Abstract
The efficiency and mechanism of orthophosphate—soluble reactive phosphorus (SRP)—inactivation in eutrophic lakes using controlled resuspension and calcite application into the sediment were investigated in this study. Two calcite materials, industrially produced precipitated calcium carbonate (PCC) and natural ground limestone (GCC), were tested in short-term batch experiments and long-term sediment incubations under oxic and anoxic conditions. Maximum SRP adsorption capacity calculated using Langmuir model for PCC (3.11 mg PO43− g−1) was 6 times higher than of GCC (0.43 mg PO43− g−1), reflecting substantial difference in the surface area of calcite materials (12.36 and 1.72 m2 g−1, respectively). PCC applied into the sediment during controlled resuspension reduced SRP release by 95% (oxic) and 78% (anoxic incubation) at medium dose (0.75 kg m−2) and suppressed it completely at high dose (1.5 kg m−2) for at least 3 months, irrespectively of incubation conditions. The maximum achieved reduction of SRP release using GCC was also meaningful: 78% under oxic and 56% under anoxic conditions, but this required very high doses of this material (6 kg m−2). Mechanisms of SRP inactivation by calcites were: (1) adsorption of SRP during application into the resuspended sediment and (2) precipitation of calcium-phosphate compounds (Ca-PO4) during subsequent incubation, which was reflected in a substantial increase in the HCl-P fraction (phosphorus extractable in 0.5 M HCl) in sediments enriched with calcite, irrespectively of oxygen presence. However, anoxia strongly promoted the formation of this fraction: the rise of HCl-P was 2–6 times higher in anoxic than in oxic conditions, depending on the dose and form of calcite applied. The results showed that SRP inactivation using the controlled resuspension method is only successful if highly efficient reactive materials are used, due to large amount of SRP being released from sediment during resuspension. Thus, calcite materials exhibiting high adsorption capacity should be used in this lakes’ restoration technology to ensure fast and sufficient SRP inactivation. The rise in the HCl-P fraction in sediment suggests SRP inactivation through precipitation of relatively stable Ca-PO4 minerals, which makes calcite a suitable agent for sustainable, long term SRP inactivation. As anoxic conditions promoted formation of these compounds, calcite seems to be a promising SRP inactivation agent in highly reductive sediments.
1. Introduction
Chemical inactivation of phosphorus (P) has been applied worldwide as restoration method in eutrophic lakes to reduce concentration of orthophosphate—soluble reactive phosphorus (SRP)—in the lake water [1]. SRP inactivation agents, mainly coagulants in form alum and iron salts [2,3,4,5,6] are applied to the water column where they form flocs removing dissolved SRP from the lake water and reduce its release from the sediment after they had settled on the lake bottom. High short- and long-term efficiency of this treatment in improving P retention in the bottom sediments was reported in numerous studies [7,8,9]. The flocs layer formed on the sediment surface may, however, be easily disturbed during resuspension events, which strongly limits its efficiency [10,11,12] and results in relocation of the flocs outside the target area [13,14]. The use of iron and alum salts has also been broadly discussed especially in relation to toxic effects on aquatic biota [15,16,17]. That is why calcite, the most stable polymorph of calcium carbonate, naturally occurring in many aquatic ecosystems, has attracted significant attention as potential agent for P inactivation in lakes. Calcite naturally precipitates in surface waters mainly during spring and summer because of oversaturation in the productive zone caused by CO2 assimilation during photosynthesis leading to a pH increase [18,19,20]. As orthophosphate is co-precipitated with newly forming calcite crystals and is removed from the water column during their settling, this process is an important self-purification mechanism [19,21,22,23,24]. To mimic this phenomenon, lake marl—calcite rich sediment deposits—was excavated in two German lakes and distributed evenly on the lakes’ surface to immobilize P [25,26]. However, subsequent comparative studies have shown that pure calcite is much more efficient than the lake marl [27].
Other available calcite materials that can be used for this technique (as SRP inactivation agents in lakes) are powdered limestones, naturally occurring carbonate rocks processed by grinding, and calcite minerals resulting from a controlled precipitation (industrially produced). The calcite materials applied show a high variability of surface properties, grain size and anion sorption capacity [28,29,30,31,32,33,34,35,36,37,38]. Different studies using powdered calcites clearly showed that most of the synthetic precipitates are more efficient in SRP binding than ground limestones, due to usually higher specific surface area, but higher cost may limit their large-scale application [35,39,40].
Efficiency and stability of SRP binding onto calcite is also dependent on the sorption mechanism [30,41]. The type of binding mechanism is mainly controlled by the initial saturation of the solution with respect to calcite. In the case of undersaturation, heterogeneous precipitation of calcium-phosphates (Ca-PO4) most likely takes place due to the at least partial dissolution of applied calcite material resulting in an increase in Ca2+ concentration [34,39,42,43]. In calcite oversaturated systems different processes are possible. At low SRP concentration, co-precipitation of phosphate takes place, leading to incorporation of part of the previously adsorbed phosphate ions into newly growing calcite crystals [44,45]. At high SRP concentration calcite growth becomes inhibited, but Ca-PO4 formation maintains SRP removal from the solution [45,46]. If the system is in equilibrium with calcite, the prevailing mechanism is the adsorption at low SRP concentrations [38,41], but with increasing initial SRP concentration and pH, precipitation of Ca-PO4 contributes more and more to SRP removal from the solution [38]. Adsorption may also take place in a wide range of saturation levels depending on surface properties of the calcite material, initial SRP concentration and the dose of calcite applied [34,39,46]. As adsorption is highly reversible [30,47], (co)precipitation processes are the more sustainable and thus, more favorable removal mechanism in the aquatic environment. Since Ca-PO4 phases are less soluble than calcite and stable in a wide range of environmental conditions [48,49], SRP bound in Ca-PO4 remains permanently fixed [30,50]. Numerous laboratory and small scale on-site experiments have shown a high efficiency of calcite, applied in form of an intact barrier, in preventing SRP release from sediments [35,39,51,52,53,54]. However, the long-term stability of calcite barriers in shallow lakes was strongly limited due to in-lake processes such as bioturbation and wind induced resuspension, which mechanically destabilized the barrier [32,39,53].
The aim of this study was to test, characterize and optimize calcite application into the lake using the controlled resuspension method originally proposed by Ripl [55] for oxidant injection into the sediment and further successfully developed by Wiśniewski [56] and Wiśniewski et al. [12] for coagulant application. The principle of this method is the addition of the P inactivation agent, in our case calcite, into (and not onto) the disturbed surficial sediment layer during artificially induced resuspension caused by controlled air injection. This method of agent application has been reported to better secure longevity of P inactivation and has been recommended for common P removing agents, but not yet for calcite, especially for shallow lakes, characterized by highly hydrated organic sediment [12,56]. Schütz et al. [57], who fluidized the sediment using lake water delivered under pressure, have recently used a similar approach for application of alum salt into the sediment.
In our study, we determined the efficacy, stability and mechanisms of SRP inactivation in lake sediments using calcite materials and the controlled resuspension method as proposed by Wiśniewski [56]. This was the first time calcite was applied to the sediment using the controlled resuspension. Two calcite materials were tested as potential SRP inactivation agents: an industrially produced precipitate and powdered limestone, differing in grain size, surface area, availability and cost. Short-term experiments in the pore water were conducted to evaluate sorption efficiency of calcites and identify sorption mechanism applying actual pore water chemistry. Incubation experiments in sediments were performed to (1) determine the efficiency of calcites in reducing internal SRP release, (2) assess the long-term stability and (3) identify mechanisms of SRP inactivation over a wide range of calcites doses. Oxic and anoxic incubations were employed to simulate changing redox conditions during stagnation and circulation periods in lakes, and check their effect on the calcite performance.
2. Materials and Methods
2.1. Materials
Two different calcites were used to investigate the effect of calcite properties on SRP removal efficacy and mechanisms: precipitated calcium carbonate (PCC) and ground calcium carbonate (GCC). PCC is synthetically produced, whereas GCC was obtained from deposits of Devonian limestone (Sancy, France) and contains trace amounts of Fe, Al, Mg and Si (Table S1 in Supplementary material). XRD analysis confirmed that both materials are dominated by calcite (Figure 1). PCC is very fine material with grain characteristic diameter d50 = 0.08 µm, whereas GCC is much larger in grain size with d50 = 11.04 µm (Figure 1). This is reflected in substantial difference in their specific surface area measured with the BET method (SSABET), which is 12.36 (PCC) and 1.72 m2 g−1 (GCC), respectively. Methodological information on measurements of particle size and SSABET as well as on determination of phase-mineral composition is provided in the Section 2.3. The given PCC was selected for the study due to its relatively high SSABET at relatively low cost (12.36 m2 g−1, ~500 EUR t−1, respectively) as compared to other potentially available PCCs (4.2–70 m2 g−1 and 450–2250 EUR t−1 [35,40,58,59,60]). Both materials were provided by the Rheinkalk Company (Rheinkalk Eifel Sauerland GmbH and Co. KG. Branch Akdolit, Lhoist Group, Pelm, Germany).
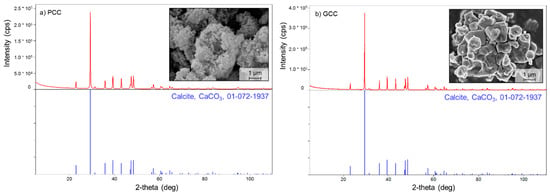
Figure 1.
XRD patterns and SEM pictures of calcite materials: (a) precipitated calcite (PCC) and (b) ground calcite (GCC).
Lake water and sediments of Lake Zdworskie, located in central Poland, were used in this study. The lake is shallow and polymictic, with surface area of 343 ha, mean depth of 2.12 m and maximum depth of 4.55 m [61]. According to monitoring data collected by the Environmental Protection Directorate (Warsaw, Poland) and Directorate of Amelioration and Water Structures (Płock, Poland), Lake Zdworskie is classified as eutrophic to hypertrophic [62]. The surficial profundal sediments are characterized by a high content of water (73–96%) and organic matter (47–56% DW), causing semi-fluid structure and low consolidation of the bottom deposits [62,63]. The surficial, 15-cm thick layer of sediments was sampled in September and October for batch and incubation experiments, respectively, from the mean depth area of the lake, using an Ekman grab. The sediments were characterized by a grey-brown color. Sediments were immediately transported into the lab and further processed, as described in Section 2.2.
2.2. Experimental Design
2.2.1. Batch Experiments
Batch experiments on SRP removal by calcite materials were performed using pore water of the Lake Zdworskie to assess sorption efficiency and identify mechanism of SRP sorption under actual pore water chemistry. This is very important as composition of the solution strongly affects sorption processes [38,41,44,64]. The pore water was separated from the solid phase of the sediment by centrifugation at 5000 rpm for 20 min. Prior to the main experiment, the pore water was filtered through cellulose filter paper (≤40 µm) to remove the majority of sediment particles. The SRP concentration in the pore water was 3.8 mg PO43− L−1 and was slightly spiked with KH2PO4 to reach a SRP content of appr. 4 mg PO43− L−1, which was the mean value achieved in the overlying lake water during trial resuspension experiments with the Lake Zdworskie sediments conducted to design this study. The pore water used for the batch experiments was slightly oversaturated with calcite (Table 1), which corresponds to conditions prevailing over the year in the mean depth area of the Lake Zdworskie.

Table 1.
Selected physio-chemical parameters and the pH in interstitial water before addition of calcite materials; PCC—batch experiment with precipitated calcite, GCC—batch experiment with ground calcite; SICC—saturation index for calcite.
The experiments were performed in centrifuge tubes on a temperature controlled shaker at room temperature (21 ± 1°C) with shaking speed of 150 rpm for 24 h. PCC and GCC were added to 50 mL of the pore water in 18 different doses ranging from 0 to 40 g L−1. After shaking, the suspension was centrifuged at 4000 rpm for 20 min and filtered (polytetrafluoroethylene syringe filters, 0.45 µm, were used for calcium ions; mixed cellulose membrane filters, 0.45 µm, were used for other analysis) for subsequent measurements of pH, SRP, Ca2+ concentration and alkalinity, which was assumed to approximately equal HCO3− concentration. Methodological information on these measurements is given in Section 2.4. Three of the calcite doses were tested in duplicates to check reproducibility of the results. For all the measured parameters (SRP and Ca2+ concentration, pH and alkalinity) differences between duplicates did not exceed 5%.
