Abstract
Substance-based medical devices (SBMDs) are increasingly used in wound care due to their favorable safety profile, physicochemical mechanisms of action, and therapeutic effectiveness. These products often incorporate biopolymers such as hyaluronic acid or chitosan, alone or in combination with antimicrobial agents like silver nanoparticles (AgNPs) or silver sulfadiazine (SSD), offering hydration, tissue protection, and control of microbial burden in both acute and chronic wounds. Despite their widespread clinical use, the regulatory classification of SBMDs under Regulation (EU) 2017/745 (MDR) remains one of the most challenging and debated areas within the current European framework. This review analyzes the scientific and regulatory context of topical SBMDs, with particular emphasis on borderline products that share similarities with medicinal products in terms of formulation, composition, or claimed effects. The discussion focuses on the application of MDR Annex VIII, specifically Rule 21 for substance-based devices and Rule 14 for devices incorporating medicinal substances with ancillary action, together with interpretative guidance provided by MDCG 2022-5 Rev.1 and the Association of the European Self-Care Industry (AESGP) Position Paper. Particular attention is given to the identification of the critical role of the primary mode of action (MoA) as the determining criterion for regulatory qualification, especially for products containing antimicrobial substances. Through selected examples and case analyses, the review highlights inconsistencies in classification across Member States and underscores the need for a more harmonized, evidence-based, and proportionate regulatory approach. Overall, SBMDs challenge traditional regulatory boundaries and call for a framework capable of accommodating complex, multifunctional products while ensuring patient safety and regulatory coherence.
1. Introduction
The application of topical products in healthcare has deep historical roots, originating from ancient civilizations that utilized natural extracts and botanicals for therapeutic, protective, and cosmetic purposes [1]. Throughout the 20th century, advancements in pharmaceutical science, dermatology, and formulation technology significantly transformed topical products, facilitating the introduction of sophisticated dosage forms such as creams, gels, ointments, and transdermal patches, designed to enhance bioavailability and controlled substance delivery [2]. Parallel to these technological advancements, the regulatory landscape also evolved to address the complexity and increasing overlap among medicinal products and medical devices, each governed by distinct European legislations: Directive 2001/83/EC for medicinal products and, more recently, the Medical Device Regulation (EU) 2017/745 (MDR) for medical devices. Medical devices (MDs) and medicinal products (MPs) constitute the essential therapeutic tools available in contemporary healthcare and are required to address the increasingly complex demands posed by both acute and chronic conditions. Although both regulatory frameworks aim to guarantee product safety and efficacy, they are based on fundamentally different principles and target distinct categories of products. The development of innovative health solutions is only achievable within an appropriate regulatory framework [3]. In this context, the qualification of certain products is not always straightforward, leading to extensive discussions at the European level regarding so-called borderline products. Due to their inherent characteristics, these products do not clearly fall within a specific regulatory category, making it challenging to determine the applicable legal framework [4].
The implementation of Regulation (EU) 2017/745 has significantly increased the complexity of product qualification at the interface between medical devices and medicinal products, making substance-based medical devices (SBMDs) a paradigmatic case of borderline classification under the MDR, where regulatory determination critically depends on the identification of the principal mode of action (MoA).
Among the most common borderline products are Integral Drug-Device Combinations (IDDCs), Drug-Device Combinations (DDCs), and Substance-Based Medical Devices (SBMDs). These categories, although distinct, often present classification challenges due to their overlapping characteristics with both MDs and MPs [2].
This review aims to provide a comprehensive overview of a specific category of borderline product: substance-based medical devices (SBMDs) for topical applications. It focuses on their regulatory definitions, classification criteria, and key distinguishing features in comparison to topical MPs. The review addresses their mechanisms of action, clinical applications, market landscape, and the current challenges associated with their classification under the European regulatory framework. Through selected examples and case studies, particular emphasis is placed on the application of MDR Annex VIII, Rules 14 and 21, in order to illustrate the practical implications for product development and regulatory compliance. Overall, topical SBMDs exemplify how formulation complexity and multifunctionality can amplify regulatory uncertainty, highlighting the need for a scientifically robust and consistent approach to classification under the MDR.
2. Substance-Based Medical Devices (SBMDs)
Under Regulation (EU) 2017/745, which became fully applicable in May 2021, the definition of a medical device is given in Chapter 1, Article 2. According to this definition, a product qualifies as a medical device if it meets two key conditions: (1) it must serve a specific medical purpose on a human being, and (2) its principal intended action must not be achieved by pharmacological, immunological or metabolic (PhIM) means. Essentially, any tool or apparatus used in medical practice in humans that achieves its intended purpose neither as a medicinal product nor as a nutritional product (such as parenteral nutrition) can be classified as a medical device [5,6]. In this regard, the MDCG 2022-5 Rev. 1—“Guidance on borderline between medical devices and medicinal products” gives the definitions of pharmacological, immunological, and metabolic means and provides some useful examples to clarify these terms in the context of identifying the products’ principal mode of action (MoA). MoA is described in the guidance as the means by which the product achieves its main intended effect; it must be determined objectively using the most current scientific evidence. According to the regulatory framework, the pharmacological MoA focuses only on the initial interaction (binding) without explicitly mentioning signal transduction [7,8].
Substance-based medical devices (SBMDs) fall within this definition when their therapeutic effect is primarily driven by physicochemical mechanisms, such as barrier formation, lubrication, hydration, adsorption, or modulation of the local microenvironment. SBMDs fall within this definition when their therapeutic effect is primarily driven by physicochemical mechanisms, such as barrier formation, lubrication, hydration, adsorption, or modulation of the local microenvironment. In this context, the regulatory qualification of SBMDs does not depend solely on their composition or formulation but on the identification of the principal mode of action through which the intended medical effect is achieved. Consequently, the presence of medicinal substances with pharmacological properties does not preclude classification as a medical device, provided that such activity remains ancillary and does not constitute the principal intended action of the product, according to Annex VIII, Rule 14 or Rule 21 (Figure 1).
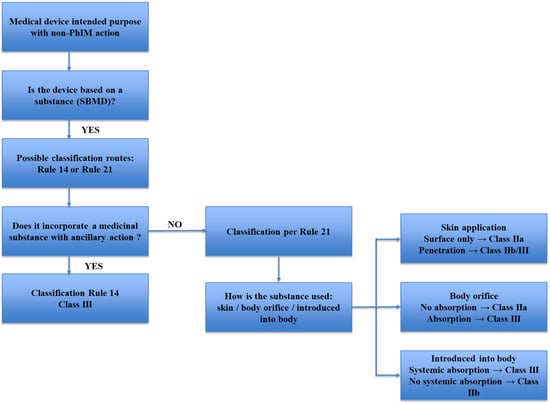
Figure 1.
Decision flowchart for the classification of substance-based medical devices in accordance with Regulation (EU) 2017/745, Annex VIII, Rules 21 and 14. The diagram illustrates the stepwise assessment of the route of administration, degree of absorption and presence of medicinal substances with ancillary action, supporting the determination of the applicable risk class and conformity assessment pathway.
Rule 14 of the MDR specifically addresses MDs incorporating medicinal substances with an ancillary function, i.e., substances that support the medical device in achieving its intended purpose without exerting the principal mode of action: “All devices incorporating, as an integral part, a substance which, if used separately, can be considered to be a medicinal product, as defined in point 2 of Article 1 of Directive 2001/83/EC, including a medicinal product derived from human blood or human plasma, as defined in point 10 of Article 1 of that Directive, and that has an action ancillary to that of the devices, are classified as class III.” [2,7].
Compared to the previous regulatory framework, Directive 93/42/EEC on Medical Devices (MDD), the MDR introduced a more explicit focus, omitting the phrase “liable to act”, on the relationship between the device’s principal intended action and the role of the incorporated substance, thereby reinforcing the importance of a robust scientific justification of the ancillary nature of any pharmacological activity but consequently raising concerns among industry stakeholders and Notified Bodies [2,7].
In this context, the determination of whether a substance exerts an ancillary or principal action is closely linked to the identification of the product’s principal MoA (Figure 2). As clarified by the MDCG 2022-5 Rev.1, the presence of a substance with pharmacological properties does not automatically trigger classification as a medicinal product, provided that such activity is secondary and not essential for achieving the intended medical effect of the device. The regulatory assessment must therefore be based on objective scientific evidence demonstrating that the primary therapeutic effect is achieved through physical or physicochemical mechanisms, with the medicinal substance acting solely in a supportive role. This clarification broadened the scope of Rule 14 to include cases such as substances exerting effects in blood bags or wound exudates. For legacy devices previously certified under the MDD, re-consultations with Competent Authorities are required under MDCG 2020-12 to verify the continued compliance of the ancillary medicinal substance [9]. This process involves submitting updated documentation on the substance itself, its method of incorporation, manufacturing processes, and any design elements that could impact the substance’s quality, safety, or functionality within the device. The Notified Body must also provide a declaration regarding any changes affecting these aspects.
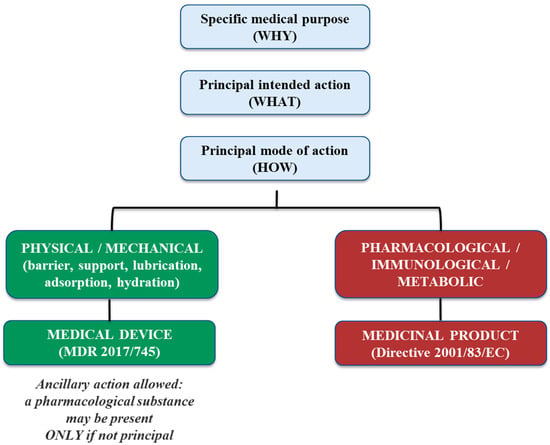
Figure 2.
Conceptual decision framework for the determination of the Principal Mode of Action (PMOA) in accordance with Regulation (EU) 2017/745 and MDCG 2022-5 Rev.1. The diagram illustrates the hierarchical distinction between specific medical purpose (WHY), principal intended action (WHAT) and principal mode of action (HOW), highlighting the PMOA as the determining criterion for regulatory qualification of borderline products.
While Rule 21 outlines a risk-based framework based on the site of application and extent of absorption, as reported below: “Devices that are composed of substances or of combinations of substances that are intended to be introduced into the human body via a body orifice or applied to the skin and that are absorbed by or locally dispersed in the human body are classified as:
- -
- class III if they, or their products of metabolism, are systemically absorbed by the human body in order to achieve the intended purpose;
- -
- class III if they achieve their intended purpose in the stomach or lower gastrointestinal tract and they, or their products of metabolism, are systemically absorbed by the human body;
- -
- class IIa if they are applied to the skin or if they are applied in the nasal or oral cavity as far as the pharynx, and achieve their intended purpose on those cavities; and
- -
- class IIb in all other cases.”
