Abstract
A new class of Schiff base/ester compounds: ICln, 4-((2′-chlorophenylimino)methyl)phenyl-4″-alkoxy benzoates, were synthesized and their mesophase characteristics and thermal behavior were evaluated. Differential scanning calorimetry (DSC) was used to study mesophase transitions, and polarized optical microscopy was carried out to identify the phases (POM). The results show that all compounds are monomorphic, and enantiotropic nematic (N) phases were seen at all side chains. It was found that lateral Cl atoms in the terminal benzene ring influence both conformation and mesomorphic properties. Comparisons between the present investigated lateral Cl derivatives and their laterally neat, as well as their isomeric, compounds have been briefly discussed. Results revealed that the insertion of lateral Cl substituent in the molecular structure impacts the type and stability of the formed mesophases. The exchanges of the ester-connecting moiety improve their thermal nematic stability than their previously prepared structurally isomeric derivatives. These compounds exhibit a broad absorption in the UV-Visible region, including a peak in UV region and a tail around 550 nm, and there were observed to be absorption tail increases and energy band gap decreases with the increase of the alkoxy side chain length. The photoluminescence (PL) intensity was noted to be quenched for the bulky alkoxy group ascribed to non-radiative recombination through the defect states. Moreover, time resolved fluorescence decay spectra reveal that both the radiative and non-radiative recombination lifetime increases with the increase of alkoxy side chain length.
1. Introduction
Liquid crystalline materials with a Schiff base group in the mesogenic core of monomers, dimers, oligomers, or compounds polymeric in nature are frequently studied [1,2,3,4,5,6,7,8,9,10] because of their fascinating optical properties, which allow for numerous electro-optical applications such as switching, holography, and optical storage devices. For such materials, the photochromic azomethine group phenomena are used to influence the phase behavior and optical characteristics [11,12,13,14,15] of several LC materials. By creating molecular ordering in the trans isomer as a result of the reversible trans-cis isomerization brought on by ultraviolet radiation, the LC’s mesophase structure is stabilized, whereas the phase structure is disrupted by disordering the cis isomer. The cis isomer will therefore have a lower clearing point than the trans one [15,16,17].
When utilized as a linking group in liquid crystal molecules, Schiff base linker groups have various benefits. They are simple to synthesize and can be used in a variety of mesogenic architectures. The imine bond is also reasonably stable, supporting the stability of the liquid crystal phase over a broad temperature range. Furthermore, by adjusting the linker group, it is possible to change the liquid crystal’s electro-optical sensibility and phase transition temperature. Last but not least, the azomethine linker group’s capability to undergo light-induced isomerization, which changes their form and orientation inside a liquid crystal phase, is one of its major characteristics. Based on their formation [8] and the observation of liquid crystal characteristics at ambient temperature, liquid crystalline compounds containing a Schiff base linkage also exhibit considerable promise and significant applications. The mesogenic core, terminal groups, and an adequate length of the flexible chain are three parameters that have an impact on the molecular structure of thermotropic liquid crystal compounds [18]. It will always be difficult to create new, inexpensive liquid crystalline materials with great thermal stability and a wide mesophase temperature range. However, chemically changing geometry has proven to be one of the most efficient methods for developing innovative, low-cost liquid crystal materials with specific features [12]. Even relatively minor modifications to the molecular shape of the molecule, such as the presence of heteroatoms or a variety of lateral substituted atoms or groups, can result in significant changes to the mesomorphic properties, molecular geometry, transition temperature, conformational preferences, and other crucial physical properties required for the design and development of new low-cost liquid crystal materials with enhanced properties suitable for display technologies [15].