2.2.2. Incubation Experiment
Sediments used in the experiment were characterized by high organic matter content (58% DW), with total P concentration of 1150 mg kg−1 DW and total calcium concentration of 35 g kg−1 DW. The lake water was in equilibrium with respect to calcite, whereas the pore water was slightly oversaturated due to a high Ca2+ and HCO3− concentration. The pore water was also characterized by a high SRP concentration and negative redox potential (Table 2). For information on measurements of sediment properties and physio-chemical parameters of the pore water see Section 2.4.

Table 2.
Selected physio-chemical parameters and the pH value of interstitial and lake water used in the incubation experiments; SICC—saturation index for calcite; ORP—redox potential.
Fresh sediments were carefully mixed to homogenize initial conditions in all treatments and placed in 16 plastic chambers (13 × 15 × 35 cm). The chambers were then carefully filled with the lake water, previously filtered through filter paper (≤40 µm). In each microcosm (chamber), sediment to overlying water ratio was 2.1:1, which corresponds to a real sediment and water layer thickness of 17 and 8 cm, respectively. All chambers were placed in a dark, temperature controlled room (10 ± 1°C) for 2 weeks of acclimatization. Microcosms used for subsequent anoxic incubations (8 chambers) were kept closed to avoid additional aeration due to contact with air prior to actual incubation. After acclimatization and first settling of the sediment, resuspension with gas injection was initiated. This procedure simulated the controlled resuspension method tested by Wiśniewski [56]. To investigate calcite performance under oxic and anoxic conditions, resuspension was initiated by injection of air and gaseous N2, respectively. The force of the resuspension was controlled by constant gas pressure. In total, the time of injection was 5 min. During resuspension of the sediment, the individual dosages of calcite powders ranging from 0.25 to 1.5 kg m−2 for PCC and from 1.5 to 6.0 kg m−2 for GCC were added to chambers. Calcite treatments were denoted as PCC1–PCC3 and GCC1–GCC3 (numbers indicate increasing doses of added material; Table 3). The high dose of PCC (PCC3) and the low dose of GCC (GCC1) were the same to directly compare performance of both calcite materials. Remaining doses of GCC were much higher than of PCC as batch tests revealed distinctly lower efficiency of GCC in SRP binding. In each experimental set (oxic/anoxic), one chamber was subjected to resuspension only (no calcite addition; denoted by RES) and one chamber remained untreated and served as control (no resuspension, no calcite addition; CONTROL) (Table 3). Results from the chambers with resuspended sediments without calcite addition (RES) were used to assess changes in SRP mobility at the sediment-water interface and to identify possible changes in sediment properties caused by calcites. Not resuspended sediments without any additions (CONTROL) served as control to evaluate the overall effect of the combined resuspension and calcite application, as a potential lake restoration technique. Following resuspension and application of calcite, sediments were incubated for 105 days in a dark, temperature controlled room (10 ± 1°C) under oxic and anoxic conditions maintained in the overlying water. Aerobic chambers were kept opened, while anaerobic ones were stored closed. N2 was systematically supplied as small bubbles into anoxic chambers to assure anoxic conditions without disturbance of the sediment-water interface.

Table 3.
Sediment treatments denotations; PCC—precipitated calcite, GCC—ground calcite.
For chemical analysis water samples (6 mL) were taken during incubation from the overlying water every 3–8 days (except for the period between days 69 and 97, when sampling took place each 2 weeks) using a syringe introduced through holes in the wall of the microcosms at a depth of 1cm above sediment surface and afterwards replaced with an aliquot of the lake water. After incubation, the samples from sediments were collected for subsequent analysis (3 sub-samples from each chamber) using a miniaturized Kajak sampler. 3 g of each sub-sample (fresh sediment) was then stored frozen for P-fraction analysis. The pore water was extracted from remaining fresh sediment samples by centrifugation at 5000 rpm for 20 min and filtered through syringe filters as described in Section 2.2.1 for subsequent determination of pH, SRP, Ca2+ and alkalinity. Details on the methods used to characterize lake water, pore water and sediments properties are provided in Section 2.4.
2.3. Characterization of Calcite Materials
Specific surface area (SSABET) and grain size of calcites were measured for 3 subsamples of each material with the N2 adsorption method using the Autoflow BET+ Analyzer (Quantachrome, USA) and with laser diffraction method using Mastersizer 3000 Analyzer after ultrasound treatment (Malvern Panalytical, Malvern, UK), respectively. Phase composition of PCC and GCC was determined using SmartLab X-Ray diffractometer (Rigaku, Tokyo, Japan).
2.4. Chemical Analysis
SRP concentration in the lake and pore water was measured using the molybdenum blue method at the wavelength of 880 nm [65] with a Helios Alpha spectrophotometer (Unicam, Cambridge, England). Ca2+ concentration in the lake and pore water was determined by ion chromatography (DIONEX 1000, Sunnyvale, CA, USA) and bicarbonate alkalinity by direct titration of the water samples with 0.1 M HCl (Merck No. 11109, Darmstadt, Germany). pH and redox potential were measured potentiometrically using standard electrodes (WTW – Xylem Analytics Germany Sales, Weilheim in Oberbayern, Germany and Mettler Toledo, Greifensee, Switzerland, respectively). Dissolved oxygen was measured using Intellical LDO101 Luminescent/Optical sensor (Hach Lange, Düsseldorf, Germany).
Water and organic matter content in the sediment were determined gravimetrically as the mass loss after drying at 105 °C (to a constant weight) and at 550 °C for 24 h, respectively [66]. Calcium content in sediment was measured by flame atomic absorption spectroscopy (AAS, PGI990, PG Instruments Ltd., Lutterworth, UK) with lanthanum chloride as modifier to avoid interferences with phosphate, at the wavelength of 422.7 nm [67], after wet microwave digestion in HNO3 at 175 °C [68]. Total phosphorus in sediments was determined after mineralization of sediments with 4% K2S2O8 at 120 °C for 2 h [69]. Phosphorus fractions in sediments were determined using a modified Psenner method [70]. The considered pools and their extraction methods were: a) NH4Cl-P—loosely bound P (dissolved and adsorbed), extracted by double shaking (2 × 30 min) with 1 M NH4Cl bubbled with N2 (1 h) prior to extraction to remove O2; b) BD-P—redox sensitive P bound to Fe(III) and Mn(IV) (hydr)oxides, extracted by sediment heating at 40 °C for 30 min after adding a mixture (1:1) of 0.11 M NaHCO3 and 0.11 M Na2S2O4 and two shaking steps (60 min, 5 min); c) NaOH-P—P bound to Al- and Fe-oxides P and d) NR-P—organic matter (non-reactive) P, both extracted by two shaking steps (16 h, 5 min) with 1.0 M NaOH; e) HCl-P—P bound with Ca compounds, extracted by two shaking steps (16 h, 5 min) with 0.5 M HCl; f) RES-P—the residual P determined after mineralization of sediments with 4% K2S2O8 at 120 °C for 2 h [69]. In all the extracts, SRP was measured using molybdenum blue method [65] after filtration through cellulose acetate filters (0.45 μm) with the following additional pre-treatments: aeration of BD-P extracts for 1h and neutralization of NaOH-P, HCl-P, NR-P and Res-P extracts. In NaOH extracts, beside SRP, total P was determined and SRP was considered as NaOH-P, whereas remaining P—as NR-P. Total P in these extracts was determined after total mineralization of all P bearing compounds applying 4% K2S2O8 at 120 °C for 2 h [69]. The subsequent SRP measurement was done as previously described. For each extractant SRP was determined based on a separate calibration curve. As recommended by Hupfer et al. [71] for calcite rich sediments, we employed enhanced volume-to-solid ratio (50:1) in the fractionation procedure, which is at least 2 times higher as originally proposed (12.5:1–25:1; [70]). This helps to avoid shifts from mobile fractions to the HCl-P pool. Due to addition of calcite, sediments were “diluted” with calcium carbonate and thus, P content expressed per unit mass of sediments enriched with PCC and GCC decreased, depending on the dose of material added. Thus, to assure comparability of the results between different treatments (sediments without calcite addition and sediments treated with different doses of PCC and GCC), P fractions were recalculated, taking into account the dilution factor resulting from calcite enrichment. The dilution factor was determined based on the change in total calcium content in the sediment which served as proxy for content of calcite materials.
2.5. Data Analysis
Loads of SRP fixed by unit mass of calcite were calculated based on difference between initial and equilibrium SRP concentration according to the equation:
where q (mg PO43− g−1) is the amount of SRP adsorbed per unit mass of calcite, and Co and Ci are the initial and equilibrium SRP concentration (mg PO43− L−1), respectively, V is solution volume (L) and m is mass of calcite (g).
In order to quantitatively describe the adsorption of SRP onto calcite, the Langmuir monolayer adsorption model was used. The linear form of the Langmuir isotherm model is given by the following equation [72]:
where q′ (mg PO43− g−1) is the amount of SRP adsorbed per unit mass of calcite at equilibrium, qmax is the maximum adsorption (mg PO43− g−1), k is adsorption energy coefficient (L mg−1) and ci is the SRP concentration at equilibrium (mg PO43− L−1).
The flux of SRP through the sediment/water interface was calculated using the following equation:
where J is the flux (mg PO43− m−2 d−1), Ci+1 and Ci are SRP concentrations in the overlying water (mg PO43− L−1) at time ti+1 and ti, respectively (d); V is the volume of the overlying water (L); A is the surface area of the sediment (m2) and d is day.
The saturation indices (SI) were calculated using the PHREEQC software for calcite and the Ca-PO4 minerals. The stoichiometry of Ca-PO4 compounds and solubility products used in the calculations are presented in Table 4. At SI = 0, the solution is in equilibrium with respect to the solid phase, whereas SI > 0 indicates oversaturation and SI < 0 indicates undersaturation. However, due to analytical and thermodynamic uncertainties, equilibrium conditions are assumed at SI values −0.5 ≥ SI ≤ +0.5 [73,74,75].

Table 4.
Stoichiometry and solubility of Ca-PO4 compounds and calcite.
3. Results
3.1. Batch Experiments
3.1.1. Sorption Capacity
After 24 of shaking, SRP concentration in the pore water without calcite slightly dropped (from 4.10 mg PO43− L−1 (Table 1) to 3.91–3.94 mg PO43− L−1 (in the experimental series for PCC and GCC, respectively; Figure 2) suggesting autogenous sorption of SRP. In samples treated with calcite, the concentration of SRP in the pore water generally decreased as the dose of calcite increased (Figure 2). PCC eliminated more than 90% of SRP after addition of 2.2 g L−1, whereas higher doses resulted in almost complete SRP removal (98–99% with minimum final SRP concentration of 0.008 mg PO43− L−1). As compared to PCC, removal of about 90% by GCC required a 18 times higher dosage (40 g L−1). Differences in sorption potential of both calcites were also expressed in SRP loads recorded in the experiments: maximum load of PCC (4.21 mg PO43− g−1) was almost 4 times higher than obtained for GCC (1.15 mg PO43− g−1) (Figure 3).
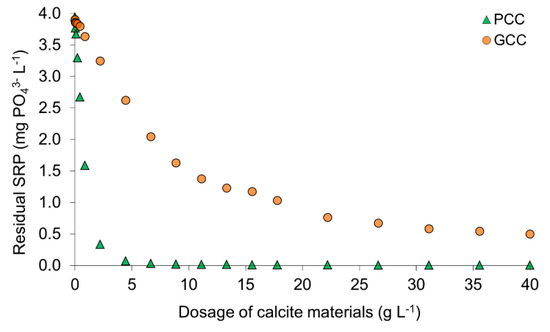
Figure 2.
SRP removal from interstitial water at initial SRP concentration of 4.1 mg PO43− L−1 after equilibration for 24 h with PCC (precipitated calcite) and GCC (ground calcite).
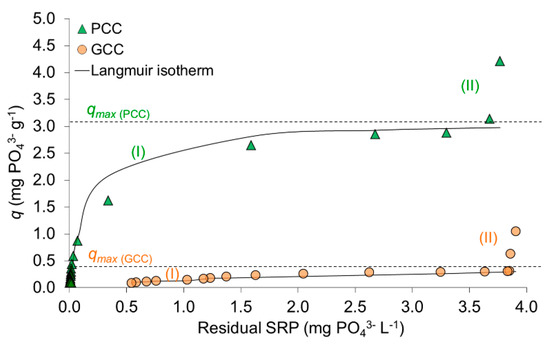
Figure 3.