According to the MDCG 2022-5 Rev.1, such devices typically act through mechanisms like physical barrier formation, lubrication, or modulation of the local microenvironment, distinguishing them from MPs whose action is pharmacological, immunological, or metabolic [7].
The application of Rule 21 requires a careful scientific assessment of the product’s physicochemical properties and mechanisms of action, particularly for topical formulations used in wound care. In this setting, SBMDs typically exert their intended effect through physical or physicochemical mechanisms, such as barrier formation, hydration, adsorption of exudate, modulation of the wound microenvironment, or control of microbial burden. However, the coexistence of such mechanisms with antimicrobial substances may complicate the distinction between physical action and pharmacological effect, especially when antimicrobial efficacy contributes significantly to the clinical performance of the device.
As a result, the interpretation of Rule 21 is closely interconnected with the identification of the principal mode of action and, where applicable, with the assessment of ancillary pharmacological activity under Rule 14 (Figure 1). Inconsistent interpretation of these criteria across Member States has contributed to divergent classification outcomes for similar SBMDs, underscoring the need for a harmonized, evidence-based application of Rule 21 aligned with MDCG guidance and scientific state of the art.
As emphasized by the Association of the European Self-Care Industry (AESGP), the application of Rule 21 must be based on a case-by-case assessment, considering both product composition and clinical context, including the use of substances with a well-established safety profile in food or cosmetics [10].
Before the new regulation, SBMDs were included in Directive 93/42 but were not clearly defined at the time. As a result, they were often considered “borderline” products, somewhere between medical devices and medicinal products, until the MDR was introduced [11].
SBMDs include devices made from chemically defined substances as well as those derived from biological sources. Although the MDR still does not provide a formal definition for them, it offers detailed rules for their regulation. These are found in General Safety and Performance Requirements (GSPRs) in Annex I of the MDR (e.g., 12.2, 13.3, 23.2(r), 23.4(t)) and a dedicated certification process (Annex IX, Section 5.4) [12].
Topical Substance-Based Medical Devices
Topically applied SBMDs constitute an increasingly relevant therapeutic category whose regulatory qualification under the MDR primarily hinges on the demonstration of a non-pharmacological principal mode of action, distinguishing them from topical medicinal products (Figure 2) [13]. SBMDs have been successfully employed across various therapeutic areas, notably dermatology, wound care, mucosal protection, and gastrointestinal disorders. In dermatology, topical SBMDs address skin conditions such as eczema, psoriasis, and dermatitis by reinforcing barrier integrity and hydration. In wound care, these products facilitate wound healing through the physical protection of tissues and maintenance of an optimal microenvironment, thus supporting natural repair mechanisms. Furthermore, SBMDs play an essential role in mucosal protection, alleviating symptoms of irritation or inflammation by creating a soothing protective layer over mucous membranes [13,14,15]. These topical SBMDs commonly act through physical or physicochemical means such as the formation of protective barriers, lubrication, hydration, or adsorption onto biological tissues, facilitating conditions optimal for healing or symptom relief related to gastrointestinal disorders [4]. Additionally, they may function through adsorption or mucoadhesion, ensuring the retention of active substances at the target site, thus enhancing local efficacy without systemic absorption [14,16]. Other examples include osmotic effects, viscosity modification, and modulation of local pH, further distinguishing these devices from topical pharmaceuticals and cosmetics [15].
Under Regulation (EU) 2017/745, Annex VIII, Rule 21 formally establishes a risk-based classification framework for topical SBMDs, whereby regulatory qualification is driven by the degree of absorption and intended site of action, with significant implications for evidentiary requirements related to safety, biocompatibility, and clinical performance (Figure 1).
3. Market Dynamics
According to recent industry reports, the European medical technology sector was valued at approximately €160 billion in 2023, with a sustained annual growth rate of 5–6% over the previous decade [17]. Market data from five European countries (Italy, Spain, France, Poland, and Germany) highlight the growing relevance of substance-based medical devices (SBMDs) in the European self-medication sector. Based on data mainly from IQVIA, the combined market value of SBMDs in these countries reached approximately €3.2 billion, representing 304 million units sold as of mid-2022 (Source: IQVIA Flexview Multichannel Italia—MKT Moving Annual Total Apr 2022; [13]). This corresponds to a growth rate of +18%, outperforming the overall self-medication sector, which grew by +13.5%. These devices now account for 11% of the self-medication market, with an average price per unit of €10.41, compared to €7.47 for the total self-medication sector, suggesting a perceived higher value and innovation level. Italy, considered a benchmark market, demonstrates particularly strong figures: in April 2022, over 650 companies were active in the SBMDs sector, generating €1.1 billion in sales. SBMDs, especially those derived from natural substances, are becoming a core element of the EU health ecosystem. Their success reflects both market demand and the ability of the newest MDR framework to support innovation, safety, and therapeutic diversity in addressing unmet medical needs [13]. Such dynamic growth highlights consumer preference for innovative, multifunctional topical products, particularly in therapeutic areas traditionally dominated by medicinal products, such as dermatology and wound care.
The historical evolution and regulatory developments underscore a notable shift towards sophisticated topical delivery systems, driving significant investment and innovation. Indeed, the rise of SBMDs illustrates the interplay between consumer demand, scientific advancement, and regulatory clarity. Their emergence not only reshapes the topical therapeutic landscape but also challenges existing classifications, necessitating continuous refinement of regulatory frameworks to accommodate innovation without compromising safety and effectiveness [13].
4. Topical Medicinal Products
Directive 2001/83 defines MPs as “any substance or combination of substances” intended to exert a therapeutic effect by modifying physiological functions through a pharmacological, immunological, or metabolic mechanism of action [18].
These products typically involve active pharmaceutical ingredients (API) that interact with skin or mucosal receptors or enzymes to modulate physiological processes. A clear example is topical corticosteroids, which act as glucocorticoid receptor agonists, regulating gene expression to suppress inflammation and immune activity [16,19]. The clinical use of corticosteroids for inflammatory dermatoses like eczema and psoriasis demands careful benefit-risk evaluation, especially in terms of potency and duration of use, to minimize systemic side effects such as hypothalamic–pituitary–adrenal axis suppression and skin atrophy [20].
Because their primary action is pharmacological, topical MPs are regulated under Directive 2001/83/EC, which mandates a rigorous marketing authorization process that involves EMA. This includes submission of comprehensive quality, safety, and efficacy data, derived from nonclinical and clinical trials, to demonstrate a favorable benefit-risk profile [18]. Randomized controlled trials (RCTs) offer the highest level of clinical evidence; for instance, combination therapies such as lebrikizumab plus topical corticosteroids have undergone robust RCT evaluation for atopic dermatitis, focusing not only on efficacy but also on safety endpoints [21].
In contrast to SBMDs, which act through physical or chemical means, topical MPs inherently rely on the biological activity of APIs. This reliance translates into stricter pre-market requirements, including evidence of pharmacokinetics, systemic exposure, and long-term toxicity. For example, guideline-driven clinical evaluations must address efficacy endpoints, adverse event profiles, and risk mitigation strategies (e.g., potency limitation, treatment duration controls) to satisfy regulatory obligations under the EMA Committee for Medicinal Products for Human Use (CHMP) and national authorities [22]. As mentioned, SBMDs, which may contain a medicinal product as an ancillary substance, differ both technically and regulatorily from pure MPs. While the ancillary substance may contribute to the overall performance, its action must remain secondary to the non-pharmacological mechanism of the device. Consequently, these products are assessed under the MDR framework, with involvement of a Notified Body and a mandatory consultation with EMA or national competent authorities for the ancillary substance. Unlike medicinal products, where full pharmacokinetic, systemic exposure, and efficacy data are required, SBMDs focus on demonstrating the safety, quality, and compatibility of the ancillary component and on ensuring that it does not pose disproportionate risks. Clinical evaluation therefore aims at confirming safety and performance of the device, while the ancillary substance is scrutinized mainly for safety and supportive contribution, rather than as the primary determinant of therapeutic efficacy.
5. Innovations in Wound Dressings: From Traditional Approaches to SBMDs
Traditional wound care strategies include a variety of approaches, ranging from non-invasive therapies and pharmacological treatments to surgical interventions. Among non-invasive methods, topical formulations, wound dressings, and skin substitutes are widely used [23]. Wound dressings play a central role in both acute and chronic wound management by maintaining a favorable microenvironment that supports tissue regeneration and reduces the risk of infection. In recent years, advances in materials science, biotechnology, and nanotechnology have led to the development of a wide range of innovative dressings tailored to different wound types and clinical needs [23].
Currently, more than 3000 wound dressing products are available on the market, generally classified as traditional, biological, or synthetic, depending on their composition and mechanism of action. The most used are film-based, hydrogel-based, and polymer-based formulations, often incorporating antimicrobial agents, particularly for the treatment of acute or infected wounds [24]. Conventional dressings such as gauze, cotton pads, and bandages primarily act as passive barriers, offering limited biological support. In contrast, modern dressings, such as hydrogels, hydrocolloids, alginates, foams, and films, are specifically designed to preserve the wound microenvironment by maintaining moisture, temperature, and pH balance, thus promoting re-epithelialization, protecting surrounding tissues, and reducing bacterial colonization [24,25,26].
An ideal wound dressing should meet multiple criteria: it should ensure a moist environment; allow gas exchange; conform to the wound bed; absorb excess exudate; promote cell migration and proliferation (key for angiogenesis and tissue regeneration); provide hemostatic control; maintain thermal insulation; prevent bacterial contamination; be occlusive yet non-adherent; exhibit biocompatibility, non-toxicity, and mechanical stability; allow atraumatic removal; facilitate debridement; minimize scar formation; and be readily available and cost-effective [24].