Recently, it was found that adding −CH=N connections to rigid structures, such as phenyl rings, helps those compounds to keep their structural linearity and boosts mesophase stability [19]. The molecule exhibits liquid crystalline properties because of its linear molecular structure and high level of polarization in rod-like mesogenic compounds with aromatic rings substituted at the para-position [20]. In most cases, molecular-molecular interactions that are primarily influenced by the geometry and space-filling nature of the molecules, polarizability anisotropy of the polar substituent, the stereo electronic characteristics of the entire molecule, and the lateral adhesion that increases with the length of the alkoxy chain determine how a calamitic mesogen behaves during mesophase. The mesophase behavior is influenced by a number of factors [20]. The presence of bulky groups makes it easier for space to be filled at the molecular terminal, which in turn promotes an increase in the mesophase stability. Furthermore, the presence of polarizable atoms increases the intermolecular interaction between molecules.
Some studies suggest that the liquid crystal characteristics of these compounds depend on the nature of the terminal groups [20,21]. All of these compounds show liquid crystalline properties when heated and cooled over different thermal ranges. In another study, the synthesis of non-symmetric dimers with different terminal group substitutions was covered. In all of these dimers, an enantiotropic nematic phase was detected. However, the substituted dimers containing halogens and ethyl groups do display SmA behavior in addition to the nematic phase [21]. For example, the impact of either a methyl or a methoxy terminal group on Schiff base compounds was investigated by Naoum and his team as well as Hagar et al. [22,23]. The thermal ranges of the compounds’ enantiotropic nematic phases were 150 °C (OCH3) and 85 °C, respectively (CH3). The outcomes demonstrated that the more electron-donating substituent increases the nematic range.
Several features of liquid crystal materials are significantly enhanced by the introduction of lateral groups (F, I, Br, CN, NO2, CH3, or OCH3). The molecular packing disruption that lowers the melting temperature and decreases the stability of liquid crystal phases may be responsible [24,25,26,27,28,29,30,31,32]. Both the features required for technical usage and the alterations to the liquid crystals’ attributes may be advantageous for mesomorphism. The melting point decreases as the length of the alkyl chains rises. In other words, as the length of the alkyl chain increases, a compound’s tendency to be nematic decreases while its propensity to display smectic characteristics increases. As a result, one may predict that the homologous series will eventually reach a point when no nematic properties are displayed and the system is only smectogenic. The smectic mesophase will transition into the isotropic liquid at this point in the homologous series, most likely because the terminal intermolecular interactions are insufficient to preserve the parallel molecular orientation needed for the nematic mesophase. It should be underlined that, at higher temperatures, a nematic mesophase does not give rise to a smectic mesophase. Since the smectic mesophase is a more highly organized state than the nematic mesophase, it represents a system with lower kinetic and potential energy. Therefore, it is less likely that a nematic–smectic transition will occur as temperature rises because doing so would require switching from a higher to a lower energy system.
The chlorine atom that extends out from the side of the molecules will sterically force the molecules apart and disturb the smectic molecular packing, which causes Cl replacement at a lateral position within the core to have a minor steric effect [33,34]. Most of the time, the melting temperature will be lowered, and occasionally the stability of the liquid crystalline phase will remain. As a result, lateral substituents tend to increase a molecule’s lateral dipole, which may encourage tilted smectic phases such as the SmC phase [35,36] and hold promise for the synthesis of ferroelectric compounds. The steric character and polarity of lateral substituent effects can be combined to produce some noticeable changes in the physical properties without significantly disrupting the molecular packing.
The goal of the work we are performing now is to investigate the mesomorphic, optical, and photophysical properties of produced lateral chloro liquid crystalline derivatives (ICln, Figure 1) by recoding the absorption, steady-state PL and time-resolved fluorescence spectra. Knowledge of the optical absorption and emission properties is vital to find their applications in optoelectronic devices.
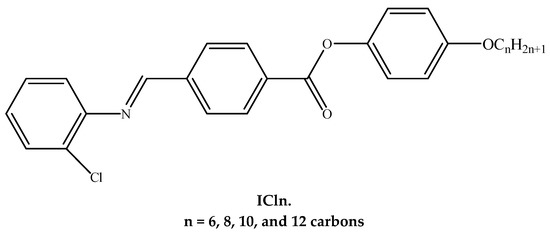
Figure 1.
Molecular structure of prepared materials.
