Abstract
Tulsi (Ocimum sanctum L.) is a sacred plant of medicinal and spiritual significance in many cultures. Medicinal properties of Tulsi are ascribed to its phytochemicals with antioxidant capabilities. The current study was undertaken to screen a large seed population of Tulsi to select germplasm lines with high antioxidant potential and to standardize protocols for micropropagation and biomass production to produce a phytochemically consistent crop. A total of 80 germplasm lines were established under in vitro conditions and screened for their antioxidant potential determined with the 2,2-diphenyl-1-picrylhydrazyl (DPPH) bioassay. The micropropagation of a selected line, named Vrinda, was established using nodal cultures grown on Murashige and Skoog medium containing benzylaminopurine (1.1 µM), gibberellic acid (0.3 µM), and activated charcoal (0.6%). The antioxidant phytohormones melatonin and serotonin were quantified in the field and greenhouse grown tissues of Vrinda and melatonin levels were found to be consistent in both conditions with higher serotonin levels under field conditions. This integrated approach combining the in vitro selection and propagation offers potential applications in the development of safe, effective, and novel natural health products of Tulsi, and many other medicinal plant species.
1. Introduction
Ayurveda, the ancient text of Indian traditional medicine including Charak Samhita, Susrut Samhita, and Rigveda (3500–1600 BCE) has described Tulsi as a “Rasayana” [1,2]. The term Rasayana refers to the means of achieving homeostasis by preventing diseases and retarding the process of aging through optimum nutritional dynamics to rejuvenate the body and mind. Tulsi (Ocimum sanctum L.) is a member of the Lamiaceae family native to South Asia and North Africa. It is known by ≈390 common names including Holy basil, Raihan, and Tulsi [3]. The plant is referred to as “the Queen of herbs” and the “Elixir of life” in India and is highly valued for its use as a spiritual and religious plant, an adaptogen, and a tonic for stress reduction [4,5,6]. Nearly 300 studies have reported the enormous potential of Tulsi in the discovery of novel treatments for multiple diseases including chronic inflammation, fevers, digestive issues, viral, fungal and bacterial infections, and many other [5,6,7,8,9,10,11]. Studies have also shown the potential of Tulsi as an immunomodulator, antioxidant, anti-inflammatory, anti-pyretic, analgesic, antiasthamatic, and bronchodilator for asthma [12].
Tulsi is normally sold as seeds for propagation and there is a wide variability in the chemical composition of individual plants. Traditionally, some families share cuttings as well as seeds of plants they believe have greater health benefit or divine properties. Elite germplasm as either genotypes, F1 hybrids, or cultivars is not commonly available and potential exists for the selection of germplasm with specific phytochemical profiles and medicinal properties. In vitro technologies such as micropropagation can facilitate the production of physiologically uniform plants. The micropropagation of Tulsi has been successfully accomplished using various explants such as: Inflorescence [13], leaf [14,15,16,17], and axillary, or nodal segments [15,18,19,20,21,22]. In vitro cultures of Ocimum basilicum L. and Ocimum tenuiflorum L. were also shown to have greater total phenolic content than the field-grown intact organs [23].
Additionally, we examined the presence of two indoleamine neurotransmitters, melatonin (N-acetyl-5-methoxytryptamine) and serotonin (5-hydroxytryptamine). These compounds, in addition to being recognized as potent antioxidants [24,25,26,27], regulate a myriad of physiological functions in both humans and plants [28,29,30]. Melatonin in particular, is used in the treatment of circadian rhythm and reproductive disorders in humans [31,32] and was recently identified as a promising treatment for the prevention of COVID19 and influenza [33,34,35] indicating the potential of phytomelatonin-enriched products [36,37] as natural antiviral remedies. The objective of our research was to identify and select Tulsi germplasm lines with medicinal potential. In the present communication, we describe the selection of germplasm lines with high antioxidant potential and the development of an efficient clonal micropropagation method.
2. Materials and Methods
2.1. Plant Materials and Germplasm Line Development
Seeds were collected from a range of sources including temples in Ontario, Canada, locally grown plants, and suppliers in Canada (Richter’s Seeds; Goodwood, ON, Canada). Tulsi is a popular plant and has been maintained in religious centers and temples across Canada with frequent seed exchange to raise fresh plants for distribution to devotees. Tulsi plants are commonly identified by the morphological traits of the leaves and inflorescence and aromatic characteristics as described in ancient texts. Little information on molecular identification practices is currently available. Seeds were surface sterilized with 10% (v/v) solution of commercial bleach (Clorox®, Oakland, CA, USA, 5.4% sodium hypochlorite) for 12 min and rinsed for 3 min with autoclaved deionized water 4 times. Seeds were plated on semi-solid medium consisting of Murashige and Skoog (MS) basal salts with vitamins [38], 3% sucrose, and 2.2 g L-1phytagel (Sigma-Aldrich, Oakville, ON, Canada). A minimum of 5 and maximum of 27 seedlings from each source were randomly selected to assemble a pool of 79 in vitro seedlings, referred to as “lines”. Each line was maintained under in vitro conditions in Magenta GA7 vessels containing 50 mL of the semisolid medium.
2.2. Determination of Antioxidant Potential Using DPPH Bioassay
Leaves were collected from 4-week-old in vitro grown seedlings and 3 samples were prepared from each line. After weighing, the fresh tissue samples were flash frozen in liquid nitrogen and stored at −80 °C. The average antioxidant potential of the leaf samples was determined using a method modified from those described earlier [39,40]. Briefly, dried leaves were ground using a vortex and ball bearings in a 15 mL centrifuge tube (Fisher Scientific, Nepean, ON, Canada), and 75% acetone was added at a ratio of 100 µL:10 mg of tissue. The extraction process was completed by keeping the centrifuge tubes in a sonicating water bath (Branson 3510, Danbury, CT, USA) for 3 h. The tubes were then centrifuged for 10 min at 1500 rpm and the supernatant was transferred to new tubes and diluted to a 1:100 ratio with the extraction solvent. The sample extract (25 μL each) or standard was transferred to individual wells of a flat bottom 96-well microplate (Costar, Corning Inc., Corning, NY, USA) to which 200 μL of 150 μM 2,2-diphenyl-1-picrylhydrazyl (DPPH) in 80% methanol was added. The microplate was covered with a lid and incubated for 120 min. The reduction in absorbance at 517 nm was measured using a microplate reader (Synergy H1 hybrid reader, BioTek Inc., Winuski, VT, USA). Trolox standards (0, 62.5, 125, 250, and 500 μM) were assayed for each microplate along with the samples (diluted to 1:16 v/v) in triplicate. The absorbance of each sample (25 μL), along with 200 μL of methanol blank, was recorded in triplicate as sample blanks to eliminate interference in the absorbance at 517 nm. The calculation of the antioxidant potential was made using the Trolox standard curve (R2 > 0.9807). Results are expressed as a Trolox equivalence (TE μM) and data reported as means ± standard deviations of three replicates.
