Ionomic Profile and Nutrient Use Efficiency in Sunflower Plants Treated with Plant-Derived Biostimulant Rich in Trigonelline
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Treatment Description and Experimental Design
2.3. Plant Sampling and Biomass Measurement
2.4. Plant Analysis
2.4.1. Leaf Area
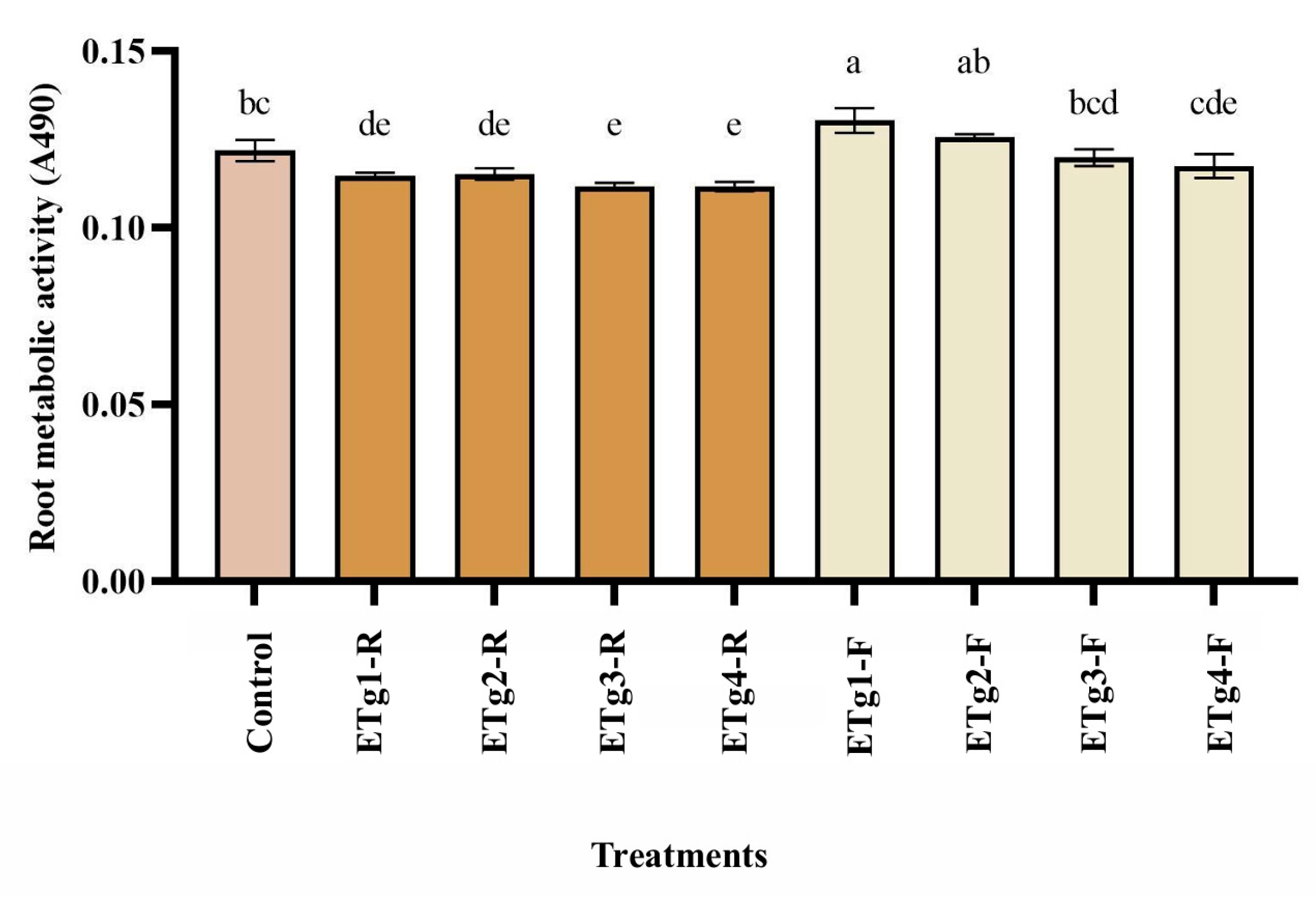
2.4.2. Root Metabolic Activity
2.4.3. Minerals and Nutrients Use Efficiency
2.5. Statistical Analysis
3. Results
3.1. Plant Growth
3.2. Root Metabolic Activity
3.3. Minerals
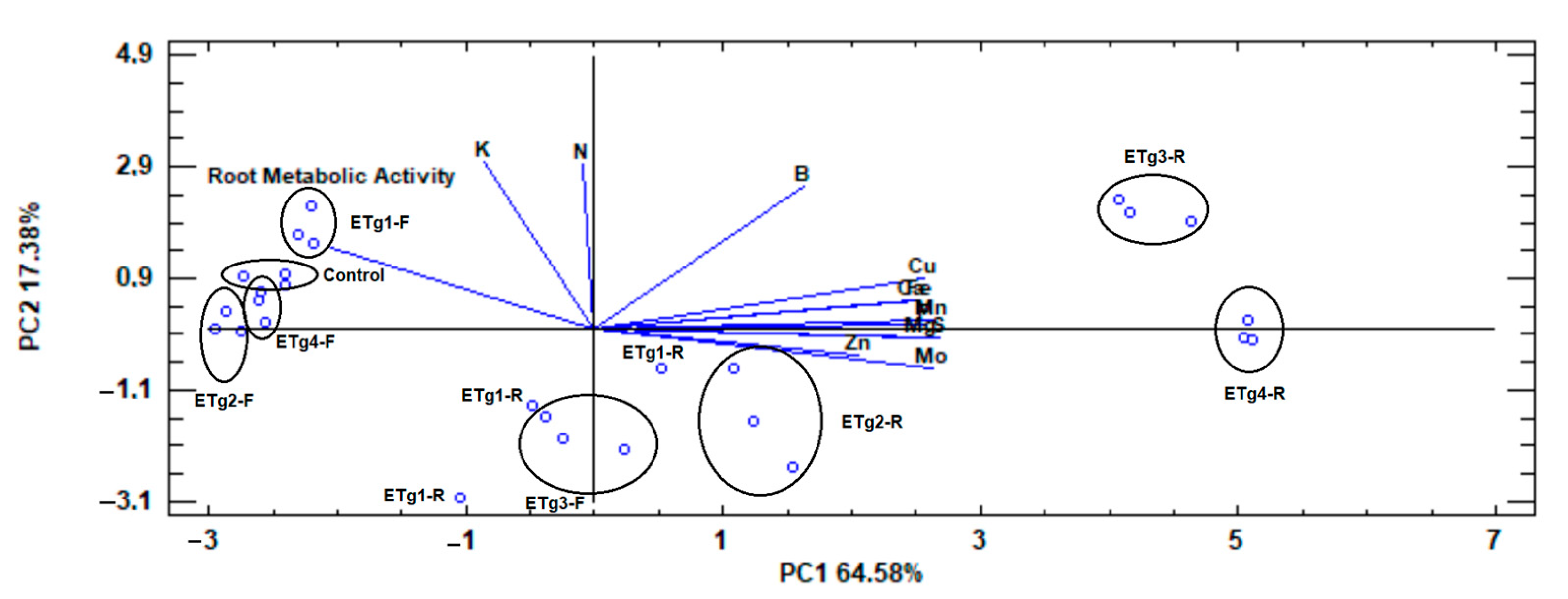
3.4. Principal Component Analysis
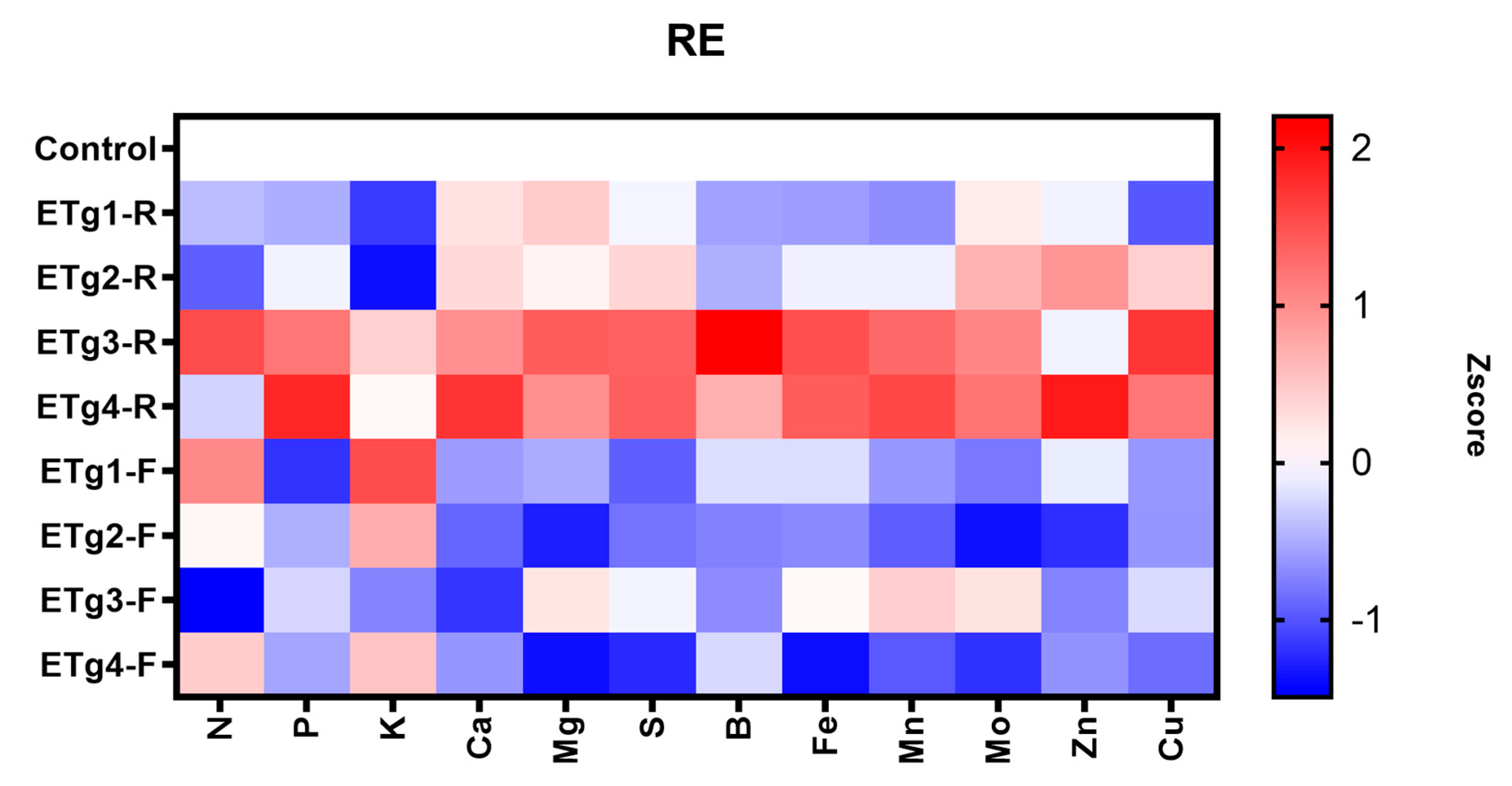
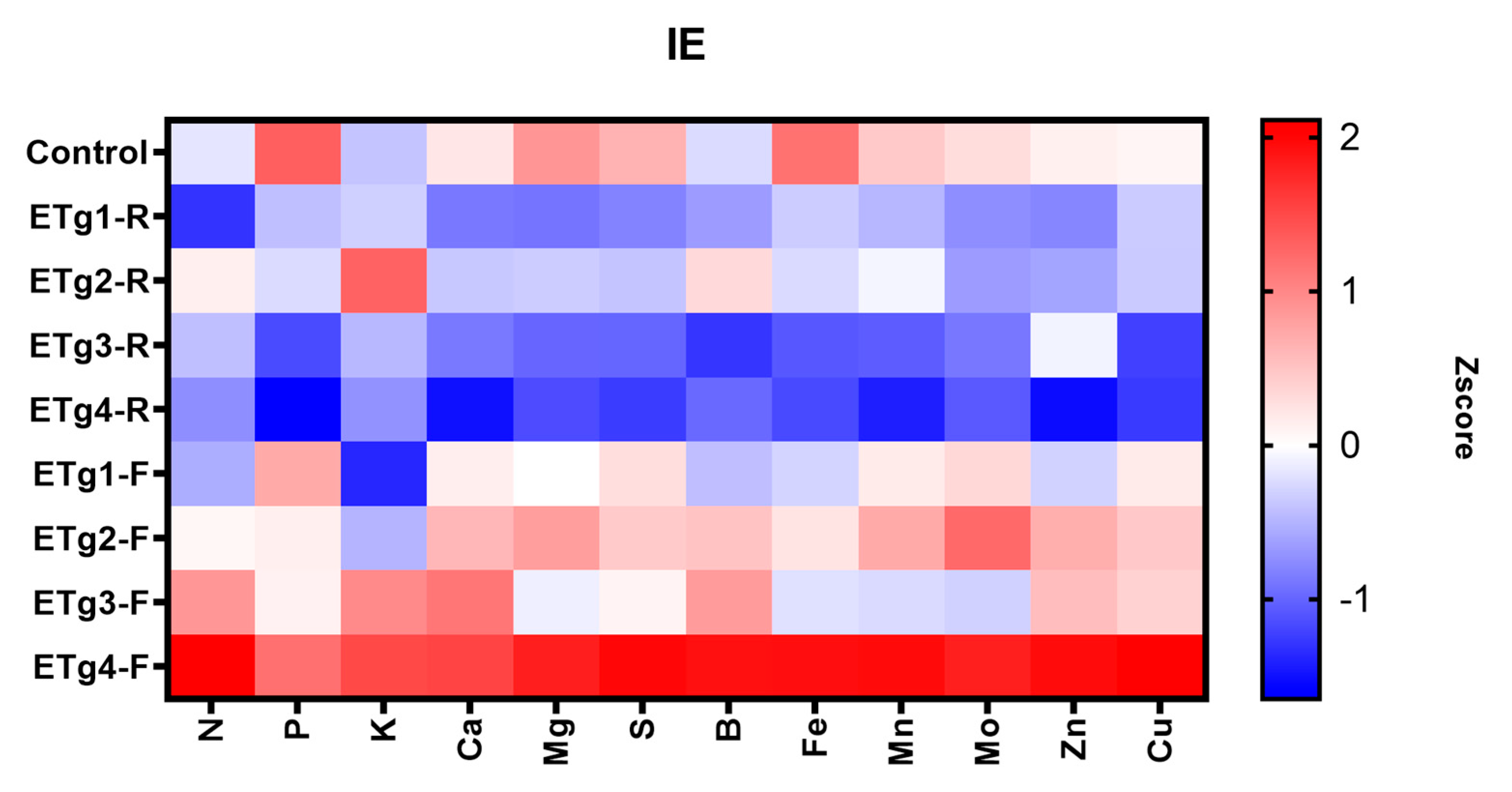
