Recovery Potential of Rare Earth Elements (REEs) from the Gem Mining Waste of Sri Lanka: A Case Study for Mine Waste Management
Abstract
1. Introduction
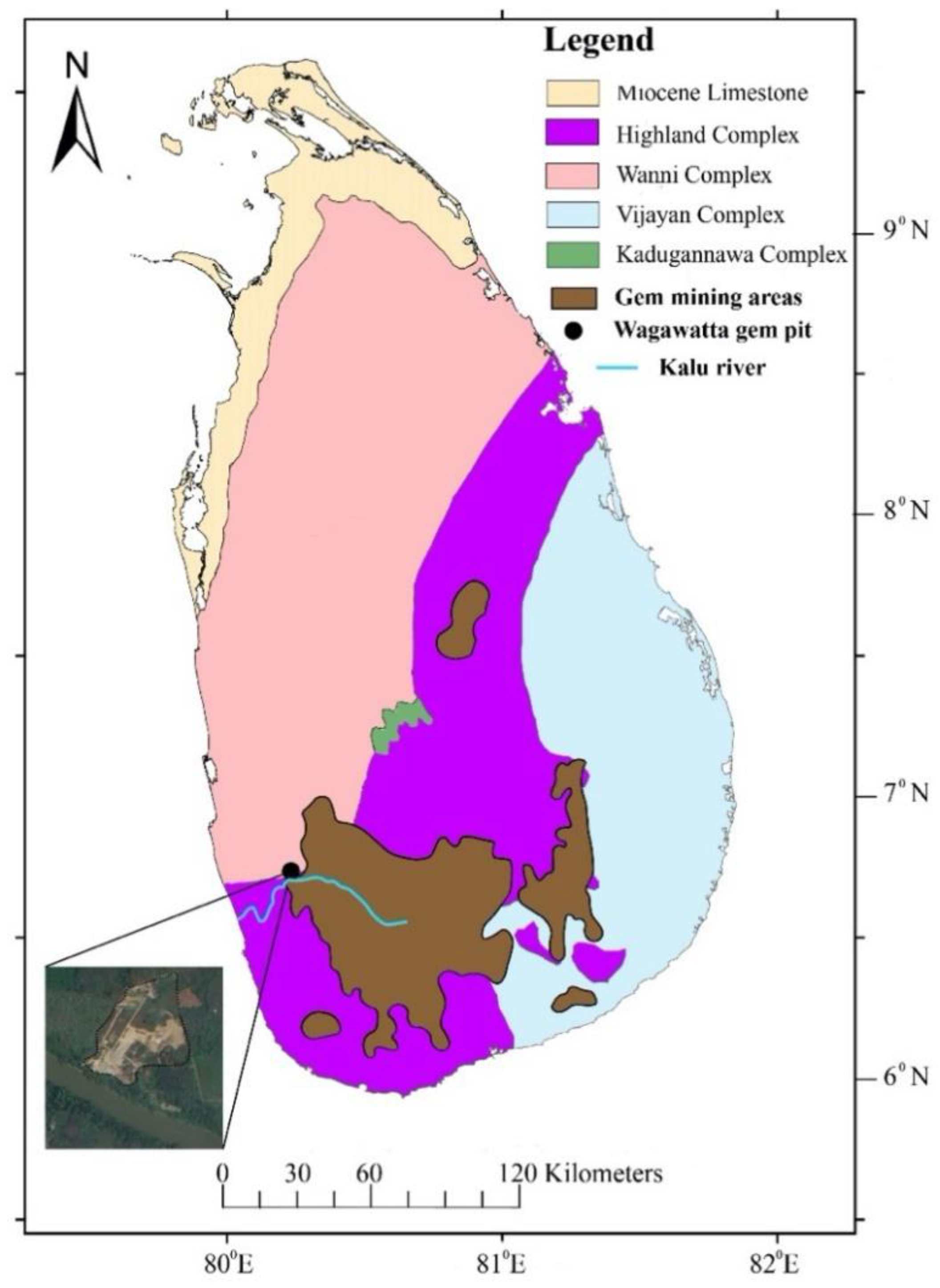
2. Study Area
3. Materials and Methods
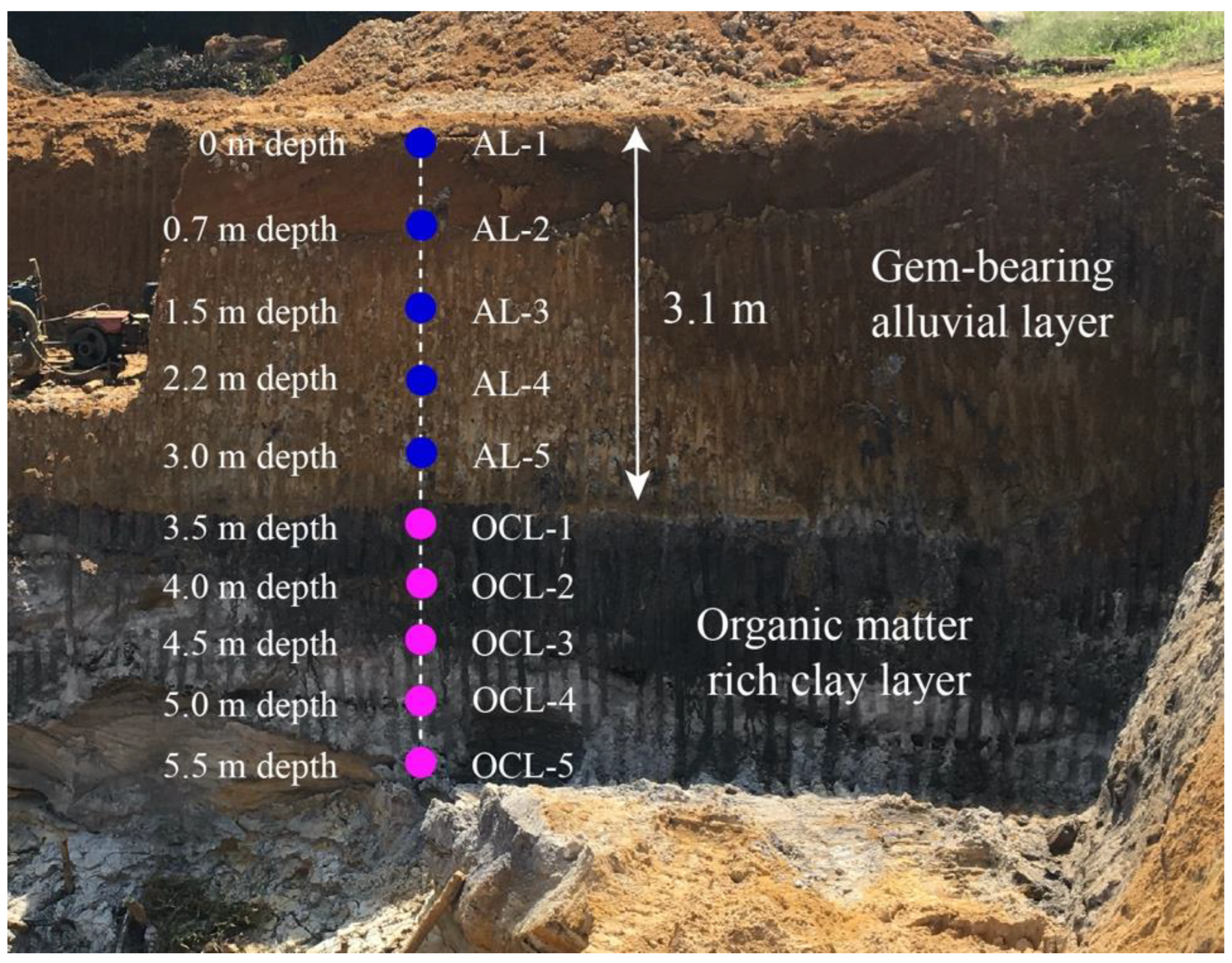
3.1. Sample Collection
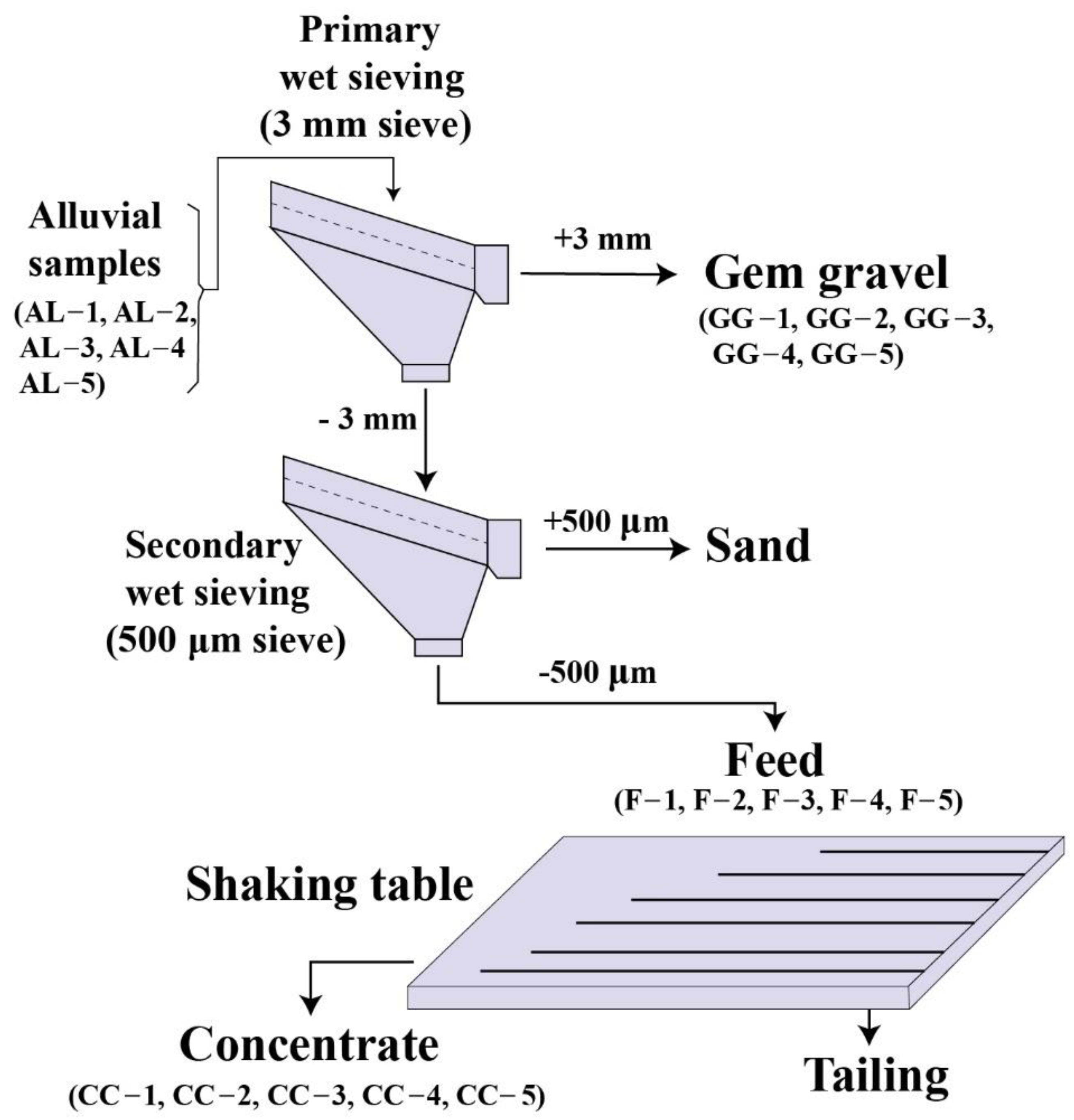
3.2. Physical Separation
3.3. Sample Analysis
3.3.1. REE and U Analysis
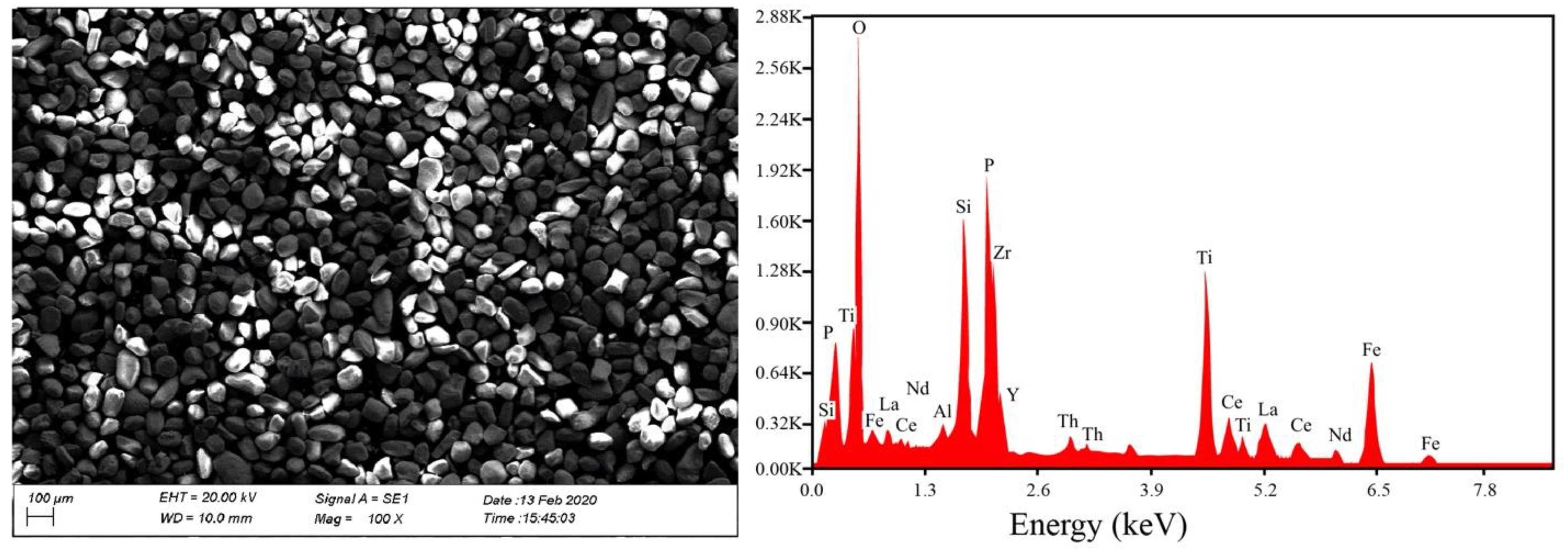
3.3.2. Mineralogical Analysis of Concentrated Samples
4. Results and Discussion
4.1. REE Concentrations of Alluvial and Gem Gravel Samples
4.2. REE Concentrations of Organic-Rich Clay Samples
4.3. REE Concentrations of Concentrated Samples
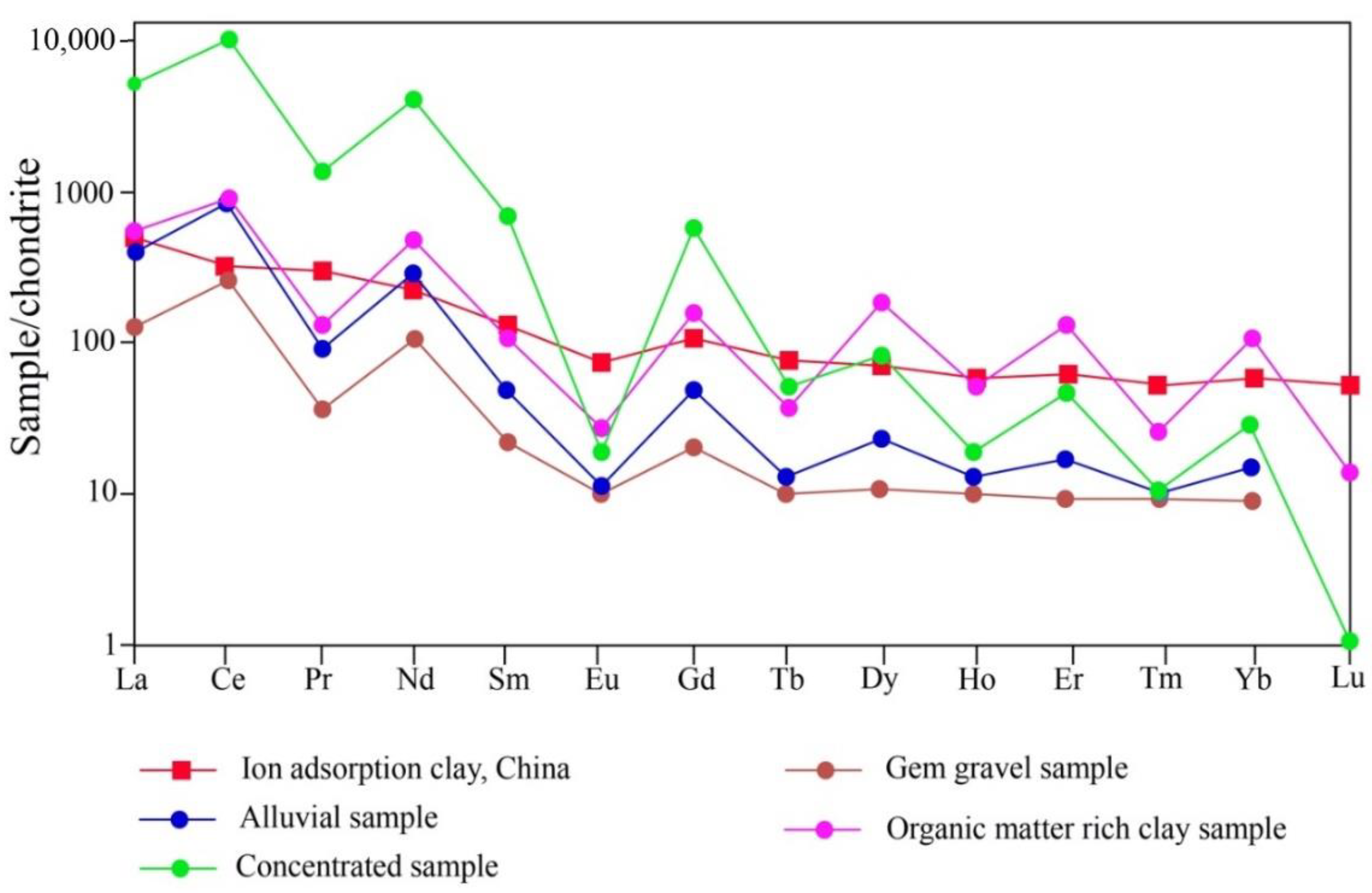
4.4. REE Patterns of Collected Samples
4.5. REE Yield, Grade, and Recovery of Separation Experiments
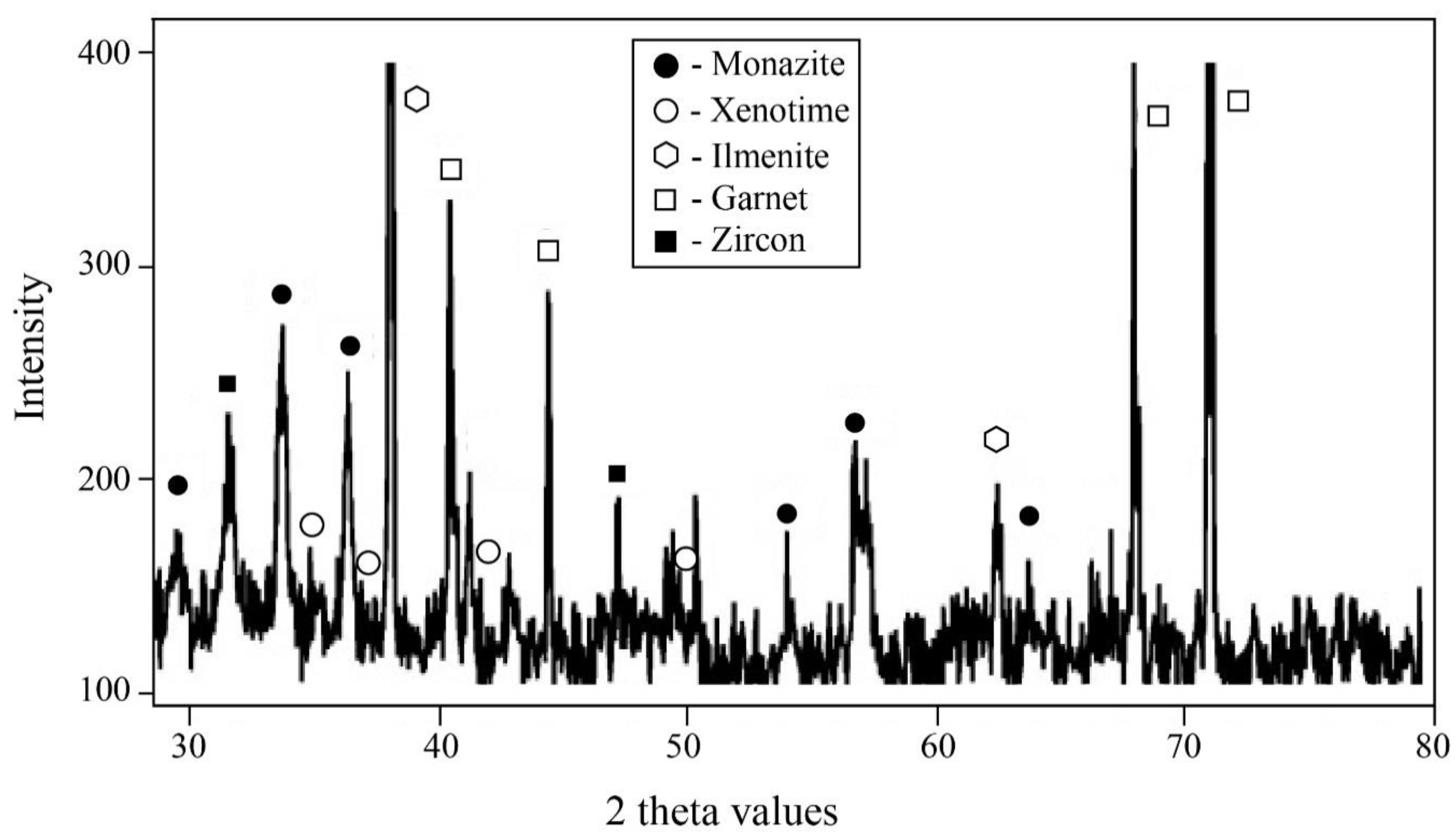
4.6. REE-Bearing Minerals in the Concentrate
4.7. Mine Waste, Value Recovery and Presence of U
