Abstract
The dissociation of the C-NO2 bond is the initial step in the process of the detonation of nitroaromatic explosives. The strength of the C-NO2 bond is significantly influenced by the relative position of the nitro group with respect to the aromatic ring plane since the planar arrangement enables the delocalization of electron density, which strengthens this bond. In this study, we have combined a statistical analysis of geometrical parameters extracted from crystal structures of trinitroaromatic molecules with ab initio calculations of non-covalent index plots and Wiberg bond index values for selected trinitroaromatic molecules to elucidate the influence of nearby substituents on the relative position of nitro groups with respect to the aromatic ring plane. The results of the analysis showed that neighboring substituents have a significant impact on the geometry of nitro groups. The results also showed that this influence arises from the repulsive interaction of voluminous substituents, attractive non-covalent contacts, and the electronic effects of substituents.
1. Introduction
The design of high-energy materials (HEMs) with improved detonation characteristics has been the focus of numerous theoretical and experimental studies in recent years [1,2,3,4,5,6,7,8,9,10]. The ultimate goal in that regard is achieving a balance between two main HEM features: the detonation efficiency and sensitivity of explosives with respect to external stimuli [6,7]. It is known that the high efficiency of the majority of explosives is usually accompanied by high sensitivity towards detonation. However, a careful analysis of all underlying factors can offer a path for fine-tuning those features, leading to a satisfactory detonation performance and the moderate or low sensitivity of the designed explosive. A major obstacle in the HEM design process is the fact that all the factors that affect the detonation performance and sensitivity of HEMs are not completely understood yet. All known factors that affect the detonation features of HEMs can be classified into two groups: electronic and geometric factors. The most important electronic factors in that regard are bond dissociation energies of the weakest bonds in the HEM molecule (“trigger-bonds”), HOMO/LUMO gap values, and the presence of strong, positive electrostatic potential in the central region of the HEM molecule [11]. This is especially important in the case of nitroaromatic explosives since, in these molecules, it is relatively easy to define the central region of the molecular surface. It is considered that strong positive values of electrostatic potential in the central parts of the molecular surface are related to the high sensitivity of explosives towards detonation [6,7]. This trend was noticed among the HEM compounds of the same type and confirmed both theoretically and experimentally.
Among the geometric factors, the most important are the length of “trigger-bonds” in HEMs and the deviation of the NO2 group from the aromatic ring plane (in the case of nitroaromatic explosives). The role of the bond length is rationalized by the fact that it is often related to the bond strength and in many cases can be an indicator of the BDE of the “trigger-bond”. Another useful indicator of the bond strength is the Wiberg bond index value [11].
In the case of the tilting of the NO2 group with respect to the aromatic ring, quantum chemical studies show that this type of deviation weakens or even breaks delocalization between the nitro group and aromatic ring, leading to the weakening of the “trigger-bonds” and making it prone to bond breaking [12]. The geometry of the NO2 group in nitroaromatic molecules is often affected by non-covalent interactions between these groups and neighboring substituents [12,13]. While electronic factors affecting detonation properties of nitroaromatic HEMs were studied in detail using both experimental and theoretical methods, geometrical factors have not yet been systematically studied due to the presence of various structural features and substituents in nitroaromatic compounds.
Substituents may affect detonation properties in different ways: by changing their electrostatic potential, by forming intramolecular non-covalent interactions that affect the overall stability of the molecule, and via repulsive intramolecular interactions which lead to the decreased stability of the HEM molecule. A study of halogen-containing polycyclic aromatic hydrocarbons with nitro substituents showed that voluminous halogen atoms in the proximity of nitro groups can weaken C-N bonds and affect their mechanical sensitivities. However, all of these influences were noticed in specific cases of particular HEM molecules and were not systematically studied in crystal structures. While the influence of substituents on the electrostatic potential and impact sensitivity of HEM molecules was mainly assessed through quantum chemical calculations, the latter two influences could be assessed by analyzing geometrical parameters (like the tilting of the NO2 group with respect to an aromatic ring) in the crystal structures of selected HEM molecules. In this work, we present a systematic analysis of geometric parameters involving substituents in crystal structures of trinitroaromatic HEM molecules. Geometrical parameters were analyzed and discussed in the context of their potential influence on the detonation properties of the analyzed molecules. Our analysis of crystal structures was further supported by the results of the DFT calculations.
2. Materials and Methods
The Cambridge Structural Database (CSD) was searched for all structures containing trinitroaromatic fragments using the ConQuest software (version 2023.3.0) [14,15]. The following filters were applied in the frame of the CSD search: 3D coordinates determined, with an R factor ≤ 0.05, only non-disordered organic structures and structures without errors were extracted, and polymeric structures and structures containing ions were excluded from the results. The selected crystal structures were used as starting geometries for quantum chemical calculations. Geometry optimizations, the Wiberg bond index (WBI), and the Non-Covalent Interactions Index (NCI) were calculated at the M06/cc-PVDZ level of theory. Vibrational frequencies were calculated for all optimized geometries to confirm that these geometries are true energetic minima. WBI and NCI values were calculated using Multiwfn software (version 3.8.), and NCI plots were visualized using VMD software (version 1.9.4a53) [16,17,18]. Three-dimensional representations of optimized geometries and geometries extracted from crystal structures were visualized using the Avogadro and Mercury (version 2023.3.1.) program packages [19,20]. Bond dissociation energies (BDEs) were calculated at the M06-2X/6-311G** level of theory since it was shown in previous studies that M06-2X is a reliable option for BDE calculations of nitro species [21].