2.3. Culture Establishment and Propagation
The plant with the highest antioxidant potential was selected for further study on micropropagation. This germplasm line hereafter referred to as Vrinda was used to optimize micropropagation methods and acclimatization conditions in the greenhouse. In vitro shoot cultures of Vrinda were derived from nodal explants with single shoot buds collected from one-year old plant grown in the greenhouse. The greenhouse compartment was programmed to have a constant temperature of 23 °C during the day and 18 °C at night with a 16 h photoperiod, and a light intensity of 250 µmol m−2 s−1. Explants were cleaned under running tap water for 60 min and surface sterilized by dipping in 70% (v/v) ethanol for one min followed by 3 min of rinsing in sterile deionized water. Nodes were further disinfected in 15% (v/v) solution of commercial bleach (Clorox®, Oakland, CA, USA, 5.4% sodium hypochlorite) for 10 min with intermittent agitation. Disinfected explants were rinsed 3 times with sterile distilled water, each lasting 4 min, and the buds were excised at the basal end and cultured in test-tubes, each containing 10 mL of semisolid or liquid medium comprised of MS basal salts with vitamins, 3% (w/v) sucrose, and solidified with 2.2 g L−1 Phytagel (Sigma-Aldrich, Oakville, ON, Canada). The pH of the medium was adjusted to 5.7 before autoclaving for 20 min at 121 °C and 118 kPa. The cultures were maintained in a growth room at 25 ± 2 °C under a 16h photoperiod (40 µmol m−2 s−1) provided by cool white fluorescent lamps (Osram Sylvania Ltd., Mississauga, ON, Canada).
2.4. Shoot Multiplication
After 4 weeks of culture establishment, nodal explants were subcultured on a semisolid medium containing MS basal salts with vitamins and supplemented with various concentrations (0, 0.5, 1.0, and 2.0 µM) of 6-benzylaminopurine (BA), kinetin (Kn), or thidiazuron (TDZ) (all obtained from Phytotechnology, Lenexa, KS, USA) to determine the optimal shoot growth medium. Cultures grown in the presence of BA showed a relatively better growth thus, BA was used in further experiments to optimize the multiplication medium. For this, shoots developed after 4 weeks, the MS basal medium were excised from the basal end and transferred into Magenta GA7 culture vessels, each containing 50 mL of semisolid basal MS medium supplemented with BA (0.0, 1.1, 2.2 or 4.4 µM). Four explants were placed in each magenta box with 3 replications. Observations were recorded after 4 weeks of culture for shoot height, number of shoots per explant, and number of internodes per shoot. Thereafter, shoots were maintained on the optimized shoot development medium containing MS basal ingredients as described above and supplemented with BA (1.1 µM), gibberellic acid (GA3, 0.3 µM), and 2.2 g L−1 phytagel (all obtained from Phytotechnology, Shawnee Mission, KS, USA). The addition of GA3 (0.3 µM) to the optimized medium showed a positive effect on plant growth and was included in the final composition of the growth medium. To overcome the liquification of the semi-solid medium during shoot multiplication, nodal segments were subcultured on MS basal medium supplemented with BA (1.1 µM) and GA3 (0.3 µM), and activated charcoal (0.0, 0.4, 0.6, or 0.8%; w/v) and maintained in standard growth conditions for 8 weeks. Four explants per Magenta GA7 culture vessels (PhytoTechnology), with four boxes for each treatment, were maintained in a growth chamber. Observations recorded included the degree of liquification of media, number of shoots, number of internodes, and shoot height.
2.5. Rooting and Acclimatization in the Greenhouse
Individual micro-shoots with 4–5 internodes were transferred into rooting media supplemented with different levels of indole-3-butyric acid (0, 0.5, 2.5, and 5.0 µM IBA) with and without activated charcoal (0.6%). The percentage of explants that developed roots, the number and length of roots, and the shoot length were recorded after 4 weeks of culture. Micro-shoots were cultured on the optimized rooting medium (basal MS medium supplemented with 0.5 μM IBA) to obtain a sufficient number of plants for acclimatization trials. In vitro rooted micro-shoots were removed from the culture medium and washed gently under running tap water. A total of 54 plantlets were transplanted into 18-cell trays filled with Sunshine professional growing media (Sun Gro Horticulture, Vancouver, British Columbia). All trays were placed in the mist bed (80% relative humidity, sprayed with water for 15 s every 35 min during the day and every 4 h at night) for a week and later transferred to greenhouse conditions as described above where watering occurred once every 3 days. The percentage survival of the plantlets was recorded after 3 weeks. A total of 25 plants were transferred into field conditions and were watered when required.
2.6. Folin-Ciocalteu Phenolic Assay
The total phenolics of five greenhouse grown Vrinda plants were measured according to the Folin–Ciocalteu method [41] using leaves tissue. For the gallic acid standard, 10 µL aliquots of 80% methanol and water blanks were pipetted into clear, flat bottom, 96-well microplates (Corning, Corning, NY, USA) with 4 replicates each. Sample extracts were pipetted into the same plate at a volume of 10 µL, replicated 3 times with an additional sample blank for each. To all standard and sample wells, 100 µL of FC reagent (MP Biomedicals, Santa Ana, CA, USA) was added. After 5 min, the same wells received 80 µL of 0.25 M Na2CO3. To all methanol, water and sample blank wells, 180 µL of distilled water was added. After a dark incubation period for an hour, microplates were read using the Synergy H1 microplate reader (Biotek, Winooski, VT, USA) at 715 nm. The absorbance values of tissue extracts were compared to the gallic acid equivalent standard curve (62.5–1000 mg/L) to determine the gallic acid equivalency (GAE). Based on the dry weight, volume, and dilution factor, these values were used to estimate the phenolic content in the plant tissue. Samples at the 1:50 dilution fell within the linear range and were used for quantification.
