Design and Synthesis of a New Photoluminescent 2D Coordination Polymer Employing a Ligand Derived from Quinoline and Pyridine
Abstract
1. Introduction
2. Materials and Methods
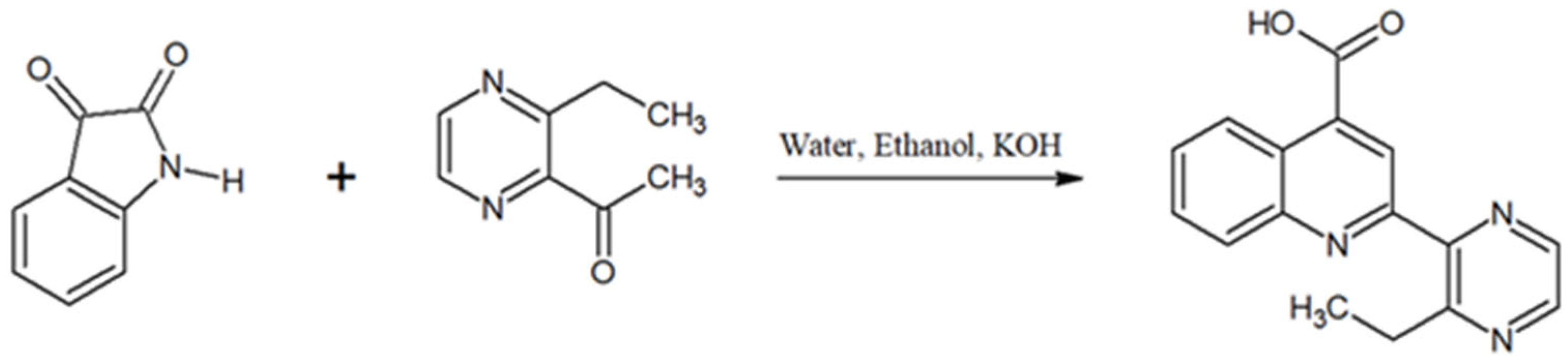
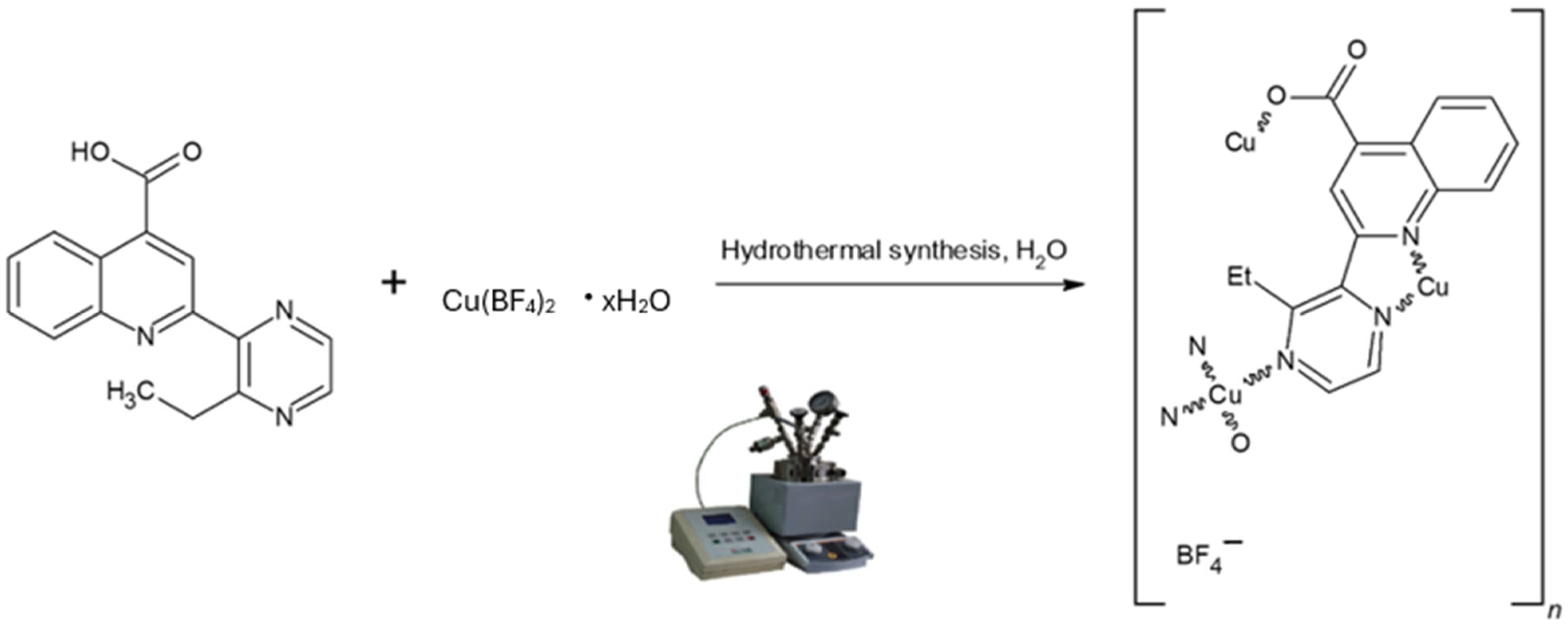
2.1. Synthesis of Crystalline Materials
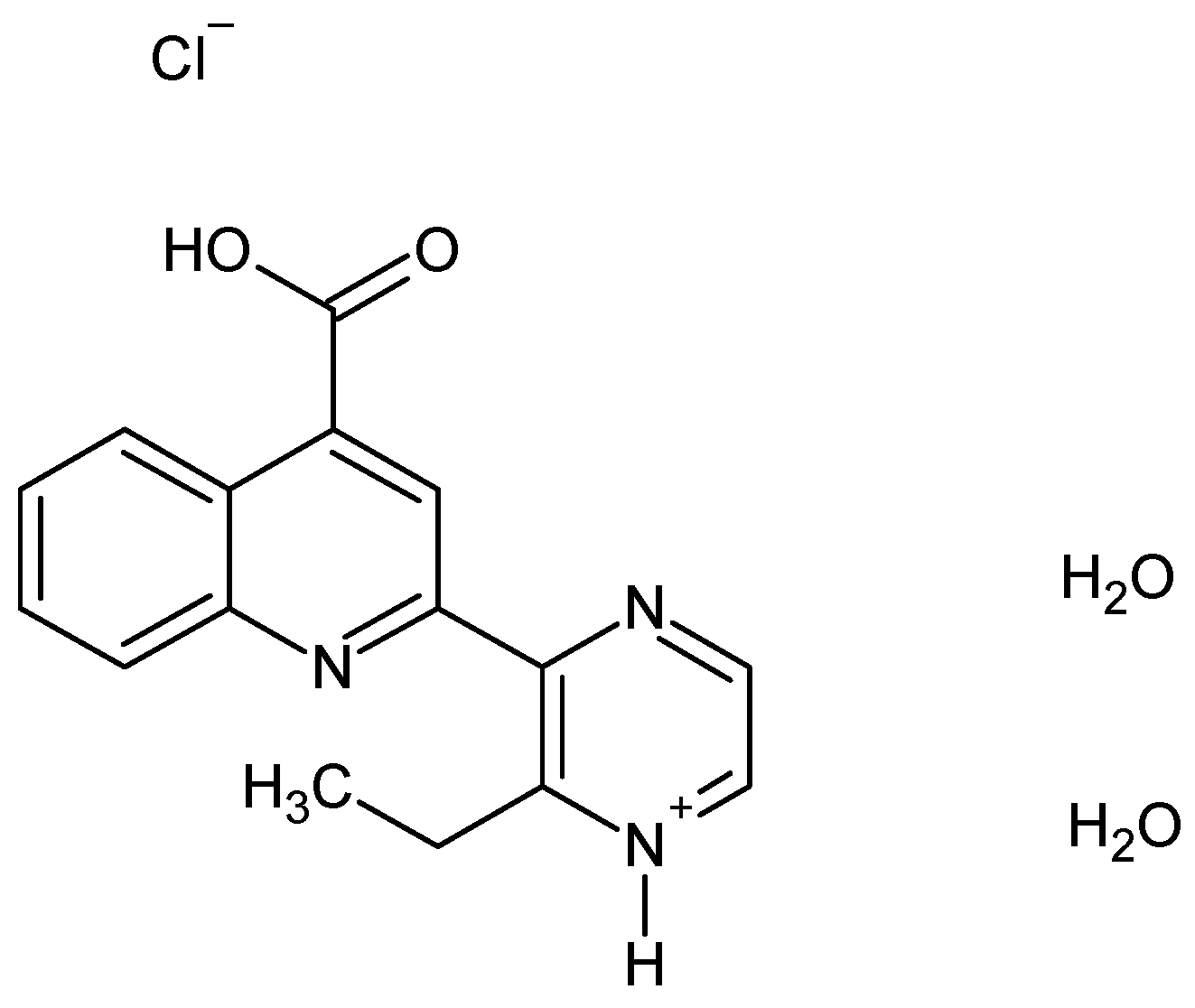
2-(3-Ethyl-pyrazin-2-yl)quinoline-4-carboxylate acid [LH] 1
2.2. IR Spectroscopy
2.3. X-Ray Diffraction Studies
2.3.1. X-Ray Diffraction Experiment
2.3.2. Crystal Structure Refinement
2.4. Photoluminescence Spectra
2.5. Studies of the Magnetic Properties
2.6. NMR
2.7. Computational Methodology
3. Results and Discussion
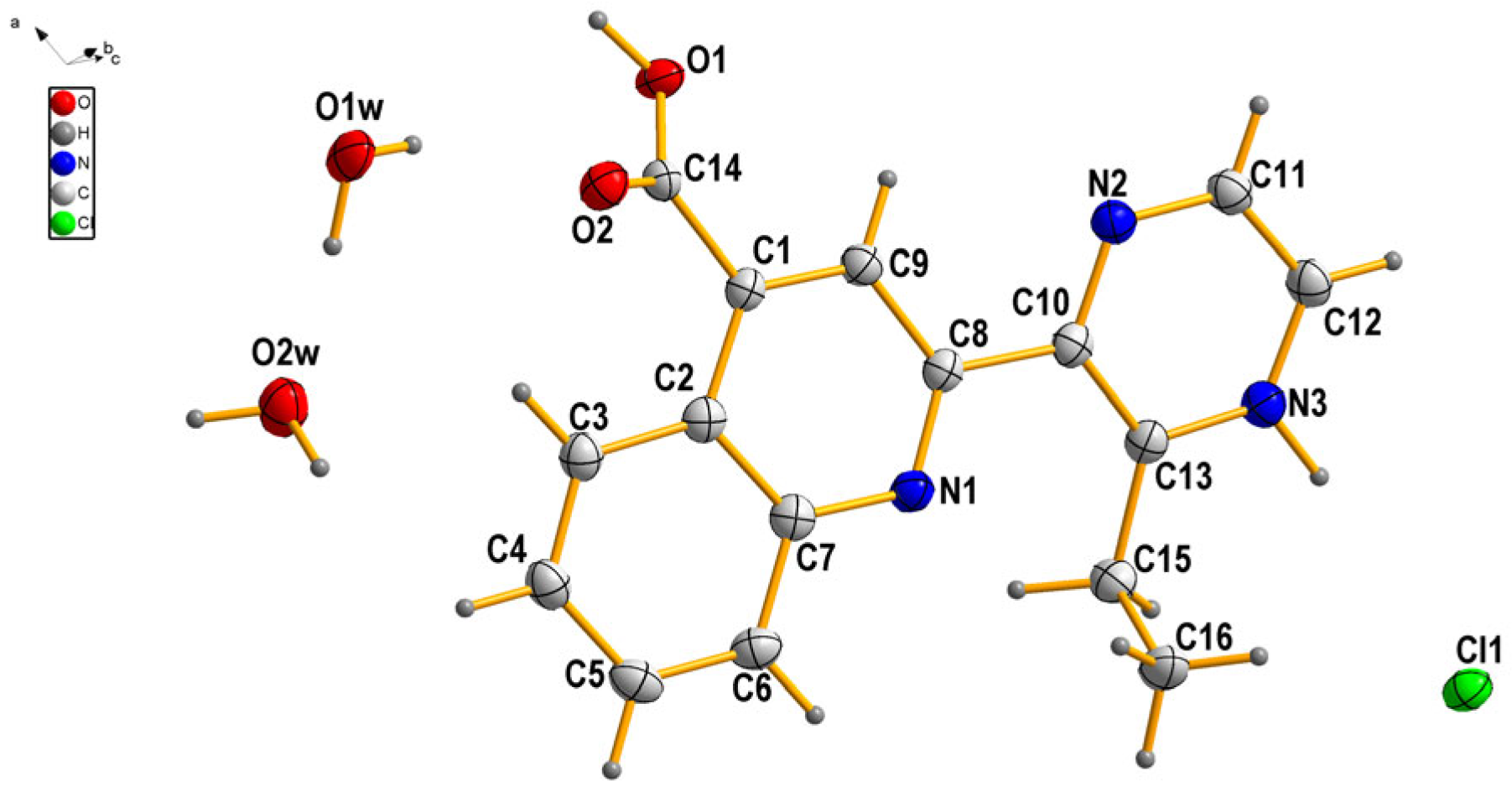
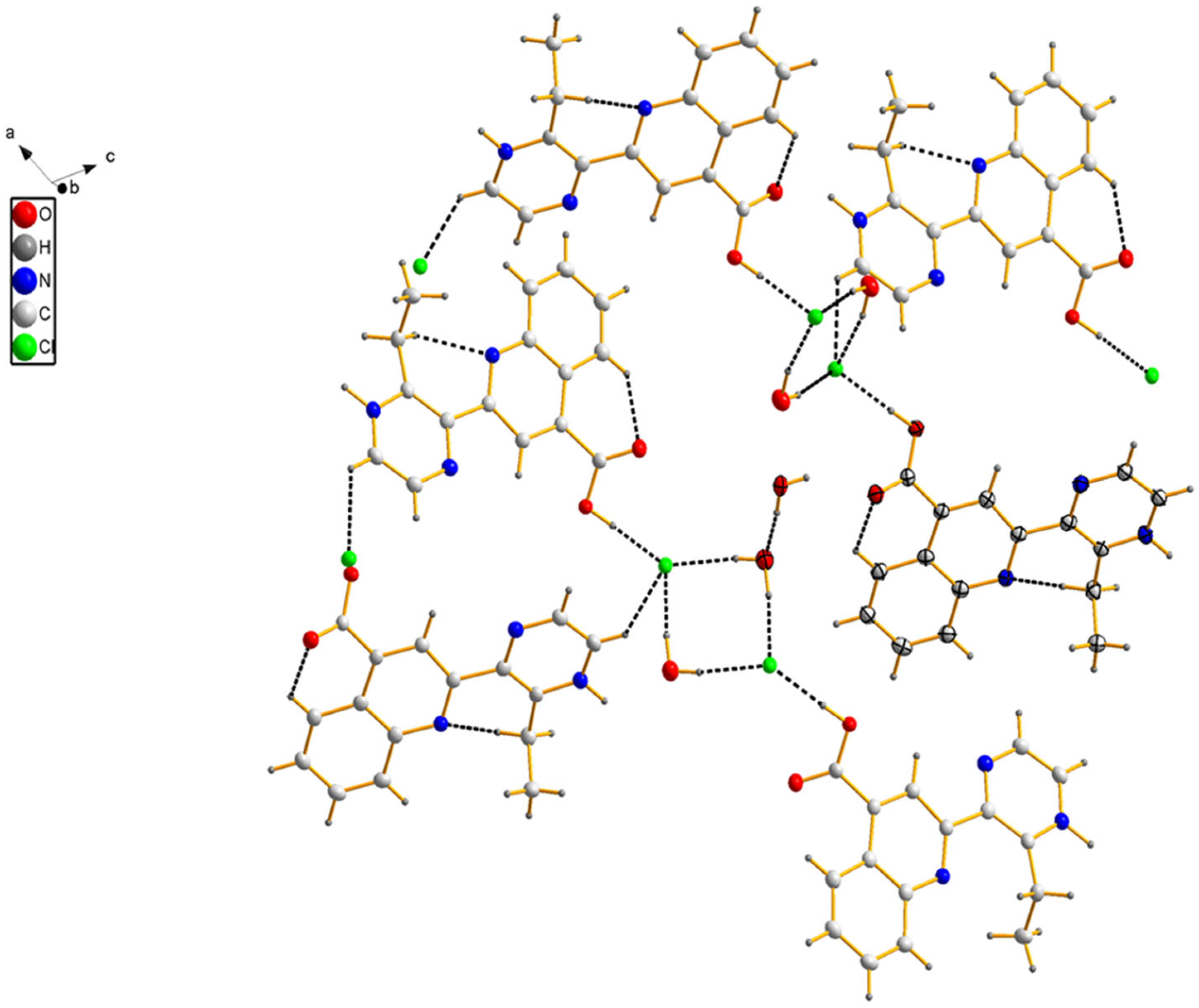
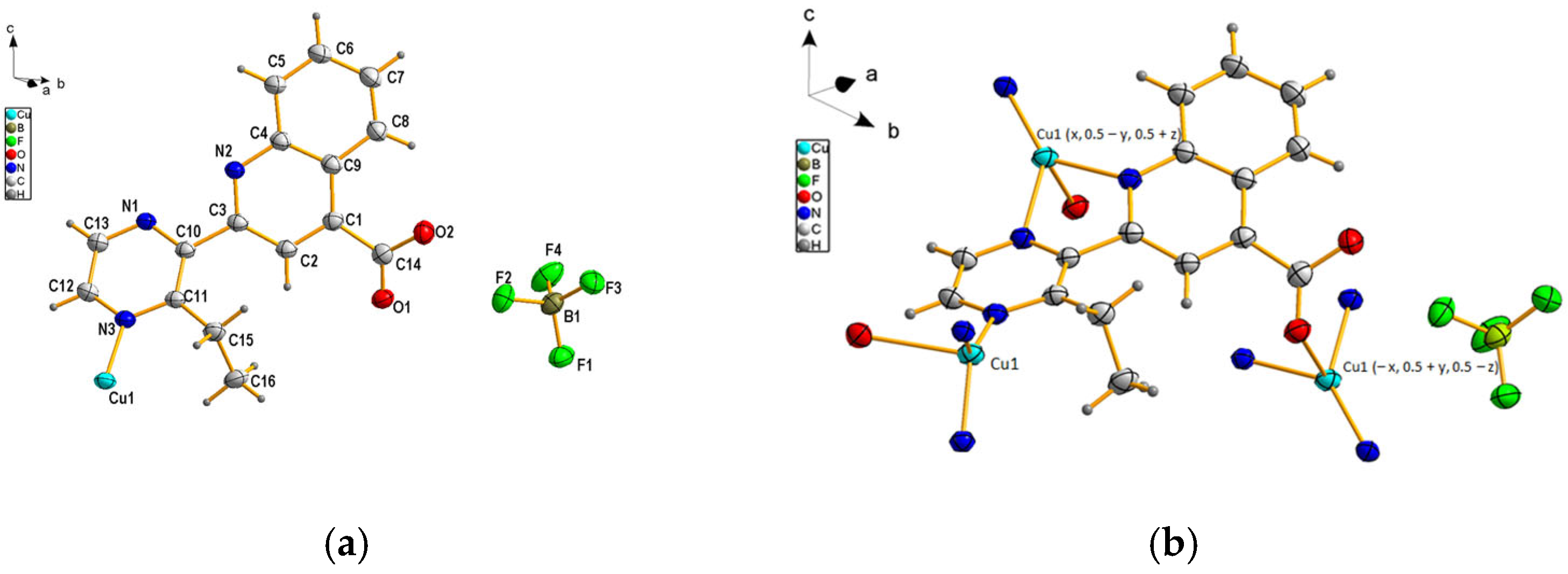
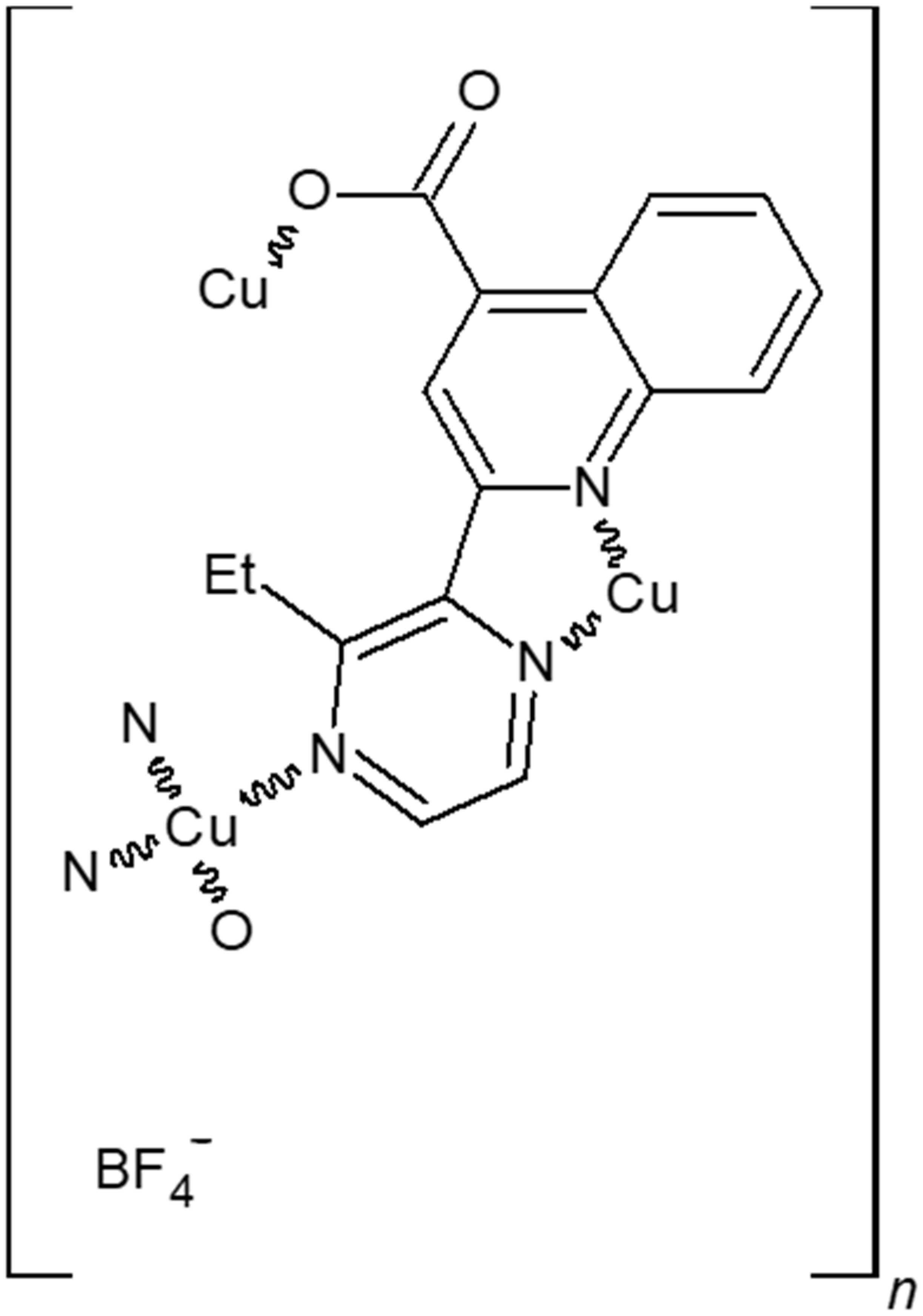
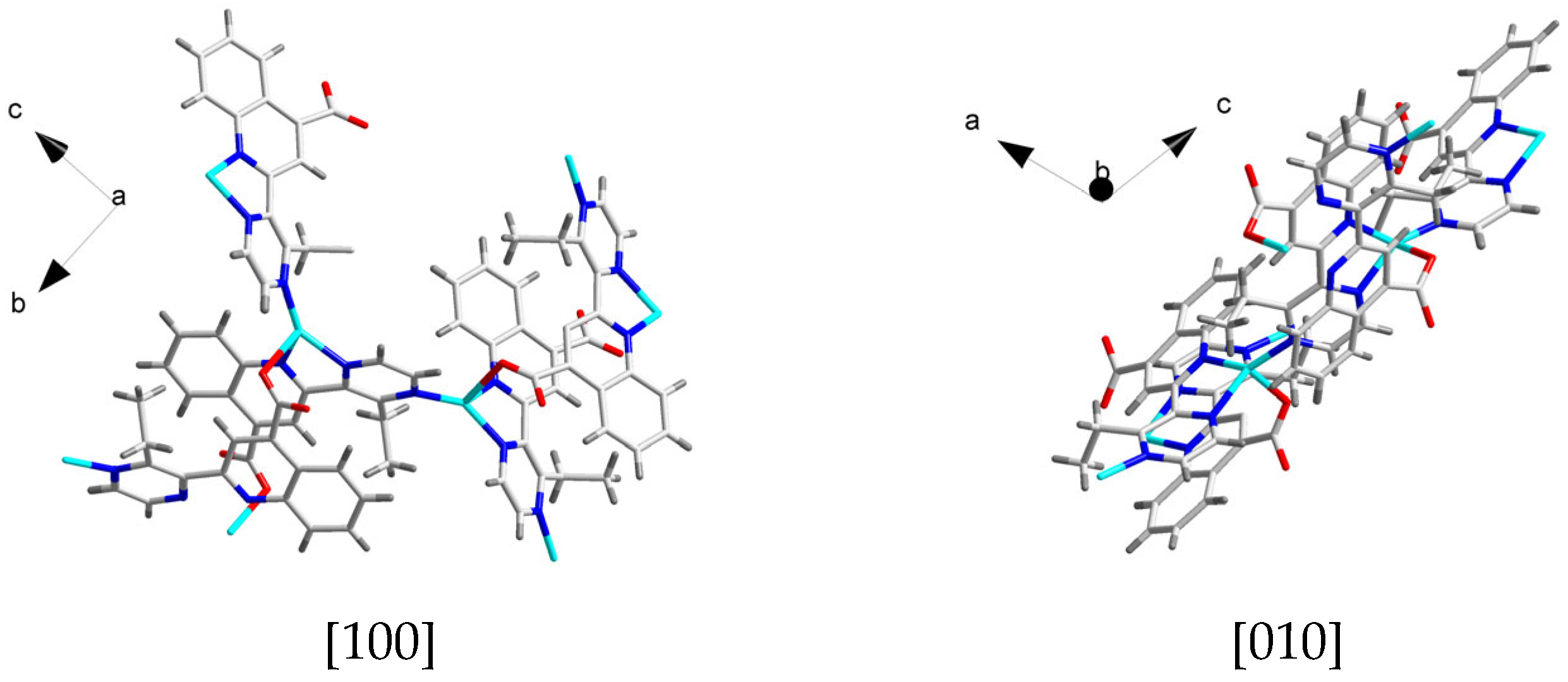
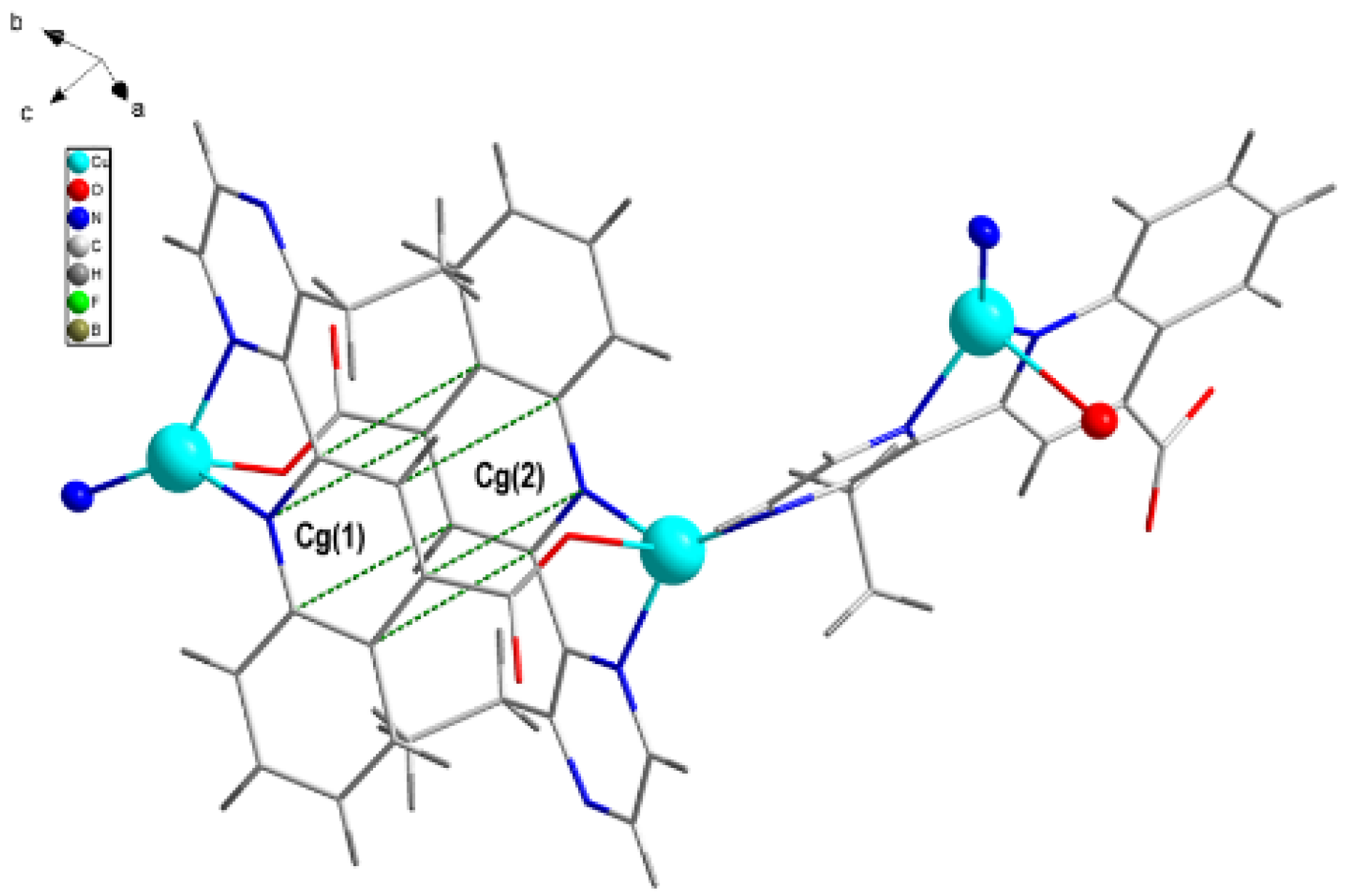
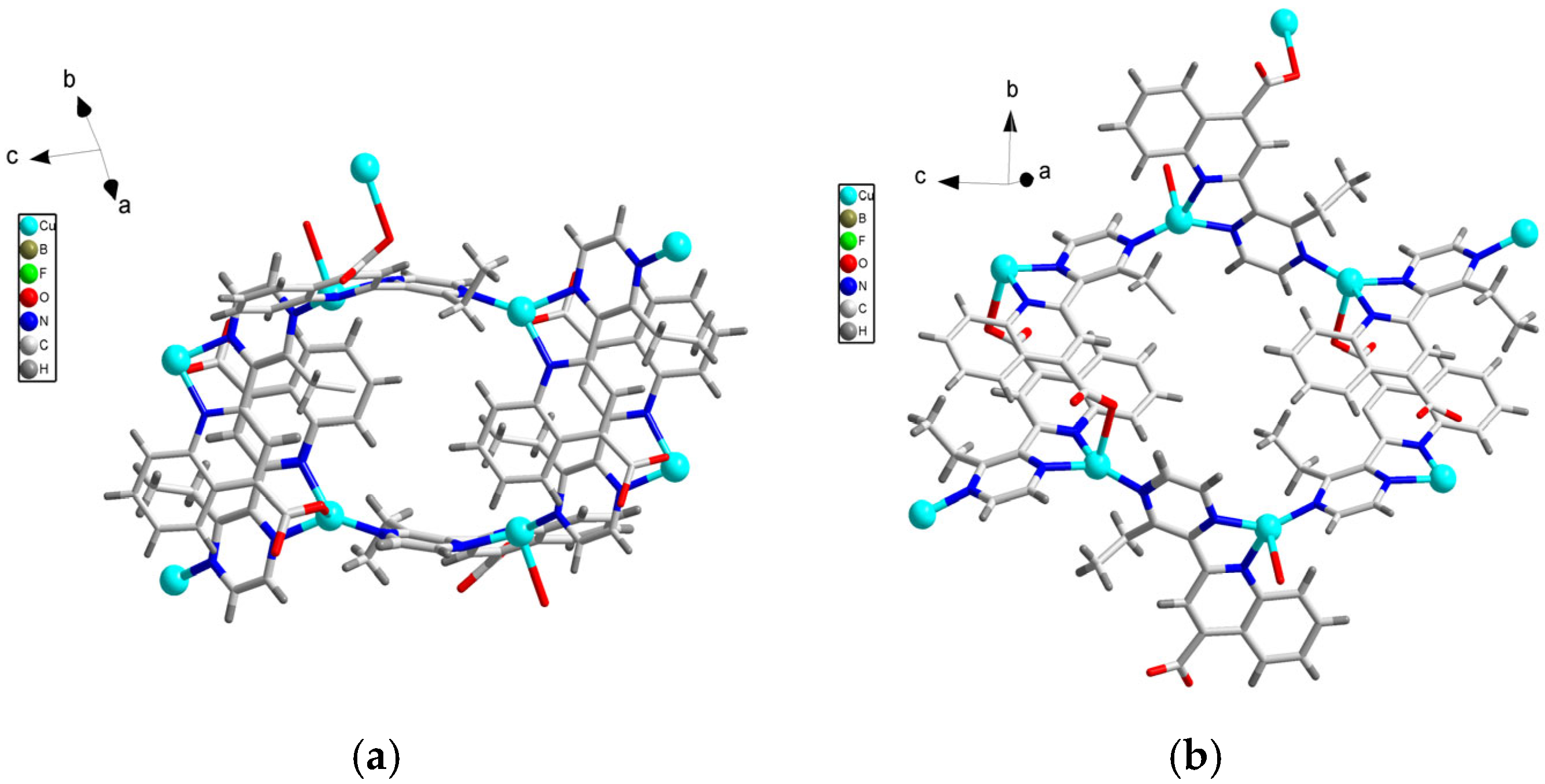
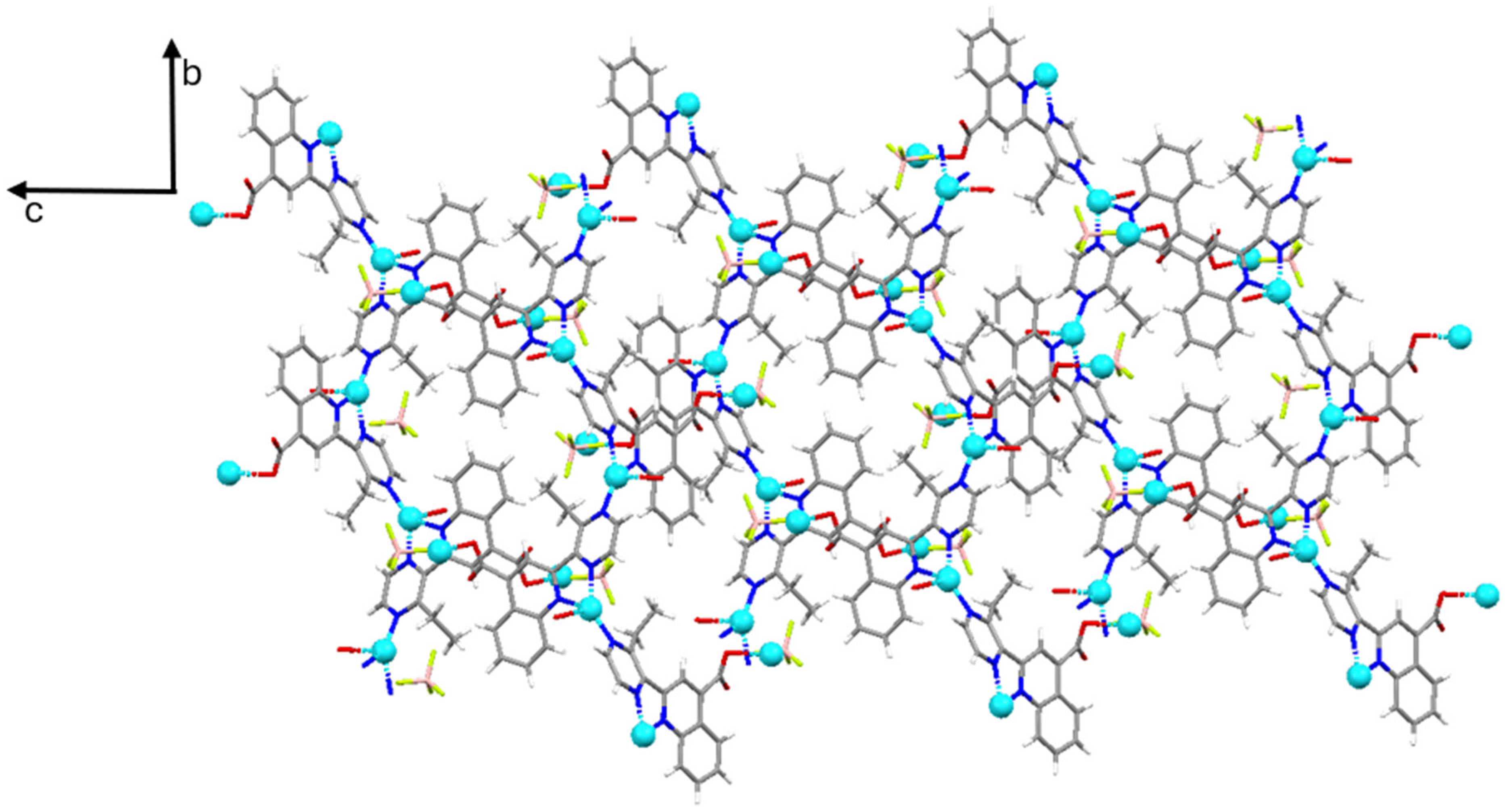
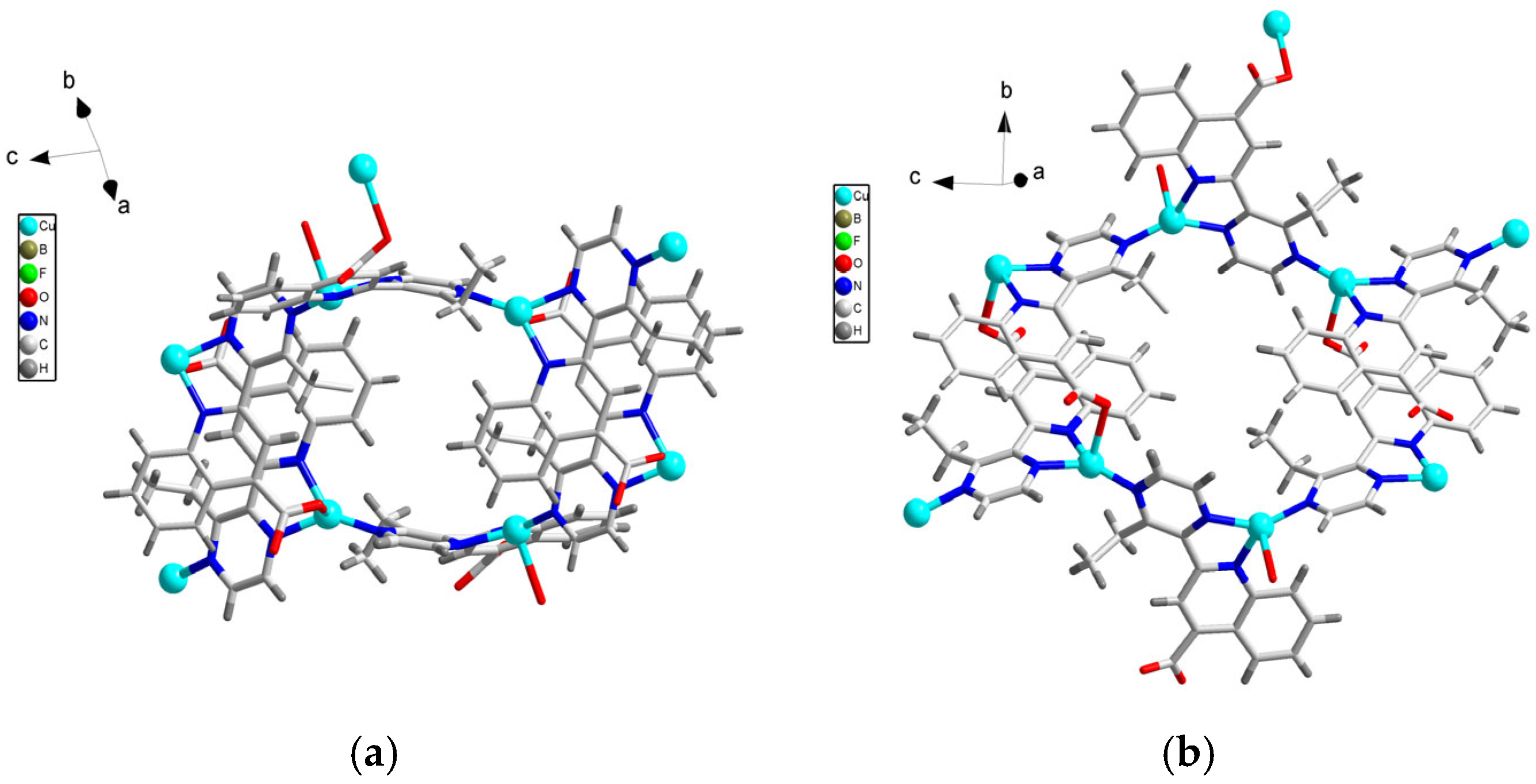
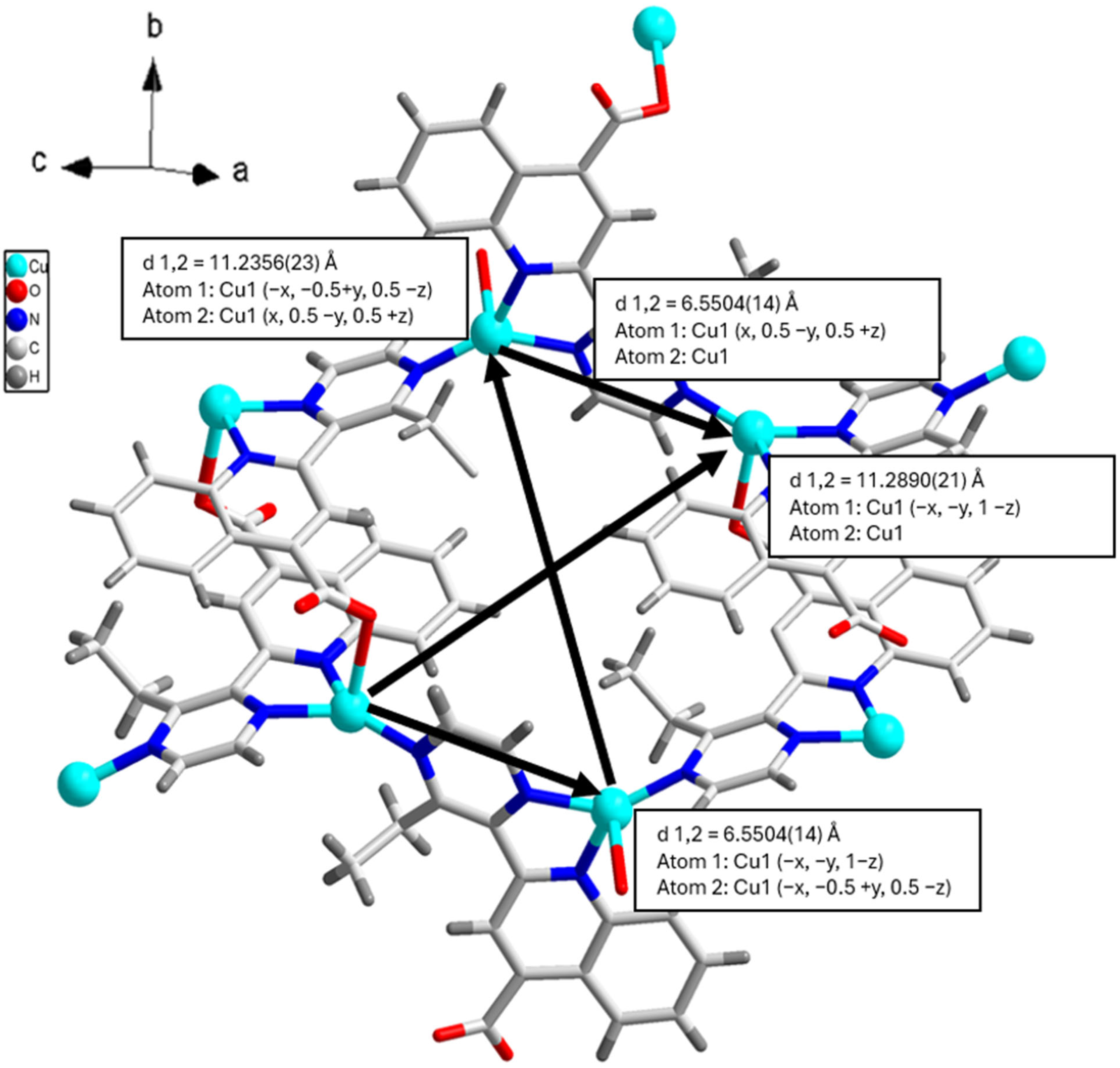
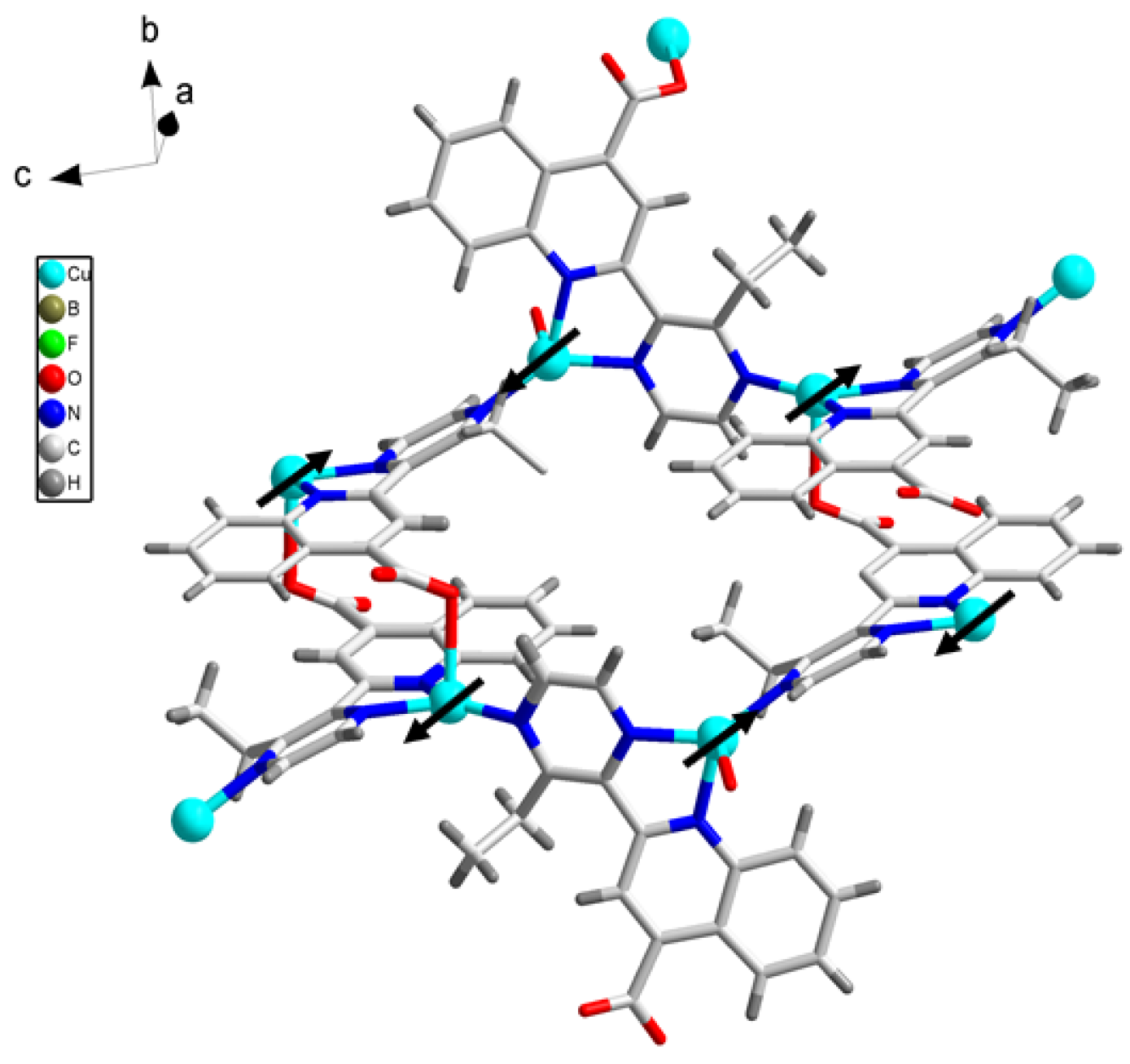
3.1. Crystal Structure
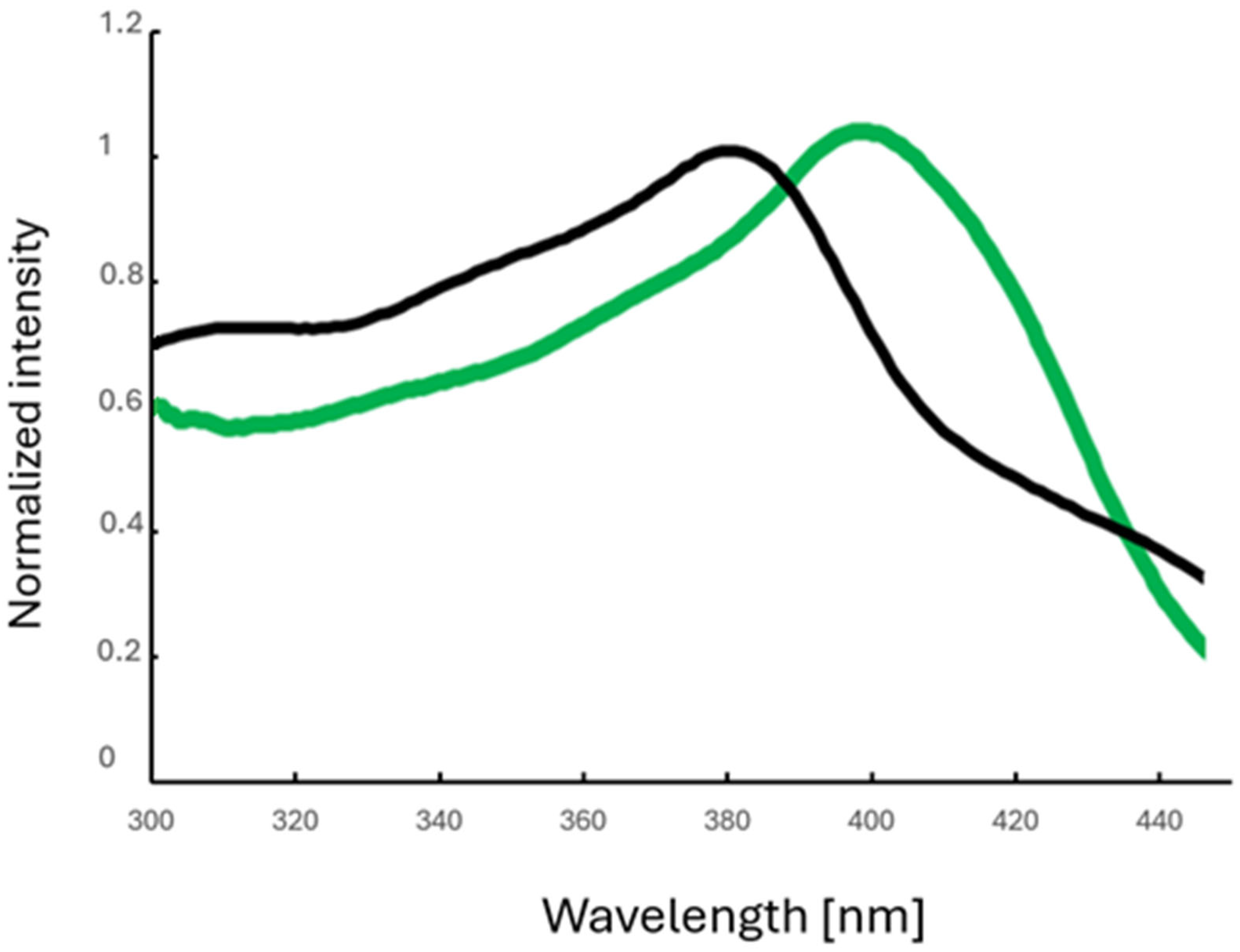
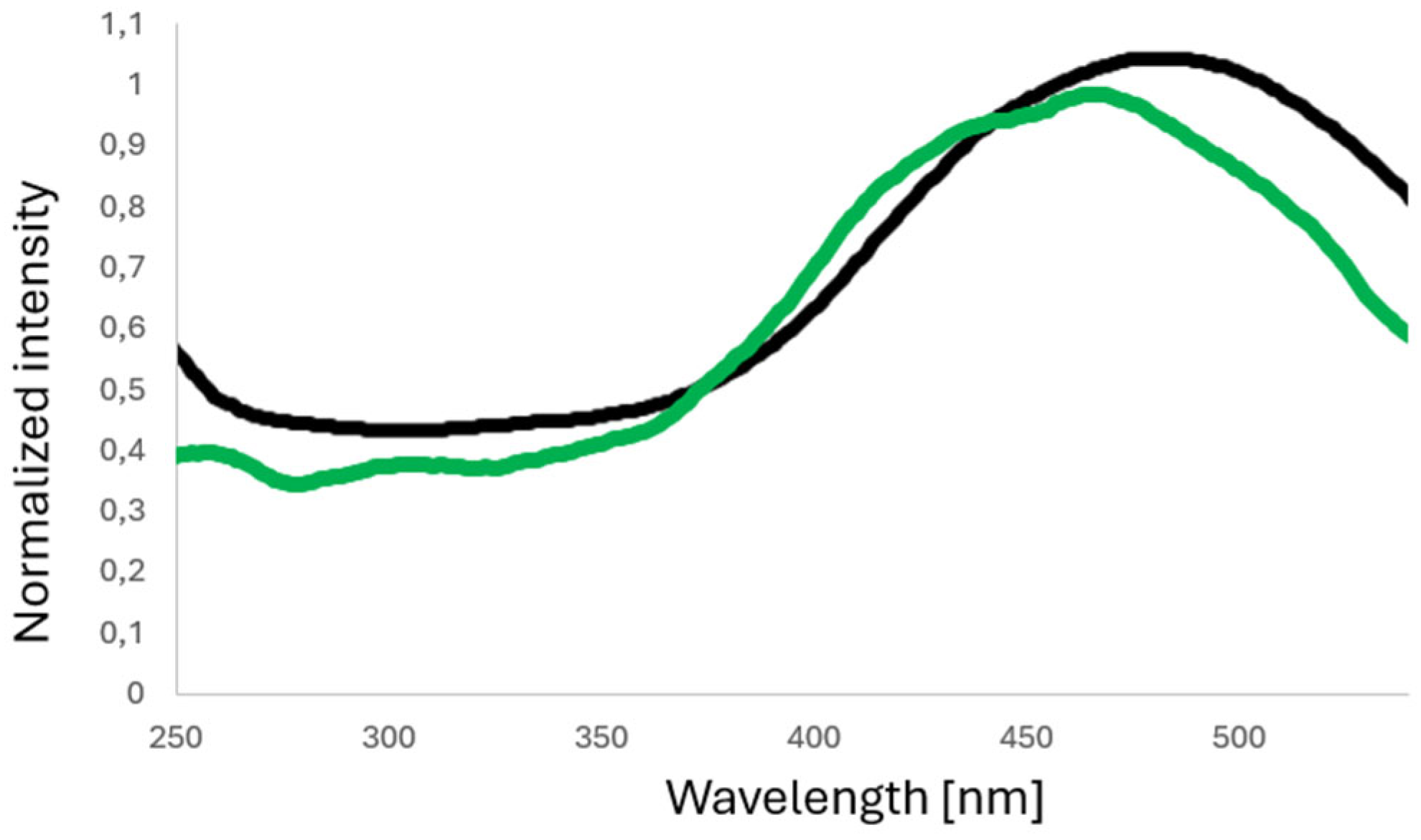
3.2. Photoluminescence Properties
