Abstract
Membrane properties are determined by their morphology, which may be symmetric (dense) or asymmetric (dense/porous). Two membrane types based on the poly[(4,4′-oxydiphenylene)pyromelliteimide] (symmetric dense and asymmetric dense/porous) were prepared for a comparative study of morphology, physical properties, and transport characteristics in the pervaporation of methanol/MTBE mixture over a wide range of concentrations including the azeotropic composition. The asymmetric membrane is a good example of improving the transport properties of the polyimide by creating structure composed of a thin dense top layer on the surface of sponge-like microporous substrate. It was found that the use of the asymmetric membrane allows increasing the total flux in separation of azeotropic mixture by 15 times as compared with the dense membrane.
1. Introduction
The socio-economic development in both industrialized and developing nations creates an increase of the energy demand, expected to double by 2050 [1]. Consequently, there is an urgent need to implement more efficient energy resources which will help to reduce the emissions of contaminant gases to the atmosphere. In this regard, the actual task is the isolation of pure organic substances (alcohols and ethers), including methyl tert-butyl ether (MTBE), which are used as gasoline additives to produce ecological fuel with high octane levels [1]. Due to the high oxygen content, these compounds increase the detonation resistance of the fuel, and also reduce its consumption. Blended with gasoline, MTBE increases an octane level and decreases carbon monoxide emissions, acting as an excellent oxygenated additive.
The MTBE is synthesized from isobutylene and methanol in the presence of an acid catalyst; an excess of methanol is often added to increase the yield of the reaction. Purification of the final MTBE from methanol admixtures was usually carried out by distillation; however, this method is not energy-saving and its application is difficult due to the formation of azeotropic methanol/MTBE mixture containing 14.3 wt% methanol at 20 °C, 760 mm Hg [2].
Recently, membrane technologies for liquid mixture separation have been successfully used. The pervaporation method is a promising alternative to distillation and azeotropic rectification, since it is characterized by low energy costs and makes it possible to carry out the separation of azeotropic mixtures without introducing additional components [3]. For the purification of MTBE from methanol by pervaporation, hydrophilic polymers have been mainly used as membrane materials, they are poly(vinyl alcohol) [4], poly(vinyl alcohol)/cellulose acetate [5], poly(vinyl pyrrolidone) [6], polyarylethersulfone with cardo [7], poly(ether ether ketone) [8].
Polyimides are promising membrane materials due to their high thermal stability, mechanical strength, and good film-forming properties [9]. Matrimid membranes has been studied in pervaporation of azeotropic methanol/MTBE mixture in [10]. With increasing temperature to 45 °C, the total flux and the separation factor increased, reaching 73 g·m−2·h−1 and 21, respectively, which exceeds the performance of some previously studied polymer membranes: (poly(ether ether ketone) and crosslinked poly(vinyl alcohol). Membranes based on Matrimid modified with graphene oxide (1–4 wt%) showed an increased total flux, but a decreased selectivity in pervaporation of the azeotropic methanol/MTBE mixture [11].
In our previous work [12], two dense flat membranes composed of polymer-metal complexes based on 2,2/-biquinoline-8,8/-dicarboxylic acid with Cu(I) and its thermally rearranged polybenzoxazinoneimide-8,8/-Cu(I) have been developed and studied in pervaporation of a methanol/MTBE mixture. These membranes were enough selective with respect to methanol, but they had moderate total flux (15 g·m−2·h−1) in separation of azeotropic mixture.
In the present paper, for the first time, membranes based on commercially available infusible poly[(4,4′-oxydiphenylene)pyromelliteimide] (PI-PM), marked in literature as PMDA-ODA or Kapton, are studied for pervaporation separation of a methanol/MTBE mixture. An important aspect of membrane properties prediction is its morphology that may be symmetric (dense) or asymmetric (dense/porous). As a rule, when examining new polymer or composite materials, researchers employ dense symmetric membranes with a thickness of about 20–150 μm. However, only highly permeable asymmetric membranes are suitable for an industrial application. An asymmetric membrane consists of a very thin, dense top layer (0.1–5 μm) casted on a porous substrate with a thickness of about 150 μm. It combines both the high selectivity of a dense membrane with the high permeability of a very thin membrane. Asymmetric dense/porous membrane is obtained by phase inversion in which the polymer is transferred from a solution to a solid state in a controlled manner, most often by precipitation upon immersion. The industrial production of asymmetric membranes has evolved considerably in recent years [13].