Sorption isotherms of precipitated (PCC) and ground (GCC) calcite at initial SRP concentration of 4.1 mg PO43− L−1 after equilibration time of 24h (qmax—maximum adsorption calculated using the Langmuir model). Numbers (I) and (II) indicate different sections of the isotherms.
3.1.2. Sorption Isotherms
Isotherms for both calcites are characterized by similar shape and indicate two ways of SRP interactions with calcite (Figure 3). Within section (I), showing a logarithmic shape, experimental data could be satisfactorily fitted with the Langmuir model (R2 = 0.996 for PCC and R2 = 0.945 for GCC) with a theoretical maximum adsorption load of qmax = 3.11 mg PO43− g−1 for PCC and qmax = 0.43 mg PO43− g−1 for GCC (Figure 3). The obtained maximum loads of SRP in section (I) nearly reach the plateau of the theoretical maximum adsorption calculated using the Langmuir model. After the maximum adsorption load is exceeded, isotherms change into an exponential form (section (II) in Figure 3), characterized with much higher and strongly increasing SRP loads. In both calcite series, the inflection of the isotherm takes place at a similar remaining SRP concentration of 3.68 mg PO43− L−1 (PCC) and 3.83 mg PO43− L−1 (GCC), which corresponds to calcite additions of 0.10 and 0.22 g L−1, respectively.
3.1.3. Saturation versus Ca-PO4 Compounds
Irrespectively of the dose of added calcite materials and residual SRP concentration, interstitial water after batch experiment was in equilibrium (GCC) or slightly oversaturated with calcite (PCC), oversaturated with respect to SC and HAp and undersaturated with respect to DCPD and DCPA (both calcite materials, Figure 4a,b). After addition of GCC, interstitial water reached oversaturation with respect to ACP and OCP at residual SRP concentration lying in the region of the isotherms inflection; similar change for OCP was observed in case of PCC at slightly lower residual SRP (Figure 4a,b). Concentration of Ca2+, alkalinity and pH values in the pore water, used for the calculation of SI for calcite and Ca-PO4 minerals, are presented in the Figure S1.
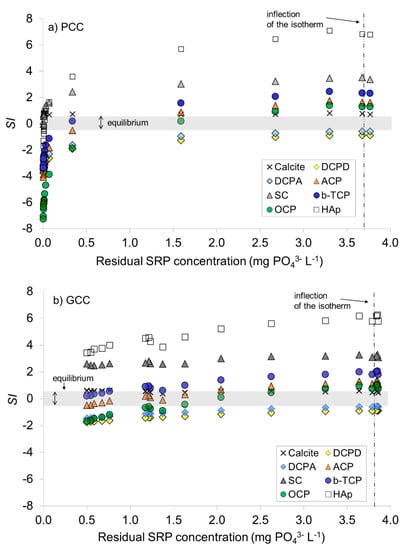
Figure 4.
Saturation index (SI) for calcite and Ca-PO4 compounds in the pore water after equilibration for 24 h with (a) PCC—precipitated calcite, (b) GCC—ground calcite at an initial SRP concentration of 4.1 mg PO43− L−1. Dashed line indicates residual SRP concentration at the inflection of the isotherm in the Figure 3. For abbreviations of Ca-PO4 compounds see Table 4.
3.2. Incubation Experiments
3.2.1. SRP in the Overlying Water and SRP Fluxes at the Sediment-Water Interface
Initial SRP concentration of 0.47–0.52 mg PO43− L−1 (oxic) and 0.64–0.75 mg PO43− L−1 (anoxic) in the overlying water (Figure 5a,b) was substantially higher after the acclimatization period of 2 weeks as compared to the fresh lake water (0.02 mg PO43− L−1, Table 2), showing, that SRP was released from sediment during preparation of the experiment and subsequent acclimatization, and that the release was higher in microcosms acclimatized closed. During the actual incubation, the control sediment still continuously released SRP during the entire incubation period in oxic and anoxic conditions (Figure 5a,b). Flux of SRP into the water column in the control sediments, calculated for the entire incubation period, was 6 times higher under anoxia (2.30 mg PO43− m−2 d−1) than under oxygen presence (0.37 mg PO43− m−2 d−1) (Figure 6c). Resuspended sediments without calcite addition released large amounts of SRP causing a sharp increase in SRP concentration in the overlying water within 7 days: from 0.55 to 2.08 mg PO43− L−1 and from 0.75 mg PO43− L−1 to 2.88 mg PO43− L−1 in oxic and anoxic incubations, respectively (Figure 5a,b).
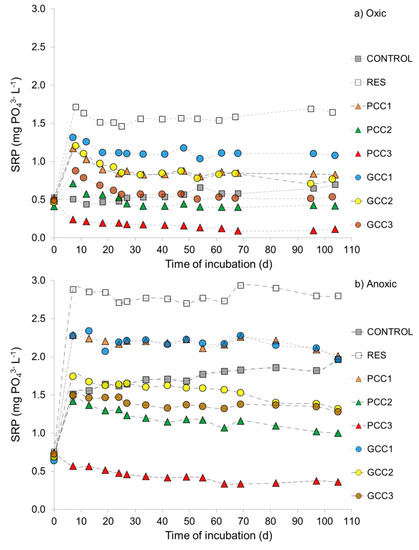
Figure 5.
Concentration of SRP in the overlying water of (a) oxic and (b) anoxic microcosms subjected to resuspension only (RES), resuspension and SRP inactivation using calcite materials (PCC1–PCC3 and GCC1–GCC3) and control (CONTROL)—microcosms not subjected to resuspension and SRP fixation. Dashed lines were used for better visibility of the trend. Zero on Y axis refers to pre-treatment conditions; resuspension and SRP inactivation were conducted on the 1st day of the experiment. For treatment abbreviations see Table 3.
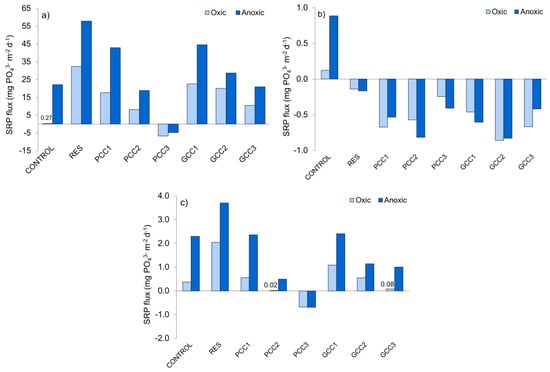
Figure 6.
Average SRP flux at the sediment water interface: (a) during resuspension (calculated based on difference between initial (day 0) and post-resuspension (day 7) SRP concentration); (b) during incubation following resuspension (calculated based on difference between post-resuspension (day 7) and final (day 105) SRP concentration); (c) during the entire experiment (calculated based on difference between initial (day 0) and final (day 105) SRP concentration). For treatment abbreviations see Table 3.
In microcosms subjected to resuspension with calcite addition, SRP concentration in the overlying water increased too, except for PCC3 treatments, but was substantially lower, as compared to resuspended sediments without calcite, and decreased with increasing dosage in case of both calcite materials. As compared to resuspended sediments without calcite, SRP concentration in the overlying water was reduced by 32–86% (oxic PCC1–PCC3), 23–49% (oxic GCC1–GCC3), 21–80% (anoxic PCC1–PCC3) and 21–48% (anoxic GCC1–GCC3) (Figure 5a,b). This is reflected in SRP fluxes at the sediment water-interface which decreased when the dose of PCC and GCC increased (Figure 6a). However, except for the medium dose of PCC (PCC2) and the high dose of GCC (GCC3) under oxic conditions as well as the high dosage of PCC (PCC3) in oxic and anoxic incubations, SRP concentration in the overlying water remained much higher (1.7–3.2 times) than before resuspension in all calcite treatments and exceeded SRP concentration observed at the same time in the control microcosms (Figure 5a,b). Consequently, the calculated SRP fluxes were, in most treatments, higher as compared to control sediments (Figure 6a). Negative SRP flux after PCC3 amendment (Figure 6a) results from the fact that SRP concentration in the overlying water was lower after resuspension and PCC addition, as compared to initial conditions and shows, that this treatment secured efficient SRP inactivation. When applied in the same dosage as GCC, PCC was about 5 times more effective in SRP removal during resuspension, which demonstrates again the much better short-term sorption properties of PCC (Figure 5a,b and Figure 6a).
Unit loads of SRP fixed by calcites during resuspension were 0.05–0.11 mg PO43− g−1 and 0.08–0.12 mg PO43− g−1 for PCC1–PCC3 in oxic and anoxic incubations, respectively (calculated based on difference in the SRP concentration in the overlying water before and after resuspension). Corresponding loads obtained for GCC1–GCC3 were at least 5 times lower (the recorded range was 0.01–0.02 mg PO43− g−1 in oxic and anoxic incubations). These values reflect differences in sorption capacity of PCC and GCC and show that incubation conditions (oxic/anoxic) did not noticeably affect removal efficiency during calcite application to disturbed sediments.
During the post-resuspension phase of incubation (>7 days of incubation), further changes in the overlying water were observed. In microcosms subjected to resuspension only, SRP did not show a constant tendency (Figure 5a,b), but negative fluxes calculated for this incubation phase (−0.14 and −0.16 mg PO43− m−2 d−1 in oxic and anoxic incubation, respectively; Figure 6b) demonstrate a re-transfer of small amounts of SRP into the sediment. In the microcosms treated with calcite, irrespectively of incubation conditions, SRP concentration showed a decreasing trend with time (Figure 5a,b). In the microcosms treated with low doses of calcites (PCC1 and GCC1) some short term increase in SRP took place. This corresponds to similar changes observed in the resuspended microcosms without calcite. On the other hand, in the microcosms treated with medium and high doses of calcite (PCC2–PCC3, GCC2–GCC3), SRP concentration almost continuously decreased (Figure 5a,b), which shows a high stability of SRP sorption in the sediments and its further binding for about 3 months after treatment. Negative SRP fluxes calculated for the post-resuspension period (Figure 6b), which were about 2–6 times higher than in resuspended sediments without calcite, show that all calcite dosages resulted in enhanced SRP transfer from the overlying water into the sediment during the 98 days of incubation. In sediments enriched with PCC, the SRP flux into the sediment decreased with increasing calcite dosage in oxic conditions. In anoxic PCC treatments as well as in oxic and anoxic GCC treatments, the SRP flux increased with increasing dosages only for the low and medium additions of calcite materials (PCC1-PCC2 and GCC1-GCC2), whereas high additions (PCC3, GCC3) resulted in the lowest SRP flux (Figure 6b). At the same time, no clear effect of incubation conditions can be observed: for example in GCC3 treatments the oxic flux was 1.6 times higher than the anoxic one, whereas in PCC3—the difference was the same (1.6 times) but opposite (Figure 6b).
The overall effect of sediment treatment with calcite applied during resuspension was assessed based on SRP fluxes through the sediment-water interface calculated for the entire incubation period (Figure 6c). SRP release was the lower, the higher was the addition of calcite materials (Figure 6c). As compared to control sediments, SRP flux was reduced when using medium and high dosages of calcites, with the following reduction rates: 51% in anoxic GCC2 treatment (no reduction in oxic conditions), 78% and 56% in oxic and anoxic GCC3 treatments and 96% and 78% in respective PCC2 treatments. PCC3 treatments in oxic and anoxic conditions completely inhibited SRP release and caused SRP transport from the overlying water into the sediments, which is reflected in negative SRP fluxes (Figure 6c).
It should be stressed, that higher reduction of SRP flux in oxic incubations results from lower fluxes observed in control experiments and not from a promoting effect of oxic conditions on SRP inactivation by calcites.
