Abstract
This study was undertaken to determine the effects of various substances on soil contaminated with cobalt (Co) on the mass and content of cobalt in the main crop—spring barley (Hordeum vulgare L.)—and the after-crop—white mustard (Synapis alba L.). Manure, clay, charcoal, zeolite, and calcium oxide were used for phytostabilization. Cobalt was applied in the form of CoCl2 in doses of 0, 20, 40, 80, 160, and 320 mg/kg soil. Amendments in the form of manure, clay, charcoal, and zeolite were applied in an amount of 2% in relation to the weight of the soil in a pot, with calcium oxide at a dose of 1.30 g CaO/kg of soil. The highest cobalt doses resulted in a significant reduction in yield of both plants and in tolerance index for cobalt. Increasing contamination of soil with cobalt resulted in a major and significant increase in its content in plants and a reduction in cobalt translocation factor in both plants. Amendments used in phytostabilization had a significant effect on growth and development of oat and content of cobalt in plants. The strongest effect on the yield of above-ground parts was exerted by manure (both plants) and calcium oxide (white mustard), while the strongest effect on weight of roots was exerted by calcium oxide (both plants) and zeolite (white mustard). The addition of manure, zeolite and calcium oxide to soil caused an increase of the tolerance index for both plants, while the addition of clay only had a positive effect for white mustard. All substances used in phytostabilization (except zeolite) decreased cobalt content of roots, and manure and calcium oxide in above-ground parts of spring barley; manure and zeolite only in above-ground parts, and calcium oxide in both organs of white mustard. Most of them also reduced bioconcentration of cobalt in above-ground parts, calcium oxide decreased cobalt content in roots of both plants, and manure in roots of spring barley. The effect on cobalt translocation was less clear, but most substances used in phytostabilization increased the transfer of cobalt from the soil to plants. White mustard had higher ability to accumulate cobalt than spring barley.
1. Introduction
Soil degradation has now become a global problem, primarily due to deforestation, erosion and, particularly, contamination with trace elements [1]. The occurrence of excessive contents of trace elements in the soil contributes to their intense uptake by microorganisms and plants, which enables those elements to penetrate into other living organisms [2]. Therefore, it poses a significant hazard to the proper growth and development of plant and also to the subsequent links of the food chain, with humans at the top of the chain [3].
The occurrence of large amounts of trace elements in the soil may also have a toxic effect on plants. An example of such an element is cobalt. The natural cobalt content of soil is up to 40 mg/kg [4]. Cases of exceeding its permissible content in the soil primarily result from the intensive extraction of this element due to its wide application in various industries [5]. The highest contamination of the soil environment associated with its extraction is found in Central-Southern Africa (DR Congo and Zambia) [6]. In Europe, since cobalt resources are small, its permissible content in the soil is exceeded more rarely. The areas that are most exposed to the occurrence of high cobalt contents of the soil of anthropogenic origin are mainly industrial and transport areas [6].
Cobalt is taken up by plants in various forms, most often in the form of di- and trivalent cations [7]. Cobalt is characterised by significant mobility in plants, but it depends on the species. Usually, its largest amounts accumulate in the roots, smaller amounts in the stems, and the smallest amounts in the leaves [8]. Cobalt toxicity is closely related with the acidity of the soil. The most frequent symptoms resulting from the high accumulation of cobalt in plants is reduced plant growth and the emergence of necroses as well as disorders in the uptake of nutrients. Cobalt toxicity for plants is most frequently found in light soils with a poorer sorption complex than in heavy soils [9].
To immobilise or eliminate trace elements from soils, various types of phytoremediation treatments which make use of the properties of plants are applied [10,11]. Phytostabilisation is one of the methods of the phytoremediation. Phytostabilisation is based on the application of various amendments to soil to reduce the availability and uptake of trace elements by plants [12].
The research hypotheses were as follows: soil contamination with cobalt has a negative effect on plants, amendments reduce the effect of cobalt on plants. In view of the above, the study was undertaken to determine the effects of various substances on soil contaminated with cobalt (Co) on the mass and content of cobalt in the main crop—spring barley (Hordeum vulgare L.)—and the after-crop—white mustard (Synapis alba L.). Manure, clay, charcoal, zeolite, and calcium oxide were used for phytostabilization.