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Araya, N.; Kraslawski, A.; Cisternas, L.A. Towards Mine Tailings Valorization: Recovery of Critical Materials from Chilean Mine Tailings. J. Clean. Prod. 2020, 263, 121555. [Google Scholar] [CrossRef]
- Rosado, S.; Gullón, L.; Martínez, L.F.M.; Llamas Borrajo, J.F. Potential Uses of Copper Wastes in the Building Sector: Inertization and Added Value Solutions. Mater. Proc. 2021, 5, 25. [Google Scholar]
- Nwaila, G.T.; Ghorbani, Y.; Zhang, S.E.; Frimmel, H.E.; Tolmay, L.C.K.; Rose, D.H.; Nwaila, P.C.; Bourdeau, J.E. Valorisation of Mine Waste-Part I: Characteristics of, and Sampling Methodology for, Consolidated Mineralised Tailings by Using Witwatersrand Gold Mines (South Africa) as an Example. J. Environ. Manag. 2021, 295, 113013. [Google Scholar] [CrossRef] [PubMed]
- Tunsu, C.; Menard, Y.; Eriksen, D.Ø.; Ekberg, C.; Petranikova, M. Recovery of Critical Materials from Mine Tailings: A Comparative Study of the Solvent Extraction of Rare Earths Using Acidic, Solvating and Mixed Extractant Systems. J. Clean. Prod. 2019, 218, 425–437. [Google Scholar] [CrossRef]