The aim of the work is to prepare and to compare symmetric dense and asymmetric dense/porous membranes based on PI-PM during the pervaporation of methanol/MTBE mixture in a wide range of concentrations, including the azeotropic point. In the course of the work, the morphology, physical properties, and transport characteristics of PI-PM membranes are estimated.
2. Materials and Methods
2.1. Materials
The poly(pyromellitic dianhydride-co-4,4′-oxydianiline) amic acid) (PAA-PM) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) (in the form of 15 ± 5 wt% solution in N-methylpyrrolidone (NMP)/aromatic hydrocarbons (80%/20% solvent ratio). NMP, ethanol and hexane were obtained from commercial suppliers and used without further purification. Methanol and MTBE of chemically pure (CP) grade were purchased from Vekton (St.Petersburg, Russia) and were used as received. Distilled water was used throughout the experiments.
2.2. Preparation of Asymmetric and Dense Membranes
The 12 wt% PAA-PM casting solution in NMP containing two moles of benzimidazole (BI) per one mole-unit of PAA-PM was prepared and used for asymmetric and dense membrane formation. The chemical composition of the casting solution and a coagulating bath were optimized in [14].
To obtain asymmetric dense/porous membrane by phase inversion technique, the PAA-PM casting solution was poured onto a glass plate using a casting knife with a nominal thickness of 0.3–0.4 mm. After two minutes of preforming in the air, the glass plate was immersed into a coagulating bath with ethanol/water mixture containing 40 wt% ethanol, where the membrane was formed by the phase inversion method for 5 h. The membrane was then washed by immersing in methanol and hexane. Finally, the PAA-PM membrane was annealed in a stepwise manner: at 120 °C for 30 min; at 140 °C for 20 min; at 160 °C for 20 min; at 180 °C for 20 min; at 200 °C for 30 min in a “SNOL 7.2/1100, Lithuania” electrical (Umega Group, AB, Utena, Lithuania) furnace in an argon atmosphere. The asymmetric membrane thickness was about 130 µm. Figure 1 shows transformation of the PAA-PM chemical structure into the PI-PM. The PAA-PM conversion into insoluble PI-PM was controlled by IR spectroscopy using a Bruker Vertex-70 [14].
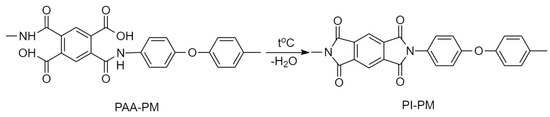
Figure 1.
Transformation of the PAA-PM chemical structure into the PI-PM.
To obtain dense symmetric membranes, the PAA-PM casting solution in NMP was poured onto a glass plate which was placed in a thermostat to evaporate the solvent at 80 °C. Finally, the PAA-PM membrane was annealed in a stepwise manner (up to 200 °C) as described above to convert it into insoluble PI-PM membrane. The dense membrane thickness was about 30 µm.
2.3. Membrane Characterizations
Images of surface and cross-section of membranes were observed by a Zeiss SUPRA 55VP scanning electron microscope (SEM) (Carl Zeiss, Oberkochen, Germany) equipped with in-lens SE and SE2 secondary electron detectors, a secondary electron detector for low vacuum mode (VPSE), and a four-quadrant backscattered electron detector (AsB). The membrane samples were cut into 0.5 cm2, attached with double-side tape to steel stabs. For cross-sectional micrographs the membranes were immersed in liquid nitrogen and then fractured. Before tests, all samples were coated with a 20 nm thick carbon layer using a Quorum 150 cathode sputtering installation (Quorum Technologies Ltd., Lewes, UK).
