Abstract
Composite electrolytes for applications in batteries and supercapacitors, i.e., in electrochemical energy technology, are gaining growing attention. In the absence of a commonly accepted definition, binary and ternary combinations of materials, e.g., a polymer with an electrolyte salt or electrolyte salt solution and a third conductivity- and performance-enhancing constituent, are assumed as a definition of a composite electrolyte in the following review. Relevant fundamentals and reported research results, including explanations of the described effects of added ingredients and achieved improvements, are reviewed. Future perspectives and directions of further research are sketched.
1. Introduction
The ionically conducting phase between the two electrodes of a supercapacitor or a battery is frequently and somewhat imprecisely called the electrolyte. According to the commonly accepted definition, an electrolyte is composed of ions (a true electrolyte according to standard textbooks [], e.g., NaCl) or of molecules, which dissociate into ions upon interaction with a suitable solvent (so-called potential electrolytes, e.g., HCl in water), and accordingly, in most cases, electrolyte solutions or molten electrolytes (salts) are employed [,,]. Ionic liquids (ILs; in this report, commonly established acronyms [] are used independently of some authors’ different, but confusing, suggestions) composed of ions only and liquid already at room temperature (room-temperature ionic liquids RTIL) are the rare example in the latter group. In the following text, this distinction between electrolyte and electrolyte solution is ignored for the sake of simplicity and convenience, but mostly because solutions play hardly any role at all, except in the preparation.
Initially, because of safety concerns and risks associated with leakage of devices, the use of liquid electrolytes (solution) was not welcome in most applications []. Consequently, numerous means to replace them with non-liquid, semi-solid, or even solid ion-conducting materials have been proposed and examined; for an overview of recent driving forces, trends, and new opportunities, see []. These attempts started with a general “wish list” for an electrolyte system [,]:
- Wide available electrode potential window or window of electrochemical stability;
- High ionic conductivity at common operating temperatures;
- Sufficient chemical and electrochemical stability;
- Compatibility with electrode and separator materials;
- Thermal stability;
- Environmental compatibility;
- Low price;
- Sustainable resources.
Given the stated flaws, limitations, and challenges of liquid electrolytes, attempts to improve on those via particular solid electrolytes have implicitly or explicitly addressed the following approaches:
- Enhanced ionic conductivity;
- Wider range of operating temperatures;
- Improved mechanical stability;
- Better long-term stability;
- Increased thermal stability.
The crucial and persistent problem of establishing and maintaining electrolyte/electrode interfaces between solid electrodes and solid electrolytes is surprisingly addressed only infrequently; for overviews, see, e.g., [,].
Initially single-constituent solid electrolytes were studied mostly for high-temperature applications in, e.g., sodium/sulfur or sodium/metal chloride batteries. Frequently encountered/employed solid ion conductors for this application and beyond are as follows []:
- Perovskites, e.g., (Li,La)TiO3;
- Garnet-like Li5La3M2O12 (with M = transition metal);
- Mostly amorphous glasses of lithium nitrides, sulfides, borates, or phosphates like lithium phosphoroxydnitride (LiPON);
- “Super ion conductors” of the LISICON or NASICON type: Li(Na)M2(PO4)3 (M = Ti(IV), Zr(IV), Ge(IV));
- Lithium salts like LiI in Li/I2 batteries [].
Unfortunately, these materials frequently show inadequate ionic conductivity; in addition, problems in difficult processing and slow deterioration because of, e.g., phase changes limit their suitability. Combining them with further ingredients into composite materials was identified as a promising option. A very early report by Liang [] from 1973 provided convincing evidence that the addition of around 45 mol% of Al2O3 to LiI resulted in a very significant increase in ionic conduction to around σRT~10−5 S/cm. Solid-state batteries—in particular, high-temperature batteries—of the type Li/LiI(Al2O3)/metal iodide were prepared and tested []. Electronic conductivity of the created composite electrolyte was negligible; the dominant ionic conduction mechanism was proposed to proceed along cation vacancies. Similar observations with other electrolytes, e.g., AgI with Al2O3 [] or LiI with SiO2 [], have been reported later. An understanding of the conductivity enhancement could not be reached. Acceleration of charge carrier movement along the interfaces between electrolyte and filler was suggested []. First hints suggested space-charge-layer formation and a contribution to accelerated ion transport in LiBr hydrate []; surprisingly, earlier, these considerations were elaborated in great detail for the electrolyte CuCl2/Al2O3 []. In later studies, this increase has been attributed to the creation of space charges at the filler/matrix interface, in particular, by surface-active but otherwise inert additives []. Another interpretation and explanation based on lattice considerations and Monte Carlo simulations appears to be less helpful when studying polymer/filler composites []. Effects of Lewis acid/Lewis base interactions at said interfaces and with anions and/or cations of the mobile charge carriers have been considered, and they may be of particular interest in understanding changes in transference numbers caused by, e.g., selective adsorption of only one kind of mobile ion []. Actually, initial observations of enhanced ionic conductivity caused by admixing a second component into an ionic crystal date back to Jander []. Only recently, the special role of filler–bulk interfaces and the conditions influencing ion transport therein has been transformed into the creation of continuous interfaces in the bulk of a solid ion conductor, yielding ion conductors with remarkably high conductivities []. Mechanochemical synthesis methods of solid electrolytes have been reviewed [].
With the discovery of various polymeric materials also suitable as ionic (or ion) conductors, in particular, after the addition of electrolytes, i.e., salts (for the earliest example, see []), the question was asked again about the cause of increased conductivity upon the addition of a filler to a polymer with added salt. Early observations indicated no enhancing effect at, e.g., less than 10 wt.% Al2O3 to poly(ethylene oxide) (PEO; for details, see below) with LiClO4 as the salt []. In any case, crystallinity of the polymer was identified as a major cause of poor conductivity, and lower crystallinity increased conductivity—thus, added materials that decrease crystallinity are welcome fillers. For polymer-based solid electrolytes, for some time, the crystallinity argument pushed the space charge argument promoted for inorganic crystalline conductors completely into the shadows. Only later was this contribution rediscovered for polymeric electrolytes together with further effects related to chemical interactions between the filler surface and the molecular structure of the polymers and the ions of the electrolyte commonly added to the polymers [,,,].
Up to this point, the common definition of a composite as a material wherein two phases can be discerned at least on the microscopic level, not necessarily on the macroscopic one, was tacitly and unreflectedly applied. Solutions of several salts in a solution or addition of one more solute did not and do not make a composite. Depending on the type and extent/intensity of interactions between the constituents, the more general term “composite” may be correctly replaced with the more focused term “hybrid”. Because there is no generally accepted and clear-cut separation between both terms, the usage by the original authors is followed in this contribution without discussing the adequacy of this terminology in every case; an extended review on “hybrid electrolytes” is available []. Actually, the phase boundaries established between the surface of filler nanoparticles and polymeric matrices frequently provide extra fast pathways for ion transport—this may be called a typical case of an interaction calling for the term hybrid, as in [], whereas it remains somewhat confusing to call a mixture of salts and liquids simply a “hybrid electrolyte” [] or to call just everything beyond a solid electrolyte a “hybrid electrolyte”, as in []. Certainly, it is wrong to attribute this—as is the case in []—to the authors of []: the claimed figure is nowhere to be found in the latter report. Clearly, this is a minority opinion anyway; nevertheless, the search string “(“hybrid electrolyte” OR “solid electrolyte”) AND (sodium OR magnesium OR potassium OR zinc OR aluminium OR calcium) AND battery” was tried in our literature searches. These interactions have been reviewed with particular attention to NMR as an experimental tool in a previous study [].
Another option for increased conductivity is the use of additives: If a small amount of another constituent is added, the result may also be called a composite; for examples, see []. Similar imprecision can be found when an electrolyte solution containing several electrolytes is called a composite electrolyte, for examples, see [,,,,,,,,,,,,,], or, with again an additive, a “solvation structure composite electrolyte” in []. “Mixture” is certainly correct and more appropriate, though unfortunately less fashionable.
Essentially, the same arguments prevailed when interest in solid electrolytes as replacements for liquid electrolyte solutions in lithium-ion batteries almost exploded, stimulated at least in part by spectacular battery failures []. But similar limitations were also readily registered []. The same approaches toward improvement were tried: Composite materials are one option.
Finally, the limitations of lithium-ion battery technology and its economy, despite its many impressive advantages, stimulated research into alternatives, so-called post-lithium or non-lithium batteries. Although it appears that both terms are sometimes almost synonyms, they need proper usage: Post-lithium commonly means everything beyond the omnipresent lithium-ion battery based on the rocking-chair principle and thus can include lithium–air and lithium–sulfur [], whereas the term non-lithium is much better defined—and applied here. Composite electrolytes for the former lithium-ion batteries are the subject of a review elsewhere []; materials for the latter, much wider but less densely populated family are the subject of this report. Earlier reviews covering a few details of the present contribution are available [,].
Early reports on composite materials, mostly on solid/solid composites, as solid electrolytes for batteries were already collected in a conference proceedings volume in 1988 [], followed by a conference report on a composite material used as a ceramic solid-state electrolyte for a sodium–sulfur battery (1990) [], and a report in 1995 on solid-state batteries with a negative magnesium electrode and various compounds as the positive electrode, with a solid composite electrolyte as a glue in between []. But only in the 2010 years was there growing general interest in non-lithium battery research, and accordingly, publication activity picked up, as depicted in Figure 1.
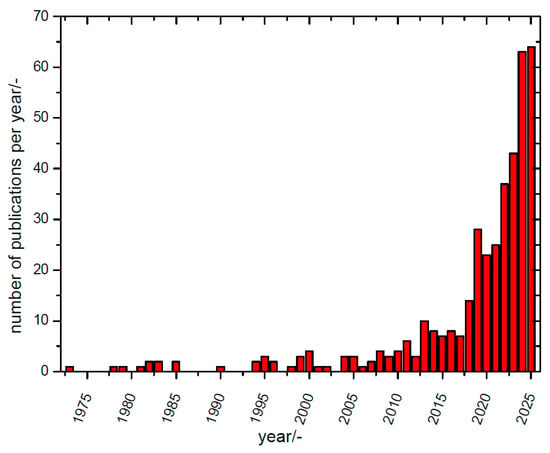
Figure 1.
A search with the string ““composite electrolyte” AND (battery OR batteries) NOT lithium” found anywhere in the title, keywords, or abstract (data from Scopus® and Web of Science®) performed on 2 August 2025 initially yielded 304 hits. Reports about, e.g., lithium-ion conductors with the term “lithium” somewhere in the text but not in the title, keywords, or abstract were removed, even those on Li. Reports dealing with electrolytes actually meeting the definition suggested above without mentioning the term “composite electrolyte” were included when detected after extended literature searches. Cases where (composite) “separator” was considered a synonym of (composite) “electrolyte” are also included (for a typical example, see []), and cases where a “composite separator” was actually a modified separator only (for examples, see [,,,,,,,,,,]; for a review, see []) were not included. Modified membranes considered as separators and called composites like [,] are not included; neither are reports dealing with “moderate electrolytes” [] nor electrolyte solutions of salt mixtures [,,,,,,,,,,,,,,], solid electrolyte interphases (SEIs), or similar electrode coatings, as in [,,,,,,,,] or reports wherein “composite electrolyte” is placed only as a teaser in the abstract [,]. This changed the total to 394 reports.
Although the majority of composite electrolytes are solids—in line with the general ambitions of working towards all-solid-state batteries—liquid electrolytes avoiding the problem of flammability are still studied. Eutectic electrolytes based on experiences with deep eutectic solvents [,,,,,,,,] are a prominent example, which have been reviewed []. Fibrous materials as scaffolds for composite electrolytes/membranes/separators have also been surveyed []. Lignocellulose as a typical renewable raw material has been discussed with respect to applications in batteries and composite electrolytes [], and respective overviews on the use of nanocellulose [] and cellulose [] are also available. Composites of metal–organic frameworks as part of solid electrolytes have been reviewed [].
The following report collects the most relevant details of original reports on composite electrolytes studied or at least proposed for the currently researched non-lithium batteries. It is organized along these metals, and in cases of frequently studied metals, there are subsections using the mostly polymeric host material as a criterion. This should provide the reader with a quick overview of previously examined options and materials and serve to identify promising avenues of further studies.
2. Electrolyte Tasks and Challenges
In conventional electrolytes as well as electrolyte solutions, the roles played by the ingredients are perfectly clear in almost every case [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. Once a further ingredient is added into an electrolyte, e.g., a further ionic or non-ionic compound to a salt, there should be a reason specified together with the suggestion of the addition. Unfortunately, as in many examples presented below, this is not always be the case, and the reader is left wondering about conceivable ideas behind the addition. Depending on the battery chemistry and the operating principle, high general ionic conductivities and, in particular, high conductivities (i.e., high transference number t+ ≈ 1) of the (mostly metal) ion shuttling between the electrodes are highly welcome. This selectivity may also be helpful by suppressing cation concentration gradients and thus suppress dendrite formation when using metal (e.g., lithium, sodium) electrodes. When authors address this detail, it is highlighted below.
2.1. Composite Electrolytes
Beyond the trivial statement that any combination of two materials enabling ionic conduction without showing electronic conduction—this may even qualify as the definition of an electrolyte—may be called a composite (wherein these two constituents stay separate in two phases at least on the microscopic level, as illustrated below in Figure 2), the goal of combining materials is commonly pursued with particular improvements in mind. These may range from clear improvements in terms of the electrochemical properties listed above in the wish list to further technical and even economical improvements. When examining composites with a polymer, the mechanical scaffold provided by the macromolecular material—serving as a welcome stiff material rendering a separator possibly superfluous—combined with an improvement of the ionic conductivity of the polymer the focuses of attention. In the case of composites composed of two or more non-polymeric materials, the welcome mechanical contribution of a polymeric scaffold remains absent; another advantage beyond an improved ionic conductance will be the driving force behind mixing. Use of the sometimes-encountered term “filler” for the added ingredient (see above) appears to be a bit unclear, or at least undefined. The distinction between passive (insulating) fillers not providing internal ionic conduction and active (conducting) fillers suggested in [,] has apparently not found widespread use. To call the salt added to a polymer to provide ions a “filler” appears to be positively confusing []; neither makes the term “composite electrolyte” reasonable for a combination of an insulating polymer with a salt, as in [].
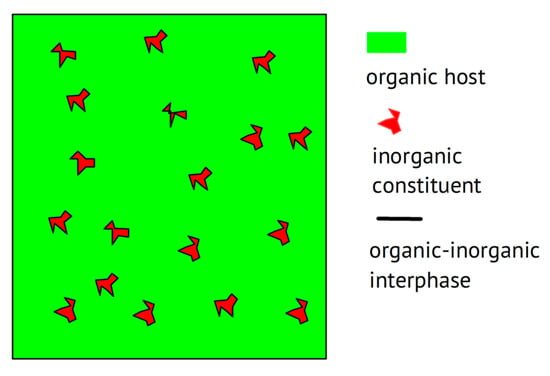
Figure 2.
Schematic of a composite with a polymer host.
2.1.1. Composites with Polymer Hosts
Overviews on polymer electrolytes are available [,,,,]. Starting a composite electrolyte with a polymer or a polymer electrolyte is very rational: The polymer will provide the mechanical support (or backbone, but this should not be confused with the chemical structure or molecular backbone) to form a scaffold, desired for an application not needing an extra separator. The added (composite) constituent may, e.g., reduce the crystallinity of a polymer like PEO, thus increasing ionic conductivity [,], or may provide particularly transport-accelerating particle/host interfaces, as demonstrated in [], essentially along the lines already indicated in []. A schematic representation is shown in Figure 2, highlighting these interfacial or interphase regions.
There is also evidence that effects may depend on the particle size, with small particles increasing conductivity only and larger ones increasing the conductivity and transport number []. This applies to all added particular materials. In a further highly unsystematic study of several inorganic components with different particle sizes and dielectric constants, no coherent conclusions were obtained []. Ion-conducting materials may add further ionic conduction pathways inside the particles. Possibilities of enhanced ionic conductivity mechanisms have been reviewed []. Further advantages may be gained by starting from a polymer blend of, e.g., poly(vinylalcohol) PVA (Figure 3) and PEO (Figure 4). Beneficial effects were also observed upon the addition of BaTiO3 as a filler to a polymer blend of PVA and poly(ethylene glycol) PEG (Figure 5), of Li0.5La0.5TiO3 to a blend of PEO and polyvinylidene fluoride (PVDF, see Figure 6), or of ZrO2 to poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP, see Figure 6). Actual modes of operation of the added ceramic may differ from material to material depending on the exact chemical composition. The case of PEO with inorganic fillers has been reviewed in detail []. In addition, attention has been paid to effects of surface modifications of inorganic fillers added to PEO [].
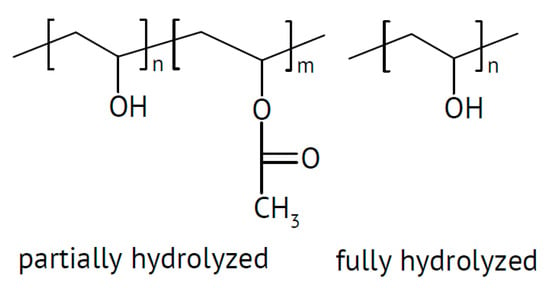
Figure 3.
Molecular structures of poly(vinyl alcohol).
Figure 4.
PEO and a scheme of interactions ---- between a sodium ion and PEO.
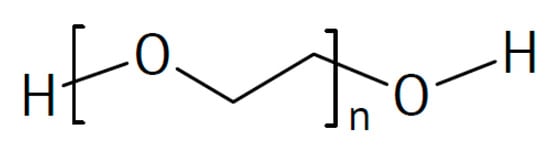
Figure 5.
Molecular structures of poly(ethylene glycol).
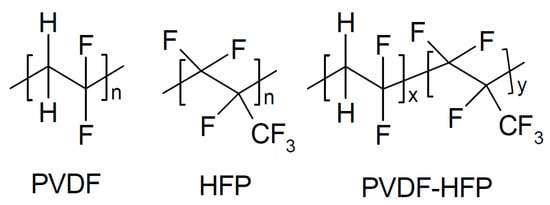
Figure 6.
PVDF, HFP, and PVDF-HFP (this acronym is chemically slightly incorrect but is—with very few (see below) misleading exceptions—firmly established and thus used here) [].
Some general procedural aspects of polymer and composite polymer electrolytes (preparation and handling) have been summarized in []. Tuning of the interface between the polymer matrix and embedded mostly inorganic particles (filler) in a solid electrolyte, preferably for photovoltaic devices, has been reviewed []. (Gel) Electrolytes obtained by plastification (gelling) of a polymer with a suitable solvent (this may be a solvent or a solvent mixture but also an electrolyte solution) have been called composite electrolytes infrequently (for an example, see []). Addition of a plasticizer to a polymer appears to be so popular that it is hardly mentioned in the reports, and the same applies to the essential addition of a source of ions, e.g., a sodium salt, either during the preparation of the solid or by soaking the prepared solid in an electrolyte solution—wherein the solvent may also act as a plasticizer. Again, these are details the interested reader should search for in an attempt to avoid experimental failures.
An overview covering PEO-based composite electrolytes with a major focus on batteries is available [], and composites with Li7La3Zr2O12 as an inorganic filler have been discussed in []. Beneficial effects of added small molecules in a polymer–ceramic solid electrolyte are discussed in general terms in []. Segmental motion of the polymer and interfacial polarization are coupled and determine ion transport jointly. A porous inorganic scaffold of freeze-cast Li7La3Zr2O12 for filling with a polymer yielding a composite has been reported []. Further polymer–inorganic filler composites have been reviewed with respect to mass production of solid-state batteries [], and early overviews on polymer–ceramic electrolytes already highlighting the three overarching issues ionic conductivity, transference number, and interfacial aspects are available [,]. Fast and efficient discovery of solid organic–inorganic composite electrolytes supported by unsupervised learning has been discussed in detail []. Approaches to reaching an optimized inorganic filler content enabling better electrolyte–electrode contact supported by machine learning have been discussed []. The beneficial, ion conductivity-increasing effect of nanosized particles of TiO2 added to amorphous polyether with either LiClO4 or LiTFSI as a salt has been studied []. At most studied concentrations, no effect was observed; only at 1.5 m LiClO4 was an increase observed and attributed (somewhat surprisingly) to an effect on crystallinity and disruption of ion aggregation.
Further general aspects of polymer-based composite electrolytes have been discussed [,,].
2.1.2. Binary Composites
Instead of a polymer acting as a host and providing in most cases the major fraction of a composite electrolyte, an inorganic material like fumed silica may act as a highly porous host material, which is subsequently soaked with an electrolyte solution, yielding a solid material (for the concept of the “soggy sand” electrolyte, see [,]), which may not possess the mechanical properties enabling its use as a separator or similar; for a scheme, see Figure 7.
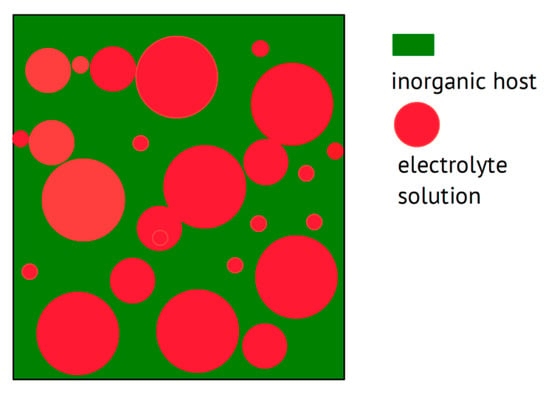
Figure 7.
Scheme of a porous inorganic host filled with an electrolyte solution.
Ultrafast high-temperature sintering as a method for preparing an inorganic scaffold for a composite electrolyte has been described [].
Given the wide variations in terms of employed compounds and constituents as well as battery chemistries, organization of the material making it easily accessible for the reader was based on a rough discrimination between room-temperature (RT) and elevated-temperature applications, with a further classification according to the metal used in a given system for the RT batteries and a further distinction based on the polymer host material.
The presumably central challenge of a sufficiently intimate contact between electrolyte and electrode—in particular, positive ones—is somewhat surprisingly addressed only sometimes, while the traditional sandwiching of electrodes with an electrolyte in between is still popular. Because this interface has been identified as a major problem [,], the following attempts to enhance this interface are highlighted.
The most prominent property of an ionic conductor is its ionic conductivity. Accordingly, measured data attract the most attention. For a given application, the actual Ohmic resistance caused by the ionic phase between the electrodes is much more relevant—but rarely reported, only sometimes included in some cell resistance and cell impedance data. A highly conducting material that can be applied only as a relatively thick layer may thus be less attractive than a relatively poorer conducting material that can be made into a very thin film. Nevertheless, data—when reported—are included below to provide at least a first number. Given the pronounced temperature dependency of ionic conductivity, most authors provide values—if at all—for a specified temperature, like room temperature σRT or σ60°C; when this basic detail is missing, σ? is provided. Temperatures like T = 50 or 60 °C are still found in the part of this report dealing with room-temperature systems because the authors do not explicitly mention the use of the reported materials at elevated or high temperatures. Some general information on experimental testing aspects can be found [], and some early materials not assigned to a particular battery chemistry have been described []. The use of NMR and EPR spectroscopy for in situ studies of rechargeable batteries has been reviewed [].
3. The Materials
3.1. Room-Temperature Systems
The careful distinction between lithium and lithium-ion or more generally metal and metal-ion batteries with the rocking-chair principle operative in the latter ones may be applicable also for post-lithium batteries. Evidence suggests otherwise and confusion reigns, and the following assignments picked by the authors were used when specified at all. These include the explicit use of the term “metal-free” (like sodium-metal-free in []), which so far appears to be reserved for batteries where essentially a negative metal electrode formed only by metal deposition from the electrolyte solution/dissolution without the solid metal being present at the start (enabling simplified manufacturing) is used (see for examples, [,,,,]) and not for a cell with a host electrode. Actually, even these cells are not called metal-free (which appears to be a rather striking characterization anyway) but “zero excess metal”, “reservoir-free”, or “anode-free” (with the precision in describing the facts decreasing in this sequence).
3.1.1. Sodium and Sodium-Ion Batteries
Inorganic Materials
A composite of NaAlCl4 with embedded particles of Al2O3 was prepared by mechanochemical synthesis []. The latter provides additional conduction along an oxychloride interface between the two constituents, yielding σRT > 0.1 × 10−3 S/cm. An all-solid-state battery demonstrated electrolyte suitability. An overview on sodium-conducting halides as potential solid electrolyte materials is available []. Also, by mechanochemical synthesis, a composite of NaI and Na2.88Sb0.88W0.12S4 has been prepared, where the formed solid electrolyte Na2.88Sb0.88W0.12S4·0.5NaI showed σ = 3.6.1 × 10−2 S/cm and was tested in an all-solid-state battery []. For enhanced ion conduction of Na3Zr2Si2PO12 (called NACICON instead of NASICON or NASICON-like for unknown reasons), a glass of 35Na2O-5Cr2O3-30TiO2-30P2O5 was infiltrated into the porous ceramic, and use of the product in sodium-ion batteries was proposed []. Increased ionic conductivity of Na3Zr2Si2PO12 by adding NaF into the precursor mixture during preparation has been reported, where conductivity increased from σRT = 4.5 × 10−4 S/cm to σRT = 1.7 × 10−3 S/cm []. A porous pellet of Na2ZnSiO4 (halloysite) with an ionic liquid N-butyl-N-methylpyrrolidinium bis(trifluoromethyl sulfonyl) imide (PYR14TFSI, see Figure 8) and NaTFSI yielded a solid electrolyte with σ300°C = 1.1 × 10−3 S/cm and t+ = 0.5, suggesting to the authors its use in sodium-ion batteries []. A composite of clay and cellulose nanocrystals has been described as a sodium-ion-conducting electrolyte with conductivities around 10−3 S/cm, without revealing its preparation details []. NaNH2 was reacted with B10H14, yielding solid electrolyte core–shell composites of NaBH4 and Na2B12H12 with σ100°C = 1 × 10−4 S/cm []. A solid-state sodium-ion battery with this composite electrolyte was run for 100 cycles.
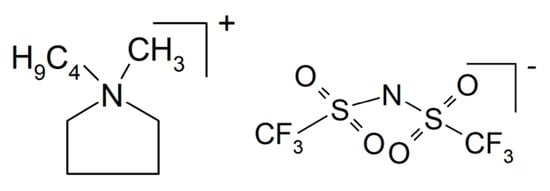
Figure 8.
Molecular structures of N-butyl-N-methylpyrrolidinium bis(trifluoromethyl sulfonyl) imide.
PVA-Based Materials
To PVA with NaClO4 as the salt (presumably not the filler as claimed in the text), Y2O3 was added as an inorganic filler, affording a solid composite electrolyte with σRT = 3.25 × 10−4 S/cm and t+ = 0.919 at the optimum composition with 3 wt.% filler (because the total composition of this material was given as 123%, uncertainty remains, and that is also the case regarding other barely comprehensible claims in this report) []. High conduction and t+ were attributed to lower crystallinity caused by the filler and strong interactions between it and the salt anion. For the assembled complete cell, the typical zinc electrode reaction was provided for the negative electrode, and for the positive one, the PbO2 and the V2O5 reactions. The decrease in crystallinity of PVA by adding various amounts of salts like MgSO4 or Li2SO4 has been demonstrated []. The effect was more pronounced with the lithium salt. At concentrations beyond around 20 wt.%, crystallization of the added salt starts, and conductivity decreases again.
Addition of sulfuric acid to a mixture (PVA)0.7(NaI)0.3 yielded a proton-conducting solid electrolyte tested in a sodium/MnO2 battery []. The addition of acid resulted in σRT = 10−3 S/cm attributed to disruption of the semi-crystalline structure of the mixed solid. Electrical properties of a polymer blend of PVA and methylcellulose with NaI as the electrolyte have been studied [].
PEO-Based Materials
The notorious tendency of PEO to crystallize (for a study of correlations between crystallinity and ionic conductivity as a function of the fraction of added ceramic filler, see []) has been decreased by grafting yielding a hyperbranched polyether, and with NaTFSI (see Figure 9), it showed an increased conductivity σRT = 5.7 × 10−4 S/cm []. A cell with a sodium-metal electrode (called a sodium-ion battery) nevertheless kept 92% of its initial capacitance after 300 cycles. A solid composite electrolyte of PEO and NaTFSI with added NASICON-type Na3.4Zr1.8Mg0.2Si2PO12 with σ80°C = 2.8 × 10−3 S/cm was used in a sodium/Na3V2(PO4)3 (NVP) battery operated for 80 cycles without significant losses []. To a mixture of PEO and NaFSI (see Figure 9) with the mass ratio 20:1 (this is presumably meant with the rather uncommon designation P(EO)20NaFSI and later EO:Na = 20:1) the researchers dissolved in acetonitrile various amounts of PYR14FSI (see Figure 10), yielding after evaporation of the solvent a solid electrolyte with σRT = 1.15 × 10−4 S/cm (without the ionic liquid σRT = 2.85 × 10−6 S/cm) tested in a sodium-metal/NVP battery at T = 60 °C []. A capacitance retention 85% after 300 cycles was reported. Conductivity improvement by the added ionic liquid was attributed to increased amorphicity and stronger interactions between NaFSI and the PEO oxygen atoms. With respect to desired sodium-ion transport and electrostatics, presumably interactions between said functionalities and the anion FSI− were meant.

Figure 9.
Molecular structures of sodium bis(trifluoromethylsulfonyl) imide NaTFSI and sodium bis(fluorosulfonyl) imide NaFSI.
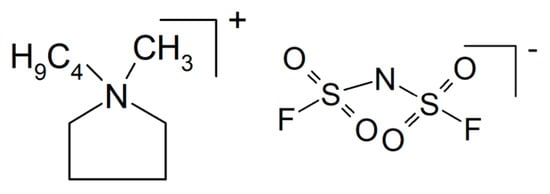
Figure 10.
Molecular structures of N-butyl-N-methylpyrrolidinium bis(fluorosulfonyl) imide PYR14FSI.
A composite electrolyte was prepared from PEO, NaTFSI, and zeolite A (LTA, presumably its sodium aluminosilicate form) with σ30°C > 1.42 × 10−4 S/cm and t+ = 0.44 and used in a sodium/NVP battery with 88% capacity retention after 100 cycles at T = 60 °C []. A Cu-metal organic framework on PEO combined with PAN yielded a solid electrolyte with σRT = 0.103 × 10−3 S/cm and t+ = 0.58 for sodium batteries []. A full cell showed a stable performance for 2000 cycles. A Zr-metal organic framework material was combined with PEO, yielding a solid electrolyte for a sodium battery with 97% capacitance retention after 1000 cycles []. σ60°C = 6.62 × 10−4 S/cm and t+ = 0.67 were attributed to adsorption of anions on zirconium sites. A composite electrolyte has been prepared from PEO, NaCF3SO3, and as a filler MOF MIL-53(Al) and has been used in a sodium/sulfur battery []. At 3.24 wt.% of the filler, t+ = 0.4 and σ50°C = 1.5 × 10−5 S/cm were recorded, and without filler, the value decreased by almost an order of magnitude. Operated at T = 60 °C, the battery kept 75% of the initial capacitance after 50 cycles. Promising results for lithium/sulfur batteries obtained with a cubic-garnet Li7La3Zr2O12 applied in a bilayer arrangement to ameliorate electrolyte/lithium incompatibilities may indicate a direction of further development [].
The problem of agglomeration of one (mostly the inorganic) constituent in a polymer/inorganic filler composite electrolyte has been addressed in detail [] and experimentally verified for some composites [], and similar observations with composites of polymers with graphene, graphene oxide, etc., are discussed elsewhere []. Surface modification of the inorganic filler Na3Zr2Si2PO12 with polydopamine improved the wettability, with the polymer component PEO enabling an all-solid-state sodium-metal battery running stably for 1350 cycles at T = 60 °C. According to results of modeling the surface modification of the inorganic filler, this also influenced sodium ion transport, helping to suppress dendrite formation. An optimized Na3.3Zr1.7La0.3Si2PO12 with σRT = 3.4 × 10−3 has been used in a sodium-metal battery []. For improved electrode/electrolyte interaction at the positive electrode, a small amount of organic liquid electrolyte or ionic liquid was soaked into the porous electrode body. Following the initial considerations provided above, no reason to call this a composite electrolyte is evident.
A laminated two-layer electrolyte has been proposed for sodium-ion batteries []. Facing the negative sodium-metal electrode a PEO film with added succinonitrile (see Figure 11) for better sodium-ion conduction is placed, and on the other side, there is a polyacrylonitrile (PAN) film (see Figure 12) with added NASICON-type Na3Zr2Si2PO12 for the same purpose. A value σRT = 1.36 × 10−4 S/cm has been reported.