3. Results
3.1. Analysis of Crystal Structures of Trinitro Aromatic Molecules
CSD was searched using the criteria provided in Section 2. Based on these criteria, 268 structures were extracted and selected for further analysis. Since the angle between NO2 and C6 planes (P1/P2 angle) significantly affects the detonation properties of nitroaromatic molecules, the distribution of the P1/P2 angle was studied for trinitroaromatic molecules for different substituents in the ortho position to the NO2 group (Figure 1).
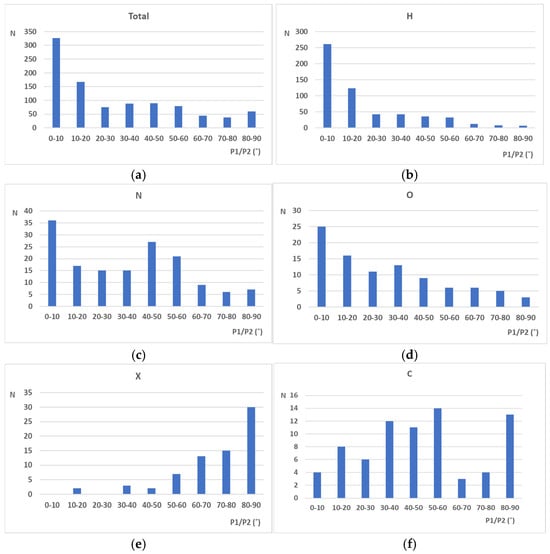
Figure 1.
Distributions of P1/P2 angles for structures with different atoms in the ortho position with respect to the studied NO2 groups. Distribution for (a) all NO2 groups in extracted structures, (b) NO2 groups with a H atom as the atom in the ortho position, (c) NO2 groups with a N atom as the atom in the ortho position, (d) NO2 groups with O atom as atom in the ortho position, (e) NO2 groups with a halogen atom (X) as the atom in the ortho position, and (f) NO2 groups with a C atom as the atom in the ortho position were separately analyzed.
A histogram showing the total distribution of all P1/P2 angles in the extracted crystal structures is given in Figure 1a. An analysis of this histogram shows that, in numerous structures, the plane of the nitro group is coplanar with the plane of the aromatic ring (P1/P2 angle values between 0 and 10°). However, it can be noticed that in the majority of cases, these values are in the interval between 10 and 90°, indicating significant deviation from planarity. To analyze the influence of substituents on the P1/P2 angle values, separated histograms were made for structures containing H, N, O, halogen, and C atoms in the ortho position with respect to the studied nitro group (Figure 1b–f). The histogram in Figure 1b shows the distribution of P1/P2 angle values for structures containing hydrogen atoms in the ortho position with respect to the NO2 group. An analysis of this histogram shows that, in the majority of cases, the P1/P2 angle values fall within the range from 0 to 10°, indicating that the NO2 group is coplanar with the aromatic ring plane. In the case of structures in which N and O atoms are in the ortho position to the NO2 group (Figure 1c,d), in many structures, the P1/P2 angle values are between 0 and 10°, indicating that the NO2 group is coplanar with respect to the aromatic ring. However, in the majority of structures, P1/P2 values are between 10 and 90°, indicating a non-planar arrangement between the aromatic plane and the NO2 group plane. A specific case is depicted in Figure 1e, which shows the distribution of P1/P2 angle values for trinitroaromatic fragments containing halogen atoms in the ortho position to the NO2 group. An analysis of this histogram shows that, in the majority of structures, P1/P2 angle values are close to 90°, indicating the perpendicular orientation of the NO2 group plane with respect to the aromatic ring. In this case, there were no structures with P1/P2 angle values between 0 and 10°. The histogram for structures in which the carbon atom was in the ortho position to the NO2 group (Figure 1f) does not show a clear preference towards either coplanar or perpendicular geometry of the NO2 group with respect to the aromatic ring plane.
To analyze the influence of substituents on the geometry of the NO2 groups in these systems in more detail, histograms showing the dependence of the number of atoms connected to the atom in the ortho position (Figure S1) on P1/P2 angle were analyzed (Figure 2).
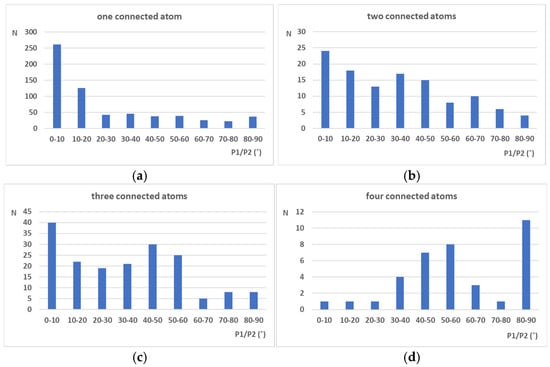
Figure 2.