2. Materials and Methods
2.1. Methodological Design
The study was based on a pot experiment carried out at a greenhouse owned by the University of Warmia and Mazury in Olsztyn (Poland). Soil was used whose granulometric composition corresponded to that of loamy sand. The chemical composition of soil was presented in Table 1. The methods of soil analysis prior to setting up the experiment were provided in a previously published study [13]. The experiment was carried out in polyethylene pots, each containing 9 kg soil, in six series (1—without amendments, 2—with added bovine manure granules, 3—with added clay, 4—with added charcoal, 5—with added zeolite of the clinoptilolite type, and 6—with added 50% calcium oxide). Cobalt was applied in the form of CoCl2 in doses of 0, 20, 40, 80, 160, and 320 mg/kg soil. Amendments in the form of manure, clay, charcoal and zeolite were applied in an amount of 2% in relation to the weight of the soil in a pot, with calcium oxide at a dose of 1.30 g CaO/kg of soil. The chemical composition of amendments was shown in Table 2. In addition, the following were added on a one-off basis to the soil in each pot: 100 mg N (NH4NO3), 35 mg P (KH2PO4), 100 mg K (KCl), 50 mg Mg (MgSO4 7H2O), 0.33 mg B (H3BO3), 5 mg Mn (MnCl2 4H2O), and 5 mg Mo ((NH4)6Mo7O24 4H2O) per kg of soil. The experiment was carried out in three replications. The applied soil amendments were primarily selected due to their frequent use in remediation treatments, while cobalt doses were based on the Polish country standards which are currently in force (Regulation of Minister of the Environment of 9 September 2002 on the quality standards for soil and quality standards for land, and of the Regulation of Minister of the Environment of 1 September 2016 on the procedures for the assessment of land surface contamination).

Table 1.
Chemical composition of soil.

Table 2.
Chemical composition of amendments.
In the prepared soil, seeds of spring barley (Hordeum vulgare L.) of the Mercada variety were sown. The sowing density was 15 plants per pot. After 52 days from sowing, in the heading stage, the above-ground parts and roots of spring barley were harvested. The above-ground parts of spring barley were cut, and the roots were separated from the soil on the sieve. The soil was returned to each of the pots. This was followed by the sowing of seeds of white mustard (Sinapis alba L.) of the Bamberka variety to the same soil. The after-crop was cultivated with a sowing density of eight plants per pot. The above-ground parts and roots of white mustard were harvested 36 days after sowing, following the completion of the flowering stage. Main environmental parameters were typical to the period May–July (average air temperature—15.6 °C, average air humidity—76.5%, length of daytime—from 13 h 3 min to 16 h 31 min.). The soil moisture was kept at 60% of water capillary capacity using distilled water. Cultivation of two plants (main crop and after-crop) resulted from the application of a system typical for field crops. Spring barley was primarily selected because it is sensitive to high contamination of soils with trace elements; in turn, white mustard is classified as a hyperaccumulator plant and it is recommended as an aftercrop following the cultivation of cereals.
2.2. Methods of Laboratory and Statistical Analyses
During the harvest, the average weights of particular organs of the plants in each pot were determined. The collected plant material was dried at a temperature of 60 °C and then ground. Next, it was “wet” mineralised in concentrated nitric acid (HNO3 of analytical grade, density of 1.40 g/cm3) in HP500 Teflon vessels in a MARS 5 microwave oven (CEM Corporation, Matthews, NC, USA). Total content of cobalt was determined using the method of flame atomic absorption spectroscopy (FAAS) in an air-acetylene flame [14]. The study results were compared with certified reference material NCS ZC 73030, originating from the Chinese National Analysis Centre (Beijing, China) for Iron and Steel 2014 and with Fluka standard solutions with the following symbol: Co 119785.0100. Additionally, the following factors were calculated for cobalt: bioconcentration factor (BCF) = Cplant part/Csoil for the above-ground parts and the roots of both test plants, translocation factor (TF) = Cabove-ground parts/Croots, and transfer factor (TFr) = Cplant/Csoil, in which C denotes the content of a particular element, expressed in mg/kg [15,16]. Moreover, calculations were made of the values of tolerance indices Ti using the formula: Ti = yield of the plant biomass from the Co-enriched soil/yield of the plant biomass from the control soil. Ti < 1 indicates a negative effect of Co, Ti = 1 indicates no effect of Co, and Ti > 1 indicates the positive effect of Co on the growth and development of the tested plants. The test results were statistically processed using ANOVA two-factor variance analysis and the Duncan test as well as the correlation coefficients from the Statistica 13 package (StatSoft, Inc., Tulsa, OK, USA) [17]. The least squares deviation (LSD) and Tukey’s HSD (honest significant difference) as a post hoc tests were applied. Significance levels * P ≤ 0.05 and ** P ≤ 0.01 were used to assess the significance of differences between the tested factors.
3. Results
An analysis of the results presented in this paper clearly indicates that the applied doses of cobalt and the applied substances in phytostabilization have a significant effect on the productivity of spring barley and white mustard, tolerance index, cobalt content and the bioconcentration, translocation, and cobalt transfer coefficients.