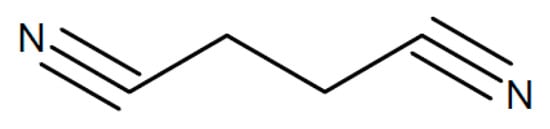
Figure 11.
Molecular structures of succinonitrile (SN).
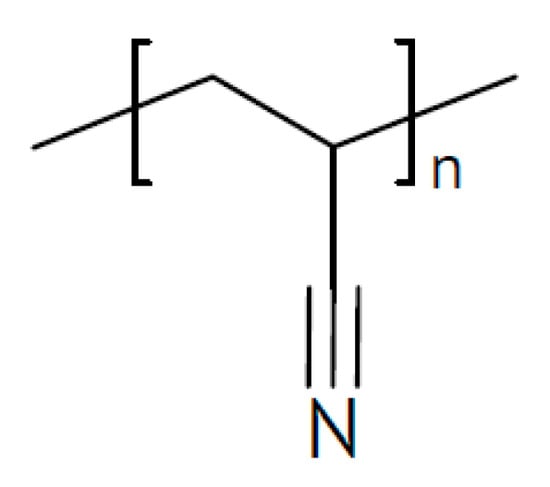
Figure 12.
Molecular structures of polyacrylonitrile.
An asymmetric composite electrolyte with a harder (or tougher) side facing the sodium-metal electrode (for better dendrite suppression) and a softer side for the positive electrode, enabling formation of a better interface, has been proposed []. The tougher side was prepared from PEO, NaTFSI, and a metal–organic framework ZIF-67, and upon this, more PEO/NaTFSI was deposited. Overall conductivity was σRT = 5.23 × 10−4 S/cm, and a complete cell showed 0.12% capacitance loss per cycle for 300 cycles. Mixtures of PEO and NaTFSI have been studied, and with growing fraction of the salt, the mix becomes more gum-like, suggesting plasticizer-like properties of the salt []. Somewhat surprisingly—when considering the effects of this filler in other composites—5 wt.% of nanosized SiO2 did not change the ionic conductivity. The interfacial resistance with the sodium electrode slightly increased, where t+ increased from t+ = 0.39 to t+ = 0.51. A membrane made from a mixture of a PEO-like polymer and Al2O3 (2:1 by weight) soaked in a solution of 1 M NaClO4 in a carbonate solvent mixture yielded a composite electrolyte tested in a sodium/Na2MnFe(CN)6 battery []. The filler blocked dendrite formation, and the cell showed no capacitance loss after 400 cycles. Suitability of a composite electrolyte prepared from PEO and NaPF6 with TiO2 (4 wt.%) as a filler for a solid-state sodium battery Na/NaTi2(PO4)3 has been studied []. Without filler, σRT ~ 0.013 × 10−3 S/cm, and at T = 80 °C, 0.2 × 10−3 S/cm was measured (for comparison, see []); upon filler addition, the latter value increased to 0.44 × 10−3 S/cm with t+ = 0.68, and at T = 80 °C, the battery showed a stable capacitance during 110 cycles. A solid composite electrolyte of PEO with NaClO4 as a salt and 5 wt.% TiO2 as a filler showed an increase in the conductivity from σRT = 1.35 × 10−4 S/cm to σRT = 2.62 × 10−4 S/cm and good compatibility with an electrode of Na2/3Co2/3Mn1/3O2 [].
Addition of 50% succinonitrile (SN, Figure 11) to a solid electrolyte of PEO and NaTFSI increased the ionic conductivity to σRT ~ 1.1 × 10−4 S/cm, about 45 times the value without SN []. SN has been called a “non-ionic plastic crystal” apparently because it is an organic crystalline solid at room temperature, with specific properties found in more details elsewhere [,].
A composite of PEO, NaTFSI, and Na6[Re4As2S2(CN)12] with a cubane-like anion showing σRT = 1.05 × 10−5 S/cm was tested successfully in a sodium/NaVPO4 cell []. A composite of PEO with partially hydrolyzed NaBH4 has been examined as a solid electrolyte for a sodium-ion battery []. σ45°C = 11.6 × 10−3 S/cm and t+ = 0.54 were found. Functionalized carbon carboxylate composited with sodium bis(oxalate)-borate yielding an electrolyte for sodium-ion batteries has been prepared []. A porous disk of alumina was soaked with a slurry of PEO, SiO2, and NaClO4 (for its preparation, see []), yielding a solid composite electrolyte with σRT = 1.6 × 10−4 S/cm for a sodium-metal battery (although according to a displayed figure, the electrolyte is permeable for lithium ions!) []. A ceramic/polymer composite has been proposed for use in a sodium-ion battery at T = 80 °C []. NASICON-type powders of Na3Zr2Si2PO12/Na3.4Zr1.8Mg0.2Si2PO12 as the ceramic constituent were combined with PEO (see Figure 4) and NaTFSI, where the obtained film was sandwiched between the negative sodium ion and the positive Na3V2(PO4)3 electrode. Sodium-ion conductivity of 2.4 mS·cm−1 was reported. PEO with NaTFSI was composited with Na3Zr2Si2PO12, yielding a composite electrolyte with σRT = 1.4 × 10−4 S/cm at the optimum composition []. A solid electrolyte based on Na3Zr2Si2PO12 and PEO with NaClO4 and some PEG “for better film formation” and σ55°C > 10−4 S/cm has been described []. A cell with a sodium-metal electrode kept 98% of the initial capacitance after 100 cycles. The same composite electrolyte was used in a sodium-ion battery of symmetric design with two Na3V2(PO4)3, showing 86.4% of its initial capacitance after 650 cycles []. When using a positive Prussian blue electrode instead, 0.005% capacity loss per cycle during 9000 cycles was observed. To PEO with NaClO4 as a salt, Na3Zr2Si2PO12 has been added, affording a solid composite electrolyte with increased conductivity σ30°C = 2.1 × 10−5 S/cm and enhanced dendrite suppression []. It was used in a sodium-metal-Prussian Blue-type positive electrode battery providing 0.05% capacitance loss per cycle during 300 cycles. To a solid electrolyte of PEO with sodium percarbonate, Na3Zr2Si2PO12 was added, yielding a conductivity σRT = 2.6 × 10−4 S/cm (the unit SCm−1 remains a mystery) []. High conductivity was attributed to lower crystallinity, and sodium dendrite formation was suppressed, yielding 73% capacitance retention after 100 cycles. A polyether-based composite electrolyte with NaTFSA as a salt and Na3Zr2Si2PO12 as an inorganic filler showed increasing conductivity with a decreasing filler content, e.g., σRT = 1.03 × 10−5 S/cm (t+ = 0.04) at 30 wt.% filler []. This behavior was attributed to improved segmental mobility of the polyether at a lower filler content, promoting dissociation of the added salt. A PEO-based solid electrolyte with added NASICON-type Na3Zr2Si2PO12 doped with Sc and Ge and enriched ceramic content near the electrolyte surface has been used in a sodium-ion battery []. Overall conductivity of the electrolyte was σ30°C = 4 × 10−5 S/cm. A complete cell with a negative electrode Sn4P3CNT/C and a NVP positive one provided 100 cycles with 88% capacitance retention. A further increase in sodium-ion conductivity as well as transference number t+ were obtained by doping NASICON-type ceramic Na3Zr2Si2PO12 with Mg2+ and Sc3+; similar to the preceding example, the ceramic was mixed with NaTFSI and PEO, yielding a composite electrolyte with t+ ≈ 0.998 and σRT = 7.96 × 10−5 S/cm were reported with an optimized composition of the ceramic []. A full sodium ion cell was operated at T = 60 °C stably for 80 cycles. Using as a salt NaClO4 instead, a cell with a sodium-metal electrode retaining 97% of its initial capacitance after 100 cycles was reported []. PEO with NaClO4 added as a salt was infiltrated into a skeleton of electro-spun Na3Zr2Si2PO12, yielding σRT = 4.43 × 10−4 S/cm and t+ = 0.61 []. A full cell with a sodium electrode kept 83% of its initial capacitance after 1500 cycles. A composite of this ceramic with polyethylene glycol diacrylate and succinonitrile as a plasticizer has been suggested as an electrolyte for sodium batteries []. The ceramic enhanced ionic conductivity (σRT = 4.5 × 10−4 S/cm) and suppressed dendrite formation, contributing to 87% capacitance retention after 100 cycles.
A glass fiber mat coated with polyethylene glycol diacrylate was soaked with PEG in the presence of NaClO4, yielding a composite electrolyte (σRT = 0.8 × 10−4 S/cm), and tested in a Na/NVP battery, showing 99% capacity retention after 1100 cycles [].
A solid electrolyte of PEO with NaTFSI as a salt and hydroxyapatite added as a filler, yielding about σ70°C ~ 10−4 S/cm and t+ = 0.38, was tested in a sodium/Na4CrFe(PO4)3 battery []. A composite of PEO with NaClO4 with BaTiO3 filler for a sodium-ion battery has been reported []. At 5 wt.% filler, σRT ~ 1 × 10−5 S/cm was reported, and reduced crystallinity was noted as the cause of increased conductance. Addition of electrospun fibers of MgAl2O4 to PEO with NaClO4 yielded a composite electrolyte with increased ionic conductivity (σ55°C = 1.89 × 10−4 S/cm with t+ = 0.55) and higher mechanical and thermal stability []. The increased conductivity is attributed to lower crystallinity and stronger interaction between the filler and the anions of the salt.
To PEO with NaTFSI as salt, Ga-doped Na2Zn2TeO6 filler at various concentrations was added, yielding a solid composite electrolyte with σ30°C = 4 × 10−5 S/cm at 50 wt.% filler from σ30°C = 1 × 10−6 S/cm without filler, with the increase ascribed to the high ionic conductivity of the filler itself and suppressed crystallization []. A sodium/NVP battery was assembled; at T = 80 °C, cells with both micro- and nanosized NVP lost about 10% of the initial capacity during 100 cycles.
A PEO-based (not cellulose-based as claimed in the title) polymer electrolyte with NaClO4 as a salt blended with sodium carboxymethyl cellulose (CMC) has been prepared, characterized, and tested in Na/TiO2 and Na/NaFePO4 batteries []. The composite showed σRT < 10−6 S/cm and t+,60°C = 0.15, and batteries were run for 12 and 20 cycles, respectively.
A polymer blend of PEO and polyvinyl pyrrolidone (PVP, Figure 13) with NaIO4 as a salt composited with TiO2 nanoparticles has been prepared for a sodium []. At 10 wt.% salt and 3 wt.% filler, σRT = 9.82 × 10−6 S/cm was found, and as a reason, the increase in the dielectric constant of the material causing an increase in the concentration of mobile ions and their mobility was suggested.
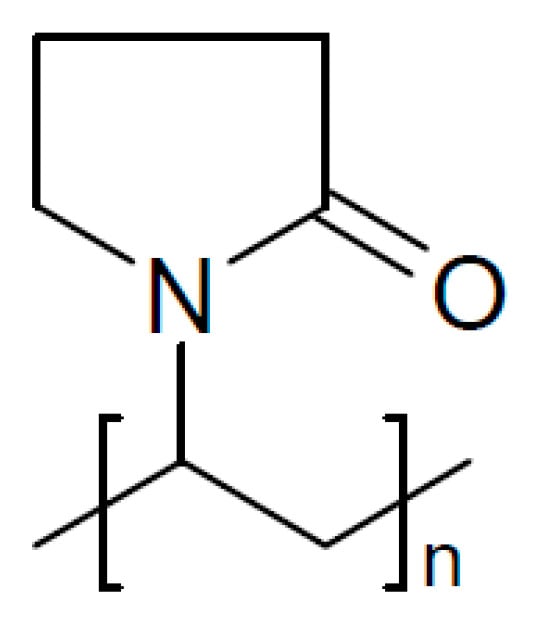
Figure 13.
Molecular structures of polyvinyl pyrrolidone.
Ionic conductivity of a PEO/PVP blend with added NaF has been studied, where a maximum conductivity σ30°C = 1.19 × 10−7 S/cm was reported []. A polymer blend of PEO and PEMA with NaIO4 with ZnO as a filler and PEG as plasticizer has been prepared and studied []. Growing filler content resulted in higher conductivity.
To a blend of PEO/polycaprolactone (see Figure 14), graphitic C3N4 and Na-β-Al2O3 have been added, yielding a composite electrolyte tested at T = 50 °C in a Na/NVP battery, showing a stable capacity for 55 cycles [].
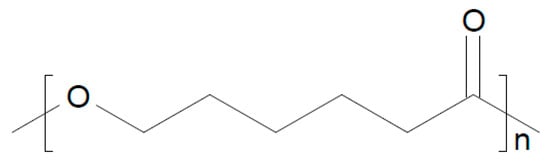
Figure 14.
Molecular structures of polycaprolactone.
Although inhibition of crystallization by adding a plasticizer seems to be a popular and well-established approach to soften hard and/or brittle plastics, the title of a report highlighting plasticizing as something special suggests otherwise []. Adding N-propyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide (PYR13FSI, see Figure 15) as a plasticizer to PEO with NaClO4 as an electrolyte yielded a composite electrolyte (with this term used generously in a somewhat wider sense, see above) with σRT = 6.8 × 10−5 S/cm and t+ = 0.44 at 40 wt.% of the ionic liquid. A Na/NVP battery kept 86% of the initial capacity after 70 cycles.
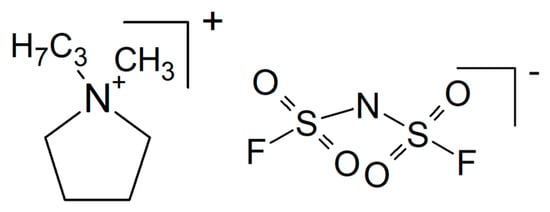
Figure 15.
Molecular structures of N-propyl-N-methylpyrrolidinium bis(fluorosulfonyl) imide.
PVDF-Based Materials
A PVDF-based composite electrolyte with NaTFSI as a salt and an inorganic filler of β″-Al2O3 has been prepared, characterized, and tested in Na/CFx and Na/NaNi1/3Mn1/3Fe1/3O2 batteries []. σRT = 4.55 × 10−4 S/cm, and the six-fold increase in comparison to the conductivity of the solid electrolyte without filler was attributed to a “destruction of the ordered arrangement of PVDF chains”, i.e., reduced crystallinity. Lower Coulomb efficiency of the solid-state cell in comparison to a conventional cell with liquid electrolyte solution was attributed to a poor electrolyte/electrode interface in the solid-state cell, as already addressed above. Capacity retention was 87% after 50 cycles for the Na/NaNi1/3Mn1/3Fe1/3O2 battery. Na/CFx cells with carbon fluorides of different degrees of fluoridation performed poorly.
To PVDF with succinonitrile as a plasticizer and NaTFSI as a salt, Na3.4Zr1.8Ni0.2Si2PO12 was added for improved sodium-ion conduction (σRT = 1.02 × 10−3 S/cm), yielding a solid electrolyte for a sodium-metal battery with 90% capacitance retention after 700 cycles []. A solid electrolyte of PVDF with Na3Zr2Si2PO12 and NaClO4 as salt (σRT = 1.1×10−4 S/cm, for plain PVDF σRT = 3.1 × 10−5 S/cm) was used as a composite electrolyte in a sodium metal/Na0.67MnO2 battery []. Wetting the porous positive electrode with a very small amount of a liquid organic electrolyte solution helped in establishing a good electrode/electrolyte interface. At an optimized content of liquid a capacitance retention of 100% after 100 cycles was observed. A solid electrolyte of PVDF with NaCF3SO3 as salt and SiO2 as a filler yielded a solid composite electrolyte with σRT = 0.06 × 10−3 S/cm []. The complete Na/NVP battery kept 70% of its initial capacitance after 100 cycles, for better contact between electrolyte and positive electrode some liquid electrolyte solution was added to the latter.
Cyclic stability was attributed to prevented dendrite formation at the sodium electrode and inhibited manganese dissolution at the positive electrode. Electrospun fibers of PVDF/NaFSI with a shell of Na3Zr2Si2PO12 were made into a separator/composite electrolyte with σ60°C = 6.6 × 10−4 S/cm, enabling a sodium-metal battery showing relatively poor specific capacity, attributed to the poor electrode/electrolyte contact []. Into a solid electrolyte of PVDF with NaClO4 as a salt Na3Zr2Si2PO12 was added, yielding a composite electrolyte with σRT = 1.069 × 10−4 S/cm tested in a sodium-ion battery keeping 95% of its initial capacitance after 290 cycles []. Into a solid electrolyte of PVDF with NaPF6 sodium β”, alumina was added []. The modified composite electrolyte had σRT = 0.19 × 10−3 S/cm and t+ = 0.91, and a complete sodium/carbon nanofiber (CNF) cell kept 95% of its initial capacitance after 100 cycles. A composite of this ceramic with PVDF-HFP with “Salt-Ionic liquid” (this apparently means an ionic liquid EMIMTFSI as a plasticizer and NaTFSI as a salt) has been prepared, yielding σRT = 1 × 10−3 S/cm and t+ = 0.57 [].
A composite electrolyte was obtained by coating both sides of a glass fiber mat first with PVDF-HFP and then with polydopamine and by finally soaking the material in a 1 M solution of NaClO4 []. σRT = 4.6 × 10−3 S/cm was reported, and with polydopamine coating, the value increased to σRT = 5.4 × 10−3 S/cm; a sodium/Na2MnFe(CN)6 battery showed 89% capacity retention after 100 cycles.
Into a porous skeleton of PVDF-HFP and Na3Zr2Si2PO12, an interpenetrating network of poly(ether-acrylate), PEO, and NaPF6 was embedded, providing a composite electrolyte with σ60°C = 1.32 × 10−4 S/cm and t+ = 0.63 at T = 60 °C []. Operated at this temperature, sodium-metal cells and various positive electrode materials showed stable capacities over 100 cycles. The multilayer structure of the electrolyte frequently claimed in the report is nowhere evident.
A solid electrolyte Na3Zr2Si2PO12 with added BaTiO3 (in the abstract, it is called reinforcement, but in the report, this claim does not show up again) better prevents dendrite formation, enabling a full cell to keep 84.4% of its initial capacitance after 400 cycles []. A composite sheet of Na3Zr2Si2PO12 and PVDF-HFP soaked in an electrolyte solution of sodium triflate and TEGDME (presumably tetraethylene glycol dimethyl ether, see Figure 16) showed σ0°C = 1.2 × 10−4 S/cm, σRT = 3.6 × 10−4 S/cm, and t+ = 0.92 [].
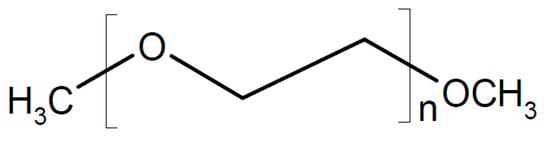
Figure 16.
Molecular structures of tetraethylene glycol dimethylether (TEGDME).
In a complete battery, only a slight capacitance loss was observed over 200 cycles.
A composite of PVDF-HFP and Na3Zr2Si2PO12 soaked in a solution of 1 M NaPF6 in a mixed carbonate solvent was used as an electrolyte (σRT = 7.94 × 10−4 S/cm) in a Na/Sn-C battery []. The high conductivity was attributed to the liquid electrolyte solution trapped inside the porous composite. After initial major capacity losses (about 50%), the cell capacity stayed constant for the following 80 cycles.
PVDF-HFP coated on both sides of a disk of Na2.5Zr1.95Ce0.05Si2.2P0.8O11.3F0.7 (σRT = 1.7 × 10−3 S/cm) was used as a solid electrolyte in a sodium-metal/Na0.67Mn0.47Ni0.33Ti0.2O2 battery, showing a stable performance over 300 cycles []. A similar ceramic Na3.2Zr1.9Ca0.1Si2PO12 combined with PVDF-HFP showing σRT = 1.32 × 10−4 S/cm has been suggested as solid electrolyte for a sodium/CO2 battery []. A solid composite electrolyte was prepared from PVDF-HFP with NaClO4 and Na3.2Zr1.9Mg0.1Si2PO12 added as a filler for use in a sodium/CO2 battery []. Partial substitution of Zr by Mg resulted in σRT = 1.16 × 10−3 S/cm. A polymer composite membrane of PVDF-HFP and Na3Zr2Si2PO12 filled with poly(methyl methacrylate) (PMMA, see Figure 17) was used as a solid electrolyte with σRT = 2.78 × 10−3 S/cm and t+ ~ 0.63, which enabled a stable cycling performance of a sodium-ion battery for 600 cycles [].
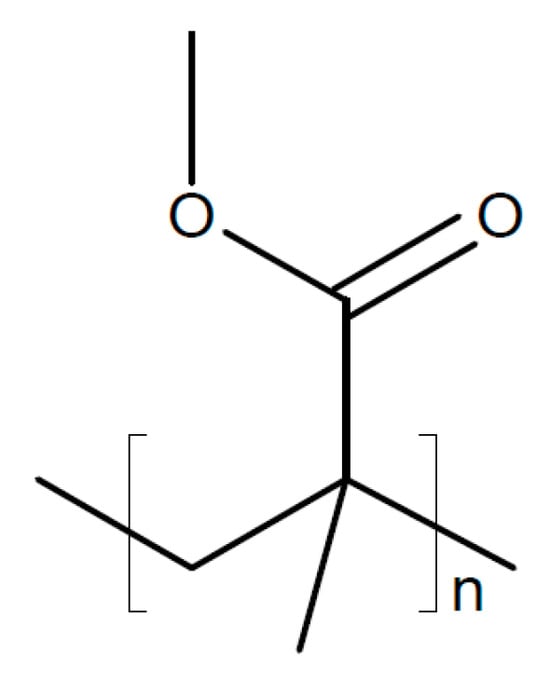
Figure 17.
Molecular structures of poly(methyl methacrylate) (PMMA).
A composite of PVDF-HFP as the matrix and a sodium-rich anti-perovskite/perovskite material has been suggested as electrolyte for sodium batteries []. σRT = 1.11 × 10−4 S/cm and stability over 500 cycles were reported. To improve the low-temperature ionic conductivity of PVDF-HFP (not PVDF as suggested in the title of the report) with NaClO4 as the salt, graphitic C3N4 has been added []. This addition increased conductivity by enhancing salt dissociation and reducing crystallinity of the copolymer. σRT = 5.171 × 10−5 S/cm without g-C3N4 increased to σRT = 1.67 × 10−4 S/cm, and t+ improved from 0.61 to 0.78. In a sodium-metal battery, 98% of the initial capacitance was retained after 200 cycles; with the addition, this decreased to 69%. A polymer blend of PVDF-HFP and PMMA was dissolved and β-alumina nanopowder was added, possibly (the report leaves this detail open) the blend was soaked in a 1 M NaClO4 in a blend of carbonate solvents []. Solution uptake was highest at 4 wt.% filler content, conductivity was highest at this composition with σRT = 3.39 × 10−3 S/cm attributed to lower crystallinity, t+ = 0.58 was attributed to a weaker interaction between fluoride atoms in the polymer and sodium ions caused by the added filler. In a test with this composite electrolyte a sodium/Na3V2(PO4)3 battery kept 85% of its initial capacitance after 300 cycles.
To a polymer blend of PVDF-HFP and PMMA with NaCF3SO3 dissolved in a carbonate solvent mixture presumably also acting as a plasticizer and as a salt, Al2O3 was added as a filler, providing a gel electrolyte with σRT ~ 1.5 × 10−3 S/cm and t+ ~ 0.29. 0.33 (not T as reported) at a 6 wt.% optimum filler content []. Enhanced conductivity caused by addition of PMMA was attributed to the amorphous nature of this polymer, whereas the added filler increased salt dissociation. PVDF-HFP combined with β-Al2O3 powder surface modified with silane for better compatibility with the polymer matrix yielded a solid electrolyte after soaking with an electrolyte solution of NaPF6 in a mixed carbonate solvent for a sodium-metal battery with σ20°C = 1.37 × 10−3 S/cm and t+ = 0.424, enabling 92% capacitance retention after 1000 cycles []. A copper-based MOF HKUST-1 [] was used as inorganic filler for PVDF-HFP (confusingly named PH), and the obtained membrane soaked in a 1 M NaClO4 solution in ethylene carbonate with 5% fluoroethylene carbonate yielded a composite electrolyte with σ30°C = 3.2 × 10−4 S/cm and t+ = 0.64 []. When tested in a sodium/Na3V2O2(PO4)2 battery, 75% of the initial capacitance was kept after 400 cycles. Inhibition of dendrite formation was attributed to the inorganic filler; in addition, the filler enhanced liquid electrolyte solution absorption and amorphicity.
A ternary polymer blend of PVDF-HFP, PEO, and PMMA has been combined with 10 wt.% silica fillers and soaked in an electrolyte solution of NaPF6 in propylene carbonate, yielding a composite electrolyte subsequently tested in a sodium/Na3V2(PO4)2F3 []. σRT = 0.88 × 10−3 S/cm and t+ = 0.86 were found, or without filler, only σRT = 0.63 × 10−3 S/cm and t+ = 0.74. The cell kept 93% of its initial capacitance after 100 cycles.
Using Sb2O3 instead of Al2O3 as a filler for a polymer mixture of PVDF-HFP and PVP yielded a highly flexible composite membrane, which was soaked with an electrolyte solution of 1 M NaClO4 in a carbonate solvent mixture []. The result, called a separator, had a “large electrolyte window”—whatever that means, it blocked dendrite formation. The same approach has been reported with SiO2 as a filler material []. Here, 6 wt.% filler was found as the optimum fraction, yielding an electrolyte with σRT = 0.71 × 10−3 S/cm and stable performance in a symmetric Na//Na cell for 200 cycles. Into a polymer blend PVDF-HFP and PEO with NaClO4 as salt and EMIMFSI as plasticizer, microtubular Na3Zr2Si2PO12 was added, yielding a solid electrolyte with σRT = 6.93 × 10−4 S/cm and t+ = 0.882 for a sodium-metal battery [].
Nanoparticles of a zeolitic imidazolate framework ZIF-67 impregnated into a fibrous membrane of PVDF-HFP and PAN yielded a sodium-ion-conducting membrane with σRT = 1.42 × 10−3 S/cm and t+ = 0.58 []. The interface established between this membrane and the sodium-metal electrode prevented sodium dendrite formation during cycling. A complete cell showed 0.052% capacitance decline per cycle during 100 cycles.
Other Polymer-Based Materials
An overview of polymer electrolytes focused on sodium batteries is available []. An epoxy-reinforced ceramic sheet of NASICON type of Na3Zr2Si2PO12 has been suggested for a solid-state sodium-ion battery []. The beneficial effect of the added polymer beyond improving mechanical strength is illustrated in Figure 18, showing the ionic conductivity as a function of the polymer/ceramic composition. The porous sintered ceramic pellet was filled with the polymer. Sintering of the inorganic components before combining the constituents was found to be highly advantageous in terms of conductivity of the product.
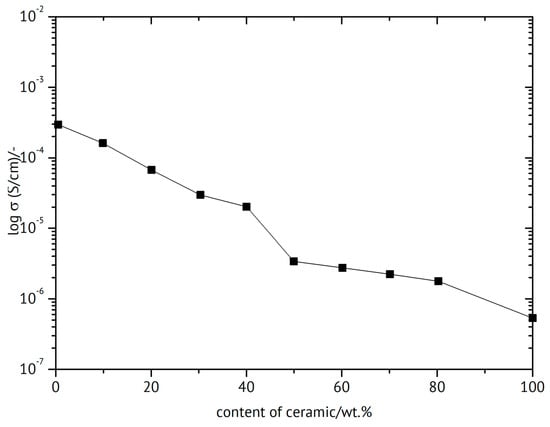
Figure 18.
Ionic conductivity of a ceramic/polymer composite as a function of composition at room temperature, based on data in [].
A highly sodium-ion-conducting SiO2/polymer hybrid has been reported []. For an increased value of t+, anions were immobilized by grafting anions of 2-[(trifluoromethane-sulfonylimido)-N-4-sulfonylphenyl]ethyl onto SiO2 particles, and this hybrid material was dispersed in a polymer matrix of polyethylene oxide (PEO, see Figure 4) or polyethylene glycol dimethylether (PEGDME, see Figure 19), yielding a composite electrolyte.
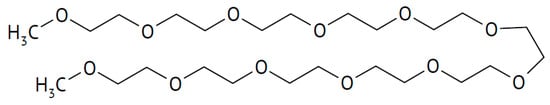
Figure 19.
Molecular structures of polyethylene glycol dimethylether.
Conductivities σRT > 10−5 S/cm were found. A matrix of electrospun SiO2 fibers was filled with a copolymer of 2-(methacryloyloxy) ethyl acetoacetate and N,N′-methylenebisacrylamide, yielding a composite electrolyte with σ−30°C = 0.153 × 10−3 S/cm []. A final coating with AlF3 suppressed sodium dendrite formation. Capabilities of this electrolyte were demonstrated by 5500 h steady sodium plating/stripping. How a pouch half-cell (elsewhere in the text, full cell) was powered by several LEDs was not explained; a cell kept 94% of its initial capacitance after 475 cycles. With Al2O3 as a filler, an acrylate-based polyester polymer yielded an electrolyte for a sodium-metal battery with σRT = 5.59 × 10−3 S/cm []. A composite of polysulfone-sodium sulfonate and poly(polyethylene glycol methacrylate) with nanosized hexagonal boron nitride for sodium-ion batteries has been reported []. σ100°C = 5.5 × 10−5 S/cm was noted.
A copolymer of acrylonitrile and polyethylene glycol methacrylate with NaClO4 as the salt and hexagonal boron nitride as the filler has been developed []. An optimum composition of σ? = 3.6 × 10−4 S/cm at an unknown temperature (possibly, according to a displayed figure, T = 100 °C) was reported, and the proposed use in a sodium-ion battery was not described. A composite electrolyte of poly(ethylene glycol) diacrylate (see below) reinforced with glass fibers and σ? = 1.38 × 10−3 S/cm/t+ = 0.79 has been tested in a sodium-metal battery, yielding 91% capacitance retention after 1000 cycles []. Felts of electrospun ceramic fibers surface-modified with acyl amino groups for enhanced interfacial sodium ion transport were filled with a deep eutectic electrolyte for long-range ion transport []. A value of σ26°C = 3.29 × 10−4 S/cm and 98% capacity retention after 1000 cycles were reported. Cross-linked β-alumina nanowires provided the scaffold for a PVDF-HFP-based gel polymer electrolyte in a sodium-metal battery []. The mechanical rigidity of the electrolyte supported smooth sodium deposition, resulting in 95% capacitance retention after 1000 cycles.
A gel polymer composite electrolyte prepared from cellulose triacetate and a polyionic liquid has enabled a sodium-metal cell to run for more than 800 cycles []. How the electrolyte powered the cell was not explained. A PEO-based gel polymer electrolyte with 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl) imide as the gelling agent and NaTFSI as the salt was modified with surface-modified sepiolite []. Conductivities’ depended on ionic liquid content, with large fractions of the liquid σRT = 0.95 × 10−3 S/cm found, which, with lower fractions this value, decreased to 0.39 × 10−3 S/cm. The influence of sepiolite on ionic conductivity was rather moderate.
A NASICON-type sodium-ion conductor of unspecified composition with a dense core and porous outer layers has been prepared []. The porous layer facing the sodium electrode was filled with PEO, and the one facing the positive Na3V2(PO4)2F3 with PAN for better compatibility with the respective electrodes. σ30°C = 4.3 × 10−4 S/cm was attributed to long-range ionic pathways, and lower interfacial resistance to the better compatibility between electrodes and the modified inorganic solid. A full cell provided 81% capacity retention after 460 cycles.
A sodium ion-conducting solid electrolyte based on PEG and NaBr with 5 nm silica nanoparticles has been developed []. At 5 wt.%, added silica conductivity enhancement was largest at σRT = 8 × 10−5 S/cm. Adsorption of anions (i.e., Br−) on the silica was upheld as the main reason of conductivity increase.
A solid electrolyte of a copolymer poly(ethylene glycol)-co-ureidopyrimidinone with NaTFSI as the salt was modified by adding hollow mesoporous SiO2, yielding a solid composite electrolyte with σRT = 2.37 × 10−5 S/cm and t+ = 0.17 []. A sodium/NVP battery assembled with this electrolyte kept 77% of its initial capacity after 200 cycles at T = 60 °C, and increased conductivity was ascribed to fast ion pathways along the surfaces of the silica spheres.
Poly(diallyldimethylammonium) bis(fluorosulfonyl)imide with NaFSI (see Figure 9) as the salt dissolved in PYR14TFSI (see Figure 8) soaked into a glass fiber mat yielded a composite electrolyte with σ70°C = 2.1 × 10−3 S/cm, and when tested in a Na/NVP battery, 75% of its initial capacity was retained after 2000 cycles [].