2.7. Detection and Quantification of Neurotransmitters
Melatonin and serotonin levels were analyzed to determine the concentrations in roots and leaves from greenhouse and field grown Vrinda plants. Samples were prepared according to previously described methods [42,43]. In brief, tissues were harvested into pre-weighed 1.5 mL Eppendorf tubes, weighed for accurate weight and prepared individually in a dark room under red light to avoid light degradation. Tissues were homogenized in a solution of 80% methanol (Fisher Optima Grade, Fisher Scientific, Mississauga, ON, USA) and 20% 0.1 N trichloroacetic acid (TCA; Sigma, Mississauga, ON, USA) in ePure water (18 MΩ; Millipore). The homogenizing solution was added to each sample in a 1:4 (w/v) ratio and samples were macerated with a disposable tissue grinder (Kontes Pellet Pestle; Fisher Scientific). Extracts were centrifuged (13,000× g) for 3 min and the supernatant was filtered (0.2 mm, Ultrafree-MC filtered centrifuge tubes; Millipore) before chromatography. Serotonin (RT 0.77), melatonin (RT 2.49), and related metabolites were separated on a reverse phase column (Waters BEH C18 column (2.1 × 150 mm, 1.7 μm)) using a Waters Acquity I-class UPLC (Waters, Mississauga, ON) over a gradient of 0.1% formic acid (Eluent A) and acetonitrile (Eluent B) (A%:B%): 0.0–0.5 min, 90:10; 0.5–3.5 min, 40:60; 3.5–4.2 min, 5:95; 4.2–6.5 min, 5:95; 6.5–7.0 min, 90:10) with a flow rate of 0.3 mL/min. Analytes were quantified with a tandem mass spectrometer (Xevo TQ-S; Waters). The capillary voltage was 3500, the desolvation gas rate was 800 L/h, the cone gas rate was 150 L/h, the desolation temperature was 550 °C, and the source temperature was 150 °C for all analyses with a dwell time of 0.02 s. Parent and daughter ions were detected using the appropriate optimized multiple reaction monitoring (MRM) transitions (Table 1). For method development, a 16-dilution standard curve of each compound spanned 5 pg–15 μg on column. A linear range of 5 concentrations was determined with a limit of detection (LOD) ≈10 pg on column for all compounds measured. Linear range standards (0.025–400 ng/mL) were analyzed in triplicate for 3 successive days to determine intraday and interday variability and precision. Interday precision was <10% relative standard deviation (RSD) but between day variability was >10% RSD due to an instability of melatonin and serotonin. The dynamic range of the data spread over 4 orders of magnitude, data for the external standard curve underwent a LOG transformation for linearity.

Table 1.
Optimized parameters for detection and quantification of neurotransmitters in Tulsi.
2.8. Statistical Analysis
All the experiments were conducted using a completely randomized design with at least three replications and analysis of variance (ANOVA) was conducted using JMP version 9.0.2 for Windows (SAS Institute Inc., Cary, NC, USA). Means were compared using Tukey’s test (p < 0.05). Figures present mean values with standard error. Values followed by different letters are significantly different at p < 0.05.
3. Results
A total of 80 plants grown from diverse seed populations were analyzed for antioxidant potential using the DPPH bioassay. The antioxidant potential ranged from 1452.08 to 27.3 (Table S1) with 20 lines from five different seed lots showing high antioxidant values (1452.08 to 172.0 Trolox Equivalents (mg/mL)) (Figure 1). Out of these 20 plants, five plants with the highest antioxidant potential (1427.0–1227.0 µmol TE/g) were from a single seed lot and one which displayed a normal growth pattern was selected for further multiplication and characterization.
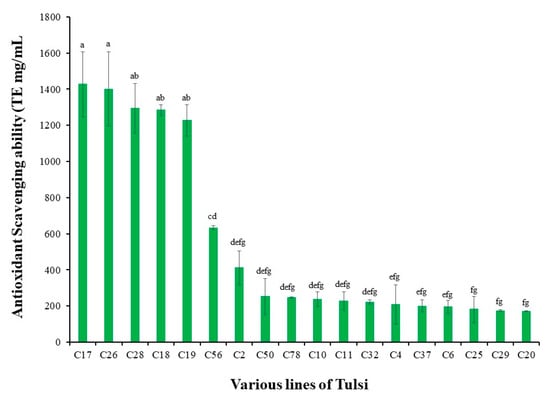
Figure 1.
Average antioxidant scavenging activity of 18 lines of Tulsi compared to a Trolox equivalent (µmol TE/g) standard using 2,2-diphenyl-1-picrylhydrazyl (DPPH) antioxidant assay. Names on the x-axis represents an individual line from different seed lots. Vertical bar with same letters indicates no significant difference between treatments at (p > 0.05). Data are displayed as mean, with bars representing standard error.
This germplasm line L17 named Vrinda was micropropagated using nodal explants. Nearly 42 shoot buds were collected from the greenhouse grown Vrinda plant (Figure 2A). About 5% of the buds were found to be contaminated during culture on the MS basal medium however, 95% shoot bud initiation was observed among the clean surviving shoot buds (Figure 2B). There was no significant difference between liquid and semisolid culture conditions for shoot bud initiation, however, shoot development from axillary bud in semisolid culture condition was better than in the liquid culture which caused vitrification. The MS basal medium supplemented with BA (1.1 µM) was the most effective treatment for shoot proliferation compared to other commonly used growth regulators including kinetin and thidiazuron (Figure S1). While all concentrations of BA tested showed an increase in the number of shoots compared to the control, the response was highest in with BA at 1.1 µM (Figure 3A). The highest number of internodes (5.8) was also observed with the medium containing BA at 1.1 µM (Figure 3B) and shoot length showed a similar pattern for 1.1 µM BA (Figure 3C). The shoots in the control and those treated with 1.1 µM BA were significantly taller than those grown on 2.2 µM and 4.4 µM BA. Overall, a higher multiplication rate was obtained at 1.1 µM BA (Figure 3A–C). Higher levels of BA adversely affected shoot proliferation with smaller leaves and shorter internodes. The addition of GA3 to the BA enriched culture medium stimulated visually better growth of cultures, however, the growth and multiplication patterns were not significantly different.