Driven by these complex requirements, research has increasingly focused on dressings based on biopolymers and bioactive compounds, formulated in novel pharmaceutical-like formats such as hydrogels, scaffolds, fibers, sponges, membranes, and films. Common biopolymers, whose advantages and disadvantages are described in Table 1, used in these formulations include chitosan, polyvinyl alcohol, cellulose, polycaprolactone, gelatin, starch, collagen, hyaluronic acid, alginate, polylactic acid and carrageenan. Notably, chitosan, a natural cationic polysaccharide derived from chitin, has demonstrated excellent antimicrobial properties, biocompatibility, biodegradability, non-toxicity, and wound-healing capabilities [25,27,28]. Due to the presence of amine groups, it becomes positively charged at pH values below 6. This positive charge enables chitosan to electrostatically interact with negatively charged sites on microbial membranes. As a result, chitosan can attach to the bacterial cell surface and disrupt membrane integrity, leading to the leakage of intracellular contents and the inhibition of nutrient transport into the cell [29,30]. An injectable hydrogel composed of two natural polymers, chitosan and konjac glucomannan, crosslinked through Schiff base bonds, demonstrated strong antibacterial activity against both Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli, achieving killing efficiencies of 96% and 98%, respectively [31]. In another approach, chitosan/bacterial cellulose semi-interpenetrating hydrogels were prepared by blending the two polymers, followed by crosslinking with glutaraldehyde. These hydrogels also exhibited antibacterial activity against the same bacterial strains, with the level of antibacterial effectiveness depending on the chitosan-to-cellulose ratio [32].
Another important example is hyaluronic acid (HA), a naturally occurring polysaccharide extensively used in hydrogel-based dressings due to its high hydrophilicity, biocompatibility, and ability to retain moisture and support tissue repair [33,34,35]. Topical dressings that combine HA with antimicrobial agents such as metallic silver or silver sulfadiazine (SSD) are among the most representative examples of substance-based medical devices (SBMDs). These formulations blur the regulatory line between medical devices and medicinal products. In particular, HA/AgNPs (silver nanoparticle) nanocomposites have shown strong antibacterial activity against Gram-negative bacteria such as Escherichia coli, with high biocompatibility and no cytotoxic effects on keratinocyte cell lines. Histological analyses confirmed enhanced wound healing compared to HA-only or untreated controls [36]. The natural biocompatibility of hydrogels also enables the incorporation of small active molecules, such as antibiotics, which can exert bacteriostatic or bactericidal effects through concentration-dependent mechanisms targeting microbial structures and metabolic processes [27].
Among antimicrobial agents reported in Table 2, silver has been used for centuries in infection control and remains a cornerstone in modern wound care. Its broad-spectrum activity covers both Gram-positive and Gram-negative bacteria, making it suitable for managing heavily infected or drug-resistant wounds [37,38]. Silver ions act on multiple bacterial targets, including membranes, cytoplasmic organelles, and DNA, which makes resistance development rare. Silver is often embedded in advanced dressing materials like foams, alginates, hydrogels, and hydrofibers, enabling sustained release in moist environments and effective exudate management while preventing periwound maceration [39]. Silver nanoparticles are typically incorporated at 0.05–0.5 wt% relative to the dry polymer matrix (i.e., 500–5000 ppm), a range chosen to provide sufficient antibacterial activity while limiting cytotoxicity, with final optimization based on silver ion release and cell viability testing [40,41].
Silver sulfadiazine (SSD), synthesized from silver nitrate and sulfadiazine, has been a standard topical antimicrobial since the 1970s, particularly for burn wounds. Its dual-action mechanism, combining the antimicrobial effects of silver and the bacteriostatic activity of sulfadiazine, has proven superior to other topical antiseptics like PVP-I [42]. Silver sulfadiazine is generally incorporated at around 1 wt% of the gel or polymer matrix, within the established 1% w/w range used in SSD hydrogels and topical burn preparations, with possible exploration up to 2.5–5% w/w in experimental studies if justified by safety data [43,44,45]. However, challenges related to its solubility and stability have limited its broader application in controlled drug delivery systems. In response, silver nanoparticles (AgNPs) have gained attention as a next-generation alternative, demonstrating effective biofilm disruption, membrane damage, and enhanced cell migration in wound models. Their integration into collagen- or chitosan-based scaffolds further improves healing performance [46].
In this context, Rule 14 of the MDR is particularly relevant, as it applies to medical devices that incorporate, as an integral part, a substance that, if used separately, would be considered a medicinal product according to Directive 2001/83/EC, and whose action is ancillary to that of the device. This rule is especially important when assessing wound dressings containing SSD or silver nanoparticles.
According to the AESGP Position Paper on Rule 14 (2018), the classification of such products requires a case-by-case assessment that considers not only the presence and amount of the active substance but also whether it is capable of appreciably restoring, correcting, or modifying physiological functions through a pharmacological, immunological, or metabolic (PhIM) action. If these conditions are met and the action remains ancillary, the product falls under Rule 14 and is classified as a Class III medical device. However, if the PhIM action is deemed primary, the product must be regulated as a medicinal product.
This distinction is critical for topical antimicrobial formulations, where the line between physical/chemical barrier function (typical of SBMDs under Rule 21) and active pharmacological antimicrobial action (indicative of a medicinal product) is often blurred. As the AESGP notes, scientific justification must support the manufacturer’s claim regarding the principal mode of action, and such claims cannot contradict current scientific evidence or pharmacological plausibility [47].

Table 1.
Comparative table outlining the advantages and disadvantages of polymer matrices employed in wound dressings.
Table 1.
Comparative table outlining the advantages and disadvantages of polymer matrices employed in wound dressings.
| Polymer | Advantages | Disadvantages | References |
|---|---|---|---|
| Chitosan | Antimicrobial, hemostatic, biocompatible and biodegradable | Solubility limitations, mechanical constraints, heterogeneity and batch variability | [48,49,50,51,52] |
| Polyvinylalcohol | Biocompatible, water solubility, hemostatic, high-water uptake, low production costs | Non-antimicrobial, limited mechanical properties | [53,54,55,56,57,58,59] |
| Cellulose | Biocompatible, hemostatic, water absorption capacity | Non-antimicrobial, limited mechanical properties | [60,61,62,63] |
| Polycaprolactone | Biocompatible, mechanical strength, controllable degradation rate, cost-effectiveness and scalability | Non-antimicrobial, hydrophobicity, poor absorption capacity | [64,65,66,67] |
| Gelatin | Biocompatible, hemostatic, versatile formulation capability, water absorption capacity | Non-antimicrobial, limited mechanical properties, thermosensitive, batch variability, high production costs | [68,69,70,71] |
| Starch | Biocompatible, water absorption capacity, cost-effectiveness, versatile formulation capability, excellent water vapor transmission rate | Non-antimicrobial, limited mechanical properties, environmental sensitivity, batch variability, high production costs and scalability challenges | [72,73,74,75,76] |
| Collagen | Biocompatible, hemostatic, angiogenic, versatile formulation capability, biodegradable, promote fibroblast proliferation and collagen deposition | Non-antimicrobial, high production costs, manufacturing complexity, weak mechanical properties, immunogenicity and allergic sensitization, ethical/religious limitations | [77,78,79,80,81,82,83] |
| Hyaluronic acid | Biocompatible, anti-inflammatory, antioxidant, optimal moist wound management, angiogenic, versatile formulation capability | Weak mechanical properties, high production costs, batch variability, maceration risk for high swelling ratio, non-antimicrobial | [34,84,85,86,87,88,89,90,91] |
| Alginate | Biocompatible, exudate absorption, moisture management, hemostatic, anti-inflammatory, versatile formulation capability, cost-effectiveness | Weak mechanical properties, non-antimicrobial, rapid enzymatic degradation, complex production, high variability | [92,93,94,95,96,97,98,99] |
| Polylactic acid | Biocompatible, superior mechanical properties, promotes angiogenesis and collagen deposition, optimal moisture management, versatile formulation capability | Slow degradation rate, acid degradation by-products, inherent hydrophobicity, low cell affinity, non-antimicrobial, high production costs, | [100,101,102,103,104,105,106] |
| Carrageenan | Biocompatible, superior exudate absorption and moisture management, hemostatic, versatile formulation capability, antioxidant, anti-inflammatory | Weak mechanical properties, non-antimicrobial, sourcing and batch variability, high production costs, batch | [107,108,109,110,111,112,113] |

Table 2.
Comparative table of the advantages and disadvantages of various antimicrobial agents employed in wound dressings.
Table 2.
Comparative table of the advantages and disadvantages of various antimicrobial agents employed in wound dressings.
| Antimicrobial Agents | Advantages | Disadvantages | References |
|---|---|---|---|
| Silver | Broad-spectrum antimicrobial, bactericidal, anti-inflammatory | Cytotoxicity, argyria and systemic silver accumulation | [114,115,116,117,118,119,120] |
| Metals and metal oxides nanoparticles (silver, zinc oxide, iron oxide, cerium dioxide, titanium dioxide) | Broad-spectrum antimicrobial, anti-inflammatory, antioxidant | Dispersion and accumulation in different organs of the body, leading to toxicity | [121,122,123,124,125] |
| Non-metal nanoparticles (dendrimers, ferritins, micelles, liposomes) | Broad-spectrum antimicrobial, low immunogenicity, and encapsulation capacity | High production costs | [38,126,127,128,129] |
| Iodine | Broad-spectrum antimicrobial, bactericidal, fungicidal, rapid efficacy, anti-inflammatory, cost-effectiveness, | Local tissue toxicity and irritation, thyroid dysfunction risk | [130,131,132,133,134,135,136] |
| Biguanides: polyhexamethylene biguanide (PHMB), chlorhexidine | Cationic emulsifier and broad-spectrum antimicrobial, bactericidal, virucidal, cysticidal, promotes tissue granulation and wound healing | Possibly cytotoxic, repeated prolonged exposure may cause sensitization | [38,137,138,139,140,141] |
| Plant-derived natural compounds (oregano, tea tree oil, lavender) | Broad-spectrum antimicrobial, bactericidal, insecticidal, analgesic, antioxidant, and anti-inflammatory effects | Frequent application and/or the use of high concentrations may be necessary, batch variability | [142,143,144,145,146,147,148] |
| Antimicrobial peptides (AMPs) | Broad-spectrum antimicrobial, biofilm penetration and disruption, minimal resistance development | Some AMPs might be sensitive to light, heat, and moisture; proper storage conditions are crucial to maintain their efficacy and cytotoxicity at higher concentrations. High production costs | [149,150,151,152,153,154] |
6. Examples of Registered Topical Substance-Based Medical Devices
Examples of topical dressings, based on substances, available on the market are given in Table 3. Some of the products are briefly commented on below.

Table 3.
Examples of topical dressings registered as SBMDs and available on the market; trade name, manufacturer, risk class and applicable legislation are mentioned.
Table 3.