Addition of an antioxidant 4-trifluoromethylphenylboronic acid to a cross-linked gel polymer electrolyte helped to avoid electrolyte decomposition at the positive electrode of a high-voltage sodium-ion battery [].
A composite of Na3Zr2Si2PO12 with cellulose acetate obtained by a simple solution-casting method with σRT = 1.73 × 10−3 S/cm was tested in a solid-state sodium battery, enabling 80% capacity retention after 800 cycles [].
For a sodium-metal battery, a solid electrolyte of porous Na3SbS4 filled with a polymer PPP of PEG and PPG (the meaning of the acronyms is nowhere revealed; evidence suggests that PPP may be a mixture of poly(ethylene glycol) (Figure 5) and poly(propylene glycol) (Figure 20) with NaTFSI as salt has been proposed []. The performance of the sodium-metal electrode was significantly improved, enabling operation for 550 cycles.
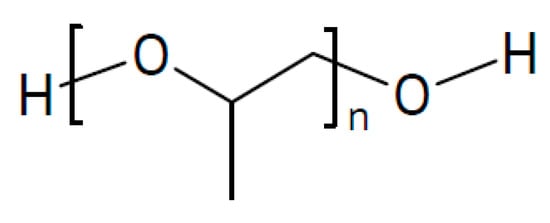
Figure 20.
Molecular structures of poly(propylene glycol).
Na3SbS4 is a good sodium-ion conductor but unfortunately chemically incompatible with sodium metal []. Nanoparticles of this material coated with an ionic liquid BMPTFSI (1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl) imide, see the common acronym PYR14TFSI Figure 8) were soaked into a porous PVDF membrane, subsequently serving as separator and solid electrolyte, yielding a conductivity σRT = 3.18 × 10−3 S/cm and enabling a good cell performance over 400 cycles. In another attempt to utilize this sodium-ion conductor, the sulfide is deposited onto a glass fiber network, and the product is soaked with an ionic liquid []. Good cycling behavior both of the sodium-metal electrode and the positive FeS2 electrode in contact with this electrolyte were reported. Another approach to utilize the attractive conductivity of this sulfide by combining it with sodium carboxymethyl cellulose (7.5 wt.% and 92.5 wt.% sulfide) has been reported []. Because the thickness of the electrolyte could by decreased to 1/5 in comparison to the sulfide alone, effective sodium-ion conduction grew roughly 5-fold. A sodium/Te-C cell showed about 40% capacitance loss after 100 cycles. A hybrid composite electrolyte prepared from Na3SbS4 and cross-linked pentaerythritol tetra acrylate with σRT = 0.047 × 10−3 S/cm has been tested in a Na3SbS4/S cell running for 90 cycles [].
Air stability of ion-conducting sulfides has been reviewed []. In a further attempt, Na3SbS3Se nanoparticles were embedded in a PVDF-HFP polymer matrix with NaPF6 as a salt, yielding a composite electrolyte with σRT = 1.31 × 10−4 S/cm at an optimum sulfide content 10 wt.% []. A Na/TiS2 battery kept 56% of its initial capacitance after 300 cycles. A core/shell composite has been proposed for use in solid-state sodium batteries []. A core of Na3PS4 is coated with a shell of Na2.25Y0.25Zr0.75Cl6, yielding σ? = 0.44 × 10−3 S/cm at an unspecified temperature. Particles of Na3Zr2Si2PO12 were embedded in Na3PS4, yielding at 70% of the ceramic, a solid electrolyte with σ100°C = 1.1 × 10−3 S/cm []. A composite electrolyte was prepared from PEO, NaClO4, Na2S, and P2S5 dissolved in acetonitrile, yielding Na3PS4/PEO/NaClO4 with σRT = 9.4 × 10−5 S/cm (only Na3PS4 σRT = 6.4 × 10−5 S/cm) at 4 wt.% of PEO and NaClO4, and tested in a Na/SnS2 battery []. A thin layer of PEO on Na3PS4 inhibited the detrimental chemical reaction of Na3PS4 with sodium and enabled more reversible sodium cycling, and greater fractions of PEO and NaClO4 resulted in a growing cell impedance because of a larger resistance of said coating.
A structural sodium-ion battery with a PEO-based composite electrolyte with NaClO4 as the salt and added Na3Zr2Si2PO12 showing σ60°C = 1.02 × 10−4 S/cm and t+ = 0.44 kept 80% of its initial capacitance after 225 cycles [].
The transition from liquid to solid electrolytes including hybrid systems for sodium-ion batteries has been surveyed; the acronym NIB (instead of the commonly accepted and used SIB) is certainly a highlight in this contribution in addition to many other inaccuracies [], further aspects of this development are discussed in [,,]. The knowledge of gel electrolytes for sodium-ion batteries has been surveyed in []. Electrolytes including composite ones based on NaCl for fuel cells and electrolyzers have been reviewed [,].
3.1.2. Potassium and Potassium-Ion Batteries
As reported, KTFSI (actually this is potassium bis(trifluoromethanesulfonyl)imide) is not suitable for potassium-ion batteries because of its low melting point, making it mechanically unstable and thus not useful as a “separator” in addition to being the electrolyte []. Inorganic fillers Al2O3 and SiO2 were tried, and they increase the mechanical stability without negatively affecting ionic conductivity. Actually, mixtures or composites of KTFSI and PEO with added fillers were studied. Conductivities above the melting point of the composite (presumably not very helpful for practical application) were not affected by the mechanical filler; at actual operating temperatures, no numbers were provided. A full cell kept 93% of its initial capacitance after 100 cycles. A powder mixture of PEO, potassium β-alumina, and potassium trifluoromethanesulfonimide was pressed into a pellet and heated; in a separate procedure, these compounds were dissolved/suspended in DMF, yielding a membrane after solvent evaporation []. For a trilayer solid electrolyte, the latter liquid was poured on both sides of the pellet and dried. An interfacial resistance but no conductivity or performance in an actual cell were reported.
A composite of potassium polystyrenesulfonate and a polyoxovanadate (HK5[V10O28]·10H2O) was used as an electrolyte in a potassium/Prussian Blue battery [].
A composite electrolyte of polypropylene carbonate with KFSI dissolved in ethylene carbonate/diethyl carbonate as the electrolyte and a nonwoven cellulose membrane as the filler and reinforcement has been prepared (σ20°C = 1.36 × 10−5 S/cm) and tested in a potassium/3,4,9,10-perylene-tetracarboxylicacid-dianhydride battery []. The rapid capacity fading was attributed to the solubility of the active material in the positive electrode, but how the conclusion of a stable cycling performance could be reached remains mysterious.
A solid electrolyte for a potassium-ion battery was obtained by dissolving a powder of perfluorinated resin in DMF and adding some KPF6-containing electrolyte solution in ethylene carbonate and diethyl carbonate []. Using it in a complete cell yielded a sodium-ion battery with a capacitance decay of 0.26% per cycle during 200 cycles.
A solid electrolyte based on polyvinyl butyral with KCl and σ30°C = 1.87 × 10−5 S/cm has been prepared and tested in a potassium-metal/iodine battery [].
3.1.3. Magnesium and Magnesium-Ion Batteries
Inorganic Electrolytes
Along the line reported by Liang [], composites of Mg(NO3)2 and Al2O3 were prepared and their ionic conductivity examined []. With the composition 0.9Mg(NO3)2/0.1Al2O3, σRT~10−4 S/cm was observed and attributed to the presence of MgO. A composite of Mg(BH4)2 with THF and MgO added for stabilization of the composition Mg(BH4)2·1.5THF-MgO(75 wt.%) yielded σ70°C~10−4 S/cm with t+ = 0.99 and was tested in a Mg/TiS2 battery []. A composite of Mg(BH4)2·CH3NH2BH3 with 75 wt.% MgO added for solidification of the initially viscous liquid showed σRT~10−5 S/cm []. Borohydride−amide composites have been prepared by mechanochemical (ball milling) and thermal procedures (annealing with various protective gas atmospheres) and characterized in view of possible applications in magnesium batteries []. Prepared samples always contained Mg(BH4)2·(NH2) and some other phase, and conductivity reached σ100°C = 3 × 10−6 S/cm, higher than earlier reported values of σ150°C = 10−6 S/cm and σ100°C = 10−8 S/cm []. The difference in reported conductivities—certainly less than the three orders of magnitude claimed in []—was attributed to some additional ionic phase.
Various cyclopropylamine borohydrides and some of their composites with Al2O3 have been prepared, characterized, and examined as solid electrolytes []. The composite electrolyte Mg(BH4)2·(CH2)2CHNH2·Al2O3 (50:50 by weight) had the highest conductivity σRT = 1.8 × 10−5 S/cm and t+ = 0.99999. A gel composite electrolyte composed of magnesium borohydride Mg(BH4)2, MgCl2, and polyethylene glycol (Figure 6) has demonstrated high thermal stability and ionic conductivity (σRT = 1.01 × 10−4 S/cm, t+ = 0.74) and good compatibility with a magnesium electrode []. Over 1000 cycles, 92.6% of the initial capacitance was retained.
Composite electrolytes based on silicate tetraethylene glycol hybrids with various magnesium salts have been prepared and characterized []. Conductivities around 10−4 to 10−3 S/cm were observed.
Composites of Mg(BH4)2 and various amounts of isopropylamine have been tested as magnesium-ion conductors [,]. The highest ionic conductivity of σ45°C = 2.7 × 10−4 S/cm was found for the composition Mg(BH4)2·1.5(CH3)2CHNH2. Materials start to soften around 50 °C, and addition of 75 wt.% of MgO increased ionic conductivity as well as thermal stability, suggesting a contribution of hydrophobic interactions to increased conductivity. Mg(BH4)2·1.47CH3)2CHNH2 confined in mesoporous silica SBA-15 yielded σ32°C = 9.1 × 10−6 S/cm [].
PEO-Based Electrolytes
In a mechanochemical procedure, a mixture of PEO, Mg(ClO4)2, and EMIMFSI in acetone was ball-milled, which yielded a composite electrolyte after solvent evaporation with σRT = 0.5 × 10−5 S/cm [].
To PEO with magnesium triflate as the salt, urea has been added, yielding a solid electrolyte with σ? = 6.3 × 10−5 S/cm, with possible use in magnesium batteries suggested []. The increase in conductivity caused by added urea was attributed to faster segmental motion in PEO and lower viscosity of the medium. A composite electrolyte of PEO with MgBr2 as the salt and added starch nanocrystals was prepared with σRT = 7.8 × 10−8 S/cm []. Increased conductivity was attributed to additional ion transport channels formed by the starch nanocrystals. To a PEO-Mg(ClO4)2 solid electrolyte, nanochitosan was added as a filler, increasing the ionic conductivity by about two orders of magnitude around σ60°C ≈ 10−3 S/cm with 10 wt.% salt and 10 wt.% filler []. A PEO-based composite electrolyte with Mg(OH)2 as a plasticizer also containing LiTFSI with σRT ≈ 1.6−5 S/cm has been reported []. A liquid (!) composite electrolyte of PEO with magnesium acetate and added nanoparticles of MgO showed σRT = 3.63 × 10−3 S/cm after γ-irradiation [].
PVA-Based Electrolytes
A solid electrolyte of PVA with various amounts of Mg(ClO4)2 has been prepared and characterized, and an optimum conductivity σ30°C ≈ 10−4 S/cm was recorded with PVA/salt composition 0.6/0.4 []. A PVA-based solid electrolyte with MgBr2 as a salt and phosphomolybdic acid (this is presumably the compound called phosphomolbidic acid throughout the report) and TEGDME has been prepared and tested in a Mg/TiO2 battery []. The highest observed ionic conductivity was σ303 K~10−6 S/cm, t+ = 0.4. A polymer electrolyte of PVA with MgBr2 and tetraethylene glycol dimethyl ether as a plasticizer was combined with Li2O, yielding a composite electrolyte with σRT ≈ 10−5 S/cm and t+ = 0.7 at 0.04 wt.% optimum filler content []. It was tested in a Mg/V2O5 battery.
A composite electrolyte of a blend of PVA and SN with a magnesium electrolyte has been characterized and tested in a Mg/TiO2 battery [].
Structural and ion transport properties of a polymer blend of PVA and hydroxypropyl methylcellulose with Mg(NO3)2 as a salt (hardly as dopant as suggested in the report) have been studied []. At the optimum composition (PVA:HPMC = 0.4:0.6, 40 wt.% salt), σRT = 2.48 × 10−4 S/cm was measured, and said mixture had the lowest crystallinity based on XRD data, certainly supporting high ionic conductivity. Unfortunately, the mentioned conductivity value does not match tabulated data; this may be due to the use of NaNO3 for measurement of the tabulated data. A most uncommon use of transference number tion = 0.995 has been reported, and for the electrons, te = 0.005 has been listed. The clearly large value of tion is highlighted, but the cause of electronic conduction is not even mentioned. Presumably, parasitic currents are a cause. Back to standard practice, t+ = 0.247 was finally obtained. A primary Mg/MnO2 battery with this optimized composite electrolyte was assembled and discharged once.
PVDF-Based Electrolytes
To dissolved PVDF, MgBr2 was added as an electrolyte and some TEGDME as a plasticizer, yielding a composite electrolyte with σRT = 1.2 × 10−6 S/cm and t+ = 0.55, showing initially reversible magnesium plating both in a symmetric Mg/Mg and a battery-type Mg/graphene nanoplatelet cell []. XRD data show an almost complete loss of crystallinity of the polymer, and decreasing reversibility of magnesium plating was attributed to a growing “interfacial resistance”.
A membrane of a polymer blend of PVDF and polyurethane with MgO as a filler was soaked in a carbonate solvent-based electrolyte solution with Mg(ClO4) σ60°C = 3.4 × 10−6 S/cm, yielding a gel composite electrolyte with σRT = 4.6 × 10−3 S/cm at the optimum filler content of 7 wt.% []. A mixture of PVDF-HFP, Mg(ClO4)2, and PYR14TFSI with 10 wt.% TiO2 as a filler has been made into a composite electrolyte with σRT = 0.16 × 10−3 S/cm []. In a symmetric Mg/Mg cell, highly reversible magnesium plating was observed.
A composite electrolyte from PVDF-HFP with Mg(ClO4)2 dissolved in a carbonate solvent mix with various amounts of fumed silica as a filler with σRT~1.1 × 10−2 S/cm at optimum filler content 7 wt.% has been prepared and tested in a Mg/MoO3 battery []. Space-charge layers formed at the filler/polymer interfaces were suggested as the cause of the enhanced conductivity. The cell ran for more than 10 (!) cycles.
Into a blend of PVDF-HFP/polyvinyl acetate with Mg(ClO4)2 as the salt, geikeilite (MgTiO3) was added as an inorganic filler at various percentages []. The highest observed ionic conductivity σ30°C ~ 5.80 × 10−3 S/cm and t+ = 0.34 were found at the optimum composition (6 wt.% of MgTiO3), and a complete cell retained 86% of its initial capacitance after 30 cycles.
With PVDF-HFP with magnesium triflate as a salt, and SN and some 1-ethyl-3-methylimidazolium trifluoromethanesulfonate for stabilization, a composite electrolyte with σ26°C = 4 × 10−3 S/cm was obtained and tested in a Mg-C/MnO2 battery, showing substantial capacity fading within eight cycles (!) []. The different effects of active and passive fillers (see also []) on magnesium-ion conductivity of PVDF-HFP with Mg(Tf)2 in a mixture of ethylene and propylene carbonate with magnesium-containing and thus possibly actively participating compounds like MgO or MgAl2O4 and passive fillers like TiO2, Al2O3, or SiO2 have been studied []. Whether the absence of peaks in diffractograms clearly attributable to MgAl2O4 or Al2O3 proves complete dissolution appears to be questionable (see also []); more likely is a very fine distribution of the nanoparticles of the filler. With an optimum content of 30 wt.% Al2O3, σRT = 3.3 × 10−4 S/cm was found, and with 30 wt.% MgAl2O4, σRT = 4 × 10−4 S/cm. Addition of fillers resulted in significant increases in transference numbers to t+ = 0.52 in the former and t+ = 0.66 in the latter case. The remarkable difference between changes afforded by active vs. passive fillers was not resolved.
A PVDF-HFP-based composite electrolyte with Mg(ClO4)2 dissolved in a mixture of ethylene carbonate and propylene carbonate (which stayed in the gel) and MgO as inorganic filler yielded σRT~6 × 10−3 S/cm and t+ = 0.39 at optimum composition 10 wt.% filler and was tested in a Mg/V2O5 battery for ten (!) cycles []. Why the even higher conductivity at 3 wt.% filler was not considered the optimum (see Figure 21) was not addressed. Such observation of two maxima has been reported elsewhere [,,,]. The first maximum has been attributed to enhanced dissociation of ion aggregates and/or undissociated salt whereas the second one has been attributed to space charge effects discussed above [,,,]. At higher filler concentrations, the particles hinder ion movement and thus decrease conductivity [].
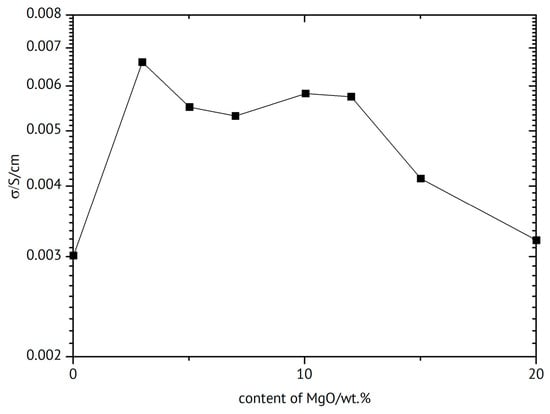
Figure 21.
Ionic conductivity vs. MgO content (based on data in []).
Other Polymer-Based Electrolytes
Into a PEG-based electrolyte with magnesium acetate as salt various amounts of TiO2 have been added []. At optimum filler content 10 wt.% σRT = 5.01 × 10−5 S/cm was found, without filler it was σRT = 1.07 × 10−6 S/cm.
To a solid electrolyte of methyl cellulose and magnesium acetate, Co3O4 was added yielding a composite electrolyte with σRT = 5.93 × 10−4 S/cm at the optimum composition tested in a Mg/MnO2 battery []. For a magnesium/iodine battery, a PEG-based electrolyte with magnesium acetate as the salt and CeO2 as the inorganic filler has been prepared and tested []. Up to 15 wt.% of added filler, an increase in conductance to σ60°C = 3.4 × 10−6 S/cm with t+ ≈ 0.97 was observed. Into a PEG-based electrolyte with Mg(NO3)2, TiO2 was added as an inorganic filler, providing a composite electrolyte with σRT = 1.06 × 10−4 S/cm and t+ = 0.98 at an optimum filler content of 10 wt.% tested in a Mg/I2 battery []. To a PEG-based polymer electrolyte with magnesium acetate, TiO2 was added as a ceramic filler, yielding a composite electrolyte with σRT = 5 × 10−5 S/cm []. To a chitosan-based polymer electrolyte with MgCl2, various amounts of V2O5 were added as inorganic filler, yielding a composite electrolyte tested in a Mg/MnO2 battery []. Added filler decreased the crystallinity of the composite, with 1 wt.% showing the most pronounced effect, which in turn increases ion mobility, i.e., conductivity. Indeed, conductivity (σRT = 1.4 × 10−3 S/cm and t+ = 0.96) was highest with this composition.
On a glass-fiber substrate, a glycerol α,α′-diallyl ether-3,6-dioxa-1,8-octanedithiol-based gel polymer electrolyte with σRT = 1.19 × 10−4 S/cm and t+ ≈ 0.704 was prepared and tested in a magnesium/Mo6S8 battery []. A composite electrolyte with nanosized MgO dispersed in poly(ethyl methacrylate) with MgTf2 and EMITFSI has been prepared []. Effects of various inorganic fillers on PEO-based composite electrolytes with Mg(CF3SO3)2 on ion transport properties have been studied, and the greatest increase in conductivity was found with 5 wt.% of MgO, where t+ = 0.38 was also the largest []. Performance improvements were largest with an active (Mg-containing) filler.
For a magnesium/sulfur battery, a gel electrolyte was prepared by a cross-linking reaction between Mg(BH4)2, Li(BH4), and polytetrahydrofurane reinforced with glass fiber separator (σ0°C = 0.15 × 10−3 S/cm) and tested in magnesium batteries with various positive electrode materials []. With Mg/TiS2, a stable capacitance over 70 cycles was achieved.
Recent progress in magnesium battery research also addressing some composite electrolytes has been reviewed []. Progress and prospects of solid electrolytes for magnesium batteries are discussed in []. The effect of Na2SiO3 on the behavior of an AZ31B magnesium alloy anode in a mixed aqueous electrolyte solution with Na2SO4 and NaNO3 has been examined [].
3.1.4. Calcium and Calcium-Ion Batteries
Various urea calciumtetrahydridoborate compositions have been synthesized and characterized []. The highest ionic conductivity was found for the composition Ca(BH4)2·3.30CO(NH2)2 at σRT = 2.46 × 10−7 S/cm with t+ = 0.997.
3.1.5. Zinc and Zinc-Ion Batteries
Rechargeable zinc/MnO2 batteries (the approved acronym is RAM for rechargeable alkaline manganese, not eAZMB as suggested by the authors in []) have been pursued for some time but discontinued, mostly because of problems related to the zinc electrode [,]. Based on advances with reversible zinc electrodes, there may be a renaissance. As an electrolyte for such systems, a composite of a gelatin hydrogel with fumed silica has been studied []. The addition of SiO2 increased porosity and ionic conductivity, and zinc corrosion was reduced and zinc deposition proceeded more uniformly; 10%wt. of SiO2 was the optimum fraction. Hierarchically porous ZIF-67 filled with PVP combined with nanocellulose yielded a composite with σRT = 4.31 × 10−3 S/cm, used as separator in an aqueous zinc battery []. To cellulose acetate with NH4BF4, SiO2 was added as a filler, yielding a composite electrolyte with σRT = 7.89 × 10−3 S/cm at 3 wt.% filler addition, tested in a Zn/MnO2 battery []. Ammonium ions were suggested as mobile charge carriers, but why the device is called a proton battery remains mysterious. Into an anhydrous PVDF-HFP-based gel polymer electrolyte with an ionic liquid with conductivity σRT = 24.32 × 10−3 S/cm and t+ = 0.42 for a zinc/MnO2 battery, copper ions were immobilized in PAA (nowhere spelled out, most likely poly acrylic acid) for accelerated zinc ion movement and deposition []. The cell kept its capacitance for 1000 cycles.
The 2 M ZnSO4 electrolyte solution for an aqueous electrolyte has been modified by the addition of various tetraazacyclotetradecane compounds at concentrations ranging from 10 to 100 mM, resulting in impressively increased cycle numbers of the zinc electrode []. Modification of the coordinative environment of the zinc ions was suggested as the reason for suppression of dendritic zinc deposition. Zinc ion transport in a gel electrolyte prepared from poly(vinyl chloride) and poly(ethyl methacrylate) with 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (EMIMTFSI) as the gelling agent and Zn(OTf)2 as the salt modified with SnO2 nanoparticles yielded an optimum value σRT = 4.92 × 10−4 S/cm at 3 wt.% filler []. A very similar approach with fumed silica nanoparticles instead of tin oxide providing σRT = 6.71 × 10−4 S/cm has been reported by these authors [], with ZrO2 yielding σRT = 3.63 × 10−4 S/cm at a 3 wt.% filler content [] or with PVDF-HFP instead yielding σRT = 4.6 × 10−4 S/cm at a 7 wt.% filler content [].
To PVDF-HFP (abbreviated quite unconventionally and thus confusing PVHF) dissolved in acetone EMIMBF4 (again confusingly abbreviated as EMIBF4 except for the common acronym in the title), Zn(OTF)2 and Ti3C2Tx were added (the meaning of “suspended above the polymer solution” remains mysterious), yielding a composite electrolyte (σ60°C = 2.11 × 10−3 S/cm and σ20°C = 1.75 × 10−3 S/cm), which was tested in a zinc/NVP battery []. In addition to 80% capacitance retention after 1000 cycles, “controlled self-discharge” appears to be the most prominent feature of this battery.
A liquid water-in-polymer salt electrolyte has been prepared from potassium polyacrylate (see Figure 22) and Zn(TFSI)2 and tested in a zinc/lignin battery, retaining 80% of its initial capacitance after 8000 cycles [].
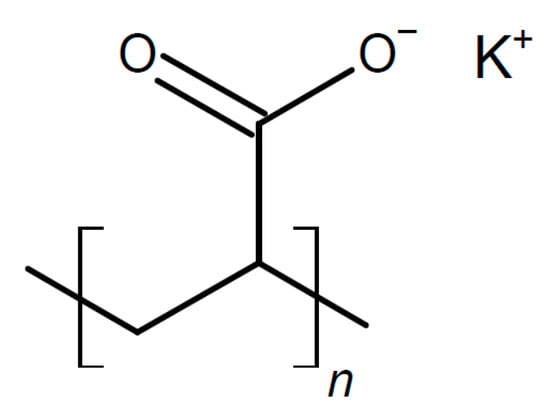
Figure 22.
Molecular structures of potassium polyacrylate.
PVA-Based Electrolytes
A PVA-based gel electrolyte with ZnSO4 was made into a composite by adding ZnO nanopowder, increasing ionic conductivity from σRT = 2 × 10−4 S/cm to σRT = 1.3 × 10−3 S/cm []. A PVA-based gel with KOH as the electrolyte and added functionalized carbon nanotubes (CNTs) (graphene oxide is nowhere found in the experimental part) showed σRT = 6.2 × 10−2 S/cm and tion = 0.9, suggesting substantial electronic conduction, which is even greater with tion = 0.55 without any filler, leaving the question of what provides electronic conduction in the filler-free gel electrolyte (for the GO-containing version, σRT = 6.9 × 10−2 S/cm and tion = 0.87) []. How the data suggest that hydroxyl ions are the charge carriers provided by KOH remains unclear. The electrolyte was tested in a zinc/Ag2O battery.
A PVA-based composite gel with added sulfonated cellulose and sepiolite was soaked in a 2 M ZnSO4 solution (σRT = 2.2 × 10−2 S/cm) and used in a zinc/I2 battery, showing 83% capacity retention after 10,000 cycles [].
Hydroxyethyl cellulose (HEC) and montmorillonite (MMT) were added into PVA, yielding a composite electrolyte after soaking in an aqueous solution of 2 M ZnSO4 and 0.1 M MnSO4 with σRT = 24.6 × 10−3 S/cm at the optimum composition (3 wt.% HEC and 2 wt.% MMT) []. A zinc/MnO2 battery with this electrolyte kept 91% of its initial capacitance after 450 cycles. A PVA-based gel electrolyte with Zn(CF3SO3) was combined with Prussian Blue for an enhanced zinc ion transference number and better water retention, yielding a composite electrolyte with σRT = 16.3 × 10−3 S/cm and t+ = 0.63 for a zinc/ammonium vanadate (NVO) battery []. PVA modified with an inorganic filler ZnO, poly(m-phenylene isophthalamide) as reinforcement, glycerol as antifreeze agent, and Zn(CF3SO3)2 had σ20°C = 9.7 × 10−3 S/cm and was used as a composite electrolyte in a zinc/MnO2 battery, losing 10% of its initial capacitance after 1200 cycles []. A PVA-based gel reinforced with nanofibers of polyurethane-poly(m-phenylene isophthalamide) used as a solid electrolyte in a Zn/MnO2 battery enabled operation for 6500 h []. A PVA-based gel electrolyte with Zn(OTf)2 was combined with aramid nanofibers into a composite electrolyte with σRT = 42.2 × 10−3 S/cm and t+ = 0.78 and tested in a zinc/polyaniline battery, keeping 78% of its initial capacitance after 9100 cycles [].
A membrane of electrospun poly(m-phenylene isophthalamide) fibers was decorated with ZnO nanorods and filled with PVA, yielding a zinc ion-conducting solid electrolyte (σRT = 18.3 × 10−3 S/cm) for a Zn/MnO2 battery running for 1000 cycles []. High conduction was attributed to transport channels along the ZnO nanorods. A mechanically stable and recyclable hydrogel P3B2Zx has been prepared by cross-linking PVA (P) with borate (borax B) and ZnCl2 (Z) as the salt []. Depending on the salt content, conductivity varied from σRT = 3.5 × 10−3 S/cm to σRT = 33.8 × 10−3 S/cm when going from P3B2Z1 to P3B2Z2.5. A full battery kept 77% of its capacity after 1500 cycles.
PAM-Based Electrolytes
A gel based on polyacrylamide PAM (see Figure 23) with ZnSO4 as salt, EMIMBF4 (see Figure 24) (the salt is certainly no co-solvent as claimed in the report), and a deep eutectic Zn(ClO4)/EG with t+ = 0.915 was tested in a zinc/VO2 cell, keeping its initial capacitance for 200 cycles []. The claimed exceptional ionic conductivity was unfortunately not reported in numerical terms.
Figure 23.
Molecular structures of polyacrylamide PAM.
Figure 24.
Molecular structures of 1-ethyl-3-methylimidazolium tetrafluoroborate EMIMBF4.
A composite solid electrolyte prepared from PAM and an unspecified salt, presumably ZnSO4, with coffee silverskin cellulose added as a filler (why and how the resulting material was combined with ZnSO4 remains unclear) with σRT = 36.51 × 10−3 S/cm (without filler, σRT = 25.85 × 10−3 S/cm) was used in a zinc/MnO2 battery []. PAM was prepared in an aqueous solution also containing 1 M ZnSO4, yielding a polymer electrolyte into which a small amount of graphitic C3N4 was added []. This resulted in less corrosion of the zinc electrode and a much smoother zinc deposition, the zinc ion transference number increased from t+ = 0.383 to t+ = 0.631, the composite electrolyte enabled a zinc/carbon cell with 87% capacitance retention after 1000 cycles, and without the added g-C3N4, only 55% was retained. PAM was polymerized from a monomer-containing solution with carboxymethylcellulose and dissolved ZnSO4, and dispersed graphene oxide (not graphene as claimed in the title of the report) []. The composite electrolyte showed σRT = 45.5 × 10−3 S/cm, and a complete zinc/NVO battery kept 82% of its initial capacitance after 800 cycles. A hydrogel electrolyte based on a polymer blend of PAM and diacetone acrylamide with dickite (Al2Si2O5(OH)4) as a filler was soaked in an aqueous solution of 2 M ZnSO4 and 0.1 M MnSO4, yielding a composite electrolyte with σRT = 20.7 × 10−3 S/cm at the optimum composition []. Smooth zinc deposition was attributed to the added dickite, resulting in a stable capacitance of a Zn/MnO2 battery over at least 700 cycles.
A PAM-gel with hemp-derived cellulose fibers was soaked in a solution of 2 M ZnSO4 and 0.5 M MnSO4, affording a composite electrolyte with σRT = 59.6 × 10−3 S/cm at 1 wt.% fibers and was tested in a zinc/CoMn2O4 battery, keeping 56% of its initial capacitance after 500 cycles [].
A PAM-based gel reinforced with a lamellar–porous polyimide covalent organic framework and ZnSO4 as an electrolyte with σRT = 21.7 × 10−3 S/cm (with reinforcement σRT = 10.9 × 10−3 S/cm) and t+ = 0.74 has been tested in a zinc/NVO battery with 96% capacitance retention after 1500 cycles []. For a cathode-less zinc/manganese fiber battery, Zn2+-containing neutral, weakly acidic, and acidic PAM-based gel electrolytes were prepared []. The weakly acidic one was used between the electrodes, and the polymer-coated zinc wires were immersed into the neutral one, whereas the acidic one was placed close to the cathode of the cathode-less (!) device. The battery kept 76% of its initial capacitance after 2700 cycles. A hydrogel prepared from agar and PAM (5:1) with ZnSO4 dissolved in ethylene glycol as the electrolyte with t+ = 0.339 has been tested in a zinc/V2O5 battery [].
A polymer blend of PAM and CMC modified and made into a composite electrolyte from an aqueous solution of 2 M ZnSO4 and 0.1 M MnO4 with 2 g/L sodium dodecyl sulfate (SDS) (σRT = 28.05 × 10−3 S/cm; without SDS, σRT = 26.07 × 10−3 S/cm; PAM only, σRT = 9.15 × 10−3 S/cm; t+ in this sequence 0.82; 0.57; 0.27) was tested in a zinc/MnO2 battery, keeping 66% of its initial capacitance after 350 cycles []. Dominant zinc deposition on the 002-plane was attributed to the added SDS, which also reduces parasitic hydrogen evolution.
