Improving the Biocontrol Potential of Bacterial Antagonists with Salicylic Acid against Brown Rot Disease and Impact on Nectarine Fruits Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Pathogen Preparation
2.2. Antagonist Preparation
2.3. Chemical Substances
2.4. Fruit Preparation
2.5. In Vitro Effects of SA, Antagonists, and Their Combined Treatments on Fungal Mycelial Growth
2.6. In Vivo Effects of SA, Antagonists, and Combined Treatments on Brown Rot Disease
2.7. Effect of Treatments on Fruit Quality Parameters
2.7.1. Weight Loss
2.7.2. Total Soluble Solids
2.7.3. Titratable Acidity
2.7.4. Maturity Index
2.8. Population Dynamics of Antagonists (SF14 and ACBC1) in Fruit Wounds
2.9. Statistical Analysis
3. Results
3.1. In Vitro Effects of SA, Antagonists, and Their Combined Treatments on Fungal Growth
3.2. In Vivo Effects of SA, Antagonists, and Their Combined Treatments on Brown Rot Disease
3.3. Effect of Treatments on Fruit Quality Parameters
3.3.1. Weight Loss
3.3.2. Total Soluble Solids
3.3.3. Titratable Acidity
3.3.4. Maturity Index
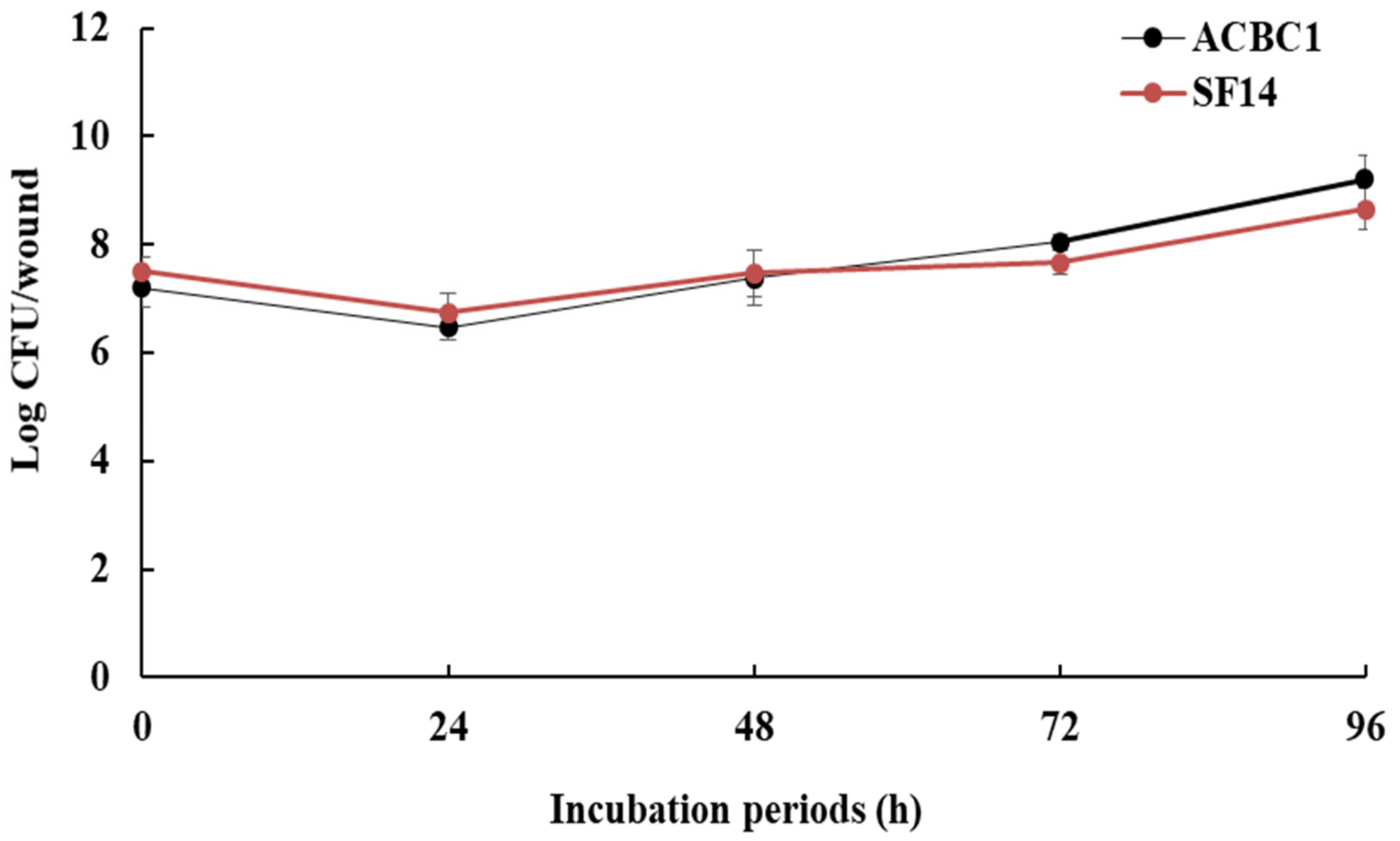
3.4. Population Dynamics of Antagonists on Fruit Wounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Matetić, M.; Zhou, H.; Zhang, X.; Jemrić, T. Postharvest Quality Monitoring and Variance Analysis of Peach and Nectarine Cold Chain with Multi-Sensors Technology. Appl. Sci. 2017, 7, 133. [Google Scholar] [CrossRef]
- Larena, I.; Torres, R.; De Cal, A.; Liñán, M.; Melgarejo, P.; Domenichini, P.; Bellini, A.; Mandrin, J.F.; Lichou, J.; de Eribe, X.O.; et al. Biological control of postharvest brown rot (Monilinia spp.) of peaches by field applications of Epicoccum nigrum. Biol. Control 2005, 32, 305–310. [Google Scholar] [CrossRef]
- Martini, C.; Mari, M. Monilinia fructicola, Monilinia laxa (Monilinia Rot, Brown Rot). In Postharvest Decay; Academic Press: Cambridge, MA, USA, 2014; pp. 233–265. [Google Scholar] [CrossRef]
- Xu, X.-M.; Bertone, C.; Berrie, A. Effects of wounding, fruit age and wetness duration on the development of cherry brown rot in the UK. Plant Pathol. 2007, 56, 114–119. [Google Scholar] [CrossRef]
- Batra, R.L. World Species of Monilinia (Fungi): Their Ecology, Biosystematics, and Control; Cramer, J., Ed.; CABI: Wallingford, UK, 1991; Volume 16. [Google Scholar]
- Teixidó, N. Brown Rot. 2018. Available online: http://www.biocomes.eu/ (accessed on 19 January 2018).