Distributions of P1/P2 angles for structures with different numbers of atoms connected to the atom in ortho position with respect to the studied NO2 groups. Distribution for the substituents in which (a) one atom is connected to the atom in the ortho position, (b) two atoms are connected to the atom in the ortho position, (c) three atoms are connected to the atom in the ortho position, and (d) four atoms are connected to the atom in the ortho position were separately analyzed.
In the case of crystal structures containing only one atom in the ortho position (connected only to the C atom from the aromatic ring), the histogram (Figure 2a) shows that there is a preference for the coplanar arrangement of NO2 groups and aromatic rings (P1/P2 values in the interval 0–10°). This is not unexpected since substituents with one connected atom are usually not voluminous. The histogram shows a similar distribution compared to the histogram in Figure 1b since many of these structures contain hydrogen atoms in the ortho position. However, it is important to note that, in a significant number of structures, the P1/P2 values indicate a non-planar arrangement of the NO2 group and aromatic ring (Figure 2a).
The distributions for structures that contain atoms in the ortho position with two or three connected atoms (Figure 2b,c) are not as conclusive as the distribution shown in Figure 2a. In these structures, an atom in the ortho position is connected to one C atom from an aromatic ring and to one or two additional atoms. Depending on the nature of the connected atoms, interactions between these substituents and the nitro group may be attractive or repulsive, which significantly affect the P1/P2 angle values and geometry of the nitro group. Distribution of the P1/P2 values for structures in which an atom in the ortho position is connected to four other atoms (one C atom from the aromatic ring and three additional atoms), show preference for the P1/P2 values in the interval between 80 and 90°. This indicates the existence of strong repulsive interactions between the bulk substituent and the nitro group.
3.2. Visual Analysis of Crystal Structures
To elucidate the influence of various substituents on the geometry of nitro groups, we have analyzed selected crystal structures with different types of substituents in ortho positions. The three-dimensional structure of the 1,3,5-trinitrobenzene (TNB) molecule extracted from the TNBENZ10 crystal structure is shown in Figure 3.
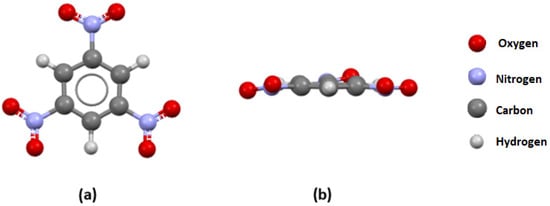
Figure 3.
(a) A front view and (b) a side view of the TNB molecule extracted from the TNBENZ10 crystal structure.
This structure is an excellent candidate for the analysis since it was obtained through a neutron diffraction experiment, and the position of all atoms (including hydrogen atoms) is very reliable. In the TNBENZ10 structure, the substituent in the ortho position to the nitro group is a hydrogen atom connected to the one C atom from the aromatic ring, which corresponds to the distributions shown in Figure 1b and Figure 2a. As expected, all three nitro groups in this structure are mostly coplanar with the aromatic ring plane, with P1/P2 values of 5.96, 9.45, and 10.2°, which aligns with the general trends in Figure 1b and Figure 2a.
Another interesting example is a molecule of 2,4,6-Trinitrophenol (TNP) extracted from the PICRAC12 crystal structure (Figure 4).
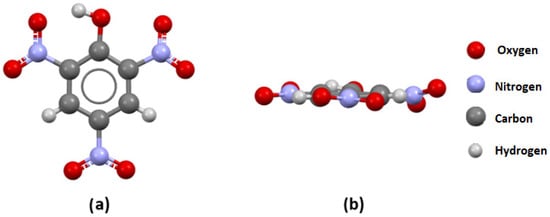
Figure 4.
(a) A front view and (b) a side view of the TNP molecule extracted from the PICRAC12 crystal structure.
In the PICRAC12 structure, the substituent in the ortho position to the nitro group is the OH group (O atom connected to the one C atom from the aromatic ring and one H atom, which corresponds to the distributions shown in Figure 1b,d). The OH substituent significantly affects the geometries of both neighboring NO2 groups in this molecule. The value of the P1/P2 angle between the planes of the aromatic ring, and the NO2 group involved in the hydrogen bond with the OH group is only 3.15°, indicating a planar arrangement. On the other hand, the value of the P1/P2 angle between the planes of the aromatic ring and the other neighboring NO2 group (non-involved in the hydrogen bond) is 21.9°, indicating significant deviation from planarity.
The 2,4,6-Trinitrophenylmethylnitramine molecule (TETRYL) extracted from the MTNANL crystal structure is depicted in Figure 5.