3.2.2. Physiochemical Conditions in the Interstitial Water
SRP concentrations in the pore water differed depending on incubation conditions and treatment method (Figure 7a). Under oxic conditions, resuspension without calcite addition caused an increase in pore water SRP (as compared to control sediments), whereas under anoxia SRP concentrations were slightly lowered. In calcite treated sediments, pore water concentrations of SRP generally declined with increasing dosages of calcite and, except for GCC1 under anoxia, were substantially lower than in resuspended sediments not subjected to calcite enrichments. The reduction rates for PCC were 75–92% (oxic PCC1-PCC3) and 69–94% (anoxic PCC1-PCC3), whereas for GCC they were lower and accounted for 34–75% (oxic GCC1-GCC3) and 45–68% (anoxic GCC2–GCC3 treatments). This shows that PCC, when applied in the same dosage, was almost 3 times more efficient in reducing SRP concentrations than GCC.
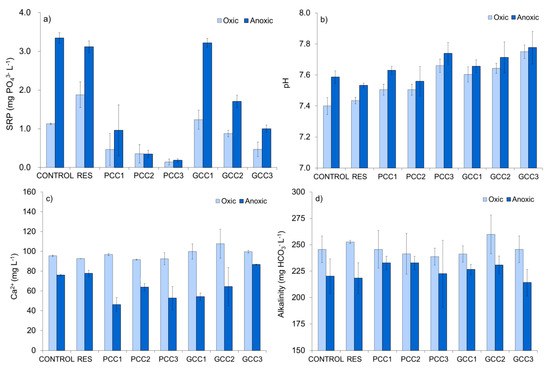
Figure 7.
Physio-chemical conditions in the interstitial water after incubation: (a) SRP concentration, (b) pH, (c) calcium concentration, (d) alkalinity. For treatment abbreviations see Table 3. The values are mean (± standard deviation) of three sediment subsamples collected from each microcosm.
Pore water pH was neutral to slightly alkaline (7.3–7.9) in all experiments (Figure 7b). However, pH values in oxic microcosms were clearly lower than in anoxic ones. In resuspended sediment under oxic and anoxic conditions, the pH remained similar as compared to control chambers (the mean recorded differences were +0.04 and −0.06). In chambers subjected to resuspension combined with most of the calcite treatments (except for PCC2 under anoxia) pH was slightly higher as compared to control sediments and resuspended sediments without calcite (Figure 7b). The mean pH values increased by 0.2–0.4 in oxic conditions and by 0.1–0.2 under anoxia, as compared to resuspended microcosms without calcite, indicating that calcite has been partly dissolved in the sediments and the dissolution rate was slightly higher in oxic treatments. In the presence of GCC, pH tended to increase with increasing calcite dosage (from 7.6 in GCC1 to 7.8 in GCC3, in oxic and anoxic conditions, Figure 7b). No clear trend was recorded in PCC amended sediments, although the highest pH was observed after addition of high PCC dosage (PCC3) (Figure 7b). Irrespectively of oxygen presence, pore water pH in PCC3 treatments was slightly higher than in corresponding GCC1 treatments (Figure 7b), which suggests higher dissolution rate in case of PCC.
The pore water concentration of Ca2+ varied depending on incubation conditions and treatment method (Figure 7c). In all chambers, the mean Ca2+ content was substantially lower under anoxia than in corresponding oxic treatments. For example in PCC2, the mean Ca2+ concentration was 92 and 64 mg L−1 in oxic and anoxic sediments, respectively. Moreover, under anoxia, mean Ca2+ concentration decreased in calcite treated sediments (except for GCC3) as compared to resuspended and control microcosms (Figure 7c). The mean decrease of Ca2+ concentration observed in anoxic calcite treatments was 12–30 mg L−1, which accounts for 16–39%, when compared to resuspended sediment without any additions. In contrast, in oxic chambers, Ca2+ concentration increased or remained similar (Figure 7c). This demonstrates that both, anoxia as well as calcite addition, caused a decline of Ca2+ concentration in the pore water. There was no clear dependency of Ca2+ concentration in the pore water and calcites dosage, except for GCC under anoxia, where mean Ca2+ increased with increasing amount of added calcite. PCC and GCC applied in the same dosage did not differ in terms of their effect on Ca2+ concentration.
Alkalinity in the pore water was affected by incubation conditions and was lower under anoxia than in oxic conditions (Figure 7d). The noted difference ranged from 9 to 33 mg L−1 and accounted for 5–13%. Alkalinity did not change due to sole resuspension (recorded increase in anoxia was 3% and can be neglected). Changes due to addition of calcites were inconsiderable. In anoxia, except for GCC3, mean alkalinity increased by 5–15 mg L−1 (2–7%), as compared to resuspended sediments, whereas in oxic conditions it decreased, except for GCC2, by 7–14 mg L−1 (3–6%) (Figure 7d). Alkalinity did not show an obvious trend with regard to amount of calcite added, except for PCC amendments in oxic conditions, where it declined with decreasing PCC dosage (Figure 7d).
3.2.3. Saturation versus Calcite and Ca-PO4 Compounds
The pore water of the sediment was in equilibrium state with respect to calcite (Table 5). However, under anoxia, the saturation with respect to calcite was lower due to lower calcium concentration and alkalinity (Figure 7c,d). The saturation index for calcite increased with increasing dosages of calcites, indicating some dissolution of calcite materials, which affected the solution pH (Figure 7b).

Table 5.
Saturation indices for calcite (SICC) and Ca-PO4 compounds in the interstitial water after incubation of sediments with PCC and GCC. The values represent range (min–max) calculated for three sediment subsamples collected from each microcosm (one value indicates cases where SI was the same in all of the subsamples). For treatment abbreviations see Table 3. For abbreviations of Ca-PO4 compounds see Table 4.
The saturation index for calcium phosphates was generally higher in anoxic than in oxic conditions (Table 5) due to higher pore water SRP concentration under anoxia (Figure 7a). The exception was saturation versus surface complex (SC) in calcite treated sediments (Table 5) due to joined effect of lower Ca2+ content and lower alkalinity in anoxic treatments as compared to the oxic ones (Figure 7c,d). In calcite treated sediments saturation indices show oversaturation with respect to SC (all treatments), ß-TCP (GCC1 under anoxia), HAp (PCC1 and PCC2 in oxic conditions together with PCC1 in anoxic conditions and all GCC treatments) and generally decreased with increasing dosages of PCC and GCC due to decline in SRP concentration (Figure 7a).
3.2.4. Phosphorus Fractions in Sediment
NH4Cl-P fraction (Figure 8a), considered here as pore water SRP and adsorbed SRP, accounted for 20% and 18% (oxic and anoxic incubation, respectively; mean values) of the total P pool in control sediments, showing a large potential of P release. Under anoxia, a slight increase in NH4Cl-P was recorded in sediments treated with resuspension alone (by 25 mg g−1 DW, which accounts for 15% of the fraction content in control sediment, Figure 8a). In sediments treated with PCC mean NH4Cl-P concentration inconsiderably increased or remained unchanged as compared to resuspended sediments without calcite enrichments, except for PCC3, where slight increase in this pool took place (by 9 and 7% in oxic and anoxic conditions, respectively). In contrast, in GCC treatments a decline in NH4Cl-P pool was recorded by 20–31 mg g−1 DW (10–16%) and 25–42 mg g−1 DW (13–21%) in oxic and anoxic conditions, respectively, as compared to resuspended sediments without calcite (Figure 8a). Based on the SRP concentration in the pore water (Figure 7a), we calculated that in oxic conditions adsorbed P constituted 89% (mean value) of the total NH4Cl-P pool in resuspended sediment without calcite, whereas it increased to 97–99% in PCC treatments and to 93–95% in GCC treatments. Respective values for anoxia were 85% (resuspension without calcite addition), 95–99% (PCC treatments) and 92% (all GCC treatments). Remaining portion was pore water SRP. In PCC3 treatments NH4Cl-P was distinctly higher than in GCC1, irrespectively on incubation conditions, which suggests, that PCC applied in the same dose as GCC contributed more to formation of this P pool in sediment.
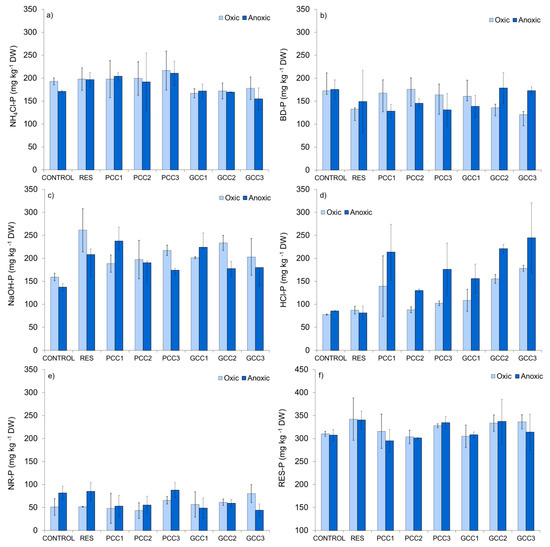
Figure 8.
Phosphorus fractions in sediments: (a) NH4Cl-P, (b) BD-P, (c) NaOH-P, (d) HCl-P, (e) NR-P, (f) RES-P. For treatment abbreviations see Table 3. The values are mean (± standard deviation) of three sediment subsamples collected from each microcosm. Note different scale range for the RES-P fraction.
BD-P and NaOH-P, which stand for other possibly mobile fractions (Figure 8b,c), had a similar share in the total P pool: BD-P accounted for 18% in oxic and anoxic conditions (control sediments, mean values) and NaOH-P for 17% and 14% (oxic and anoxic, respectively; mean values for the control sediments). Mean content of BD-P decreased due to resuspension alone (Figure 8 b) by 40 and 26 mg g−1 DW in oxic and anoxic conditions, respectively, which corresponds to 23% and 15% of the fraction content in the control sediment. As compared to resuspended sediments without calcite, calcite additions resulted in BD-P rise by 28–43 mg g−1 DW (21–32%) under oxic conditions, except for GGC2 and GCC3 (Figure 8b). Under anoxia, an adverse change can be observed in PCC1, PCC3 and GCC1 treatments, with decline in this fraction by 10–21 mg g−1 DW (7–14%; Figure 8b). In most of the treatments with calcite, except for GCC2 and GCC3, mean BD-P content was noticeably lower under anoxia as compared to corresponding oxic treatments (Figure 8b).
NaOH-P substantially increased due to resuspension under oxic and anoxic conditions (Figure 8c). Addition of calcites had no clear effect on this pool. In oxic sediments treated with calcite, the mean content of this fraction was 30–73 mg g−1 DW (11–28%) lower than in resuspended sediments without calcite (Figure 8c). Under anoxia, such a decrease was recorded in chambers treated with medium and high calcite additions (PCC2, PCC3, GCC2, GCC3) and accounted for 18–34 mg g−1 DW (9–16%) (Figure 8c). Concentrations of NaOH-P were slightly lower in some of the anoxic treatments (PCC2, PCC3, GCC2, GCC3 and sediments not treated with calcite) as compared to corresponding oxic treatments (Figure 8c).
HCl-P (Figure 8d) constituted 8% (oxic) and 9% (anoxic) of the total P pool in control sediments. Resuspension without calcite additions did not cause an important change in the content of this pool (Figure 8d). However, resuspension combined with calcite addition influenced the results. A clear increase in the mean content of the HCl-P fraction, as compared to control and resuspended sediments without calcite, was detected in all treatments with calcite (except for PCC2 under oxic conditions) (Figure 8d). Given by mass, the mean rise in HCl-P concentration was 52 mg g−1 DW (PCC1), 14 mg g−1 DW (PCC3), 21 mg g−1 DW (GCC1), 68 mg g−1 DW (GCC2) and 91 mg g−1 DW (GCC3) in oxic treatments (the values are given as comparison to resuspended sediments without calcite) (Figure 8d). Under anoxia the observed increase by mass was 2–6 times higher, with 132 mg g−1 DW (PCC1), 49 mg g−1 DW (PCC2), 95 mg g−1 DW (PCC3), 74 (GCC1), 139 mg g−1 DW (GCC2) and 163 mg g−1 DW (GCC3), respectively, resulting in distinctly higher HCl-P concentration in anoxic treatments (Figure 8 d). This suggests a promoting effect of anoxia on HCl-P formation in calcite enriched sediments. However, share and concentration of the HCl-P pool tended to increase with increasing dosage only in GCC treatments (Figure 8 d). In PCC3 treatments HCl-P content was lower under oxic and higher under anoxic conditions, as compared to GCC1.