- FAO. Food and Agriculture Data. Available online: www.fao.org/statistics/faostatagriculture (accessed on 20 September 2019).
- Lahlali, R.; Aksissou, W.; Lyousfi, N.; Ezrari, S.; Blenzar, A.; Tahiri, A.; Ennahli, S.; Hrustić, J.; MacLean, D.; Amiri, S. Biocontrol activity and putative mechanism of Bacillus amyloliquefaciens (SF14 and SP10), Alcaligenes faecalis ACBC1, and Pantoea agglomerans ACBP1 against brown rot disease of fruit. Microb. Pathog. 2020, 139, 103914. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.W.; Ogawa, J.M. The Chemical Control of Postharvest Diseases: Subtropical and Tropical Fruits. Annu. Rev. Phytopathol. 1985, 23, 421–454. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance in Crop Pathogens: How Can It Be Managed? Crop Protection Federation: Brussels, Belgium, 2007; Volume 56. [Google Scholar]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Adaskaveg, J.E.; Förster, H. New Developments in Postharvest Fungicide Registrations for Edible Horticultural Crops and Use Strategies in the United States. In Postharvest Pathology; Prusky, D., Gullino, M.L., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 107–117. [Google Scholar] [CrossRef]
- Bazioli, J.M.; Belinato, J.R.; Costa, J.H.; Akiyama, D.Y.; Pontes, J.G.D.M.; Kupper, K.C.; Augusto, F.; de Carvalho, J.E.; Fill, T.P. Biological Control of Citrus Postharvest Phytopathogens. Toxins 2019, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Hernandez, S.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of Postharvest Fruit Fungal Diseases by Bacterial Antagonists: A Review. Agronomy 2019, 9, 121. [Google Scholar] [CrossRef]
- Lastochkina, O.; Seifikalhor, M.; Aliniaeifard, S.; Baymiev, A.; Pusenkova, L.; Garipova, S.; Kulabuhova, D.; Maksimov, I. Bacillus spp.: Efficient Biotic Strategy to Control Postharvest Diseases of Fruits and Vegetables. Plants 2019, 8, 97. [Google Scholar] [CrossRef]
- Lastochkina, O.; Baymiev, A.; Shayahmetova, A.; Garshina, D.; Koryakov, I.; Shpirnaya, I.; Pusenkova, L.; Mardanshin, I.D.; Kasnak, C.; Palamutoglu, R. Effects of Endophytic Bacillus Subtilis and Salicylic Acid on Postharvest Diseases (Phytophthora infestans, Fusarium oxysporum) Development in Stored Potato Tubers. Plants 2020, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-Z.; Zeng, J.-K.; Tang, H.; Zhou, Y.; Li, W. The chemical treatments combined with antagonistic yeast control anthracnose and maintain the quality of postharvest mango fruit. J. Integr. Agric. 2019, 18, 1159–1169. [Google Scholar] [CrossRef]