2. Experimental
2.1. Materials
The following chemicals were bought from Sigma Aldrich (Schnelldorf, Germany): 4-hydroxybenzaldehyde, 4-decyloxybenzoic acid, 4-octoyloxybenzoic acid, 4-dodecyloxybenzoic acid, 4-hexadecyloxybenzoic acid, and 2-chloroaniline. Aldrich (Milwaukee, WI, USA) provided the dichloro-methane, N,N′-dicyclohexylcarbodiimide (DCC), ethanol, and 4-dimethylaminopyridine (DMAP).
2.2. Synthesis
The materials ICln were formed according to the following Scheme 1:
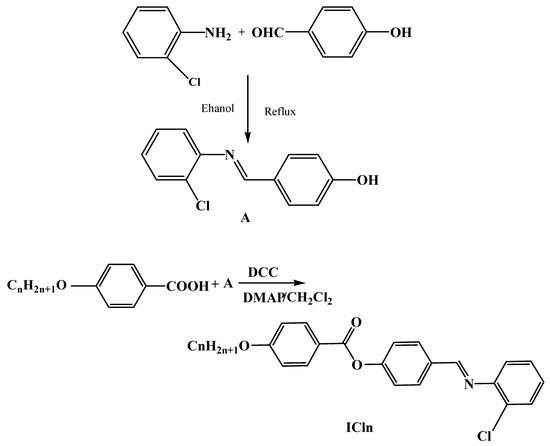
Scheme 1.
Synthesis of 4-((2′-chlorophenylimino)methyl)phenyl-4″-alkoxy benzoates, ICln.
2.2.1. Synthesis of 4-((2′-Chlorophenylimino)methyl)phenol (A)
Equimolar amounts of 4-hydroxybenzaldehyde (4.0 mmol) and 2-chloroaniline (4.0 mmol) in ethanol (10 mL) were refluxed for two hours. The reaction mixture was allowed to cool, and the separated product was filtered. The obtained solid was recrystallized from ethanol.
2.2.2. Synthesis of 4-((2′-Chlorophenylimino)methyl)phenyl-4″-alkoxy Benzoates, ICln
Compounds A and 4-alkoxybenzoic acid were dissolved in dry methylene chloride (DCM) (25 mL) as their molar equivalents (0.01 mol). The reaction mixture was then given a 0.02 molar addition of N,N′-dicyclohexylcarbodiimide (DCC) and a minute amount of 4-dimethylaminopyridine (DMAP). For 72 h, the reaction was stirred at room temperature. Dicyclohexylurea (DCU), a separated byproduct, was filtered out. After the filtrate was evaporated, ethanol was used to recrystallize the resulting product (SD, Figures S1–S4).
3. Results and Discussion
3.1. Liquid Crystalline Behavior
The synthesized materials’ transition temperatures and corresponding enthalpies were determined from the DSC scan and are reported in Table 1 and schematically shown in Figure 2. The transition temperatures and enthalpy values are estimated using the second heating scans. The stability of the DSC curves for heating and cooling demonstrated the thermal stability of all derivatives. The DSC heating and cooling lines of compound ICl8 are shown as an example in Figure 3. POM measurements revealed textures that helped in the formation of mesophases (Figure 4). All of the compounds in the ICln group exhibit enantiotropic mesomorphic phases, as seen in Table 1. Moreover, depending on the length of the terminal side chain, they all display thermal mesophases at various temperatures. The transition temperatures of all the studied derivatives were graphically represented in Figure 2 to show the impact of the terminal alkoxy chain length on the mesophase behavior in prepared series.

Table 1.
Transition temperatures (°C), enthalpy in kJ/mol and normalized entropy of transitions as well as mesophase range of series ICln.
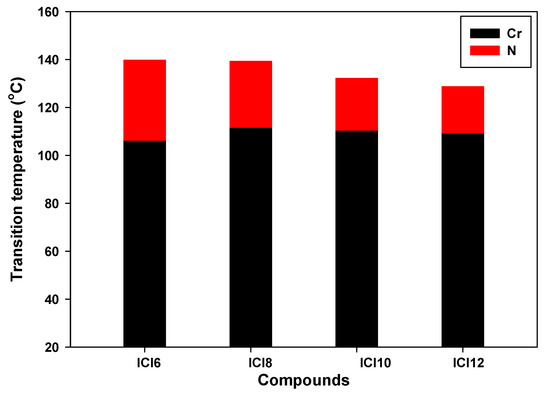
Figure 2.