To estimate the hydrophilicity of the membranes, the contact angles of water and ethanol on the dense PI-PM membrane were measured via the sessile drop technique using a DSA 10 drop shape analyzer (KRÜSS GmbH, Hamburg, Germany) at room temperature and atmospheric pressure. Before the experiment, the samples were kept in a desiccator to remove moisture adsorbed from the atmosphere. Surface tension () of water and ethanol are equal to 72.4 mN/m and 21.4 mN/m, respectively.
Membrane surface tension (σs) was calculated by the Owens–Wendt method [15]. In accordance with this method, the surface tension includes two components:
where is polar, and is the dispersion component of the surface tension of the film. Using the cosine () of contact angles for two measured fluids and table data of polar () and dispersion () components of the liquids surface tension, the following equation was solved:
2.4. Sorption Research
The sorption experiment was carried out by the immersion of the dense PI-PM membrane samples in a pure liquid at atmospheric pressure and ambient temperature ~25 °C. Samples were removed from the liquid at regular intervals and wiped with a tissue paper, and then they were weighed on an analytical balance with an accuracy of 10−4 g. The experiment continued until equilibrium state was achieved.
The sorption degree (S) was determined as the difference between the weights of the swollen and dry membranes after a desorption experiment, referred to the mass of the dry membrane:
where Ms is the weight of the swollen membrane, Md is the weight of the dry membrane.
To study the diffusion properties of penetrant molecules through the polymer matrix of membranes, kinetic desorption curves were plotted in the coordinates Mt/M∞ and t1/2/l, where Mt is the amount of desorbed substance over time t, M∞ is the equilibrium amount of desorbed substance, defined as the difference between the weight of the swollen membrane and the weight of the membrane dried to constant weight, l is the thickness of the membrane. From the slope of the linear portion of the obtained curves, the diffusion coefficients (D) were calculated (in this case, the initial moments of the diffusion flow time when Mt/M∞ ~ 0.4 were considered) [16,17]:
2.5. Pervaporation
Pervaporation experiments were conducted via lab-scale apparatus with stirring (Figure 2). The effective area of the membrane supported by a metal disk with small gaps was approximately 14.8 cm2. Downstream pressure below 10–2 mm Hg was maintained on the permeate side with vacuum pump MD 1C (Vacuubrand GMBH, Wertheim, Germany). The permeate was collected into a trap immersed in liquid nitrogen, and weighted with the balance Mettler Toledo ME204 (Mettler Toledo, Columbus, Ohio, US). The feed and the permeate concentration was analyzed with a Chromatec–Crystal 5000.2 gas chromatograph (Yoshkar-Ola, Chromatec, Russia) with thermal conductivity detector. The experiments were repeated three times and the average value of the results was considered with an accuracy of ±0.1 wt%.
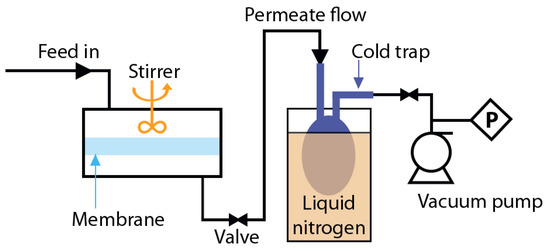
Figure 2.
Scheme of a pervaporation test setup.
Total flux (J) through membrane was determined as follows:
where Q is the weight of permeate (g), A is the effective membrane area (m2), and t is the operating time (h).
The separation factor αMethnol/MTBE was defined according to the equation:
where Y and X are the weight fraction of component in the permeate and feed, respectively.
3. Results
Two types of membranes (asymmetric dense/porous and symmetric) composed of infusible and insoluble poly[(4,4′-oxydiphenylene)pyromelliteimide] (PI-PM) were prepared from its prepolymer by casting 12 wt% PAA-PM solution in NMP on a glass plate. After formation of asymmetric or dense structure, the membranes were heated for solid phase conversion of PAA-PM into insoluble PI-PM. The effective catalytic method [18] using addition of BI catalyst in the casting solution allows low-temperature (200 °C) conversion of the PAA-PM into PI-PM to preserve porous structure in asymmetric membranes [19].