NR-P, considered as non-mobile organic fraction (Figure 8e), had a similar share of the total P as HCl-P (5 and 9% in oxic and anoxic incubation, respectively, mean values) in control sediments. This pool was not affected by resuspension alone (Figure 8e). In oxic sediments with calcite addition, the mean NR-P content remained similar or slightly increased as compared to control and resuspended sediment without calcite. In contrast, under anoxia mean NR-P concentration decreased (except for PCC3) by 26–41 mg g−1 DW (30–48%) as compared to resuspended sediments without calcite (Figure 8e).
Another non mobile P pool, RES-P (Figure 8f), representing refractive organic compounds, constituted 32% of total P (oxic and anoxic incubation, mean value). The mean content of this fraction in resuspended sediments without calcite increased slightly as compared to control sediments in oxic as well as anoxic conditions (by 32 and 33 mg g−1 DW, which corresponds to 10% of this pool in control sediment; Figure 8f). In chambers treated with resuspension and calcites, mean RES-P concentration was 2–13% and 2–11% lower as compared to resuspended sediment without calcite (the difference was 6–37 mg g−1 DW and 3–45 mg g−1 DW in oxic and anoxic conditions, respectively) (Figure 8f).
4. Discussion
4.1. Sorption Efficiency of Calcites as Revealed by Batch Experiments
Our study revealed large difference in the sorption capacity of both calcites for SRP with a clear dominance for PCC that shows a 7 times higher sorption compared to GCC (Figure 2 and Figure 3). One explanation could be the slightly higher initial pH in the experiment with PCC compared to GCC (7.9 and 7.7, respectively; Table 1), as higher pH promotes adsorption of phosphate onto calcite [41] and Ca-PO4 formation [79]. However, this effect probably plays a minor role due to very small difference in the initial pH. The main reason that explains the higher performance of PCC in sorption efficacy is the difference in SSABET (12.36 m2 g−1 versus 1.72 m2 g−1 for GCC). Berg et al. [39] have reported that SSA strongly influences the P binding capacity of calcite in oversaturated conditions due to adsorption and/or co-precipitation processes.
The maximum SRP adsorption capacity of GCC (qmax = 0.43 mg PO43− g−1) calculated based on batch experiments using Langmuir model, is in the same range as obtained for other ground calcites having comparable SSABET, which were reported as 0.27–0.53 mg PO43− g−1 at SSABET = 1.00 m2 g−1 [40] and 0.30 mg PO43− g−1 at SSABET = 1.50 m2 g−1 and 0.38–0.40 PO43− g−1 at SSABET = 1 m2 g−1 [29]. However, maximum adsorption of PCC obtained in our study (qmax = 3.11 mg PO43- g−1) is substantially lower than for other precipitated calcites. Xu et al. [59] reported a very high qmax of 95 mg PO43− g−1 for PCC with a relatively low SSABET of 4.17 m2 g−1. These experiments however, are not fully comparable to our results due to extremely high SRP concentrations used (up to 750 mg PO43− g−1; [59]). Even higher adsorption, given as nominal binding capacity of 150 mg PO43− g−1, was reported for another PCC – Algalblock® [52]. However this sorption capacity has to be compared with care due to the unknown method of its determination and lack of SSABET data for this material. Other lakes remediation studies testing PCCs for SRP inactivation [35,39,40] did not present maximum adsorption data. The above discussion clearly shows a lack in comparable data on adsorption capacities of PCCs with respect to SRP, especially under freshwater relevant conditions.
4.2. SRP Removal Mechanism as Revealed by Sorption Isotherms
Mechanism of SRP removal by calcite can be derived from the sorption isotherms, which give important hints about the nature of sorption processes [30,80,81]. In the logarithmic part (section (I)) of isotherms obtained for both calcite materials (Figure 3), corresponding to very low calcite doses (<0.22 g L−1 and <0.10 g L−1 for GCC and PCC, respectively), SRP binding is assumed to proceed via adsorption, whereas inflection of the isotherm (Section (II) in Figure 3) indicates calcium phosphate formation as the dominating mechanism [40,59,82]. This conclusion is supported by the fact that the inflection of the isotherms obtained for both calcite materials takes place after reaching the maximum adsorption load, so that a supersaturation of calcium phosphate phases at the mineral surface is highly conceivable. Thus, the formation of Ca-PO4 compounds is likely near the inflection point of the isotherm because of the shift from undersaturation to oversaturation for OCP and/or ACP (Figure 4). Both compounds have been reported to form as a result of calcite and SRP interaction as precursors of HAp [38,50,59,83]. At initial SRP concentrations of ~4 mg PO43− L−1, our results clearly indicate that the favorable precipitation of Ca-PO4 compounds is the most likely mechanism of SRP sorption within the time of our experiment (24 h) if very small calcite dosages (<0.22–0.10 g L−1) are applied. At higher doses of calcite, which is recommended for field applications as further discussed and which results in much higher SRP reduction rates (Figure 2), less favorable adsorption is to be expected as the dominating removal process in a short term perspective.
4.3. Mechanism of P Inactivation in Sediments
After resuspension without calcite addition and sediment stabilization, SRP concentrations in the overlying water tended to decrease. Similar results were obtained in other studies on sediment resuspension [84,85]. This was probably caused by diffusive flux of SRP from the overlying water into the sediment, as SRP concentration in the pore water (Figure 7a) was lower than in the overlying water in the final stage of incubation (Figure 5). Resedimentation in the initial stage of the incubation (which we observed as stepwise decrease of water turbidity in all of the microcosms within 2 weeks after resuspension) probably also contributed to this decrease, as SRP has a high affinity to fine sediment particles [86].
Mechanisms of SRP inactivation by calcite are complex. During calcite application, which means contact of calcite with the mixture of lake water and sediments, adsorption should be dominant due to a short interaction time: visible settlement of calcite particles was observed to last for about 10 min in case of both calcite materials studied. According to Kuo and Lotse [87], 80% of the phosphate ions should be adsorbed onto calcite within 10 s. Sø et al. [41] reported that adsorption reaches equilibrium after 3 h.
After resuspension (>7 days of incubation), all PCC and GCC treatments greatly enhanced SRP transfer into the sediment (Figure 5, Figure 6b). This indicates that SRP was removed from the overlying water through interaction with calcite, probably as adsorption, present in the surficial layer of the sediment. Such an effect was also observed by Ding et al. [52] and Yu et al. [54], who investigated P retention in sediments covered with an intact barrier consisting of calcite. Another possible mechanism of enhanced SRP flux into the sediment is the diffusive transport due to the high gradient of SRP between overlying and pore water caused by removal of SRP from the pore water by calcites during incubation. This is confirmed by the fact, that the lowest flux into the sediment was caused by PCC3 treatment (Figure 6b), characterized by the smallest difference of SRP concertation between the overlying and the pore water at the end of the incubation experiment.
Within the sediments, the main mechanism of SRP inactivation by both calcite materials was probably precipitation of Ca-PO4 and/or co-precipitation with calcite, which is shown by a substantial increase in HCl-P in calcite treatments as compared to sediments without calcite addition (Figure 8d), as this pool may be explained by Ca-PO4 compounds and P bound to carbonates [70]. The rise in HCl-P corresponds with a decrease in the share of fractions: NaOH-P and RES-P (oxic and anoxic treatments) and NR-P and BD-P (only anoxic treatments), as compared to resuspended sediments without calcite addition (Figure 8 a,c,e,f), which suggests that P released from these pools contributed to HCl-P formation.
The nature of the HCl-P fraction formed as a result of calcite presence in sediments could not have been directly determined within the study. However, co-precipitation of P with calcite does not seem to play an important role in our system, despite initial oversaturation with respect to calcite (SICC = 0.8; Table 2), due to high SRP concentration in the pore water (1.88 and 3.35 mg PO43− L−1 in resuspended sediments without calcite addition in oxic and anoxic incubation, respectively), which probably prevented calcite growth. The growth of calcite crystals ceases or becomes strongly retarded at initial SRP concentrations as low as 0.10–0.21 mg PO43− L−1 [45,88].
Therefore, we suggest that this pool originates from surface precipitation of Ca-PO4 induced by preceding calcite dissolution, as was observed by several authors in phosphate bearing solutions [30,37,42,45,83]. This is especially conceivable in GCC treatments, where HCl-P pool increased with increasing pH of the pore water, which suggests that Ca-PO4 formation was related to the rate of calcite dissolution. Dissolution of calcite might have promoted Ca-PO4 formation via Ca2+ supply and/or pH increase as solubility of Ca-PO4 decreases with increasing pH [79]. This mechanism is likely to take place both, in oxic and anoxic conditions, as in both incubations the pH increased in sediments treated with calcite as compared to resuspended sediments without calcite (Figure 7b). Based on the calculated saturation indices of different Ca-PO4 phases in the pore water (Table 5) the formation of two different phases is possible: SC and HAp. Precipitation of SC seems likely as this compound was proved to form in the Tiefenwarensee as a result of calcium hydroxide application [89] and in sediments of hardwater lakes as naturally occurring mineral phase [78,90]. Taking into account the duration of our experiment (~100 days), formation of HAp, which takes place via transformation of less stable Ca-PO4 precursor phases, is also possible: Klasa et al. [43] observed a transformation of amorphous Ca-PO4 to HAp within 5 weeks.
It is interesting to note, that Ca-PO4 compounds formed preferentially under anoxia, which is reflected in substantially higher HCl-P concentrations in anoxic incubations, as compared to the oxic ones (Figure 7d) and further confirmed by a distinct depletion of Ca2+ in the pore water of anoxic sediments treated with calcite (Figure 7c) (the exception was GCC3, where Ca2+ concentration slightly increased, suggesting, that Ca-PO4 precipitation did not consume all the Ca2+ ions supplied to the system due to calcite dissolution). The promoting effect of anoxia on formation of Ca-PO4 compounds had probably two drivers. Firstly, higher pH of the anoxic pore water should be considered (Figure 7b), because Ca-PO4 solubility decreases with the pH rise [79], as already mentioned. One likely reason for higher pore water pH under anoxia is the anaerobic decomposition of organic matter, as this process generates less H+ and dissolves less calcite than aerobic mineralization pathways [80,91]. This is in agreement with slightly lower calcites dissolution rates observed in anoxia (based on pH increase in the pore water as compared to sediment without calcite, Figure 7b). However, if the dissolution of calcite initiates Ca-PO4 formation, as previously discussed, lower dissolution in anoxia must have been overcome by other factors which promoted Ca-PO4 precipitation under these conditions. Another explanation for differences in the pore water pH, resulting from incubation conditions, could be that in oxic treatments oxidation of Fe2+ and followed precipitation of FeOOH took place. This reaction produces H+ and thus led to an acidifying effect on the pore water in oxic chambers. The second driver promoting Ca-PO4 formation under anoxia was probably the higher concentration of pore water SRP, which can be seen in sediments not treated with calcite (Figure 7a), as it represents a pool accessible for Ca-PO4 precipitation processes. One source of this SRP under anoxia was probably reductive dissolution of Fe(III) minerals, leading to liberation of previously sorbed P. This is confirmed by slightly lower concentration of the BD-P fraction under anoxia in some of the treatments (Figure 8b) and also by the relatively high Fe2+ concentration and very low redox potential in the pore water of anoxic chambers (up to 0.5 mg Fe2+ L−1 and−150–−280 mV, data not shown). The recorded redox potential is far lower than values attributed to Fe(III) reduction (<200 mV; [92,93]). This conclusion is in agreement with other studies, which stressed, that calcium compounds may only play a dominant role in P inactivation in sediment, when binding to Fe (hydro)oxides becomes unfavorable due to reductive conditions [93,94,95]. Similarly, Ann et al. [96] reported very high performance of calcite in P binding under reductive conditions in flooded soil. Another source of high SRP in the pore water of anoxic chambers could be the NaOH-P fraction, representing P bound to Al-an Fe-oxides, which is sensitive to elevated pH [70]. NaOH-P concentration was lower under anoxia than in oxic incubations in most of the treatments, which can be explained by higher pH under anoxia.
In GCC treatments, a decline in NHC4Cl-P of up to 21% was observed, compared to resuspended sediments without calcite (Figure 8a). This fraction can be explained by two pools: SRP dissolved in the pore water and adsorbed onto sediment particles. As already given, SRP in the pore water constituted barely 5–7% of NH4Cl-P in GCC treatments, so the observed decrease in pore water SRP concentration (Figure 7a) could not have been responsible for the recorded NH4Cl-P loss. One possible explanation could be that previously adsorbed SRP was transformed into more stable compounds in HCl-P fraction during subsequent incubation.