In the series without amendments, the highest doses of cobalt (320 mg Co/kg soil) resulted in a decrease in the weight of the above-ground parts by almost 100% and the roots of spring barley by 90% and completely prevented the growth and development of white mustard, as compared to the soil not contaminated with this element. A lower dose of cobalt (160 mg Co/kg soil) decreased the weight of the above-ground parts and roots of the main crop by 98% and 90% and of the after-crop by 99% and 90%, respectively (Table 3).

Table 3.
Yield of above-ground parts and mass of roots of spring barley and white mustard (g FW/pot).
Amendments used in phytostabilization had a significant and positive effect on the weight of the roots of both plants. The application of most of the substances had a positive effect on the yield of the above-ground parts of white mustard. Manure and calcium oxide had a positive effect on the yield of the above-ground parts of spring barley. The application of manure had the strongest effect on an increase in the average yield of the above-ground parts by 58%.
The application of calcium oxide had the strongest effect on the weight of the roots of spring barley (an increase of 171%). The application of manure and calcium oxide had the strongest effect on the yield of the above-ground parts (an increase of 50%). The application of calcium oxide and zeolite had the strongest effect on the weight of the roots of white mustard (an increase of 68–69%), compared to the series without amendments. The beneficial effect of manure and calcium oxide on the increase in the yield of both test plants are particularly confirmed in the soil with doses of 160 and 320 mg Co/kg soil. The addition of charcoal to the soil had a negative (but small) effect on the yield of the above-ground parts of white mustard and, to a small extent, of spring barley (Table 3).
In the series without amendments, increasing doses of cobalt decreased the tolerance index (Ti) from 1 to 0.006 for spring barley and from 1 to 0 for white mustard (Figure 1). The application of all substances to the soil resulted in an increase in the tolerance index. Of all the applied substances, manure and calcium oxide exhibited the strongest effect on the increase in the tolerance index Ti. A value of Ti exceeding 1 indicates the inhibition of the growth of plants, which was observed in the control series.
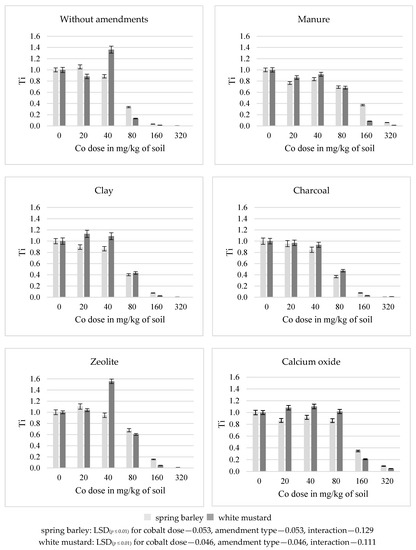
Figure 1.
Tolerance index (Ti) for plants.
In the soil without amendments, the increasing contamination of the soil with cobalt resulted in a significant increase in its content in both plants (Table 4). Compared to the control (non-contaminated with cobalt), the increase was up to 38-fold in the above-ground parts and 816-fold in the roots of spring barley and 397-fold in the above-ground parts and 16-fold in the roots of white mustard. The positive effect of these substances applied to the soil on cobalt content was most evident for the roots of spring barley (Table 4).

Table 4.
Content of cobalt in spring barley and white mustard (mg/kg d. m.).
All of the substances (except zeolite) used in phytostabilization had a beneficial effect on the cobalt content of the roots of spring barley (Table 2). Under the influence of these substances, a decrease in the content of cobalt in the roots of spring barley varied on average from 11% (charcoal) to 42% (manure), compared to the series without amendments. An analogous effect was exerted by manure and calcium oxide through decreasing the content of cobalt (by 44% and 31%, respectively) in the above-ground parts of spring barley, and by manure, zeolite and calcium oxide through decreasing its accumulation (on average by 41%, 29%, and 74%) in the above-ground parts of white mustard. In other cases, the substances either had no effect or contributed to an increase in cobalt accumulation in plants. Increasing effect of amendments on cobalt content in plants in some objects of experiment was higher in white mustard than in spring barley.