3.1. Membrane Morphology
Scanning electron microscopy was used to study the membrane structure. Figure 3 and Figure 4 show the micrographs of top layer and cross-section of symmetric dense and asymmetric membranes and bottom of asymmetric membranes. As can be seen from Figure 3, a surface of the dense PI-PM membrane has a homogeneous defect-free structure. Some creases can be seen on the cross-section image. Probably, the sample was damaged during fractioning. The asymmetric membrane (Figure 4) exhibits an anisotropic structure consisting of a thin dense top layer (3 µm) and a sponge-like microporous substrate, the size of the substrate pores is 0.1–0.15 mm. Pores in the bottom layer are open but not through. The formation of such asymmetric structure leads to fabrication of membrane materials with improved performance. The dense top layer provides selective transport while defect-free sponge-like microporous substrate promotes a higher flux through the asymmetric membrane as compared to the symmetric dense membrane.
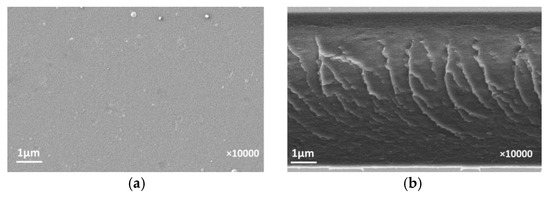
Figure 3.
SEM images of symmetric dense membrane: (a) top layer and (b) cross-section.

Figure 4.
SEM images of asymmetric membrane: (a) top layer, (b) cross-section, and (c) bottom.
3.2. Membrane Characterization
Contact angle is the most widespread characteristic of solid surface tension. The water contact angle is 73.4° suggesting the relatively hydrophilic surface. Thus, the PI-PM membrane has a considerable methanol capacity when separating the methanol/MTBE solutions. In our previous works [12,20], water contact angles were measured for various polyimide membranes: P84 (72.0°) and thermally rearranged polyimide (73.1°). It is indicating the similar wettability of polyimide membranes, which confirmed the data validity for PI-PM membrane.
The surface of PI-PM membrane was characterized by measuring contact angles of water and ethanol which were used to calculate polar (σp) and dispersion (σd) contributions to the critical surface tension (σs) (Table 1). It is known that the surface free energy and surface tension should be the sum of the contributions from different intermolecular forces, like van der Waals, dipole-dipole, and ion-dipole forces, or hydrogen-bonding [15]. Since the PI-PM membrane possesses hydrophilic properties, the contribution of polar forces is bigger than dispersive ones. It revealed that the membrane interacts with a liquid mixture mostly by hydrogen bonding during the pervaporation experiment.

Table 1.
Contact angles and surface tension of the PI-PM membrane.
3.3. Transport Properties
The PI-PM membranes were tested in the pervaporation system for a methanol/MTBE mixture to examine a separation efficiency. Table 2 lists some physical properties of methanol and MTBE, such as molecular weight, density, molecular volume, boiling point, dipole moment, and solubility parameters. It should be underlined that methanol and MTBE form an azeotropic mixture composed of 14.3 wt% methanol and 85.7 wt% MTBE which cannot be separated by distillation [12,21].

Table 2.
Properties of penetrants.
The mass transfer in pervaporation occurs according to the solution–diffusion mechanism. Separation is achieved through preferred sorption and dissolution of particular component of a mixture by the membrane material and owing to different diffusion rate of the penetrants. Mass transfer through PI-PM membranes was studied by sorption experiments for individual liquids (methanol and MTBE) and pervaporation tests toward their mixture.
Sorption
Sorption research was carried out in individual liquids (methanol, MTBE), as well as their mixtures using the samples of the dense PI-PM membrane. Table 3 shows the data on equilibrium sorption degrees and diffusion coefficients. The PI-PM membrane exhibits a high sorption activity towards methanol but it does not adsorb MTBE. The high sorption of methanol should be attributed to the close solubility parameters of methanol (29.7 (J/cm3)1/2) and PI-PM (34.6 (J/cm3)1/2) [22]. It should be noted that methanol has enough good diffusion activity due to the lower molar volume, which should contribute to its permeability through the membrane.