Figure 2.
A mature Vrinda plant growing in the greenhouse (A) used for the culture initiation, (B) multiplication, and (C) under in vitro condition. Healthy shoots growing in rooting medium (D) and rooted plantlets after four weeks of growth (E). Greenhouse acclimatization (F,G) and field grown Vrinda plants (H).
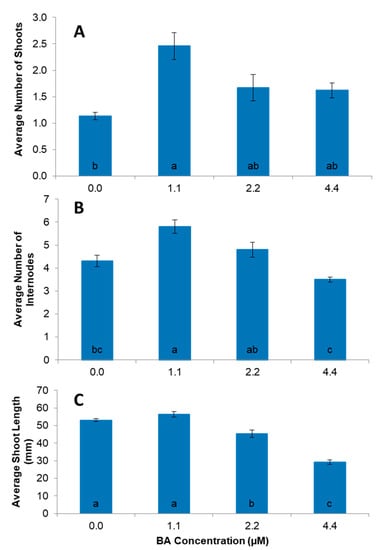
Figure 3.
The effect of various concentrations of BA added to Murashige and Skoog basal (MS) medium on average number of shoots (A), number of internodes (B) and shoot length (C) of ‘Vrinda’ nodal explants after 4 weeks of culture. Data represent mean ± standard error from about 36 explants per treatment and three replications. Means from all the treatments were compared with each other using Tukey’s test. Vertical bar with same letters indicates no significant difference between treatments at (p > 0.05).
A noticeable issue with proliferating shoots cultures of Vrinda was the start of liquification of culture medium after 3–4 weeks of growth. The addition of activated charcoal (AC) not only maintained the semi-solid state of the culture medium even after 7 weeks, it also improved average shoot length and visual appearance of the growing shoots. A similar effect of the AC was observed in shoot cultures of other lines of Tulsi.
Shoots from nodal cultures developed roots when induced with IBA (0.5–5.0 µM) in the presence and absence of activated charcoal. Overall, the MS medium containing IBA at 0.5 µM in combination with 0.6% activated charcoal was found to be the most effective to stimulate the development of a healthy root system compared to the control (Figure 4A and Figure 2E). The highest number of roots (10.3), root length (40.2 mm), and percentage of rooting (88.9%) were obtained on medium supplemented with 0.5 µM IBA with and without 0.6% activated charcoal (Figure 4A–C and Figure 2D,E). The addition of activated charcoal had positive effects on the rooting percentage, numbers of root, and root length when compared to medium without activated charcoal. These values were all significantly greater than the control with and without activated charcoal. The average number of roots on shoots cultured with 2.5 µM IBA was not significantly different than the control and 5.0 µM IBA was significantly less effective than the control (Figure 4B).
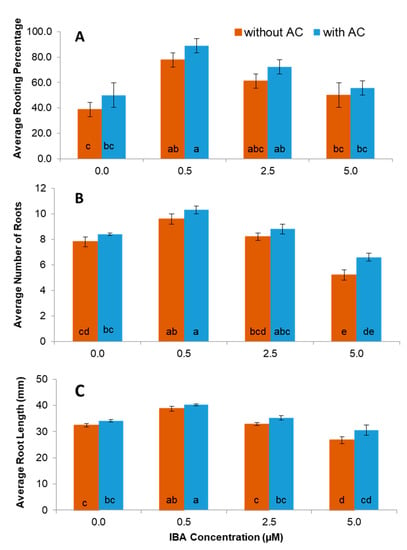
Figure 4.
Effect of different concentrations of IBA (indole-3-butyric acid) added to Murashige and Skoog basal (MS) medium on the percentage of rooting (A), number of roots (B) and root length (C) of shoots developed from in vitro grown nodal explants of ‘Vrinda’ compared to control. Means from all the treatments were compared with each other using Tukey’s test and data represent mean ± standard error from 30 explants per treatment and three replications. Vertical bar with same letters indicates no significant difference between treatments at (p > 0.05).
Rooted plantlets were acclimatized in the misting bed for a week (Figure 2F) and then transferred in the greenhouse where they showed 83% survival after four weeks of growth (Figure 2E). Interestingly rooted plantlets that were acclimatized in the growth chamber showed a relatively poor survival (65%). The two-month-old greenhouse acclimatized plants survived in the field condition with an efficiency of 100% (Figure 2H).
Micropropagated Tulsi lines grown in greenhouse and field conditions were analyzed for total phenolic compounds and the indoleamines, melatonin, and serotonin. The phenolic contents of greenhouse grown mature plants were found in the range of 66.6 and 93.4 GAE mg/g of plant tissue. The antioxidant capacity of the greenhouse grown mature plants ranged between 6741.77–7717.59 µmol TE/g. There was no significant difference observed in terms of antioxidant activity between the field and greenhouse grown plants (Figure S2).
Melatonin concentration was measured in the in vitro grown plantlets of three lines with high antioxidant potential and 12 wildtype germplasm lines to test the hypothesis that high antioxidant lines produce significantly more melatonin than other germplasm lines. In vitro grown shoots of the Vrinda germplasm line were found to consistently contain ≈50% more melatonin throughout the 18-month test period (Table 2). The highest melatonin concentration measured was 132.1 ng/g in Vrinda shoot cultures (Table 2). Interestingly, the average serotonin levels were also decreased in Vrinda as compared to the other germplasm lines but the decline in serotonin was not significantly different throughout the culture period (Table 2).

Table 2.
Average concentrations of melatonin (ng/g) and serotonin (ng/g) in in vitro grown leaf tissues of the selected line Vrinda and a representative wildtype line with three biological replications. Columns with different letter indicate significant difference between two lines (Tukey’s test, p < 0.05).
