A20 and TNIP-3 Reduce NF-κB-Mediated Paracrine Responses to Hypoxia/Hyperglycemia-Induced Endothelial Senescence
Highlights
- Hypoxia, alone or combined with hyperglycemia, induces endothelial cell senescence without activating the classical pro-inflammatory SASP.
- This condition is associated with the upregulation of A20 and TNIP-3, suggesting a deviation from canonical senescence programs.
- The non-canonical senescence profile observed under hypoxia indicates that endothelial senescence may be more heterogeneous than previously recognised.
- The functional significance of A20 and TNIP-3 upregulation in this context remains to be clarified and represents an important direction for future studies.
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Experimental Design for Glucose and Hypoxia Treatments
2.3. Cell Viability Assays
2.4. Senescence-Associated β-Galactosidase Activity by Flow Cytometry
2.5. Multiplex Cytokine Quantification
2.6. Quantitative Reverse Transcription Polymerase Chain Reaction
2.7. Statistical Analysis
3. Results
3.1. Preliminary Assessment of EC Viability Under Hyperglycemia, Osmolarity and Hypoxia
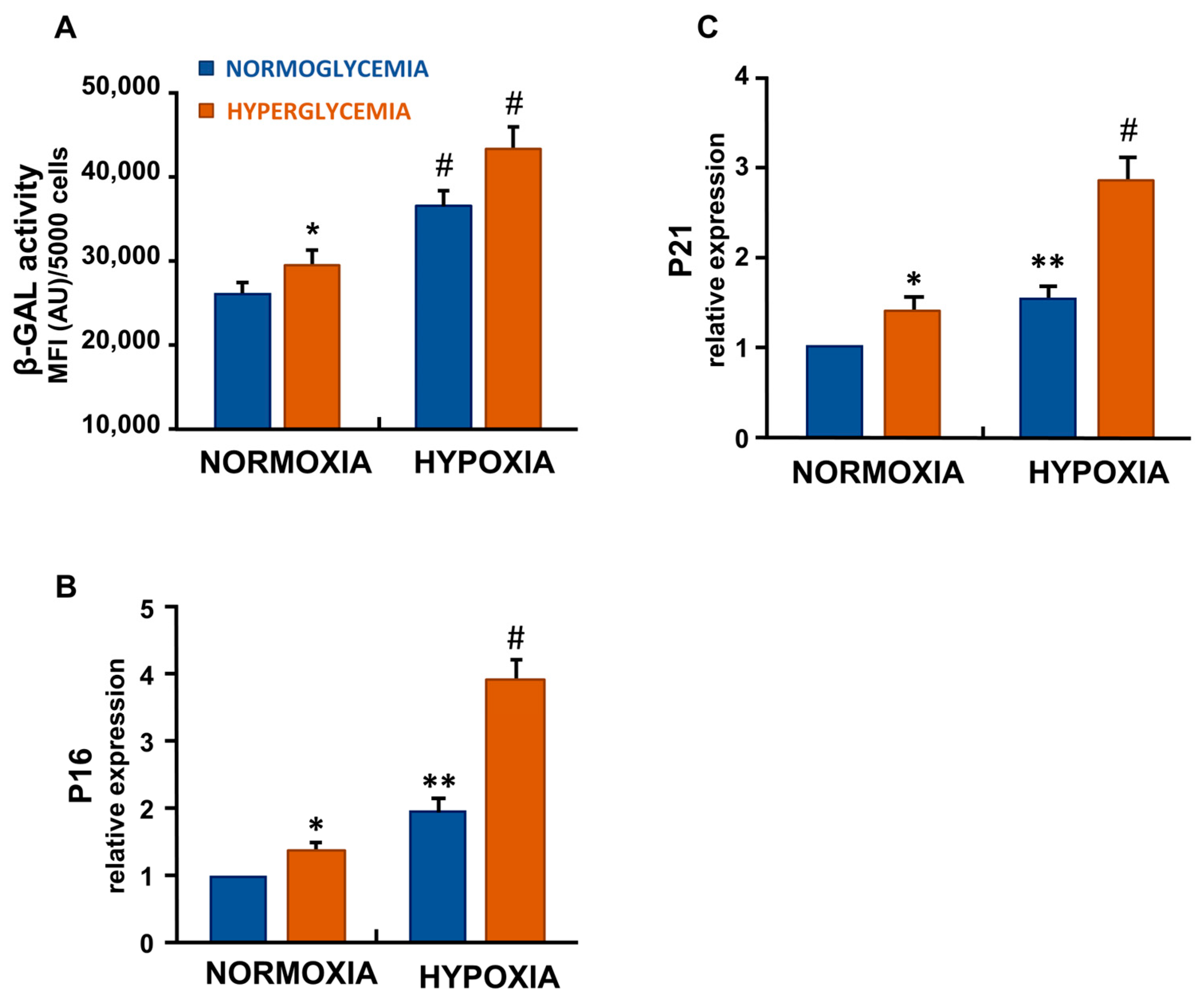
3.2. Hypoxia and Hyperglycemia Increased Senescence-Associated β-Galactosidase Activity in ECs
3.3. Hypoxia and High Glucose Increased the Gene Expression of p16 and p21 in ECs
3.4. Hypoxia Alone and Hypoxia Combined with High Glucose Did Not Elicit a Canonical Pro-Inflammatory SASP in ECs
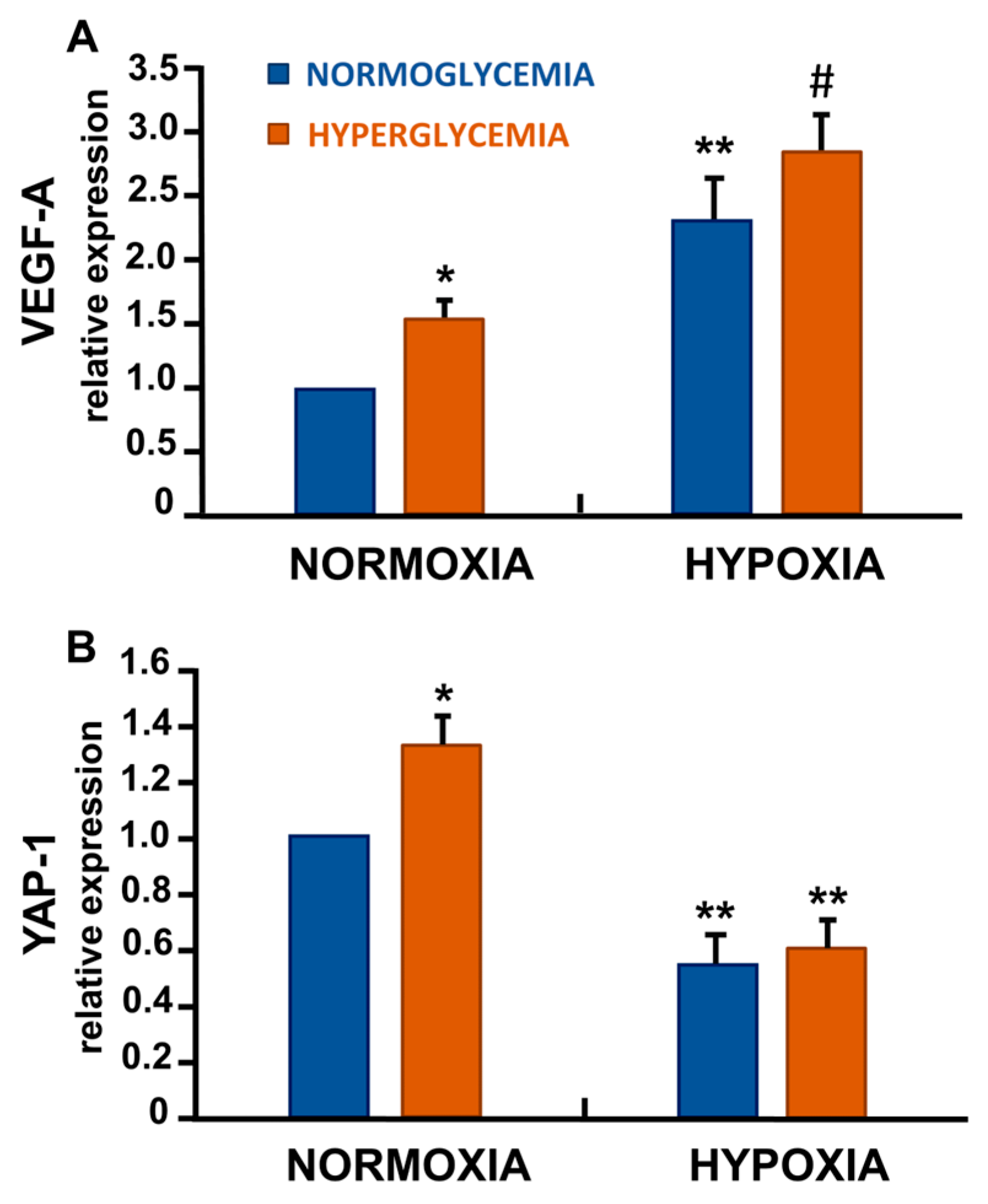
3.5. Differential Regulation of VEGF-A and Yes-Associated Protein-1 (YAP-1) in Response to Hypoxia and High Glucose
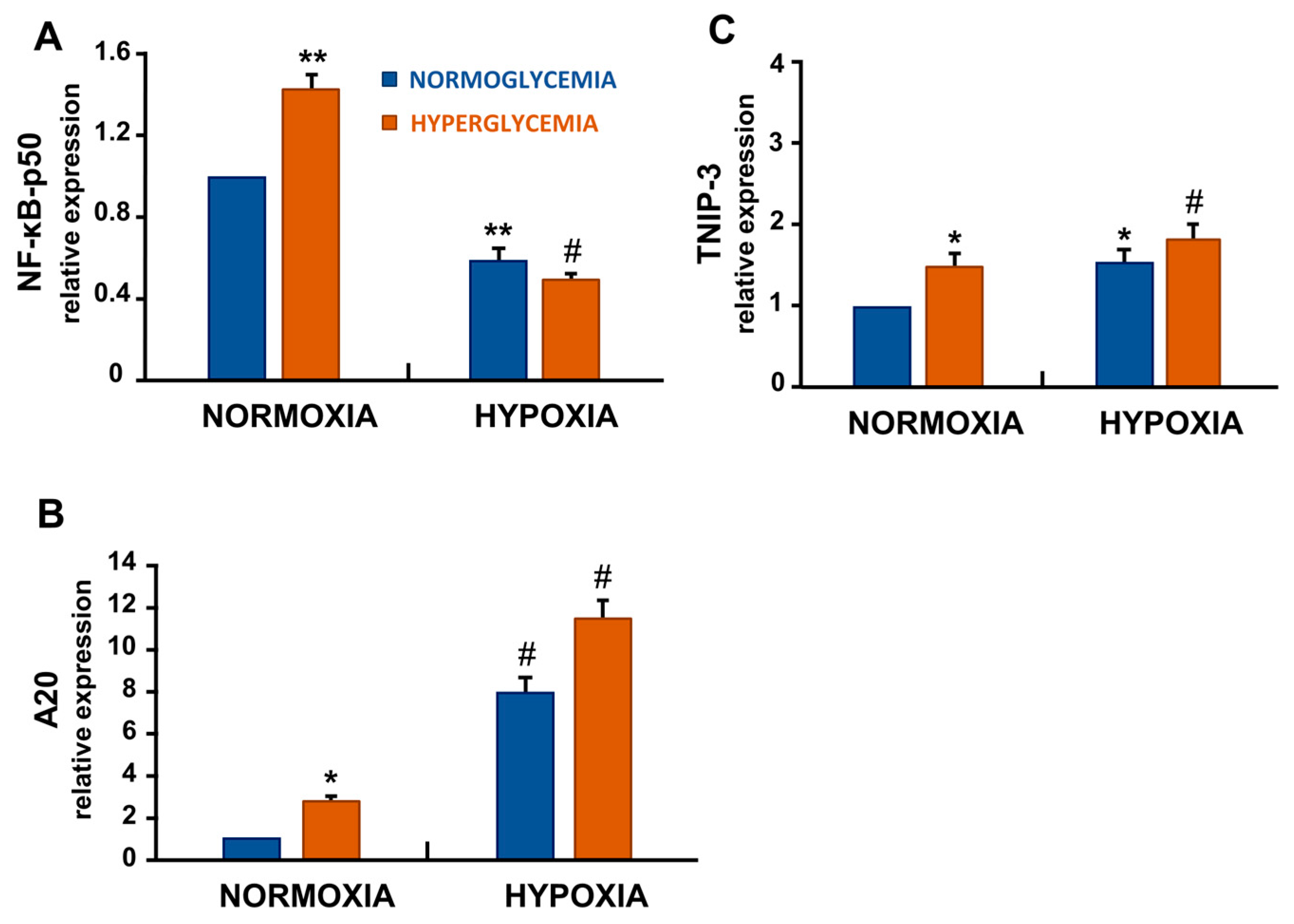
3.6. Hypoxia and High Glucose Shifted NF-κB Signalling by Decreasing p50 and Increasing A20/TNIP-3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EC | Endothelial Cell |
| SASP | Senescence-Associated Secretory Phenotype |
| NF-κB-p50 | Nuclear Factor-kappa B p50 subunit |