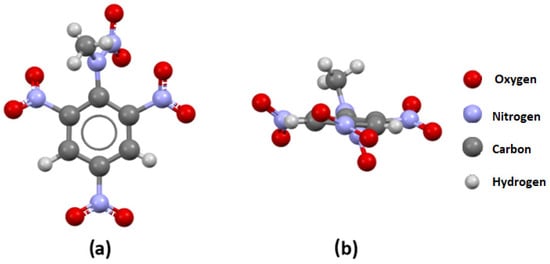
Figure 5.
(a) A front view and (b) a side view of the TETRYL molecule extracted from the MTNANL crystal structure.
In this structure, there are two nitro groups in the ortho position to the bulk substituent. The P1/P2 angles between the planes of these two nitro groups and the aromatic ring have values of 43.5° and 24.7°, respectively. This indicates the existence of strong repulsive interactions between the bulk substituent and studied nitro groups.
3.3. Density Functional Theory Calculations
To better understand the role of intramolecular attractive and repulsive interactions within trinitroaromatic molecules on the geometry of nitro groups, a Non-Covalent Index (NCI) analysis was performed on the optimized geometries (Tables S1 and S5) of selected molecules of nitroaromatic explosives, and NCI plots were visualized (Figure 6).
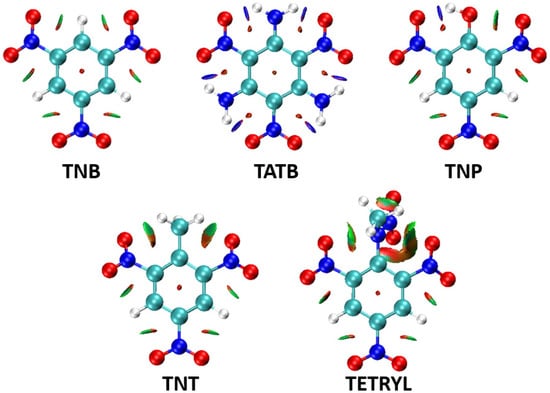
Figure 6.
Calculated NCI plots for optimized geometries of TNB (1,3,5-Trinitrobenzene), TATB (2,4,6-triamino-1,3,5-trinitrobenzene), TNP (2,4,6-Trinitrophenol), TNT (2,4,6-Trinitrotoluene), and the TETRYL (2,4,6-Trinitrophenylmethylnitramine) molecules. Blue surfaces represent strongly attractive areas of non-covalent interactions, green surfaces represent moderately attractive interactions, and brown areas represent repulsive interactions (the selected isosurface value was 0.5, while the color range was in the interval of −0.035 to 0.02). Red atoms represent oxygen, dark blue atoms nitrogen, pale blue atoms carbon and white atoms hydrogen.
An analysis of the calculated NCI plots showed that the influence of substituents on the geometry of nitro groups can be rationalized in terms of non-covalent interaction patterns. In the TNB, areas of weak attractive (green) and repulsive (red) non-covalent interactions are evenly distributed throughout the molecule. There are no voluminous substituents that could induce strong repulsive interactions and lead to the tilting of nitro groups. However, there are also no strong hydrogen bonds that could “lock” nitro groups in the same plane with an aromatic ring. This type of interaction exists in the TATB molecule, in which NH2 substituents form strong hydrogen bonds (blue regions) with the oxygen atoms from nitro groups, leading to the geometry in which all nitro groups are in the same plane with an aromatic ring plane. In the case of the TNP molecule, this type of hydrogen bonding also exists, between the hydrogen atom from the OH substituent and the oxygen atom from the neighboring nitro group. However, there is also an area of repulsive interaction between the oxygen atom from the OH substituent and the oxygen atom from the other neighboring nitro group, which explains the significant deviation from the planarity of this group (P1/P2 angle value is 21.9°, Figure 4b). The influence of repulsive interactions can also be observed in the structures of TNT and TETRYL molecules (Figure 6). In both molecules, bulk substituents are involved in repulsive interactions with neighboring nitro groups (red areas).
To quantitatively describe the influence of these substituents on the strength of C-NO2 “trigger” bonds, Wiberg bond index calculations were performed. Calculated WBI values can provide detailed insight into electron delocalization between the aromatic ring and the nitro group, which is prevented in cases where the nitro group plane is tilted with respect to the aromatic ring plane. These values can also be used for an indirect assessment of the bond strength (higher WBI values indicate stronger covalent bonds). The results of WBI calculations are presented in Figure 7.
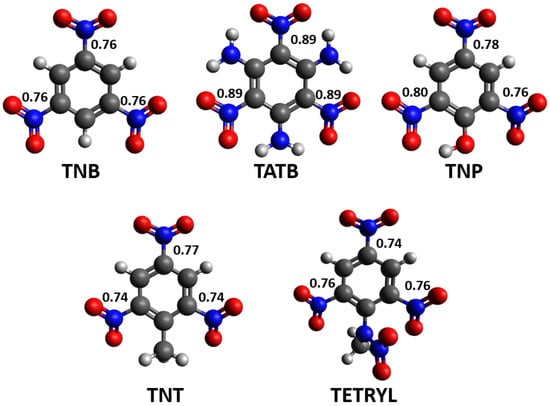
Figure 7.