The average levels of melatonin in leaves and roots of fresh weight in plants grown under greenhouse or field condition ranged from 327–378 ng/g. No significant differences in melatonin content were observed between roots and shoots grown in the greenhouse or field-grown plants (Table 3). Serotonin levels in the leaves and roots were higher than the melatonin levels in the same organs and ranged from 497–685 ng/g in the greenhouse and field grown plants. The levels of both melatonin and serotonin provided in Table 3 are representative of average contents in vegetative tissues of non-flowering plants. Over the course of all experiments, a wide variation of 127–1200 ng/g of tissue was observed at different stages of growth, reproduction, and climatic conditions.

Table 3.
Average concentrations of melatonin (ng/g) and serotonin (ng/g) in leaf and root tissues of greenhouse and field grown plants with five biological replications. Columns with same letter indicate no significant difference between field and greenhouse (Tukey’s test, p > 0.05).
4. Discussion
The ancient texts of Ayurveda describe Tulsi (Ocimum sanctum L.) as one of the most sacred medicinal plants in India which is widely used as a nervine tonic, adaptogen, and medicine for improving health when faced with multiple diseases [2,11]. The objective of this study was to develop an integrated strategy for the selection and propagation of Tulsi germplasm with potential health benefits. Our approach consisted of three components: identification and isolation of a germplasm line with high antioxidant potential; development of an efficient micropropagation system; and characterization of indoleamines of potential medicinal importance in plants grown in greenhouse and field conditions. Phytochemical profiles vary in seedlings from diverse seed populations and this chemo-diversity offers an opportunity to select for a range of characteristics including compounds of medicinal interest. In the screening of a population of 80 seedlings, we were able to identify five germplasm lines with high antioxidant potential and ability to grow in in vitro conditions, for further multiplication. One of these five high antioxidant potential lines, named Vrinda, was selected for further studies based on consistent plant growth during micropropagation.
In animals and humans, the oxidative stress caused by reactive oxygen species (ROS) is a key factor in the pathogenesis of various diseases and disorders through cell damage, DNA alterations, and metabolic malfunctions [44]. Antioxidants in humans, animals, and plants act as defense molecules and are known to play an important role in adaptations to diverse stressful environments. On this basis, the use of plant antioxidants has been proposed as an effective remedy to prevent or reduce the severity of medical conditions caused by the deleterious effects of ROS. Therefore, antioxidant potential is considered an effective marker for the medicinal efficacy of plants although it may not be the sole criterion of their medicinal efficacy. Plants contain a range of antioxidants known to prevent various disorders and the plants of the Lamiaceae family, to which Tulsi belongs, are particularly known for higher phenolic and antioxidant compounds [44]. A number of studies have demonstrated the presence of pharmacologically important compounds in Tulsi [8,10]. The compounds ocimumoside A and B isolated from Tulsi leaves were shown to possess antistress potential [45] and normalize the chronic unpredictable stress in a rat model system. Interestingly, the efficacy of these compounds in restoring alterations in the antioxidant systems was comparable to that of melatonin [8,45]. Thus, the presence of melatonin and serotonin, two principle compounds involved in brain functions, observed in our study further strengthens the potential of Tulsi in the treatment of stress-induced neurological disorders. It is noteworthy that both melatonin and serotonin were also found to be present in the roots, which opens the possibility of the large-scale bioreactor-based production of roots for developing root-based formulations of Tulsi. Leaves are the most commonly used tissues in Tulsi formulations, presumably for ease of availability and processing. To our knowledge, the occurrence of indoleamines, melatonin, and serotonin has not been reported in Tulsi tissues. Roots of medicinal plants can also provide an efficient system for harvesting physiologically uniform biomass for producing safe and efficacious natural health products [46,47,48,49].
Micropropagation is crucial to this strategy of integrated plant production in controlled environments. We developed a nodal explant-based micropropagation method that allows the clonal propagation of naturally occurring superior clones to provide physiologically consistent plant biomass. The micropropagation method developed in this study relied on the use of BA as a cytokinin along with GA3 and activated charcoal as additional supplements. BA is the most common cytokinin used for shoot development and multiplication in a range of species [18,50,51]. For Tulsi, a better response with BA was observed in an earlier study which compared shoot multiplication induced by BA and Kn [15]. Similarly, BA was found to be suitable for shoot induction from various explants of several Ocimum species including O. sanctum and O. basilicum, O. Americanum, and O. gratissimum [50,52,53]. In Tulsi, a lower level of Kn (2.32 µM) has also been used as the only cytokinin source for inducing shoot development [20]. The efficacy of cytokinins is generally variable depending on the specific species with regards to its optimal concentration and type [54,55,56], probably due to endogenous levels of plant hormones, the type of the explant used, and the genetic variability among genotypes and species. Further optimization of the micropropagation method revealed that the addition of 0.3 µM of gibberellic acid (GA3) to the BA supplemented medium improved shoot multiplication and maintenance. Although the addition of GA3 did not produce significant differences in the proliferation rate of Tulsi in this study, the development and growth of shoots was superior compared to the cultures raised with BA alone. The combination of cytokinins and gibberellic acid is known to improve shoot growth in the in vitro grown cultures, likely due to increased cell elongation [50,57].
Rooting could be successfully achieved with the exogenous application of auxin. In our study, a maximum of 89% Tulsi shoots formed roots in the presence of IBA at 0.5 µM along with 0.6% activated charcoal which is a significantly higher percent of the rooting compared hormone-free medium. Other studies on Tulsi have reported root development on both hormone-free and on media supplemented with a wide range of auxin levels [15,20,50]. The observed differences in shoot and root development in cultures of Tulsi in various studies may have resulted from the variation in the seed population, genotypes, and ecotypes, as well as inthe conditions of growth and manipulation of plants and explants.
The destabilization of the media matrix in vitro after four weeks was an issue encountered in our study of Tulsi micropropagation, limiting its long-term maintenance in culture. Activated charcoal (AC) was used to effectively resolve this issue. The use of 0.6% (w/v) AC maintained the integrity of the semi-solid matrix for up to eight weeks and improved the shoot multiplication with reduced browning of leaves, increased survival rates, and better overall growth. Additionally, the improvement in root development observed in this study with a combination of IBA and activated charcoal compared to the control suggests a modulation of hormonal balance due to the adsorptive ability of the AC. The AC particles have a large oxidized surface area free of non-carbon impurities [58] and have large pores enhancing the adsorption capacity. AC is commonly used in vitro to decrease browning, stabilize pH, adsorb harmful chemicals produced by the medium or plant tissues, and provide a dark environment for in vitro cultures, though it has not previously been used in the micropropagation of Tulsi [59].