- Lahlali, R.; Mchachti, O.; Radouane, N.; Ezrari, S.; Belabess, Z.; Khayi, S.; Mentag, R.; Tahiri, A.; Barka, E.A. The Potential of Novel Bacterial Isolates from Natural Soil for the Control of Brown Rot Disease (Monilinia fructigena) on Apple Fruits. Agronomy 2020, 10, 1814. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Korsten, L. Biological control of postharvest diseases of fruits. Annu. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef]
- Talibi, I.; Boubaker, H.; Boudyach, E.H.; Aoumar, A.A.B. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17. [Google Scholar] [CrossRef]
- Qin, G.Z.; Tian, S.P.; Xu, Y.; Wan, Y.K. Enhancement of biocontrol efficacy of antagonistic yeasts by salicylic acid in sweet cherry fruit. Physiol. Mol. Plant Pathol. 2003, 62, 147–154. [Google Scholar] [CrossRef]
- Farahani, L.; Etebarian, H.R. Enhancement of the efficacy of two antagonistic yeasts with salicylic acid against Penicillium expansum. Arch. Phytopathol. Plant Prot. 2012, 45, 260–267. [Google Scholar] [CrossRef]
- Da Rocha Neto, A.C.; Luiz, C.; Maraschin, M.; Di Piero, R.M. Efficacy of salicylic acid to reduce Penicillium expansum inoculum and preserve apple fruits. Int. J. Food Microbiol. 2016, 221, 54–60. [Google Scholar] [CrossRef]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar] [CrossRef]
- Meena, B.; Marimuthu, T.; Velazhahan, R. Salicylic acid induces systemic resistance in groundnut against late leaf spot caused by Cercosporidium personatum. J. Mycol. Plant Pathol. 2001, 31, 139–145. [Google Scholar]
- Bürger, M.; Chory, J. Stressed Out About Hormones: How Plants Orchestrate Immunity. Cell Host Microbe 2019, 26, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha Neto, A.C.; Maraschin, M.; Di Piero, R.M. Antifungal activity of salicylic acid against Penicillium expansum and its possible mechanisms of action. Int. J. Food Microbiol. 2015, 215, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J. Use of plant-defense hormones against pathogen-diseases of postharvest fresh produce. Physiol. Mol. Plant Pathol. 2020, 111, 101521. [Google Scholar] [CrossRef]