Calculated WBI values for C-NO2 bonds in selected nitroaromatic molecules. Red atoms represent oxygen, blue atoms nitrogen, gray atoms carbon and white atoms hydrogen.
In the case of the TNB molecule, the WBI values for all three C-NO2 bonds are 0.76, which is expected for symmetric molecules like this. Similar conclusions can be made for very symmetric TATB molecules. In the TATB, the WBI indices values for all three C-NO2 bonds are calculated to be 0.89. Higher WBI values compared to TNT molecules could be rationalized in the context of the intramolecular hydrogen bond network that keeps nitro groups in TATB in the same plane with an aromatic ring. A more interesting case of intramolecular hydrogen bonding can be observed in the TNP molecule. In this structure, the WBI value of the C-NO2 bond involving the hydrogen-bonded nitro group is 0.80, while the WBI value of the C-NO2 bond involving the nitro group that forms repulsive interactions with an oxygen atom of the OH substituent is 0.76. A good example of the influence of the bulk substituent on the geometry of the nitro group is the TNT molecule. Both C-NO2 bonds in the vicinity of the relatively bulky methyl substituent have WBI values of 0.74, which is smaller compared to the C-NO2 bond in the para-position of the molecule (WBI value 0.76). The most complicated situation can be observed in the structure of the TETRYL molecule. Here, the bulk substituent indeed induces the significant tilting of neighboring nitro groups, but it also forms non-covalent interactions with these groups. As a result, both C-NO2 bonds in the vicinity of the bulk substituent in the TETRYL molecule have moderate WBI values (0.76). Also, the electronic effect of every substituent present in these molecules should be taken into account when the results are discussed.
To further evaluate the influence of voluminous substituents on the nitro group geometry, we have calculated BDE values for three model systems containing a voluminous iodine substituent: o-iodonitrobenzene, m-iodonitrobenzene, and p-iodonitrobenzene (Table 1).

Table 1.
Calculated BDE energies for three nitroaromatic molecules with iodine substituents: o-iodonitrobenzene, m-iodonitrobenzene, and p-iodonitrobenzene.
The results of the BDE calculations showed that the C-NO2 bond involving the nitro group in ortho position to the voluminous iodine substituent is the weakest (65.92 kcal/mol), while the C-NO2 bond involving the nitro group in para-position to the voluminous iodine substituent is the strongest (71.87 kcal/mol).
4. Discussion
A statistical analysis of crystallographic data extracted from the CSD presented in Figure 1 shows a clear relation between the size of neighboring substituents and the arrangement of nitro groups with respect to the aromatic ring. In the case of small substituents like hydrogen atoms (Figure 1b) neighboring nitro groups tend to stay in the same plane with the aromatic ring (P1/P2 angle values between 0 and 10°), enabling electron delocalization between this ring and nitro group. This has an overall positive effect on the strength of the C-NO2 bonds, making them less prone to dissociation. Since the dissociation of these “trigger’’ bonds represents the first step in the detonation of the nitroaromatic explosives, strong C-NO2 bonds are usually related to the low sensitivity of explosives towards detonation. The opposite situation can be observed in the case of bulk substituents as halogen atoms (Figure 1e) or substituents with carbon atoms directly connected to the aromatic ring (Figure 1f). Repulsive interactions between these voluminous substituents and nitro groups can lead to the tilting of the nitro group with respect to the aromatic ring (P1/P2 angle values between 10 and 90°), which leads to the weakening of the respective C-NO2 bonds. In the case of halogen substituents, a clear peak can be observed in Figure 1e in the region 80–90°. On the other hand, the situation with the C-substituents (Figure 1f) is more complicated due to the possible other types of interactions between nitro groups and atoms connected to the C atom. However, since these substituents are also voluminous, in the majority of cases, P1/P2 angles are in the interval 10–90°, with a pronounced peak between 50 and 60°, as well as 80 and 90°. The significance of the substituent size for the geometry of the nitro group can also be observed in histograms presented in Figure 2a–d. The histogram, including substituents consisting of only one atom (Figure 2a), shows a pronounced peak in the interval 0–10°, indicating a co-planarity of the nitro group and aromatic ring. On the other hand, in histogram related to substituents connected to two, three, and four atoms (voluminous substituents), the majority of structures have angle P1/P2 values in the interval 10–90°, which corresponds to the non-planar orientation of the nitro group with respect to the aromatic ring plane.