PAM was polymerized inside a network of agarose and CMC in an aqueous solution also containing 1 M ZnSO4 and 0.1 M MnSO4, yielding a composite gel electrolyte with σRT = 23.1 × 10−3 S/cm at the optimum composition and was subsequently tested in a zinc/MnO2 battery, keeping 80% of its initial capacitance after 800 cycles [].
Biopolymer-Based Electrolytes
A biocompatible gel of a blend of agarose and sodium alginate soaked in an aqueous solution of 2 M ZnSO4 and 0.1 M MnSO4, yielding an electrolyte with σRT = 25.05 × 10−3 S/cm and t+ = 0.75, was tested in a flexible zinc/MnO2 battery, which kept 83% of its initial capacitance after 800 cycles []. For stabilization of the water in a sodium alginate gel electrolyte, the disaccharide trehalose was added, yielding σRT = 12.4 × 10−3 S/cm; it was tested successfully in a zinc/polyaniline battery, with 70.4% capacitance retention after 500 cycles []. A sodium alginate hydrogel electrolyte modified with urea was used in a biocompatible printable flexible zinc-ion battery []. Urea regulated zinc nucleation kinetics. A cotton pad treated with sodium alginate and soaked with aqueous zinc acetate solution provided σRT = 1.96 × 10−3 S/cm when treated with 1 wt.% alginate solution, and this separator provided the best zinc cycling []. A composite hydrogel of sodium alginate and carrageenan with a zinc-ion transference number t+ = 0.58 and σRT = 5.89 × 10−2 S/cm has been tested in a zinc/NH4V4O10 battery for 600 cycles []. A sodium alginate-based gel electrolyte with added nano-SiO2 has been reported []. When used in a Zn/MnO2 battery with an aqueous neutral ZnSO4 gel electrolyte also containing some MnSO4 and Cs2SO4, the added filler increased the available cycle number from 1000 to 1800 with 78% capacitance retention in the latter case. A polymer blend of sodium alginate and PAM (presumably this is meant, with the acronym PAAM nowhere explained in the report) with ZnCl2 as the electrolyte and graphene oxide as the filler with σRT = 12.12 × 10−3 S/cm was used as a composite electrolyte in a zinc/LiFePO4 battery, with 63% capacitance retention after 500 cycles []. Into a hydrogel of cross-linked sodium alginate and acrylamide, PVP-coated nanosheets of alumina were added; the obtained composite electrolyte membrane was soaked in an aqueous solution of zinc trifluoromethanesulfonate (Zn(OTf)), providing σRT = 17 × 10−3 S/cm []. A complete zinc/PVO (vanadium pentoxide with poly(3,4-ethylenedioxythiophene)) battery kept 83% of its initial capacitance over 1000 cycles. A blend of PVA, sodium alginate, and polyvinyl pyrrolidone gel polymer electrolyte showed σRT = 9.46 × 10−3 S/cm (presumably due to the immersion of the gel in a mixed solution of ZnSO4 and Li2SO4), and when tested in a zinc/LiFePO4 battery, showing 68% capacitance retention after 1000 cycles []. A sodium alginate electrolyte with ZnSO4 as the salt has been modified by adding tannic acid, yielding a composite electrolyte (σRT = 24 × 10−3 S/cm; without tannic acid, σRT = 20 × 10−3 S/cm), tested in a zinc/NH4V4O10 battery with 97% capacitance retention after 200 cycles []. An improved performance in terms of more reversible zinc plating achieved by added tannic acid was stated.
Chitosan mixed with NH4NO3 and ethylene carbonate as a plasticizer yielded a composite electrolyte used in a zinc/MnO2 battery [].
From lignin and fumed silica, with the addition of an aqueous electrolyte solution of 2 M ZnSO4 and 0.2 M MnSO4, a composite gel electrode was prepared, and it was tested in a zinc/MnO2 battery with an almost constant capacity during 3000 cycles []. This performance suggests strong dendrite formation suppression, low zinc corrosion, and less byproduct formation, as confirmed by analytical verification.
To a mixture of carboxy methylcellulose and poly(N-isopropylacrylamide) with ethylene carbonate as plasticizer and zinc triflate as a salt, Ti3AlC2 (MAX) was added, yielding a composite electrolyte for a Zn/NVO battery []. Strong interaction between MAX and the triflate anion was suggested as a reason for the unspecified high ionic conductivity and transference number. A hydrogel electrolyte of sodium CMC and sodium alginate soaked in a 1.5 M ZnSO4 and 0.1 M MnSO4 solution was tested in a zinc/MnO2 battery, keeping 48% of its initial capacitance after 500 cycles [].
A hydrogel of gelatin and γ-polyglutamic acid soaked in an aqueous solution of 2.5 M ZnSO4 and 0.3 M MnSO4 yielded a composite electrolyte with σRT = 12.6 × 10−3 S/cm, which was tested in a zinc/MnO2 battery, keeping 70% of its initial capacitance after 1000 cycles []. The added γ-polyglutamic was identified as the reason for smooth zinc deposition, less corrosion, and byproduct formation.
Miscellaneous Electrolytes
A composite of 70 parts P2O5·5H2O and 30 parts of a mix of 92 parts SiO2 and 8 parts of Al2O3 served as proton-conducting solid electrolyte in a battery with a negative zinc/zinc sulfate and a positive MnO2/graphite electrode []. A composite electrolyte based on a non-woven fabric soaked with a slurry of PTFE and Mg(OH)2 particles was used in a zinc/carbon battery (also called a hybrid capacitor in the report) [].
A hydrogel prepared from polyethylene-g-poly(acrylic acid) with added organo-montmorillonite was used with ZnCl2 as a salt as electrolyte in a Zn/carbon battery hydrogel []. Comparable data on performance and stability were not provided.
A porous sponge of cross-linked bacterial cellulose was filled with a hydrogel of poly(sodium 4-styrenesulfonate)/poly(dimethyl diallyl ammonium chloride), which was “loaded” either with a solution of 6 M KOH or 3 M KI, yielding σRT,KOH = 42.5 × 10−3 S/cm and σRT,KI = 29.0 × 10−3 S/cm []. The cellulose sponge without gel filling provided higher conductivities as expected.
A UV-curable gel electrolyte processable by ink-jet printing for a conformal zinc-ion battery has been reported [].
Addition of CuCl2 and poly(N-diallyldimethylammonium chloride) to an aqueous ZnSO4 electrolyte solution resulted in a smoother zinc deposition []. For a zinc/iodine battery, the zinc electrode was coated with a zinc-ion selective electrolyte layer of poly(ether-block-amide) modified with graphene oxide []. The zinc ion transference number was t+ = 0.77; more than 36,000 cycles with 95.5% of the initial capacitance retained were recorded.
A mixture of several compounds (called for unknown reasons a composite) has been added to a presumably aqueous electrolyte solution for improved performance of a zinc-ion battery []. A membrane prepared from cellulose fibers surface-modified with tannic acid providing phenolic hydroxyl groups has been suggested for use in aqueous zinc-ion batteries []. By supporting a uniform distribution of zinc ions, high reversibility of the zinc electrode in various electrolyte solutions was enabled, yielding 83% capacitance retention after 1000 cycles.
Hydrogel-based electrolytes suggested for zinc-ion batteries show some deterioration during operation, attributed to densification resulting in poor electrolyte/electrode contact; a modified hydrogel showing stronger adhesion has been reported [].
The separator of a zinc/LiMn2O4 battery has been soaked with a mixed electrolyte solution of ZnSO4 and Li2SO4, which was subsequently gelled with fumed silica []. Gelling of the liquid electrolyte solution resulted in a substantial improvement of cycling performance, a well-known observation with lead-acid batteries.
Zinc–Air Batteries
A hydroxyl-ion-conducting composite based on a Bakelite-type polymer with covalently attached viologen units coated onto filter paper was tested as an electrolyte/separator for a zinc/air battery []. Another strategy towards an improved interface by surface polymerization on the air electrode has been suggested []. In addition to proper zinc deposition, dehydration of water-containing electrolytes is a further challenge for zinc/air batteries.
A sodium polyacrylate gel electrolyte modified with graphene oxide or graphene oxide nanoribbons and cellulose nanofibers soaked in a solution of 6 M KOH and 0.2 M zinc acetate, yielding a composite electrolyte with σRT = 268 × 10−3 S/cm (with GO only σRT = 188 × 10−3 S/cm), was tested in a flexible zinc–air battery []. GO and GO nanoribbons were considered as plasticizers without further explanation and not supported by the displayed XRD results.
A PAA-based gel electrolyte with Al2O3 added as filler yielded a composite electrolyte after soaking in an aqueous solution of 6 M KOH and 0.2 M zinc acetate []. At the optimum filler content (30 wt.%) σRT = 186 × 10−3 S/cm, unfortunately, at this composition, water retention was slightly inferior to the composite with 20 wt.%. Presumably this property also enabled the slightly longer lifetime of a battery with this electrolyte composition.
A sodium CMC-PAA blend with KOH as a salt has been prepared, characterized, and suggested as an electrolyte for a zinc/air battery []. A ternary composite hydrogel of CMC, polyacrylamide, and graphene oxide has been proposed as a remedy []. A solid composite electrolyte based on CMC blended with either PVA or PAA yielded σRT = 231 × 10−3 S/cm at a composition of CMC:PAA 1:2 when the solid was soaked in aqueous KOH solution; it was proposed for use in zinc/air batteries []. A hydrogel prepared from CMC, PAM, and cellulose nanofibers (the acronym CNF used in this report appears to stand for carbon nanofibers in the rest of the world), with KOH or KCl as electrolytes, with σRT = 290 × 10−3 S/cm in the former case (and σRT = 163 × 10−3 S/cm without the cellulose nanofibers) and σRT = 171 × 10−3 S/cm in the latter case, was tested in a zinc/air cell [].
A composite gel electrolyte of PVA with embedded cross-aligned polyacrylic nanofibers soaked in aqueous KOH solution provided σRT = 235 × 10−3 S/cm and has been proposed for use in zinc/air batteries []. Into a porous PVA gel (using PEG as pore-forming agent), various amounts of silica were added, yielding after soaking in 6 M KOH a composite electrolyte, tested in a flexible zinc/air battery []. At the optimum silica content, σRT = 57.3 × 10−3 S/cm was found, and this value decreased rapidly within 22 h; nevertheless, the best performance of a battery with this electrolyte was claimed. A composite electrolyte of PVA functionalized with quaternary ammonium groups combined with chitosan and MoS2 has been prepared, which (not mentioned in the experimental part) was finally soaked in an aqueous KOH-solution []. Addition of chitosan caused a higher uptake of the KOH solution. At 0.5 wt.% MoS2, σRT = 87.3 × 10−3 S/cm, where presumably MoS2 enhanced hydroxide ion mobility and increased longevity. A test in a zinc/air battery ran for 465 cycles. A gel of PVA with carboxylated nanocellulose fibrils soaked in aqueous 6 M KOH afforded a composite electrolyte with σRT = 312 × 10−3 S/cm, tested in a zinc/air battery []. A PVA gel reinforced with electrospun polyetherimide nanofibers apparently swollen in a KOH electrolyte, yielding a composite electrolyte with σRT = 13 × 10−3 S/cm, was tested in a rechargeable zinc/air battery []. Inhibition of zincate crossover deemed essential for a secondary zinc/air battery was observed. A near-neutral gel polymer composite electrolyte of PVA with functionalized MXene prepared in an aqueous KOH solution with σRT = 58.5 × 10−3 S/cm and a good water retention caused by the functionalized filler was tested in a rechargeable zinc/air battery []. In a somewhat mysterious procedure, a PVA-based gel with added Lyocell fibers was made into a composite electrolyte and suggested as replacement of an unspecified “commercialized composite separator” for a primary zinc/air battery []. A gel of PVA reinforced with epoxy resin as a composite electrolyte (σRT = 77.6 × 10−3 S/cm) was cast on the negative electrode composite and subsequently soaked in aqueous KOH solution, establishing a tight contact between electrolyte and electrode in a zinc/air battery [].
A PVA gel with ZnSO4 and MnSO4 as salts was reinforced with bacterial cellulose fibers, yielding a composite electrolyte with σRT = 80.8 × 10−3 S/cm at an optimum filler content of 6 wt.% []. In a flexible secondary zinc–air battery, 650 cycles were run with only a minute deterioration of performance. The composite electrode could handle internal pressure changes during cycling, kept hydration water well, and suppressed dendrite formation and corrosion.
A PVA gel was modified with recycled polyurethane particles, onto which flexible polyurethane chains had been grafted and polydopamine had been deposited, yielding a flexible composite electrolyte with improved mechanical properties and ionic conductivity after soaking in a 6 M KOH solution with 0.2 M zinc acetate []. The composite showed σRT = 175 × 10−3 S/cm, plain PVA only σRT = 108 × 10−3 S/cm, and the composite with polyurethane particles only 131 × 10−3 S/cm, suggesting a beneficial effect of the grafted chains and the deposited amine, whereas the extended cell lifetime and improved zinc deposition also indicate an improved zinc ion distribution.
Into a mixed organic network of sodium polyacrylate (see Figure 25) and PVP, nanoparticles of TiO2(NH2) were added, yielding a gel polymer electrolyte for a zinc/air battery with enhanced water retention properties, capable of suppressing zinc dendrite formation []. Addition of the filler increased the electrolyte solution uptake to 13.8 times the solid’s weight and σRT = 272 × 10−3 S/cm.
Figure 25.
Molecular structures of sodium polyacrylate.
The influence of the size of SiO2 nanospheres on the ionic conductivity of a composite with a blend of PVA and PAA, yielding a composite claimed to be porous (presumably the composite, not the polymer blend) and capable of reducing dendrite formation in a zinc/air battery, has been studied []. To a polymer blend of PVA and PAA, cellulose was added, and the composite was soaked in a solution of KOH and zinc acetate, yielding a composite electrolyte with σRT = 123 × 10−3 S/cm, tested in a secondary zinc/air battery [].
A composite electrolyte of sodium polyacrylate with added nanocellulose and graphene oxide and σRT = 178 × 10−3 S/cm was used in a zinc/air battery after soaking in a mixed aqueous electrolyte solution of zinc acetate and potassium hydroxide []. For a rechargeable zinc/air battery, a hydrogel of polyacrylamide-carboxymethyl chitosan-ethylene glycol polymer blend was soaked in aqueous KOH/zinc acetate solution with σRT = 278 × 10−3 S/cm (which declined quickly within hours) and tested in a battery for 54 h []. A composite of PAM with various amounts of mesoporous SiO2 was soaked in aqueous KOH, yielding a composite electrolyte with σRT = 337 × 10−3 S/cm at an optimum filler content of 5 wt.% (this is presumably misleadingly called 5 wt.% mPAM in the report) and tested in a zinc/air battery []. To a PAM-based hydrogel with Zn(OTf)2 as a salt, fumed silica was added for enhanced water retention as a solid electrolyte in a zinc/air battery [].
The influence of silica nanoparticles and PEG (see Figure 5) added into a polymer blend of PAM and CMC immersed presumably in an electrolyte solution of 4 M KOH or 4 M KOH + 2 M KI in water, yielding a composite electrolyte, has been studied []. Addition of KI resulted in a major increase in ionic conductivity from σRT = 135 × 10−3 S/cm to σRT = 312 × 10−3 S/cm, where the use of 2.8 M NH4Cl + 0.6 M ZnCl2 or 2.8 M KCl yielded σRT = 161 × 10−3 S/cm and σRT = 69 × 10−3 S/cm, respectively. The claimed benefits of the added PEG and silica are impossible to appreciate in the absence of comparable data; the mitigation of the “carbonate problem” expected with an alkaline electrolyte by use of a near-neutral electrolyte may possibly balance the somewhat worse overall performance.
A composite electrolyte of PEO, PVA, and KOH with a glass fiber mat has been prepared, characterized, and tested in a primary zinc/air battery []. With the optimum polymer mixture (50:50), σ30°C = 47.5 × 10−3 S/cm was observed.
A composite electrolyte based on surface-functionalized nonwoven polypropylene/polyethylene fabric was coated with PVA and with additional acrylic acid monomer, yielding a composite electrolyte with σRT = 0.16. 0.21 × 10−3 S/cm and t− = 0.83. 0.91 for a zinc–air battery (called a metal–air fuel cell) []. A composite of PVA and up to 2.5 wt.% mesoporous silica MCM-41 soaked in an aqueous solution of KOH (σRT = 380 × 10−3 S/cm; without silica, σRT = 340 × 10−3 S/cm) was tested in a zinc/air battery running for 145 cycles []. Without the filler, 163 cycles were achieved. To overcome the claimed insufficient stability of PVA-KOH electrolytes, a composite of sulfonated cassava starch, nano-attapulgite, and polyacrylamide finally ion-exchanged with aqueous KOH was prepared, enabling a flexible zinc/air battery running for 450 h [].
A polymer blend of PAM and CMC was reinforced with hyaluronic acid (HA), yielding a gel, which was soaked in an electrolyte solution of unspecified composition, later stated as aqueous 6 M KOH with 0.2 M zinc acetate, yielding a composite electrolyte with σRT = 321 × 10−3 S/cm, or without HA, only σRT = 180 × 10−3 S/cm []. The displayed “power density curves of the electrolyte” actually refer to a full zinc/air battery. A gel composed of guar gum and sodium alginate soaked in a water/ethylene glycol solution of 2 M ZnSO4 as a salt with σRT = 25.37 × 10−3 S/cm was tested as a composite electrolyte in a zinc/MnO2 battery, keeping 91% of its initial capacitance after 1000 cycles []. Further general considerations on zinc/air battery development have been collected [].
A wider overview on prospects of anion exchange membranes known from fuel cells and electrolyzers in metal-ion batteries is available [].
Electrolytes for Structural Batteries
A composite electrolyte of poly(ethylene glycol) diacrylate (Figure 26) reinforced with glass fibers for structural carbon fiber zinc-ion batteries has been described; it enabled a zinc-ion battery to run stably for 1000 cycles [].
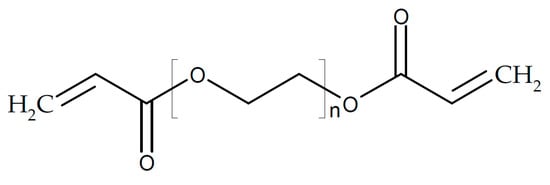
Figure 26.
Molecular structures of poly(ethylene glycol) diacrylate.
A “biomimetic” electrolyte of PEO with Zn(CF3SO3)2 as a salt and with branched aramid nanofibers (σRT = 2.5 × 10−5 S/cm) as a filler and reinforcement has been tested in a structural zinc/MnO2 battery, showing 96% capacitance retention after 50 cycles [].
To a glass-fiber reinforced PVDF-HFP, an unspecified substance KL and KL-Z, presumably kaolin, was added, yielding a structural electrolyte with Zn(CF3SO3)2 as a salt (σRT = 0.44 × 10−3 S/cm and t+ = 0.78), subsequently tested in a zinc/ammonium vanadate battery []. The battery kept 95% of its initial capacitance after 500 cycles.
An epoxy-based solid electrolyte with EMIMTFSI and Zn(TFSI)2 was tested in a structural zinc/NH4V4O10 battery []. With the optimum composition, the battery barely showed any degradation after 140 cycles.
A biomimetic composite electrolyte based on quaternary ammonium-functionalized PVA deposited into an Aramid nanofiber membrane (σRT = 61 × 10−3 S/cm) for use in a structural zinc–air battery for robotic devices has been developed [].
3.1.6. Aluminum and Aluminum-Ion Batteries
An aqueous electrolyte solution containing a mixture of Al(OTF)3, HOTF, and Zn(OTF)2 for a battery with a negative Al-Zn electrode and a positive AlxMnO2·nH2O has been developed []. The battery ran for 100 cycles.
Addition of 3 wt.% of cellulose nanofibrils (the acronym CNF appears to be a confusing assignment) to polyacrylic acid with aqueous KOH solution as the gelling agent caused a 100% increase in ionic conductivity and major improvement of mechanical strength []. The electrolyte was tested successfully in an Al/air battery.
3.1.7. Further Battery Chemistries
For a tin–zinc/graphite battery, a solid composite electrolyte composed of magnesium silicate and sodium phosphate pressed into a PET cloth has been described [].
For a fluoride-ion battery, a composite electrolyte composed of NH4HF2, PEO, and β-PbSnF4 has been prepared []. It was tested in a cell with a positive CuF2 and a negative Pb/PbF2 electrode for up to 50 cycles.
A solid composite electrolyte of PVA cellulose acetate with ammonium triflate as a salt and nano-Al2O3 as an inorganic filler for a proton battery with σRT = 2.012 × 10−3 S/cm and t+ = 0.9684 has been reported []. Without filler, σRT = 2.93 × 10−4 S/cm was obtained.
A mixed salt of AgI and AgCl was composited with Al2O3, yielding a solid electrolyte for a silver/I2 battery, with the highest ionic conductivity σ27°C = 9.2 × 10−4 S/cm at a fraction of 0.3 Al2O3 []. These researchers obtained similar results with another filler, where the highest conduction σRT = 1.5 × 10−3 S/cm was observed at a fraction of 0.2 Fe2O3 []. The reason for the higher effect of Fe2O3 as compared to Al2O3 was not specified.
3.1.8. General Aspects and Miscellaneous Observations
The viscosity and dynamics of a mixture (called a composite by the authors) of pectin and an ionic liquid BMIMPF6 considered as a possible electrolyte for batteries have been studied with extensive simulations []. A composite (actually, following the proposed narrower definition of a composite above, it is a mixture) of PEO and E8 nematic liquid crystals has been studied as a possible electrolyte for solid-state batteries []. Properties of mixtures of PEO and variable fractions of the nematic liquid crystal E8 have been investigated, and no charge carriers were identified []. Although clearly no DC conductivities were measured, a result of σRT = 4.36 × 10−9 S/cm apparently derived from AC impedance measurements for the most conductive composition was stated. Why this should lead the researchers to recommend the described materials for application in batteries remains blurred. Even with 10 wt.% added NaIO4 the resulting value of σRT = 1.05 × 10−7 appears not very attractive [].
Composite electrolytes based on polyurethane with silica for application in MEMS have been reported []. In a general study of blends of PEO and PVP (see Figure 13) with added inorganic fillers ZnO and TiO2, evidence was found suggesting the use of some of the studied combinations in energy storage devices []. A composite electrolyte of PEO (50 parts) and AgCF3SO3 (1 part) with 2 wt.% SnO2 as the inorganic filler and various amounts of ethylene carbonate as the plasticizer has been prepared and studied []. Without plasticizer, σRT = 3.1 × 10−6 S/cm was found, and upon addition, the value went up to 5.4 × 10−5 S/cm; at 30 wt.% added liquid with growing plasticizer fraction.
Nanofibers of ceramic NASICON Li1.5Al0.5Ge1.5(PO4)3 were prepared by electrospinning and composited with PEO, and the composite electrolyte had double the ionic conductivity of the plain PEO []. An electrolyte of PVA with 5 wt.% KBr (why the salt is called a composite electrolyte remains unclear) showed an increased ionic conductivity of σ? = 6.24 × 10−2 S/cm after electron irradiation. Whether the mentioned temperature applies to the conductivity measurement or the electron irradiation remains unclear. In a high-throughput study of more than 700 ceramics derived from Na2ZnSiO4-based compounds soaked with an electrolyte solution of NaTFSI in PYR14TFSI, relatively high ionic conductivities were observed, but unfortunately, all materials were highly incompatible with sodium commonly used in sodium batteries [].
Filling mesoporous silica with an ionic liquid EMIMBF4 (Figure 23) and combining the product with PEO to form a composite electrolyte yielded a 3-fold increase in ionic conductivity when only 5 wt.% of the IL was incorporated []. This increase was attributed to fast ion movement inside the IL-filled pores and channels. The ionic conductivity of a composite of PVdF-HFP and an IL increased by an order of magnitude upon addition of organic solvents like propylene carbonate as a plasticizer []. This increase has been attributed to an increase in the concentration of free ions and their higher mobility because of the decreased viscosity of the medium.
Details of modeling polymer/ceramic composite electrolytes at the molecular level have been discussed, with particular attention to the ceramic/polymer interface as the assumed main contributor affecting overall ionic conductivity []. Mathematical models have been applied, working towards the calculation of effective conductivities of particular polymer composite electrolytes []. Computational prediction methods for dielectric properties relevant for behavior of these materials as electrolytes have been discussed []. Computational tools for the development of soft solid composite electrolytes with enhanced ionic conductivities have been presented []. Results of a theoretical study of PVA/cellulose composite electrolytes are available [].
Phase-change materials suitable both as a solid electrolyte and as heat storage materials have been reviewed [].
A composite electrolyte of Sn0.9In0.1P2O7 combined with either PTFE or sulfonated polystyrene-b-poly(ethylene/butylene)-b-polystyrene for a device called a “rechargeable fuel cell”, essentially a cell able to run as an electrolyzer or a fuel cell, has been reported [].
Composite materials have been applied also in redox flow batteries [,].
3.2. Elevated Temperature Systems
In batteries employing molten metals as well as other molten electrode materials (e.g., sulfur or metal halides), solid electrolytes have been essential prerequisites to consider such systems. Even in the very specific case of the all-liquid metal accumulator [] with a molten salt as an electrolyte, advances with solid-ion conductors have attracted attention because their use may avoid some difficulties encountered with molten salt electrolytes. The essential role of solid electrolytes as well as their inherent drawbacks and limitations sketched above have attracted considerable research efforts in this field before the focus has shifted to their use in ambient temperature systems as a replacement of liquid electrolyte (solutions).
Composite solid electrolytes for HT sodium batteries of Na-β″-alumina and YSZ have been prepared by vapor-phase synthesis []. Conductivity was σRT = 1.6 × 10−3 S/cm and σ300°C = 1.3 × 10−1 S/cm with a fully converted (i.e., α-Alumina + YSZ composite into Na-β″AY) material. Improved mechanical properties of the composite β″-Al2O3-ZrO2 as compared to plain β″-Al2O3 have been reported []. Improved conduction behavior of a two-layer solid electrolyte comprising a dense support of β″-Al2O3 has been stated []. A β″-Al2O3 –glass composite electrolyte with a resistance of about 20 Ω·cm at 250 °C chemically stable vs. sodium has been described [].
A composite of gadolinium-doped ceria/magnesia with an optimized composition has been developed for intermediate-temperature solid oxide fuel cells []. A composite electrolyte of Ce0.85Gd0.15O2 (CGO) and Sc2O3-doped ZrO2 (ScSZ) for an SOFC has been prepared, where the cell performance with a simple single-layer CGO electrolyte was increased substantially in terms of open circuit voltage and peak power density when a second layer of ScSZ was added [].
4. Conclusions
Given the reported examples of various added components finally yielding a wide range of composite electrolytes for metal and metal-ion batteries with widely varying properties of the negative electrode, several tasks and beneficial effects of these additions can be discerned, which are arranged in the order of importance and relevance—from the author’s perspective:
- Enhanced ionic conductivity;
- Increased transference number of the ion (mostly the cation) of interest;
- Stronger adherence to electrode(s);
- Better inhibition of dendrite formation at the negative electrode;
- Better water retention for hydrogels;
- Higher thermal and mechanical stability;
- Self-healing.
Achieved specific conductivities vary widely; sometimes acceptable values are reached only at temperatures so high above room temperature that practical application appears to be unlikely. Actually, contributions of an electrolyte to the internal cell resistance depend on the thickness of the electrolyte or electrolyte and separator and on properties of the established electrolyte/electrode interface. The latter value is more relevant than a specific value but more difficult to specify for a given cell. At least in correctly evaluated impedance measurements, the Ohmic component of the impedance at f → ∞ Hz should provide a value that can even be converted into an aerial one when cell and electrode dimensions are well-defined. The general wish to achieve higher specific values is certainly dominant and appropriate, but even more attractive is a value of the latter resistance that is as small as possible. Reporting such data in future communications would be highly helpful. Equally important but not at all always reported is the cell performance—in particular, cell behavior as a function of current/current density. This numerical value will implicitly allow researchers to report about the establishment of an adequate electrolyte/electrode interface. In particular, with porous electrodes—and most of the positive electrodes are of this type—the establishment of an intimate contact between is a challenge, which goes way beyond the approach sometimes encountered to “just apply a high enough mechanical pressure”.