- El Khetabi, A.; Lahlali, R.; Askarne, L.; Ezrari, S.; El Ghadaroui, L.; Tahiri, A.; Hrustić, J.; Amiri, S. Efficacy assessment of pomegranate peel aqueous extract for brown rot (Monilinia spp.) disease control. Physiol. Mol. Plant Pathol. 2020, 110, 101482. [Google Scholar] [CrossRef]
- Bahadou, S.A.; Ouijja, A.; Karfach, A.; Tahiri, A.; Lahlali, R. New potential bacterial antagonists for the biocontrol of fire blight disease (Erwinia amylovora) in Morocco. Microb. Pathog. 2018, 117, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xiao, H.; Xue, C.; Yu, Z.; Yang, R.; Cai, Z.; Si, L. Biocontrol of gray mold in grapes with the yeast Hanseniaspora uvarum alone and in combination with salicylic acid or sodium bicarbonate. Postharvest Biol. Technol. 2015, 100, 160–167. [Google Scholar] [CrossRef]
- Saikia, R.; Singh, T.; Kumar, R.; Srivastava, J.; Srivastava, A.K.; Singh, K.; Arora, D.K. Role of salicylic acid in systemic resistance induced by Pseudomonas fluorescens against Fusarium oxysporum f. sp. ciceri in chickpea. Microbiol. Res. 2003, 158, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.-H. Exogenous treatment with salicylic acid attenuates occurrence of citrus canker in susceptible navel orange (Citrus sinensis Osbeck). J. Plant Physiol. 2012, 169, 1143–1149. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Tian, S.; Norelli, J.; Hershkovitz, V. Effect of heat treatment on inhibition of Monilinia fructicola and induction of disease resistance in peach fruit. Postharvest Biol. Technol. 2012, 65, 61–68. [Google Scholar] [CrossRef]
- Geng, P.; Chen, S.; Hu, M.; Rizwan-ul-Haq, M.; Lai, K.; Qu, F.; Zhang, Y. Combination of Kluyveromyces marxianus and sodium bicarbonate for controlling green mold of citrus fruit. Int. J. Food Microbiol. 2011, 151, 190–194. [Google Scholar] [CrossRef]
- Sangwanich, S.; Sangchote, S.; Leelasuphakul, W. Biocontrol of citrus green mould and postharvest quality parameters. Int. Food Res. J. 2013, 20, 3381–3386. [Google Scholar]
- Yu, T.; Zheng, X.D. Salicylic Acid Enhances Biocontrol Efficacy of the Antagonist Cryptococcus laurentii in Apple Fruit. J. Plant Growth Regul. 2006, 25, 166–174. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Analytical Chemists International: Washington, DC, USA, 1995. [Google Scholar]