- Ilankoon, I.M.S.K.; Dushyantha, N.P.; Mancheri, N.; Edirisinghe, P.M.; Neethling, S.J.; Ratnayake, N.P.; Rohitha, L.P.S.; Dissanayake, D.; Premasiri, H.M.R.; Abeysinghe, A.M.K.B.; et al. Constraints to Rare Earth Elements Supply Diversification: Evidence from an Industry Survey. J. Clean. Prod. 2022, 331, 129932. [Google Scholar] [CrossRef]
- Balaram, V. Rare Earth Elements: A Review of Applications, Occurrence, Exploration, Analysis, Recycling, and Environmental Impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Dushyantha, N.; Batapola, N.; Ilankoon, I.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The Story of Rare Earth Elements (REEs): Occurrences, Global Distribution, Genesis, Geology, Mineralogy and Global Production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Dushyantha, N.P.; Ratnayake, N.P.; Premasiri, H.M.R.; Ilankoon, I.M.S.K.; Hemalal, P.V.A.; Jayawardena, C.L.; Chandrajith, R.; Rohitha, L.P.S.; Abeysinghe, A.; Dissanayake, D. Leaching of Rare Earth Elements (REEs) from Lake Sediments around Eppawala Phosphate Deposit, Sri Lanka: A Secondary Source for REEs. Hydrometallurgy 2021, 205, 105751. [Google Scholar] [CrossRef]
- Jha, A.; Lahiri, A.; Kumari, E.J. Beneficiation of Titaniferous Ores by Selective Separation of Iron Oxide, Impurities and Rare Earth Oxides for the Production of High Grade Synthetic Rutile. Miner. Process. Extr. Metall. 2008, 117, 157–165. [Google Scholar] [CrossRef]
- Peelman, S.; Sun, Z.H.I.; Sietsma, J.; Yang, Y. Hydrometallurgical Extraction of Rare Earth Elements from Low Grade Mine Tailings. In Rare Metal Technology 2016; Springer: Cham, Switzerland, 2016; pp. 17–29. [Google Scholar]
- Peelman, S.; Sun, Z.H.I.; Sietsma, J.; Yang, Y. Leaching of Rare Earth Elements: Review of Past and Present Technologies. In Rare Earths Industry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 319–334. [Google Scholar]