Table 3.
Data of the sorption research.
Figure 5 shows the dependence of the sorption degree on the methanol concentration in the methanol/MTBE mixture for the PI-PM membrane. The sorption degree increases with the growth of methanol content in the feed, because the membrane has good affinity to methanol. The observed shape of the sorption curve corresponds to type L3 in accordance with the classification system of Giles and Smith for solution adsorption isotherms [23] (or type II according to the BET adsorption theory [24]), which corresponds to the case of multimolecular adsorption of a component from a solution on a non-porous sorbent (membrane). The isotherm has a convex part relative to the axis of concentration, which indicates a stronger interaction between the molecules of the adsorbent (membrane) and adsorbate (methanol) than between the adsorbed molecules.
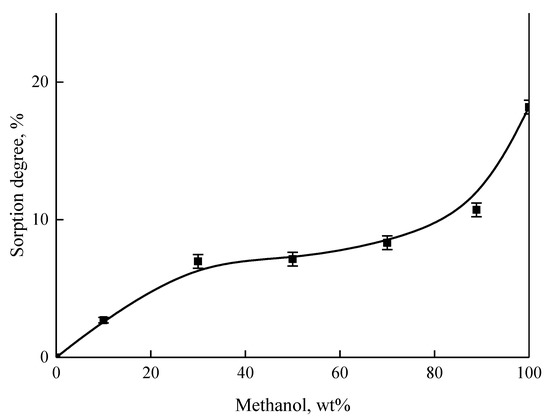
Figure 5.
The dependence of the sorption degree on the methanol content in the methanol/MTBE mixture for dense PI-PM membrane.
3.4. Pervaporation of the Methanol/MTBE Mixture
The pervaporation of the methanol/MTBE mixture was studied in a wide range of methanol concentrations at 50 °C using asymmetric and dense membranes. It was found that both membranes are preferably permeable to methanol and exhibit high selectivity in separation of methanol/MTBE mixture, including mixture compositions near the azeotropic point. Figure 6 shows the results of the pervaporation as well as the vapor-liquid equilibrium (VLE) curve of methanol and MTBE mixture at 50 °C and 760 mmHg. It can be seen that pervaporation curves are located higher than VLE curve, and this fact testifies the advantage of pervaporation technique over classical separation method of distillation.
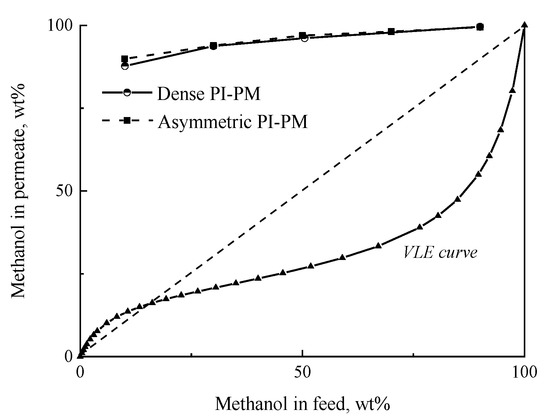
Figure 6.
The dependence of the methanol concentration in the permeate on the methanol concentration in the feed in the pervaporation of the methanol/MTBE mixture using asymmetric and dense membranes, 50 °C. The vapor-liquid equilibrium curve of methanol/MTBE at 50 °C.
It should be mention that the fabrication of asymmetric membrane does not worsen the effectivity of separation what is the fundamental challenge. It is probably due to the selection of optimal conditions of asymmetric membrane formation.
Figure 7 shows the dependence of total flux on the methanol concentration in the feed for asymmetric and dense membranes. It was found that total flux through the membranes increases with increasing alcohol concentration in the feed. An increase in methanol content leads to the enhancement of the penetrant solubility in the membrane due to the greater sorption activity of the membrane with respect to alcohol; as a result, an increase in performance takes place.