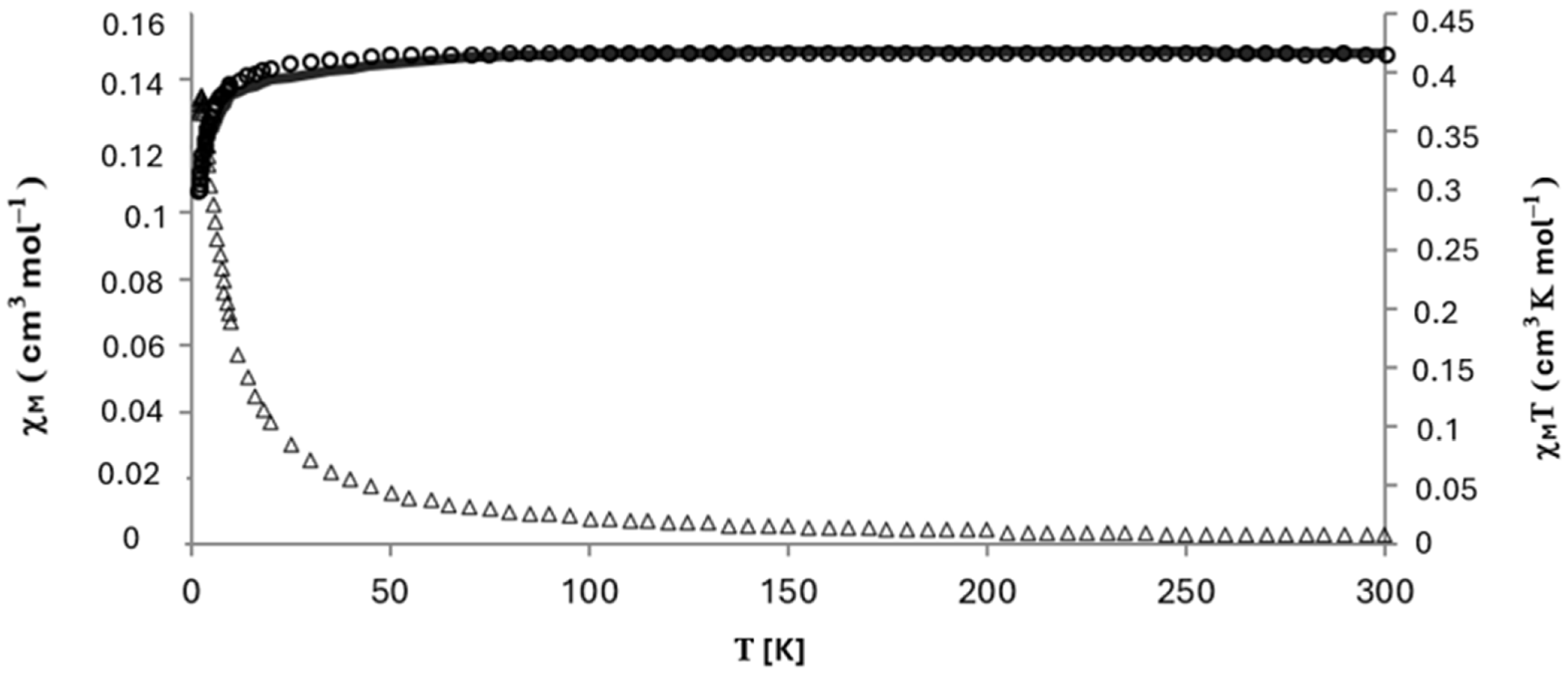
3.3. Magnetic Properties
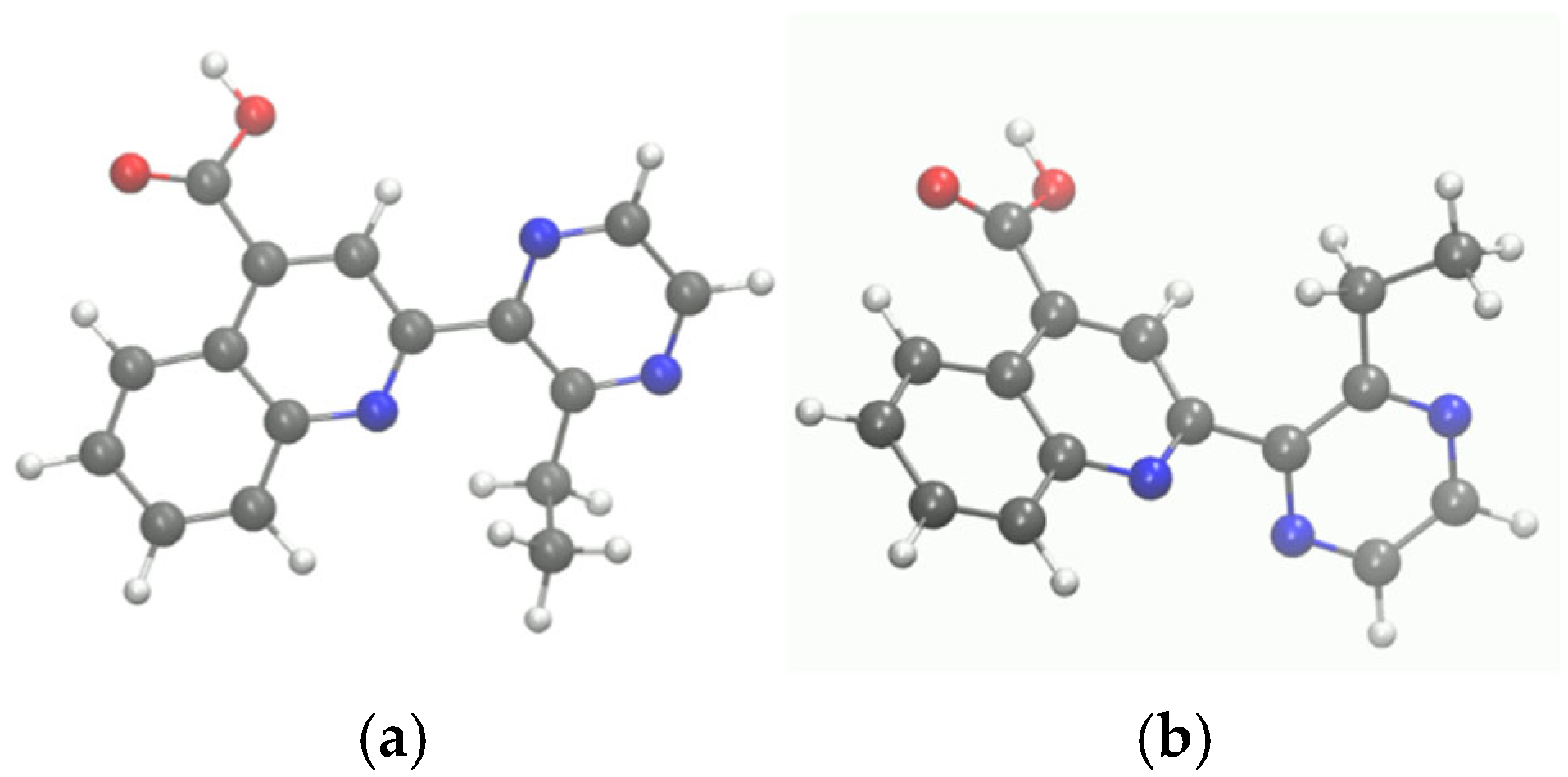
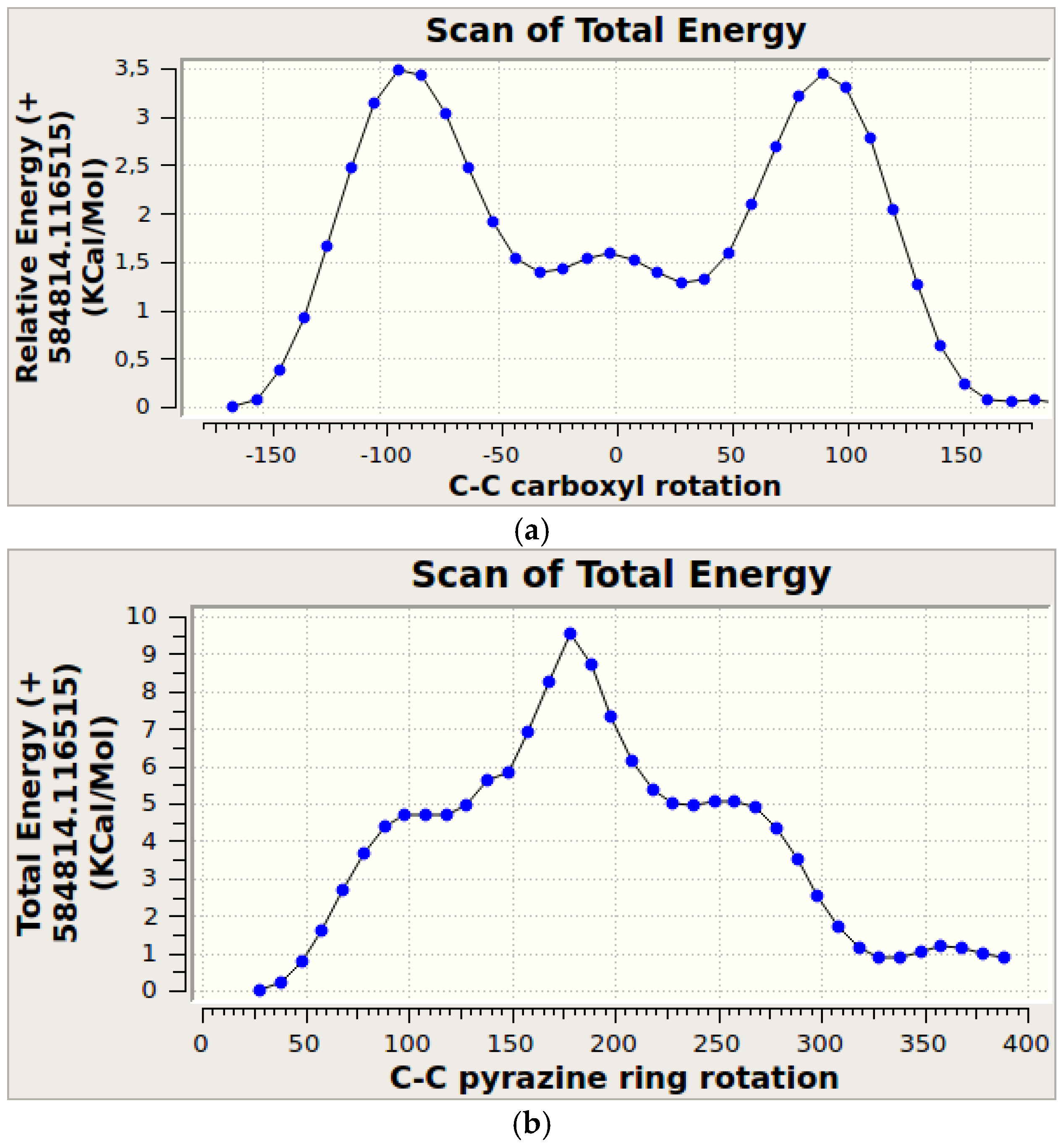
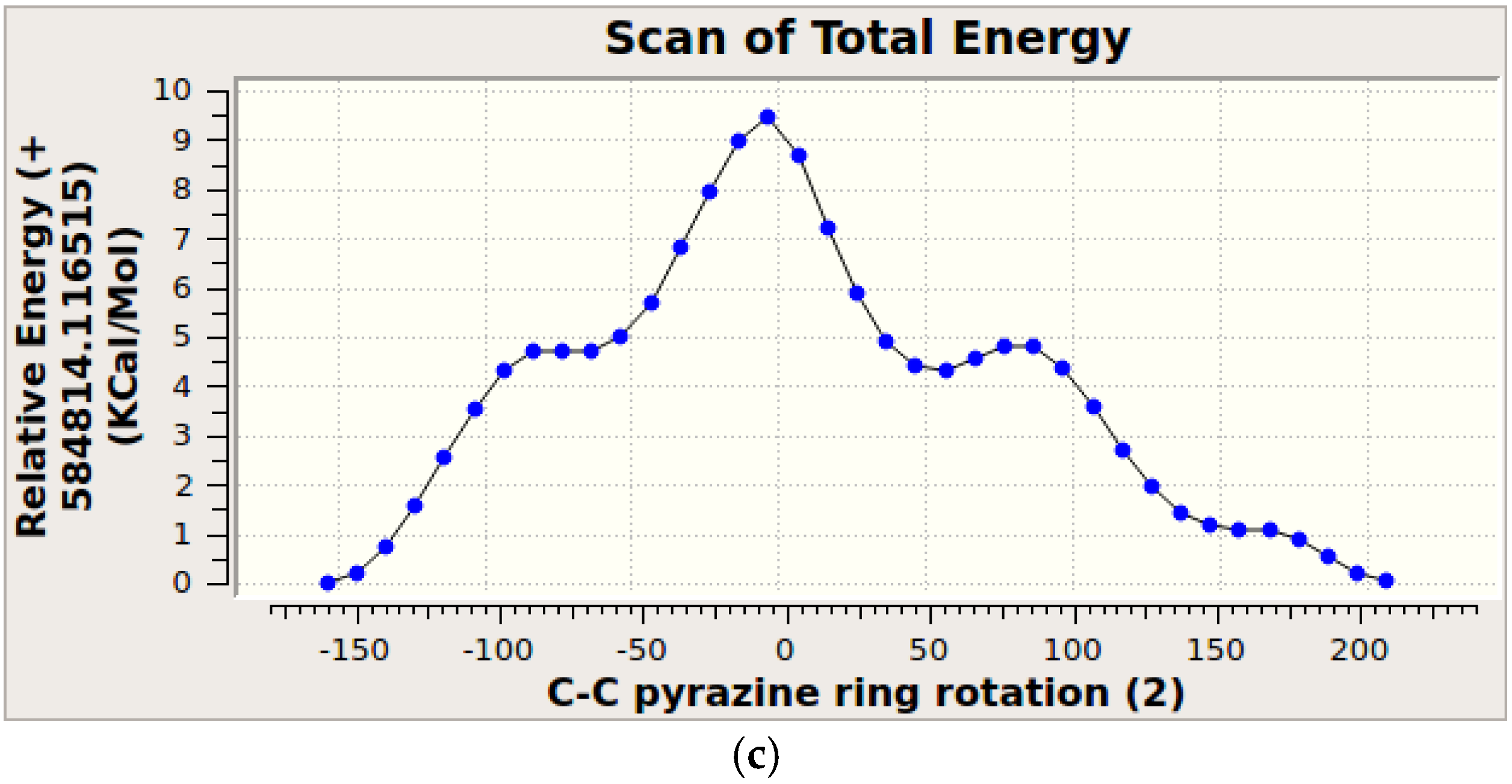
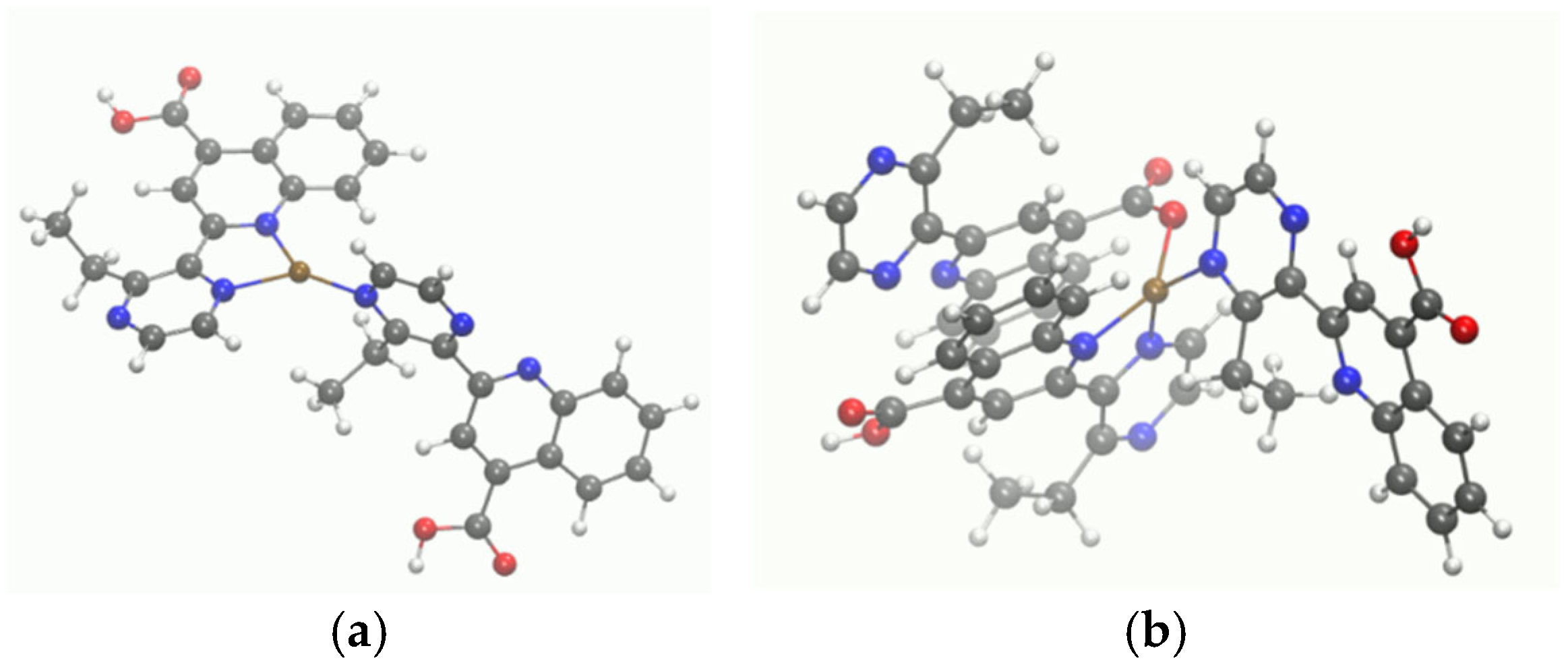
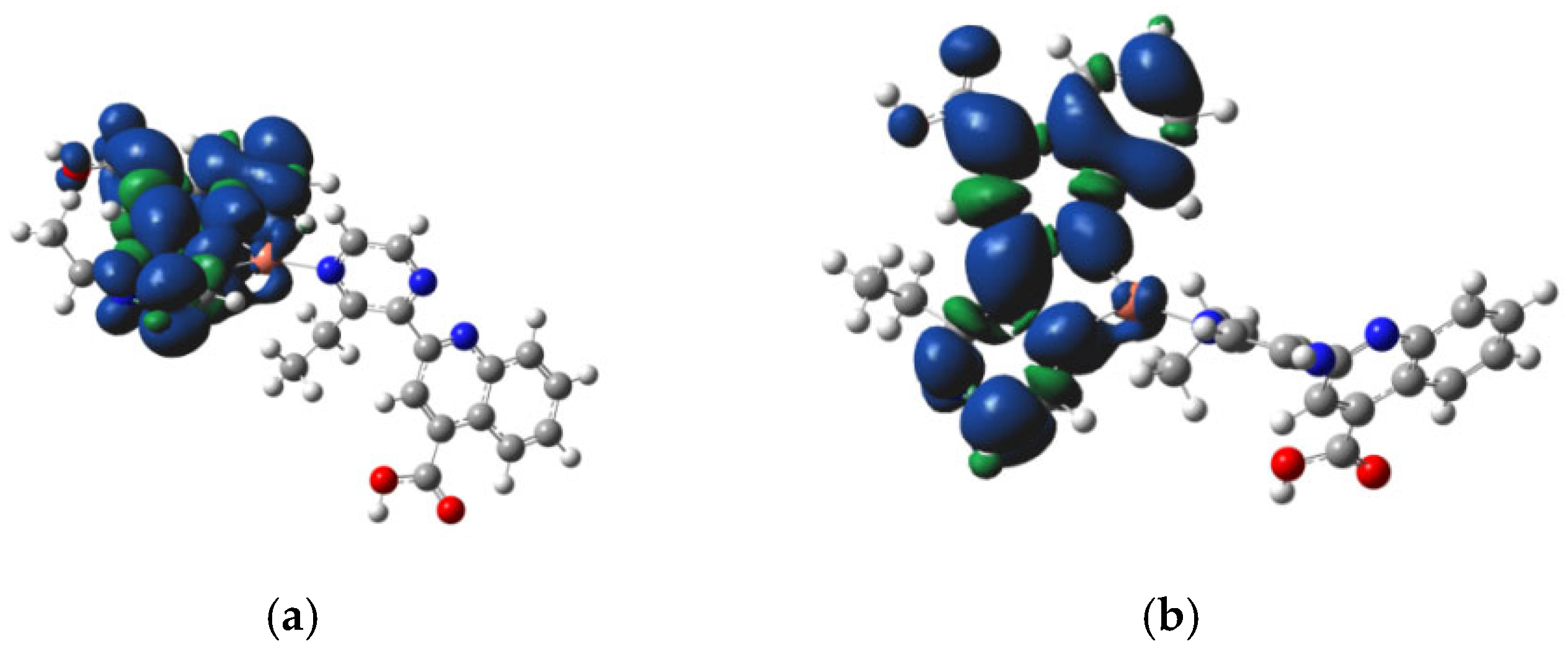
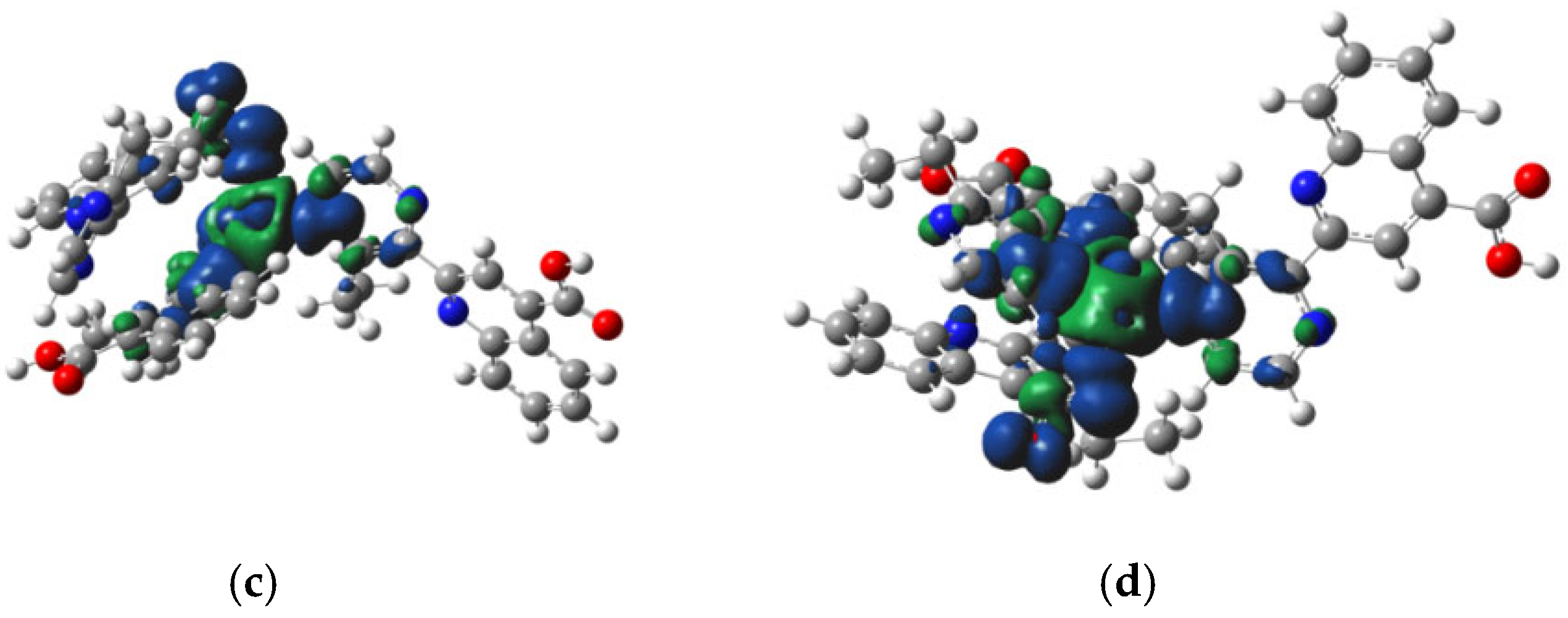
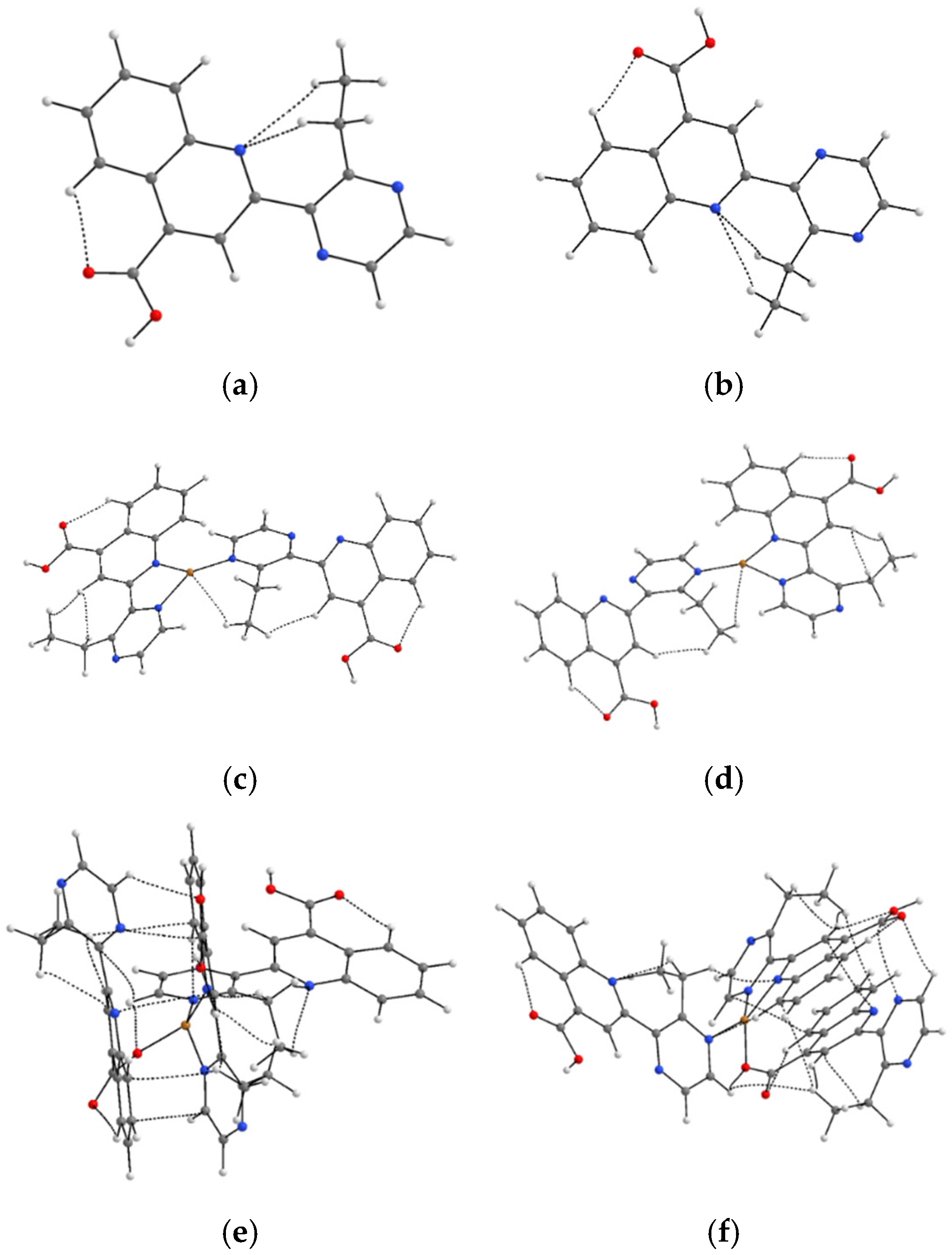
3.4. Molecular Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noro, S.; Kitagawa, S.; Kondo, M.; Seki, K. A New Methane Adsorbent, Porous Coordination Polymer [{CuSiF6(4,4′-bipyridine)2}n]. Angew. Chem. Int. Ed. 2000, 39, 2082–2084. [Google Scholar] [CrossRef]
- Peppas, A.; Sokalis, D.; Perganti, D.; Schnakenburg, G.; Falaras, P.; Philippopoulos, A.I. Sterically demanding pyridine-quinoline anchoring ligands as building blocks for copper(I)-based dye-sensitized solar cell (DSSC) complexes. Dalton Trans. 2022, 51, 15049–15066. [Google Scholar] [CrossRef]
- Appleby, M.V.; Cowin, R.A.; Ivalo, I.I.; Peralta-Arriaga, S.L.; Robertson, C.C.; Bartlett, S.; Fitzpatrick, A.; Dent, A.; Karras, G.; Diaz-Moreno, S.; et al. Ultrafast electronic, infrared, and X-ray absorption spectroscopy study of Cu(I) phosphine diimine complexes. Faraday Discuss. 2023, 244, 391–410. [Google Scholar] [CrossRef]