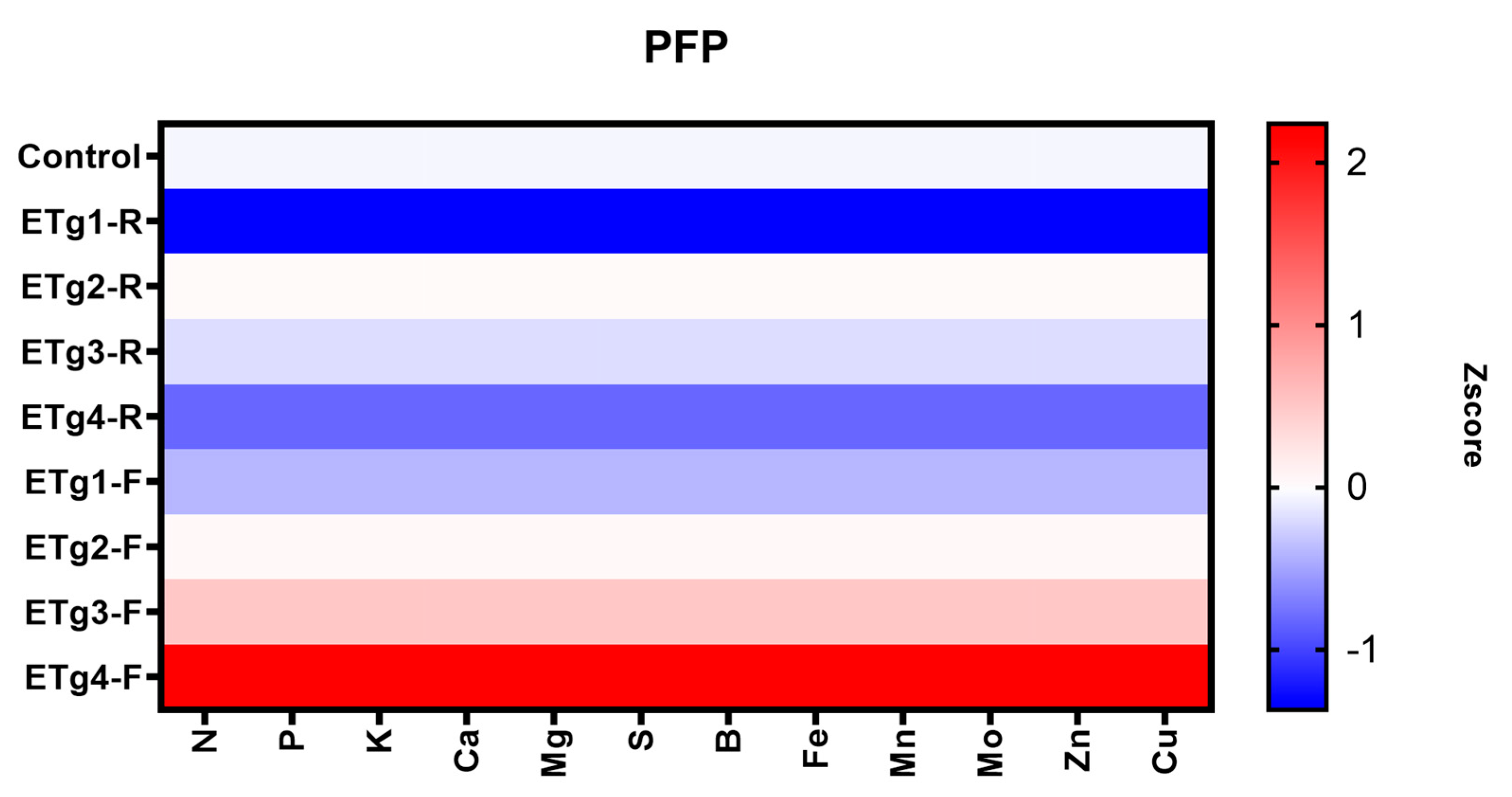
3.5. Parameters in Nutrient Use Efficiency
4. Discussion
4.1. Plant Growth
4.2. Root Metabolic Activity
4.3. Minerals
4.4. Principal Component Analysis
4.5. Parameters in Nutrient Use Efficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DW | Dry Weight |
| ETg1-R | Extract Trigonelline 1–Root |
| ETg2-R | Extract Trigonelline 2–Root |
| ETg3-R | Extract Trigonelline 3–Root |
| ETg4-R | Extract Trigonelline 4–Root |
| ETg1-F | Extract Trigonelline 1–Foliar |
| ETg2-F | Extract Trigonelline 2–Foliar |
| ETg3-F | Extract Trigonelline 3–Foliar |
| ETg4-F | Extract Trigonelline 4–Foliar |
| FW | Fresh Weight |
| IE | Internal Utilization Efficiency of the Applied Nutrient |