The results of the visual analysis of selected crystal structures are in agreement with the results of the statistical analysis of crystallographic data. In TNB molecules, the lack of voluminous substituents in the proximity of nitro groups can explain the predominantly coplanar arrangement between the nitro group plane and aromatic ring (the largest P1/P2 value is 10.2°). In the TNP molecule, the OH substituent “locks” one of the neighboring nitro groups in the same plane with an aromatic ring through a strong N-O…H hydrogen bond (Figure 4). The deviation of this nitro group from planarity is only 3.15°. However, the deviation of the other nitro group in the vicinity of the OH substituent with respect to the aromatic ring plane is much larger (P1/P2 angle value of 21.9°). This is the consequence of the repulsive interactions between the oxygen atom from the OH substituent and the oxygen atom from the nitro group. In the TETRYL molecule, the situation is more complex. Although the voluminous N-methylnitramine group significantly affects the geometries of neighboring nitro groups through repulsive interactions (the P1/P2 angle values are 43.5 and 24.74°), the hydrogen atoms from this group can also affect overall geometry by forming hydrogen bonds with oxygen atoms from nitro groups. The largest deviations of P1/P2 angle values in these molecules are related to experimentally measured h50 values (indicators of sensitivity). A higher h50 value is related to the lower sensitivity, and a lower h50 value is related to the higher sensitivity of explosives to impacts. The largest deviations in the series TNB (10.2°) < TNP (21.9°) < TETRYL (43.5) are in good agreement with experimentally obtained h50 values for these molecules: TNB (100 cm) > TNP (87 cm) > TETRYL (32 cm). The relation between experimentally obtained h50 values [11,22,23,24] for selected trinitroaromatic compounds and the corresponding P1/P2 angle values from crystal structures is given in Figure 8.
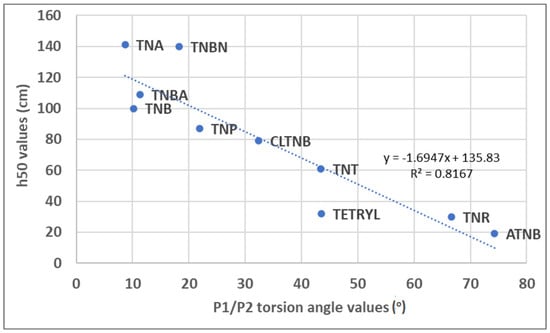
Figure 8.
Scatterplot representing relation between experimental h50 values and P1/P2 angles for 1,3,5-trinitrobenzene (TNB), 2,4,6-trinitrophenol (TNP), 2,4,6-trinitrophenylmethylnitramine (TETRYL), 2,4,6-trinitrotoluene (TNT), 2,4,6-trinitroanline (TNA), 2-azido-1,3,5-trinitrobenzene (ATNB), 2,4,6-trinitrobenzonitrile (TNBN), chloro-2,4,6-trinitrobenzene (CLTNB), 2,4,6-trinitrobenzoic acid (TNBA), and 2,4,6-trinitroresorcinol (TNR).
The analysis of the results presented in Figure 8 shows that, with the increase in the deviation of the nitro group with respect to the aromatic ring plane, h50 values decrease and mechanical sensitivity increases. This is in agreement with the results of the quantum chemical calculations presented in this study. It should be noted that experimentally obtained h50 values strongly depend on experimental conditions and, for this reason, may significantly differ for the same compounds in the literature. The mechanical sensitivity values analyzed in this study were obtained using the same drop weight (2.5 kg).
An analysis of the calculated NCI plots confirms the significance of attractive and repulsive non-covalent interactions for the geometry of nitro groups in the vicinity of the substituents. Small areas of weak attractive and repulsive interactions in the TNB molecule (Figure 7) align with the observed planarity of nitro groups in this structure. Blue areas on the NCI plot of TATB and TNP molecules indicate the presence of strong hydrogen bonds that, in these cases, “lock” neighboring nitro groups in the same plane with an aromatic ring. However, in the TNP molecule, there is only one H atom in the OH substituent available for hydrogen bond formation. The other neighboring nitro group in this molecule is involved in a repulsive interaction with the O atom from the OH substituent, which leads to the deviation of the nitro group from the aromatic ring plane. In the case of TNT and TETRYL molecules, the voluminous methyl and N-methylnitramine groups are involved in repulsive interactions (red areas) with neighboring nitro groups, although weak attractive interactions (green areas) also occur.
Calculated WBI values are in agreement with the analysis of NCI values. In the TNB molecule, an even distribution of areas of weak attractive and repulsive interactions corresponds to the calculated WBI values, which are the same for all three C-NO2 fragments (0.76). A similar situation is with the TATB molecule, where an even distribution of areas of strong hydrogen bonds and weak repulsive interactions explains the same WBI values for all three C-NO2 groups (0.89). In the case of the TNP molecule, a higher WBI value was calculated for the C-NO2 fragment involved in the hydrogen bond with OH substituents than for the nitro group involved in a repulsive interaction with oxygen atom from the OH substituent (0.76). This demonstrates the significance of both attractive and repulsive intramolecular interactions for the stability and strength of “trigger bonds” in nitroaromatic molecules. In the case of the TNT molecule, two C-NO2 fragments in the vicinity of the voluminous methyl group have lower WBI values (0.74) than the C-NO2 fragment in para-position to the methyl group (0.77). The results of WBI calculations for TETRYL molecules show that, although the N-methylnitramine group induces significant deviations from planarity for two neighboring nitro groups, the calculated WBI values for these fragments (0.76) are still higher compared to the C-NO2 fragment in the para-position (0.74). This is the consequence of the electron-withdrawing effect of nitro groups and one N-methylnitramine group, combined with the stabilization of nitro groups in the ortho position due to the formation of attractive non-covalent interactions with the N-methylnitramine group. Since hydrogen atoms from the N-methylnitramine group are out of the aromatic ring plane, hydrogen bonds involving these hydrogen atoms can tilt the nitro group and break delocalization between the nitro group and the aromatic ring. It should be noted that, while the WBI data is internally consistent within a single molecule, the values are not transferable from one molecule to another.