- Zhao, X.; Su, B.; Zhao, Q.; Lv, W.; Chen, L.; Huang, L.; Li, X.; Liao, S. Construction of pyridine-functionalized metal–organic frameworks for the detection of flazasulfuron. Acta Cryst. 2024, C80, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Noro, S.; Kitaura, R.; Kondo, M.; Kitagawa, S.; Ishii, T.; Matsuzaka, H.; Yamashita, M. Framework Engineering by Anions and Porous Functionalities of Cu(II)/4,4‘-bpy Coordination Polymers. J. Am. Chem. Soc. 2002, 124, 2568–2583. [Google Scholar] [CrossRef]
- Blake, A.J.; Hill, S.J.; Hubberstey, P.; Li, W.S. Rectangular grid two-dimensional sheets of copper(II) bridged by both co-ordinated and hydrogen bonded 4,4′-bipyridine (4,4′-bipy) in [Cu(µ-4,4′-bipy)(H2O)2(FBF3)2]·4,4′-bip. J. Chem. Soc. Dalton Trans. 1997, 6, 913–914. [Google Scholar] [CrossRef]
- Kitaura, R.; Fujimoto, K.; Noro, S.I.; Kondo, M.; Kitagawa, S. A pillared-layer coordination polymer network displaying hysteretic sorption: [Cu2(pzdc)2(dpyg)]n (pzdc = pyrazine-2,3-dicarboxylate; dpyg=1,2-Di(4-pyridyl)glycol). Angew. Chem. Ed. Engl. 2002, 41, 133–135. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3D-linked molecular rods. A reappraisal of the zinc cyanide and cadmium cyanide structures and the synthesis and structure of the diamond-related frameworks [N(CH3)4][CuIZnII(CN)4] and CuI[4,4′,4″,4‴tetracyanotetraphenylmethane]BF4.xC6H5NO2. J. Am. Chem. Soc. 1990, 112, 1546–1554. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Lee, E.; Kim, J.; Heo, J.; Whang, D.; Kim, K. A Two-Dimensional Polyrotaxane with Large Cavities and Channels: A Novel Approach to Metal–Organic Open-Frameworks by Using Supramolecular Building Blocks. Angew. Chem. 2001, 113, 413–416. [Google Scholar] [CrossRef]
- Phuengphai, P.; Youngme, S.; Mutikainen, I.; Gamez, P.; Reedijk, J. A series of related 2D coordination polymers based on [copper(II)-4,4′-bpy-carboxylato] building blocks. Polyhedron 2012, 42, 10–17. [Google Scholar] [CrossRef]
- Geng, J.; Qin, L.; He, C.; Cui, G. Synthesis, crystal structures and catalytic properties of copper(II) and cobalt(II) coordination polymers based on a flexible benzimidazole ligand. Transit. Met. Chem. 2012, 37, 579–585. [Google Scholar] [CrossRef]
- Formica, M.; Fusi, V.; Giorgi, L.; Micheloni, M. New fluorescent chemosensors for metal ions in solution. Coord. Chem. Rev. 2012, 256, 170–192. [Google Scholar] [CrossRef]
- Sinzger, K.; Hunig, S.; Jopp, M.; Bauer, D.; Beitsch, W.; Wolf, C.H.; Kremer, R.K.; Metzenthin, T.; Bau, R.; Khan, S.I.; et al. The organic metal (Me2-DCNQI)2Cu: Dramatic changes in solid-state properties and crystal structure due to secondary deuterium effects. J. Am. Chem. Soc. 1993, 115, 7696–7705. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Bader Richard, F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, MS, USA, 1994; ISBN 978-0-19-855865-1. [Google Scholar]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Computer Programs: CrysAlis PRO, Rigaku OD, 2015; Rigaku Oxford Diffraction Ltd.: Abingdon, UK, 2020.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr.; Hay, P.J. Modern Theoretical Chemistry; Schaefer, H.F., III, Ed.; Plenum: New York, NY, USA, 1977; Volume 3, pp. 1–28. [Google Scholar]
- Igel-Mann, G.; Stoll, H.; Preuss, H. Pseudopotentials for main group elements (IIIA through VIIA). Mol. Phys. 1988, 65, 1321–1328. [Google Scholar] [CrossRef]
- Andrae, D.; Haeussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the 2nd and 3rd row transition-elements. Theor. Chem. Acc. 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Keith, T.A. Gristmill T.K. AIMAll, Version 19.10.12; TK Gristmill Software: Overland Park, KS, USA, 2019. Available online: https://aim.tkgristmill.com (accessed on 4 June 2025).