To determine whether the generalization “better a stable long-term performance at slightly higher internal resistance than a low-resistance cell failing after ten cycles” is acceptable, there is a definite need to test the cell performance for higher cycle numbers. This means, for secondary batteries in portable applications (e.g., mobile phones), 500 to 1000 (Chinese standard GB/T 18287 ≥ 400, with an EU directive aiming at 2000); for cars (standard GB/T 31484), ≥1000; and for stationary storage (Chinese standard GB/T 36276), ≥6000.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
Preparation of this review has been supported in various ways by National Key R & D Program of China (Grant No. 2021YFB2400400), by the Alexander von Humboldt-Foundation, Deutscher Akademischer Austauschdienst, Fonds der Chemischen Industrie, Deutsche For¬schungsgemeinschaft, National Basic Research Program of China, Natural Science Foundation of China (Grant No. 52131306), Project on Carbon Emission Peak and Neutrality of Jiangsu Province (Grant No. BE2022031-4), and by Grants Nos. 26455158 and 70037840 within research projects at St. Petersburg State University. Patient support in literature retrieval and management by H. Trapp (Chemnitz University of Technology Library) is gratefully appreciated.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kortüm, G. Lehrbuch der Elektrochemie, 4th ed.; Verlag Chemie: Weinheim, Germany, 1966. [Google Scholar]
- Robinson, R.A.; Stokes, R.H. Electrolyte Solutions; Academic Press Inc.: New York, NY, USA, 1955. [Google Scholar]
- Harned, H.S.; Owen, B.B. Physical Chemistry of Electrolyte Solutions, 3rd ed.; Reinhold Publishing Corp.: New York, NY, USA, 1957. [Google Scholar]
- Wright, M.R. An Introduction to Aqueous Electrolyte Solutions; WILEY: Chichester, UK, 2007. [Google Scholar]
- Holze, R. Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology—New Series; Group IV: Physical Chemistry; Volume 9: Electrochemistry, Sub-Volume B: Ionic Conductivities of Liquid Systems, Part 1: Molten Salts and Ionic Liquids; Martienssen, W., Lechner, M.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Holze, R. Between the Electrodes of a Supercapacitor: An Update on Electrolytes. Adv. Mater. Sci. Technol. 2024, 6, 0627771. [Google Scholar] [CrossRef]
- Fan, X.; Zhong, C.; Liu, J.; Ding, J.; Deng, Y.; Han, X.; Zhang, L.; Hu, W.; Wilkinson, D.P.; Zhang, J. Opportunities of Flexible and Portable Electrochemical Devices for Energy Storage: Expanding the Spotlight onto Semi-solid/Solid Electrolytes. Chem. Rev. 2022, 122, 17155–17239. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Holze, R. Polymer Electrolytes for Supercapacitors. Polymers 2024, 16, 3164. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; He, Q.; Zhou, J.; Chang, Z.; Pan, A.; Zhou, H. Solid-State Electrolytes and Electrode/Electrolyte Interfaces in Rechargeable Batteries. ChemSusChem 2024, 17, e202301268. [Google Scholar] [CrossRef]
- Murugan, R.; Weppner, W. (Eds.) Solid Electrolytes for Advanced Applications; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Mallela, V.S.; Ilankumaran, V.; Rao, N.S. Trends in cardiac pacemaker batteries. Ind. Pac. Electrophysiol. J. 2004, 4, 201–212. [Google Scholar]
- Liang, C.C. Conduction characteristics of lithium iodide aluminum oxide solid electrolytes. J. Electrochem. Soc. 1973, 120, 1289–1292. [Google Scholar] [CrossRef]
- Liang, C.C.; Joshi, A.V.; Hamilton, N.E. Solid-state storage batteries. J. Appl. Electrochem. 1978, 8, 445–454. [Google Scholar] [CrossRef]
- Shahi, K.; Wagner, J.B. Ionic-conductivity and thermoelectric-power of pure and Al2O3-dispersed Agl. J. Electrochem. Soc. 1981, 128, 6–13. [Google Scholar] [CrossRef]
- Phipps, J.B.; Whitmore, D.J. Interfacial conduction in lithium iodide containing inert oxides. J. Power Sources 1983, 9, 373–378. [Google Scholar] [CrossRef]
- Phipps, J.B.; Whitmore, D.H. Ion-transport in LiI-SiO2 composites. Solid State Ion. 1983, 9–10, 123–130. [Google Scholar] [CrossRef]
- Nakamura, O.; Goodenough, J.B. Conductivity enhancement of lithium bromide monohydrate by AL2O3 particles. Solid State Ion. 1982, 7, 119–123. [Google Scholar] [CrossRef]
- Jow, T.; Wagner, J.B. Effect of dispersed alumina particles on the electrical-conductivity of cuprous chloride. J. Electrochem. Soc. 1979, 126, 1963–1972. [Google Scholar] [CrossRef]
- Maier, J. Space-charge regions in solid 2-phase systems and their conduction contribution—I. Conductance enhancement in the system ionic conductor-inert phase and application on agcl-AL2O3 and agcl-SIO2. J. Phys. Chem. Sol. 1985, 46, 309–320. [Google Scholar] [CrossRef]
- Bunde, A.; Dietrich, W.; Roman, E. Dispersed ionic conductors and percolation theory. Phys. Rev. Lett. 1985, 55, 5–8. [Google Scholar] [CrossRef]
- Wieczorek, W.; Stevens, J.R.; Florjańczyk, Z. Composite polyether based solid electrolytes. The Lewis acid-base approach. Solid State Ion. 1996, 85, 67–72. [Google Scholar] [CrossRef]
- Jander, W. Neuere Forschungen über Diffusion und elektrische Leitfähigkeit fester Salze. Z. Angew. Chem. 1929, 42, 462–467. [Google Scholar] [CrossRef]
- Hu, C.; Shen, Y.; Shen, M.; Liu, X.; Chen, H.; Liu, C.; Kang, T.; Jin, F.; Li, L.; Li, J.; et al. Superionic Conductors via Bulk Interfacial Conduction. J. Am. Chem. Soc. 2020, 142, 18035–18041. [Google Scholar] [CrossRef]
- Burazer, S.; Popovic, J. Mechanochemical Synthesis of Solid-State Electrolytes. Inorganics 2024, 12, 54. [Google Scholar] [CrossRef]
- Wright, P.V. Electrical Conductivity in Ionic Complexes of Poly(ethylene oxide). Br. Polym. J. 1975, 7, 319–327. [Google Scholar] [CrossRef]
- Weston, J.E.; Steele, B.H. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly(ethylene oxide) polymer electrolytes. Solid State Ion. 1982, 7, 75–79. [Google Scholar] [CrossRef]
- Croce, F.; Curini, R.; Martinelli, A.; Persi, L.; Ronci, F.; Scrosati, B.; Caminiti, R. Physical and chemical properties of nanocomposite polymer electrolytes. J. Phys. Chem. B 1999, 103, 10632–10638. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Croce, F.; Persi, L.; Ronci, F.; Scrosati, B. Transport and interfacial properties of composite polymer electrolytes. Electrochim. Acta 2000, 45, 1481–1490. [Google Scholar]
- Croce, F.; Persi, L.; Scrosati, B.; Serraino-Fiory, F.; Plichta, E.; Hendrickson, M.A. Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrochim. Acta 2001, 46, 2457–2461. [Google Scholar]
- Popovic, J.; Brandell, D.; Ohno, S.; Hatzell, K.B.; Zheng, J.; Hu, Y.Y. Polymer-based hybrid battery electrolytes: Theoretical insights, recent advances and challenges. J. Mater. Chem. A 2021, 9, 6050–6069. [Google Scholar] [CrossRef]
- Kim, J.K.; Lim, Y.J.; Kim, H.; Cho, G.B.; Kim, Y. A hybrid solid electrolyte for flexible solid-state sodium batteries. Energy Environ. Sci. 2015, 8, 3589–3596. [Google Scholar] [CrossRef]
- Patel, D.; Dharmesh, A.; Sharma, Y.; Rani, P.; Sharma, A.K. Hybrid electrolyte with biomass-derived carbon host for high-performance aqueous Zn-S battery. Chem. Eng. J. 2024, 479, 147722. [Google Scholar]
- Toprakci, H.A.K.; Toprakci, O. Recent Advances in New-Generation Electrolytes for Sodium-Ion Batteries. Energies 2023, 16, 3169. [Google Scholar] [CrossRef]
- Peljo, P.; Girault, H.H. Electrochemical potential window of battery electrolytes: The HOMO-LUMO misconception. Energy Environ. Sci. 2018, 11, 2306–2309. [Google Scholar] [CrossRef]
- García, Y.; O’Dell, L.A. Understanding the interfacial region in organic ionic plastic crystal composite electrolyte materials by solid-state NMR. Curr. Opin. Colloid Interf. Sci. 2022, 61, 101632. [Google Scholar]
- Patil, A.M.; You, H.M.; Jadhav, A.A.; Hong, J.; Das, S.K.; Dhas, S.D.; Lim, T.J.; Lee, E.; Chung, K.Y.; Kim, K.; et al. Dual Strategies of Na+ Electrolyte Additives and Dendrites Protective Ti3C2TX-MXene/Zn Anode with 2D MXene Nanosheet Encased Niobium Pyrophosphate (NbP2O7) Composite Binder-Free Cathode for Stable Zinc-Ion Storage. Adv. Energy Mater. 2025, 15, 2403322. [Google Scholar] [CrossRef]
- Hu, X.; Matios, E.; Zhang, Y.; Wang, C.; Luo, J.; Li, W. Deeply Cycled Sodium Metal Anodes at Low Temperature and in Lean Electrolyte Conditions. Angew. Chem. Int. Ed. 2021, 60, 5978–5983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Zhao, F.; Gu, C.; Liu, Y.; Sun, Q. Effect of Na2SiO3 and TEA Electrolyte Additives on the Corrosion Inhibition of Zinc Electrodes and Electrochemical Performance of Rechargeable Alkaline Manganese Batteries. J. Electrochem. Soc. 2023, 170, 110507. [Google Scholar] [CrossRef]
- Getie, F.A.; Ayele, D.W.; Habtu, N.G.; Yemata, T.A.; Yihun, F.A.; Ambaw, M.D.; Worku, A.K. Development of zinc nitrate hexahydrate salt (ZNH)/ethylene glycol (EG) binary composite deep eutectic electrolytes. Int. J. Electrochem. Sci. 2024, 19, 100616. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, X.; Li, J.; Yang, L.; Cheng, S. Achievement of advanced alkaline Ni-Zn batteries with good durability at a high areal capacity of 10 mAh cm−2 via the synergistic regulation of dual electrolyte additives. Chem. Eng. J. 2024, 499, 155757. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Z.; Cai, J.; Fan, D.; Zhao, F. Effect of Na2SiO3 and SDBS electrolyte additives on corrosion inhibition of zinc electrodes and electrochemical performance of zinc-air batteries. J. Mater. Sci. Mater. Electron. 2023, 34, 697. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, N.; Liu, J.; Guo, X.; Chen, X.; Zhou, Z.; Zhang, Z.; Li, G. Activation mechanism of conventional electrolytes with amine solvents: Species evolution and hydride-containing interphase formation. J. Energy Chem. 2024, 98, 615–622. [Google Scholar] [CrossRef]
- Qi, Y.; Xia, Y. Electrolyte Regulation Strategies for Improving the Electrochemical Performance of Aqueous Zinc-Ion Battery Cathodes. Acta Phys. Chim. Sin. 2023, 39, 2205045. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, Z.; Xu, C.; Deng, Y.; Jia, Y.; Luo, H.; Wu, H.; Cai, W.; Zhang, Y. Electrolyte Engineering via Competitive Solvation Structures for Developing Longevous Zinc Ion Batteries. Adv. Energy Mater. 2023, 13, 2302749. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, E.; Sung, Y.E.; Yu, S.H. Side Reaction Pathway Modulation for Hydrogen Evolution-Free Aqueous Zn-Ion Batteries. Adv. Funct. Mater. 2025, 35, e12884. [Google Scholar] [CrossRef]
- Chen, L.; Zhong, F.; Zan, J. Discharge property and voltage delay of AZ31B Mg alloy in Mg(NO3)2/Mg(ClO4)2 composite electrolyte. J. Chin. Soc. Corros. Prot. 2018, 38, 197–202. [Google Scholar]
- Chen, C.; Chen, J.; Tan, S.; Gao, Z.; Huang, X.; He, Z.; Huang, J.; Deng, R.; Xiong, F.; Huang, G.; et al. Semi-Chelation Solvation Enables High Interface Compatibilities with both Anode and Cathode in a Bulky Mg[B(HFIP)4]2 Electrolyte. Adv. Funct. Mater. 2025, 35, 2505843. [Google Scholar] [CrossRef]
- Dingzheng Li, C.; Li, W.; Liu, H.; Bu, X.; Zhang, T.; Li, J.; Zhang, M.; Kong, X.; Wang, C.; Wang, X. Xu Constructing a Multifunctional SEI Layer Enhancing Kinetics and Stabilizing Zinc Metal Anode. Adv. Funct. Mater. 2025, 35, 2415107. [Google Scholar]
- Chen, L.B.; Han, Y.P.; Wang, Z.W.; Li, Q.H.; Zhang, J. Outer Helmholtz Plane Regulation and In Situ Zn Surface Reconstruction for Highly Reversible Zn Anodes. Adv. Funct. Mater. 2023, 33, 2308023. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, C.; Xie, Y.; Nan, Q.; Gao, Y.; Li, F.; Rao, P.; Li, J.; Tian, X.; Shi, X. Inorganic Electrolyte Additive Promoting the Interfacial Stability for Durable Zn-Ion Batteries. Small 2024, 20, 2404237. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Li, X.; Zhou, Y.; Wang, Z.; Wu, R.; Zhou, H.; Meng, C.; Xue, Y.; Khan, I.; Yuan, A. Enhancing Zn Anode Reversibility via Solvation Structure Reconstruction by a Trimethyl Phosphate Additive. ACS Appl. Energy Mater. 2024, 7, 4385–4393. [Google Scholar] [CrossRef]
- Trivedi, M. The promise beyond lithium-ion technology: Dream vs. reality. Bunsen-Mag. 2025, 27, 201–203. [Google Scholar]
- Yuan, X.; Fu, L.; Liu, L.; Wu, Y. Solid-state batteries: Facts and fiction. Bunsen-Mag. 2025, 27, 198–200. [Google Scholar]
- Kulova, T.L.; Fateev, V.N.; Seregina, E.A.; Grigoriev, A.S. A brief review of post-lithium-ion batteries. Int. J. Electrochem. Sci. 2020, 15, 7242–7259. [Google Scholar] [CrossRef]
- Trivedi, M.; Holze, R. Composite Electrolytes for Lithium-ion Batteries. Energy Storage Appl. to be submitted.
- Ye, T.; Li, L.; Zhang, Y. Recent Progress in Solid Electrolytes for Energy Storage Devices. Adv. Funct. Mater. 2020, 30, 2000077. [Google Scholar] [CrossRef]
- Srivastava, S.; Schaefer, J.L.; Yang, Z.; Tu, Z.; Archer, L.A. 25th Anniversary article: Polymer-particle composites: Phase stability and applications in electrochemical energy storage. Adv. Mater. 2014, 26, 201–234. [Google Scholar] [CrossRef]
- Werner, W.; Heinz, S. Solid state ionics-87. In Proceedings of the 6th International Conference on Solid State Ionics, Garmisch-Partenkirchen, Germany, 6–11 September 1987; Volume 28–30, pp. 3–1783. [Google Scholar]
- Bloom, I.; Nelson, P.A.; Redey, L.; Orth, S.K.; Hammer, C.L.; Skocypec, R.S.; Dees, D.W.; Hash, M.C.; Vissers, D.R. Design considerations for the development of advanced sodium/metal-chloride cells. In Proceedings of the 25th Intersociety Energy Conversion Engineering Conference, Reno, NV, USA, 12–17 August 1990; Volume 3, pp. 341–347. [Google Scholar]
- Dalas, E.; Tsamouras, D.; Bouropoulos, N. Primary Solid State Batteries. Ionics 1995, 1, 235–241. [Google Scholar] [CrossRef]
- Park, K.; Cho, J.H.; Shanmuganathan, K.; Song, J.; Peng, J.; Gobet, M.; Greenbaum, S.; Ellison, C.J.; Goodenough, J.B. New battery strategies with a polymer/Al2O3 separator. J. Power Sources 2014, 263, 52–58. [Google Scholar]
- Wei, Z.; Huang, B.; Song, L.; Chen, Y.; Du, P.; Zhu, H.; Xiong, J.; Guo, Y. A robust, efficient ion transport polytetrafluoroethylene fibrous membrane-based separator with superior stability for ultralong-life zinc ion hybrid supercapacitors. J. Mater. Chem. C 2025, 13, 2388–2398. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, H.; Sun, C.; Ma, Z.; Zuo, X.; Nan, J. Paper-Based Composite Separator with Thermotolerant and Flame-Retardant Sodium Silicate Sheath for High-Performance Sodium-Ion Batteries. ACS Appl. Energy Mater. 2024, 7, 1212–1223. [Google Scholar] [CrossRef]
- Ford, H.O.; Merrill, L.C.; He, P.; Upadhyay, S.P.; Schaefer, J.L. Cross-Linked lonomer Gel Separators for Polysulfide Shuttle Mitigation in Magnesium-Sulfur Batteries: Elucidation of Structure-Property Relationships. Macromolecules 2018, 51, 8629–8636. [Google Scholar]
- Cao, J.; Zhang, D.; Gu, C.; Zhang, X.; Okhawilai, M.; Wang, S.; Han, J.; Qin, J.; Huang, Y. Modulating Zn deposition via ceramic-cellulose separator with interfacial polarization effect for durable zinc anode. Nano Energy 2021, 89, 106322. [Google Scholar] [CrossRef]
- Sun, Y.; Jian, Q.; Wang, T.; Liu, B.; Wan, Y.; Sun, J.; Zhao, T. A Janus separator towards dendrite-free and stable zinc anodes for long-duration aqueous zinc ion batteries. J. Energy Chem. 2023, 81, 583–592. [Google Scholar] [CrossRef]
- Nan, S.; Wei, W.; Su, X.; Zhang, Q.; Qian, L.; Dong, Y.; He, R. Imidazole-rich polymer modified glass fiber separators with low electrolyte uptake for dendrite-free and corrosion-free zinc metal batteries. Chem. Eng. J. 2025, 504, 158634. [Google Scholar] [CrossRef]
- Lei, Y.; Li, Q.; Liu, Q.; Zeng, Y.; Li, J.; Huang, W.; Wang, F.; Zhong, S.; Yan, D. Low-cost separator with dust-free fabric composite cellulose acetate toward stable dendrite-free aqueous zinc-ion batteries. Chem. Eng. J. 2024, 479, 147846. [Google Scholar] [CrossRef]
- Xiao, W.; Zhao, L.; Gong, Y.; Wang, H.; Liu, J.; Yan, C. Preparation and properties of composite separators with asymmetric structures for alkaline batteries. RSC Adv. 2015, 5, 54742–54748. [Google Scholar] [CrossRef]
- Suppanucroa, N.; Yoopensuk, W.; Thanapong-A-Morn, W.; Rukkachat, K.; Pakawanit, P.; Wu, H.L.; Mahlendorf, F.; Balz, L.; Kheawhom, S.; Somwangthanaroj, A. Quaternized PVA-MoS2 composite separator: Enhancing long-term stability and ion transport in secondary zinc-air batteries. J. Energy Storage 2025, 121, 116497. [Google Scholar]
- Cao, Y.; Bian, Y.; Xu, Y.; Fu, Q.; Zhao, P.; Sun, Y. Facile preparation of aqueous zinc-ion batteries with excellent long cycle life and anti-freezing performance. J. Energy Storage 2025, 132, 117838. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Xu, H.; Jiang, Y.; Li, F.; Xue, B. An expanded clay-coated separator with unique microporous structure for enhancing electrochemical performance of rechargeable hybrid aqueous batteries. J. Solid State Electr. 2019, 23, 215–226. [Google Scholar]
- Jana, K.K.; Lue, S.J.; Huang, A.; Soesanto, J.F.; Tung, K.L. Separator Membranes for High Energy-Density Batteries. ChemBioEng Rev. 2018, 5, 346–371. [Google Scholar] [CrossRef]
- Xu, W.; Liao, X.; Xu, W.; Zhao, K.; Yao, G.; Wu, Q. Ion Selective and Water Resistant Cellulose Nanofiber/MXene Membrane Enabled Cycling Zn Anode at High Currents. Adv. Energy Mater. 2023, 13, 2300283. [Google Scholar] [CrossRef]
- Akram, M.Z.; Qaiser, A.A.; Abid, N.; Shehzad, M.A. Electro-ionically conductive functional separators for fast ion transport in aqueous zinc ion batteries: Preparation and characterization. Mater. Chem. Phys. 2025, 340, 130863. [Google Scholar] [CrossRef]
- Li, W.; Song, Q.; Dong, Q.; Zhang, J.; Wang, J.; Wu, Y.; Yu, Y.; Li, X. Proton Storage Chemistry in Aqueous Zinc-Inorganic Batteries with Moderate Electrolytes. Adv. Mater. 2025, 37, 2414019. [Google Scholar] [CrossRef]
- Chen, T.Y.; Lin, T.J.; Vedhanarayanan, B.; Shen, H.H.; Lin, T.W. Optimization of acetamide based deep eutectic solvents with dual cations for high performance and low temperature-tolerant aqueous zinc ion batteries via tuning the ratio of co-solvents. J. Colloid Interface Sci. 2023, 629, 166–178. [Google Scholar]
- Li, M.H.; Li, C.; Lu, G.J.; Wang, R.H.; Luo, Z.C.; Li, Z.Y.; Deng, R.R.; Zheng, W.K.; Wang, W.J.; Fang, Y.H.; et al. Manipulation of the Anode Interphase by Multicomponent Composite Sodium for Fast-Charging and Low-Temperature Sodium Metal Batteries. Adv. Funct. Mater. 2025, 35, 2422892. [Google Scholar]
- Li, W.; Zhao, L.; Guo, J.; Zhu, H.; Liu, W.; Kong, W.; Ilyas, F.; Han, X.; Cui, L.; Wen, Z. Turning crisis into opportunity: Intrinsically polarised low concentration eutectic electrolytes enable highly reversible zinc anodes. Energy Storage Mater. 2024, 73, 103866. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, S.; Xu, S.; Ma, Z.F.; Wang, J.; Yuan, X. Highly effective solid electrolyte interface on SnO2@C enabling stable potassium storage performance. Chem. Eng. J. 2022, 446, 137265. [Google Scholar]
- Guo, X.; Peng, Q.; Yang, R.; Cao, G.; Wen, J.; Shin, K.; Zheng, Y.; Tunmee, S.; Zou, C.; Zheng, Y.; et al. Non-coordinating charge transfer enables ultrafast desolvation of hydrated zinc ions in the outer Helmholtz layer for stable aqueous Zn metal batteries. Nat. Sci. Rev. 2025, 12, nwaf070. [Google Scholar]
- Guo, X.; Peng, Q.; Shin, K.; Zheng, Y.; Tunmee, S.; Zou, C.; Zhou, X.; Tang, Y. Construction of a Composite Sn-DLC Artificial Protective Layer with Hierarchical Interfacial Coupling Based on Gradient Coating Technology Toward Robust Anodes for Zn Metal Batteries. Adv. Energy Mater. 2024, 14, 2402015. [Google Scholar] [CrossRef]
- Aupama, V.; Sangsawang, J.; Kao-Ian, W.; Wannapaiboon, S.; Pimoei, J.; Yoopensuk, W.; Opchoei, M.; Tehrani, Z.; Margadonna, S.; Kheawhom, S. Adaptive COF-PVDF composite artificial solid electrolyte interphase for stable aqueous zinc batteries. Electrochim. Acta 2024, 506, 145059. [Google Scholar] [CrossRef]
- Song, H.W.; Su, J.; Wang, C.X. Hybrid Solid Electrolyte Interphases Enabled Ultralong Life Ca-Metal Batteries Working at Room Temperature. Adv. Mater. 2021, 33, 2006141. [Google Scholar] [CrossRef] [PubMed]
- Rajput, N.N.; Qu, X.; Sa, N.; Burrell, A.K.; Persson, K.A. The Coupling between Stability and Ion Pair Formation in Magnesium Electrolytes from First-Principles Quantum Mechanics and Classical Molecular Dynamics. J. Am. Chem. Soc. 2015, 137, 3411–3420. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, D.; Sun, R.; Gao, Y.; Wang, D.; Li, Z.; Kang, X. Multifunctionalized Supramolecular Cyclodextrin Additives Boosting the Durability of Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2024, 16, 17626–17636. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar]
- Wei, L.; Fan, Y. Progress of deep eutectic solvents and their applications. Chem. Bull. 2011, 74, 333. [Google Scholar]
- Taneja, N.; Kumar, A.; Gupta, P.; Gupta, M.; Singh, P.; Agrawal, N.; Bocchetta, P.; Kumar, Y. Advancements in liquid and solid electrolytes for their utilization in electrochemical systems. J. Energy Storage 2022, 56, 105950. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environm. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar]
- Wu, J.; Liang, Q.; Yu, X.; Lu, Q.F.; Ma, L.; Qin, X.; Chen, G.; Li, B. Deep Eutectic Solvents for Boosting Electrochemical Energy Storage and Conversion: A Review and Perspective. Adv. Energy Mater. 2021, 31, 2011102. [Google Scholar]
- Elia, G.A.; Kravchyk, K.V.; Kovalenko, M.V.; Chacon, J.; Holland, A.; Wills, R.G.A. An overview and prospective on Al and Al-ion battery technologies. J. Power Sources 2021, 481, 228870. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, H.; Sun, P.; Liu, J.; Li, Q.; Lu, P.; Xu, Q. Recent research progress of redox flow batteries based on deep eutectic solvents (DESs). Int. J. Green Energy 2022, 22, 947–957. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, R.; Thakur, R.C.; Singh, L. An overview of deep eutectic solvents: Alternative for organic electrolytes, aqueous systems & ionic liquids for electrochemical energy storage. J. Energy Chem. 2023, 82, 592–626. [Google Scholar] [CrossRef]
- Al-Farsi, R.; Hayyan, M. Paving the way for advancement of renewable energy technologies using deep eutectic solvents: A review. Renew. Sust. Energy Rev. 2023, 184, 113505. [Google Scholar]
- Liu, Z.; Feng, F.; Feng, W.; Wang, G.; Qi, B.; Gong, M.; Zhang, F.; Pang, H. Eutectic electrolytes: A new platform for high-safety batteries. Energy Environ. Sci. 2025, 18, 3568–3613. [Google Scholar] [CrossRef]
- Kawakami, H. Development of composite electrolyte membranes with functional polymer nanofiber frameworks. Polym. J. 2025, 57, 623–633. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, D.; Lee, S.Y. Lignocellulosics as a Green Material Opportunity for Energy Storage Systems. In Lignocellulosics: Renewable Feedstock for (Tailored) Functional Materials and Nanotechnology; Filpponen, I., Peresin, M., Nypelo, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 297–343. [Google Scholar]
- Xu, T.; Du, H.; Liu, H.; Liu, W.; Zhang, X.; Si, C.; Liu, P.; Zhang, K. Advanced Nanocellulose-Based Composites for Flexible Functional Energy Storage Devices. Adv. Mater. 2021, 33, 2101368. [Google Scholar] [CrossRef]
- Farooq, A.; Wanyan, H.; Lu, S.; Mosisa, M.T.; Zhou, X.; Xiao, H.; Liu, K.; Huang, L.; Chen, L.; Wu, H. A review on cellulose-based derivatives and composites for sustainable rechargeable batteries. Int. J. Biol. Macromol. 2025, 308, 142528. [Google Scholar] [CrossRef]
- Javed, M.U.; Muhammad, S.; Wang, Z.; Li, J.; Wu, X.; Chukwuka, A.; Zhang, Y.; Wang, K.; Guo, B. Metal-Organic Framework/Polymer Composites for Solid State Electrolytes—A Critical Review. J. Power Sources 2025, 640, 236720. [Google Scholar] [CrossRef]
- Nestler, T.; Roedern, E.; Uvarov, N.F.; Hanzig, J.; Elia, G.A.; De Vivanco, M. Separators and electrolytes for rechargeable batteries: Fundamentals and perspectives. Phys. Sci. Rev. 2019, 4, 20170115. [Google Scholar] [CrossRef]
- Neha; Dalvi, A. Fast ionic PEO-NaCF3SO3-Na3Zr2Si2P3O12 membranes for all-solid-state energy storage devices. Mater. Sci. Eng. B 2023, 289, 116252. [Google Scholar] [CrossRef]
- Sharma, J.; Hashmi, S. Magnesium ion-conducting gel polymer electrolyte nanocomposites: Effect of active and passive nanofillers. Polym. Comp. 2019, 40, 1295–1306. [Google Scholar] [CrossRef]
- Chavan, C.; Bhajantri, R.F.; Bulla, S.; Ravikumar, H.B.; Raghavendra, M.; Sakthipandi, K.; Yogesh Kumar, K.; Prasanna, B.P. Ion dynamics and positron annihilation studies on polymer ceramic composite electrolyte system (PVA/NaClO4/Y2O3): Application in electrochemical devices. Ceram. Int. 2022, 48, 17864–17884. [Google Scholar] [CrossRef]
- Yoshizawa-Fujita, M.; Ota, R.; Ishii, J.; Takeoka, Y.; Rikukawa, M. Ion Conductive Behavior of Oligoether/Zwitterion Diblock Copolymers Containing Magnesium Salt. Macromol. Chem. Phys. 2022, 223, 2100363. [Google Scholar] [CrossRef]
- Bruce, P.G.; Vincent, C.A. Polymer electrolytes. J. Chem. Soc. Farad. Trans. 1993, 89, 3187–3203. [Google Scholar] [CrossRef]
- Kundu, S.; Ein-Eli, Y. A review on design considerations in polymer and polymer composite solid-state electrolytes for solid Li batteries. J. Power Sources 2023, 553, 232267. [Google Scholar] [CrossRef]
- Alvarez-Fernandez, A.; Hernández, G.; Maiz, J. Unlocking Solid Polymer Electrolytes: Advancing Materials through Characterization-Driven Insights. JACS Au 2025, 5, 3701–3715. [Google Scholar] [CrossRef]
- Inamuddin; Ahamed, M.I.; Boddula, R.; Altalhi, T. (Eds.) Polymers in Energy Conversion and Storage; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Xi, G.; Xiao, M.; Wang, S.; Han, D.; Li, Y.; Meng, Y. Polymer-Based Solid Electrolytes: Material Selection, Design, and Application. Adv. Funct. Mater. 2021, 31, 2007598. [Google Scholar] [CrossRef]
- Bakar, R.; Darvishi, S.; Senses, E. Enhanced ionic conductivity and mechanical strength in nanocomposite electrolytes with nonlinear polymer architectures. Turk. J. Chem. 2023, 47, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, S.J.; Kim, S. Thermal and electrical conducting property of sodium polymer electrolyte containing barium titanate filler. J. Nanosci. Nanotechnol. 2017, 17, 5768–5770. [Google Scholar] [CrossRef]
- Capiglia, C.; Yang, J.; Imanishi, N.; Hirano, A.; Takeda, Y.; Yamamoto, O. Composite polymer electrolyte: The role of filler grain size. Solid State Ion. 2002, 154–155, 7–14. [Google Scholar] [CrossRef]
- Arya, A.; Sadiq, M.; Sharma, A.L. Effect of variation of different nanofillers on structural, electrical, dielectric, and transport properties of blend polymer nanocomposites. Ionics 2018, 24, 2295–2319. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, I.; Ur Rahman, M.; Khan, H.; Shah, A.B.; Althomali, R.H.; Rahman, M.M. Inorganic-polymer composite electrolytes: Basics, fabrications, challenges and future perspectives. Rev. Inorg. Chem. 2024, 44, 347–375. [Google Scholar] [CrossRef]
- Syzdek, J.S.; Marcinek, M.; Marczewski, M.; Smoliński, M.; Szczęsna-Chrzan, A.; Zalewska, A.; Wieczorek, W. Structural and conductivity investigations of composite polymer electrolytes based on poly(oxyethylene). Appl. Phys. A 2023, 129, 74. [Google Scholar] [CrossRef]
- Syzdek, J.; Armand, M.; Marcinek, M.; Zalewska, A.; Zukowska, G.; Wieczorek, W. Detailed studies on the fillers modification and their influence on composite, poly(oxyethylene)-based polymeric electrolytes. Electrochim. Acta 2010, 55, 1314–1322. [Google Scholar] [CrossRef]