In the soil without amendments, a dose of 80 mg Co/kg soil had the strongest effect on the growth of bioconcentration factor (BCF) for cobalt in the above-ground parts (a five-fold increase) and in the roots (a 53-fold increase) of spring barley and in the above-ground parts (a 36-fold increase) of white mustard. Dose of 40 mg Co/kg soil had the strongest effect on its roots (a two-fold increase) (Figure 2 and Figure 3). Higher cobalt doses resulted in a decrease in the bioconcentration factor (BCF) in the tested organs of both plants, compared to the previous levels of Co contamination. The application of most substances to the soil for phytostabilization of cobalt resulted in an increase in the BCF value in the roots and a decrease in the above-ground parts of the after-crop. Manure and calcium oxide lead to a decrease in the BCF value in the above-ground parts and roots of the main crop, in relation to the series without amendments. Other substances had an opposite effect on the BCF value in this plant. The most significant increase in the BCF in the roots (by an average of 42–44%) of spring barley following the introduction of charcoal and zeolite and in roots of white mustard (257%) after application of manure was noted. Small increase (7%) in the above-ground parts of white mustard after application of clay was observed. Both in the first (the above-ground part increased by 45% and the roots increased by 27%) and in the second (the above-ground part increased by 83%, the roots increased by 36%) test plants, calcium oxide had the strongest effect on a decrease in the BCF.
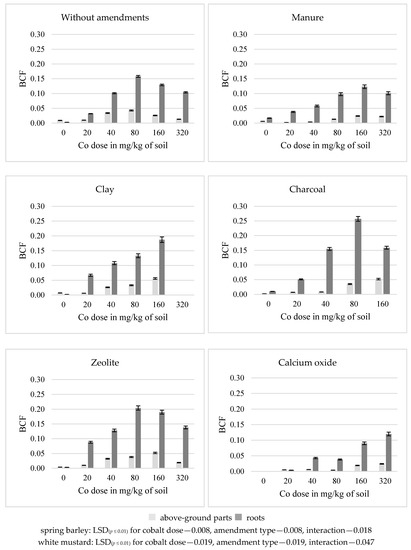
Figure 2.
Bioconcentration factor (BCF) of cobalt in spring barley above-ground parts and roots.
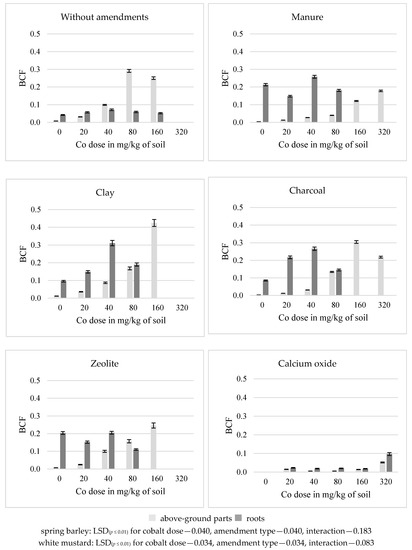
Figure 3.
Bioconcentration factor (BCF) of cobalt in white mustard above-ground parts and roots.
In the series without amendments, a 320 mg Co/kg soil resulted in a 95% decrease in the translocation coefficient of this element (TF) in spring barley, while 80 mg Co/kg soil resulted in a 25-fold increase in white mustard as compared to the control soil (Figure 4). All substances (except clay for spring barley) decreased the value of this coefficient in both plants. Manure had the strongest effect on a decrease in the TF in the main crop, on average by 74%, and in the after-crop by 95%, in relation to the series without amendments.
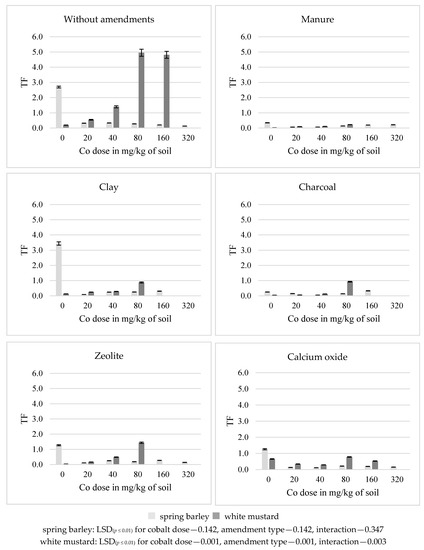
Figure 4.
Translocation factor (TF) of cobalt in spring barley and white mustard.
In the series without amendments, the coefficient of cobalt transfer from the soil to the plants (TFr) increased the most in spring barley (by 17 times) and in white mustard (by seven times) under the influence of 80 mg Co/kg soil (Figure 5). Higher cobalt doses reduced this coefficient in the plants. The application of calcium oxide to both plants, and of manure to white mustard, reduced (while the addition of the remaining substances increased) the value of the TFr. Of the used amendments, charcoal and zeolite had the greatest effect on an increase in the value of the TFr in spring barley (on average, by 35% and 37%, respectively), while clay had the greatest effect on white mustard (by 53%) as compared to the series without amendments. Calcium oxide most strongly decreased the value of TFr in both plants. Under its influence, the TFr decreased by an average of 31% in spring barley and by 72% in white mustard.