| SA-β-gal | Senescence-Associated β-galactosidase |
| p16 | protein 16 |
| p21 | protein 21 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| MCP-1 | Monocyte Chemotactic Protein-1 |
| VEGF-A | Vascular Endothelial Growth Factor-A |
| YAP-1 | Yes-Associated Protein-1 |
| TAZ | Transcriptional co-Activator with PDZ-binding motif |
| A20 | Tumor Necrosis Factor Alpha-Induced Protein 3 (TNFAIP3) |
| TNIP-3 | TNFAIP3 Interacting Protein 3 |
References
- Regina, C.; Panatta, E.; Candi, E.; Melino, G.; Amelio, I.; Balistreri, C.R.; Annicchiarico-Petruzzelli, M.; Di Daniele, N.; Ruvolo, G. Vascular ageing and endothelial cell senescence: Molecular mechanisms of physiology and diseases. Mech. Ageing Dev. 2016, 159, 14–21. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Jia, C.; Sowers, J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1802–1809. [Google Scholar] [CrossRef]
- Bloom, S.I.; Islam, M.T.; Lesniewski, L.A.; Donato, A.J. Mechanisms and consequences of endothelial cell senescence. Nat. Rev. Cardiol. 2023, 20, 38–51. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Kiss, T.; Wren, J.D.; Giles, C.B.; Griffin, C.T.; Murfee, W.L.; Pacher, P.; Csiszar, A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018, 15, 555–565. [Google Scholar] [CrossRef]
- Bertelli, P.M.; Pedrini, E.; Hughes, D.; McDonnell, S.; Pathak, V.; Peixoto, E.; Guduric-Fuchs, J.; Stitt, A.W.; Medina, R.J. Long term high glucose exposure induces premature senescence in retinal endothelial cells. Front. Physiol. 2022, 13, 929118. [Google Scholar] [CrossRef]
- Prattichizzo, F.; De Nigris, V.; Mancuso, E.; Spiga, R.; Giuliani, A.; Matacchione, G.; Lazzarini, R.; Marcheselli, F.; Recchioni, R.; Testa, R.; et al. Short-term sustained hyperglycaemia fosters an archetypal senescence-associated secretory phenotype in endothelial cells and macrophages. Redox Biol. 2018, 15, 170–181. [Google Scholar] [CrossRef]