- Pobiega, K.; Igielska, M.; Włodarczyk, P.; Gniewosz, M. The use of pullulan coatings with propolis extract to extend the shelf life of blueberry (Vaccinium corymbosum) fruit. Int. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Pobiega, K.; Przybył, J.L.; Żubernik, J.; Gniewosz, M. Prolonging the Shelf Life of Cherry Tomatoes by Pullulan Coating with Ethanol Extract of Propolis During Refrigerated Storage. Food Bioprocess Technol. 2020, 13, 1447–1461. [Google Scholar] [CrossRef]
- Srivastava, M.K.; Dwivedi, U.N. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 2000, 158, 87–96. [Google Scholar] [CrossRef]
- Rasmussen, J.B.; Hammerschmidt, R.; Zook, M.N. Systemic Induction of Salicylic Acid Accumulation in Cucumber after Inoculation with Pseudomonas syringae pv syringae. Plant Physiol. 1991, 97, 1342–1347. [Google Scholar] [CrossRef]
- Yao, H.J.; Tian, S.P. Effects of a biocontrol agent and methyl jasmonate on postharvest diseases of peach fruit and the possible mechanisms involved. J. Appl. Microbiol. 2005, 98, 941–950. [Google Scholar] [CrossRef]
- Ahima, J.; Zhang, X.; Yang, Q.; Zhao, L.; Tibiru, A.M.; Zhang, H. Biocontrol activity of Rhodotorula mucilaginosa combined with salicylic acid against Penicillium digitatum infection in oranges. Biol. Control 2019, 135, 23–32. [Google Scholar] [CrossRef]
- Spoel, S.H.; Johnson, J.S.; Dong, X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc. Natl. Acad. Sci. USA 2007, 104, 18842–18847. [Google Scholar] [CrossRef]
- Kouzai, Y.; Kimura, M.; Watanabe, M.; Kusunoki, K.; Osaka, D.; Suzuki, T.; Matsui, H.; Yamamoto, M.; Ichinose, Y.; Toyoda, K.; et al. Salicylic acid-dependent immunity contributes to resistance against Rhizoctonia solani, a necrotrophic fungal agent of sheath blight, in rice and Brachypodium distachyon. New Phytol. 2018, 217, 771–783. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, H.; Lin, Y.; Shi, J.; Xue, S.; Hung, Y.-C.; Chen, Y.; Wang, H. Effects of biocontrol bacteria Bacillus amyloliquefaciens LY-1 culture broth on quality attributes and storability of harvested litchi fruit. Postharvest Biol. Technol. 2017, 132, 81–87. [Google Scholar] [CrossRef]
- Yánez-Mendizábal, V.; Zeriouh, H.; Viñas, I.; Torres, R.; Usall, J.; de Vicente, A.; Pérez-García, A.; Teixidó, N. Biological control of peach brown rot (Monilinia spp.) by Bacillus subtilis CPA-8 is based on production of fengycin-like lipopeptides. Eur. J. Plant Pathol. 2012, 132, 609–619. [Google Scholar] [CrossRef]
- Dihazi, A.; Serghini, M.A.; Jaiti, F.; Daayf, F.; Driouich, A.; Dihazi, H.; El Hadrami, I. Structural and Biochemical Changes in Salicylic-Acid-Treated Date Palm Roots Challenged with Fusarium oxysporum f. sp. albedinis. J. Pathog. 2011, 2011, 280481. [Google Scholar] [CrossRef] [PubMed]
