, J.P. Stereology: A method for analyzing images. Prog. Neurobiol. 1991, 37, 433–474. [Google Scholar] [CrossRef]
- Sahagian, D.L.; Proussevitch, A.A. 3D particle size distributions from 2D observations: Stereology for natural applications. J. Volcanol. Geotherm. Res. 1998, 84, 173–196. [Google Scholar] [CrossRef]
- Adams, G.E.; Bishop, F.C. The olivine—clinopyroxene geobarometer: Experimental results in the CaO-FeO-MgO-SiO2 system. Contrib. Mineral. Petrol. 1986, 94, 230–237. [Google Scholar] [CrossRef]
- Beyer, C.; Frost, D.J.; Miyajima, N. Experimental calibration of a garnet–clinopyroxene geobarometer for mantle eclogites. Contrib. Mineral. Petrol. 2015, 169, 18. [Google Scholar] [CrossRef]
- Di, Y.; Tian, W.; Chen, M.; Li, Z.; Chu, Z.-Y.; Liang, J. A method to estimate the pre-eruptive water content of basalts: Application to the Wudalianchi–Erkeshan–Keluo volcanic field, Northeastern China. Am. Mineral. 2020, 105, 149–161. [Google Scholar] [CrossRef]
- Masotta, M.; Mollo, S.; Freda, C.; Gaeta, M.; Moore, G. Clinopyroxene–liquid thermometers and barometers specific to alkaline differentiated magmas. Contrib. Mineral. Petrol. 2013, 166, 1545–1561. [Google Scholar] [CrossRef]
- Pu, X.; Lange, R.; Moore, G. A comparison of olivine-melt thermometers based on D Mg and D Ni: The effects of melt composition, temperature, and pressure with applications to MORBs and hydrous arc basalts. Am. Mineral. 2017, 102, 750–765. [Google Scholar] [CrossRef]
- Wells, P.R.A. Pyroxene thermometry in simple and complex systems. Contrib. Mineral. Petrol. 1977, 62, 129–139. [Google Scholar] [CrossRef]
- Ghiorso, M.S.; Evans, B.W. Thermodynamics of Rhombohedral Oxide Solid Solutions and a Revision of the FE-TI Two-Oxide Geothermometer and Oxygen-Barometer. Am. J. Sci. 2008, 308, 957–1039. [Google Scholar] [CrossRef]
- Lepage, L.D. ILMAT: An Excel worksheet for ilmenite–magnetite geothermometry and geobarometry. Comput. Geosci. 2003, 29, 673–678. [Google Scholar] [CrossRef]
- Palma, G.; Reich, M.; Barra, F.; Ovalle, J.T.; del Real, I.; Simon, A.C. Thermal evolution of Andean iron oxide–apatite (IOA) deposits as revealed by magnetite thermometry. Sci. Rep. 2021, 11, 18424. [Google Scholar] [CrossRef]
- Neave, D.; Putirka, K. A new clinopyroxene-liquid barometer, and implications for magma storage pressures under Icelandic rift zones. Am. Mineral. 2017, 102, 777–794. [Google Scholar] [CrossRef]
- Neave, D.A.; Bali, E.; Guðfinnsson, G.H.; Halldorsson, S.A.; Kahl, M.; Schmidt, A.; Holtz, F. Clinopyroxene–Liquid Equilibria and Geothermobarometry in Natural and Experimental Tholeiites: The 2014–2015 Holuhraun Eruption, Iceland. J. Petrol. 2019, 60, 1653–1680. [Google Scholar] [CrossRef]
- Putirka, K.; Johnson, M.; Kinzler, R.; Longhi, J.; Walker, D. Thermobarometry of mafic igneous rocks based on clinopyroxene-liquid equilibria, 0–30 kbar. Contrib. Mineral. Petrol. 1996, 123, 92–108. [Google Scholar] [CrossRef]
- Putirka, K.; Mikaelian, H.; Ryerson, F.; Shaw, H. New clinopyroxene-liquid thermobarometers for mafic, evolved, and volatile-bearing lava compositions, with applications to lavas from Tibet and the Snake River Plain, Idaho. Am. Mineral. 2003, 88, 1542–1554. [Google Scholar] [CrossRef]
- Mollo, S.; Hammer, J.E.; Heinrich, W.; Abart, R. Dynamic crystallization in magmas. In Mineral Reaction Kinetics: Microstructures, Textures, Chemical and Isotopic Signatures; Heinrich, W., Abart, R., Eds.; Mineralogical Society of Great Britain and Ireland: London, UK, 2017; Volume 16, pp. 373–418. [Google Scholar]
- Mollo, S.; Masotta, M. Optimizing pre-eruptive temperature estimates in thermally and chemically zoned magma chambers. Chem. Geol. 2014, 368, 97–103. [Google Scholar] [CrossRef]
- Keiding, J.K.; Sigmarsson, O. Geothermobarometry of the 2010 Eyjafjallajökull eruption: New constraints on Icelandic magma plumbing systems. J. Geophys. Res. Solid Earth 2012, 117, B00C09. [Google Scholar] [CrossRef]
- Mollo, S.; Blundy, J.; Scarlato, P.; Serena Pia, D.C.; Tecchiato, V.; Stefano, F.; Vetere, F.; Holtz, F.; Bachmann, O. An integrated P-T-H2O-lattice strain model to quantify the role of clinopyroxene fractionation on REE+Y/HFSE patterns of mafic alkaline magmas: Application to eruptions at Mt. Etna. Earth-Sci. Rev. 2018, 185, 32–56. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).