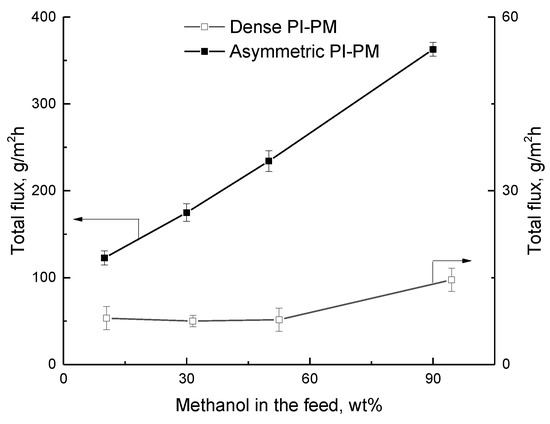
Figure 7.
The dependence of total flux on the methanol concentration in the feed in the pervaporation of methanol/MTBE mixture using asymmetric and dense membranes, 50 °C.
The much higher flux through asymmetric membrane as against symmetric membrane provides the combination of dense top layer and microporous substrate of asymmetric membrane.
The most important pervaporation application is the separation of methanol/MTBE azeotropic mixture (14.3 wt% methanol and 85.7 wt% MTBE). Figure 8 shows data on the total flux and separation factor in pervaporation of the azeotropic mixture using asymmetric and dense PI-PM membranes with overall thicknesses of 135 and 24 μm, respectively. The main transport parameters are better for asymmetric membrane as compared with dense membrane. The total flux for asymmetric membrane is greater by ~15 orders of magnitude than that for the dense membrane. These results show that the asymmetric membrane is more promising for use in the separation of methanol/MTBE mixture.
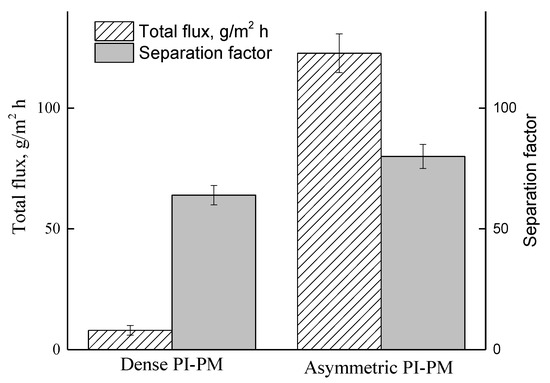
Figure 8.
Total flux and the separation factor of asymmetric and dense membranes in the pervaporation of azeotropic methanol/MTBE mixture (14.3:85.7 wt%).
The transport properties of the asymmetric PI-PM membrane were compared with published data on the separation of methanol/MTBE mixture by pervaporation. Table 4 lists data on the operating temperature, composition of the feed and permeate, as well as the values of total flux and separation factor that were obtained for different polymer membranes in a number of published works. It can be stated that dense symmetric PI-PM membrane possesses high selective properties which are comparable or superior than various dense membranes [8,10,12]. At the same time, the flux through the asymmetric PI-PM membrane is higher as compared to other membranes, except for some modified membranes [21,25,26]. In the case of asymmetric PI-PM membrane, it should be highlighted that the excellent specific productivity is observed along with good separation efficiency which should totally satisfy industrial needs. For the future more successful industrial applications, it would be promising to development hollow fibers membranes based on developed asymmetric membranes for greater increase of the flux.

Table 4.
Comparison of transport properties of polymer membranes in the pervaporation of methanol‒MTBE mixture.
4. Conclusions
In this work, commercially available infusible poly[(4,4′-oxydiphenylene)pyromelliteimide] (PI-PM) marked in literature as PMDA-ODA or Kapton was firstly studied for pervaporation separation of methanol/MTBE mixture. Two types of PI-PM membranes (asymmetric dense/porous and symmetric dense) were prepared from its prepolymer–polyamic acid solution in NMP. After formation of asymmetric or dense structure, the membranes were heated for solid phase conversion into insoluble PI-PM. The effective catalytic method using addition of benzimidazole catalyst in the casting solution allows low-temperature (200 °C) conversion into PI-PM to preserve porous structure in asymmetric membranes. SEM images of the asymmetric membrane reveal its anisotropic structure consisting of a thin dense top layer (3 µm) and a sponge-like microporous substrate.