We are aware that using chemicals to determine the fractionation of P compounds in carbonate rich sediments may cause some methodologically driven shifts between determined P pools, such as artificial formation of Ca-PO4 during NaOH extraction, observed for example by Hupfer et al. [69] during fractionation of the sediments treated with alum sulphate. However, the same authors did not detect such an effect in calcite rich sediments characterized by similar calcium content (161–185 mg Ca g−1 DW) as sediments enriched with calcite investigated in our study (70–250 mg Ca g−1 DW). To minimize such methodological artefacts in our results, we increased volume-to-sediment ratio during the fractionation procedure (Section 2.4) as suggested by Hupfer et al. [69].
In future, we intend to confirm our conclusions by using direct spectroscopic methods such as XANES, which should also enhance the insight into the chemical and mineralogical nature of the newly formed HCl-P pool. These methods develop fast and have been recently successfully applied to track qualitative P speciation transformations in the lake sediments (e.g. [97]) and to differentiate between P co-precipitated with calcite and bound in Ca-PO4 minerals [98].
4.4. Efficiency and Stability of P Inactivation in Sediments
Both calcite materials, irrespectively of their dosage, enhanced the transfer of SRP from the overlying water into the sediment after resuspension combined with calcite addition and substantially reduced SRP release as compared to control sediments. This effect was the better, the higher the dose of calcites was. PCC applied in the same dose was substantially more efficient than GCC (Figure 5, Figure 6a). However, only high addition of PCC (1.5 kg m−2) was efficient enough to fix all the SRP released during resuspension and thus secured an overall positive effect of the conducted treatment (Figure 5 and Figure 6c). This is due to the much higher SSABET of PCC as compared to GCC as previously discussed.
As shown in this study, high initial SRP concentrations in the overlying water has to be expected during and shortly after applying the controlled resuspension method due to rapid SRP release from sediments, with much higher loads released under anoxic than in oxic conditions (Figure 6a). The same was observed during laboratory and field experiments on the controlled resuspension method combined with coagulants addition [56]. High SRP release resulting from forced sediment resuspension makes the selection of P inactivation agents and its dose a crucial factor in order to successfully reach restoration goals. When calcite is chosen as a possible inactivation agent, only the materials exhibiting a high SSABET and a high adsorption capacity should be considered due to their ability to durably fix high SRP loads within a sufficient short period of time and at reasonable doses applied. This forces the use of synthetized calcium carbonates—precipitates—because these materials are typically characterized by a high SSABET ranging from 5.0 to 70 m2 g−1 [35,40,58,60], while natural ground calcites typically show much lower SSABET in the range of 1.0–4.3 m2 g−1 [29,30,34,39,40]. The use of PCC would also require much lower doses of calcite than in the case of GCC, which means less modification of the sediment composition in terms of calcium carbonate content. In this context application of PCC may be considered as more reasonable.
Stable or decreasing SRP concentration in the overlying water during incubation experiments after all calcite treatments, irrespectively of oxygen presence, indicates a high durability of P inactivation by PCC and GCC which may be attributed to Ca-PO4 precipitation as this mechanism secures long term P binding [30,99]. No limiting effect of anoxia on performance of both calcite materials was detected within our study which is in accordance with observations made by Hart et al. [35] and Lin et al. [51], who tested active calcite barriers on sediments.
A slight dissolution of calcites, suggested by a pH increase in the pore water of sediments treated with PCC and GCC (Figure 7b), did not result in P release, which may be attributed to Ca-PO4 compounds formation, as previously discussed. Dissolution of calcite is to be expected when the solution is undersaturated, whereas at the end of the experiment pore water was in the equilibrium state with respect to this mineral (Table 5), contrary to initial oversaturation recorded in the fresh pore water (Table 2). Lower saturation index after incubation was probably due to mixing of the sediments with lake water during resuspension, which limited availability of Ca2+ and HCO3- in the pore water, as lake water was characterized with much lower concentration of these ions (Table 2). Lower pH observed in the pore water after incubation (Figure 7 b), as compared to initial conditions, (Table 2) could result from decomposition of organic matter in sediments. Under equilibrium conditions, which were present in the pore water after incubation, dissolution of calcite (as well as crystallization) is possible [100]. Stronger dissolution of PCC as compared to GCC, indicated by higher pore water pH in treated sediments (Figure 7 b), is most likely related to smaller grain size of PCC [39].
4.5. Possibilities of Method Implementation in Practice
Our study revealed that the application of P inactivation agents using the controlled resuspension method requires addition of a highly efficient adsorbent. If calcite is used, synthetic precipitates seem to be a rational solution. Large scale application of PCCs might be, however, limited due to their high costs ranging from 450 to 2250 EUR t−1, which is at least 9 times more than the price of ground calcites—~50 EUR t−1 [32,34,35,39,40]. Relatively high costs of PCCs might be factors limiting potential in-lake applications of these calcite materials. However, if the method will be further developed and successfully applied, interest in these materials among potential suppliers is likely to increase, which should result in improved availability and higher cost efficiency.
The optimal dose of PCC applied by means of the controlled resuspension method (1.5 kg m−2) is in the range of PCCs doses used for creation of an active barrier on the sediment surface (0.1–4.8 kg m−2) in previous studies [35,39,52]. However, the commonly used barrier thickness of 1 cm seems to be too low due to insufficient P binding [54] or potential mechanical disturbance [39]. Consequently, for a barrier method a minimum thickness of 5 cm is recommended [39]. In that case, the dose of PCC added into the ecosystem may be as high as 20 kg m−2 and 24 kg m−2 as calculated for two PCCs—Socal U1® and SocalU3®, respectively and have the estimated cost of 11 EUR m−2 and 28 EUR m−2 [39]. Compared to that, the application of PCC using the controlled resuspension method in our study required an about 13–16 times lower dose of calcite (1.5 kg m−2), which makes this method more reasonable in terms of material consumption, at 14–37 times lower cost (0.75 EUR m−2; the price per t is appr. 500 EUR). However, a comprehensive comparison between the two discussed methods of calcite application—onto the sediment surface (active barrier) and into the sediment (controlled resuspension method)—should also consider cost of usage of special devices which are needed for applying inactivation agent using the latter method [12].
As our study showed, application of calcite as P inactivation agent may be considered especially in lakes with highly reductive conditions in the pore water because this factor promotes a high stability of P binding onto calcite through precipitation of calcium-phosphates (Figure 8d). In such lakes, the use of commonly applied iron salts may be limited due to susceptibility of iron-phosphate binding to reduction under redox values lower than 200 mV [93]. Thus, calcite can be regarded as an alternate to other P inactivation agents not sensitive to anoxia, which are lanthanum bentonite and alum salts. Nevertheless, it has to be stressed that amount of calcite applied using the controlled resuspension method has to be adjusted to specific in-lake conditions, to prevent uncontrolled SRP release. As our study showed, SRP release during sediment disturbance will depend on oxygen presence (Figure 6a). Further important factors are sorption capacity of sediment and SRP gradient between pore and lake water [56]. Thus, pre-testing of appropriate calcite dosages has to be performed. If the dose is too low, a huge amount of SRP will be released into the water column without being captured and retransferred into the sediment, leading to an unintended and potentially harmful influence on the ecosystem.
5. Conclusions
Both calcites investigated within this study were, to a various extent, efficient in SRP removal from the lake water and pore water. Sorption capacity of PCC was, however, 4–5 times higher than that of GCC, which is mainly explainable by large differences in surface area of both materials. All tested dosages of PCC and GCC substantially reduced SRP release from sediment during artificially induced resuspension and increased P binding in the sediment during subsequent incubation, under oxic and anoxic conditions, although obtained results largely differed depending on the calcite material used and the dose applied. Only the high dosage of PCC (1.5 kg m−2) was efficient enough to fix all SRP released during resuspension and secured an overall positive effect of the treatment. Therefore, when application of calcite for P inactivation is considered by means of the controlled resuspension method, only large surface area materials may be suitable due to sufficient sorption efficiency at relatively small doses.
SRP was inactivated by both calcite materials via adsorption in the first step (during application into the disturbed sediment) and precipitation processes during subsequent incubation in sediment. As calcite presence resulted in an increase of the relatively stable HCl-P pool, most likely due to precipitation of Ca-PO4 phases, the use of calcite materials should lead to long term and stable SRP binding in the sediment. The promoting effect of anoxia on P precipitation with calcium compounds shows that calcite may be a suitable tool to prevent sediment P release especially under anoxic conditions. In stratified lakes, suffering from anoxia in the bottom layer during stagnation, application of calcite during stagnant conditions will result in higher stability of SRP inactivation.
According to our study, application of calcite into the sediment by controlled resuspension requires a much smaller amount of reactive material than formation of active barriers from this mineral, which makes this method a promising solution in shallow lakes, where active barriers are mechanically disturbed due to wind mixing. However, one fundamental restriction for successful application of calcite into the sediment has to be addressed beforehand, which is to determine necessary amount of calcite needed, to prevent unintended P release during controlled resuspension. The appropriate dosage will strongly depend on oxygen presence and pore water SRP concentration, as these factors determine SRP release during resuspension.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-163X/10/3/223/s1, Table S1: Composition of ground calcite; Figure S1: Physio-chemical conditions in the pore water after equilibration with PCC and GCC for 24 h: (a) pH, (b) Ca2+, (c) alkalinity.
Author Contributions
Conceptualization, A.B.-S. & Z.P.; methodology, A.B.-S., E.E., A.B., Z.P.; formal analysis, A.B.-S.; investigation, A.B.-S., A.B.; resources, A.B.-S., Z.P., U.F., A.B.; data curation, A.B.-S.; writing—original draft preparation, A.B.-S.; writing—review and editing, E.E., A.B., Z.P., U.F.; visualization, A.B.-S.; supervision, Z.P.; project administration, A.B.-S. and Z.P.; funding acquisition, A.B.-S. and Z.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Polish Ministry of Science and Higher Education/National Science Centre, Poland [grant No. NN305 1515 33]. The publication of the article was financed by the Polish National Agency for Academic Exchange as part of the Foreign Promotion Program.