- Rupasinghe, M.S.; Dissanayake, C.B. The Rare-Earth Element Abundance in the Sedimentary Gem Deposits of Sri Lanka. Lithos 1984, 17, 329–342. [Google Scholar] [CrossRef]
- Dissanayake, C.B.; Chandrajith, R.; Tobschall, H.J. The Geology, Mineralogy and Rare Element Geochemistry of the Gem Deposits of Sri Lanka. Bull. Soc. Finl. 2000, 72, 5–20. [Google Scholar] [CrossRef]
- Dharmaratne, P.G.R. Gem Industry of Sri Lanka, 2nd ed.; The Geological Survey & Mines Bureau, Sri Lanka: Colombo, Sri Lanka, 2016; ISBN 9559370010.
- Moon, J.W. The Mineral Industry of Sri Lanka. Available online: https://www.usgs.gov/media/files/mineral-industry-sri-lanka-2017-18-pdf (accessed on 20 October 2022).
- Cartier, L.E. Environmental Stewardship in Gemstone Mining: Quo Vadis? Available online: https://www.uvm.edu/~shali/egems.pdf (accessed on 20 October 2022).
- Thu, K. Gem Mining and Sustainability in Myanmar. Available online: https://sgiinstitute.com/wp-content/uploads/2019/10/Gem-Mining-and-Sustainability-in-Myanmar-presentation.pdf (accessed on 20 October 2022).