- Humphrey, W.; Dalke, A.; Schulten, K. VMD-Visual Molecular Dynamics. J. Mol. Graphics. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.1.1; Semichem Inc.: Shawnee Mission, KS, USA, 2019.
- Zhu, A.X.; Xu, Q.Q.; Liu, F.Y.; Qi, X.L. Synthesis, Characterization, and Photoluminescence Properties of a Planar Copper Triazolate Coordination Polymerwith Cyanide as Co-Ligand, Zeitschrift Für Anorg. Und Allg. Chem. 2011, 637, 502–505. [Google Scholar] [CrossRef]
- Rani, V.S.V.; Dhanasekaran, T.; Jayathuna, M.; Narayanan, V.; Jesudurai, D. Synthesis, characterization of Cu (II) Schiff base complexes: Optical and magnetic studies. Mater. Today Proc. 2018, 5, 8784–8788. [Google Scholar] [CrossRef]
- Hietsoi, O.; Filatov, A.S.; Dubceac, C.; Petrukhina, M.A. Structural diversity and photoluminescence of copper(I) carboxylates: From discrete complexes to infinite metal-based wires and helices. Coord. Chem. Rev. 2015, 295, 125–138. [Google Scholar] [CrossRef]
- Zink, D.M.; Bächle, M.; Baumann, T.; Nieger, M.; Kühn, M.; Wang, C.; Klopper, W.; Monkowius, U.; Hofbeck, T.; Yersin, H.; et al. Synthesis, structure, and characterization of dinuclear copper(I) halide complexes with P^N ligands featuring exciting photoluminescence properties. Inorg. Chem. 2013, 52, 2292–2305. [Google Scholar] [CrossRef]