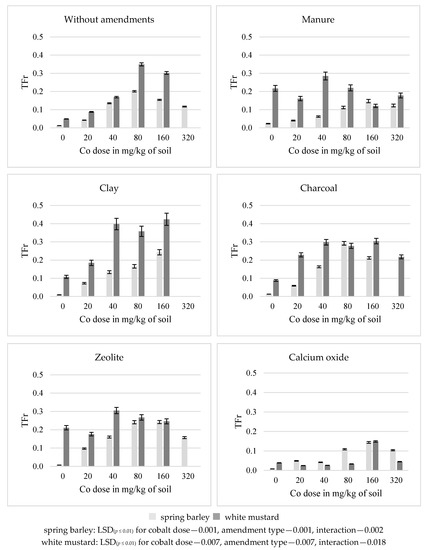
Figure 5.
Transfer factor (TFr) of cobalt in spring barley and white mustard.
4. Discussion
The toxicity of trace elements to plants is determined by many factors, which mainly include the species of a particular plant, the type of soil and its physico-chemical properties, the type of an element, and primarily their content in the soil [18]. Cobalt is a trace element which, at low concentrations in the soil, has a positive effect on plant growth, while at high concentrations it exhibits adverse effects [9]. The highest yield of the first and the second test plants was reached in soil non-contaminated with cobalt and with an added dose of 20 mg/kg soil, while in other soil contaminated with high doses of this element, the growth of plants was partially or completely stopped. The above relationships were also confirmed in the other authors’ studies. Khalid and Ahmed [19] found that the application of 30 mg Co/L had a positive effect on the growth of Nigella sativa L., Sarma et al. [20] found that the application of 100 and 200 mg Co in a cultivation nutrient solution and sand using Hoagland solution had a stimulating effect on the height, number and surface area of the leaves and the wheat dry matter content, while 300, 400, and 500 mg Co exhibit a harmful effect. An adverse effect of cobalt doses of over 300 mg on chlorophyll a and b contents (and on its stability index) was also found [20]. According to Wallace et al. [8] an increase in cobalt content in the leaves to 43 μg Co/g and 142 μg Co/g dry matter significantly contributes to the emergence of severe chloroses. The inhibition of the increase in biomass of Hordeum vulgare L., Brassica napus L., and Lycopersicon esculentum L. under the influence of cobalt contamination is demonstrated by a study by Li et al. [21]. According to Shaukat et al. [22], the stoppage of seed germination may result from the occurrence of an osmotic effect caused by a high cobalt content of the soil.
The reduction in the negative effects of high contamination of soil on plants can be obtained through the introduction of various mineral and organic substances into the soil in phytostabilization process. A beneficial effect of charcoal on the growth and development of various plant species was demonstrated by Kuzyakov et al. [23], zeolites and dolomite on maize by Kovacevic and Rastija [24], calcium oxide and compost on: maize by Wyszkowski and Radziemska [25] and Wyszkowski and Ziółkowska [26,27], oats by Wyszkowski and Ziółkowska [26], and yellow lupine by Wyszkowski and Ziółkowska [27].
Phytostabilization of soil contaminants through the application of various substances has a significant effect on the content of trace elements in the soil and, at the same time, on the uptake by plants [28]. According to Pichtel and Bradway [29], the introduction of bovine manure to the soil results in a reduction in heavy metal uptake from the soil by plants. With the short duration of its mineralisation, the availability of nutrients increases, which may, at the same time, explain its favourable effect on the growth and quality of plants [30]. In turn, Skwaryło-Bednarz et al. [31] found that an appropriate content of basic nutrients provided during fertilisation has an effect on the yield of plants. The content of nutrients in plants is strongly correlated with the physico-chemical properties of the soil.
Application of an alkaline fertiliser to the soil increased the pH and reduced solubility in the soil and the availability of cobalt and other trace elements for plants [32,33]. The cobalt content in the soil and other soil properties after the plants cultivation in this experiment were published in other papers [34,35]. Kosiorek and Wyszkowski [34] found that following the introduction of calcium oxide, the pH value of the soil increased (>7). They also noted that manure and calcium oxide used in phytostabilization had the most favourable effect on an increase in the yield and had a significant effect on a reduction in the contents of cobalt, lead, zinc, copper, and manganese in selected parts of the plants, which confirms the above assumptions. According to Ciećko et al. [36], manure, brown coal, and calcium oxide are effective in reducing cadmium uptake by plants. In an experiment by Sivitskaya and Wyszkowski [13], the introduction of calcium oxide resulted in an increase in the contents of most trace elements in maize. This was also confirmed by the authors’ own study, since the greatest increase in cobalt content of both test plants was noted in the series with the addition of calcium oxide, which resulted in the greatest increase in the pH value of the soil and could have a toxic effect on the development of the test plants.