| PFP | Partial Factor Productivity of the Applied Nutrient |
| RE | Apparent Crop Recovery Efficiency of the Applied Nutrient |
References
- Kocira, S.; Szparaga, A.; Findura, P.; Treder, K. Modification of yield and fiber fractions biosynthesis in Phaseolus vulgaris L. by treatment with biostimulants containing amino acids and seaweed extract. Agronomy 2020, 10, 1338. [Google Scholar] [CrossRef]
- Searchinger, T.; Hanson, C.; Ranganathan, J.; Lipinski, B.; Waite, R.; Winterbottom, R.; Heimlich, R. Installment 1 of “Creating a Sustainable Food Future” The Great Balancing Act; World Resources Institute: Washington, DC, USA, 2013. [Google Scholar]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef]
- Hamedani, R.S.; Rouphael, Y.; Colla, G.; Colantoni, A.; Cardarelli, M. Biostimulants as a tool for improving environmental sustainability of greenhouse vegetable crops. Sustainability 2020, 12, 5101. [Google Scholar] [CrossRef]
- López-Padrón, I.; Martínez-González, L.; Pérez-Domínguez, G.; Reyes-Guerrero, Y.; Núñez-Vázquez, M.; Cabrera-Rodríguez, J.A. Uso de bioestimulantes en el cultivo del garbanzo. Cultiv. Trop. 2021, 42, e13. [Google Scholar]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- European Parliament and the Council. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products. Off. J. Eur. Union 2019, L 170, 1–114.