DSC transitions of the phase behavior upon heating of designed ICln derivatives.
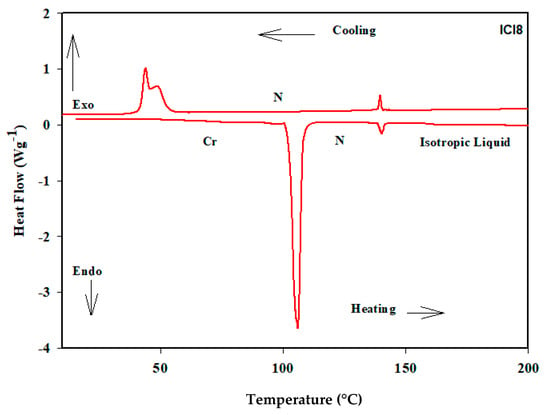
Figure 3.
DSC thermograms of ICl8 derivatives recorded from second heating and cooling at a rate of 10 °C/min.
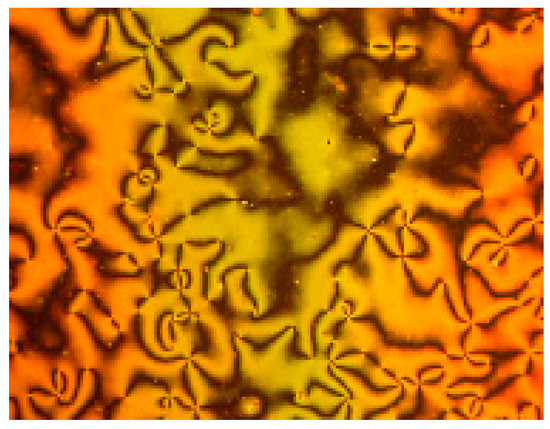
Figure 4.
Nematic texture of ICl8 compound during heating at 120 °C.
All of the formed compounds confirmed the nematic mesophase’s presence, as shown in Table 1 and Figure 2. Increasing the polarizability and/or polarity of the entire molecule’s mesogenic component, in general, improves mesophase stability. Table 1 and Figure 2 show the irregular pattern of the melting points (Cr to N). The homologues ICl6 and ICl8 have different melting points, with ICl6 having the lowest (Cr-N = 106.1 °C) and ICl8 having the highest (Cr-N = 111.5 °C) values. The synthesized group displays only N mesophases and according to previous descriptions [37,38], N phase stability decreases with length of side carbon chain (n). Compound ICl6 has a broad N phase range of 33.8 °C. The impact of the length of the terminal alkoxy group is another significant factor affecting the difference in transition temperature from mesophase to isotropic phase. Yet, the reduced stiffness of the rod-shaped molecule results in an increase in the molecule’s anisotropic characteristics, which in turn improves mesophase stability [37]. Moreover, a decrease in stability is predicted because an increase in the alkoxy terminal chain will dilute the mesogenic core. According to the data, the diluting effect predominates the thermal stability decline with increasing alkoxy chain length. Moreover, the microphase separation between the aromatic cores and the alkyl chains play important roles for the formation of the mesophase.
For all compounds (ICln), the entropy change related to the nematic-isotropic transition, TN-I, expressed as a dimensionless quantity ∆S/R, was calculated. The data are shown in Table 1. As shown in Table 1, all entropies of transitions demonstrated reduced ∆S/R values regardless of the length of the alkoxy chain (n) or the polarity/size of the lateral Cl substituent. The low value of entropy was likely due in part to the mesogenic group’s increased biaxiality and decreased positional order, which led to a reduction in conformational entropy. This can be explained by the lateral unit’s improved biaxiality, which results in lower ∆S/R values with higher mesogenic group biaxiality [38], compared to more typical rod-like mesogenic units.