The favourable effect of zeolites used in phytostabilization on the increase in the pH value of the soil (and thus an increase in the immobilisation of micronutrients) was also demonstrated by Querol et al. [37]. Zeolite applied in this study failed to exert the assumed effects in the remediation of the soil contaminated with cobalt, as it led to an increase in the cobalt content in the above-ground parts and in the roots of the main crop. Zeolites have strong adsorption properties in relation to both cationic forms and to anionic trace elements occurring in the soil [38].
Deenik et al. [39] reported that the content of volatile substances in charcoal has a significant effect on the reduction in the growth of Lactuca sativa L. and Zea mays L., cultivated in tropical soils under greenhouse conditions, and on nitrogen transformations in the soil. As reported by Yamato et al. [40], the introduction of charcoal to the soil reduces the content of trivalent aluminium cations in the soil and exhibits a significantly more favourable effect on the properties of a low-quality soil than on highly fertile soils. It resulted in an almost 50% increase in the yield of Zea mays L., Vigna unguiculata L., and Arachis hypogaea L. According to Kolb et al. [41], the presence of charcoal in the soil contributes to an increase in the activity of bacteria and microorganisms in the soil, and to an increase in the availability of nutrients necessary to plants. In the authors’ own study, the introduction of charcoal into the soil resulted in a slight decrease in the yield of the above-ground parts of spring barley and white mustard. It also had an effect on the increase in cobalt content of the above-ground parts and the roots of white mustard.
The roots of the plants, as compared to the stems and leaves, accumulate the greatest amounts of cobalt from the soil [8]. Sarma et al. [20] found that the introduction of 100 mg Co/kg and 200 mg Co/kg to the nutrient solution has a considerably stronger effect on the cobalt content of the grains of wheat than a dose of 500 mg of this element on kg of soil. In the authors’ own study, cobalt was accumulated in greater amounts in the roots than in the above-ground parts of the barley. In turn, a reverse trend was demonstrated for white mustard. According to Abdel-Sabour and Al-Salama [42], the cultivation of Brassica napus may eliminate up to 40% cobalt from the soil. Tappero et al. [43], using Allysum murale L., indicated the value of the bioconcentration factors (BCF) for cobalt at a level of 532 (the Co + Ni series) and 702 (the Co + Ni + Zn series) and the value of the translocation factor expressed by the ratio between the cobalt content of the above-ground parts and this content in the roots (3.0). In this study, the highest average translocation factor of 2.379 in the series without amendments was found in the white mustard. Therefore, it can be concluded that this plant is more effective than spring barley in the process of remediation of a soil contaminated with cobalt, for which the average TF in an analogous series amounted to 0.658.
Majority of amendments used in phytostabilization in our study had a positive effect and reducing the negative influence of cobalt contamination on both plants, especially spring barley.
5. Conclusions
In the series without amendments, the highest cobalt doses resulted in a significant reduction in both the yield of the above-ground parts and the roots of both plants and in the tolerance index. In the series without amendments, increasing contamination of the soil with cobalt resulted in a major and significant increase in its content in the tested organs of the plants (the highest levels of cobalt were in the above-ground parts of the main crop, and the lowest levels were in the roots of the after-crop), and a reduction in the translocation factor in both plants. Medium doses of cobalt had a similar effect on the bioconcentration and transfer factors, in contrast to high doses of cobalt.
All substances used in phytostabilization reduced the adverse effect of cobalt. The strongest effect on the yield of the above-ground parts was exerted by manure (both plants) and calcium oxide (white mustard), while the strongest effect on the weight of the roots was exerted by calcium oxide (both plants) and zeolite (white mustard). The addition of manure, zeolite and calcium oxide to the soil had a positive effect through increasing the tolerance index for both plants, while the addition of clay only had a positive effect for white mustard. All substances used in phytostabilization (except zeolite) decreased cobalt content of the roots, and manure and calcium oxide decreased the cobalt content of the above-ground parts of spring barley; manure and zeolite decreased the cobalt content only in the above-ground parts, and calcium oxide decreased cobalt content in both organs of white mustard. Most of them also reduced the bioconcentration of cobalt in the above-ground parts, calcium oxide decreased cobalt content in the roots of both plants, and manure decreased cobalt content in the roots of spring barley. The effect on the cobalt translocation was less clear, but most substances increased the transfer of cobalt from the soil to the plants.
White mustard had higher ability to accumulate cobalt than spring barley. It can be used for remediation of areas which are contaminated with this element.