- Csik, B.; Nyul-Toth, A.; Gulej, R.; Patai, R.; Kiss, T.; Delfavero, J.; Nagaraja, R.Y.; Balasubramanian, P.; Shanmugarama, S.; Ungvari, A.; et al. Senescent Endothelial Cells in Cerebral Microcirculation Are Key Drivers of Age-Related Blood-Brain Barrier Disruption, Microvascular Rarefaction, and Neurovascular Coupling Impairment in Mice. Aging Cell 2025, 24, e70048. [Google Scholar] [CrossRef] [PubMed]
- Novo, J.P.; Gee, L.; Caetano, C.A.; Tome, I.; Vilaca, A.; von Zglinicki, T.; Moreira, I.S.; Jurk, D.; Rosa, S.; Ferreira, L. Blood-brain barrier dysfunction in aging is mediated by brain endothelial senescence. Aging Cell 2024, 23, e14270. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Morgan, R.G.; Walker, A.E.; Lesniewski, L.A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell Cardiol. 2015, 89, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Meex, R.C.R.; Goossens, G.H. The role of tissue oxygenation in obesity-related cardiometabolic complications. Rev. Endocr. Metab. Disord. 2025, 26, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.W.; Marsch, E.; Treps, L.; Baes, M.; Carmeliet, P. Endothelial cell metabolism in health and disease: Impact of hypoxia. EMBO J. 2017, 36, 2187–2203. [Google Scholar] [CrossRef]
- Bitar, M.S. Diabetes Impairs Angiogenesis and Induces Endothelial Cell Senescence by Up-Regulating Thrombospondin-CD47-Dependent Signaling. Int. J. Mol. Sci. 2019, 20, 673. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, D.; Chen, S.; Zhang, S.; Gu, Q.; Shen, Y.; Xu, L.; Xu, X.; Wei, F.; Wang, N. UCP2-SIRT3 Signaling Relieved Hyperglycemia-Induced Oxidative Stress and Senescence in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2024, 65, 14. [Google Scholar] [CrossRef]
- Gao, H.; Nepovimova, E.; Heger, Z.; Valko, M.; Wu, Q.; Kuca, K.; Adam, V. Role of hypoxia in cellular senescence. Pharmacol. Res. 2023, 194, 106841. [Google Scholar] [CrossRef]