- Kumar, G.; Singh, R.; Chand, P.; Kumar, M.; Singh, R.R.; Kumar, A. A review on polymer electrolyte materials in context to modifications in PVDF-HFP polymer host. J. Solid State Electrochem. 2025, 29, 4493–4528. [Google Scholar] [CrossRef]
- Wang, W.M. Preparation and characterization of composite polymer electrolyte. Adv. Mater. Res. 2012, 571, 17–21. [Google Scholar] [CrossRef]
- Vedhanarayanan, B.; Lakshmi, K.C.S.; Lin, T.W. Interfacial Tuning of Polymeric Composite Materials for High-Performance Energy Devices. Batteries 2023, 9, 487. [Google Scholar] [CrossRef]
- Teramoto, D.; Yokoyama, R.; Kagawa, H.; Sada, T.; Ogata, N. A Novel Ionic Liquid-Polymer Electrolyte for the Advanced Lithium Ion Polymer Battery. In Molten Salts and Ionic Liquids: Never the Twain? Gaune-Escard, M., Seddon, K.R., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 367–388. [Google Scholar]
- Xi, J.; Qiu, X.; Zhu, W.; Tang, X. Enhanced electrochemical properties of poly(ethylene oxide)-based composite polymer electrolyte with ordered mesoporous materials for lithium polymer battery. Micropor. Mesopor. Mater. 2006, 88, 1–7. [Google Scholar] [CrossRef]
- Langer, F.; Kun, R.; Schwenzel, J. Li7La3Zr2O12 and poly(Ethylene Oxide) based composite electrolytes. In Solid Electrolytes for Advanced Applications; Murugan, R., Weppner, W., Eds.; Springer: Cham, Switzerland, 2019; pp. 195–215. [Google Scholar]
- Huang, Y.; Mei, X.; Guo, Y. Segmental and interfacial dynamics quantitatively determine ion transport in solid polymer composite electrolytes. J. Appl. Polym. Sci. 2022, 139, e52143. [Google Scholar] [CrossRef]
- Buannic, L.; Naviroj, M.; Miller, S.M.; Zagorski, J.; Faber, K.T.; Llordés, A. Dense freeze-cast Li7La3Zr2O12 solid electrolytes with oriented open porosity and contiguous ceramic scaffold. J. Am. Ceram. Soc. 2019, 102, 1021–1029. [Google Scholar] [CrossRef]
- Fan, L.Z.; He, H.; Nan, C.W. Tailoring inorganic-polymer composites for the mass production of solid-state batteries. Nat. Rev. Mater. 2021, 6, 1003–1019. [Google Scholar] [CrossRef]
- Kumar, B.; Scanlon, L.G. Polymer-ceramic composite electrolytes. J. Power Sources 1994, 52, 261–268. [Google Scholar] [CrossRef]
- Kumar, B.; Scanlon, L.G. Polymer-ceramic composite electrolytes: Conductivity and thermal history effects. Solid State Ion. 1999, 124, 239–254. [Google Scholar] [CrossRef]
- Tao, K.; Wang, Z.; Han, Y.; Li, J. Rapid discovery of inorganic-organic solid composite electrolytes by unsupervised learning. Chem. Eng. J. 2023, 454, 140151. [Google Scholar] [CrossRef]
- Xiong, Y.; Lin, Z.; Li, J.; Li, Z.; Cheng, A.; Zhang, X. Design of Low-Resistance Composite Electrolytes for Solid-State Batteries Based on Machine Learning. Acta Mech. Solida Sin. 2025, 38, 549–557. [Google Scholar] [CrossRef]
- Best, A.S.; Ferry, A.; MacFarlane, D.R.; Forsyth, M. Conductivity in amorphous polyether nanocomposite materials. Solid State Ion. 1999, 126, 269–276. [Google Scholar] [CrossRef]
- Bosquez-Caceres, M.F.; Hidalgo-Bonilla, S.; Cordova, V.M.; Michell, R.M.; Tafur, J.P. Nanocomposite Polymer Electrolytes for Zinc and Magnesium Batteries: From Synthetic to Biopolymers. Polymers 2021, 13, 4284. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, X.; Wang, H. Solid Composite Electrolytes for Solid-State Alkali Metal Batteries. ACS Symp. Ser. 2022, 1413, 395–423. [Google Scholar]
- Qi, D.; Jiang, Z.; Yu, J.; Gao, Z. Preparation process and application of novel solid-state composite polymer electrolytes. Mater. Rev. 2016, 30, 144–149. [Google Scholar]
- Jarosik, A.; Pfaffenhuber, C.; Bunde, A.; Maier, J. Electrochemical investigations of polyethylene glycol-based “soggy sand” electrolytes—From the local mechanism to the overall conduction. Adv. Funct. Mater. 2011, 21, 3961–3966. [Google Scholar] [CrossRef]
- Bhattacharyya, A.J.; Maier, J.; Bock, R.; Lange, F.F. New class of soft matter electrolytes obtained via heterogeneous doping: Percolation effects in “soggy sand” electrolytes. Solid State Ion. 2006, 177, 2565–2568. [Google Scholar] [CrossRef]
- Wang, R.; Dong, Q.; Wang, C.; Hong, M.; Gao, J.; Xie, H.; Guo, M.; Ping, W.; Wang, X.; He, S.; et al. High-Temperature Ultrafast Sintering: Exploiting a New Kinetic Region to Fabricate Porous Solid-State Electrolyte Scaffolds. Adv. Mater. 2021, 33, 2100726. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.M. Study on all solid-state composite polymer electrolyte. Adv. Mater. Res. 2012, 571, 13–16. [Google Scholar] [CrossRef]
- Yong, X. Research on the preparation & performance of polymer electrolyte composite materials. Adv. Mater. Res. 2012, 548, 68–72. [Google Scholar] [CrossRef]
- Shi, Y.; Tang, M. NMR/EPR investigation of rechargeable batteries. Acta Phys. Chim. Sin. 2019, 36, 1905004. [Google Scholar]
- Ran, L.; Tao, S.; Gentle, I.; Luo, B.; Li, M.; Rana, M.; Wang, L.; Knibbe, R. Stable Interfaces in a Sodium Metal-Free, Solid-State Sodium-Ion Battery with Gradient Composite Electrolyte. ACS Appl. Mater. Interfaces 2021, 13, 39355–39362. [Google Scholar] [CrossRef]
- Holze, R. Hot electrochemistry: Ceramics and solid ion conductors. Bunsen-Mag. 2025, 27, 211. [Google Scholar]
- Lowack, A.; Grun, P.; Anton, R.; Auer, H.; Nikolowski, K.; Partsch, M.; Kusnezoff, M.; Michaelis, A. Sputtered Zero-Excess Electrodes with Metallic Seed Layers for Solid-State Sodium Batteries. Batter. Supercaps 2024, 7, e202400364. [Google Scholar] [CrossRef]
- Zheng, X.; Bommier, C.; Luo, W.; Jiang, L.; Hao, Y.; Huang, Y. Sodium metal anodes for room-temperature sodium-ion batteries: Applications, challenges and solutions. Energy Stor. Mater. 2019, 16, 6–23. [Google Scholar] [CrossRef]
- Yang, T.Z.; Luo, D.; Liu, Y.Z.; Yu, A.P.; Chen, Z.W. Review Anode-free sodium metal batteries as rising stars for lithium-ion alternatives. iScience 2023, 26, 105982. [Google Scholar] [CrossRef]
- Heubner, C.; Maletti, S.; Auer, H.; Hüttl, J.; Voigt, K.; Lohrberg, O.; Nikolowski, K.; Partsch, M.; Michaelis, A. From Lithium-Metal toward Anode-Free Solid-State Batteries: Current Developments, Issues, and Challenges. Adv. Funct. Mater. 2021, 31, 2106608. [Google Scholar] [CrossRef]
- Ruoff, E.; Kmiec, S.; Manthiram, A. Enhanced Interfacial Conduction in Low-Cost NaAlCl4 Composite Solid Electrolyte for Solid-State Sodium Batteries. Adv. Energy Mater. 2024, 14, 2402091. [Google Scholar] [CrossRef]
- Huang, H.; Yang, H.; Yao, Y.; Yu, Y. Progress on Halide Solid Electrolytes in All-Solid-State Sodium Batteries. J. Chin. Ceram. Soc. 2025, 53, 1561–1576. [Google Scholar]
- Takayanagi, T.; Nasu, A.; Tsuji, F.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Mechanochemically Prepared Highly Conductive Na2.88Sb0.88W0.12S4-NaI Composite Electrolytes for All-Solid-State Sodium Battery. Electrochemistry 2022, 90, 047005. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, G.; Sun, M.; Li, X.; Wang, Z. Effects of addition of sodium titanophosphate glass on structure and physical properties of Na3Zr2Si2PO12/glass-ceramic solid composite electrolytes. Ceram. Int. 2025, 51, 11220–11230. [Google Scholar] [CrossRef]
- Shao, Y.; Zhong, G.; Lu, Y.; Liu, L.; Zhao, C.; Zhang, Q.; Hu, Y.S.; Yang, Y.; Chen, L. A novel NASICON-based glass-ceramic composite electrolyte with enhanced Na-ion conductivity. Energy Storage Mater. 2019, 23, 514–521. [Google Scholar] [CrossRef]
- Johari, N.S.M.; Adnan, S.B.R.S.; Ahmad, N. Sodium-ion nanoionic hybrid solid electrolyte: Extended study on enhanced electrical and electrochemical properties. Solid State Ion. 2022, 377, 115882. [Google Scholar]
- Mandal, S.; Tom, C.; Singh, Y.; Khoder, H.; Launois, P.; Paineau, E.; Grage, S.L.; Schaller, M.; Alwarappan, S.; Singh, R.S.; et al. Domain Size Modulation of Clay–Nanocellulose Composites for Enhanced Na-Ion Transport. ACS Appl. Energy Mater. 2025, 8, 6071–6086. [Google Scholar] [CrossRef]
- He, L.; Wang, Z.; Li, Y.; Lin, H.; Li, J.; Cheng, T.; Zhu, Q.; Shang, C.; Lu, Z.; Floriano, R.; et al. Electrolyte precursor-free approach to prepare composite electrolyte for all-solid-state Na-ion battery. Mater. Today Chem. 2023, 31, 101607. [Google Scholar] [CrossRef]
- Rathore, M.; Dalvi, A. Electrical Characterization of PVA-MgSO4 and PVA-Li2SO4 Polymer Salt Composite Electrolytes. Mater. Today Proc. 2019, 10, 106–111. [Google Scholar] [CrossRef]
- Badr, S.; Sheha, E.; Bayomi, R.M.; El-Shaarawy, M.G. Structural and electrical properties of pure and H2SO4 doped (PVA)0.7(NaI)0.3 solid polymer electrolyte. Ionics 2010, 16, 269–275. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Aziz, S.B.; Saeed, S.R. Structural and electrical properties of polyvinyl alcohol (PVA):Methyl cellulose (MC) based solid polymer blend electrolytes inserted with sodium iodide (NaI) salt. Arab. J. Chem. 2021, 14, 103388. [Google Scholar] [CrossRef]
- Mei, X.; Wu, Y.; Gao, Y.; Zhu, Y.; Bo, S.H.; Guo, Y. A quantitative correlation between macromolecular crystallinity and ionic conductivity in polymer-ceramic composite solid electrolytes. Mater. Today Commun. 2020, 24, 101004. [Google Scholar] [CrossRef]
- Chen, G.; Ye, L.; Zhang, K.; Gao, M.; Lu, H.; Xu, H.; Bai, Y.; Wu, C. Hyperbranched polyether boosting ionic conductivity of polymer electrolytes for all-solid-state sodium ion batteries. Chem. Eng. J. 2020, 394, 124885. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, K.; Rong, X.; Hu, Y.S.; Li, H.; Huang, X.; Chen, L. Na3.4Zr1.8Mg0.2Si2PO12 filled poly(ethylene oxide)/Na(CF3SO2)2N as flexible composite polymer electrolyte for solid-state sodium batteries. J. Power Sources 2017, 372, 270–275. [Google Scholar] [CrossRef]
- Chiekezi, O.; He, X.; Wang, H.; Adebisi, T.R.; Ayodele, O.; Deng, T.; Reed, D.; Wang, C.; Lu, X. Highly Conductive Poly(ethylene oxide)-Based Composite Polymer Electrolyte for Sodium Battery Applications. ACS Appl. Energy Mater. 2023, 6, 8434–8442. [Google Scholar] [CrossRef]
- Qu, S.; Cai, G.; Qiao, X.; Li, G.; Sun, J. Enhancing Sodium-Ion Transport in LTA Zeolite/PEO Composite Polymer Electrolytes through Cation Adsorption. ACS Appl. Energy Mater. 2024, 7, 8964–8972. [Google Scholar] [CrossRef]
- Tian, W.; Lin, G.; Yuan, S.; Jin, T.; Wang, Q.; Jiao, L. Competitive Coordination and Dual Interphase Regulation of MOF-Modified Solid-State Polymer Electrolytes for High-Performance Sodium Metal Batteries. Angew. Chem. Int. Ed. 2025, 64, e202423075. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Jian, Z.; Liao, X.; Liao, W.; Huang, Y.; Chen, D.; Li, R.K.Y.; Liu, C. Tailored architecture of composite electrolyte for all-solid-state sodium batteries with superior rate performance and cycle life. Nano Res. 2024, 17, 4171–4180. [Google Scholar] [CrossRef]
- Ge, Z.; Li, J.; Liu, J. Enhanced electrochemical performance of all-solid-state sodium-sulfur batteries by PEONaCF3SO3-MIL-53(Al) solid electrolyte. Ionics 2020, 26, 1787–1795. [Google Scholar] [CrossRef]
- Shi, C.M.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; O’Neill, J.; Dura, J.A.; Wachsman, E.D. High Sulfur Loading and Capacity Retention in Bilayer Garnet Sulfurized-Polyacrylonitrile/Lithium-Metal Batteries with Gel Polymer Electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Peng, C.; Huang, S.; Shen, X.; Ding, J.; Luo, J.; Du, J.; Xia, Z.; Zhang, X.; Chen, J. An amphiphilic interface for constructing a uniform composite solid-state electrolyte towards long-life all-solid-state sodium metal batteries. J. Mater. Chem. A 2024, 12, 23485–23494. [Google Scholar] [CrossRef]
- Zhang, S.; Lee, J.Y.; Hong, L. Visualization of particle distribution in composite polymer electrolyte systems. J. Power Sources 2004, 126, 125–133. [Google Scholar] [CrossRef]
- Liu, D.; Xie, X.; Trivedi, M.; Chen, X.; Holze, R. Graphene-containing composite coatings for corrosion protection. J. Compos. Sci. 2025; to be submitted. [Google Scholar]
- Zhang, Z.; Zhang, Q.; Shi, J.; Chu, Y.S.; Yu, X.; Xu, K.; Ge, M.; Yan, H.; Li, W.; Gu, L.; et al. A Self-Forming Composite Electrolyte for Solid-State Sodium Battery with Ultralong Cycle Life. Adv. Energy Mater. 2017, 7, 1601196. [Google Scholar] [CrossRef]
- Yu, X.; Xue, L.; Goodenough, J.B.; Manthiram, A. Ambient-Temperature All-Solid-State Sodium Batteries with a Laminated Composite Electrolyte. Adv. Funct. Mater. 2021, 31, 2002144. [Google Scholar] [CrossRef]
- Guo, J.; Cai, L.; Wang, R.; Zhou, K.; Zhang, J.; Chen, S.; Liu, T. Facile Design of a Soft-Tough Asymmetric Composite Electrolyte for Stable All-Solid-State Sodium Batteries. ACS Sustain. Chem. Eng. 2025, 13, 8184–8192. [Google Scholar] [CrossRef]
- Serra Moreno, J.; Armand, M.; Berman, M.B.; Greenbaum, S.G.; Scrosati, B.; Panero, S. Composite PEOn:NaTFSI polymer electrolyte: Preparation, thermal and electrochemical characterization. J. Power Sources 2014, 248, 695–702. [Google Scholar] [CrossRef]
- Peta, G.; Bublil, S.; Alon-Yehezkel, H.; Breuer, O.; Elias, Y.; Shpigel, N.; Fayena-Greenstein, M.; Golodnitsky, D.; Aurbach, D. Toward high performance all solid-state Na batteries: Investigation of electrolytes comprising NaPF6, poly(ethylene oxide) and TiO2. J. Electrochem. Soc. 2021, 168, 110553. [Google Scholar] [CrossRef]
- Hashmi, S.A.; Chandra, S. Experimental investigations on a sodium-ion-conducting polymer electrolyte based on poly(ethylene oxide) complexed with NaPF6. Mater. Sci. Eng. B 1995, 34, 18–26. [Google Scholar] [CrossRef]
- Lailun Ni’mah, Y.; Cheng, M.Y.; Cheng, J.H.; Rick, J.; Hwang, B.J. Solid-state polymer nanocomposite electrolyte of TiO2/PEO/NaClO4 for sodium ion batteries. J. Power Sources 2015, 278, 375–381. [Google Scholar]
- Patel, M.; Chandrappa, K.G.; Bhattacharyya, A.J. Increasing ionic conductivity of polymer-sodium salt complex by addition of a non-ionic plastic crystal. Solid State Ion. 2010, 181, 844–848. [Google Scholar] [CrossRef]
- Alarco, P.J.; Abu-Lebdeh, Y.; Abouimrane, A.; Armand, M. The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors. Nat. Mater. 2004, 3, 476–481. [Google Scholar] [CrossRef]
- Sherwood, J. (Ed.) The Plastically Crystalline State; Wiley: London, UK, 1979. [Google Scholar]
- Li, H.; Ermolaev, A.V.; Pronin, A.S.; Zheng, J.; Huang, H.; Zhang, H.; Li, L.; An, B.; Mironov, Y.V.; Sun, C. Synthesis of a cubane-like anion [Re4As2S2(CN)12]6− for coordinate regulation of Na+ ion transport. Inorg. Chem. Front. 2024, 11, 6671–6678. [Google Scholar] [CrossRef]
- Luo, X.; Aguey-Zinsou, K.F. NaBH4-Poly(Ethylene Oxide) Composite Electrolyte for All-Solid-State Na-Ion Batteries. Batteries 2024, 10, 316. [Google Scholar] [CrossRef]
- Wang, L.; Han, W.; Ge, C.; Zhang, R.; Bai, Y.; Zhang, X. Functionalized Carboxyl Carbon/NaBOB Composite as Highly Conductive Electrolyte for Sodium Ion Batteries. ChemistrySelect 2018, 3, 9293–9300. [Google Scholar] [CrossRef]
- Song, S.; Yang, S.; Zheng, F.; Zeng, K.; Duong, H.M.; Savilov, S.V.; Aldoshin, S.M.; Hu, N.; Lu, L. Poly(ethylene oxide)-Immobilized Ionogel with High Ionic Liquid Loading and Superior Ionic Conductivity. J. Electrochem. Soc. 2016, 163, A2887–A2889. [Google Scholar] [CrossRef]
- Song, S.; Dong, Z.; Fernandez, C.; Wen, Z.; Hu, N.; Lu, L. Nanoporous ceramic-poly(ethylene oxide) composite electrolyte for sodium metal battery. Mater. Lett. 2019, 236, 13–15. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Ren, C.; Luo, F.; Ma, Q.; Hu, Y.S.; Zhou, Z.; Li, H.; Huang, X.; Chen, L. A ceramic/polymer composite solid electrolyte for sodium batteries. J. Mater. Chem. A 2016, 4, 15823–15828. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Sun, J.; Zheng, F.; Kotobuki, M.; Wu, T.; Zeng, K.; Lu, L. Flexible, stable, fast-ion-conducting composite electrolyte composed of nanostructured Na-super-ion-conductor framework and continuous Poly(ethylene oxide) for all-solid-state Na battery. J. Power Sources 2020, 454, 227949. [Google Scholar] [CrossRef]
- Niu, W.; Chen, L.; Liu, Y.; Fan, L.Z. All-solid-state sodium batteries enabled by flexible composite electrolytes and plastic-crystal interphase. Chem. Eng. J. 2020, 384, 123233. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Xu, X.; Oh, S.J.A.; Sun, J.; Zheng, F.; Lu, X.; Xu, C.; Yan, B.; Huang, G.; et al. Ultra-Stable Sodium-Ion Battery Enabled by All-Solid-State Ferroelectric-Engineered Composite Electrolytes. Nano-Micro Lett. 2024, 16, 254. [Google Scholar] [CrossRef]
- Yu, X.; Xue, L.; Goodenough, J.B.; Manthiram, A. A High-Performance All-Solid-State Sodium Battery with a Poly(ethylene oxide)-Na3Zr2Si2PO12 Composite Electrolyte. ACS Mater. Lett. 2019, 1, 132–138. [Google Scholar] [CrossRef]
- Kannadasan, M.; Sathiasivan, K.; Pandurangan, I.; Balakrishnan, M. Synergistic nanocomposite polymer electrolytes for advanced all-solid-state sodium-ion batteries. Int. J. Hydrogen Energy 2024, 78, 634–641. [Google Scholar] [CrossRef]
- Hiraoka, K.; Kato, M.; Kobayashi, T.; Seki, S. Polyether/Na3Zr2Si2PO12 Composite Solid Electrolytes for All-Solid-State Sodium Batteries. J. Phys. Chem. C 2020, 124, 21948–21956. [Google Scholar] [CrossRef]
- Dwivedi, S.; Vasudevan, S.; Balaya, P. A dual aliovalent ion doped NASICON ceramic filler embedded in the PEO-NaTFSI polymer matrix for high-performance solid-state sodium-ion batteries. J. Mater. Chem. A 2024, 12, 22867–22882. [Google Scholar] [CrossRef]
- Zhao, L.; Hou, M.; Ren, K.; Yang, D.; Li, F.; Yang, X.; Zhou, Y.; Zhang, D.; Liu, S.; Lei, Y.; et al. Hot-Pressing Enhances Mechanical Strength of PEO Solid Polymer Electrolyte for All-Solid-State Sodium Metal Batteries. Small Methods 2024, 8, 2301579. [Google Scholar] [CrossRef]
- He, K.Q.; Liao, X.G.; Lian, H.J.; Guan, X.; Chen, D.Z.; Su, Y.K.; Li, R.K.Y.; Liu, C. Endowing rapid Na+ conduction by architecture design of Na3Zr2Si2PO12 in composite electrolytes for ultralong lifespan quasi-solid-state sodium metal batteries. Rare Met. 2025, 44, 3795–3805. [Google Scholar] [CrossRef]
- Yu, X.; Xue, L.; Goodenough, J.B.; Manthiram, A. All-Solid-State Sodium Batteries with a Polyethylene Glycol Diacrylate-Na3Zr2Si2PO12 Composite Electrolyte. Adv. Energy Sustain. Res. 2021, 2, 2000061. [Google Scholar] [CrossRef]
- Luo, C.Z.; Li, Q.Y.; Shen, D.Y.; Zheng, R.H.; Huang, D.; Chen, Y. Enhanced interfacial kinetics and fast Na+ conduction of hybrid solid polymer electrolytes for all-solid-state batteries. Energy Storage Mater. 2021, 43, 463–470. [Google Scholar] [CrossRef]
- Abouricha, S.; Ihechmyen, A.; Aziam, H.; Ait Said, H.; Ouarga, A.; Oueldna, N.; Sabi, N.; Noukrati, H.; Lahcini, M.; Saadoune, I.; et al. From bone tissue to batteries: Hydroxyapatite as a filler to enhance the mechanical, thermal, and electrochemical properties of electrolytes for solid-state sodium-ion batteries. J. Energy Storage 2024, 91, 111967. [Google Scholar] [CrossRef]
- Kuang, J.; Li, X.; Li, Y.; Zhong, Y.; Gu, C.; Xia, X.; Wang, X.; Tu, J. Robust polymer electrolyte with enhanced ionic conductivity realized by the incorporation of electrospun MgAl2O4 nanofibers. J. Solid State Electrochem. 2023, 27, 3315–3324. [Google Scholar] [CrossRef]
- Wu, J.F.; Yu, Z.Y.; Wang, Q.; Guo, X. High performance all-solid-state sodium batteries actualized by polyethylene oxide/Na2Zn2TeO6 composite solid electrolytes. Energy Storage Mater. 2020, 24, 467–471. [Google Scholar] [CrossRef]
- Colò, F.; Bella, F.; Nair, J.R.; Destro, M.; Gerbaldi, C. Cellulose-based novel hybrid polymer electrolytes for green and efficient Na-ion batteries. Electrochim. Acta 2015, 174, 185–190. [Google Scholar] [CrossRef]
- Koduru, H.K.; Kondamareddy, K.K.; Iliev, M.T.; Marinov, Y.G.; Hadjichristov, G.B.; Karashanova, D.; Scaramuzza, N. Synergetic effect of TiO2 nano filler additives on conductivity and dielectric properties of PEO/PVP nanocomposite electrolytes for electrochemical cell applications. J. Phys. Conf. Ser. 2017, 780, 012006. [Google Scholar] [CrossRef]
- Kiran Kumar, K.; Ravi, M.; Pavani, Y.; Bhavani, S.; Sharma, A.K.; Narasimha Rao, V.V.R. Investigations on the effect of complexation of NaF salt with polymer blend (PEO/PVP) electrolytes on ionic conductivity and optical energy band gaps. Phys. B 2011, 406, 1706–1712. [Google Scholar] [CrossRef]
- Naveen Kumar, P.; Sasikala, U.; Sekhar, P.C.; Sharma, A.K. Enhancement of Electrical Conductivity and Ion Transport in Plasticized Polymer Blend Electrolytes (PEO/PEMA) by Addition of Nanoscale Inorganic Oxides. Asian J. Chem. 2013, 25, S111–S113. [Google Scholar]
- Wang, W.; Song, R.; Li, T.; Xu, R.; Zhao, Y.; Zhang, L. Enhancement of Electrochemical Performance of Polyoxyethylene-Polycaprolactone Blended Na-Ion Solid Electrolyteby Incorporating g-C3N4. J. Chin. Ceram. Soc. 2022, 50, 47–54. [Google Scholar]
- Chen, G.; Bai, Y.; Gao, Y.; Wang, Z.; Zhang, K.; Ni, Q.; Wu, F.; Xu, H.; Wu, C. Inhibition of Crystallization of Poly(ethylene oxide) by Ionic Liquid: Insight into Plasticizing Mechanism and Application for Solid-State Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 43252–43260. [Google Scholar] [CrossRef]
- Liu, W.; Yan, T.F.; Wang, Y.; Li, Y.; Luo, Y.; Guo, R.; Zhou, L.Z.; Yang, C.; Pei, H.J.; Xie, J.Y. Matching Poly(vinylidene fluoride) and β”-Al2O3 for Hybrid Electrolyte Membrane for Advanced Solid-State Sodium Batteries. J. Electrochem. Soc. 2021, 168, 080541. [Google Scholar] [CrossRef]
- Pei, L.; Han, Y.; Dong, J.; Wang, Y.; Liu, Y.; Liu, X.; Diao, Z.; Liu, J.; Wang, X. High-performance solid-state sodium-ion batteries for lightweight electric vehicles: A closed-loop feedback-optimized composite electrolyte design. eTransportation 2025, 25, 100439. [Google Scholar] [CrossRef]
- Lyu, Y.Q.; Yu, J.; Wu, J.; Effat, M.B.; Ciucci, F. Stabilizing Na-metal batteries with a manganese oxide cathode using a solid-state composite electrolyte. J. Power Sources 2019, 416, 21–28. [Google Scholar] [CrossRef]
- Bag, S.; Zhou, C.; Reid, S.; Butler, S.; Thangadurai, V. Electrochemical studies on symmetric solid-state Na-ion full cell using Na3V2(PO4)3 electrodes and polymer composite electrolyte. J. Power Sources 2020, 454, 227954. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, N.; Lin, J.; Guo, X. Flexible and ultra-thin membrane electrolyte with polymer-reinforced-ceramic ion-conducting framework for sodium metal batteries. J. Energy Chem. 2025, 104, 576–584. [Google Scholar] [CrossRef]
- Ruan, Y.; Huang, X.; Lei, H.; Liu, Y.; Wang, J.; Sun, W.; Song, S. A flexible and free-standing composite solid electrolyte with a pomegranate-like structure for solid-state sodium-ion batteries. Solid State Ion. 2023, 403, 116406. [Google Scholar] [CrossRef]
- Fang, R.; Li, Y.; Wu, N.; Xu, B.; Liu, Y.; Manthiram, A.; Goodenough, J.B. Ultra-Thin Single-Particle-Layer Sodium BetaAlumina-Based Composite Polymer Electrolyte Membrane for Sodium-Metal Batteries. Adv. Funct. Mater. 2023, 33, 2211229. [Google Scholar] [CrossRef]
- Tiwari, A.; Kumar, R. Singh Na3Zr2Si2PO12-Polymer Composite Electrolyte for Solid State Sodium Batteries. ChemPhysChem 2024, 25, e202400620. [Google Scholar] [CrossRef]
- Gao, H.; Guo, B.; Song, J.; Park, K.; Goodenough, J.B. A Composite Gel-Polymer/Glass-Fiber Electrolyte for Sodium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1402235. [Google Scholar] [CrossRef]
- Ling, W.; Fu, N.; Yue, J.; Zeng, X.X.; Ma, Q.; Deng, Q.; Xiao, Y.; Wan, L.J.; Guo, Y.G.; Wu, X.E. A Flexible Solid Electrolyte with Multilayer Structure for Sodium Metal Batteries. Adv. Energy Mater. 2020, 10, 1903966. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Y.; Ni, Q.; Liu, Y.; Sun, C.; Li, J.; Jin, H. Solid-State Na Metal Batteries with Superior Cycling Stability Enabled by Ferroelectric Enhanced Na/Na3Zr2Si2PO12 Interface. Small 2022, 18, 2200716. [Google Scholar] [CrossRef]
- Yu, H.; Seomoon, K.; Kim, J.; Kim, J.K. Low-cost and highly safe solid-phase sodium ion battery with a Sn-C nanocomposite anode. J. Ind. Eng. Chem. 2021, 100, 112–118. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, W.; Zhao, Z.; Zhou, P.; Zhang, P.; Ding, M.; Bai, J.; Wenig, J. Sandwiched composite electrolyte with excellent interfacial contact for high-performance solid-state sodium-ion batteries. J. Colloid Interface Sci. 2023, 652, 132–141. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, L.; Sun, C. A Durable Solid-State Na-CO2 Battery with Solid Composite Electrolyte Na3.2Zr1.9Ca0.1Si2PO12-PVDF-HFP. Energy Technol. 2023, 11, 2201383. [Google Scholar] [CrossRef]
- Lu, L.; Sun, C.; Hao, J.; Wang, Z.; Mayer, S.F.; Fernandez-Diaz, M.T.; Alonso, J.A.; Zou, B. A High-Performance Solid-State Na-CO2 Battery with Poly(Vinylidene Fluoride-co-Hexafluoropropylene)-Na3.2Zr1.9Mg0.1Si2PO12 Electrolyte. Energy Environ. Mater. 2023, 6, e12364. [Google Scholar] [CrossRef]
- Yi, Q.; Zhang, W.; Li, S.; Li, X.; Sun, C. Durable Sodium Battery with a Flexible Na3Zr2Si2PO12-PVDF-HFP Composite Electrolyte and Sodium/Carbon Cloth Anode. ACS Appl. Mater. Interfaces 2018, 10, 35039–35046. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Z.; Zhou, W.; Liu, L.; Tu, C.; Tang, M.; Xie, H.; Lu, Y.; Yan, X.; Ding, Z.; et al. A Cross-Linked Flexible Metaferroelectrolyte Regulated by 2D/2D Perovskite Heterostructures for High-Performance Compact Solid-State Sodium Batteries. Adv. Sci. 2025, 12, 2416662. [Google Scholar] [CrossRef]
- Shu, K.; Zhou, J.; Wu, X.; Liu, X.; Sun, L.; Wang, Y.; Tian, S.; Niu, H.; Duan, Y.; Hu, G.; et al. A PVDF/g-C3N4-Based Composite Polymer Electrolytes for Sodium-Ion Battery. Polymers 2023, 15, 2006. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Chen, J.; Tang, Y.; Mao, Z.; Wang, D. Gel Polymer Electrolyte Membranes Boosted with Sodium-Conductive a-Alumina Nanoparticles: Application for Na-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 623–632. [Google Scholar] [CrossRef]
- Mishra, K.; Arif, T.; Kumar, R.; Kumar, D. Effect of Al2O3 nanoparticles on ionic conductivity of PVdF-HFP/PMMA blend-based Na+-ion conducting nanocomposite gel polymer electrolyte. J. Solid State Electr. 2019, 23, 2401–2409. [Google Scholar] [CrossRef]
- Lai, H.; Lu, Y.; Zha, W.; Hu, Y.; Zhang, Y.; Wu, X.; Wen, Z. In situ generated composite gel polymer electrolyte with crosslinking structure for dendrite-free and high-performance sodium metal batteries. Energy Storage Mater. 2023, 54, 478–487. [Google Scholar] [CrossRef]
- Shen, T.Y.; Liu, T.C.; Mo, H.Q.; Yuan, Z.C.; Cui, F.; Jin, Y.X.; Chen, X.J. Cu-based metal-organic framework HKUST-1 as effective catalyst for highly sensitive determination of ascorbic acid. RSC Adv. 2020, 10, 22881–22890. [Google Scholar] [CrossRef]
- Lei, Y.; Yue, L.; Qi, Y.; Niu, Y.; Bao, S.; Song, J.; Xu, M. Metal-Organic Framework Enabling Poly(Vinylidene Fluoride)-Based Polymer Electrolyte for Dendrite-Free and Long-Lifespan Sodium Metal Batteries. Energy Environ. Mater. 2024, 7, e12511. [Google Scholar] [CrossRef]
- Shi, J.; Xiong, H.; Yang, Y.; Shao, H. Nano-sized oxide filled composite PEO/PMMA/P(VDF-HFP) gel polymer electrolyte for rechargeable lithium and sodium batteries. Solid State Ion. 2018, 326, 136–144. [Google Scholar] [CrossRef]
- Ansari, Y.; Guo, B.; Cho, J.H.; Park, K.; Song, J.; Ellison, C.J.; Goodenough, J.B. Low-cost, dendrite-blocking polymer-Sb2O3 separators for lithium and sodium batteries. J. Electrochem. Soc. 2014, 161, A1655–A1661. [Google Scholar] [CrossRef]
- Das, A.; Melepurakkal, A.; Sreeram, P.; Gireesh, K.T.; Balakrishnan, N.T.M.; Jabeen Fatima, M.J.; Pullanchiyodan, A.; Ahn, J.H.; Shelke, M.V.; Raghavan, P. Exceptional cyclability of thermally stable PVdF-co-HFP/SiO2 nanocomposite polymer electrolytes for sodium ion batteries. J. Energy Storage 2023, 73, 109026. [Google Scholar] [CrossRef]