Author Contributions
M.W. and M.K. framed the methodology, conceived the ideas, designed the paper, wrote the paper, prepared the tables and figures, and collected the data. M.W. reviewed the manuscript. All authors contributed significantly to the discussion of the results and the preparation of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Ministry of Science and Higher Education funds for statutory activity. Project financially supported by Minister of Science and Higher Education in the range of the program entitled “Regional Initiative of Excellence” for the years 2019–2022, Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Biro, K.; Pradhan, B.; Buchroithner, M.; Makeschin, F. Land use/land cover change analysis and its impact on soil properties in the Northern part of Gadarif region, Sudan. Land Degrad. Dev. 2013, 24, 90–102. [Google Scholar] [CrossRef]
- Zaborowska, M.; Kucharski, J.; Wyszkowska, J. Biological activity of soil contaminated with cobalt, tin and molybdenum. Environ. Monit. Assess. 2016, 188, 398. [Google Scholar] [CrossRef] [PubMed]
- Timsina, J.; Connor, D.J. Productivity and management of rice-wheat cropping systems: Issues and challenges. Field Crop. Res. 2001, 69, 93–132. [Google Scholar] [CrossRef]
- Blume, H.-P.; Brümmer, G.W.; Fleige, H.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.-M. Scheffer/Schachtschabel Soil Science; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–618. [Google Scholar]
- Kim, J.H.; Gibb, H.J.; Howe, P.D. Cobalt and Inorganic Cobalt Compounds; Concise International Chemical Assessment Document, No 69; World Health Organization: Geneva, Switzerland, 2006; pp. 1–82. [Google Scholar]
- Report on Critical Raw Materials for the EU; European Comission: Brussels, Belgium, 2014.
- Pilon-Smits, E.A.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of benefical elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.; Alexander, G.V.; Chaudhry, F.M. Phytotoxicity of cobalt, vanadium, titanium, silver, and chromium. Commun. Soil Sci. Plant Anal. 2008, 8, 751–756. [Google Scholar] [CrossRef]
- Feng, N.; Dagan, R.; Bitton, G. Toxicological approach for assessing the heavy metal binding capacity of soils. Soil Sed. Cont. 2007, 16, 451–458. [Google Scholar] [CrossRef]
- Alkorta, I.; Hernández-Allica, J.; Becerril, J.; Amezaga, I.; Albizu, I.; Garbisu, C. Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead, and arsenic. Rev. Environ. Sci. Biotechnol. 2004, 3, 71–90. [Google Scholar] [CrossRef]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, R.; Sas-Nowosielska, A.; Małkowski, E.; Japenga, J.; Kuperberg, J.M.; Pogrzeba, M.; Krzyżak, J. The use of indigenous plant species and calcium phosphate for the stabilization of highly metal-polluted sites in southern Poland. Plant Soil 2005, 273, 291–305. [Google Scholar] [CrossRef]
- Sivitskaya, V.; Wyszkowski, M. Effect of heating oil and neutralizing substances on the content of some trace elements in maize (Zea mays L.). Ecol. Chem. Eng. A 2013, 20, 323–331. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods for Analysis and Evaluation of Soil and Plant Properties; Institute of Environmental Protection: Warsaw, Poland, 1991; pp. 1–334. (In Polish) [Google Scholar]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals-concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Mleczek, M.; Gąsecka, M.; Drzewiecka, K.; Goliński, P.; Magdziak, Z.; Chadzinikolau, T. Copper phytoextraction with willow (Salix viminalis L.) under various Ca/Mg ratios. Part 1. Copper accumulation and plant morphology changes. Acta Physiol. Plant 2013, 35, 3251–3259. [Google Scholar] [CrossRef]
- Dell Inc. Dell Statistica (Data Analysis Software System), Version 13. 2016. Available online: http://software.dell.com (accessed on 6 April 2020).