- Dharmaratne, P.G.R.; Premasiri, H.M.; Dillimuni, D. Sapphires from Thammannawa, Kataragama Area, Sri Lanka. Gems Gemol. 2012, 48, 98–107. [Google Scholar] [CrossRef]
- Cooray, P.G. The Precambrian of Sri Lanka: A Historical Review. Precambrian Res. 1994, 66, 3–18. [Google Scholar] [CrossRef]
- Gunaratne, H.S.; Dissanayake, C.B. Gems and Gem Deposits of Sri Lanka; National Gem and Jewellery Authority of Sri Lanka: Colombo, Sri Lanka, 1995; ISBN 9559371002.
- Ampitiyawatta, A.D.; Guo, S. Precipitation Trends in the Kalu Ganga Basin in Sri Lanka. J. Agric. Sci. 2009, 4, 10–18. [Google Scholar] [CrossRef]
- Kumara, S.; Premsiri, R.; Hewawasam, T. Morphological Features and Depositional Patterns of Gem-Bearing Sediments in the Kalu Ganga Basin, Sri Lanka. J. Geol. Soc. Sri Lanka 2015, 17, 125–138. [Google Scholar]
- Dushyantha, N.P.; Hemalal, P.V.A.; JAayawardena, C.L.; Ratnayake, A.S.; Premasiri, H.M.R.; Ratnayake, N.P. Nutrient Characteristics of Lake Sediments Around Eppawala Phosphate Deposit, Sri Lanka. J. Geol. Soc. Sri Lanka 2017, 18, 33–42. [Google Scholar]
- Dushyantha, N.P.; Hemalal, P.V.A.; Jayawardena, C.L.; Ratnayake, A.S.; Ratnayake, N.P. Application of Geochemical Techniques for Prospecting Unconventional Phosphate Sources: A Case Study of the Lake Sediments in Eppawala Area Sri Lanka. J. Geochem. Explor. 2019, 201, 113–124. [Google Scholar] [CrossRef]
- Ratnayake, A.S.; Dushyantha, N.; De Silva, N.; Somasiri, H.P.; Jayasekara, N.N.; Weththasinghe, S.M.; Samaradivakara, G.V.I.; Vijitha, A.V.P.; Ratnayake, N.P. Sediment and Physicochemical Characteristics in Madu-Ganga Estuary, Southwest Sri Lanka. J. Geol. Soc. Sri Lanka 2017, 18, 43–52. [Google Scholar]
- Subasinghe, C.S.; Ratnayake, A.S.; Roser, B.; Sudesh, M.; Wijewardhana, D.U.; Attanayake, N.; Pitawala, J. Global Distribution, Genesis, Exploitation, Applications, Production, and Demand of Industrial Heavy Minerals. Arab. J. Geosci. 2022, 15, 1–28. [Google Scholar] [CrossRef]
- Moldoveanu, G.A.; Papangelakis, V.G. Recovery of Rare Earth Elements Adsorbed on Clay Minerals: I. Desorption Mechanism. Hydrometallurgy 2012, 117–118, 71–78. [Google Scholar] [CrossRef]
- Ram, R.; Becker, M.; Brugger, J.; Etschmann, B.; Burcher-Jones, C.; Howard, D.; Kooyman, P.J.; Petersen, J. Characterisation of a Rare Earth Element-and Zirconium-Bearing Ion-Adsorption Clay Deposit in Madagascar. Chem. Geol. 2019, 522, 93–107. [Google Scholar] [CrossRef]
- Bao, Z.; Zhao, Z. Geochemistry of Mineralization with Exchangeable REY in the Weathering Crusts of Granitic Rocks in South China. Ore Geol. Rev. 2008, 33, 519–535. [Google Scholar] [CrossRef]
- Sun, S.-S.; McDonough, W.F. Chemical and Isotopic Systematics of Oceanic Basalts: Implications for Mantle Composition and Processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Ilankoon, I.M.S.K.; Tang, Y.; Ghorbani, Y.; Northey, S.; Yellishetty, M.; Deng, X.; Mcbride, D. The Current State and Future Directions of Percolation Leaching in the Chinese Mining Industry: Challenges and Opportunities. Miner. Eng. 2018, 125, 206–222. [Google Scholar] [CrossRef]
- Canadian Council of Ministers of the Environment. Canadian Soil Quality Guidelines for Uranium: Environmental and Human Health—Scientific Supporting Document; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2007; ISBN 9781896997643. [Google Scholar]
- Subasinghe, H.C.S.; Ratnayake, A.S.; Sameera, K.A.G. State-of-the-Art and Perspectives in the Heavy Mineral Industry of Sri Lanka. Miner. Econ. 2021, 34, 427–439. [Google Scholar] [CrossRef]