- Conway, W.S.; Leverentz, B.; Janisiewicz, W.J.; Saftner, R.A.; Camp, M.J. Improving biocontrol using antagonist mixtures with heat and/or sodium bicarbonate to control postharvest decay of apple fruit. Postharvest Biol. Technol. 2005, 36, 235–244. [Google Scholar] [CrossRef]
- Obagwu, J.; Korsten, L. Integrated control of citrus green and blue molds using Bacillus subtilis in combination with sodium bicarbonate or hot water. Postharvest Biol. Technol. 2003, 28, 187–194. [Google Scholar] [CrossRef]
- Cao, J.; Yan, J.; Zhao, Y.; Jiang, W. Effects of postharvest salicylic acid dipping on Alternaria rot and disease resistance of jujube fruit during storage. J. Sci. Food Agric. 2013, 93, 3252–3258. [Google Scholar] [CrossRef] [PubMed]
- Mekbib, S.B.; Regnier, T.J.C.; Korsten, L. Efficacy and mode of action of yeast antagonists for control of Penicillium digitatum in oranges. Trop. Plant Pathol. 2011, 36, 233–240. [Google Scholar]
- Song, M.; Yun, H.Y.; Kim, Y.H. Antagonistic Bacillus species as a biological control of ginseng root rot caused by Fusarium cf. incarnatum. J. Ginseng Res. 2014, 38, 136–145. [Google Scholar] [CrossRef]
- Lahlali, R.; Hamadi, Y.; Guilli, M.E.; Jijakli, M.H. Efficacy assessment of Pichia guilliermondii strain Z1, a new biocontrol agent, against citrus blue mould in Morocco under the influence of temperature and relative humidity. Biol. Control 2011, 56, 217–224. [Google Scholar] [CrossRef]
- Hong, P.; Hao, W.; Luo, J.; Chen, S.; Hu, M.; Zhong, G. Combination of hot water, Bacillus amyloliquefaciens HF-01 and sodium bicarbonate treatments to control postharvest decay of mandarin fruit. Postharvest Biol. Technol. 2014, 88, 96–102. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Tian, Z.; He, H.; Long, C.-A.; Jiang, F. Bacillus species as potential biocontrol agents against citrus diseases. Biol. Control 2020, 151, 104419. [Google Scholar] [CrossRef]
- Roberts, D.P.; Kobayashi, D.Y.; Dery, P.D.; Short, N.M., Jr. An image analysis method for determination of spatial colonization patterns of bacteria in plant rhizosphere. Appl. Microbiol. Biotechnol. 1999, 51, 653–658. [Google Scholar] [CrossRef]