5. Conclusions
In this work, a systematic analysis of the influence of substituents on nitro group geometries was performed on crystal structures of trinitroaromatic molecules. Since the deviation of the nitro group from the aromatic ring plane prevents delocalization and weakens the C-NO2 “trigger” bond, this geometrical characteristic can be used to predict the sensitivity of nitroaromatic molecules towards detonation. An analysis of crystallographic data, combined with the results of DFT calculations, showed that there are three main ways in which the substituent can affect the strength of the weakened C-NO2 “trigger” bond and detonation characteristics of nitroaromatic molecules: (1) through repulsive interaction with neighboring nitro groups that induce the tilting of the nitro group, (2) through the formation of attractive non-covalent interactions, and (3) through the electronic effect of a particular substituent, which can result in the depletion of electron density in the C-NO2 bond region.
An analysis of crystallographic data showed that voluminous ligands in the proximity of the nitro group induce deviation of this group from planarity, which prevents delocalization and weakens the C-NO2 bond. On the other hand, hydrogen bonds between substituents and nitro groups in molecules like TATB or TNP can “lock” certain nitro groups in the same plane with the aromatic ring, enhancing the delocalization and preventing corresponding C-NO2 bonds from dissociation. An analysis of NCI plots and WBI values supports the conclusion drawn from the analysis of geometrical parameters in crystal structures. WBI index values calculated for C-NO2 bonds in proximity to voluminous substituents are generally lower compared to the WBI values of other C-NO2 bonds, indicating weaker delocalization over these bonds. The general trend shows that steric hinderance causes a deviation from the planarity of the nitro group that is not unexpected. However, in cases where more complex substituents are present in the molecular structure, the explanation of the overall effect of the substituent on the strength of the C-N bond has to be discussed in the context of other possible influences, including electronic effects and possible non-covalent interactions. In special cases like that of the TETRYL molecule, the combination of repulsive and attractive interactions, as well as the electron-withdrawing properties of the N-methylnitramine substituent, defines the overall strength of C-NO2 bonds and the detonation characteristic of these molecules. The results of BDE calculations for three nitroaromatic molecules with voluminous substituents (o-iodonitrobenzene, m-iodonitrobenzene, and p-iodonitrobenzene) showed that the weakest C-NO2 bonds are present in fragments in the ortho position to the voluminous substituent.
While the results presented in this paper can be helpful for better understanding the mechanical sensitivities in selected cases of nitroaromatic explosives, it should be pointed out that previous studies showed that C-NO2 homolysis cannot be used to explain trends in sensitivity since the activation energy is relatively insensitive to the ring substituents [25]. Also, the parameters that control the mechanical sensitivity of nitroaromatic explosives only partially depend on the molecular structure of these HEM compounds, which was pointed out in the review article by T. B. Brill and K. J. James [26].
The results of this study can contribute to the design of new types of nitroaromatic high-energy materials with improved performance and stability.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cryst15080692/s1: Table S1: Coordinates for optimized TNB molecule; Table S2: Coordinates for optimized TATB molecule; Table S3: Coordinates for optimized TNP molecule; Table S4: Coordinates for optimized TNT molecule; Table S5: Coordinates for optimized TETRYL molecule. Figure S1: Examples of crystal structures with atom in the ortho position with respect to the nitro group connected to (a) only one atom (Cl atom in CLNOBE02 crystal structure), (b) two atoms (O atom from OH group in crystal structure BAKLII), (c) three atoms (N atom from triazole group of MUKRAM crystal structure), and (d) four atoms (C atom from methyl group of TNOXYL01 crystal structure).