Examples of topical dressings registered as SBMDs and available on the market; trade name, manufacturer, risk class and applicable legislation are mentioned.
| Trade Name | Manufacturer | Risk Class | Applicable Legislation | Reference |
|---|---|---|---|---|
| Bioepithelia base crema | Kethema farmaceutici srl | Class I | Reg. UE 2017/745 | [155] |
| Alovex ferite spray | Nirial pharma s.r.l. | Class IIa | Reg. UE 2017/745 | [156] |
| Ialuset | Ibsa farmaceutici italia srl | Class IIb | Reg. UE 2017/745 | [157] |
| Iodosorb ointment | Smith and nephew medical limited | Class III | Reg. UE 2017/745 | [158] |
| Sofargen repair | Sofar s.p.a. | Class IIa | Directive 93/42/EEC on Medical Devices | [159] |
| Fitostimoline plus crema | Farmaceutici damor s.p.a. | Class IIb | Directive 93/42/EEC on Medical Devices | [160] |
| Connettivinabio plus crema | Fidia farmaceutici s.p.a. | Class III | Directive 93/42/EEC on Medical Devices | [161] |
| ialuset plus | Ibsa farmaceutici italia srl | Class III | Directive 93/42/EEC on Medical Devices | [162] |
Taking into consideration Ialusetcare, for example, is a medical device indicated for the treatment of various skin lesions, including non-infected wounds, cuts, burns, leg ulcers, hand fissures, fistulas, pressure ulcers, abrasive dermatitis, scars, and excoriations. It is also recommended for moderate to severe skin irritation and dehydration, such as that occurring during radiotherapy. Additionally, the cream may be used to support re-epithelialization in difficult-to-heal wounds and major scars, including post-surgical ones, helping to restore the physiological condition of the skin [162]. Connettivina® bio plus is recommended for managing skin lesions at high risk of infection. It is particularly suitable for both acute and chronic wounds, including first- and second-degree burns, vascular and metabolic ulcers, and pressure sores. This formulation helps maintain a moist, microbe-free wound environment [161]. Another product, Connettivinasilver® Plus spray, is a topical powder spray composed of sodium hyaluronate and metallic silver. It is indicated for the temporary topical treatment of non-infected skin injuries such as abrasions, excoriations, fissures, cuts, minor surgical wounds, and localized first- and second-degree burns. The device promotes a humid wound environment while protecting the lesion from external microbial contamination [161].
All these medical devices are composed of substance-based formulations containing hyaluronic acid (HA), alone or in combination with silver-based antimicrobial agents, and share the same non-PhIM mechanism of action. HA, owing to its strong hygroscopic properties, plays a key role in maintaining a favorable microenvironment for wound healing by supporting skin hydration and elasticity in the presence of tissue damage. The hydrogel-based matrix, typically containing over 95% water, ensures continuous moisture at the wound site, thereby promoting the physiological healing process and facilitating the autolytic debridement of necrotic tissue.
In formulations that include silver sulfadiazine (SSD), a metal–organic compound obtained by reacting silver nitrate with sulfadiazine, an additional antimicrobial effect is provided, helping to prevent and control microbial contamination within the dressing and maintaining a wound environment free from exogenous pathogens. The overall formulation creates optimal conditions for tissue regeneration, acting as a physical barrier to microbial penetration while remaining easy to remove through gentle rinsing with saline solution.
In addition, several clinical trials were retrieved to support the safety and efficacy of wound healing through use of HA dressing. The study conducted by Gazzabin et al. (2019) evaluated the medical device Hyalosilver®, a topical spray powder containing HA and metallic silver [163]. The findings indicate that the spray is effective not only in reducing wound size from the first application but also in lowering the bacterial load. Quantitative analyses demonstrated a significant reduction in bacterial presence at both one and seven days after treatment, although complete eradication was not achieved in any of the cases [163]. The study by Dereure et al. (2012) was based on the hypothesis that the HA-containing formulation (Ialuset® cream) would demonstrate superior clinical efficacy compared to its neutral vehicle (an identical formulation lacking HA) [164]. The primary endpoint, percentage reduction in wound size at day 45, was evaluated as a potential surrogate marker for leg ulcer healing [164]. The study by Barrois et al. (2007) evaluated the use of Ialuset® cream (a 0.2% hyaluronic acid-based topical formulation) and Ialuset® gauze pads (containing 0.05% hyaluronic acid) in the treatment of pressure ulcers [165]. Over a three-week period, complete wound healing was observed in only 1 out of 20 patients, suggesting that extended treatment durations and follow-up are necessary to fully assess the therapeutic potential of Ialuset®. Additionally, the study reported a significant reduction in the mean percentage of fibrous tissue within the wounds, along with a near-significant increase in granulation tissue [165]. In a single-center, observational, and retrospective study conducted by De Francesco et al. (2022) involving eighty patients, both cream and gauze formulations containing low molecular weight HA (200 kDa) and 1% SSD were well tolerated [166]. Most participants reported high levels of satisfaction with the treatments. The findings underscore the value and efficacy of appropriately designed wound dressings, such as Connettivina® Bio Plus cream and gauze pads. The presence of LMW hyaluronic acid was shown to promote granulation tissue formation, offer protection against bacterial biofilms, and improve the condition of the perilesional skin. The observed enhancement in ulcer healing appears to result from the synergistic effect of HA and SSD. HA contributes to the creation of a moist wound environment that supports cell viability, tissue regeneration, and fluid retention, key factors in granulation tissue development and subsequent wound closure.
In combination, SSD adds further therapeutic benefit by inhibiting bacterial proliferation and reducing infection risk. The study demonstrated a strong correlation between bacterial control, effective wound bed management, and improvement of the periwound area, an essential aspect in the treatment of chronic ulcers. Overall, the treatment was associated with a notable reduction in discomfort, erythema, and edema [166]. The study conducted by Russo et al. (2022) aimed to evaluate and compare the efficacy and tolerability of two advanced wound dressings, Fitostimoline® Plus and Connettivina® Bio Plus, in the management of acute superficial skin lesions; both treatment groups demonstrated efficacy in reducing fibrin within the lesion [167]. These results align with existing literature, which consistently demonstrates that fibrin reduction facilitates the removal of a degraded extracellular matrix and supports the formation of a functional provisional matrix, thereby promoting a more physiological wound healing process [167].
7. Conclusions
The growing interest in SBMDs for topical wound care reflects a broader trend in biomedical innovation: the convergence of pharmaceutical-like formulations with medical device principles. Products containing biopolymers such as hyaluronic acid and chitosan, often combined with silver-based agents (e.g., silver sulfadiazine, AgNPs), demonstrate significant therapeutic potential while relying on physical or physicochemical mechanisms of action, a hallmark of SBMDs under Rule 21 of the MDR.
However, the regulatory classification of these products remains highly complex. The boundary between SBMDs under Rule 14 and MPs is often blurred, particularly when antimicrobial substances are incorporated. As highlighted in MDCG 2022-5 Rev.1 and reaffirmed by the AESGP position paper, a thorough, evidence-based assessment of the principal mode of action is essential. Yet, interpretations vary across Member States, creating uncertainty for developers and inconsistencies in market access.
The paradox is clear: a product classification may depend less on its clinical performance than on the regulatory lens through which it is viewed. A cream that hydrates and protects the wound is a device until it also claims to “kill bacteria,” at which point it may be seen as a drug. This binary logic, rooted in the separation between pharmacological and physical mechanisms, no longer reflects the complexity of modern therapeutic products, particularly those based on natural or multifunctional substances.
To foster innovation without compromising safety, a shift is needed from rigid categorization toward a more integrated and harmonized regulatory approach. This includes clearer guidance on the role of ancillary substances (Rule 14), greater acceptance of complex modes of action, and consistent application of borderline classification principles across Europe.
In conclusion, topical SBMDs represent not just a therapeutic opportunity but a regulatory challenge that forces us to rethink how we define, evaluate, and legitimize medical technologies. After all, a dressing should not become a drug merely because it works well, and the future of wound care depends on our ability to recognize that.
8. Key Takeaways
- -
- Topical substance-based medical devices offer significant therapeutic potential in wound care, particularly when formulated with hydrating biopolymers (e.g., hyaluronic acid) and antimicrobial agents (e.g., silver sulfadiazine, AgNPs).
- -
- The principal mode of action is the defining criterion for classification under MDR, impacting whether a product is regulated as a medical device (Rule 21), a drug-device combination (Rule 14), or a medicinal product.
- -
- Products containing silver or SSD exemplify borderline challenges, as their antimicrobial activity may be ancillary (Rule 14) or primary (MPs), depending on concentration, presentation, and intended purpose.
- -
- The interpretation of Rule 14 has been clarified by MDCG 2022-5 Rev.1 and the AESGP Position Paper, which stress the need for a case-by-case assessment and scientific justification of the mode of action.
- -
- Inconsistent classification practices across EU Member States delay market access, increase regulatory burden, and hinder innovation, particularly for complex, multifunctional, or natural-origin substances.
- -
- A more harmonized, flexible, and evidence-based regulatory approach is urgently needed to support the development of effective, safe, and innovative wound care solutions.
Author Contributions
Conceptualization, D.I. and M.M.; methodology, D.I. and M.M.; investigation, D.I. and M.M.; data curation, D.I., C.V. and M.R.; writing—original draft preparation, D.I. and M.M.; writing—review and editing, D.I., M.M. and S.R.; visualization, D.I., B.V. and C.V.; supervision, S.R.; project administration, S.R. and B.V.; funding acquisition, S.R. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by PNRR funded by the European Union—NextGenerationEU DM 352/2022—CUP F13C22000960005—fellow-ship n. 38-033-22-DOT1322889-3527.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
Author Michela Mori was employed by the company 1Med SAX. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Roberts, M.S.; Cheruvu, H.S.; Mangion, S.E.; Alinaghi, A.; Benson, H.A.E.; Mohammed, Y.; Holmes, A.; van der Hoek, J.; Pastore, M.; Grice, J.E. Topical drug delivery: History, percutaneous absorption, and product development. Adv. Drug Deliv. Rev. 2021, 177, 113929. [Google Scholar] [CrossRef] [PubMed]
- Jeary, T.; Sutch, J. An Overview of the Impact of the MDR (EU) 2017/745 for Combination Products and Substance-Based Devices. BSI Group. 2025. Available online: https://www.bsigroup.com (accessed on 15 October 2025).