- Yu, W.; Zhai, Y.; Yang, G.; Yao, J.; Song, S.; Li, S.; Tang, W.; Hu, N.; Lu, L. A composite electrolyte with Na3Zr2Si2PO12 microtube for solid-state sodium-metal batteries. Ceram. Int. 2021, 47, 11156–11168. [Google Scholar] [CrossRef]
- Yuan, W.; Ding, M.; Weng, J.; Xue, T.; Xie, M.; Zhang, P.; Mu, J.; Zhou, P. Composite electrolyte membrane with continuous Na+ transport for high-performance quasi-solid-state sodium batteries. Chem. Eng. J. 2025, 514, 163142. [Google Scholar] [CrossRef]
- Kumar, S.; Raghupathy, R.; Vittadello, M. Sodium Polymer Electrolytes: A Review. Batteries 2024, 10, 73. [Google Scholar] [CrossRef]
- Lim, Y.J.; Han, J.; Kim, H.W.; Choi, Y.; Lee, E.; Kim, Y. An epoxy-reinforced ceramic sheet as a durable solid electrolyte for solid state Na-ion batteries. J. Mater. Chem. A 2020, 8, 14528–14537. [Google Scholar] [CrossRef]
- Villaluenga, I.; Bogle, X.; Greenbaum, S.; Gil de Muro, I.; Rojo, T.; Armand, M. Cation only conduction in new polymer-SiO2 nanohybrids: Na+ electrolytes. J. Mater. Chem. A 2013, 1, 8348–8352. [Google Scholar] [CrossRef]
- Wu, S.; Yu, Z.; Nie, X.; Wang, Z.; Huang, F.; Wie, Q. Fast Ion Conduction Nanofiber Matrix Composite Electrolyte for Dendrite-Free Solid-State Sodium-Ion Batteries with Wide Temperature Operation. Adv. Energy Mater. 2022, 12, 2202930. [Google Scholar] [CrossRef]
- Wang, Q.; He, X.; Zhang, D.; Li, Z.; Sun, H.; Sun, Q.; Wang, B.; Fan, L.Z. A composite electrolyte based on aluminum oxide filler/polyester polymer via in situ thermal polymerization for long-cycle sodium metal batteries. Inorg. Chem. Front. 2024, 11, 2300–2311. [Google Scholar] [CrossRef]
- Hamisu, A.; Çelik, S.Ü. Polymer composite electrolyte of SPSU(Na)/PPEGMA/hBN for sodium-ion batteries. Polym. Polym. Compos. 2019, 27, 419–428. [Google Scholar] [CrossRef]
- Hamisu, A.; Çelik, S.Ü. Poly(AN-co-PEGMA)/hBN/NaClO4 composite electrolytes for sodium ion battery. e-Polymers 2017, 17, 507–515. [Google Scholar] [CrossRef]
- Guo, W.; Zhou, X.; Liu, L.; Fang, M.; Zhang, B.; Li, J.; Zhang, L.; Li, J.; Yang, Q. A novel gel electrolyte with a wide electrochemical window and high stability for sodium metal batteries. Chem. Phys. Lett. 2025, 870, 142099. [Google Scholar] [CrossRef]
- Wu, S.; Tang, F.; Zhang, K.; Zhang, L.; Huang, F. Synergistic Long- and Short-Range Sodium-Ion Transport Pathways for Enhanced Low-Temperature Performance in Ceramic-DEE-Polymer Electrolytes. Adv. Funct. Mater. 2025, 35, 25011107. [Google Scholar] [CrossRef]
- Lei, D.; He, Y.B.; Huang, H.; Yuan, Y.; Zhong, G.; Zhao, Q.; Hao, X.; Zhang, D.; Lai, C.; Zhang, S.; et al. Cross-linked beta alumina nanowires with compact gel polymer electrolyte coating for ultra-stable sodium metal battery. Nat. Commun. 2019, 10, 4244. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Che, Y.; Jabeen, M.; Lu, C.; Liao, X.Z.; Ma, Z.F. Cellulose Derivative and Polyionic Liquid Crosslinked Network Gel Electrolytes for Sodium Metal Quasi-Solid-State Batteries. Adv. Funct. Mater. 2025, 35, 2422162. [Google Scholar] [CrossRef]
- Gregorio, V.; Garc¡a, N.; Tiemblo, P. Addressing manufacturability and processability in polymer gel electrolytes for Li/Na batteries. Polymers 2021, 13, 2093. [Google Scholar] [CrossRef]
- Ran, L.; Li, M.; Cooper, E.; Luo, B.; Gentle, I.; Wang, L.; Knibbe, R. Enhanced Safety and Performance of High-Voltage Solid-State Sodium Battery through Trilayer, Multifunctional Electrolyte Design. Energy Storage Mater. 2021, 41, 8–13. [Google Scholar] [CrossRef]
- Praveen, D.; Bhat, S.V.; Damle, R. Role of silica nanoparticles in conductivity enhancement of nanocomposite solid polymer electrolytes: (PEGxNaBr):ySiO2. Ionics 2013, 19, 1375–1379. [Google Scholar] [CrossRef]
- Lin, Y.; Li, X.; Zheng, W.; Gang, Y.; Liu, L.; Cui, X.; Dan, Y.; Chen, L.; Cheng, X. Effect of SiO2 microstructure on ionic transport behavior of self-healing composite electrolytes for sodium metal batteries. J. Membr. Sci. 2023, 672, 121442. [Google Scholar] [CrossRef]
- Edison, E.; Abbott, D.F.; Ramakrishna, S.N.; Mougel, V.; Niederberger, M. Non-flammable polymeric ionic liquid hybrid electrolytes for high-stability solid-state sodium metal batteries. Chem. Eng. J. 2025, 522, 167657. [Google Scholar] [CrossRef]
- Lin, J.; Peng, H.; Huang, P.; Naren, T.; Liang, C.; Kuang, G.; Chen, L.; Zhang, C.; Wie, W. Electrically Coupled Electrolyte Engineering Enables High Interfacial Stability for High-Voltage Sodium-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2307061. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, C.; Li, X.; Zheng, Z.; Shu, Z.; Zhou, J.; Zhu, Y. Na3Zr2Si2PO12/cellulose acetate composite solid electrolyte unlocking high ionic conductivity and long cycle life in solid-state sodium batteries. J. Mater. Chem. A 2025, 13, 23805–23815. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.; Halacoglu, S.; Graff, K.; Zhang, C.; Fu, K.; Xiong, H.; Wang, H. Self-Templated 3D Sulfide-Based Solid Composite Electrolyte for Solid-State Sodium Metal Batteries. Small 2024, 20, 2406298. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Shang, X.; Wang, W.; Yan, X.; Yu, C.; Wang, L.M. Enhanced electrochemical performance enabled by ionic-liquid-coated Na3SbS4 electrolyte encapsulated in flexible filtration membrane. Chem. Eng. J. 2022, 428, 132094. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, H.; Zhang, L. Preparation and electrochemical properties of ionic-liquid-modified Na3SbS4 membrane composite electrolytes. J. Mater. Sci. 2021, 56, 10565–10574. [Google Scholar] [CrossRef]
- Dong, S.; Xie, G.; Xu, S.; Tan, X.; Chaudhary, M.; Zhang, Y.; Wu, R.; Wen, F.; Ayranci, C.; Michaelis, V.K.; et al. Cellulose-Encapsulated Composite Electrolyte Design: Toward Chemically and Mechanically Enhanced Solid-Sodium Batteries. ACS Nano 2024, 18, 16285–16296. [Google Scholar] [CrossRef]
- Ren, Y.X.; Hortance, N.; McBride, J.; Hatzell, K.B. Sodium-Sulfur Batteries Enabled by a Protected Inorganic/Organic Hybrid Solid Electrolyte. ACS Energy Lett. 2021, 6, 345–353. [Google Scholar] [CrossRef]
- Lu, P.; Wu, D.; Chen, L.; Li, H.; Wu, F. Air Stability of Solid-State Sulfide Batteries and Electrolytes. Electrochem. Energy Rev. 2022, 5, 3. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.; Wang, H. Ultrathin Sulfide/PVDF-HFP Composite Electrolyte for Solid-State Sodium Metal Batteries. ACS Appl. Energy Mater. 2024, 7, 1008–1014. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, J.; Wei, Z.; Yao, S.; Chen, G.; Du, F. Core-Shell Structured Composite Solid Electrolyte Enables High-Rate All-Solid-State Sodium Batteries. Angew. Chem. Int. Ed. 2025, 64, e202507247. [Google Scholar] [CrossRef]
- Noi, K.; Nagata, Y.; Hakari, T.; Suzuki, K.; Yubuchi, S.; Ito, Y.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. Oxide-Based Composite Electrolytes Using Na3Zr2Si2PO12/Na3PS4 Interfacial Ion Transfer. ACS Appl. Mater. Interfaces 2018, 10, 19605–19614. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Cheng, J.; Hou, G.; Nie, X.; Ai, Q.; Dai, L.; Feng, J.; Ci, L. Composite solid electrolyte of Na3PS4-PEO for all-solid-state SnS2/Na batteries with excellent interfacial compatibility between electrolyte and Na metal. J. Energy Chem. 2020, 41, 73–78. [Google Scholar] [CrossRef]
- Iyer, V.; Petersen, J.; Geier, S.; Wierach, P. Development and Multifunctional Characterization of a Structural Sodium-Ion Battery Using a High-Tensile-Strength Poly(ethylene oxide)-Based Matrix Composite. ACS Appl. Energy Mater. 2024, 7, 3968–3982. [Google Scholar] [CrossRef]
- Darjazi, H.; Falco, M.; Colò, F.; Balducci, L.; Piana, G.; Bella, F.; Meligrana, G.; Nobili, F.; Elia, G.A.; Gerbaldi, C. Electrolytes for Sodium Ion Batteries: The Current Transition from Liquid to Solid and Hybrid systems. Adv. Mater. 2024, 36, 2313572. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, X.; Yang, Q.; Hou, L.; Mu, D.; Tan, G.; Li, L.; Chen, R.; Wu, F. The Progress in the Electrolytes for Solid State Sodium-Ion Battery. Adv. Mater. Technol. 2023, 8, 2200822. [Google Scholar] [CrossRef]
- Voss, P.; Gruber, B.; Mitterfellner, M.; Plöpst, J.D.; Degen, F.; Schmuch, R.; Lux, S. Benchmarking state-of-the-art sodium-ion battery cells—Modeling energy density and carbon footprint at the gigafactory-scale. Energy Environ. Sci. 2025, 18, 8104–8129. [Google Scholar] [CrossRef]
- Zheng, J.; Li, W.; Liu, X.; Zhang, J.; Feng, X.; Chen, W. Progress in Gel Polymer Electrolytes for Sodium-Ion Batteries. Energy Environ. Mater. 2023, 6, e12422. [Google Scholar] [CrossRef]
- Wei, W.; Xu, J.; Chen, W.; Mi, L.; Zhang, J. A review of sodium chloride-based electrolytes and materials for electrochemical energy technology. J. Mater. Chem. A 2022, 10, 2637–2671. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, D.; Ren, K.; Li, F.; Dong, P.; Zhang, J.; Yang, B.; Liang, F. All Solid-State Sodium Batteries and Its Interface Modification. Prog. Chem. 2023, 35, 1177–1190. [Google Scholar]
- Khudyshkina, A.D.; Rauska, U.C.; Butzelaar, A.J.; Hoffmann, M.; Wilhelm, M.; Theato, P.; Jeschull, F. Impact of Nano-sized Inorganic Fillers on PEO-based Electrolytes for Potassium Batteries. Batter. Supercaps 2024, 7, e202300404. [Google Scholar] [CrossRef]
- Elgin, J.; Wu, Y. Extracting Interfacial Resistance between Potassium beta-Alumina and Polymer Electrolytes for Composite Electrolytes. ACS Appl. Energy Mater. 2025, 8, 9319–9327. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, A.M.; Chen, Y.F.; Xie, J.; Xin, Z.F.; Chen, Y.J.; Kan, Y.H.; Li, S.L.; Lan, Y.Q.; Zhang, Q. Polyoxovanadate-polymer hybrid electrolyte in solid state batteries. Energy Storage Mater. 2020, 29, 172–181. [Google Scholar] [CrossRef]
- Fei, H.; Liu, Y.; An, Y.; Xu, X.; Zeng, G.; Tian, Y.; Ci, L.; Xi, B.; Xiong, S.; Feng, J. Stable all-solid-state potassium battery operating at room temperature with a composite polymer electrolyte and a sustainable organic cathode. J. Power Sources 2018, 399, 294–298. [Google Scholar] [CrossRef]
- Du, G.; Tao, M.; Liu, D.; Aslam, M.K.; Qi, Y.; Jiang, J.; Li, Y.; Bao, S.J.; Xu, M. Low-operating temperature quasi-solid-state potassium-ion battery based on commercial materials. J. Colloid Interface Sci. 2021, 582, 932–939. [Google Scholar] [CrossRef]
- Nivetha, K.; Vijaya Kumar, K.; Krishna Jyothi, N.; Venkataratnam Kamma, K. Exploring the synergistic potential of PVB: KCl composite electrolyte films for enhanced performance in solid-state potassium batteries. Bull. Mater. Sci. 2024, 47, 250. [Google Scholar] [CrossRef]
- Sulaiman, M.; Rahman, A.A.; Mohamed, N.S. Structural, thermal and conductivity studies of magnesium nitrate—Alumina composite solid electrolytes prepared via sol-gel method. Int. J. Electrochem. Sci. 2013, 8, 6647–6655. [Google Scholar] [CrossRef]
- Skov, L.N.; Grinderslev, J.B.; Rosenkranz, A.; Lee, Y.S.; Jensen, T.R. Towards Solid-State Magnesium Batteries: Ligand-Assisted Superionic Conductivity. Batter. Supercaps 2022, 5, e202200163. [Google Scholar] [CrossRef]
- Mazzucco, A.; Tricerri, N.; Lamacchia, L.; Francesco Sgroi, M.; Baricco, M.; Filinchuk, Y. Mg(BH4)2-CH3NH2BH3@MgO solid state electrolyte for magnesium batteries. Mater. Renew. Sustain. Energy 2025, 14, 2. [Google Scholar] [CrossRef]
- Le Ruyet, R.; Berthelot, R.; Salager, E.; Florian, P.; Fleutot, B.; Janot, R. Investigation of Mg(BH4)(NH2)-Based Composite Materials with Enhanced Mg2+ Ionic Conductivity. J. Phys. Chem. C 2019, 123, 10756–10763. [Google Scholar] [CrossRef]
- Higashi, S.; Miwa, K.; Aoki, M.; Takechi, K. A novel inorganic solid state ion conductor for rechargeable Mg batteries. Chem. Commun. 2014, 50, 1320–1322. [Google Scholar] [CrossRef]
- Amdisen, M.B.; Lee, Y.S.; Jensen, T.R. Cyclopropylamine Magnesium Borohydrides as Solid-State Electrolytes. Inorg. Chem. 2025, 64, 3696–3706. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Zheng, K.; Xiong, F.; Chen, Y.; Sheha, E.; Yang, J.; Wang, J.; NuLi, Y. Facilely synthesized crosslinked gel polymer electrolytes for high-performance quasi-solid-state rechargeable magnesium batteries. Inorg. Chem. Front. 2025, 12, 6166–6177. [Google Scholar] [CrossRef]
- Mitra, S.; Sampath, S. Sol-gel derived, magnesium based ionically conducting composites. J. Mater. Chem. 2002, 12, 2531–2537. [Google Scholar] [CrossRef]
- Kristensen, L.G.; Amdisen, M.B.; Skov, L.N.; Jensen, T.R. Fast magnesium ion conducting isopropylamine magnesium borohydride enhanced by hydrophobic interactions. Phys. Chem. Chem. Phys. 2022, 24, 18185–18197. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.G.; Amdisen, M.B.; Andersen, M.; Jensen, T.R. Synthesis, Structure and Mg2+ Ionic Conductivity of Isopropylamine Magnesium Borohydride. Inorganics 2023, 11, 17. [Google Scholar] [CrossRef]
- Dansirima, P.; Kristensen, L.G.; Grinderslev, J.B.; Skibsted, J.; Utke, R.; Jensen, T.R. Nanoconfinement of an ammine magnesium borohydride composite electrolyte in a mesoporous silica scaffold. Commun. Mater. 2024, 5, 160. [Google Scholar] [CrossRef]
- Song, S.; Kotobuki, M.; Zheng, F.; Li, Q.; Xu, C.; Wang, Y.; Li, W.D.Z.; Hu, N.; Lu, L. A Composite Polymer Electrolyte for Safer Mg Batteries. J. Electrochem. Soc. 2017, 164, A741–A743. [Google Scholar] [CrossRef]
- Mishra, K.; Kanchan, D.K.; Gohel, K.; Sharma, P.; Kumar, D. Urea-assisted ion-transport behavior in magnesium ion conducting solid polymer electrolyte membranes intended for magnesium batteries. Chem. Pap. 2022, 76, 827–839. [Google Scholar] [CrossRef]
- Marinov, Y.G.; Koduru, H.K.; Exner, G.K.; Ivanov, G.R.; Scaramuzza, N. Peo/starch-nanocrystals based solid polymer electrolyte membranes for magnesium ion conducting applications. Compt. R. Acad. Bulg. Sci. 2023, 76, 689–697. [Google Scholar] [CrossRef]
- Sridevi, N.A.; Karuppasamy, K.; Vani, C.V.; Balakumar, S.; Sahaya Shajan, X. Characterization of nanochitosan incorporated solid polymer composite electrolytes for magnesium batteries. Adv. Mater. Res. 2013, 678, 316–320. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhan, H.; Liu, S.Q.; Huang, K.L.; Zhou, Y.H. Nanosized Flame Retarded Hydroxide Magnesium/Poly(ethylene-oxide) Composite Polymer Electrolyte. Acta Phys. Chim. Sin. 2010, 26, 2387–2391. [Google Scholar]
- Zaky, M.M.; Eyssa, H.M.; Sadek, R.F. Improvement of the magnesium battery electrolyte properties through gamma irradiation of nano polymer electrolytes doped with magnesium oxide nanoparticles. J. Vinyl Addit. Technol. 2019, 25, 243–254. [Google Scholar] [CrossRef]
- Khalil, R.; Sheha, E.M.; Eid, A. Investigation of Electrical Properties, Structure and Morphological Characterization of Mg+2 Ions Conducting Solid Polymer Electrolyte Based on Poly(vinyl alcohol). J. Adv. Phys. 2017, 6, 102–107. [Google Scholar] [CrossRef]
- Sheha, E. Prototype system for magnesium/TiO2 anatase batteries. Int. J. Electrochem. Sci. 2013, 8, 3653–3663. [Google Scholar] [CrossRef]
- Gamal, R.; Sheha, E.; Shash, N.; El-Shaarawy, M.G. Preparation and characterization of Mg2+-ion conducting composite based on poly(vinyl alcohol) with various concentrations of Li2O. Mater. Express 2014, 4, 293–300. [Google Scholar] [CrossRef]
- Abdel-Samiea, B.M.; Gamal, R.; Sheha, E. All-solid-state polymer electrolyte with plastic crystal materials for rechargeable magnesium battery. In Nanotechnology 2012: Bio Sensors, Instruments, Medical, Environment And Energy; CRC Press: Boca Raton, FL, USA, 2012; Volume 3, pp. 533–536. [Google Scholar]
- Kanakaraj, T.M.; Bhajantri, R.F.; Chavan, C.; Cyriac, V.; Bulla, S.S.; Ismayil. Investigation on the structural and ion transport properties of magnesium salt doped HPMC-PVA based polymer blend for energy storage applications. J. Non-Cryst. Sol. 2023, 609, 122276. [Google Scholar]
- Mesallam, M.; Kamar, E.M.; Sharma, N.; Sheha, E. Synthesis and characterization of polyvinylidene fluoride/magnesium bromide polymer electrolyte for magnesium battery application. Phys. Scrip. 2020, 95, 115805. [Google Scholar] [CrossRef]
- Wu, N.; Wang, W.; Wei, Y.; Li, T. Studies on the Effect of Nano-Sized MgO in Magnesium-Ion Conducting Gel Polymer Electrolyte for Rechargeable Magnesium Batteries. Energies 2017, 10, 1215. [Google Scholar] [CrossRef]
- Deivanayagam, R.; Cheng, M.; Wang, M.; Vasudevan, V.; Foroozan, T.; Medhekar, N.V.; Shahbazian-Yassar, R. Composite Polymer Electrolyte for Highly Cyclable Room-Temperature Solid-State Magnesium Batteries. ACS Appl. Energy Mater. 2019, 2, 7980–7990. [Google Scholar] [CrossRef]
- Pandey, G.P.; Agrawal, R.C.; Hashmi, S.A. Magnesium ion-conducting gel polymer electrolytes dispersed with fumed silica for rechargeable magnesium battery application. J. Solid State Electr. 2011, 15, 2253–2264. [Google Scholar] [CrossRef]
- Ponmani, S.; Selvakumar, K.; Ramesh Prabhu, M. The effect of the geikeilite (MgTiO3) nanofiller concentration in PVdF-HFP/PVAc-based polymer blend electrolytes for magnesium ion battery. Ionics 2020, 26, 2353–2369. [Google Scholar] [CrossRef]
- Sharma, J.; Hashmi, S.A. Plastic crystal-incorporated magnesium ion conducting gel polymer electrolyte for battery application. Bull. Mater. Sci. 2018, 41, 147. [Google Scholar] [CrossRef]
- Modrow, H. X-Ray Methods for the Characterization of Nanoparticles. In Nanofabrication Towards Biomedical Applications; Kumar, C.S.S.R., Hormes, J., Leuschner, C., Eds.; Wiley: Weinheim, Germany, 2005; pp. 163–196. [Google Scholar]
- Pandey, G.P.; Agrawal, R.C.; Hashmi, S.A. Performance studies on composite gel polymer electrolytes for rechargeable magnesium battery application. J. Phys. Chem. Sol. 2011, 72, 1408–1413. [Google Scholar] [CrossRef]
- Choi, B.K.; Shin, K.H. Effects of SiC fillers on the electrical and mechanical properties of (PEO)16LiClO4 electrolytes. Solid State Ion. 1996, 86–88, 303–306. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Sahu, D.K.; Mahipal, Y.K.; Ashrafi, R. Investigations on ion transport properties of hot-press cast magnesium ion conducting Nano-Composite Polymer Electrolyte (NCPE) films: Effect of filler particle dispersal on room temperature conductivity. Mater. Chem. Phys. 2013, 139, 410–415. [Google Scholar] [CrossRef]
- Hashmi, S.A.; Upadhayaya, H.M.; Thakur, A.K. Sodium ion conducting composite polymer electrolyte for battery applications. In Solid State Ionics—Materials and Devices; Chowdari, B.V.R., Wang, W., Eds.; World Scientific: Singapore, 2000; pp. 461–466. [Google Scholar]
- Pandey, G.P.; Hashmi, S.A.; Agrawal, R.C. Hot-press synthesized polyethylene oxide based proton conducting nanocomposite polymer electrolyte dispersed with SiO2 nanoparticles. Solid State Ion. 2008, 179, 543–549. [Google Scholar] [CrossRef]
- Maier, J. Ionic-conduction in-space charge regions. Prog. Solid State Chem. 1995, 23, 171–263. [Google Scholar] [CrossRef]
- Maier, J. Defect chemistry at interfaces. Solid State Ion. 1994, 70, 43–51. [Google Scholar] [CrossRef]
- Kumar, B. From colloidal to composite electrolytes: Properties, peculiarities, and possibilities. J. Power Sources 2004, 135, 215–231. [Google Scholar] [CrossRef]
- Kumar, B.; Nellutla, S.; Thokchom, J.S.; Chen, C. Ionic conduction through heterogeneous solids: Delineation of the blocking and space charge effects. J. Power Sources 2006, 160, 1329–1335. [Google Scholar] [CrossRef]
- Polu, A.R.; Kumar, R.; Kumar, K.V.; Jyothi, N.K. Effect of TiO2 Ceramic Filler on PEG-Based Composite Polymer Electrolytes for Magnesium Batteries. Solid State Phys. 2013, 1512, 996–997. [Google Scholar]
- Jayalakshmi, K.; Ismayil; Hegde, S.; Sanjeev, G.; Murari, M.S. Improving electrolyte performance: Nanocomposite solid polymer electrolyte films with cobalt oxide nanoparticles. Polym. Compos. 2024, 45, 137–150. [Google Scholar] [CrossRef]
- Polu, A.R.; Kumar, R. Preparation and characterization of PEG-Mg(CH3COO)2-CeO2 composite polymer electrolytes for battery application. Bull. Mater. Sci. 2014, 37, 309–314. [Google Scholar] [CrossRef]
- Polu, A.R.; Kumar, R.; Rhee, H.W. Effect of ceramic fillers on polyethylene glycol-based solid polymer electrolytes for solid-state magnesium batteries. High Perform. Polym. 2014, 26, 628–631. [Google Scholar] [CrossRef]
- Polu, A.R.; Kumar, R. Mg2+-ion conducting poly(ethylene glycol)-TiO2 composite polymer electrolytes for solid-state batteries. Mater. Express 2014, 4, 79–84. [Google Scholar] [CrossRef]
- Helen, P.A.; Ajith, K.; Diana, M.I.; Lakshmi, D.; Selvin, P.C. Chitosan based biopolymer electrolyte reinforced with V2O5 filler for magnesium batteries: An inclusive investigation. J. Mater. Sci. Mater. Electron. 2022, 33, 3925–3937. [Google Scholar] [CrossRef]
- Sun, Y.; Pan, M.; Wang, Y.; Hu, A.; Zhou, Q.; Zhang, D.; Zhang, S.; Zhao, Y.; Wang, Y.; Chen, S.; et al. A Facile Strategy for Constructing High-Performance Polymer Electrolytes via Anion Modification and Click Chemistry for Rechargeable Magnesium Batteries. Angew. Chem. Int. Ed. 2024, 63, e202406585. [Google Scholar] [CrossRef]
- Zain, N.F.; Zainal, N.; Mohamed, N.S. The Effects of MgO Nanofiller to the Physicochemical and Ionic Liquid Retention Properties of PEMA-MgTf2-EMITFSI Nanocomposite Polymer Electrolytes. Polym. Comp. 2018, 39, 1500–1506. [Google Scholar] [CrossRef]
- Wang, P.; Trueck, J.; Hacker, J.; Schlosser, A.; Kuester, K.; Starke, U.; Reinders, L.; Buchmeiser, M.R. A design concept for halogen-free Mg2+/Li+-dual salt-containing gel-polymer-electrolytes for rechargeable magnesium batteries. Energy Storage Mater. 2022, 49, 509–517. [Google Scholar] [CrossRef]
- Pandeeswari, J.; Jenisha, G.; Walle, K.Z.; Kotobuki, M. Recent Research Progress on All-Solid-State Mg Batteries. Batteries 2023, 9, 570. [Google Scholar] [CrossRef]
- Sun, Q.; Luo, S.; Huang, R.; Liu, Q.; Yan, S.; Lin, X. Insights on solid electrolytes for solid-state magnesium batteries: Progress and prospects. Energy Storage Mater. 2024, 70, 103508. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Q.; Su, C.; Li, C. Electrochemical behavior of Mg electrode in sodium salt electrolyte system. Front. Chem. 2022, 10, 992400. [Google Scholar] [CrossRef]
- Amdisen, M.B.; Jensen, T.R. Urea Calcium Borohydrides as Ca2+ Solid-State Electrolytes. Chem. Mater. 2025, 37, 1183–1194. [Google Scholar] [CrossRef]
- Xiong, W.; Xie, Q.; Zhang, H.; Alam, M.A.; Zhu, C.; Wang, L.; Xu, J. Development of flexible Zn/MnO2 secondary batteries using a fumed silica-doped hydrogel electrolyte. RSC Adv. 2024, 14, 37512–37520. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Holze, R. Electrochemical Energy Conversion and Storage; Wiley: Weinheim, Germany, 2022. [Google Scholar]
- Holze, R. Elektroenergie: Elektrochemisch Nutzen, Speichern und Wandeln; Walter de Gruyter: Berlin, Germany, 2025. [Google Scholar]
- Zhao, T.; Yu, J.; Xiao, P.; Nie, S.; Peng, S.; Chen, J.; Luo, F.; Janiak, C.; Chen, Y. Preparation of Hierarchical Porous ZIF-67 and Its Application in Zinc Battery Separator. Chemistry 2024, 6, 1363–1373. [Google Scholar] [CrossRef]
- Johari, N.A.; Kudin, T.I.T.; Ali, A.M.M.; Winie, T.; Yahya, M.Z.A. Studies on cellulose acetate-based gel polymer electrolytes for proton batteries. Mater. Res. Innov. 2009, 13, 232–234. [Google Scholar] [CrossRef]
- Hu, J.; Qu, Y.; Shi, F.; Wang, J.; He, X.; Liao, S.; Duan, L. Enhancing the Kinetics of Zinc Ion Deposition by Catalytic Ion in Polymer Electrolytes for Advanced Zn-MnO2 Batteries. Adv. Funct. Mater. 2022, 32, 2209463. [Google Scholar] [CrossRef]
- Shang, J.; Wang, Y.; Chen, S.; Zhang, J. Solvation structure regulation of zinc ions with nitrogen-heterocyclic additives for advanced batteries. Nanoscale 2024, 17, 2121–2129. [Google Scholar] [CrossRef]
- Prasanna, C.M.S.; Suthanthiraraj, S.A. Improved zinc ion transportation in gel polymer electrolyte upon the addition of nano-sized SnO2. Polym. Polym. Comp. 2020, 28, 54–65. [Google Scholar] [CrossRef]
- Murali, S.P.C.; Samuel, A.S. Zinc ion conducting blended polymer electrolytes based on room temperature ionic liquid and ceramic filler. J. Appl. Polym. Sci. 2019, 136, 47654. [Google Scholar] [CrossRef]
- Prasanna, C.M.S.; Suthanthiraraj, S.A. PVC/PEMA-based blended nanocomposite gel polymer electrolytes plasticized with room temperature ionic liquid and dispersed with nano-ZrO2 for zinc ion batteries. Polym. Comp. 2019, 40, 3402–3411. [Google Scholar] [CrossRef]
- Johnsi, M.; Austin, S. Suthanthiraraj Compositional effect of ZrO2 nanofillers on a PVDF-co-HFP based polymer electrolyte system for solid state zinc batteries. Chin. J. Polym. Sci. 2016, 34, 332–343. [Google Scholar] [CrossRef]
- Kumar, D.R.; Kanagaraj, I.; Sukanya, R.; Karthik, R.; Hasan, M.; Thalji, M.R.; Dhakal, G.; Milton, A.; Prakash, A.S.; Shim, J.J. Ti3C2Tx Filled in EMIMBF4 Semi-Solid Polymer Electrolytes for the Zinc-Metal Battery. ACS Appl. Mater. Interfaces 2024, 16, 33294–33306. [Google Scholar] [CrossRef]
- Kumar, D.; Franco, R.; Abdou, N.; Shu, R.; Martinelli, A.; Moyses Araujo, C.; Gladisch, J.; Gueskine, V.; Crispin, R.; Khan, Z. Water-in-Polymer Salt Electrolyte for Long-Life Rechargeable Aqueous Zinc-Lignin Battery. Energy Environ. Mater. 2025, 8, e12752. [Google Scholar] [CrossRef]
- Sun, B.; Zong, Y.; Bao, K.; Wang, M.; Wang, P.; Xu, H.; Jin, Y. Activating Gel Polymer Electrolyte Based Zinc-Ion Conduction with Filler-Integration for Advanced Zinc Batteries. ACS Appl. Mater. Interfaces 2023, 15, 37916–37924. [Google Scholar] [CrossRef]
- Karaman, E.S.; Wang, Z.; Chen, K.; Siddiqui, Z.; Cheng, Y.; Basuray, S.; Kumar, V.; Mitra, S. Functionalized carbon nanotube doped gel electrolytes with enhanced mechanical and electrical properties for battery applications. Mater. Chem. Phys. 2021, 264, 124448. [Google Scholar] [CrossRef]
- Li, J.X.; Li, C.X.; Ren, J.F.; Li, P.X.; Zhang, K.; Wu, T.T.; Wang, L. Synergistic effect of organic-inorganic hybrid electrolyte for ultra-long Zn-I2 batteries. Int. J. Hydrogen Energy 2023, 48, 21985–21995. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Z.; Wang, H.; Ma, Z.; Yi, T.; Li, Y.; Yang, K.; Li, F.; Xue, B. Synergistic crosslinking effect of hydroxyethyl cellulose and montmorillonite on the improvement of mechanical and electrochemical properties of polyvinyl alcohol hydrogel. Mater. Today Chem. 2024, 37, 101996. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, L.; Hu, Z.; Tang, Z.; Lu, H.; Gao, Y.; Wang, J.; Song, Y.; Han, C.; Li, W. Altering the Zn2+ Migration Mechanism Enables the Composite Hydrogel Electrolytes with High Zn2+ Conduction and Superior Anti-Dehydration. Adv. Funct. Mater. 2025, 35, 2504782. [Google Scholar] [CrossRef]
- Ju, J.; Zhang, T.; Wang, H.; Liu, Y.; Zhang, Y.; Wang, Z.; Li, L.; Li, Y.; Kang, W. Nanofiber-reinforced composite hydrogel with flexibility and anti-freezing for high-performance zinc-ion batteries. Chem. Eng. J. 2025, 521, 166983. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Ju, J.; Wang, Y.; Zhang, Z.; Kang, W. Nanofiber-Reinforced Composite Gel Enabling High Ionic Conductivity and Ultralong Cycle Life for Zn Ion Batteries. Small 2024, 20, 2305140. [Google Scholar] [CrossRef]
- Du, Z.; Shen, S.; Su, X.; Zhuang, Y.; Chen, M.; Zhang, X.; Lin, Z.; Yu, L.; Zhou, P.; Wu, M.; et al. A Robust and Tough Composite Hydrogel Electrolyte with Anion-Locked Supramolecular Network for Zinc Ion Batteries. Adv. Mater. 2025, 37, 2502328. [Google Scholar] [CrossRef]