Melatonin and serotonin are a novel class of phytohormones which interact with established phytohormones including auxins, cytokinin, and gibberellins in the regulation of plant growth, reproduction, and adaptation to biotic and abiotic stress [28,60,61,62]. Khan et al. (2017) demonstrated that exposure of an in vitro grown callus of the related species Ocimum basilicum with melatonin enhanced the capacity of the tissue to biosynthesize silver nanoparticles, thereby demonstrating the utility of Ocimum species for the production of novel industrial chemicals. Melatonin application during O. basilicum seed priming and growth under salt stress increased shoot growth and phytochemicals [63] and also increased phenolic metabolites along with the mitigation of UV-C induced damage [64]. Due to the strong antioxidant potential, it is possible that the selection of Vrinda as a high antioxidant line also leads to selection of a high melatonin line as has been reported in other species [65], making it a potentially valuable novel phytomelatonin source. Additionally, it was found that growth in the greenhouse or the field did not have a significant effect on melatonin content of the cultures suggesting either would be an effective cultivation strategy if Tulsi were to be used as the basis for a phytomelatonin product.
In conclusion, 80 accessions of Tulsi were analyzed from different sources for their antioxidant potential. Of these, an individual plant named “Vrinda”, which showed the highest antioxidant potential was selected and clonally propagated with an efficient protocol developed in this investigation for micropropagation. The Vrinda plants were successfully grown in the greenhouse and later in the field with a high survival. In addition to the phenolics, these plants also had indoleamine neurotransmitters both in the leaves as well as in the roots. Further research may find new roles for Tulsi formulations rich in these metabolites in improving human health conditions. Collectively, these accomplishments have significant potential for the commercial scale production of high-quality Tulsi biomass free of chemical and biological contaminants.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/11/2/207/s1, Figure S1: The effect of various concentrations (0, 0.5, 1.0, 2.0 μM) of 6-benzylaminopurine (BA), kinetin (Kn), or thidiazuron (TDZ) added to Murashige and Skoog basal (MS) medium on average number of shoots (A) and shoot height (B) of ‘Vrinda’ nodal explants after 4 weeks of culture. Data represent mean ± standard error from about ten explants per treatment and three replications. Means from all the treatments were compared with each other using Tukey’s test. Vertical bar with same letters indicates no significant difference between treatments at (p > 0.05); Figure S2: Average antioxidant scavenging activity of greenhouse (GH) and field grown “Vrinda” plants compared to a Trolox equivalent (μmolTE/g) standard using DPPH antioxidant assay. Names on the x-axis represents sample tissue of flower, leaf and stem of ‘Vrinda’ plant in greenhouse (GH) and field. Data are displayed as mean, with bars representing standard error. Means from all the treatments were compared with each other using Tukey’s test and vertical bar with same letters indicates no significant difference between treatments at (p > 0.05), Table S1: Antioxidant scavenging activity of individual “Tulsi” lines (total 79) grown in vitro when compared to a trolox equivalent (μmol TE/g) standard using DPPH antioxidant assay. Means from all the lines were compared with each other using Tukey’s test. Columns with same letter indicate no significant difference (p < 0.05).
Author Contributions
M.R.S. and P.K.S. conceptualized and designed in vitro propagation study, A.K. conducted the experiments and collected the in vitro growth data. A.K. and M.R.S. collected and analyzed the data. C.E.T. and L.A.E.E. conducted biochemical analysis. P.K.S., M.R.S., S.J.M., J.A.S. organized and supervised the study. M.R.S. prepared the manuscript and all authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Gosling Foundation, grant number 050294.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Award of research grants from the Gosling Foundation, Guelph, Ontario and the discovery grant program of the Natural Sciences and Engineering Council of Canada (NSERC) is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bano, N.; Ahmed, A.; Tanveer, M.; Khan, G.; Ansari, M. Pharmacological evaluation of Ocimum sanctum. J. Bioequivalence Bioavailab. 2017, 9, 387–392. [Google Scholar] [CrossRef]
- Dafni, A.; Petanidou, T.; Vallianatou, I.; Kozhuharova, E.; Blanche, C.; Pacini, E.; Peyman, M.; Stevanoic, Z.D.; Franchi, G.G.; Benitez, G. Myrtle, basil, rosemary, and three-lobed sage as ritual plants in the monotheistic religions: An Historical-ethnobotanical comparison. Econ. Bot. 2020, 74, 330–355. [Google Scholar] [CrossRef]
- Ved, D.K.; Sureshchandra, S.T.; Barve, V.; Srinivas, V.; Sangeetha, S.; Ravikumar, K.; Kartikeyan, R.; Kulkarni, V.; Kumar, A.S.; Venugopal, S.N.; et al. FRLHT’s ENVIS Centre on Medicinal Plants, Bengaluru. Available online: Envis.frlht.org/frlhtenvis.nic.in (accessed on 8 November 2020).