- Marletta, M. Regulation 2017/745 on medical devices, two major innovations: 1) the physiological action of devices consisting of natural materials such as vegetal matrices; 2) the chemical-physical-mechanical action of devices made of “substances”, which as such are artificial derivatives. Front. Drug Saf. Regul. Front. Media SA 2024, 4, 1389406. [Google Scholar] [CrossRef]
- Leone, M.G. Medical devices made of substances: Regulatory perspectives and market challenges. Front. Drug Saf. Regul. 2022, 2, 952013. [Google Scholar] [CrossRef]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- Aronson, J.K.; Ferner, R.E. Clarification of Terminology in Drug Safety. Drug Saf. 2005, 28, 851–870. [Google Scholar] [CrossRef]
- MDCG MDCGD. MDCG 2022-5 Rev. 1 Guidance on Borderline Between Medical Devices and Medicinal Products Under Regulation (EU) 2017/745 on Medical Devices; European Commission: Brussels, Belgium, 2024. [Google Scholar]
- Racchi, M.; Govoni, S.; Lucchelli, A.; Capone, L.; Giovagnoni, E. Insights into the definition of terms in European medical device regulation. Expert Rev. Med. Devices 2016, 13, 907–917. [Google Scholar] [CrossRef]
- MDCG MMDCGD. Guidance on Transitional Provisions for Consultations of Authorities on Devices Incorporating a Substance Which May be Considered a Medicinal Product and Which Has Action Ancillary to that of the Device, as Well as on Devices Manufactured Using TSE Susceptible Animal Tissues; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- AESGP. Association of the European Self-Care Industry AESGP Position Paper on Classification Rule 21. 2018. Available online: www.aesgp.eu (accessed on 24 July 2025).
- European Commission. MEDDEV 2.1/3 Rev. 3—Guidelines on Borderline Products, Drug-Delivery Products and Medical Devices Incorporating an Ancillary Medicinal Substance or an Ancillary Human Blood Derivative. 2009. Available online: https://ec.europa.eu/docsroom/documents/10328/attachments/1/translations (accessed on 30 October 2025).
- European Commission. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/ 385/EEC and 93/42/EEC; Official Journal of the European Union; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Giovagnoni, E. Substance-based medical devices made of natural substances: An opportunity for therapeutic innovation. Front. Drug Saf. Regul. 2022, 2, 998114. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M. Mucoadhesive polymers in substance-based medical devices: Functional ingredients or what else? Front. Drug Saf. Regul. 2023, 3, 1227763. [Google Scholar] [CrossRef]
- Marletta, M. The new regulation 2017/745: An opportunity for innovation. Pharmadvances 2020, 1. [Google Scholar] [CrossRef]
- Racchi, M.; Govoni, S. The concept of non-pharmacological mechanism of action in medical devices made of substances in practice: What pharmacology can do to promote the scientific implementation of the European medical device regulation. Pharmadvances 2020, 1, 4–12. [Google Scholar] [CrossRef]
- MedTech Europe. European Medical Technology Market Statistics. 2023. Available online: https://www.medtecheurope.org/datahub/market (accessed on 30 October 2025).
- European Commission. Directive 2001/83/EC of the European Parliament and of the Council on the Community Code Relating to Medicinal Products for Human Use. 2001. Available online: https://eur-lex.europa.eu/eli/dir/2001/83/oj (accessed on 30 October 2025).
- Uva, L.; Miguel, D.; Pinheiro, C.; Antunes, J.; Cruz, D.; Ferreira, J.; Filipe, P. Mechanisms of Action of Topical Corticosteroids in Psoriasis. Int. J. Endocrinol. 2012, 2012, 561018. [Google Scholar] [CrossRef]
- Axon, E.; Chalmers, J.R.; Santer, M.; Ridd, M.J.; Lawton, S.; Langan, S.M.; Grindlay, D.J.C.; Muller, I.; Roberts, A.; Ahmed, A.; et al. Safety of topical corticosteroids in atopic eczema: An umbrella review. BMJ Open 2021, 11, 46476. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Guttman-Yassky, E.; Thaçi, D.; Irvine, A.D.; Stein Gold, L.; Blauvelt, A.; Simpson, E.L.; Chu, C.-Y.; Liu, Z.; Lima, R.G.; et al. Two Phase 3 Trials of Lebrikizumab for Moderate-to-Severe Atopic Dermatitis. N. Engl. J. Med. 2023, 388, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Life Sciences Law Review—European Union Chapter. 2017. Available online: https://www.cov.com/-/media/files/corporate/publications/2017/03/life_sciences_law_review_european_union_2017.pdf (accessed on 15 October 2025).
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N. In Vitro and In Vivo Characterization Methods for Evaluation of Modern Wound Dressings. Pharmaceutics 2023, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.M.; Leite, J.M.d.S.; Medeiros, K.d.A.; Assis, K.M.A.d.; Borges, J.C.; Santana, L.M.B.; de Carvalho Moreira, L.M.C.; Alves, L.P.; de Oliveira, T.K.B.; de Souza da Silveira, J.W.; et al. Pentoxifylline/Chitosan Films on Wound Healing: In Vitro/In Vivo Evaluation. Pharmaceutics. Pharmaceutics 2023, 15, 1122. [Google Scholar] [CrossRef]
- Torres, A.; Rego, L.; Martins, M.S.; Ferreira, M.S.; Cruz, M.T.; Sousa, E.; Almeida, I.F. How to Promote Skin Repair? In-Depth Look at Pharmaceutical and Cosmetic Strategies. Pharmaceuticals 2023, 16, 573. [Google Scholar] [CrossRef]
- Kapusta, O.; Jarosz, A.; Stadnik, K.; Giannakoudakis, D.A.; Barczyński, B.; Barczak, M. Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review. Int. J. Mol. Sci. 2023, 24, 2191. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Vazifehdoost, M.; Alhashemi, S.H.; Davoudi, H.; Zarrabi, A.; Dehshahri, A.; Fekri, H.S.; Mohammadinejad, R.; Thakur, V.K. Next-Generation Hydrogels as Biomaterials for Biomedical Applications: Exploring the Role of Curcumin. ACS Omega 2023, 8, 8960. [Google Scholar] [CrossRef]
- Khalil, A.M.; Abdel-Monem, R.A.; Darwesh, O.M.; Hashim, A.I.; Nada, A.A.; Rabie, S.T. Synthesis, Characterization, and Evaluation of Antimicrobial Activities of Chitosan and Carboxymethyl Chitosan Schiff-Base/Silver Nanoparticles. J. Chem. 2017, 2017, 1434320. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Hu, X.H.; Chu, L.Q.; Jia, S.R.; Xie, Y.Y.; Zhong, C. Development of bacterial cellulose/chitosan based semi-interpenetrating hydrogels with improved mechanical and antibacterial properties. Int. J. Biol. Macromol. 2019, 122, 380–387. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Hyaluronic Acid-Based Scaffolds as Potential Bioactive Wound Dressings. Polymers 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Shao, K.; Huang, S.; Chen, C. Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: A review. Int. J. Biol. Macromol. 2023, 240, 124321. [Google Scholar] [CrossRef]
- Sudhakar, K.; Ji, S.M.; Kummara, M.R.; Han, S.S. Recent Progress on Hyaluronan-Based Products for Wound Healing Applications. Pharmaceutics 2022, 14, 2235. [Google Scholar] [CrossRef]
- Brouillard, C.; Bursztejn, A.C.; Latarche, C.; Cuny, J.F.; Truchetet, F.; Goullé, J.P.; Schmutz, J.L. Silver absorption and toxicity evaluation of silver wound dressings in 40 patients with chronic wounds. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2295–2299. [Google Scholar] [CrossRef]
- Yousefian, F.; Hesari, R.; Jensen, T.; Obagi, S.; Rgeai, A.; Damiani, G.; Bunick, C.G.; Grada, A. Antimicrobial Wound Dressings: A Concise Review for Clinicians. Antibiotics 2023, 12, 1434. [Google Scholar] [CrossRef]
- Sibbald, R.G.; Elliott, J.A.; Verma, L.; Brandon, A.; Persaud, R.; Ayello, E.A. Update: Topical Antimicrobial Agents for Chronic Wounds. Adv. Ski. Wound Care 2017, 30, 438–450. [Google Scholar] [CrossRef]
- Pangli, H.; Vatanpour, S.; Hortamani, S.; Jalili, R.; Ghahary, A. Incorporation of Silver Nanoparticles in Hydrogel Matrices for Controlling Wound Infection. J. Burn Care Res. 2020, 42, 785. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Constantin, M.; Solcan, G.; Ichim, D.L.; Rata, D.M.; Horodincu, L.; Solcan, C. Composite Hydrogels with Embedded Silver Nanoparticles and Ibuprofen as Wound Dressing. Gels 2023, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Punjataewakupt, A.; Napavichayanun, S.; Aramwit, P. The downside of antimicrobial agents for wound healing. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gálvez, J.; Martínez-Isasi, S.; Gómez-Salgado, J.; Rumbo-Prieto, J.M.; Sobrido-Prieto, M.; Sánchez-Hernández, M.; García-Martínez, M.; Fernández-García, D. Cytotoxicity and concentration of silver ions released from dressings in the treatment of infected wounds: A systematic review. Front. Public Health 2024, 12, 1331753. [Google Scholar] [CrossRef]
- Jodar, K.S.P.; Balcão, V.M.; Chaud, M.V.; Tubino, M.; Yoshida, V.M.H.; Oliveira, J.M.; Vila, M.M. Development and Characterization of a Hydrogel Containing Silver Sulfadiazine for Antimicrobial Topical Applications. J. Pharm. Sci. 2015, 104, 2241–2254. [Google Scholar] [CrossRef]
- Liu, X.; Gan, H.; Hu, C.; Sun, W.; Zhu, X.; Meng, Z.; Gu, R.; Wu, Z.; Dou, G. Silver sulfadiazine nanosuspension-loaded thermosensitive hydrogel as a topical antibacterial agent. Int. J. Nanomed. 2018, 14, 289–300. [Google Scholar] [CrossRef]
- Dutt, Y.; Pandey, R.P.; Dutt, M.; Gupta, A.; Vibhuti, A.; Raj, V.S.; Chang, C.-M.; Priyadarshini, A. Silver Nanoparticles Phytofabricated through Azadirachta indica: Anticancer, Apoptotic, and Wound-Healing Properties. Antibiotics 2023, 12, 121. [Google Scholar] [CrossRef]
- AESGP. AESGP Position Paper on Classification Rule 14. 2018. Available online: https://aesgp.eu/content/uploads/2020/07/AESGP-position-paper-on-Rule-14_October-2018.pdf (accessed on 15 September 2025).