- Food and Agriculture Organization (FAO). Sustainable Food Systems: Concept and Framework; FAO: Rome, Italy, 2018.
- Martínez-Lorente, S.E.; Martí-Guillén, J.M.; Pedreño, M.Á.; Almagro, L.; Sabater-Jara, A.B. Higher plant-derived biostimulants: Mechanisms of action and their role in mitigating plant abiotic stress. Antioxidants 2024, 13, 318. [Google Scholar] [CrossRef]
- Dalal, A.; Bourstein, R.; Haish, N.; Shenhar, I.; Wallach, R.; Moshelion, M. Dynamic physiological phenotyping of drought-stressed pepper plants treated with “productivity-enhancing” and “survivability-enhancing” biostimulants. Front. Plant Sci. 2019, 10, 905. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann. Appl. Biol. 2021, 178, 169–191. [Google Scholar] [CrossRef]
- Bitarafan, Z.; Asghari, H.R.; Hasanloo, T.; Gholami, A.; Moradi, F.; Khakimov, B.; Liu, F.; Andreasen, C. The effect of charcoal on medicinal compounds of seeds of fenugreek (Trigonella foenum-graecum L.) exposed to drought stress. Ind. Crops Prod. 2019, 131, 323–329. [Google Scholar] [CrossRef]
- Naeem, M.; Aftab, T.; Khan, M.M.A. Fenugreek. Biology and Applications; Springer: Singapore, 2021. [Google Scholar]
- Syed, Q.A.; Rashid, Z.; Ahmad, M.H.; Shukat, R.; Ishaq, A.; Muhammad, N.; Rahman, H.U.U. Nutritional and therapeutic properties of fenugreek (Trigonella foenum-graecum): A review. Int. J. Food Prop. 2020, 23, 1777–1791. [Google Scholar] [CrossRef]
- Amiri, H.; Zamani, Z.; Arnao, M.B.; Ismaili, A.; Gavyar, P.H.H.; Khodayari, H. Optimal concentration of melatonin enhances drought stress tolerance in fenugreek. Acta Physiol. Plant. 2024, 46, 15. [Google Scholar] [CrossRef]
- Ashihara, H. Trigonelline (N-methylnicotinic acid) biosynthesis and its biological role in plants. Nat. Prod. Commun. 2008, 3, 1934578X0800300906. [Google Scholar] [CrossRef]
- Li, Y.; Ding, M.; Cui, C.; An, Q.; Wu, J.; Zhou, G.; Li, X.; Yu, S.; Bao, W. Overexpression of a gene encoding trigonelline synthase from Areca catechu L. promotes drought resilience in transgenic Arabidopsis. Plants 2022, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Minorsky, P.V. Trigonelline: A diverse regulator in plants. Plant Physiol. 2002, 128, 7–8. [Google Scholar] [CrossRef]
- Parwez, R.; Nabi, A.; Mukarram, M.; Aftab, T.; Khan, M.M.A.; Naeem, M. Various Mitigation Approaches Applied to Confer Abiotic stress tolerance in Fenugreek (Trigonella foenum-graecum L.): A review. In Fenugreek: Biology and Applications; Naeem, M., Aftab, T., Khan, M.M.A., Eds.; Springer: Singapore, 2021; pp. 137–185. [Google Scholar]
- Al-Khateeb, I.H. A New Method for the Isolation and Purification of Trigonelline as Hydrochloride from Trigonella foenum-graecum L. Baghdad Sci. J. 2019, 16, 2. [Google Scholar]
- McMichael, B.L.; Burke, J.J. Metabolic activity of cotton roots in response to temperature. Environ. Exp. Bot. 1994, 34, 201–206. [Google Scholar] [CrossRef]
- Wolf, B. An improved universal extracting solution and its use for diagnosing soil fertility. Commun. Soil Sci. Plant Anal. 1982, 13, 1005–1033. [Google Scholar] [CrossRef]