- Kong, Q.; Shan, S.; Liu, Q.; Wang, X.; Yu, F. Biocontrol of Aspergillus flavus on peanut kernels by use of a strain of marine Bacillus megaterium. Int. J. Food Microbiol. 2010, 139, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-M.; Zhu, S.; Kachroo, P.; Kachroo, A. Signal regulators of systemic acquired resistance. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
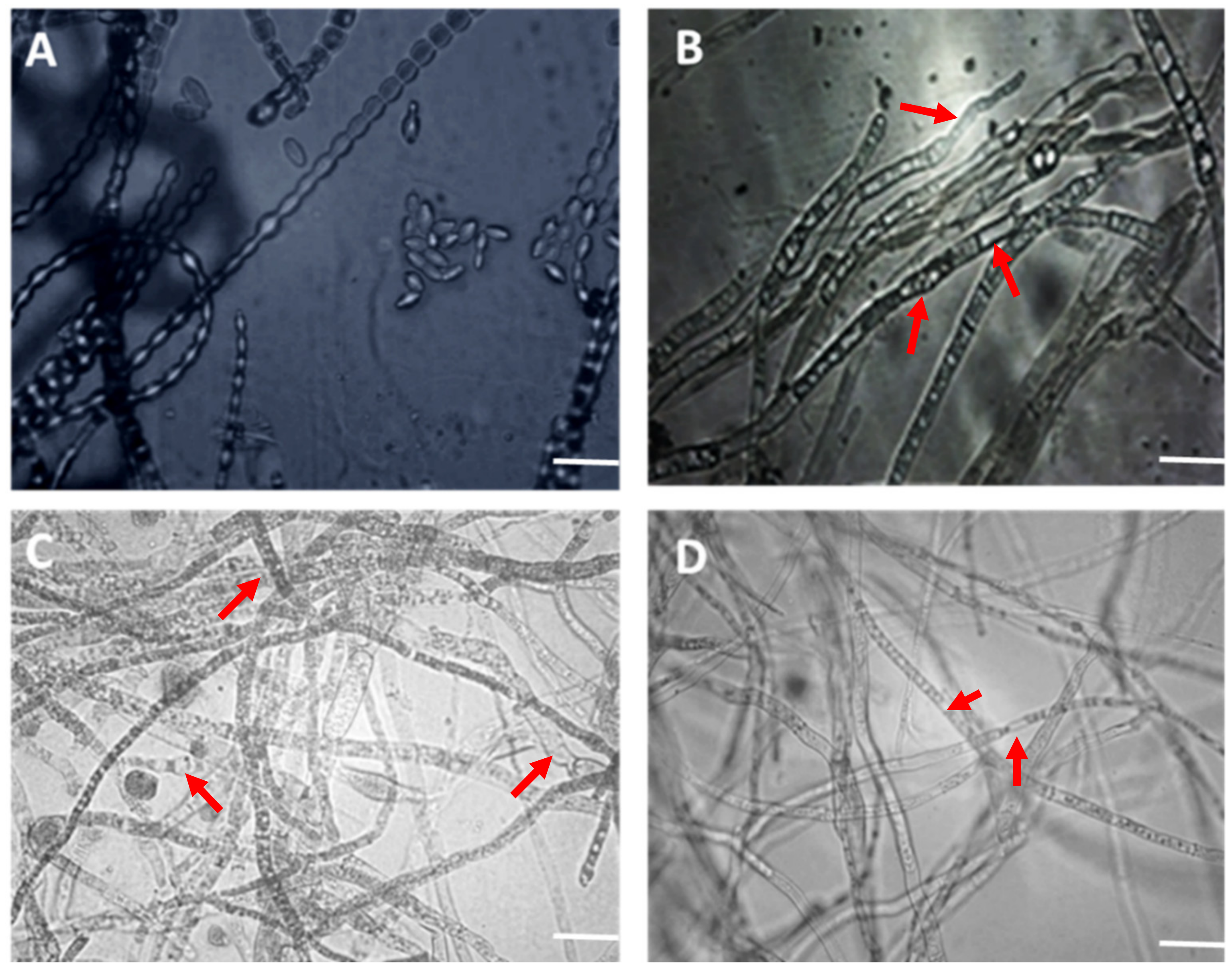

| Treatments | 5 Days of Incubation | 10 Days of Incubation | ||
|---|---|---|---|---|
| Colony Diameter (mm) | IR (%) | Colony Diameter (mm) | IR (%) | |
| Untreated Control | 54.03 j | 0.00 | 82.75 j | 26.28 |
| 0.5 SA | 48.74 i | 9.79 | 61.00 i | 26.28 |
| 2 SA | 45.43 h | 15.92 | 55.18 h | 33.32 |
| 3.5 SA | 34.10 g | 36.89 | 44.30 g | 46.47 |
| 5 SA | 12.25 b | 77.33 | 35.35 f | 57.28 |
| SF14 | 11.56 b | 78.60 | 16.74 c | 79.77 |
| 0.5 SA + SF14 | 13.95 bc | 74.18 | 15.55 bc | 81.21 |
| 2 SA + SF14 | 23.57 e | 56.38 | 24.52 e | 70.37 |
| 3.5 SA + SF14 | 15.11 c | 72.03 | 16.82 c | 79.67 |
| 5 SA + SF14 | 16.63 d | 69.22 | 17.64 c | 78.68 |
| ACBC1 | 18.00 f | 67.34 | 20.13 de | 75.82 |
| 0.5 SA + ACBC1 | 8.38 a | 84.80 | 13.71 b | 83.53 |
| 2 SA + ACBC1 | 17.68 e | 67.92 | 18.97 d | 77.21 |
| 3.5 SA + ACBC1 | 8.36 a | 84.83 | 9.97 a | 88.02 |
| 5 SA + ACBC1 | 12.25 b | 77.78 | 15.46 bc | 81.43 |
| Treatments | 5 Days of Incubation | 10 Days of Incubation | ||
|---|---|---|---|---|
| Lesion Diameter (mm) | DS (%) | Lesion Diameter (mm) | DS (%) | |
| Untreated control | 58.08 j | 100.00 | 68.27 f | 100.00 |
| 0.5 SA | 21.46 i | 36.95 | 35.44 e | 51.91 |
| 2 SA | 11.04 f | 19.01 | 21.08 d | 30.88 |
| 3.5 SA | 0.00 a | 0.00 | 6.39 ab | 9.36 |
| 5 SA | 0.00 a | 0.00 | 2.41 a | 3.53 |
| SF14 | 11.49 f | 19.78 | 18.16 cd | 26.60 |
| 0.5 SA + SF14 | 7.73 d | 13.31 | 14.43 bc | 21.14 |
| 2 SA + SF14 | 10.72 f | 18.46 | 15.19 bc | 22.25 |
| 3.5 SA + SF14 | 16.01 h | 27.57 | 26.71 de | 39.12 |
| 5 SA + SF14 | 15.42 h | 26.55 | 22.27 d | 32.62 |
| ACBC1 | 5.35 c | 9.21 | 10.3 b | 15.08 |
| 0.5 SA + ACBC1 | 5.00 c | 8.60 | 10.24 b | 14.99 |
| 2 SA + ACBC1 | 9.78 e | 16.83 | 14.07 bc | 20.60 |
| 3.5 SA + ACBC1 | 13.27 g | 22.84 | 18.91 cd | 27.70 |
| 5 SA + ACBC1 | 10.96 f | 18.87 | 16.92 c | 24.78 |
| MT | 3.23 b | 5.56 | 5.75 ab | 8.42 |
| Treatments | Weight Loss (WL) | Total Soluble Solids (TSSs) | Titratable Acidity (TA) | Maturity Index (MI) |
|---|---|---|---|---|
| Untreated control | 0.16 ± 0.01 xc | 10.6 ± 0.17 cde | 10.18± 0.00 fg | 1.04 |