3.2. Comparison with Related Materials
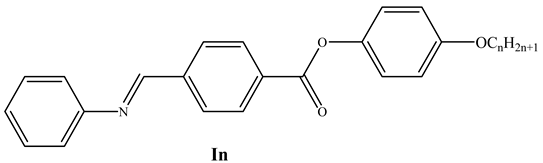
It is exciting to compare the examined compounds with homologues materials previously reported but without any lateral substitution in order to understand the impact of the lateral chlorine substitution on the phase behavior of the researched compounds. The temperatures and phase types of the neat compounds, which retain the same aromatic core as compound In but absent chlorine substitution (In) [5], are shown in Table 2 and Figure 5.

Table 2.
Chemical structure, transition temperatures (°C) and enthalpy of transitions in kJ/mol of the non-chlorinated compounds In [5].
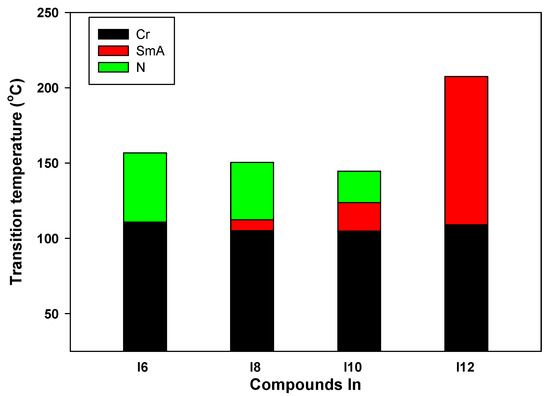
Figure 5.
DSC transitions of the phase behavior upon heating of designed In derivatives.
According to the length of the side chains, all compounds are mesomorphic and have either one or two LC phases, as shown in Table 1 or Table 2. The lateral Cl substitution lowers the melting temperature and mesophase range for n = 6, but only the N phase is seen for both homologues, so the mesophase type is unaffected (compare compounds ICl6 and I6, Table 1 and Table 2). With an increase in n from 6 to 12 carbons for compounds ICln, the nematic phase is maintained. The melting point is significantly lower for the laterally neat compounds I8, I10 and I12, leading to a larger range of SmA phases.
Overall, this study shows that lateral Cl causes less ordered mesophases because of its steric influence; as a result, in most instances, the SmA phases are replaced by N phases in addition to stabilizing the nematic phases.
It has been demonstrated [39] that, within the same series of compounds, the dipole moments of its individuals are determined by the nature of the substituent since the mesophase stability of a liquid crystalline compound depends primarily upon intermolecular attractions in which molecular polarity plays a significant role. The amount of conjugation changes the polarisability of the molecule and the resulting dipole moment. Moreover, it has been proven [40] that, regardless of the length of the alkoxy chain, all members of a homologous series have similar dipole moments. This conclusion is confirmed by the fact that, regardless of their length, alkoxy groups have similar polarity and do not change the degree of conjugative interactions between the alkoxy oxygen and the ester carbonyl moiety.
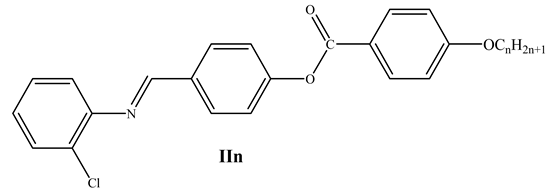
A series compounds of the type 4(4′-alkoxybenzoyloxy)benzylidene-2-chloroanilin (IIn) were prepared, in which the ester groups (OOC) in the previously investigated isomeric derivatives are inverted to COO in the present investigation (ICln). In a molecule of type IIn, inversion of the ester group produces an isomer that reacts mesomerically in a different way and subsequently affects their mesophase behavior in a different way.