- Abdullaev, I.; Gayibov, U.; Omonturdiev, S.; Fotima, S.; Gayibova, S.; Aripov, T. Molecular pathways in cardiovascular disease under hypoxia: Mechanisms, biomarkers, and therapeutic targets. J. Biomed. Res. 2025, 39, 254–269. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Alique, M.; Sanchez-Lopez, E.; Bodega, G.; Giannarelli, C.; Carracedo, J.; Ramirez, R. Hypoxia-Inducible Factor-1alpha: The Master Regulator of Endothelial Cell Senescence in Vascular Aging. Cells 2020, 9, 195. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Martic, I.; Cavinato, M.; Weinberger, B. Detecting Senescence in T Cells by Flow Cytometry Using the SA-beta-Galactosidase Assay. Methods Mol. Biol. 2025, 2906, 73–81. [Google Scholar] [CrossRef]
- Adewoye, A.B.; Tampakis, D.; Follenzi, A.; Stolzing, A. Multiparameter flow cytometric detection and quantification of senescent cells in vitro. Biogerontology 2020, 21, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.X.; McElhaney, J.E.; Walston, J.D.; Xie, D.; Fedarko, N.S.; Kuchel, G.A. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Melandro, F.; Babboni, S.; Del Turco, S.; Ndreu, R.; Torri, F.; Martinelli, C.; Silvestrini, B.; Peris, A.; Lazzeri, C.; et al. An extensive evaluation of hepatic markers of damage and regeneration in controlled and uncontrolled donation after circulatory death. Liver Transpl. 2023, 29, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Khan, D.; Hussain, S.M.; Gerdes, N.; Hagenbeck, C.; Rana, M.; Cornelius, J.F.; Muhammad, S. Colchicine prevents oxidative stress-induced endothelial cell senescence via blocking NF-kappaB and MAPKs: Implications in vascular diseases. J. Inflamm. 2023, 20, 41. [Google Scholar] [CrossRef]