Comparative research of homogeneous dense and asymmetric porous membranes was carried out in the pervaporation of methanol/MTBE mixture over a wide range of concentrations including the azeotropic point (14.3 wt% methanol and 85.7 wt% MTBE). The main transport parameters: total flux and separation factor of asymmetric membrane are better than that of dense membrane. The total flux for asymmetric membrane is greater by ~15 orders of magnitude than that for dense membrane. These results show that the polyimide asymmetric porous membrane is more promising for use in the purification of MTBE oxygenate by pervaporation.
Author Contributions
Conceptualization: A.P. and G.P.; investigation: A.P., M.T., I.F., and V.R.; methodology: A.P., and G.P.; writing—original draft: A.P., M.T., I.F., V.R., and G.P.; writing—review and editing: A.P., M.T., I.F., V.R., and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
The investigation of structure and transport properties of the membranes was funded the Russian Foundation for Basic Research (grant 18-33-01203). Authors would like to thank the Russian Science Foundation (RSF) (grant 18-79-10116) for financial support of asymmetric membrane formation studies.
Acknowledgments
Equipment of Resource Centers of St. Petersburg State University, namely, the Interdisciplinary Resource Center “Nanotechnologies”, “Thermogravimetric and calorimetric methods of investigation”, “Centre for X-ray Diffraction Studies”, “Cryogenic Department”, and the Education Resource Centre in the direction of chemistry were used for membrane investigations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alnsworth, S.J. Booming MTBE demand draws increasing number of producers. Chem. Eng. News 1991, 69, 13–16. [Google Scholar] [CrossRef]
- Sridhar, S.; Smitha, B.; Shaik, A. Pervaporation-Based Separation of Methanol/MTBE Mixtures—A Review. Sep. Purif. Rev. 2005, 34, 1–33. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; Wiley: Chichester, UK, 2012; ISBN 9780470743720. [Google Scholar]
- Khatinzadeh, G.; Mahdyarfar, M.; Mehdizadeh, A. Pervaporation (PV) Separation of Methanol/Methyl Tert-butyl Ether Mixtures in Low Permeate Pressure Conditions. J. Pet. Sci. Technol. 2017, 7, 43–48. [Google Scholar]
- Zhou, K.; Zhang, Q.G.; Han, G.L.; Zhu, A.M.; Liu, Q.L. Pervaporation of water-ethanol and methanol-MTBE mixtures using poly (vinyl alcohol)/cellulose acetate blended membranes. J. Memb. Sci. 2013, 448, 93–101. [Google Scholar] [CrossRef]
- Wu, H.; Fang, X.; Zhang, X.; Jiang, Z.; Li, B.; Ma, X. Cellulose acetate–poly(N-vinyl-2-pyrrolidone) blend membrane for pervaporation separation of methanol/MTBE mixtures. Sep. Purif. Technol. 2008, 64, 183–191. [Google Scholar] [CrossRef]
- Han, G.L.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Pervaporation separation of methanol/methyl tert-butyl ether mixtures using polyarylethersulfone with cardo membranes. Sep. Purif. Technol. 2013, 107, 211–218. [Google Scholar] [CrossRef]