Author Contributions
Conceptualization, D.Ž.V.; methodology, D.Ž.V. and D.S.K.; software, D.Ž.V.; validation, D.S.K., A.B.Đ. and D.B.N.; formal analysis, D.Ž.V., D.S.K., A.B.Đ. and D.B.N.; investigation, D.Ž.V., D.S.K., A.B.Đ. and D.B.N.; resources, D.Ž.V.; data curation, D.Ž.V.; writing—original draft preparation, D.Ž.V.; writing—review and editing, D.S.K.; visualization, D.Ž.V., D.S.K., A.B.Đ. and D.B.N.; supervision, D.Ž.V.; project administration, D.Ž.V.; funding acquisition, D.Ž.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia (contract numbers: 451-03-136/2025-03/200168 and 451-03-136/2025-03/200288), and it contributes to the achievement of Sustainable Development Goals 9 (Industry, Innovation, and Infrastructure) and 12 (Responsible Consumption and Production). This research was supported by the Science Fund of the Republic of Serbia, PROMIS, #6066886, CD-HEM.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders played no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Richardson, P.; Kitos, A.A.; Triglav, M.; Ovens, J.S.; Laroche, I.; Delisle, S.; Jolicoeur, B.; Brusso, J.L.; Murugesu, M. Synthesis and detonation performance of novel tetrazolyl–triazine nitrogen-rich energetic materials. Mater. Adv. 2023, 4, 5775–5784. [Google Scholar] [CrossRef]
- Born, M.; Plank, J.; Klapötke, T.M. Energetic Polymers: A Chance for Lightweight Reactive Structure Materials? Prop. Explos. Pyrotech. 2022, 47, e202100368. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J. Molecular dynamics simulations for the sensitivity and moisture adsorption on the surface of a novel cocrystal: CL-20/DNAN. J. Mol. Model. 2025, 31, 178. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, J.P.; Dodke, V.S. Some Novel High Energy Materials for Improved Performance. Z. Anorg. Allg. Chem. 2021, 647, 1856–1882. [Google Scholar] [CrossRef]
- Born, M.; Karaghiosoff, K.; Klapötke, T.M.; Voggenreiter, M. Oxetane Monomers Based on the Powerful Explosive LLM-116: Improved Performance, Insensitivity, and Thermostability. ChemPlusChem 2022, 87, e202200049. [Google Scholar] [CrossRef] [PubMed]
- Nešić, J.; Cvijetić, I.N.; Bogdanov, J.; Marinković, A. Synthesis and Characterization of Azido Esters as Green Energetic Plasticizers. Propellants Explos. Pyrotech. 2021, 46, 1537–1546. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Detonation Performance and Sensitivity: A Quest for Balance. In Advances in Quantum Chemistry; Academic Press: Cambridge, MA, USA, 2014; Volume 69, pp. 1–30. [Google Scholar]
- Politzer, P.; Murray, J.S. High Performance, Low Sensitivity: Conflicting or Compatible? Propellants Explos. Pyrotech. 2016, 41, 414–425. [Google Scholar] [CrossRef]
- Kretić, D.S.; Maslarević, M.I.; Veljković, D.Ž. Small Deviations in Geometries Affect Detonation Velocities and Pressures of Nitroaromatic Molecules. Organics 2025, 6, 17. [Google Scholar] [CrossRef]
- Kretić, D.S.; Veljković, I.S.; Veljković, D.Ž. Tris(3-nitropentane-2,4-dionato-κ2 O,O′) Complexes as a New Type of Highly Energetic Materials: Theoretical and Experimental Considerations. Chemistry 2023, 5, 1843–1854. [Google Scholar] [CrossRef]
- Shoaf, A.L.; Bayse, C.A. Trigger Bond Analysis of Nitroaromatic Energetic Materials Using Wiberg Bond Indices. J. Comput. Chem. 2018, 39, 1236–1248. [Google Scholar] [CrossRef]
- Aina, A.A.; Misquitta, A.J.; Phipps, M.J.S.; Price, S.L. Charge Distributions of Nitro Groups Within Organic Explosive Crystals: Effects on Sensitivity and Modeling. ACS Omega 2019, 4, 8614–8625. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wei, S.-H.; Zhang, C. Review of the Intermolecular Interactions in Energetic Molecular Cocrystals. Cryst. Growth Des. 2020, 20, 7065–7079. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualising crystal structures. Acta Crystallogr. Sect. B 2002, 58, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Kiselev, V.G. Comment on “Decomposition mechanisms of trinitroalkyl compounds: A theoretical study from aliphatic to aromatic nitro compounds”. Phys. Chem. Chem. Phys. 2015, 17, 10283–10284. [Google Scholar] [CrossRef]
- Storm, C.B.; Stine, J.R.; Kramer, J.F. Sensitivity Relationships in Energetic Materials. In Chemistry and Physics of Energetic Materials; NATO ASI Series; Bulusu, S.N., Ed.; Springer: Dordrecht, The Netherlands, 1990; Volume 309. [Google Scholar]
- Politzer, P.; Murray, J.S. Some molecular/crystalline factors that affect the sensitivities of energetic materials: Molecular surface electrostatic potentials, lattice free space and maximum heat of detonation per unit volume. J. Mol. Model 2015, 21, 25. [Google Scholar] [CrossRef]
- Rice, B.M.; Hare, J.J. A Quantum Mechanical Investigation of the Relation between Impact Sensitivity and the Charge Distribution in Energetic Molecules. J. Phys. Chem. A 2002, 106, 1770–1783. [Google Scholar] [CrossRef]
- Brill, T.B.; James, K.J. Thermal Decomposition of Energetic Materials. 61. Perfidy in the Amino-2,4,6-trinitrobenzene Series of Explosives. J. Phys. Chem. 1993, 97, 8152–8758. [Google Scholar] [CrossRef]
- Brill, T.B.; James, K.J. Kinetics and Mechanisms of Thermal Decomposition of Nitroaromatlc Explosives. Chem. Rev. 1999, 93, 2667–2692. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).