- Chen, M.; Ding, Z.; Zhang, F.; Shen, H.; Zhu, L.; Yang, H.; Chen, S. A20 attenuates hypoxia-induced pulmonary arterial hypertension by inhibiting NF-kappaB activation and pulmonary artery smooth muscle cell proliferation. Exp. Cell Res. 2020, 390, 111982. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Zhang, Y.; Liu, Y.; Zhang, H.; Wei, L.; Shen, T.; Jiang, C.; Zhu, D. A20 deficiency leads to angiogenesis of pulmonary artery endothelial cells through stronger NF-kappaB activation under hypoxia. J. Cell Mol. Med. 2016, 20, 1319–1328. [Google Scholar] [CrossRef]
- Verstrepen, L.; Carpentier, I.; Beyaert, R. The biology of A20-binding inhibitors of NF-kappaB activation (ABINs). Adv. Exp. Med. Biol. 2014, 809, 13–31. [Google Scholar] [CrossRef]
- Verhelst, K.; Verstrepen, L.; Coornaert, B.; Carpentier, I.; Beyaert, R. Cellular expression of A20 and ABIN-3 in response to Toll-like receptor-4 stimulation. Methods Mol. Biol. 2009, 517, 205–215. [Google Scholar] [CrossRef]
- Shi, H.; Yu, Y.; Li, D.; Zhu, K.; Cheng, X.; Ma, T.; Tao, Z.; Hong, Y.; Liu, Z.; Zhou, S.; et al. TNIP3 protects against pathological cardiac hypertrophy by stabilizing STAT1. Cell Death Dis. 2024, 15, 450. [Google Scholar] [CrossRef]
- van Uden, P.; Kenneth, N.S.; Rocha, S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem. J. 2008, 412, 477–484. [Google Scholar] [CrossRef]
- Murat, A.; Migliavacca, E.; Hussain, S.F.; Heimberger, A.B.; Desbaillets, I.; Hamou, M.F.; Ruegg, C.; Stupp, R.; Delorenzi, M.; Hegi, M.E. Modulation of angiogenic and inflammatory response in glioblastoma by hypoxia. PLoS ONE 2009, 4, e5947. [Google Scholar] [CrossRef] [PubMed]
- Boopathy, G.T.K.; Hong, W. Role of Hippo Pathway-YAP/TAZ Signaling in Angiogenesis. Front. Cell Dev. Biol. 2019, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Freire Valls, A.; Schermann, G.; Shen, Y.; Moya, I.M.; Castro, L.; Urban, S.; Solecki, G.M.; Winkler, F.; Riedemann, L.; et al. YAP/TAZ Orchestrate VEGF Signaling during Developmental Angiogenesis. Dev. Cell 2017, 42, 462–478.e7. [Google Scholar] [CrossRef]