- Makri, O.; Kintzios, S. Ocimum sp. (Basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Duke, J.A. The Garden Pharmacy: Basil as the Holy Hindu Highness. Altern. Complement. Ther. 2008, 14, 5–8. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdelmohsen, U.R.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Diversity, phytochemical and medicinal potential of the genus Ocimum L. (Lamiaceae). Phytochem Rev. 2020, 19, 907–953. [Google Scholar] [CrossRef]
- Engels, G.; Brinckmann, J. Holy Basil. In HerbalGram; American Botanical Council: Austin, TX, USA, 2013; pp. 1–6. [Google Scholar]
- Singh, D.; Chaudhuri, P.K. A review on phytochemical and pharmacological properties of Holy basil (Ocimum sanctum L.). Ind Crop. Prod. 2018, 118, 367–382. [Google Scholar] [CrossRef]
- Maharjan, S. Ocimum sanctum (Linn.); The queen of herbs. Eur. J. Biomed. Pharm. Sci. 2019, 6, 106–109. [Google Scholar]
- Pandey, V.K.; Upadhyay, S.N.; Mishra, P.K. Light-induced synthesis of silver nanoparticles using Ocimum tenuiflorum extract: Characterisation and application. J. Chem Res. 2020, 174751982093651. [Google Scholar] [CrossRef]
- Cohen, M.M. Tulsi—Ocimum sanctum: A herb for all reasons. J. Ayurveda Integr. Med. 2014, 5, 251–259. [Google Scholar] [CrossRef]
- Pathania, M.; Bhardwaj, P.; Pathania, N.; Rathaur, V.; Amisha. A review on exploring evidence-based approach to harnessing the immune system in times of corona virus pandemic: Best of modern and traditional Indian system of medicine. J. Fam Med. Prim. Care 2020, 9, 3826. [Google Scholar] [CrossRef]
- Singh, N.K.; Sehgal, C.B. Micropropagation of ‘Holy Basil’ (Ocimum sanctum Linn.) from young inflorescences of mature plants. Plant Growth Regul. 1999, 29, 161–166. [Google Scholar] [CrossRef]
- Shilpa, K.; Selvakkumar, C.; Senthil, A.K.; Lakshmi, B.S. In vitro root culture of Ocimum sanctum L. and evaluation of its free radical scavenging activity. Plant Cell Tissue Organ Cult. 2010, 101, 105–109. [Google Scholar] [CrossRef]
- Banu, L.; Bari, M. Protocol establishment for multiplication and regeneration of Ocimum sanctum Linn. an important medicinal plant with high religious value in Bangladesh. J. Plant Sci. 2007, 2, 530–537. [Google Scholar] [CrossRef][Green Version]
- Lim, Z.; Ling, A.; Hussein, S. Callus Induction of Ocimum sanctum and estimation of its total flavonoids Content. Asian J. Agric. Sci. 2009, 1, 55–61. [Google Scholar]
- Mishra, T. Protocol establishment for multiplication and regeneration of ‘Holy Basil’ (Ocimum sanctum Linn). An important medicinal plant with high religious value in India. J. Med. Plants Stud. 2015, 3, 16–19. [Google Scholar]
- Mandal, J.; Pattnaik, S.; Chand, P.K. Alginate encapsulation of axillary buds of Ocimum americanum L. (hoary basil), O. basilicum L. (sweet basil), O. gratissimum L. (shrubby basil), and O. sanctum. L. (sacred basil). Vitr. Cell Dev. Biol. Plant 2000, 36, 287–292. [Google Scholar] [CrossRef]
- Tyub, S.; Kamili, A. Enhanced axillary shoot proliferation in Ocimum sanctum Linn. via shoot tip culture using various concentrations of BAP. J. Res. Dev. 2008, 8, 80–85. [Google Scholar]
- Gogoi, K.; Kumara, S. Callus-Mediated plantlet regeneration of Ocimum tenuiflorum L. using axillary buds as explants. Plant Sci. 2011, v.2, 1–5. [Google Scholar]
- Girija, S.; Kavitha, S.; Deepavathi, S. Direct multiple shoot regeneration from shoot tip and nodal explants of Ocimum sanctum L. (Tulsi): A medicinal herb. Plant Cell Biotechnol. 2006, 7, 23–28. [Google Scholar]
- Aggarwal, D.; Neeti, N.; Reddy, M.S.; Kumar, A. Shoot organogenesis and assessment of clonal fidelity of regenerated plants of Ocimum tenuiflorum L.: Queen of Herbs. Vegetos 2020, 33, 420–429. [Google Scholar] [CrossRef]
- Bhuvaneshwari, K.; Gokulanathan, A.; Jayanthi, M.; Govindasamy, V.; Milella, L.; Lee, S.; Yang, D.C.; Girija, S. Can Ocimum basilicum L. and Ocimum tenuiflorum L. in vitro culture be a potential source of secondary metabolites? Food Chem. 2016, 194, 55–60. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Aguilera, Y.; Herrera, T.; Cayuelas, L.T.; Dueñas, M.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Arribas, S.M.; Martín-Cabrejas, M.A. Bioavailability of melatonin from lentil sprouts and its role in the plasmatic antioxidant status in rats. Foods 2020, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Reiter, R.J.; Manchester, L.C.; Yan, M.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Naranjo, M.I.; Moya, M.L.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Comparative evaluation of the antioxidant activity of melatonin and related indoles. J. Food Compos. Anal. 2012, 28, 16–22. [Google Scholar] [CrossRef]
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.M.; Murch, S.J.; Saxena, P.K. Identification and characterization of serotonin as an anti-browning compound of apple and pear. Postharvest Biol. Technol. 2015, 110, 183–189. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Murch, S.J.; Reiter, R.J.; Saxena, P.K. A new balancing act: The many roles of melatonin and serotonin in plant growth and development. Plant Signal. Behav. 2015, 10, e1096469-15. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Saxena, P.K. Melatonin in morphogenesis. Vitr. Cell Dev. Biol. Plant 2018, 54, 3–24. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Turi, C.E.; Saxena, P.K. Serotonin: An ancient molecule and an important regulator of plant processes. Biotechnol. Adv. 2016, 8, 1347–1361. [Google Scholar] [CrossRef]
- Basheer, M.; Rai, S.; Hsu, T.-C. Melatonin vs. phytomelatonin: Therapeutic uses with special reference to polycystic ovarian syndrome (PCOS). Cogent. Biol. 2016, 2, 1136257. [Google Scholar] [CrossRef]
- Lanfumey, L.; Mongeau, R.; Hamon, M. Biological rhythms and melatonin in mood disorders and their treatments. Pharm. Ther. 2013, 138, 176–184. [Google Scholar] [CrossRef]