In a molecule of type IIn, inversion of the ester group produces an isomer that reacts mesomerically in a different way and subsequently affects their mesophase behavior in a different way. The temperature stability and hydrolysis resistance of aromatic esters are well known [41]; in addition, conjugation interactions between the terminal substituent and the ester group via the intermediate benzene rings do result in double bond character. Table 3 and Figure 6 show that all compounds in the isomeric series IIn [32] are mesomorphic and have monomorphic (only one phase) nematic phases, except the longest chain derivative II16, which has dimorphic smectic and nematic phases. Comparing the present compounds ICln and IIn (Table 1 and Table 3), shows that the previous derivatives IIn are less stable than the prepared investigated materials ICln. Moreover, this comparison shows that the exchange of ester linkage connection in present lateral Cl materials ICln influences the mesophase thermal stability of formed nematic phases.

Table 3.
Chemical structure, transition temperatures (°C) of compounds IIn [32].
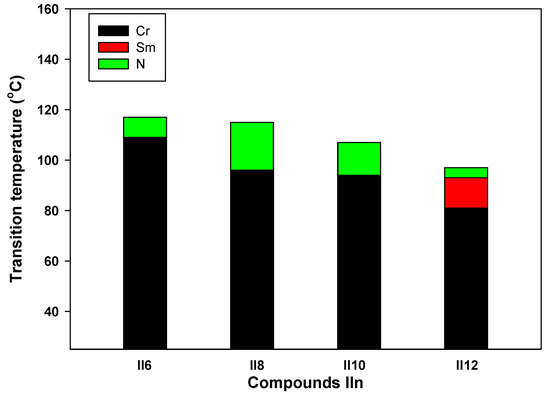
Figure 6.
DSC transitions of the phase behavior upon heating of designed IIn derivatives.
3.3. UV-Visible Absorption Spectra
On glass slides, samples’ absorption spectra were captured using an Agilent Cary 5000 UV-Vis-NIR spectrophotometer. The normalized UV-Visible absorption spectra were performed for all liquid crystalline homologue series, ICln, and shown in Figure 7. It can be noticed that the onset of absorption observed around 550 nm and absorbance increases for the lower wavelength. The absorption in the UV region is associated to the benzene ring, whereas the low energy tail in the visible region corresponds to the transition in the alkoxy side chain. The absorption was found to increase with the increase of alkyl side chain length until ICI10 and thereafter slightly decreases for the ICI12. Moreover, the low energy absorption tail becomes more prominent with increase of alkoxy side chain length as revealed from Figure that ICI6 has no tail while the sample ICI12 has more prominent tail. The direct energy bandgap (Eg) of synthesized liquid crystalline materials was evaluated through Tauc’s Equation [42,43,44,45]: , where α is the absorption coefficient evaluated by . The Tauc plot of four materials is shown in Figure 8. The energy bandgap evaluated by the intercept of tangent to the energy axis as illustrated in Figure 6. The energy bandgap of ICI6 was evaluated to be 2.168 eV and noted to be decreased with increase of alkoxy side chain length with a value of 2.022 eV, 2.012 eV, and 1.868 eV for ICl8, ICl10, and ICl12, respectively. Generally, the bulky side group increases the energy bandgap, however here it decreases. The decrease in the band gap in the present case might be due to the increase of defect states with the increase of side chain length which lowers the Urbach tail and, consequently, causes a decrease of the energy bandgap.
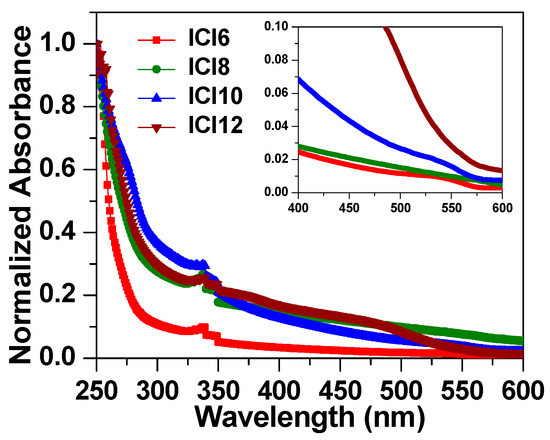
Figure 7.