- Zhang, C.; Bian, M.; Chen, X.; Jin, H.; Zhao, S.; Yang, X.; Shao, J.; Chen, A.; Guo, Q.; Zhang, F.; et al. Oroxylin A prevents angiogenesis of LSECs in liver fibrosis via inhibition of YAP/HIF-1alpha signaling. J. Cell Biochem. 2018, 119, 2258–2268. [Google Scholar] [CrossRef]
- Angom, R.S.; Kulkarni, T.; Wang, E.; Kumar Dutta, S.; Bhattacharya, S.; Das, P.; Mukhopadhyay, D. Vascular Endothelial Growth Factor Receptor-1 Modulates Hypoxia-Mediated Endothelial Senescence and Cellular Membrane Stiffness via YAP-1 Pathways. Front. Cell Dev. Biol. 2022, 10, 903047. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Chen, J.; Feng, H.; Peng, S.; Adams, U.; Bai, Y.; Huang, L.; Li, J.; Huang, J.; Meng, S.; et al. YAP/TEAD-mediated transcription controls cellular senescence. Cancer Res. 2013, 73, 3615–3624. [Google Scholar] [CrossRef]
- Mongiardi, M.P.; Merolle, M.; Fustaino, V.; Levi, A.; Falchetti, M.L. Gene expression profiling of hypoxic response in different models of senescent endothelial cells. Aging Clin. Exp. Res. 2021, 33, 1993–2001. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, L.; Babboni, S.; Del Turco, S.; Basta, G. A20 and TNIP-3 Reduce NF-κB-Mediated Paracrine Responses to Hypoxia/Hyperglycemia-Induced Endothelial Senescence. Cells 2025, 14, 1908. https://doi.org/10.3390/cells14231908
Russo L, Babboni S, Del Turco S, Basta G. A20 and TNIP-3 Reduce NF-κB-Mediated Paracrine Responses to Hypoxia/Hyperglycemia-Induced Endothelial Senescence. Cells. 2025; 14(23):1908. https://doi.org/10.3390/cells14231908
Chicago/Turabian StyleRusso, Lara, Serena Babboni, Serena Del Turco, and Giuseppina Basta. 2025. "A20 and TNIP-3 Reduce NF-κB-Mediated Paracrine Responses to Hypoxia/Hyperglycemia-Induced Endothelial Senescence" Cells 14, no. 23: 1908. https://doi.org/10.3390/cells14231908
APA StyleRusso, L., Babboni, S., Del Turco, S., & Basta, G. (2025). A20 and TNIP-3 Reduce NF-κB-Mediated Paracrine Responses to Hypoxia/Hyperglycemia-Induced Endothelial Senescence. Cells, 14(23), 1908. https://doi.org/10.3390/cells14231908