| Sample ID | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y | Sc | LREE | HREE | TREE | LREE% | HREE% | Sc% | REO% | U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AL-1 | 413 | 875 | 93 | 308 | 48 | 11 | 46 | 13 | 24 | 12 | 17 | 11 | 15 | bdl | 98 | 51 | 1746 | 237 | 2034 | 86 | 12 | 2 | - | 8.3 |
| AL-2 | 483 | 955 | 121 | 343 | 50 | 12 | 58 | 14 | 27 | 16 | 20 | 12 | 17 | bdl | 122 | 74 | 1963 | 285 | 2322 | 85 | 12 | 3 | - | 9.1 |
| AL-3 | 370 | 833 | 80 | 308 | 59 | 13 | 55 | 15 | 25 | 14 | 18 | 11 | 17 | bdl | 92 | 34 | 1662 | 246 | 1941 | 86 | 13 | 1 | - | 8.6 |
| AL-4 | 440 | 913 | 108 | 343 | 61 | 14 | 67 | 16 | 27 | 17 | 21 | 12 | 19 | bdl | 117 | 57 | 1878 | 294 | 2229 | 84 | 13 | 3 | - | 9.4 |
| AL-5 | 525 | 998 | 134 | 343 | 39 | 10 | 49 | 12 | 26 | 14 | 19 | 12 | 16 | bdl | 128 | 92 | 2047 | 275 | 2414 | 85 | 11 | 4 | - | 8.8 |
| AL-AVG | 446 | 915 | 107 | 329 | 51 | 12 | 55 | 14 | 26 | 14 | 19 | 12 | 17 | bdl | 111 | 62 | 1859 | 267 | 2188 | 85 | 12 | 3 | 0.3 | 8.8 |
| STD | 60 | 65 | 21 | 19 | 9.1 | 1.6 | 8 | 1.6 | 1.2 | 1.7 | 1.2 | 0.5 | 1.4 | - | 16 | 22 | 156 | 25 | 197 | - | - | - | - | 0.4 |
| GG-1 | 133 | 268 | 35 | 108 | 23 | 10 | 21 | 10 | 11 | 10 | 8.5 | 9 | 8.3 | bdl | 30 | 31 | 576 | 108 | 715 | 81 | 15 | 4 | - | 6.3 |
| GG-2 | 171 | 333 | 47 | 145 | 30 | 11 | 28 | 12 | 12 | 12 | 12 | 11 | 10 | bdl | 47 | 44 | 736 | 143 | 923 | 80 | 15 | 5 | - | 8.1 |
| GG-3 | 109 | 217 | 35 | 92 | 18 | 11 | 18 | 11 | 11 | 12 | 10 | 11 | 10 | bdl | 19 | 29 | 481 | 101 | 611 | 79 | 17 | 4 | - | 8.7 |
| GG-4 | 147 | 282 | 47 | 129 | 25 | 12 | 25 | 12 | 12 | 14 | 13 | 13 | 12 | bdl | 36 | 42 | 641 | 137 | 819 | 78 | 17 | 5 | - | 10.5 |
| GG-5 | 195 | 383 | 47 | 162 | 35 | 10 | 31 | 11 | 12 | 10 | 11 | 9 | 8 | bdl | 58 | 47 | 831 | 150 | 1027 | 82 | 14 | 4 | - | 5.7 |
| GG-AVG | 151 | 296 | 42 | 127 | 26 | 11 | 24 | 11 | 12 | 11 | 11 | 11 | 10 | bdl | 38 | 39 | 653 | 128 | 819 | 80 | 16 | 4 | 0.1 | 7.9 |
| STD | 33 | 64 | 6.7 | 28 | 6.4 | 0.7 | 5.2 | 0.8 | 0.4 | 1.6 | 1.7 | 1.7 | 1.7 | - | 15 | 8 | 136 | 22 | 165 | - | - | - | - | 1.9 |
| OCL-1 | 553 | 973 | 135 | 505 | 115 | 25 | 172 | 32 | 179 | 46 | 129 | 24 | 107 | 13 | 1473 | 113 | 2304 | 2174 | 4591 | 50 | 47 | 3 | - | 7.6 |
| OCL-2 | 658 | 1205 | 178 | 624 | 149 | 33 | 212 | 57 | 248 | 79 | 207 | 48 | 162 | 18 | 2105 | 150 | 2846 | 3135 | 6131 | 46 | 51 | 3 | - | 8.9 |
| OCL-3 | 493 | 850 | 123 | 450 | 93 | 31 | 147 | 30 | 171 | 39 | 86 | 11 | 85 | 15 | 1043 | 92 | 2039 | 1627 | 3758 | 54 | 43 | 3 | - | 8.4 |
| OCL-4 | 598 | 1083 | 166 | 569 | 127 | 39 | 186 | 55 | 239 | 72 | 165 | 36 | 140 | 21 | 1675 | 129 | 2581 | 2588 | 5298 | 50 | 47 | 3 | - | 9.7 |
| OCL-5 | 718 | 1328 | 190 | 679 | 171 | 27 | 238 | 58 | 256 | 85 | 250 | 61 | 183 | 16 | 2535 | 171 | 3111 | 3682 | 6963 | 42 | 55 | 3 | - | 8.1 |
| OCL-AVG | 604 | 1088 | 158 | 565 | 131 | 31 | 191 | 46 | 219 | 64 | 167 | 36 | 135 | 17 | 1766 | 131 | 2576 | 2641 | 5348 | 48 | 49 | 3 | 0.6 | 8.5 |
| STD | 88 | 188 | 29 | 91 | 30 | 6 | 35 | 14 | 40 | 20 | 64 | 20 | 40 | 3 | 575 | 31 | 425 | 803 | 1258 | - | - | - | - | 0.8 |
| Sample ID | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y | Sc | LREE | HREE | TREE | LREE% | HREE% | Sc% | REO% | U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-1 | 2854 | 6116 | 656 | 2238 | 392 | 11 | 271 | 27 | 43 | 11 | 25 | 7 | 14 | bdl | 123 | 66 | 12,266 | 521 | 12,854 | 95 | 5 | 0 | - | 457 |
| F-2 | 2671 | 6012 | 682 | 2101 | 329 | 10 | 281 | 23 | 45 | 13 | 29 | 9 | 17 | bdl | 144 | 83 | 11,805 | 521 | 12,449 | 95 | 5 | 0 | 511 | |
| F-3 | 2816 | 5928 | 671 | 2865 | 389 | 12 | 278 | 21 | 39 | 12 | 27 | 5 | 18 | bdl | 137 | 74 | 12,681 | 561 | 13,292 | 95 | 5 | 0 | 472 | |