- Zereshki, S.; Figoli, A.; Madaeni, S.S.; Simone, S.; Esmailinezhad, M.; Drioli, E. Pervaporation separation of MeOH/MTBE mixtures with modified PEEK membrane: Effect of operating conditions. J. Memb. Sci. 2011, 371, 1–9. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Wang, Y.; Chung, T.S.; Qiao, X.Y.; Lai, J.Y. Polyimides membranes for pervaporation and biofuels separation. Prog. Polym. Sci. 2009. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; Fíla, V.; Drioli, E.; Figoli, A. Matrimid®5218 dense membrane for the separation of azeotropic MeOH-MTBE mixtures by pervaporation. Sep. Purif. Technol. 2018, 199, 27–36. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; de la Iglesia, Ó.; Fíla, V.; Téllez, C.; Coronas, J.; Figoli, A. Graphene oxide—Filled polyimide membranes in pervaporative separation of azeotropic methanol–MTBE mixtures. Sep. Purif. Technol. 2019, 224, 265–272. [Google Scholar] [CrossRef]
- Pulyalina, A.; Polotskaya, G.; Goikhman, M.; Podeshvo, I.; Gulii, N.; Shugurov, S.; Tataurov, M.; Toikka, A. Preparation and characterization of methanol selective membranes based on polyheteroarylene—Cu(I) complexes for purification of methyl tertiary butyl ether. Polym. Int. 2017. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; Springer: Dordrecht, The Netherlands, 1996; ISBN 978-0-7923-4248-9. [Google Scholar]
- Polotskaya, G.A.; Meleshko, T.K.; Gofman, I.V.; Polotsky, A.E.; Cherkasov, A.N. Polyimide ultrafiltration membranes with high thermal stability and chemical durability. Sep. Sci. Technol. 2009, 44, 3814–3831. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Feng, X.; Huang, R.Y.M. Liquid Separation by Membrane Pervaporation: A Review. Ind. Eng. Chem. Res. 1997, 36, 1048–1066. [Google Scholar] [CrossRef]
- Binning, R.; Lee, R.; Jennings, J.; Martin, E. Separation of Liquid Mixtures by Permeation. Ind. Eng. Chem. 1961, 53, 45–50. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Kostereva, T.A.; Stepanov, N.G.; Shibaev, L.A.; Kuznetsov, Y.P. The effect of imidization on gas-separation properties of membranes based on poly(4,4′-oxydiphenylene)pyromellitimide. Vysokomol. Soedin. Ser.A Ser.B Ser.C Kratk. Soobshcheniya 1996, 38, 123–1238. [Google Scholar]
- Polotsky, A.; Cherkasova, V.; Potokin, I.; Polotskaya, G.; Meleshko, T. Chemically and thermally resistant polyimide ultrafiltration membranes prepared from polyamic acid. Desalination 2006, 200, 341–342. [Google Scholar] [CrossRef]
- Pulyalina, A.; Polotskaya, G.; Rostovtseva, V.; Pientka, Z.; Toikka, A. Improved Hydrogen Separation Using Hybrid Membrane Composed of Nanodiamonds and P84 Copolyimide. Polymers 2018, 10, 828. [Google Scholar] [CrossRef]
- Penkova, A.V.; Polotskaya, G.A.; Gavrilova, V.A.; Toikka, A.M.; Liu, J.-C.; Trchová, M.; Šlouf, M.; Pientka, Z. Polyamide Membranes Modified by Carbon Nanotubes: Application for Pervaporation. Sep. Sci. Technol. 2009, 45, 35–41. [Google Scholar] [CrossRef]
- Ma, X.-H.; Yang, S.-Y. Polyimide Gas Separation Membranes. In Advanced Polyimide Materials; Yang, S.-Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 257–322. ISBN 978-0-12-812640-0. [Google Scholar]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. 786. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 3973. [Google Scholar] [CrossRef]
- Brunauer, S. The adsorption of gases and vapors, vol.1; Princeton University Press: London, UK, 1943. [Google Scholar]
- Doghieri, F.; Nardella, A.; Sarti, G.C.; Valentini, C. Pervaporation of methanol-MTBE mixtures through modified poly(phenylene oxide) membranes. J. Memb. Sci. 1994, 91, 283–291. [Google Scholar] [CrossRef]
- Zereshki, S.; Figoli, A.; Madaeni, S.S.; Simone, S.; Drioli, E. Pervaporation separation of methanol/methyl tert-butyl ether with poly(lactic acid) membranes. J. Appl. Polym. Sci. 2010, 118, 1364–1371. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).