- Dobermann, A. Nutrient use efficiency–measurement and management. In Fertilizers Best Management Practices, Proceeding of the International Fertilizer Industry Association, Brussels, Belgium, 7–9 June 2007; Kraus, A., Isherwood, K., Heffer, P., Eds.; International Fertilizer Industry Association: Paris, France, 2007; pp. 1–22. [Google Scholar]
- Jindo, K.; Martim, S.A.; Navarro, E.C.; Pérez-Alfocea, F.; Hernandez, T.; Garcia, C.; Sforza, E.; Canellas, L.P. Root growth promotion by humic acids from composted and non-composted urban organic wastes. Plant Soil. 2012, 353, 209–220. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Epstein, E.; Bloom, A.J. Mineral Nutrition of Plants; Principles and Perspective; Sinauer Associates, Inc. Publishers: Sunderland, MA, USA, 2005. [Google Scholar]
- De Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef] [PubMed]
- Anschütz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R.; El-Serehy, H.A.; George, T.S.; Neugebauer, K. Linear relationships between shoot magnesium and calcium concentrations among angiosperm species are associated with cell wall chemistry. Ann. Bot. 2018, 122, 221–226. [Google Scholar] [CrossRef]
- Lea, T.D.; Duc Chua, H.; Quynh Lea, N. Improving nutritional quality of plant proteins through genetic engineering. Curr. Genom. 2016, 17, 220–229. [Google Scholar] [CrossRef]
- Kopriva, S.; Rennenberg, H. Control of sulphate assimilation and glutathione synthesis: Interaction with N and C metabolism. J. Exp. Bot. 2004, 55, 1831–1842. [Google Scholar] [CrossRef]
- Marschner, P.; Rengel, Z. Nutrient cycling in the rhizosphere. In The Rhizosphere; Academic Press: London, UK, 2018; pp. 57–89. [Google Scholar]
- Lilay, G.H.; Thiébaut, N.; du Mee, D.; Assunção, A.G.; Schjoerring, J.K.; Husted, S.; Persson, D.P. Linking the key physiological functions of essential micronutrients to their deficiency symptoms in plants. New Phytol. 2024, 242, 881–902. [Google Scholar] [CrossRef]
- Hantzis, L.J.; Kroh, G.E.; Jahn, C.E.; Cantrell, M.; Peers, G.; Pilon, M.; Ravet, K. A program for iron economy during deficiency targets specific Fe proteins. Plant Physiol. 2018, 176, 596–610. [Google Scholar] [CrossRef]
- Kroh, G.E.; Pilon, M. Regulation of iron homeostasis and use in chloroplasts. Int. J. Mol. Sci. 2020, 21, 3395. [Google Scholar] [CrossRef]
- Cakmak, I.; Pfeiffer, W.H.; McClafferty, B. Biofortification of durum wheat with zinc and iron. Cereal Chem. 2010, 87, 10–20. [Google Scholar] [CrossRef]
- Du Jardin, P. The Science of Plants Biostimulants: A Bibliographic Analysis; EU: Brussels, Belgium, 2012. [Google Scholar]
- Congreves, K.A.; Otchere, O.; Ferland, D.; Farzadfar, S.; Williams, S.; Arcand, M.M. Nitrogen use efficiency definitions of today and tomorrow. Front. Plant Sci. 2021, 12, 637108. [Google Scholar] [CrossRef] [PubMed]


| ETg1 | ETg2 | ETg3 | ETg4 | |
|---|---|---|---|---|
| Metabolites | ||||
| Acetate (mM) | 0.334 | 0.507 | 1.056 | 2.338 |
| Citrate (mM) | n.d | 2.368 | 0.497 | 0.928 |
| Glucose (mM) | 29.713 | 15.438 | 38.758 | 83.259 |