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533. [Google Scholar] [CrossRef]
- Rajinikanth, S.B.; Rajkumar, D.S.R.; Keerthika, K.; Vijayaragavan, V. Chitosan-Based Biomaterial in Wound Healing: A Review. Cureus 2024, 16, e55193. [Google Scholar] [CrossRef]
- Sheokand, B.; Vats, M.; Kumar, A.; Srivastava, C.M.; Bahadur, I.; Pathak, S.R. Natural polymers used in the dressing materials for wound healing: Past, present and future. J. Polym. Sci. 2023, 61, 1389–1414. [Google Scholar] [CrossRef]
- Ianev, D.; Vigani, B.; Valentino, C.; Sorrenti, M.; Catenacci, L.; Bonferoni, M.C.; Ruggeri, M.; Sandri, G.; Mori, M.; Rossi, S. Whey protein-chitosans complexes as sustainable and value-added biomaterials for wound healing. Carbohydr. Polym. Technol. Appl. 2025, 9, 100643. [Google Scholar] [CrossRef]
- Pino, P.; Vigani, B.; Valentino, C.; Ianev, D.; Ruggeri, M.; Boselli, C.; Cornaglia, A.I.; Grisoli, P.; Onida, B.; Bosco, F.; et al. Sustainable whey proteins-nanostructured zinc oxide-based films for the treatment of chronic wounds: New insights from biopharmaceutical studies. Int. J. Biol. Macromol. 2024, 263, 130655. [Google Scholar] [CrossRef] [PubMed]
- Dorkhani, E.; Faryabi, A.; Noorafkan, Y.; Heirani, A.; Behboudi, B.; Fazeli, M.S.; Kazemeini, A.; Keramati, M.R.; Keshvari, A.; Tafti, S.M.A. Biomedical properties and hemostatic efficacy of polyvinyl alcohol (PVA) based hydrogel in experimental rat liver injury model. J. Appl. Biomater. Funct. Mater. 2023, 21, 22808000231198803. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.M.; Arisawa, E.A.L.S.; Filho, A.L.M.M.; Figueredo-Silva, J.; Alves, N.; da Silveira, C.H.; Vieira, L. Bioabsorbable Poly(vinyl alcohol)–Citric Acid Dressings: Wound Healing Studies in an Experimental In Vivo Model. Eur. Burn. J. 2025, 6, 18. [Google Scholar] [CrossRef]
- Jin, S.G. Production and Application of Biomaterials Based on Polyvinyl alcohol (PVA) as Wound Dressing. Chem. Asian J. 2022, 17, e202200595. [Google Scholar] [CrossRef]
- Yudaev, P.; Butorova, I.; Chuev, V.; Posokhova, V.; Klyukin, B.; Chistyakov, E. Wound Gel with Antimicrobial Effects Based on Polyvinyl Alcohol and Functional Aryloxycyclotriphosphazene. Polymers 2023, 15, 2831. [Google Scholar] [CrossRef]
- Afzal, A.; Jalalah, M.; Noor, A.; Khaliq, Z.; Qadir, M.B.; Masood, R.; Nazir, A.; Ahmad, S.; Ahmad, F.; Irfan, M.; et al. Development and Characterization of Drug Loaded PVA/PCL Fibres for Wound Dressing Applications. Polymers 2023, 15, 1355. [Google Scholar] [CrossRef]
- Liang, X.; Zhong, H.J.; Ding, H.; Yu, B.; Ma, X.; Liu, X.; Chong, C.-M.; He, J. Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications. Polymers 2024, 16, 2755. [Google Scholar] [CrossRef]
- Ji, W.; Mizanur, M.; Khan, R.; Mahamudul, M.; Rumon, H. Synthesis of PVA-Based Hydrogels for Biomedical Applications: Recent Trends and Advances. Gels 2025, 11, 88. [Google Scholar] [CrossRef]
- Bukatuka, C.F.; Mbituyimana, B.; Xiao, L.; Qaed Ahmed, A.A.; Qi, F.; Adhikari, M.; Shi, Z.; Yang, G. Recent Trends in the Application of Cellulose-Based Hemostatic and Wound Healing Dressings. J. Funct. Biomater. 2025, 16, 151. [Google Scholar] [CrossRef]
- Tudoroiu, E.E.; Dinu-Pîrvu, C.E.; Kaya, M.G.A.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef]
- Zhong, Y.; Seidi, F.; Li, C.; Wan, Z.; Jin, Y.; Song, J.; Xiao, H. Antimicrobial/Biocompatible Hydrogels Dual-Reinforced by Cellulose as Ultrastretchable and Rapid Self-Healing Wound Dressing. Biomacromolecules 2021, 22, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Islam, M.S.; Christopher, L.P. Sustainable Production of Cellulose-Based Hydrogels with Superb Absorbing Potential in Physiological Saline. ACS Omega 2019, 4, 9419–9426. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, M.O.; Altun, E.; Crabbe-Mann, M.; Brako, F.; Koc, F.; Ozen, G.; Kuruca, S.E.; Edirisinghe, U.; Luo, C.J.; Gunduz, O.; et al. Cellular interactions with bacterial cellulose: Polycaprolactone nanofibrous scaffolds produced by a portable electrohydrodynamic gun for point-of-need wound dressing. Int. Wound J. 2018, 15, 789. [Google Scholar] [CrossRef] [PubMed]
- Azari, A.; Golchin, A.; Maymand, M.M.; Mansouri, F.; Ardeshirylajimi, A. Electrospun Polycaprolactone Nanofibers: Current Research and Applications in Biomedical Application. Adv. Pharm. Bull. 2021, 12, 658. [Google Scholar] [CrossRef]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun Polycaprolactone (PCL) Degradation: An In Vitro and In Vivo Study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Zeng, W.; Cheng, N.M.; Liang, X.; Hu, H.; Luo, F.; Jin, J.; Li, Y.-W. Electrospun polycaprolactone nanofibrous membranes loaded with baicalin for antibacterial wound dressing. Sci. Rep. 2022, 12, 10900. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef]
- Cao, H.; Wang, J.; Hao, Z.; Zhao, D. Gelatin-based biomaterials and gelatin as an additive for chronic wound repair. Front. Pharmacol. 2024, 15, 1398939. [Google Scholar] [CrossRef]
- Pei, Y.; Ye, D.; Zhao, Q.; Wang, X.; Zhang, C.; Huang, W.; Zhang, N.; Liu, S.; Zhang, L. Effectively promoting wound healing with cellulose/gelatin sponges constructed directly from a cellulose solution. J. Mater. Chem. B 2015, 3, 7518–7528. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Bejenaru, L.E.; Bejenaru, C.; Blendea, A.; Mogoşanu, G.D.; Biţă, A.; Boia, E.R. Advancements in Hydrogels: A Comprehensive Review of Natural and Synthetic Innovations for Biomedical Applications. Polymers 2025, 17, 2026. [Google Scholar] [CrossRef]
- Garavito, J.; Peña-Venegas, C.P.; Castellanos, D.A. Production of Starch-Based Flexible Food Packaging in Developing Countries: Analysis of the Processes, Challenges, and Requirements. Foods 2024, 13, 4096. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, H.; Singhal, N.; Vashistha, P.; Jain, R.; Bist, Y.; Gaur, A.; Wagri, N.K. Starch–biomacromolecule complexes: A comprehensive review of interactions, functional materials, and applications in food, pharma, and packaging. Carbohydr. Polym. Technol. Appl. 2025, 11, 101001. [Google Scholar] [CrossRef]
- Sringam, J.; Pankongadisak, P.; Trongsatitkul, T.; Suppakarn, N. Improving Mechanical Properties of Starch-Based Hydrogels Using Double Network Strategy. Polymers 2022, 14, 3552. [Google Scholar] [CrossRef] [PubMed]
- Eskandarinia, A.; Kefayat, A.; Rafienia, M.; Agheb, M.; Navid, S.; Ebrahimpour, K. Cornstarch-based wound dressing incorporated with hyaluronic acid and propolis: In vitro and in vivo studies. Carbohydr. Polym. 2019, 216, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yin, J.; Shi, Y.; Cheng, J.; Fang, Y.; Huang, C.; Yu, W.; Liu, M.; Yang, Z.; Zhou, H.; et al. Starch and chitosan-based antibacterial dressing for infected wound treatment via self-activated NO release strategy. Int. J. Biol. Macromol. 2022, 220, 1177–1187. [Google Scholar] [CrossRef]
- Pasaribu, K.M.; Ilyas, S.; Tamrin, T.; Radecka, I.; Swingler, S.; Gupta, A.; Stamboulis, A.G.; Gea, S. Bioactive bacterial cellulose wound dressings for burns with collagen in-situ and chitosan ex-situ impregnation. Int. J. Biol. Macromol. 2023, 230, 123118. [Google Scholar] [CrossRef]
- Salthouse, D.; Goulding, P.D.; Reay, S.L.; Jackson, E.L.; Xu, C.; Ahmed, R.; Mearns-Spragg, A.; Novakovic, K.; Hilkens, C.M.U.; Ferreira, A.M. Amine-reactive crosslinking enhances type 0 collagen hydrogel properties for regenerative medicine. Front. Bioeng. Biotechnol. 2024, 12, 1391728. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Ding, X.; Yu, Y.; Yang, C.; Wu, D.; Zhao, Y. Multifunctional GO Hybrid Hydrogel Scaffolds for Wound Healing. Research 2022, 2022, 9850743. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, Y.; Yang, Y.; Jin, M.; Lin, X.; Zhuang, Z.; Guo, K.; Zhang, T.; Tan, W. Application of Collagen-Based Hydrogel in Skin Wound Healing. Gels 2023, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Xia, Z.; Qin, X.; Wang, X.; Lu, W.; Luo, Q.; Zhang, Z.; Xiong, X. The clinical efficacy of collagen dressing on chronic wounds: A meta-analysis of 11 randomized controlled trials. Front. Surg. 2022, 9, 978407. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.; Bratu, A.G.; Niculescu, A.G.; Grumezescu, A.M. Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications. Gels 2025, 11, 271. [Google Scholar] [CrossRef]