- Bleaney, B.; Bowers, K.; Proc, K.D. Proceedings of the Royal Society of London. Ser. A Math. Phys. Sci. 1952, 214, 451–465. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; Wiley–VCH: New York, NY, USA, 1993. [Google Scholar] [CrossRef]
- Kachi-Terajjma, C.; Hagiwara, S. Synthesis, crystal structure and photophysical properties of chlorido-[(E)-3-hy-droxy-2-methyl-6-(quinolin-8-yldiazen-yl)phenolato]copper(II) monohydrate. Acta Crystallogr. E Crystallogr. Commun. 2022, 78, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Kochel, A.; Hołyńska, M.; Twaróg, K.; Jezierska, A.; Panek, J.J.; Wojaczyński, J. A new mixed-valence CuI/CuII three-dimnsional coordination polymer constructed with an N,O-donor ligand generated via solvothermal synthesis: Structural features and magnetic properties. Acta Cryst. 2022, C78, 405–413. [Google Scholar] [CrossRef]
- Gogia, A.; Novikov, E.M.; Guzei, I.A.; Fonari, M.S. Crystal structure and characterization of a new one-dimensional copper(II) coordination polymer containing a 4-amino¬benzoic acid ligand. Acta Cryst. E 2024, 80, 330–334. [Google Scholar] [CrossRef]