| Sucrose (mM) | 90.342 | 62.527 | 26.954 | 16.311 |
| Trigonelline (mM) | 0.311 | 1.576 | 2.481 | 5.317 |
| Nutrients | ||||
| C total (g 100 g−1) | 0.485 | 0.490 | 0.365 | 0.685 |
| N total (g 100 g−1) | 0.010 | 0.020 | 0.025 | 0.025 |
| P (mg L−1) | 1.755 | 9.375 | 13.760 | 25.630 |
| K (mg L−1) | 21.110 | 79.415 | 74.600 | 141.34 |
| Ca (mg L−1) | 86.94 | 86.805 | 87.705 | 87.025 |
| Mg (mg L−1) | 42.960 | 44.925 | 46.365 | 48.320 |
| S (mg L−1) | 32.470 | 25.810 | 26.210 | 26.055 |
| B (mg L−1) | 0.115 | 0.155 | 0.175 | 0.205 |
| Fe (mg L−1) | 0.050 | 0.040 | 0.230 | 0.315 |
| Mn (mg L−1) | 0.295 | 0.320 | 0.325 | 0.350 |
| Mo (mg L−1) | <0.01 | <0.01 | <0.01 | <0.01 |
| Zn (mg L−1) | 0.040 | 0.060 | 0.160 | 0.370 |
| Cu (mg L−1) | 0.030 | 0.070 | 0.090 | 0.135 |
| Other characterizations | ||||
| Choline (mM) | 0.068 | 0.373 | 0.899 | 1.751 |
| Total phenols (mg gFW−1) | 0.285 | 0.382 | 0.550 | 0.825 |
| Antioxidant capacity (Abs gFW −1) | 0.162 | 0.269 | 0.628 | 1.321 |
| Treatment | g FW Aerial Part | g DW Aerial Part | Leaf Area (cm2) | Plant Length (cm) |
|---|---|---|---|---|
| Control | 25.24 ± 1.51 e | 5.06 ± 0.47 b | 534.26 ± 26.68 c | 36.2 ± 0.7 c |
| ETg1-R | 24.61 ± 3.74 e | 5.15 ± 0.34 b | 551.61 ± 45.94 c | 34.5 ± 3.0 c |
| ETg2-R | 29.24 ± 2.33 d | 5.35 ± 0.32 b | 585.31 ± 41.14 bc | 34.6 ± 2.4 c |
| ETg3-R | 28.51 ± 1.92 d | 5.12 ± 0.17 b | 530.48 ± 22.87 c | 36.9 ± 0.6 bc |
| ETg4-R | 30.89 ± 3.41 d | 5.29 ± 0.50 b | 574.71 ± 38.34 bc | 36.7 ± 1.8 bc |
| ETg1-F | 27.91 ± 2.10 d | 5.20 ± 0.20 b | 547.42 ± 27.55 c | 36.2 ± 1.3 c |
| ETg2-F | 36.38 ± 1.67 c | 5.43 ± 0.31 b | 654.70 ± 15.22 ab | 40.8 ± 0.5 ab |
| ETg3-F | 38.95 ± 1.18 b | 5.99 ± 0.12 ab | 662.30 ± 42.85 ab | 40.6 ± 1.2 ab |
| ETg4-F | 44.30 ± 3.10 a | 6.48 ± 0.45 a | 680.22 ± 19.96 a | 42.8 ± 0.9 a |
| p-value | *** | ** | ** | *** |
| Treatment | g FW Root | g DW Root | Root Length (cm) |
|---|---|---|---|
| Control | 6.26 ± 0.46 b | 0.60 ± 0.03 b | 26.7 ± 2.5 bc |
| ETg1-R | 6.07 ± 0.44 b | 0.65 ± 0.03 b | 27.8 ± 1.8 bc |
| ETg2-R | 6.18 ± 0.35 a | 0.63 ± 0.01 b | 27.5 ± 1.8 bc |
| ETg3-R | 6.80 ± 0.46 a | 0.71 ± 0.02 a | 33.6 ± 2.0 a |
| ETg4-R | 6.20 ± 0.43 b | 0.58 ± 0.03 b | 25.9 ± 1.5 bc |
| ETg1-F | 6.03 ± 0.34 b | 0.61 ± 0.04 b | 25.7 ± 1.8 bc |
| ETg2-F | 6.11 ± 0.30 b | 0.62 ± 0.03 b | 24.8 ± 1.7 c |
| ETg3-F | 6.98 ± 0.19 a | 0.73 ± 0.02 a | 30.9 ± 1.4 ab |
| ETg4-F | 7.02 ± 0.63 a | 0.72 ± 0.02 a | 33.1 ± 1.4 a |
| p-value | ** | ** | ** |
| Treatment | N (mg g−1 DW) | P (mg g−1 DW) | K (mg g−1 DW) | Ca (mg g−1 DW) | Mg (mg g−1 DW) | S (mg g−1 DW) |
|---|---|---|---|---|---|---|
| Control | 68.4 ± 0.1 abc | 6.21 ± 0.02 d | 59.06 ± 0.23 b | 27.61 ± 0.01 d | 12.84 ± 0.20 d | 4.62 ± 0.01 c |
| ETg1-R | 67.0 ± 0.8 bc | 7.75 ± 0.16 c | 50.73 ± 1.32 d | 30.29 ± 0.92 c | 19.53 ± 0.81 b | 5.41 ± 0.10 b |
| ETg2-R | 66.8 ± 1.5 cd | 8.64 ± 0.04 b | 49.57 ± 0.63 d | 31.36 ± 0.34 c | 18.28 ± 1.04 b | 5.72 ± 0.02 b |
| ETg3-R | 69.2 ± 0.1 a | 8.30 ± 0.01 b | 48.77 ± 0.57 d | 34.43 ± 0.48 b | 18.14 ± 0.86 b | 5.46 ± 0.08 b |