- Grabowski, M.; Gmyrek, D.; Żurawska, M.; Trusek, A. Hyaluronic Acid: Production Strategies, Gel-Forming Properties, and Advances in Drug Delivery Systems. Gels 2025, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Cerminati, S.; Leroux, M.; Anselmi, P.; Peirú, S.; Alonso, J.C.; Priem, B.; Menzella, H.G. Low cost and sustainable hyaluronic acid production in a manufacturing platform based on Bacillus subtilis 3NA strain. Appl. Microbiol. Biotechnol. 2021, 105, 3075–3086. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, L.; Shen, X. Development of hyaluronic acid-based hydrogels for chronic diabetic wound healing: A review. Int. J. Biol. Macromol. 2025, 308, 142273. [Google Scholar] [CrossRef]
- Romanò, C.L.; Vecchi, E.D.; Bortolin, M.; Morelli, I.; Drago, L. Hyaluronic Acid and Its Composites as a Local Antimicrobial/Antiadhesive Barrier. J. Bone Jt. Infect. 2017, 2, 63. [Google Scholar] [CrossRef]
- Kim, D.S.; Seong, K.Y.; Lee, H.; Kim, M.J.; An, S.M.; Jeong, J.S.; Kim, S.Y.; Kang, H.-G.; Jang, S.; Hwang, D.-Y.; et al. Antiadhesive Hyaluronic Acid-Based Wound Dressings Promote Wound Healing by Preventing Re-Injury: An In Vivo Investigation. Biomedicines 2024, 12, 510. [Google Scholar] [CrossRef]
- Zhao, X.; Dai, W.; Liu, C.; An, M.; Li, S.; Guo, L.; Fan, Y.; Zhang, X. Gelatin/hyaluronic acid-based in situ forming hydrogel promotes wound regeneration by the synergy of ROS-scavenging and pro-healing activity. Regen. Biomater. 2025, 12, rbaf052. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Q.; Ning, F.; Du, C.; Chen, M.; Feng, C.; Dong, C.-M. Dynamic Hyaluronic Acid Hydrogels for Comprehensively Regulating Inflammation, Angiogenesis, and Metabolism to Effectively Proheal Diabetic Wounds. ACS Appl. Mater. Interfaces 2024, 16, 70256–70273. [Google Scholar] [CrossRef]
- Lee, H.M.; Jang, E.J.; Choi, K.H.; Na, Y.C. Comparative evaluation of hyaluronic acid-based dressing versus hydrocolloid dressing in rat dermal wound healing. Arch. Craniofac. Surg. 2024, 25, 224. [Google Scholar] [CrossRef]
- Pastrana-Alta, R.Y.; Huarote-Garcia, E.; Egusquiza-Huamani, M.A.; Baena-Moncada, A.M. Antimicrobial activity of chitosan, alginate, pectin, and cellulose-based biopolymer composites with silver, copper oxide, and zinc oxide nanoparticles. RSC Adv. 2025, 15, 35807–35843. [Google Scholar] [CrossRef]
- Ramírez-Partida, A.E.; García-Cayuela, T.; Amador-Castro, L.F.; Alper, H.S.; Carrillo-Nieves, D. Towards a biorefinery processing Sargassum seaweed: Techno-economic assessment of alginate and fucoidan production through SuperPro Designer® process simulation. Environ. Technol. Innov. 2024, 34, 103587. [Google Scholar] [CrossRef]
- Babić Radić, M.M.; Vukomanović, M.; Nikodinović-Runić, J.; Tomić, S. Gelatin-/Alginate-Based Hydrogel Scaffolds Reinforced with TiO2 Nanoparticles for Simultaneous Release of Allantoin, Caffeic Acid, and Quercetin as Multi-Target Wound Therapy Platform. Pharmaceutics 2024, 16, 372. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Bao, D.; Ta, F.; Liu, D.; Zhang, D.; Zhang, Z.; Fan, Z. Multifunctional Alginate Hydrogel Protects and Heals Skin Defects in Complex Clinical Situations. ACS Omega 2020, 5, 17152–17159. [Google Scholar] [CrossRef] [PubMed]
- Mujawar, S.S.; Arbade, G.K.; Rukwal, S.; Tripathi, V.; Mane, M.; Sharma, R.K.; Kashte, S.B. 3D printed sodium Alginate-Gelatin hydrogel loaded with Santalum album oil as an antibacterial Full-Thickness wound healing and scar reduction Scaffold: In vitro and in vivo study. Int. J. Pharm. 2025, 670, 125164. [Google Scholar] [CrossRef]
- Mazurek, Ł.; Kuś, M.; Jurak, J.; Rybka, M.; Kuczeriszka, M.; Stradczuk-Mazurek, M.; Konop, M. Biomedical potential of alginate wound dressings—From preclinical studies to clinical applications: A review. Int. J. Biol. Macromol. 2025, 309, 142908. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Froelich, A.; Jakubowska, E.; Wojtyłko, M.; Jadach, B.; Gackowski, M.; Gadziński, P.; Napierała, O.; Ravliv, Y.; Osmałek, T. Alginate-Based Materials Loaded with Nanoparticles in Wound Healing. Pharmaceutics 2023, 15, 1142. [Google Scholar] [CrossRef]
- Mou, L.; Li, J.; Lu, Y.; Li, G.; Li, J. Polylactic acid: A future universal biobased polymer with multifunctional performance—From monomer synthesis, and processing to applications: A review. J. Hazard. Mater. Adv. 2025, 18, 100757. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Weng, Q.H.; Hu, M.H.; Wang, J.F.; Hu, J.J. Enhancing the Flexibility and Hydrophilicity of PLA via Polymer Blends: Electrospinning vs. Solvent Casting. Polymers 2025, 17, 800. [Google Scholar] [CrossRef] [PubMed]
- Kayadurmus, H.M.; Rezaei, A.; Ilhan, E.; Cesur, S.; Sahin, A.; Gunduz, O.; Kalaskar, D.M.; Ekren, N. Whey protein-loaded 3D-printed poly (lactic) acid scaffolds for wound dressing applications. Biomed. Mater. 2024, 19, 045045. [Google Scholar] [CrossRef] [PubMed]
- Heydari, P.; Zargar Kharazi, A.; Shariati, L. Enhanced wound regeneration by PGS/PLA fiber dressing containing platelet-rich plasma: An in vitro study. Sci. Rep. 2024, 14, 12019. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Chen, H.L.; Chung, J.W.Y.; Yan, V.C.M.; Wong, T.K.S. Polylactic Acid-Based Biomaterials in Wound Healing: A Systematic Review. Adv. Ski. Wound Care 2023, 36, 1–8. [Google Scholar] [CrossRef]
- Ditta, L.A.; Rao, E.; Provenzano, F.; Sánchez, J.L.; Santonocito, R.; Passantino, R.; Costa, M.A.; Sabatino, M.A.; Dispenza, C.; Giacomazza, D.; et al. Agarose/κ-carrageenan-based hydrogel film enriched with natural plant extracts for the treatment of cutaneous wounds. Int. J. Biol. Macromol. 2020, 164, 2818–2830. [Google Scholar] [CrossRef]
- Khajavi, M.; Raoufi, Z.; Abdollahi, S. Investigating the potential of collagen/carrageenan trilayer sponges with optimal therapeutic and physical properties for the treatment of pressure ulcers. Int. J. Biol. Macromol. 2025, 306, 141743. [Google Scholar] [CrossRef]
- Biranje, S.S.; Madiwale, P.V.; Patankar, K.C.; Chhabra, R.; Bangde, P.; Dandekar, P.; Adivarekar, R.V. Cytotoxicity and hemostatic activity of chitosan/carrageenan composite wound healing dressing for traumatic hemorrhage. Carbohydr. Polym. 2020, 239, 116106. [Google Scholar] [CrossRef]
- P, A.; Joy, J.M.; Visnuvinayagam, S.; Remya, S.; Mathew, S. Development of κ-carrageenan-based transparent and absorbent biodegradable films for wound dressing applications. Int. J. Biol. Macromol. 2024, 282, 137084. [Google Scholar] [CrossRef]
- Rode, M.P.; Batti Angulski, A.B.; Gomes, F.A.; da Silva, M.M.; Jeremias, T.d.S.; de Carvalho, R.G.; Vieira, D.C.I.; Oliveira, L.F.C.; Maia, L.F.; Trentin, A.G.; et al. Carrageenan hydrogel as a scaffold for skin-derived multipotent stromal cells delivery. J. Biomater. Appl. 2018, 33, 422–434. [Google Scholar] [CrossRef]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.Ș.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Ianev, D.; Adami, M.; Valentino, C.; Ruggeri, M.; Boselli, C.; Cornaglia, A.I.; Sandri, G.; Rossi, S. Porous Functionally Graded Scaffold prepared by a single-step freeze-drying process. A bioinspired approach for wound care. Int. J. Pharm. 2024, 656, 124119. [Google Scholar] [CrossRef] [PubMed]
- Kostenko, V.; Lyczak, J.; Turner, K.; Martinuzzi, R.J. Impact of Silver-Containing Wound Dressings on Bacterial Biofilm Viability and Susceptibility to Antibiotics during Prolonged Treatment. Antimicrob. Agents Chemother. 2010, 54, 5120. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, T.; Nigusse, T.; Dhanaraju, M.D. Silver Nanoparticles as Real Topical Bullets for Wound Healing. J. Am. Coll. Clin. Wound Spec. 2012, 3, 82. [Google Scholar] [CrossRef]
- Liang, K.; Liu, Y.; Jiang, F. Analysis of therapeutic effect of silver-based dressings on chronic wound healing. Int. Wound J. 2024, 21, e70006. [Google Scholar] [CrossRef]
- Isak, V.; Beerli, T.; Cozzio, A.; Flatz, L. A Rare Case of Localized Argyria on the Face. Case Rep. Dermatol. 2019, 11, 23. [Google Scholar] [CrossRef]
- Steck, M.B.; Murray, B.P. Silver Toxicity; StatPearls Publishing: Orlando, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK604211/ (accessed on 17 December 2025).