Acknowledgments
The research was conducted in the Laboratory—Water Centre, WULS-SGGW (Poland). We thank Ignacy Kardel (WULS-SGGW) for help during chromatographic measurements, Katarzyna Struzik and Tomasz Kasjan for laboratory assistance and Michał Wasilewicz (WULS-SGGW) for support during sediment sampling. SEM observations were carried out at the Laboratory for Electron Microscopy at KIT—we thank Volker Zibat for his support. Dominik Małasiewicz, Lhoist Poland, is greatly acknowledged for providing data on elemental composition of GCC. SSABET and grain size of calcite materials were determined in Research and Development Centre for Protection and Restoration of Freshwater Ecosystems (APRS Ltd., Nielbark, Poland). We kindly thank Jakub Idźkowski and Łukasz Kozłowicz for performing these measurements. XRD analysis was conducted by Laboratory of Structural Research and Materials Characterization at Institute of Electronic Materials Technology (Warsaw, Poland).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cooke, G.D.; Welch, E.B.; Peterson, S.; Nichols, S.A. Restoration and Management of Lakes and Reservoirs, 3rd ed.; Taylor & Francis Group, LCC CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Cooke, G.D.; Welch, E.B.; Martin, A.B.; Fulmer, D.G.; Hyde, J.B.; Schrieve, G.D. Effectiveness of Al, Ca, and Fe salts for control of internal phosphorus loading in shallow and deep lakes. Hydrobiologia 1993, 253, 323–335. [Google Scholar] [CrossRef]
- Gibbs, M.M.; Hickey, C.W. Flocculants and Sediment Capping for Phosphorus Management. In Lake Restoration Handbook, A New Zealand Perspective, 1st ed.; Hamilton, D., Collier, K., Quinn, K., Howard-Williams, C., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Dunalska, J.A.; Wiśniewski, G. Can we stop the degradation of lakes? Innovative approaches in lake restoration. Ecol. Eng. 2016, 95, 714–722. [Google Scholar] [CrossRef]
- Huser, B.J.; Egemose, S.; Harper, H.; Hupfer, M.; Jensen, H.; Pilgrim, K.M.; Reitzel, K.; Rydin, E.; Futter, M. Longevity and effectiveness of aluminum addition to reduce sediment phosphorus release and restore lake water quality. Water Res. 2016, 97, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Parszuto, K.; Łopata, M.; Grochowska, J.; Tandyrak, R.; Augustyniak, R. Support of the Self-purification Processes in Lakes Restored in Poland. In Polish River Basins and Lakes—Part II, the Handbook of Environmental Chemistry 87, 1st ed.; Korzeniewska, E., Harnisz, M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Lewandowski, J.; Schauser, I.; Hupfer, M. Long term effects of phosphorus precipitations with alum in hypereutrophic Lake Susser See (Germany). Water Res. 2003, 37, 3194–3204. [Google Scholar] [CrossRef]
- Bakker, E.; Van Donk, E.; Immers, A.K. Lake restoration by in-lake iron addition: A synopsis of iron impact on aquatic organisms and shallow lake ecosystems. Aquat. Ecol. 2016, 50, 121–135. [Google Scholar] [CrossRef]
- Augustyniak, R.; Grochowska, J.; Łopata, M.; Parszuto, K.; Tandyrak, R.; Tunowski, J. Sorption Properties of the Bottom Sediment of a Lake Restored by Phosphorus Inactivation Method 15 Years after the Termination of Lake Restoration Procedures. Water 2019, 11, 175. [Google Scholar] [CrossRef]
- Van Hullebusch, E.; Deluchat, V.; Chazal, P.M.; Baudu, M. Environmental impact of two successive chemical treatments in a small shallow eutrophied lake: Part I. Case of aluminium sulphate. Environ. Pollut. 2002, 120, 617–626. [Google Scholar] [CrossRef]
- Egemose, S.; Wauer, G.; Kleeberg, A. Resuspension behaviour of aluminium treated lake sediments: Effects of ageing and pH. Hydrobiologia 2009, 636, 203–217. [Google Scholar] [CrossRef]
- Wiśniewski, R.; Ślusarczyk, J.; Kaliszewski, T.; Szulczewski, A.; Nowacki, P. “Proteus”, a new device for application of coagulants directly to sediment during its controlled resuspension. Verh. Int. Ver. Limnol. 2010, 30, 1421–1424. [Google Scholar] [CrossRef]
- Wagner, K.; Meringolo, D.; Mitchell, D.; Moran, E.; Smith, S. Aluminium treatments to control internal phosphorus loading in lakes on Cape Cod, Massachusetts. Lake Reserv. Manag. 2017, 33, 171–186. [Google Scholar] [CrossRef]
- Welch, E.B.; Gibbons, H.L.; Brattebo, S.K.; Corson-Rikert, H.A. Distribution of aluminium and phosphorus fractions following alum treatments in a large shallow lake. Lake Reserv. Manag. 2017, 33, 198–204. [Google Scholar] [CrossRef]
- Schumaker, R.J.; Funk, W.H.; Moore, B.C. Zooplankton responses to aluminium sulphate treatment of Newman Lake, Washington. J. Freshw. Ecol. 1993, 8, 375–387. [Google Scholar] [CrossRef]
- Randall, S.; Harper, D.; Brierley, B. Ecological and ecophysiological impacts of ferric dosing in reservoirs. Hydrobiologia 1999, 395–396, 355–364. [Google Scholar] [CrossRef]
- Immers, A.K.; Van der Sande, M.T.; Van der Zande, R.M.; Geurts, J.J.M.; Van Donk, E.; Bakker, E.S. Iron addition as a shallow lake restoration measure: Impacts on charophyte growth. Hydrobiologia 2013, 710, 241–251. [Google Scholar] [CrossRef][Green Version]
- Stabel, H.-H. Calcite precipitation in Lake Constance: chemical equilibrium, sedimentation, and nucleation by algae. Limnol. Oceanogr. 1986, 31, 1081–1093. [Google Scholar] [CrossRef]
- Danen-Louwerse, H.J.; Lijklema, L.; Coenraats, M. Coprecipitation of phosphate with calcium carbonate in Lake Veluwe. Water Res. 1995, 29, 1781–1785. [Google Scholar] [CrossRef]
- Dittrich, M.; Koschel, R. Interactions between calcite precipitation (natural and artificial) and phosphorus cycle in the hardwater lake. Hydrobiologia 2002, 469, 49–57. [Google Scholar] [CrossRef]
- Murphy, T.P.; Hall, K.J.; Yesaki, I. Coprecipitation of phosphate with calcite in a naturally eutrophic lake. Limnol. Oceanogr. 1983, 28, 58–69. [Google Scholar] [CrossRef]
- Koschel, R. Pelagic calcite precipitation and trophic state of hardwater lakes. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1990, 33, 713–722. [Google Scholar]
- De Vicente, I.; Cattaneo, K.; Cruz-Pizarro, L.; Brauer, A.; Guilizzoni, P. Sedimentary phosphate fractions related to calcite precipitation in an eutrophic hardwater lake (Lake Alserio, northern Italy). J. Paleolimnol. 2006, 35, 55–64. [Google Scholar] [CrossRef]
- Walsh, J.R.; Corman, J.R.; Munoz, S.E. Coupled long-term limnological data and sedimentary records reveal new control on water quality in a eutrophic lake. Limnol. Oceanogr. 2019, 64, S34–S48. [Google Scholar] [CrossRef]
- Klapper, H. Calcite covering of sediment as a possible way of curbing blue-green algae. In Eutrophication: Research and Application to Water Supply, 3rd ed.; Sutcliffe, D.W., Jones, J.G., Eds.; Freshwater Biological Association: Ambleside, UK, 1992; pp. 107–111. Available online: https://core.ac.uk/download/pdf/11020726.pdf (accessed on 13 December 2019).
- Stüben, D.; Walpersdorf, E.; Voss, K.; Rönicke, H.; Schimmele, M.; Baborowski, M.; Luther, G.; Elsner, W. Application of lake marl at Lake Arendsee, NE Germany: First results of a geochemical monitoring during the restoration experiment. Sci. Total Environ. 1998, 218, 33–44. [Google Scholar] [CrossRef]
- Walpersdorf, E.; Neumann, T.; Stüben, D. Efficiency of natural calcite precipitation compared to lake marl application used for water quality improvement in an eutrophic lake. Appl. Geochem. 2004, 19, 1687–1698. [Google Scholar] [CrossRef]
- Griffin, R.A.; Jurinak, J.J. The interaction of phosphate with calcite. Soil Sci. Soc. Am. J. 1973, 37, 847–850. [Google Scholar] [CrossRef]
- Holford, I.C.R.; Mattingly, G.E.G. Phosphate sorption by jurassic oolitic limestones. Geoderma 1975, 13, 257–264. [Google Scholar] [CrossRef]
- Freeman, J.S.; Rowell, D.L. The adsorption and precipitation of phosphate onto calcite. J. Soil Sci. 1981, 32, 75–84. [Google Scholar] [CrossRef]
- Hinedi, Z.R.; Goldberg, S.; Chang, A.C.; Yesinowski, J.P. A 31P and 1H MAS NMR study of phosphate sorption onto calcium carbonate. J. Colloid Interface Sci. 1992, 152, 141–160. [Google Scholar] [CrossRef]
- Berg, U. Die Kalzitapplikation als interne Restaurierungsmaβnahme für eutrophierte Seen—Ihre Optimierung und Bewertung. Karlsruher Geochem. H. 2001, 20. [Google Scholar]
- Brix, H.; Arias, C.A.; del Bubba, M. Media selection for sustainable phosphorus removal in subsurface flow constructed wetlands. Water Sci. Technol. 2001, 44, 47–54. [Google Scholar] [CrossRef]
- Donnert, D.; Berg, U.; Weidler, P.G.; Nüesch, R.; Song, Y.; Salecker, M.; Kusche, I.; Bumiller, W.; Friedrich, F. Phosphorus removal and recovery from waste water by crystallisation. Wasser Geotechnol. 2002, 3, 115–132. Available online: http://www.academia.edu/22247677/Phosphorus_removal_and_recovery_from_waste_water_by_crystallisation (accessed on 13 December 2019).
- Hart, B.; Roberts, S.; James, R.; Taylor, J.; Donnert, D.; Furrer, R. Use of active barriers to reduce eutrophication problems in urban lakes. Water Sci. Technol. 2003, 47, 157–163. [Google Scholar] [CrossRef][Green Version]
- Lyngsie, G.; Penn, C.J.; Hansen, H.C.B.; Borggaard, O.K. Phosphate sorption by three potential filter materials as assessed by isothermal titration calorimetry. J. Environ. Manag. 2014, 143, 26–33. [Google Scholar] [CrossRef]
- Wan, B.; Yan, Y.; Liu, F.; Tan, W.; Chen, X.; Feng, X. Surface adsorption and precipitation of inositol hexakisphosphate on calcite: A comparison with orthophosphate. Chem. Geol. 2016, 421. [Google Scholar] [CrossRef]
- Li, Z.; Sun, X.; Huang, L.; Liu, D.; Yu, L.; Wu, H.; Wei, D. Phosphate adsorption and precipitation on calcite under calco-carbonic equilibrium condition. Chemosphere 2017, 183, 419–428. [Google Scholar] [CrossRef]
- Berg, U.; Neumann, T.; Donnert, D.; Nüesch, R.; Stüben, D. Sediment capping in eutrophic lakes—Efficiency of undisturbed calcite barriers to immobilize phosphorus. Appl. Geochem. 2004, 19, 1759–1771. [Google Scholar] [CrossRef]
- Eiche, E.; Berg, U.; Song, Y.; Neumann, T. Fixation and phase transformation of phosphate at calcite surfaces—Implications for eutrophic lake restoration. In Proceedings of the Ninth International Congress for Applied Mineralogy (1–10), Brisbane, QLD, Australia, 8–10 September 2008. [Google Scholar]
- Sø, H.U.; Postma, D.; Jakobsen, R.; Larsen, F. Sorption of phosphate onto calcite: results from batch experiments and surface complexation modelling. Geochim. Cosmochim. Acta 2011, 75, 2911–2923. [Google Scholar] [CrossRef]
- Wang, L.; Ruiz-Agudo, E.; Putnis, C.V.; Menneken, M.; Putnis, A. Kinetics of calcium phosphate nucleation and growth on calcite: implications for predicting the fate of dissolved phosphate species in alkaline soils. Environ. Sci. Technol. 2012, 46, 834–842. [Google Scholar] [CrossRef]
- Klasa, J.; Ruiz-Agudo, E.; Wang, L.J.; Putnis, C.V.; Valsami-Jones, E.; Menneken, M.; Putnis, A. An atomic force microscopy study of the dissolution of calcite in the presence of phosphate ions. Geochim. Cosmochim. Acta 2013, 117, 115–128. [Google Scholar] [CrossRef]
- Suzuki, T.; Inomata, S.; Sawada, K. Adsorption of phosphate onto calcite. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1986, 82, 1733–1743. [Google Scholar] [CrossRef]
- Plant, L.J.; House, W.A. Precipitation of calcite in the presence of inorganic phosphate. Colloids Surf. A Phys. Eng. Asp. 2002, 203, 143–153. [Google Scholar] [CrossRef]
- Eiche, E.; Berg, U.; Neumann, T.; Nüesch, R.; Stüben, D. Multilayer fixation of dissolved phosphate on natural calcites derived from sorption experiments. Geochim. Cosmochim. Acta 2007, 71, 251. Available online: https://www.goldschmidtabstracts.info/abstracts/abstractView?id=2007004069 (accessed on 13 December 2019).
- Sø, H.U. Adsorption of Arsenic and Phosphate Onto the Surface of Calcite as Revealed by Batch Experiments and Surface Complexation Modelling. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 2011. Available online: http://orbit.dtu.dk/files/5511322/Helle%20Ugilt%20Soe%20PhD-thesis%20WWW-Version.pdf (accessed on 13 December 2019).