| ETg4-R | 67.0 ± 0.1 bc | 10.43 ± 0.24 a | 46.69 ± 0.10 d | 31.25 ± 0.35 c | 18.50 ± 0.91 b | 5.97 ± 0.03 b |
| ETg1-F | 68.6 ± 0.2 ab | 6.71 ± 0.02 d | 64.64 ± 1.11 a | 26.96 ± 0.12 d | 15.67 ± 0.22 c | 4.77 ± 0.01 c |
| ETg2-F | 67.4 ± 0.1 bc | 7.92 ± 0.05 c | 60.55 ± 0.76 b | 25.95 ± 0.50 d | 13.14 ± 0.11 d | 4.85 ± 0.04 c |
| ETg3-F | 65.1 ± 0.4 d | 10.82 ± 0.14 a | 53.52 ± 0.55 c | 34.76 ± 0.52 a | 23.07 ± 0.79 a | 6.54 ± 0.07 a |
| ETg4-F | 68.1 ± 0.1 abc | 7.81 ± 0.03 c | 59.10 ± 0.30 b | 27.00 ± 0.21 d | 12.75 ± 0.32 d | 4.58 ± 0.01 c |
| p-value | ** | *** | *** | *** | *** | *** |
| Treatment | B (μg g−1 DW) | Fe (μg g−1 DW) | Mn (μg g−1 DW) | Mo (μg g−1 DW) | Zn (μg g−1 DW) | Cu (μg g−1 DW) |
|---|---|---|---|---|---|---|
| Control | 101.06 ± 0.21 bc | 89.77 ± 0.97 e | 264.32 ± 0.81 d | 4.53 ± 0.02 d | 54.80 ± 0.55 d | 16.98 ± 0.13 d |
| ETg1-R | 92.36 ± 2.30 e | 120.08 ± 3.81 d | 277.43 ± 8.91 d | 5.75 ± 0.16 c | 55.12 ± 0.83 cd | 15.89 ± 0.50 d |
| ETg2-R | 95.32 ± 0.59 de | 135.58 ± 0.90 c | 295.53 ± 3.42 c | 6.44 ± 0.04 b | 62.18 ± 0.57 b | 18.54 ± 0.10 c |
| ETg3-R | 115.00 ± 0.65 a | 139.53 ± 0.90 c | 319.35 ± 5.54 b | 5.85 ± 0.05 c | 55.79 ± 0.48 cd | 17.06 ± 0.08 c |
| ETg4-R | 103.02 ± 1.25 b | 164.35 ± 0.71 b | 304.05 ± 2.63 b | 7.05 ± 0.02 a | 67.19 ± 0.26 a | 20.36 ± 0.06 b |
| ETg1-F | 99.91 ± 2.46 bc | 131.96 ± 5.70 c | 269.32 ± 1.08 d | 4.32 ± 0.02 e | 56.52 ± 0.02 c | 16.15 ± 0.05 d |
| ETg2-F | 93.43 ± 0.96 de | 116.29 ± 2.74 d | 255.50 ± 4.33 e | 3.55 ± 0.04 g | 51.23 ± 0.15 f | 16.13 ± 0.05 d |
| ETg3-F | 93.84 ± 1.21 de | 186.11 ± 3.59 a | 361.35 ± 5.54 a | 7.01 ± 0.11 a | 54.55 ± 0.95 de | 21.57 ± 0.27 a |
| ETg4-F | 97.10 ± 0.54 cd | 93.93 ± 0.88 e | 254.07 ± 0.86 e | 3.79 ± 0.01 f | 52.96 ± 0.43 e | 15.57 ± 0.01 d |
| p-value | *** | *** | *** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izquierdo-Ramos, M.J.; Atero-Calvo, S.; Navarro-Morillo, I.; Pérez-Millán, R.; Blasco, B. Ionomic Profile and Nutrient Use Efficiency in Sunflower Plants Treated with Plant-Derived Biostimulant Rich in Trigonelline. Agronomy 2025, 15, 2556. https://doi.org/10.3390/agronomy15112556
Izquierdo-Ramos MJ, Atero-Calvo S, Navarro-Morillo I, Pérez-Millán R, Blasco B. Ionomic Profile and Nutrient Use Efficiency in Sunflower Plants Treated with Plant-Derived Biostimulant Rich in Trigonelline. Agronomy. 2025; 15(11):2556. https://doi.org/10.3390/agronomy15112556
Chicago/Turabian StyleIzquierdo-Ramos, María José, Santiago Atero-Calvo, Iván Navarro-Morillo, Rafael Pérez-Millán, and Begoña Blasco. 2025. "Ionomic Profile and Nutrient Use Efficiency in Sunflower Plants Treated with Plant-Derived Biostimulant Rich in Trigonelline" Agronomy 15, no. 11: 2556. https://doi.org/10.3390/agronomy15112556
APA StyleIzquierdo-Ramos, M. J., Atero-Calvo, S., Navarro-Morillo, I., Pérez-Millán, R., & Blasco, B. (2025). Ionomic Profile and Nutrient Use Efficiency in Sunflower Plants Treated with Plant-Derived Biostimulant Rich in Trigonelline. Agronomy, 15(11), 2556. https://doi.org/10.3390/agronomy15112556