- Zou, S.B.; Yoon, W.Y.; Han, S.K.; Jeong, S.H.; Cui, Z.J.; Kim, W.K. Cytotoxicity of silver dressings on diabetic fibroblasts. Int. Wound J. 2012, 10, 306. [Google Scholar] [CrossRef]
- You, C.; Li, Q.; Wang, X.; Wu, P.; Ho, J.K.; Jin, R.; Zhang, L.; Shao, H.; Han, C. Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci. Rep. 2017, 7, 10489. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, D.; Nie, F.; Wang, Y.; Chong, Y. Iron Nanoparticles Open Up New Directions for Promoting Healing in Chronic Wounds in the Context of Bacterial Infection. Pharmaceutics 2023, 15, 2327. [Google Scholar] [CrossRef]
- Nosrati, H.; Heydari, M. Titanium dioxide nanoparticles: A promising candidate for wound healing applications. Burn. Trauma 2025, 13, tkae069. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Y.; Bao, S.; Yao, L.; Fu, X.; Yu, Y.; Lyu, H.; Pang, H.; Guo, S.; Zhang, H.; et al. Cerium oxide nanoparticles in wound care: A review of mechanisms and therapeutic applications. Front. Bioeng. Biotechnol. 2024, 12, 1404651. [Google Scholar] [CrossRef] [PubMed]
- Dam, P.; Celik, M.; Ustun, M.; Saha, S.; Saha, C.; Kacar, E.A.; Kugu, S.; Karagulle, E.N.; Tasoglu, S.; Buyukserin, F.; et al. Wound healing strategies based on nanoparticles incorporated in hydrogel wound patches. RSC Adv. 2023, 13, 21345–21364. [Google Scholar] [CrossRef] [PubMed]
- Rayyif, S.M.I.; Mohammed, H.B.; Curuțiu, C.; Bîrcă, A.C.; Grumezescu, A.M.; Vasile, B.Ș.; Dițu, L.M.; Lazăr, V.; Chifiriuc, M.C.; Mihăescu, G.; et al. ZnO Nanoparticles-Modified Dressings to Inhibit Wound Pathogens. Materials 2021, 14, 3084. [Google Scholar] [CrossRef]
- Tetteh-Quarshie, S.; Blough, E.R.; Jones, C.B. Exploring Dendrimer Nanoparticles for Chronic Wound Healing. Front. Med. Technol. 2021, 3, 661421. [Google Scholar] [CrossRef]
- Roy, M.; Roy, A.; Rustagi, S.; Pandey, N. An Overview of Nanomaterial Applications in Pharmacology. Biomed. Res. Int. 2023, 2023, 4838043. [Google Scholar] [CrossRef]
- Yudaev, P.; Mezhuev, Y.; Chistyakov, E. Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects. Gels 2022, 8, 329. [Google Scholar] [CrossRef]
- Ding, C.; Liu, X.; Zhang, S.; Sun, S.; Yang, J.; Chai, G.; Wang, N.; Ma, S.; Ding, Q.; Liu, W. Multifunctional hydrogel bioscaffolds based on polysaccharide to promote wound healing: A review. Int. J. Biol. Macromol. 2024, 259, 129356. [Google Scholar] [CrossRef]
- Grada, A.; Obagi, Z.; Phillips, T. Management of chronic wounds in patients with pemphigus. Chronic Wound Care Manag. Res. 2019, 6, 89–98. [Google Scholar] [CrossRef]
- Vercammen, Y.; Dauwe, D.; De Vlieger, G.; Houthoofd, S.; Desmet, L.; Casaer, M.P. Povidone Iodine Disinfection Associated with Hypothyroidism and Potentially Contributing to Prolonged Kidney Failure. Case Rep. Crit. Care 2021, 2021, 5528210. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, J.; Liu, Y.; Ben, C.; Li, H.; Cheng, D.; Sun, Y.; Guang-Yi, W.; Zhu, S. Analysis of povidone iodine, chlorhexidine acetate and polyhexamethylene biguanide as wound disinfectants: In vitro cytotoxicity and antibacterial activity. BMJ Nutr. Prev. Health 2023, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.K.; Wa, C.T.C.; Villa, M.A. Povidone iodine in wound healing: A review of current concepts and practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Naseri, E.; Ahmadi, A. A review on wound dressings: Antimicrobial agents, biomaterials, fabrication techniques, and stimuli-responsive drug release. Eur. Polym. J. 2022, 173, 111293. [Google Scholar] [CrossRef]
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Consensus on Wound Antisepsis: Update 2018. Ski. Pharmacol. Physiol. 2018, 31, 28–58. [Google Scholar] [CrossRef]
- Kanagalingam, J.; Feliciano, R.; Hah, J.H.; Labib, H.; Le, T.A.; Lin, J.C. Practical use of povidone-iodine antiseptic in the maintenance of oral health and in the prevention and treatment of common oropharyngeal infections. Int. J. Clin. Pract. 2015, 69, 1247–1256. [Google Scholar] [CrossRef]
- Hübner, N.O.; Kramer, A. Review on the Efficacy, Safety and Clinical Applications of Polihexanide, a Modern Wound Antiseptic. Ski. Pharmacol. Physiol. 2010, 23, 17–27. [Google Scholar] [CrossRef]
- Guiomar, A.J.; Urbano, A.M. Polyhexanide-Releasing Membranes for Antimicrobial Wound Dressings: A Critical Review. Membranes 2022, 12, 1281. [Google Scholar] [CrossRef]
- Günther, F.; Blessing, B.; Dapunt, U.; Mischnik, A.; Mutters, N.T. Ability of chlorhexidine, octenidine, polyhexanide and chloroxylenol to inhibit metabolism of biofilm-forming clinical multidrug-resistant organisms. J. Infect. Prev. 2020, 22, 12. [Google Scholar] [CrossRef]
- Sukakul, T.; Dahlin, J.; Pontén, A.; Antelmi, A.; Bruze, M.; Hamnerius, N.; Hauksson, I.; Isaksson, M.; Lejding, T.; Svedman, C. Contact allergy to polyhexamethylene biguanide (polyaminopropyl biguanide). Contact Dermat. 2021, 84, 326–331. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Isopencu, G.O.; Covaliu-Mierlă, C.I.; Deleanu, I.M. From Plants to Wound Dressing and Transdermal Delivery of Bioactive Compounds. Plants 2023, 12, 2661. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.F.; Durço, A.O.; Rabelo, T.K.; Barreto, R.d.S.S.; Guimarães, A.G. Effects of Carvacrol, Thymol and essential oils containing such monoterpenes on wound healing: A systematic review. J. Pharm. Pharmacol. 2019, 71, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, S.; Tsvetkova, A.; Stamova, S.; Ermenlieva, N.; Tsankova, G.; Georgieva, E.; Peycheva, K.; Panayotova, V.; Voynikov, Y. Antibacterial Effects of Bulgarian Oregano and Thyme Essential Oils Alone and in Combination with Antibiotics Against Klebsiella pneumoniae and Pseudomonas aeruginosa. Microorganisms 2025, 13, 843. [Google Scholar] [CrossRef] [PubMed]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Cuttle, L.; Kempf, M.; Kravchuk, O.; George, N.; Liu, P.Y.; Chang, H.E.; Mill, J.; Wang, X.-Q.; Kimble, R.M. The efficacy of Aloe vera, tea tree oil and saliva as first aid treatment for partial thickness burn injuries. Burns 2008, 34, 1176–1182. [Google Scholar] [CrossRef]
- Dunn, K.; Edwards-Jones, V. The role of ActicoatTM with nanocrystalline silver in the management of burns. Burns 2004, 30, S1–S9. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Trzaskowska, M.; Przekora, A. Wound Dressing Modifications for Accelerated Healing of Infected Wounds. Int. J. Mol. Sci. 2023, 24, 2193. [Google Scholar] [CrossRef]
- Zhu, A.; Chen, B.; Ma, J.; Wang, J.; Tang, R.; Liu, L.; Sun, W.; Zheng, X.; Pan, G. Application of Antimicrobial Peptides in Wound Dressings. Drug Des. Devel. Ther. 2025, 19, 8523–8539. [Google Scholar] [CrossRef]
- Adnan, S.B.; Maarof, M.; Fauzi, M.B.; Md Fadilah, N.I. Antimicrobial Peptides in Wound Healing and Skin Regeneration: Dual Roles in Immunity and Microbial Defense. Int. J. Mol. Sci. 2025, 26, 5920. [Google Scholar] [CrossRef]
- Kanaujia, K.A.; Mishra, N.; Rajinikanth, P.S.; Saraf, S.A. Antimicrobial peptides as antimicrobials for wound care management: A comprehensive review. J. Drug Deliv. Sci. Technol. 2024, 95, 105570. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front. Pharmacol. 2018, 9, 352601. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Ali, Z.; Mahfouz, M. Molecular farming for sustainable production of clinical-grade antimicrobial peptides. Plant Biotechnol. J. 2024, 22, 2282. [Google Scholar] [CrossRef] [PubMed]
- BIOEPITHELIA® BASE: Per il Trattamento Della Cute Infiammata. Available online: https://www.bioepithelia.it/bioepithelia-per-la-guarigione-delle-patologie-cutanee/bioepithelia-base/ (accessed on 31 July 2025).
- Ferite: Il Rimedio Alle Ferite Cutanee. Alovex Ferite. Available online: https://www.alovex.it/ferite-cutanee/rimedio (accessed on 7 August 2025).
- IALUSET—ialuset. Available online: https://ialuset.it/prodotti/ialuset/ (accessed on 7 August 2025).
- IODOSORB Global. Available online: https://www.smith-nephew.com/en/health-care-professionals/products/advanced-wound-management/iodosorb-global#overview (accessed on 7 August 2025).
- Alfasigma Medicazione Sofargen Gel Acido Ialuronico E Argento Sulfadiazina Tubetto 25 G. Available online: https://www.farmaciarossettisas.it/primo-soccorso-in-viaggio/57094-alfasigma-medicazione-sofargen-gel-acido-ialuronico-e-argento-sulfadiazina-tubetto-25-g.html?srsltid=AfmBOoqoupk_6ddzS5eqele-HLMsCVyri4qeD_Kgr2NRRj-4D0LoOqmP (accessed on 7 August 2025).
- Fitostimoline®Plus Crema—Damor Farmaceutici. Available online: https://www.damorpharma.it/product/fitostimoline-plus-crema/ (accessed on 7 August 2025).
- ConnettivinaBio Plus—Fidiaperlapelle. Available online: https://fidiaperlapelle.it/connettivinabio-plus/ (accessed on 7 August 2025).
- IALUSET PLUS—ialuset. Available online: https://ialuset.it/prodotti/ialuset-plus/ (accessed on 7 August 2025).
- Gazzabin, L.; Serantoni, S.; Palumbo, F.P.; Giordan, N. Hyaluronic acid and metallic silver treatment of chronic wounds: Healing rate and bacterial load control. J. Wound Care 2019, 28, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Dereure, O.; Czubek, M.; Combemale, P. Efficacy and safety of hyaluronic acid in treatment of leg ulcers: A double-blind RCT. J. Wound Care 2012, 21, 131–139. [Google Scholar] [CrossRef]
- Barrois, B.; Carles, M.; Rumeau, M.; Tell, L.; Toussaint, J.F.; Bonnefoy, M.; de Vathaire, F. Efficacy and tolerability of hyaluronan (ialuset®) in the treatment of pressure ulcers: A multicentre, non-randomised, pilot study. Drugs R D 2007, 8, 267–273. [Google Scholar] [CrossRef]
- De Francesco, F.; Riccio, M.; Jimi, S. Contribution of Topical Agents such as Hyaluronic Acid and Silver Sulfadiazine to Wound Healing and Management of Bacterial Biofilm. Medicina 2022, 58, 835. [Google Scholar] [CrossRef]
- Russo, R.; Carrizzo, A.; Barbato, A.; Rasile, B.R.; Pentangelo, P.; Ceccaroni, A.; Marra, C.; Alfano, C.; Losco, L. Clinical Evaluation of the Efficacy and Tolerability of Rigenase® and Polyhexanide (Fitostimoline® Plus) vs. Hyaluronic Acid and Silver Sulfadiazine (Connettivina® Bio Plus) for the Treatment of Acute Skin Wounds: A Randomized Trial. J. Clin. Med. 2022, 11, 2518. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.