| F-4 | 2743 | 6231 | 599 | 2003 | 401 | 14 | 269 | 29 | 46 | 25 | 21 | 7 | 15 | bdl | 116 | 90 | 11,991 | 537 | 12,609 | 95 | 5 | 0 | 443 | |
| F-5 | 2903 | 6193 | 612 | 2362 | 381 | 11 | 288 | 30 | 44 | 10 | 29 | 9 | 11 | bdl | 126 | 87 | 12,462 | 528 | 13,096 | 95 | 5 | 0 | 470 | |
| F-AVG | 2797 | 6096 | 644 | 2314 | 378 | 12 | 277 | 26 | 43 | 14 | 26 | 7 | 15 | bdl | 129 | 80 | 12,241 | 534 | 12,860 | 95 | 5 | 0 | 1.4 | 471 |
| STD | 92 | 126 | 37 | 337 | 29 | 2 | 8 | 4 | 3 | 6 | 3 | 2 | 3 | - | 11 | 10 | 352 | 17 | 344 | 0 | 0 | 0 | - | 25 |
| CC-1 | 6138 | 12,455 | 1435 | 4953 | 718 | 25 | 635 | 52 | 103 | 23 | 52 | 14 | 34 | 1.5 | 293 | 77 | 25,722 | 1206 | 27,005 | 95 | 5 | 1 | - | 794 |
| CC-2 | 5198 | 10,478 | 1768 | 3465 | 880 | 17 | 738 | 68 | 77 | 18 | 72 | 8.5 | 22 | 0.8 | 360 | 110 | 21,804 | 1364 | 23,279 | 94 | 3 | 1 | - | 806 |
| CC-3 | 6020 | 11,868 | 1235 | 5513 | 685 | 22 | 615 | 47 | 97 | 24 | 41 | 14 | 43 | 1.5 | 234 | 52 | 25,342 | 1116 | 26,510 | 96 | 5 | 1 | - | 717 |
| CC-4 | 5080 | 9890 | 1568 | 4025 | 848 | 14 | 718 | 62 | 71 | 19 | 62 | 8.3 | 32 | 0.8 | 302 | 86 | 21,424 | 1274 | 22,783 | 94 | 6 | 1 | - | 871 |
| CC-5 | 5315 | 11,065 | 1968 | 2905 | 913 | 20 | 758 | 73 | 84 | 17 | 83 | 8.8 | 13 | 0.8 | 419 | 135 | 22,185 | 1455 | 23,774 | 93 | 5 | 1 | - | 814 |
| CC-AVG | 5550 | 11,151 | 1595 | 4172 | 809 | 19 | 693 | 60 | 86 | 20 | 62 | 11 | 29 | 1.1 | 321 | 92 | 23,295 | 1283 | 24,670 | 94 | 5 | 1 | 2.8 | 800 |
| STD | 492 | 1033 | 285 | 1065 | 101 | 4 | 64 | 11 | 13 | 3 | 16 | 3 | 12 | 0.4 | 70 | 32 | 2064 | 132 | 1945 | - | 2 | - | - | - |
| Product | Yield % | LERO | HREO | TREO | |||
|---|---|---|---|---|---|---|---|
| Grade % | Recovery % | Grade % | Recovery % | Grade % | Recovery % | ||
| Feed | 100 | 1.33 | 100 | 0.06 | 100 | 1.4 | 100 |
| Concentrate | 37 | 2.65 | 74 | 0.14 | 90 | 2.8 | 74 |
| Tailing | 63 | 0.54 | 26 | 0.01 | 10 | 0.6 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dushyantha, N.; Ilankoon, I.M.S.K.; Ratnayake, N.P.; Premasiri, H.M.R.; Dharmaratne, P.G.R.; Abeysinghe, A.M.K.B.; Rohitha, L.P.S.; Chandrajith, R.; Ratnayake, A.S.; Dissanayake, D.M.D.O.K.; et al. Recovery Potential of Rare Earth Elements (REEs) from the Gem Mining Waste of Sri Lanka: A Case Study for Mine Waste Management. Minerals 2022, 12, 1411. https://doi.org/10.3390/min12111411
Dushyantha N, Ilankoon IMSK, Ratnayake NP, Premasiri HMR, Dharmaratne PGR, Abeysinghe AMKB, Rohitha LPS, Chandrajith R, Ratnayake AS, Dissanayake DMDOK, et al. Recovery Potential of Rare Earth Elements (REEs) from the Gem Mining Waste of Sri Lanka: A Case Study for Mine Waste Management. Minerals. 2022; 12(11):1411. https://doi.org/10.3390/min12111411
Chicago/Turabian StyleDushyantha, Nimila, I. M. Saman K. Ilankoon, N. P. Ratnayake, H. M. R. Premasiri, P. G. R. Dharmaratne, A. M. K. B. Abeysinghe, L. P. S. Rohitha, Rohana Chandrajith, A. S. Ratnayake, D. M. D. O. K. Dissanayake, and et al. 2022. "Recovery Potential of Rare Earth Elements (REEs) from the Gem Mining Waste of Sri Lanka: A Case Study for Mine Waste Management" Minerals 12, no. 11: 1411. https://doi.org/10.3390/min12111411
APA StyleDushyantha, N., Ilankoon, I. M. S. K., Ratnayake, N. P., Premasiri, H. M. R., Dharmaratne, P. G. R., Abeysinghe, A. M. K. B., Rohitha, L. P. S., Chandrajith, R., Ratnayake, A. S., Dissanayake, D. M. D. O. K., & Batapola, N. M. (2022). Recovery Potential of Rare Earth Elements (REEs) from the Gem Mining Waste of Sri Lanka: A Case Study for Mine Waste Management. Minerals, 12(11), 1411. https://doi.org/10.3390/min12111411