- Valsami-Jones, E. Mineralogical controls on phosphorus recovery from wastewaters. Miner. Mag. 2001, 65, 611–620. [Google Scholar] [CrossRef]
- Mekmene, O.; Quillard, S.; Rouillon, T.; Bouler, J.-M.; Piot, M.; Gaucheron, F. Effects of pH and Ca/P molar ratio on the quantity and crystalline structure of calcium phosphates obtained from aqueous solutions. Dairy Sci. Technol. 2009, 89, 301–316. [Google Scholar] [CrossRef]
- Naidu, S.; Scherer, G.W. Nucleation, Growth and Evolution of Calcium Phosphate Films on Calcite. J. Colloid Interface Sci. 2014, 435, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhan, Y.; Zhu, Z. Evaluation of sediment capping with active barrier systems (ABS) using calcite/zeolite mixtures to simultaneously manage phosphorus and ammonium release. Sci. Total Environ. 2011, 409, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Qin, B.; Xu, H.; Dong, B.; Brookes, J.D. Comparison of efficacy of two P-inactivation agents on sediments from different regions of Lake Taihu: sediment core incubations. Fundam. Appl. Limnol. 2012, 181, 271–281. [Google Scholar] [CrossRef]
- Galvez-Cloutier, R.; Saminathan, S.K.M.; Boillot, C.; Triffaut-Bouchet, G.; Bourget, A.; Soumis-Dugas, G. An evaluation of several in-lake restoration techniques to improve the water quality problem (eutrophication) of Saint-Augustin Lake, Quebec, Canada. Environ. Manag. 2012, 49, 1037–1053. [Google Scholar] [CrossRef]
- Yu, X.; Grace, M.R.; Sun, G.; Zou, Y. Application of ferrihydrite and calcite as composite sediment capping materials in a eutrophic lake. J. Soils Sediments 2018, 18, 1185–1193. [Google Scholar] [CrossRef]
- Ripl, W. Biochemical oxidation of polluted lake sediment with nitrate—A new lake restoration method. Ambio 1976, 5, 132–135. Available online: www.jstor.org/stable/4312194 (accessed on 13 December 2019).
- Wiśniewski, R. Phosphate inactivation with iron chloride during sediment resuspension. Lakes Reserv. Res. Manag. 1999, 4, 65–73. [Google Scholar] [CrossRef]
- Schütz, J.; Rydin, E.; Huser, B.J. A newly developed injection method for aluminum treatment in eutrophic lakes: Effects on water quality and phosphorus binding efficiency. Lake Reserv. Manag. 2017, 33, 152–162. [Google Scholar] [CrossRef]
- Wei, S.-H.; Mahuli, S.K.; Agnihotri, R.; Fan, L.-S. High Surface Area Calcium Carbonate: Pore Structural Properties and Sulfation Characteristics. Ind. Eng. Chem. Res. 1997, 36, 2141–2148. [Google Scholar] [CrossRef]
- Xu, N.; Chen, M.; Zhou, K.; Wang, Y.; Yin, H.; Chen, Z. Retention of phosphorus on calcite and dolomite: Speciation and modeling. RSC Adv. 2014, 4, 3525–35214. [Google Scholar] [CrossRef]
- Chang, R.; Kim, S.; Lee, S.; Choi, S.; Kim, M.; Park, Y. Calcium Carbonate Precipitation for CO2 Storage and Utilization: A Review of the Carbonate Crystallization and Polymorphism. Front. Energy Res. 2017, 5, 17. [Google Scholar] [CrossRef]
- Bańkowska, A.; Sawa, K.; Popek, Z.; Wasilewicz, M. The analysis of the Zdworskie Lake water supply in aspect of the restoration programme (in Polish). Sci. Rev. Eng. Environ. Sci. 2011, 20, 84–96. Available online: http://iks_pn.sggw.pl/z52/art2.pdf (accessed on 13 December 2019).
- Popek, Z.; Wasilewicz, M.; Bańkowska-Sobczak, A. Qualitative and Quantitative Monitoring of the Zdworskie Lake Water Supply. Tech. Rep. (Polish); Warsaw University of Life Sciences: Warsaw, Poland, 2017. [Google Scholar]
- Wiśniewski, R. Properties of the Sediment and Water of the Zdworskie Lake. Tech. Rep. (Polish); PROTE; Technologies for the Environment: Poznań, Poland, 2006. [Google Scholar]
- Bus, A.; Karczmarczyk, A. Kinetic studies on removing phosphate from synthetic solution and river water by reactive material in a form of suspended reactive filters. Desalin. Water Treat. 2018, 136, 237–244. [Google Scholar] [CrossRef]
- PN-EN ISO 6878. Water Quality. Determination of Phosphorus. Ammonium Molybdate Spectrometric Method; PKN: Warsaw, Poland, 2004. [Google Scholar]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- PN-EN ISO 7980:2002. Water Quality—Determination of Calcium and Magnesium—Atomic Absorption Spectrometric Method; PKN: Warsaw, Poland, 2002. [Google Scholar]
- U.S. EPA. Method 3051A (SW-846): Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils, Revision 1; U.S. EPA: Washington, DC, USA, 2007.
- Hupfer, M.; Gächter, R.; Giovanoli, R. Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquat. Sci. 1995, 57, 305–324. [Google Scholar] [CrossRef]
- Lewandowski, J. Untersuchungen Zum Einfluss Seeinterner Verfahren auf Die Phosphor-Diagenese in Sedimenten. Ph.D. Thesis, HU Berlin, Institut für Biologie, Berlin, Germany, 2002. [Google Scholar] [CrossRef]
- Hupfer, M.; Zak, D.; Roßberg, R.; Herzog, C.; Pöthig, R. Evaluation of a well-established sequential phosphorus fractionation technique for use in calcite-rich lake sediments: identification and prevention of artifacts due to apatite formation. Limnol. Oceanogr. Methods 2009, 7, 399–410. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Deutsch, W.J.; Siegel, R. Groundwater Geochemistry: Fundamentals and Applications to Contamination, 1st ed.; CRC Press LCC: Boca Raton, FL, USA, 1997. [Google Scholar] [CrossRef]
- Macioszczyk, A.; Dobrzyński, D. Hydrogeochemia Strefy Aktywnej Wymiany Wód Podziemnych; Wydawnictwo PWN: Warszawa, Polska, 2002. (In Polish) [Google Scholar]
- Upchurch, S.; Scott, T.M.; Alfieri, M.; Fratesi, B.; Dobecki, T.L. The Karst Systems of Florida, Understanding Karst in a Geologically Young Terrain, 1st ed.; Springer International Publishing AG: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Song, Y.; Hahn, H.H.; Hoffmann, E. The effect of carbonate on the precipitation of calcium phosphate. Environ. Technol. 2002, 23, 207–215. [Google Scholar] [CrossRef]
- Recillas, S.; Rodríguez-Lugo, V.; Montero, M.L.; Viquez-Cano, S.; Hernandez, L.; Castano, V.M. Studies on the precipitation behaviour of calcium phosphate solutions. J. Ceram. Process. Res. 2012, 13, 5–10. Available online: http://jcpr.kbs-lab.co.kr/file/JCPR_vol.13_2012/JCPR13-1/5_10.pdf (accessed on 13 December 2019).
- Avnimelech, Y. Phosphorus and calcium carbonate solubilities in Lake Kinneret. Limnol. Oceanogr. 1983, 28, 640–645. [Google Scholar] [CrossRef]
- Sanciolo, P.; Zou, L.; Gray, S.; Leslie, G.; Stevens, D. Accelerated seeded precipitation pre-treatment of municipal wastewater to reduce scaling. Chemosphere 2008, 72, 243–249. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J. Aquatic Chemistry: Chemical Equilibria and Rates. In Natural Waters, 3rd ed.; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Yagi, S.; Fukushi, K. Removal of phosphate from solution by adsorption and precipitation of calcium phosphate onto monohydrocalcite. J. Colloid Interface Sci. 2012, 384, 128–136. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y. Phosphorus-sorption characteristics of calcareous soils and limestone from the Southern Everglades and adjacent farmlands. Soil Sci. Soc. Am. J. 2001, 65, 1404–1412. [Google Scholar] [CrossRef]
- House, W.A. The physico-chemical conditions for the precipitation of phosphate with calcium. Environ. Technol. 1999, 20, 727–733. [Google Scholar] [CrossRef]
- Reddy, K.R.; Fisher, M.M.; Ivanoff, D. Resuspension and diffusive flux of nitrogen and phosphorus in a hypereutrophic lake. J. Environ. Qual. 1996, 25, 363–371. [Google Scholar] [CrossRef]
- Rosell, E.A. Influence of Resuspension on Sediment-Water Solute Exchange and Particle Transport in Marine Environments. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 2011. Available online: https://gupea.ub.gu.se/bitstream/2077/27061/1/gupea_2077_27061_1.pdf (accessed on 13 December 2019).
- Fan, C.; Zhang, L.; Qu, W.C. Lake sediment resuspension and caused phosphate release – A simulation study. J. Environ. Stud. (China) 2001, 13, 406–410. [Google Scholar]
- Kuo, S.; Lotse, E.G. Kinetics of phosphate adsorption by calcium carbonate and Ca-kaolinite. Soil Sci. Soc. Am. J. 1972, 36, 725–729. [Google Scholar] [CrossRef]
- Lin, Y.; Singer, P.C. Inhibition of calcite precipitation by orthophosphate: speciation and thermodynamic considerations. Geochim. Cosmochim. Acta 2006, 70, 2530–2539. [Google Scholar] [CrossRef]
- Koschel, R.; Casper, P.; Gonsiorczyk, T.; Rossberg, R.; Wauer, G. Hypolimnetic Al- and CaCO3-treatments and aeration for restoration of a stratified eutrophic hardwater lake (Lake Tiefwarensee, Mecklenburg-Vorpommern, Germany). Verh. Des Int. Ver. Limnol. 2006, 29, 2165–2171. [Google Scholar]
- Staudinger, B.; Peiffer, S.; Avnimelech, Y.; Berman, T. Phosphorus mobility in pore waters of sediments in Lake Kinneret, Israel. Hydrobiologia 1990, 207, 167–177. [Google Scholar] [CrossRef]
- Müller, B.; Wang, Y.; Wehrli, B. Cycling of calcite in hard-water lakes of different trophic states. Limnol. Oceanogr. 2006, 51, 1678–1688. [Google Scholar] [CrossRef]
- Golterman, H.L. The Chemistry of Phosphate nnd Nitrogen Compounds in Sediments, 1st ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Søndergaard, M. Nutrient dynamics in lakes—With emphasis on phosphorus, sediment and lake restoration. Ph.D. Thesis, (DSc), National Environmental Research Institute of Denmark, University of Aarhus, Aarhus, Denmark, 2007. Available online: https://www2.dmu.dk/pub/dsc_ms_uk.pdf (accessed on 13 December 2019).
- Golterman, H.L. The calcium- and iron-bound phosphate phase diagram. Hydrobiologia 1988, 159, 149–151. [Google Scholar] [CrossRef]
- Gonsiorczyk, T.; Casper, P.; Koschel, R. Phosphorus-binding forms in the sediment of an oligotrophic and an eutrophic hardwater lake of the Baltic Lake District (Germany). Water Sci. Technol. 1998, 37, 51–58. [Google Scholar] [CrossRef]
- Ann, Y.; Reddy, K.R.; Delfino, J.J. Influence of redox potential on phosphorus solubility in chemically amended wetland organic soils. Ecol. Eng. 1999, 14, 169–180. [Google Scholar] [CrossRef]
- Giguet-Covex, C.; Poulenard, J.; Chalmin, E.; Arnaud, F.; Rivard, C.; Jenny, J.P.; Dorioz, J.-M. XANES spectroscopy as a tool to trace phosphorus transformation during soil genesis and mountain ecosystem development from lake sediments. Geochim. Cosmochim. Acta 2013, 118, 129–147. [Google Scholar] [CrossRef]
- Adam, N. A Wet–Chemical and Phosphorus K-edge X-ray Absorption Near Edge Structure Investigation of Phosphate Adsorption on Binary Mixtures of Ferrihydrite and Calcite: Implications for Phosphorus Bioavailability. Soil Sci. Soc. Am. J. 2017, 81, 1079–1087. [Google Scholar] [CrossRef]
- Gomez, E.; Durillon, C.; Rofes, G.; Picot, B. Phosphate adsorption and release from brackisch lagoons: pH, O2 and loading influence. Water Res. 1999, 33, 2437–2447. [Google Scholar] [CrossRef]
- Song, Y.; Weidler, P.G.; Berg, U.; Nüesch, R.; Donnert, D. Calcite-seeded crystallization of calcium phosphate for phosphorus recovery. Chemosphere 2006, 63, 236–243. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).