- Zhang, T.; Su, D.; Yu, J.; Zhang, Y.; Jiang, M.; Ju, J.; Sun, Y.; Kang, W. Construction of electrospun multistage ZnO@PMIA gel electrolytes for realizing high performance zinc-ion batteries. Electrochim. Acta 2024, 507, 145124. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Y.; Zhang, Y.; Yu, M.; Zhao, Q.; Briscoe, J.; Lu, Y.; Yao, J. Tough and recyclable hydrogel electrolytes with continuous ion migration pathways for dendrite-free zinc-ion batteries under harsh conditions. J. Mater. Chem. 2025, 13, 13276–13285. [Google Scholar] [CrossRef]
- Li, T.; Liu, J.; Shu, H.; Sun, A.; Zhao, T.; Chen, Y.; Chen, Y. High-Performance Deep Eutectic/Ionic Liquid Gels for Zinc-Ion Battery and Flexible Sensor Applications in Extreme Environments. Adv. Funct. Mater. 2025, 35, e14358. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Khamsawat, J.; Danwong, P.; Buasri, A.; Pattananuwat, P. Application of Coffee Silverskin Cellulose/Polyacrylamide Gel Polymer Electrolytes for Rechargeable Zinc-Ion Batteries. Sci 2024, 6, 50. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, P.; Li, A.; Qiu, W.; Wu, J.; Cheng, M. Suppressing water reactivity and manipulating zinc ion deposition by a composite polyacrylamide/graphitic carbon nitride hydrogel electrolyte for durable aqueous zinc metal batteries. Electrochim. Acta 2025, 529, 146367. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Jia, C.; Xue, L.; Zhang, J.; Yang, C.; Liu, X.; Zhang, L. Graphene enhanced hydrogel electrolyte boost flexible zinc ion battery stability. Electrochim. Acta 2025, 538, 147009. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Y.; Qu, Z.; Ma, Z.; Yang, K.; Li, F.; Xue, B. High-performance dickite strengthened double-crosslinked hydrogel electrolytes for aqueous zinc-ion batteries. Appl. Clay Sci. 2025, 266, 107691. [Google Scholar] [CrossRef]
- Ahmed, A.; Subchokpool, T.; Khampuanbut, A.; Hongrattanavichit, I.; Okhawilai, M.; Sukmas, W.; Pattananuwat, P. Enhancing high ionic conductivity of polyacryamide/hemp cellulose nanofibers for utilizing as quasi solid electrolyte composites in flexible rechargeable zinc-ion battery with high-safety. Mater. Today Sustain. 2025, 30, 101088. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Zhang, H.; Zhang, X.; Li, J.; Shi, Y.; Geng, D.; Li, Z.; Sha, D.; Yu, G.; et al. Coupling covalent organic framework with polyacrylamide to construct a robust composite hydrogel electrolyte for dendrite-free and long-lifespan aqueous zinc-ion batteries. Chem. Eng. J. 2025, 519, 164988. [Google Scholar] [CrossRef]
- Xu, S.; Shen, P.; Shen, Z.; Chen, R.; Zhang, D.; Zhao, Z.; Zhang, Y.; Li, D.; Xiong, Y.; Wang, X.; et al. Cathode-less zinc-manganese fiber batteries with reinforced-concrete structured composite hydrogel electrolytes. Chem. Eng. J. 2025, 504, 158509. [Google Scholar] [CrossRef]
- Zheng, Z.; Shi, W.; Zhou, X.; Zhang, X.; Guo, W.; Shi, X.; Xiong, Y.; Zhu, Y. Agar-based hydrogel polymer electrolyte for high-performance zinc-ion batteries at all climatic temperatures. iScience 2023, 26, 106437. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Xu, P.; He, J.; Xu, H.; Zhang, W.; Zhu, J.; He, G.; Chen, H. Sodium dodecyl sulfonate-mediated gel electrolyte for horizontal zinc deposition in robust zinc-ion batteries. J. Mater. Sci. Technol. 2025, 230, 311–318. [Google Scholar] [CrossRef]
- Ling, W.; Mo, F.; Wang, J.; Liu, Q.; Liu, Y.; Yang, Q.; Qiu, Y.; Huang, Y. Self-healable hydrogel electrolyte for dendrite-free and self-healable zinc-based aqueous batteries. Mater. Today Phys. 2021, 20, 100458. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Ke, L.; Jiang, H.; Huang, Y.; Nie, Z.; Jin, S. Biocompatible hydrogel electrolyte with high ionic conductivity and transference number towards dendrite-free Zn anodes. J. Mater. Sci. Technol. 2025, 225, 40–48. [Google Scholar] [CrossRef]
- Bai, Q.; Meng, Q.; Liu, W.; Lin, W.; Yi, P.; Tang, J.; Zhang, G.; Cao, P.; Yang, J. Advanced electrolyte with high stability and low-temperature resistance for zinc-ion batteries. J. Mater. Chem. A 2023, 12, 277–285. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, X.; Li, J.; Zhang, H.; Li, D.; Ge, H.; Liang, S.; Lu, B.; Zhao, J.; Zhou, J. Biocompatible and stable quasi-solid-state zinc-ion batteries for real-time responsive wireless wearable electronics. Energy Environ. Sci. 2024, 17, 3878–3887. [Google Scholar] [CrossRef]
- Chiaoprakobkij, N.; Okhawilai, M.; Kasemsiri, P.; Qin, J.; Uyama, H. Eco-friendly alginate-doped cotton pad as a separator for zinc-ion batteries. Cellulose 2023, 30, 6989–7001. [Google Scholar] [CrossRef]
- Ge, H.; Qin, L.; Zhang, B.; Jiang, L.; Tang, Y.; Lu, B.; Tian, S.; Zhou, J. An ionically cross-linked composite hydrogel electrolyte based on natural biomacromolecules for sustainable zinc-ion batteries. Nanoscale Horiz. 2024, 9, 1514–1521. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Tian, Z.; Zhu, J.; Liu, Y.; Jia, L.; You, D.; Li, X.; Kang, L. A Quasi-gel SiO2/Sodium Alginate (SA) Composite Electrolyte for Long-life Zinc-manganese Aqueous Batteries. J. Inorg. Mater. 2020, 35, 909–915. [Google Scholar]
- Mi, H.; Tian, P.; Yuan, C.; Shi, F. Inorganic-polymer hydrogel electrolyte enabling aqueous Zn-ions batteries. Mater. Lett. 2023, 330, 133354. [Google Scholar] [CrossRef]
- Shin, K.H.; Ji, D.; Park, J.M.; Joe, Y.S.; Park, H.S.; Kim, J. Structural Composite Hydrogel Electrolytes for Flexible and Durable Zn Metal Batteries. Adv. Funct. Mater. 2024, 34, 2309048. [Google Scholar] [CrossRef]
- Zhong, X.; Tian, P.; Chen, C.; Meng, X.; Mi, H.; Shi, F. Preparation and interface stability of alginate-based gel polymer electrolyte for rechargeable aqueous zinc ion batteries. J. Electroanal. Chem. 2022, 927, 116968. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, L.; Fang, Y.; Chai, Y.; Xie, X.; Lu, B.; Liang, S.; Zhou, J. Tuning Zn2+ coordination tunnel by hierarchical gel electrolyte for dendrite-free zinc anode. Sci. Bull. 2022, 67, 955–962. [Google Scholar] [CrossRef]
- Ng, L.S.; Mohamad, A.A. Effect of temperature on the performance of proton batteries based on chitosan-NH4NO3-EC membrane. J. Membr. Sci. 2008, 325, 653–657. [Google Scholar] [CrossRef]
- Xu, J.; Wang, M.; Alam, M.A.; Muhammad, G.; Lv, Y.; Zhu, C.; Zhang, H.; Xiong, W. Fabricating a Gel Electrolyte Based on Lignin-Coated Nanosilica to Enhance the Reversibility of Zinc Anodes for Rechargeable Aqueous Zn/MnO2 Batteries. ACS Sustain. Chem. Eng. 2022, 10, 2063–2071. [Google Scholar] [CrossRef]
- Dueramae, I.; Okhawilai, M.; Kasemsiri, P.; Uyama, H.; Kita, R. Effect of Ti3AlC2 MAX phase on electrochemical performance of thermo-responsive copolymer electrolyte for solid state zinc-ion battery. Mater. Sci. Energy Technol. 2024, 7, 237–248. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, L.; Wu, Y.; Chen, Z.; Zhu, X.; Du, L.; Wang, Y. High voltage, toughness and improved interfacial ion deposition through a dual-crosslinked and self-healable hydrogel electrolyte for green zinc-ion batteries. Chem. Eng. J. 2024, 484, 149780. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, H.; Wang, L.; Dong, R.; Xie, W.; Alam, M.A.; Xu, J.; Xiong, W. Gelatin/γ-polyglutamic acid composite hydrogel electrolytes for high-performance aqueous zinc-ion batteries. J. Colloid Interface Sci. 2025, 697, 137989. [Google Scholar] [CrossRef] [PubMed]
- Bansod, S.M.; Bhoga, S.S.; Singh, K.; Tiwari, R.U. The role of electrolyte in governing the performance of protonic solid state battery. Ionics 2007, 13, 329–332. [Google Scholar] [CrossRef]
- Ogawa, S.; Takazawa, Y.; Harada, H.; Nogami, M. The Development of the Organic/Inorganic Composite Separator for Use in Secondary Zinc Batteries. Electrochemistry 2022, 90, 017001. [Google Scholar] [CrossRef]
- Irani, M.; Ismail, H.; Ahmad, Z. Linear low-density polyethylene-g-poly(acrylic acid)/(organo-montmorillonite) hydrogel composite as hydrogel electrolytes for zinc-carbon batteries. J. Vinyl Addit. Technol. 2016, 22, 279–284. [Google Scholar] [CrossRef]
- Sungoradee, T.; Srikulkit, K. Bacterial Cellulose/Polyelectrolyte Complex Hydrogel Separator with Thermal and Dimensional Stabilities for Dendrite Suppression in Zinc Ion Battery. ACS Omega 2024, 9, 47088–47096. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.B.; Kim, W.Y.; Lee, K.H.; Lee, S.S.; Kim, S.H.; Park, S.; Hong, Y.K.; Lee, S.Y. Enabling On-Demand Conformal Zn-Ion Batteries on Non-Developable Surfaces. Adv. Funct. Mater. 2023, 33, 2211597. [Google Scholar] [CrossRef]
- Yang, L.; Shen, Y.; Long, X.; Ma, Q.; Ruan, Z.; Xu, N.; Li, K.; Jiao, L.; Kong, Y.; Li, J.; et al. Engineering an ion-pumping solid electrolyte interphase for ultra-stable aqueous zinc-ion batteries under deep discharge conditions. Energy Environ. Sci. 2025, 18, 8667–8678. [Google Scholar] [CrossRef]
- Huang, M.; Yuan, X.; Gan, J.; Ji, F.; Wang, F.; Pan, M.; Huang, W.; Li, L.; Huang, P. Effect of Electrolyte Additives on the Performance of Zinc Ion Batteries. Int. J. Electrochem. Sci. 2022, 17, 221250. [Google Scholar] [CrossRef]
- Deng, Q.; Zhou, W.; Wang, H.; Ma, Q.; Li, C.; Wu, X.; Wu, Y. Design of a polymer electrolyte membrane for enhanced zinc anode stability in reversible aqueous zinc-ion batteries. Energy Mater. 2025, 5, 500103. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Zhao, S.; Zhao, F.; Xiong, S.; Brett, D.J.L.; He, G.; Parkin, I.P. An anti-aging polymer electrolyte for flexible rechargeable zinc-ion batteries. J. Mater. Chem. A 2020, 8, 22637–22644. [Google Scholar] [CrossRef]
- Nguyen, N.; Doan, T.N.L.; Hoang, T.K.A.; Zhao, H.; Chen, P. Effect of fumed silica concentration and separator type on the electrochemical performance of gel electrolyte in the rechargeable hybrid aqueous battery. Mater. Today Energy 2018, 8, 73–79. [Google Scholar] [CrossRef]
- Rase, D.; Illathvalappil, R.; Singh, H.D.; Shekhar, P.; Leo, L.S.; Chakraborty, D.; Haldar, S.; Shelke, A.; Ajithkumar, T.G.; Vaidhyanathan, R. Hydroxide ion-conducting viologen-bakelite organic frameworks for flexible solid-state zinc-air battery applications. Nanoscale Horiz. 2023, 8, 224–234. [Google Scholar] [CrossRef]
- Kurian, M.; Vijayakumar, V.; Manna, N.; Kanheerampockil, F.; Bhat, S.; Kurungot, S. Electrode|electrolyte interface enhancement in quasi-solid-state zinc-air batteries through an anion conducting polymer electrolyte interlayer by in situ polymerization. J. Mater. Chem. A 2023, 11, 14776–14787. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, X.; Ye, X.; Zheng, X.; Zhang, Y.; Wang, M.; Lin, X.; Liu, B.; Han, L.; Ning, Y.; et al. Graphene Oxide Nanoribbon-Based Composite Gel Polymer Electrolytes: Enhancing Mechanical Strength and Ionic Conductivity for Long Cycling Lifetime of Flexible Zinc-Air Batteries. Energy Fuels 2024, 38, 6508–6517. [Google Scholar] [CrossRef]
- Zhong, C.; Song, Z.; Liu, X.; Ding, J.; Liu, J.; Han, X.; Deng, Y.; Hu, W. Poly(acrylic acid)-Based Composite Gel Polymer Electrolytes with High Mechanical Strength and Ionic Conductivity toward Flexible Zinc-Air Batteries with Long Cycling Lifetime. ACS Appl. Mater. Interfaces 2022, 14, 49801–49810. [Google Scholar]
- Qu, S.; Liu, J.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Dynamic stretching-electroplating metal-coated textile for a flexible and stretchable zinc-air battery. Carbon Energy 2022, 4, 867–877. [Google Scholar] [CrossRef]
- Qu, X.; Xu, N.; Zhao, L.; Xu, Z. High-performance flexible zinc-air batteries enabled by carboxymethyl cellulose/graphene oxide composite hydrogel electrolytes. New J. Chem. 2025, 49, 7376–7383. [Google Scholar] [CrossRef]
- Garcia-Gaitan, E.; Frattini, D.; Ruiz de Larramendi, I.; Martinez-Ibanez, M.; Gonzalez, D.; Armand, M.; Ortiz-Vitoriano, N. Flexible Gel Polymer Electrolytes Based on Carboxymethyl Cellulose Blended with Polyvinyl Alcohol or Polyacrylic Acid for Zinc-Air Batteries. Batter. Supercaps 2023, 6, e202200570. [Google Scholar] [CrossRef]
- Qu, X.; Zhou, B.; Xu, N.; Zhao, L.; Xu, Z. CNF-reinforced ternary hydrogel electrolyte for flexible houralkaline-neutral zinc-air batteries. Polymer 2025, 336, 128919. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, H.; Choi, J.; Choi, S.J.; Yoon, K.R. Internally connected porous PVA/PAA membrane with cross-aligned nanofiber network for facile and long-lasting ion transport in zinc-air batteries. Energy Storage Mater. 2024, 71, 103594. [Google Scholar] [CrossRef]
- Fan, X.; Liu, J.; Song, Z.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Porous nanocomposite gel polymer electrolyte with high ionic conductivity and superior electrolyte retention capability for long-cycle-life flexible zinc-air batteries. Nano Energy 2019, 56, 454–462. [Google Scholar] [CrossRef]
- Suppanucroa, N.; Yoopensuk, W.; Pimoei, J.; Thanapong-A-Morn, W.; Kao-Ian, W.; Pakawanit, P.; Mahlendorf, F.; Kheawhom, S.; Somwangthanaroj, A. Enhanced long-term stability of zinc-air batteries using a quaternized PVA-chitosan composite separator with thin-layered MoS2. Electrochim. Acta 2025, 510, 145361. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Jiang, Y.; Ding, Q.; Han, W. Nanocellulose composite gel with high ionic conductivity and long service life for flexible zinc-air battery. Polym. Test. 2021, 104, 107380. [Google Scholar] [CrossRef]
- Lee, H.J.; Lim, J.M.; Kim, H.W.; Jeong, S.H.; Eom, S.W.; Hong, Y.T.; Lee, S.Y. Electrospun polyetherimide nanofiber mat-reinforced, permselective polyvinyl alcohol composite separator membranes: A membrane-driven step closer toward rechargeable zinc-air batteries. J. Membr. Sci. 2016, 499, 526–537. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.; Yang, X.; Ke, C.; Chen, H.; Li, Q.; Guo, J.; He, Y.; Guo, Z.; Liang, X. Gel polymer electrolyte with MXene to extend cycle lifespan of flexible and rechargeable Zinc-Air batteries. J. Power Sources 2022, 523, 231020. [Google Scholar] [CrossRef]
- Wang, Y.; Long, J.; Hu, J.; Sun, Z.; Meng, L. Polyvinyl alcohol/Lyocell dual-layer paper-based separator for primary zinc-air batteries. J. Power Sources 2020, 453, 227853. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Chen, D.; Sha, P.; Yong, X.; Liu, K.; Pang, B.; Zhang, Q.; Yu, J.; Yu, L.; et al. High-performance flexible zinc-air batteries with layered Zn/C3N4-carbon nanotube/polyvinyl alcohol-epoxy resin composite: In-situ crosslinking and electrode-electrolyte integration. J. Power Sources 2025, 625, 235606. [Google Scholar] [CrossRef]
- Zhao, N.; Wu, F.; Xing, Y.; Qu, W.; Chen, N.; Shang, Y.; Yan, M.; Li, Y.; Li, L.; Chen, R. Flexible Hydrogel Electrolyte with Superior Mechanical Properties Based on Poly(vinyl alcohol) and Bacterial Cellulose for the Solid-State Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2019, 11, 15537–15542. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Xiao, S.; Yu, B.; Wu, W.; Liu, B.; Wu, L. Dual-Modified Thermoset Polyurethane Particles as Active Organic Additives in Electrolytes for High-Performance Flexible Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2025, 7, 6010–6020. [Google Scholar]
- Wu, M.; Huang, N.; Lv, M.; Wang, F.; Ma, F.; Deng, Y.; Sun, P.; Zheng, Y.; Liu, W.; Ye, L. Organic-inorganic hybrid hydrogel electrolyte for high-performance quasi-solid-state zinc-air batteries. Front. Chem. Sci. Eng. 2025, 19, 15. [Google Scholar]
- Coello-Mauleon, C.; Arredondo-Espinola, A.; Alvarez-Contreras, L.; Guerra-Balcazar, M.; Arjona, N. Gel polymer electrolytes containing SiO2 spheres with tuned sizes to increase rechargeability of quasi-solid-state Zn-air batteries. Electrochim. Acta 2024, 508, 145265. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Liu, R.; Chen, W.; Zhang, H.; Zhang, Z. Gel Polymer-Based Composite Solid-State Electrolyte for Long-Cycle-Life Rechargeable Zinc-Air Batteries. ACS Sustain. Chem. Eng. 2023, 11, 3732–3739. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Zheng, X.; Wang, M.; Ye, X.; Liu, B.; Han, L.; Ning, Y.; Lu, Y.; Zhang, S.; et al. High-Performance Flexible Zinc-Air Batteries Enabled by a Sodium Polyacrylate-Based Gel Electrolyte Containing Graphene Oxide and Cellulose Nanofibers. Energy Fuels 2023, 37, 16097–16104. [Google Scholar] [CrossRef]
- Chen, S.; Zhong, J.; Zhang, Y.; Zeng, Z.; Fang, X.; Yuan, D. Efficient electrocatalysts for zinc-air batteries featuring CoFe/CoFe2O4 heterojunctions in a nitrogen-doped carbon framework. Int. J. Hydrogen Energy 2025, 159, 150580. [Google Scholar]
- Liu, Y.; Bildan, D.; Zhuge, X.; Liu, T.; Zhong, H.; Luo, Z.; Lei, H.; Luo, K.; Ren, Y.; Bayati, M.; et al. Composite Gel Polymer Electrolyte for High-Performance Flexible Zinc-Air Batteries. Small 2025, 21, 202408015. [Google Scholar]
- Qiu, K.; Wang, F.; Liao, M.; Zhu, K.; Chen, J.; Zhang, W.; Xia, Y.; Dong, X.; Wang, F. A Fumed SiO2-based Composite Hydrogel Polymer Electrolyte for Near-Neutral Zinc-Air Batteries. Acta Phys. Chim. Sin. 2024, 40, 2304036. [Google Scholar] [CrossRef]
- Qu, X.; Liu, G.; Xu, N.; Zhao, L.; Xu, Z. Study on the enhancement of flexible zinc-air battery performance with polyethylene glycol and nano SiO2 composite hydrogel. J. Electroanal. Chem. 2024, 974, 118721. [Google Scholar] [CrossRef]
- Yang, C.C.; Lin, S.J. Alkaline composite PEO-PVA-glass-fibre-mat polymer electrolyte for Zn-air battery. J. Power Sources 2002, 112, 497–503. [Google Scholar] [CrossRef]
- Wu, G.M.; Lin, S.J.; Yang, C.C. High performance composite solid polymer electrolyte systems for electrochemical cells. J. Power Sources 2013, 244, 287–293. [Google Scholar] [CrossRef]
- Nanthapong, S.; Kheawhom, S.; Klaysom, C. MCM-41/PVA Composite as a Separator for Zinc-Air Batteries. Int. J. Mol. Sci. 2020, 21, 7052. [Google Scholar] [CrossRef]
- Fan, X.; Wang, H.; Liu, X.; Liu, J.; Zhao, N.; Zhong, C.; Hu, W.; Lu, J. Functionalized Nanocomposite Gel Polymer Electrolyte with Strong Alkaline-Tolerance and High Zinc Anode Stability for Ultralong-Life Flexible Zinc-Air Batteries. Adv. Mater. 2023, 35, 2209290. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Xu, N.; Zhao, L.; Xu, Z. Hyaluronic acid-reinforced PAM/CMC/HA ternary composite hydrogel electrolyte and its application in flexible zinc-air batteries. J. Alloys Compds. 2025, 1036, 181802. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Liu, B.; Li, Z.; Zhang, J.; Yang, G.; Hiralal, P.; Jin, S.; Zhou, H. Flexible and anti-freezing zinc-ion batteries using a guar-gum/sodium-alginate/ethylene-glycol hydrogel electrolyte. Energy Storage Mater. 2021, 41, 599–605. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, L.; Dargusch, M. Mechanisms of Anode Interfacial Phenomena and Multi-perspective Optimization in Aqueous Alkaline Zinc-Air Batteries. Adv. Funct. Mater. 2025, 35, 201510263. [Google Scholar] [CrossRef]
- Tsehaye, M.T.; Alloin, F.; Iojoiu, C. Prospects for Anion-Exchange Membranes in Alkali Metal-Air Batteries. Energies 2019, 12, 4702. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, L. Multifunctional composite electrolytes for mechanically-robust and high energy density carbon fiber structural batteries. Chem. Eng. J. 2024, 497, 154786. [Google Scholar] [CrossRef]
- Wang, M.; Emre, A.; Tung, S.; Gerber, A.; Wang, D.; Huang, Y.; Cecen, V.; Kotov, N.A. Biomimetic Solid-State Zn2+ Electrolyte for Corrugated Structural Batteries. ACS Nano 2019, 13, 1107–1115. [Google Scholar] [CrossRef]
- Liu, X.; Han, Q.; Wang, J.; Shi, M.; Liu, C. Tree root-inspired structural electrolyte for laminated carbon fiber reinforced composites with high energy density and mechanically-robust properties. Chem. Eng. J. 2022, 449, 137828. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, G.; Wang, Z.; Tang, J.; Wang, S.; Qiang, J.; Cui, Y.; Chen, Y.; Zhang, L.; Deng, L.; et al. A zinc-ion battery based machinable structure energy storage material with a high specific energy and good environmental adaptability. Nano Energy 2025, 142, 111210. [Google Scholar] [CrossRef]
- Wang, M.; Vecchio, D.; Wang, C.; Emre, A.; Xiao, X.; Jiang, Z.; Bogdan, P.; Huang, Y.; Kotov, N.A. Biomorphic structural batteries for robotics. Sci. Robot. 2020, 5, eaba1912. [Google Scholar] [CrossRef]
- Li, L.; Liu, L.; Qing, Y.; Zhang, Z.; Yan, N.; Wu, Y.; Tian, C. Stretchable alkaline poly(acrylic acid) electrolyte with high ionic conductivity enhanced by cellulose nanofibrils. Electrochim. Acta 2018, 270, 302–309. [Google Scholar] [CrossRef]
- Huang, P.Y.; Hung, F.Y.; Huang, B.C. Preliminary study of novel all-solid-state tin-graphite battery based on composite solid electrolyte. Energy Storage 2024, 6, e603. [Google Scholar] [CrossRef]
- Cui, H.; Gao, X.; Guo, K.; Liu, W.; Ouyang, B.; Yi, W. Lewis Acid-Base Synergistically Enhancing Practical Composite Electrolyte for Fluoride-ion Batteries at Room Temperature. Adv. Sci. 2025, 12, 2502824. [Google Scholar] [CrossRef] [PubMed]
- Gurulakshmi, S.; Madeswaran, S.; Karthikeyan, S.; Porchezhiyan, V. Studies on composite PVA-Ca-NH4CF3SO3-Al2O3 polymer electrolyte for electrochemical devices. Asian J. Chem. 2019, 31, 1181–1188. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Gupta, R.K. Transport property and battery discharge characteristic studies on 1-x(0.75Agl:0.25AgCl): xAl2O3 composite electrolyte system. J. Mater. Sci. 1995, 30, 3612–3618. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Gupta, R.K.; Sinha, C.K.; Kumar, R.; Pandey, G.P. Transport properties and battery discharge characteristics of the Ag+ ion conducting composite electrolyte system (1-x)[0.75AgI: 0.25AgCl]: xFe2O3. Ionics 2004, 10, 113–117. [Google Scholar] [CrossRef]
- Kumari, P.; Mohapatra, S.; Halder, S.; Mogurampelly, S. Viscosity of Pectin-[BMIM][PF6] electrolytes and the interplay of ion-ion interactions. J. Mol. Liqu. 2024, 397, 124159. [Google Scholar] [CrossRef]
- Hadjichristov, G.B.; Marinov, Y.G.; Petrov, A.G.; Koduru, H.K.; Scaramuzza, N. Ion electrolytic flexible composite from poly(ethylene oxide) and E8 nematic liquid crystals. AIP Conf. Proc. 2019, 2075, 160005. [Google Scholar] [CrossRef]
- Koduru, H.K.; Marinov, Y.G.; Hadjichristov, G.B.; Petrov, A.G.; Godbert, N.; Scaramuzza, N. Polyethylene oxide (PEO)—Liquid crystal (E8) composite electrolyte membranes: Microstructural, electrical conductivity and dielectric studies. J. Non-Cryst. Sol. 2018, 499, 107–116. [Google Scholar] [CrossRef]
- Koduru, H.K.; Marinov, Y.G.; Hadjichristov, G.B.; Scaramuzza, N. Characterization of polymer/liquid crystal composite based electrolyte membranes for sodium ion battery applications. Solid State Ion. 2019, 335, 86–96. [Google Scholar] [CrossRef]
- Kim, J.N.; Park, J.W. Characteristics of polymer composite electrolyte for MEMS power system. Mater. Sci. Forum 2005, 486–487, 566–569. [Google Scholar] [CrossRef]
- Ragab, H.M. Enhancement in optical, thermal and electrical properties of Polyvinyl pyrrolidone/polyethylene oxide matrix-based nanocomposites for advanced flexible optoelectronic technologies considering nanoceramic zinc oxide/titanium dioxide filler. J. Mol. Struct. 2023, 1275, 134663. [Google Scholar] [CrossRef]
- Austin Suthanthiraraj, S.; Kumara Vadivel, M. Electrical and structural studies on (PEO)50-AgCF3SO3:SnO2 nanocomposite gel polymer electrolyte materials. Trans. Ind. Inst. Met. 2011, 64, 149–153. [Google Scholar] [CrossRef]
- la Monaca, A.; Girard, G.; Savoie, S.; Bertoni, G.; Krachkovskiy, S.; Vijh, A.; Pierini, F.; Rosei, F.; Paolella, A. Synthesis of electrospun NASICON Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte nanofibers by control of germanium hydrolysis. J. Electrochem. Soc. 2021, 168, 110512. [Google Scholar] [CrossRef]
- Johari, N.S.M.; Jonderian, A.; Jia, S.P.; Cozea, V.; Yao, E.L.S.; Adnan, S.B.R.S.; Ahmad, N.; McCalla, E. High-throughput development of Na2ZnSiO4-based hybrid electrolytes for sodium-ion batteries. J. Power Sources 2022, 541, 231706. [Google Scholar] [CrossRef]
- Tominaga, Y.; Asai, S.; Sumita, M.; Panero, S.; Scrosati, B. Fast ionic conduction in PEO-based composite electrolyte filled with ionic liquid-modified mesoporous silica. Electrochem. Solid-State Lett. 2005, 8, A22–A25. [Google Scholar] [CrossRef]
- Lalia, B.S.; Yamada, K.; Hundal, M.S.; Park, J.S.; Park, G.G.; Lee, W.Y.; Kim, C.S.; Sekhon, S.S. Physicochemical studies of PVdF-HFP-based polymer-ionic liquid composite electrolytes. Appl. Phys. A 2009, 96, 661–670. [Google Scholar] [CrossRef]
- Kozdra, M.; Brandell, D.; Araujo, C.M.; Mace, A. The sensitive aspects of modelling polymer-ceramic composite solid-state electrolytes using molecular dynamics simulations. Phys. Chem. Chem. Phys. 2024, 26, 6216–6227. [Google Scholar] [CrossRef] [PubMed]
- Kalnaus, S.; Sabau, A.S.; Newman, S.; Tenhaeff, W.E.; Daniel, C.; Dudney, N.J. Effective conductivity of particulate polymer composite electrolytes using random resistor network method. Solid State Ion. 2011, 199–200, 44–53. [Google Scholar] [CrossRef]
- Islam, M.D.; Liu, S.; Choi, D.; Guo, Z.; Ryu, J.E. Physics-based Computational Method Predicting the Dielectric Properties of Polymer Nanocomposites. Appl. Compos. Mater. 2022, 29, 1579–1595. [Google Scholar] [CrossRef]
- Khodabandehloo, N.; Mozaffari, K.; Liu, L.; Sharma, P. Micro-Structural Design of Soft Solid Composite Electrolytes with Enhanced Ionic Conductivity. J. Appl. Mech. 2022, 89, 051004. [Google Scholar] [CrossRef]
- Offia-Kalu, N.E.; Nwanonenyi, S.C.; Abdulhakeem, B.; Dzade, N.Y.; Onwalu, P.A. Theoretical investigation of electronic, energetic, and mechanical properties of polyvinyl alcohol/cellulose composite hydrogel electrolyte. J. Mol. Graph. Modell. 2024, 127, 108667. [Google Scholar] [CrossRef]
- Brechtl, J.; Ullman, A.M.; Li, K.; Yang, G.; Nanda, J.; Nawaz, K.; Sacci, R.L. Phase change electrolytes for combined electrochemical and thermal energy storage. Energy Rep. 2024, 11, 3931–3940. [Google Scholar] [CrossRef]
- Hibino, T.; Kobayashi, K.; Nagao, M.; Yamamoto, Y. Design of a rechargeable fuel-cell battery with enhanced performance and cyclability. J. Electrochem. Soc. 2016, 163, A1420–A1428. [Google Scholar] [CrossRef]
- Han, D.; Shanmugam, S. Active material crossover suppression with bi-ionic transportability by an amphoteric membrane for Zinc-Bromine redox flow battery. J. Power Sources 2022, 540, 231637. [Google Scholar] [CrossRef]
- Chola, N.M.; Bavdane, P.P.; Nagarale, R.K. “SPEEK-COF” Composite Cation Exchange Membrane for Zn-I2 Redox Flow Battery. J. Electrochem. Soc. 2022, 169, 100542. [Google Scholar] [CrossRef]
- Ge, Y.; Holze, R. All-Liquid Metal Battery. Encyclopedia 2022, 2, 1859–1865. [Google Scholar] [CrossRef]
- Zhu, L.; Virkar, A.V. Conversion Kinetics and Ionic Conductivity in Na-β″-Alumina + YSZ (Naβ″AY) Sodium Solid Electrolyte via Vapor Phase Conversion Process. Membranes 2022, 12, 567. [Google Scholar] [CrossRef]
- Wen, Z.; Gu, Z.; Xu, X.; Cao, J.; Zhang, F.; Lin, Z. Research activities in Shanghai Institute of Ceramics, Chinese Academy of Sciences on the solid electrolytes for sodium sulfur batteries. J. Power Sources 2008, 184, 641–645. [Google Scholar] [CrossRef]
- Mali, A.; Petric, A. Fabrication of a thin walled β″-alumina electrolyte cells. J. Power Sources 2011, 196, 5191–5196. [Google Scholar] [CrossRef]
- Jo, S.H.; Muralidharan, P.; Kim, D.K. Electrical properties of gadolinium-doped Ceria/Magnesia (CGO/MgO) composite electrolytes. Korean J. Mater. Res. 2008, 18, 470–474. [Google Scholar]
- You, H.; Chen, G.; Liu, R.; Zhou, Y.; Abuliti, A.; Ding, X. Preparation and electrochemical properties of NiO-CGO anode support solid oxide fuel cell with ScSZ/CGO composite electrolyte. J. Chin. Ceram. Soc. 2009, 37, 1306–1310. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).