| 0.5 SA | 0.15 ± 0.00 d | 8.06 ± 0.07 a | 10.49 ± 0.51 g | 0.77 |
| 2 SA | 0.14 ± 0.00 c | 10.2 ± 0.39 c | 11 ± 0.06 h | 0.93 |
| 3.5 SA | 0.14 ± 0.00 c | 13 ± 0.00 g | 8 ± 0.04 a | 1.62 |
| 5 SA | 0.13 ± 0.00 b | 10.5 ± 0.69 cd | 8 ± 0.18 a | 1.31 |
| SF14 | 0.14 ± 0.00 c | 9.1 ± 0.11 b | 9.62 ± 0.87 de | 0.95 |
| 0.5 SA + SF14 | 0.14 ± 0.00 c | 11.7 ± 0.46 f | 10.11 ± 0.07 f | 1.16 |
| 2 SA + SF14 | 0.14 ± 0.00 c | 12.9 ± 0.51 g | 9.47 ± 0.15 cd | 1.36 |
| 3.5 SA + SF14 | 0.12 ± 0.00 a | 9.9 ± 1.39 bc | 8 ± 0.11 a | 1.24 |
| 5 SA + SF14 | 0.16 ± 0.00 e | 10.7 ± 0.73 cde | 9.49 ± 0.19 cd | 1.13 |
| ACBC1 | 0.14 ± 0.01 c | 10.3 ± 0.65 c | 10.11 ± 0.13 f | 1.02 |
| 0.5 SA + ACBC1 | 0.14 ± 0.01 c | 11.6 ± 0.00 ef | 9.01 ± 0.04 b | 1.29 |
| 2 SA + ACBC1 | 0.16 ± 0.01 e | 12.1 ± 0.20 fg | 9.18 ± 0.07 bc | 1.32 |
| 3.5 SA + ACBC1 | 0.16 ± 0.00 e | 10.4 ± 0.56 c | 9.89 ± 0.06 ef | 1.05 |
| 5 SA + ACBC1 | 0.16 ± 0.00 e | 11.4 ± 0.58 def | 9.40 ± 0.14 bcd | 1.21 |
| MT | 0.14 ± 0.00 c | 13 ± 0.73 g | 9.53 ± 0.10 d | 1.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyousfi, N.; Lahlali, R.; Letrib, C.; Belabess, Z.; Ouaabou, R.; Ennahli, S.; Blenzar, A.; Barka, E.A. Improving the Biocontrol Potential of Bacterial Antagonists with Salicylic Acid against Brown Rot Disease and Impact on Nectarine Fruits Quality. Agronomy 2021, 11, 209. https://doi.org/10.3390/agronomy11020209
Lyousfi N, Lahlali R, Letrib C, Belabess Z, Ouaabou R, Ennahli S, Blenzar A, Barka EA. Improving the Biocontrol Potential of Bacterial Antagonists with Salicylic Acid against Brown Rot Disease and Impact on Nectarine Fruits Quality. Agronomy. 2021; 11(2):209. https://doi.org/10.3390/agronomy11020209
Chicago/Turabian StyleLyousfi, Nadia, Rachid Lahlali, Chaimaa Letrib, Zineb Belabess, Rachida Ouaabou, Said Ennahli, Abdelali Blenzar, and Essaid Ait Barka. 2021. "Improving the Biocontrol Potential of Bacterial Antagonists with Salicylic Acid against Brown Rot Disease and Impact on Nectarine Fruits Quality" Agronomy 11, no. 2: 209. https://doi.org/10.3390/agronomy11020209
APA StyleLyousfi, N., Lahlali, R., Letrib, C., Belabess, Z., Ouaabou, R., Ennahli, S., Blenzar, A., & Barka, E. A. (2021). Improving the Biocontrol Potential of Bacterial Antagonists with Salicylic Acid against Brown Rot Disease and Impact on Nectarine Fruits Quality. Agronomy, 11(2), 209. https://doi.org/10.3390/agronomy11020209