- Mildvan, A.S. Metal in enzymes catalysis. In The Enzymes; Boyer, D.D., Ed.; Academic Press: London, UK, 1970; pp. 445–536. [Google Scholar]
- Khalid, K.A.; Ahmed, A.M.A. Effect of cobalt on growth, yield and chemical constituents of Nigella sativa L. J. Mater. Environ. Sci. 2016, 7, 2201–2207. [Google Scholar]
- Sarma, B.; Devi, P.; Gogoi, N.; Devi, Y.M. Effects of cobalt induced stress on Triticum aestivum L. Crop. Asian. J. Agri. Biol. 2014, 2, 137–147. [Google Scholar]
- Li, H.F.; Gray, C.; Mico, C.; Zhao, F.J.; McGrath, S.P. Phytotoxicity and bioavailability of cobalt to plants in a range of soils. Chemosphere 2009, 75, 979–986. [Google Scholar] [CrossRef]
- Shaukat, S.S.; Mushtaq, M.; Siddiqui, Z.S. Effects of cadmium, chromium and lead on seed germination, early seedling growth and phenolic contents of Parkinsonia aculeatt L. and Pennisetum americanum (L.), Schumann. Pak. J. Biol. Sci 1999, 2, 1307–1313. [Google Scholar]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar] [CrossRef]
- Kovacevic, V.; Rastija, M. Impacts of liming by dolomite on the maize and barley grain yields. Poljoprivreda 2010, 16, 3–8. [Google Scholar]
- Wyszkowski, M.; Radziemska, M. The effect of chromium (III) and chromium (VI) on the yield and content of nitrogen compounds in plants. J. Toxicol. Environ. Heal. A 2010, 73, 1274–1282. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Ziółkowska, A. Role of compost, bentonite and calcium oxide in restricting the effect of soil contamination with petrol and diesel oil on plants. Chemosphere 2009, 74, 860–865. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Ziółkowska, A. The importance of relieving substances in restricting the effect of soil contamination with oil derivatives on plants. Fresenius Environ. Bull. 2011, 20, 711–719. [Google Scholar]
- Wyszkowski, M. Effect of contamination with copper and mineral or organic amendments on the content of trace elements in soil. Environ. Prot. Eng. 2017, 43, 165–175. [Google Scholar] [CrossRef]
- Pichtel, J.; Bradway, D. Conventional crops and organic amendments for Pb, Cd and Zn treatment at a severely contaminated site. Bioresour. Technol. 2008, 99, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.; Robinson, C.; Parker, D.B. Examples and case studies of beneficial reuse of beef cattle by-products. In Land Application of Agricultural, Industrial, and Municipal By-Products; Power, J.F., Dick, W.A., Kashmanian, R.M., Sims, J.T., Wright, R.J., Dawson, M.D., David Bezdicek, D., Eds.; Soil Science Society of America Inc.: Madison, WI, USA, 2000; pp. 387–407. [Google Scholar]
- Skwaryło-Bednarz, B.; Brodowska, M.S.; Brodowski, R. Evaluating the influence of varied NPK fertilization on yielding and microelements contents at amaranth (Amaranthus cruentus L.) depending on its cultivar and plant spacing. Acta Sci. Pol. 2011, 10, 245–261. [Google Scholar]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Milani, N.; McLaughlin, M.J.; Stacey, S.P.; Kirby, J.K.; Hettiarachchi, G.M.; Beak, D.G.; Cornelis, G. Dissolution kinetics of macronutrient fertilizers coated with manufactured zinc oxide nanoparticles. J. Agric. Food Chem. 2012, 60, 3991–3998. [Google Scholar] [CrossRef]
- Kosiorek, M.; Wyszkowski, M. Effect of neutralising substances on selected properties of soil contaminated with cobalt. J. Ecol. Eng. 2016, 17, 193–197. [Google Scholar] [CrossRef]
- Kosiorek, M.; Wyszkowski, M. Effect of neutralising substances on reducing influence of cobalt on the content of selected elements in soil. Int. Agroph. 2019, 33, 153–159. [Google Scholar] [CrossRef]
- Ciećko, Z.; Wyszkowski, M.; Krajewski, W.; Zabielska, J. Effect of organic matter and liming on the reduction of cadmium uptake from soil by triticale and spring oilseed rape. Sci. Total Environ. 2001, 281, 37–45. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; Moreno, N.; Alvarez-Ayuso, E.; Garcia-Sanchez, A.; Cama, J.; Ayora, C.; Simon, M. Immobilization of heavy metals in polluted soils by the addition of zeolitic material synthesized from coal fly ash. Chemosphere 2006, 62, 171–180. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Sorgona, A.; Rizzo, M.; Cacco, G. Cadmium adsorption on vermiculite, zeolite and pumice: Batch experimental studies. J. Environ. Manage. 2009, 90, 364–374. [Google Scholar] [CrossRef]
- Deenik, J.L.; McClellan, T.; Uehara, G.; Antal, M.J.; Campbell, S. Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci. Soc. Am. J. 2009, 74, 1259–1270. [Google Scholar] [CrossRef]
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Kolb, S.E.; Fermanich, K.J.; Dornbush, M.E. Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci. Soc. Am. J. 2008, 73, 1173–1181. [Google Scholar] [CrossRef]
- Abdel-Sabour, M.F.; Al-Salama, Y.J. Zinc and cobalt phytoextraction by different plant species. Remediat. J. 2007, 18, 109–119. [Google Scholar] [CrossRef]
- Tappero, R.; Peltier, E.; Gräfe, M.; Heidel, K.; Ginder-Vogel, M.; Livi, K.J.T.; Rivers, M.L.; Marcus, M.A.; Chaney, R.L.; Sparks, D.L. Hyperaccumulator Alyssum murale relies on a different metal storage mechanism for cobalt than for nickel. New Phytol. 2007, 175, 641–654. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).