- Reiter, R.J.; Abreu-Gonzalez, P.; Marik, P.E.; Dominguez-Rodriguez, A. Therapeutic algorithm for use of melatonin in patients with COVID-19. Front. Med. 2020, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Hardeland, R. Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: Focus on COVID-19. Melatonin Res. 2020, 3, 120–143. [Google Scholar] [CrossRef]
- Anderson, G.; Reiter, R.J. Melatonin: Roles in influenza, Covid-19, and other viral infections. Rev. Med. Virol. 2020, 30, e2109. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Llamas, F.; Hernández-Ruiz, J.; Cuesta, A.; Zamora, S.; Arnao, M.B. Development of a phytomelatonin-rich extract from cultured plants with excellent biochemical and functional properties as an alternative to synthetic melatonin. Antioxidants 2020, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.; Hernández-Ruiz, J. The potential of phytomelatonin as a nutraceutical. Molecules 2018, 23, 238. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Saremba, B.M.; Tymm, F.J.M.; Baethke, K.; Rheault, M.R.; Sherif, S.M.; Saxena, P.K.; Murch, S.J. Plant signals during beetle (Scolytus multistriatus) feeding in American elm Ulmus americana Planch). Plant Signal. Behav. 2017, 12, e1296997. [Google Scholar] [CrossRef][Green Version]
- Beilby, M.J.; Turi, C.E.; Baker, T.C.; Tymm, F.J.M.; Murch, S.J. Circadian changes in endogenous concentrations of indole-3-acetic acid, melatonin, serotonin, abscisic acid and jasmonic acid in Characeae (Chara australis Brown). Plant Signal. Behav. 2015, 10, e1082697. [Google Scholar] [CrossRef] [PubMed]
- Ulewicz-Magulska, B.; Wesolowski, M. Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant Food Hum. Nutr. 2019, 74, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, M.M.; Raza, S.S.; Javed, H.; Ashafaq, M.; Islam, F.; Safhi, M.M.; Islam, F. Ocimum sanctum attenuates oxidative damage and neurological deficits following focal cerebral ischemia/reperfusion injury in rats. Neurol. Sci. 2012, 33, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Zobayed, S.M.A.; Saxena, P.K. In vitro-grown roots: A superior explant for prolific shoot regeneration of St. John’s wort (Hypericum perforatum L. cv ‘New Stem’) in a temporary immersion bioreactor. Plant Sci. 2003, 165, 463–470. [Google Scholar] [CrossRef]
- Liu, C.Z.; Murch, S.J.; El-Demerdash, M.; Saxena, P.K. Artemisia judaica L.: Micropropagation and antioxidant activity. J. Biotechnol. 2004, 110, 63–71. [Google Scholar] [CrossRef]
- Murch, S.J.; Liu, C.; Romero, R.M.; Saxena, P.K. In vitro culture and temporary immersion bioreactor production of Crescentia cujete. Plant Cell Tissue Org. Cult. 2004, 78, 63–68. [Google Scholar] [CrossRef]
- Murch, S.J.; Saxena, P.K. St. John’s wort (Hypericum perforatum L.): Challenges and strategies for production of chemically-consistent plants. Can. J. Plant Sci. 2006, 86, 765–771. [Google Scholar] [CrossRef]
- Pattnaik, S.; Chand, P.K. In vitro propagation of the medicinal herbs Ocimum americanum L. syn. O. canum Sims. (hoary basil) and Ocimum sanctum L. (holy basil). Plant Cell Rep. 1996, 15, 846–850. [Google Scholar] [CrossRef]
- Cole, I.B.; Saxena, P.K.; Murch, S.J. Medicinal biotechnology in the genus scutellaria. Vitr. Cell Dev. Biol. Plant 2007, 43, 318–327. [Google Scholar] [CrossRef]
- Jamal, M.A.H.M.; Sharif, I.H.; Shakil, M.M.; Rahman, A.N.M.R.-B.; Banu, N.A.; Islam, M.R.; Nazmuzzaman, M. In vitro regeneration of a common medicinal plant, Ocimum sanctum L. for mass propagation. Afr. J. Biotechnol. 2016, 15, 1269–1275. [Google Scholar] [CrossRef][Green Version]
- Sahoo, Y.; Pattnaik, S.K.; Chand, P.K. In vitro clonal propagation of an aromatic medicinal herb Ocimum basilicum L. (sweet basil) by axillary shoot proliferation. Vitr. Cell Dev. Biol. Plant 1997, 33, 293–296. [Google Scholar] [CrossRef]
- Mao, A.A.; Wetten, A.; Fay, M.; Caligari, P.D.S. In vitro propagation of Clerodendrum colebrookianum Walp., a potential natural anti-hypertension medicinal plant. Plant Cell Rep. 1995, 14, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Tiwari, K.N. In vitro plant regeneration from decapitated embryonic axes of Clitoria ternatea L.—An important medicinal plant. Ind. Crop. Prod. 2012, 35, 224–229. [Google Scholar] [CrossRef]
- Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant hormones and plant growth regulators in plant tissue culture. Vitr. Cell Dev. Biol. Plant 1996, 32, 272–289. [Google Scholar] [CrossRef]
- Shukla, M.R.; Jones, A.M.P.; Sullivan, J.A.; Liu, C.; Gosling, S.; Saxena, P.K. In vitro conservation of American elm (Ulmus americana): Potential role of auxin metabolism in sustained plant proliferation. Can. J. For. Res. 2012, 42, 686–697. [Google Scholar] [CrossRef]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef]
- Pan, M.J.; Staden, J. van. The use of charcoal in in vitro culture–A review. Plant Growth Regul. 1998, 26, 155–163. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trend Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Reiter, R.; Tan, D.-X.; Zhou, Z.; Cruz, M.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting plants to survive and thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Shukla, M.R.; Singh, A.S.; Murch, S.J.; Saxena, P.K. Melatonin and serotonin: Mediators in the symphony of plant morphogenesis. J. Pineal Res. 2018, 64, e12452. [Google Scholar] [CrossRef]
- Bahcesular, B.; Yildirim, E.D.; Karaçocuk, M.; Kulak, M.; Karaman, S. Seed priming with melatonin effects on growth, essential oil compounds and antioxidant activity of basil (Ocimum basilicum L.) under salinity stress. Ind. Crop. Prod. 2020, 146, 112165. [Google Scholar] [CrossRef]
- Nazir, M.; Ullah, M.A.; Mumtaz, S.; Siddiquah, A.; Shah, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Interactive effect of melatonin and UV-C on phenylpropanoid metabolite production and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var purpurascens). Molecules 2020, 25, 1072. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Saxena, P.K. A melatonin-rich germplasm line of St John’s wort (Hypericum perforatum L.). J. Pineal Res. 2006, 41, 284–287. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).