- Scatena, R.; Massignani, S.; Lanza, A.E.; Zorzi, F.; Monari, M.; Nestola, F.; Pattinari, C.; Pandolfo, L. Synthesis of Coordination Polymers and Discrete Complexes from the Reaction of Copper(II) Carboxylates with Pyrazole: Role of Carboxylates Basicity. Cryst. Growth Des. 2022, 22, 1032–1044. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochel, A.; Hołyńska, M.; Jezierska, A.; Panek, J.J. Design and Synthesis of a New Photoluminescent 2D Coordination Polymer Employing a Ligand Derived from Quinoline and Pyridine. Crystals 2025, 15, 691. https://doi.org/10.3390/cryst15080691
Kochel A, Hołyńska M, Jezierska A, Panek JJ. Design and Synthesis of a New Photoluminescent 2D Coordination Polymer Employing a Ligand Derived from Quinoline and Pyridine. Crystals. 2025; 15(8):691. https://doi.org/10.3390/cryst15080691
Chicago/Turabian StyleKochel, Andrzej, Małgorzata Hołyńska, Aneta Jezierska, and Jarosław J. Panek. 2025. "Design and Synthesis of a New Photoluminescent 2D Coordination Polymer Employing a Ligand Derived from Quinoline and Pyridine" Crystals 15, no. 8: 691. https://doi.org/10.3390/cryst15080691
APA StyleKochel, A., Hołyńska, M., Jezierska, A., & Panek, J. J. (2025). Design and Synthesis of a New Photoluminescent 2D Coordination Polymer Employing a Ligand Derived from Quinoline and Pyridine. Crystals, 15(8), 691. https://doi.org/10.3390/cryst15080691