The Absorption spectra of liquid crystalline samples, ICln.
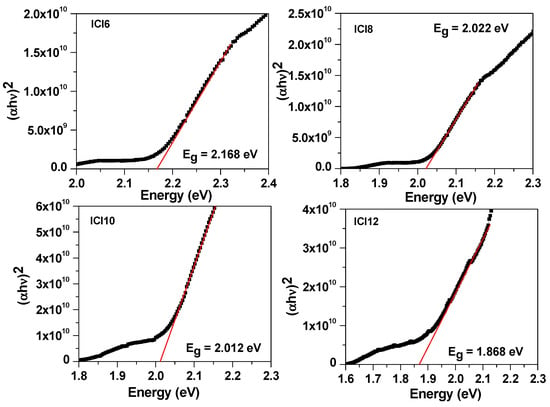
Figure 8.
The Tauc’s plot to evaluate the energy band gap of liquid crystalline materials, ICln.
3.4. Photophysical Properties
To illustrate the impact of side chain length on the photophysical properties of liquid crystalline materials, the steady-state photoluminescence (PL) spectrum was recorded by illuminating the liquid crystalline samples through a laser light of peak emission~319 nm. The steady-state emission spectrum shown in Figure 9a exhibits peak emission at 459 nm for ICl6. The PL intensity was noted to first increase for ICI8 and then decrease with the further increase of side chain length (ICl10) and was almost diminished for ICl12. Moreover, the peak emission was noted to be slightly red shifted with an increase of side chain length. The red shift in PL emission is associated to the decrease in the energy band gap as illustrated in Figure 8. The decrease in PL intensity for ICl10 and ICl12 indicates the suppression of radiative recombination with the increase of alkoxy side chain length. The reduction in radiative recombination might be associated with the increase of trap density, which provides alternative paths for the excited charge carriers to relax through the non-radiative recombination process, consequently causing a decrease in PL intensity.
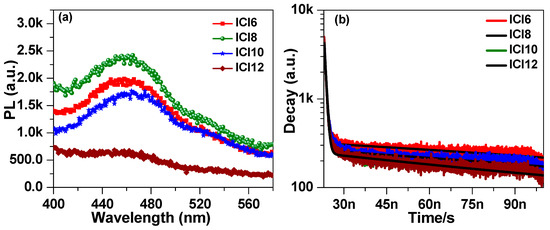
Figure 9.
Steady-state emission (a) and the time resolved fluorescence decay (b) spectra of synthesized liquid crystalline materials ICln.
To further investigate the reason for the reduction of PL intensity with the increase of alkoxy side chain length, the fluorescence decay was recorded at the peak emission wavelength. The decay spectra of liquid crystalline samples are shown in Figure 9b. The decay spectra exhibit two regions: a fast decay at short time and a second, slower, decay at higher time. The decay at shorter lifetime corresponds to radiative decay and the decay at longer time corresponds to non-radiative recombination [46]. The decay spectrum was fitted with a biexponential decay function [9,47]: , where A0 is constant, A1 and A2 are relative amplitude corresponding to the radiative and non-radiative decay, respectively, and τ1 and τ2 are lifetimes corresponding to the radiative and non-radiative recombination process, respectively. The lifetime τ2 corresponds to non-radiative recombination through the defect states. The shorter lifetime (of the order of ps) corresponds to the radiative recombination, whereas the longer (of the order of ns) one is ascribed to the non-radiative decay of the excited charge carriers. The fitting parameters and lifetime are listed in Table 4. The radiative decay lifetime of samples ICI6 was evaluated to be τ1 = 557 ps and was noted to be delayed for sample ICl8 with τ1 = 635 ps. The τ1 for sample ICl10 and ICl12 slightly decreases as compared to the ICl8. The faster non-radiative decay is ascribed to the increase of defect state in ICl10 and ICl12. The radiative recombination time of sample ICl6 was found to be 91 ns which dropped to 66 ns for ICl8 as an increase of PL intensity. The radiative decay become delayed for sample ICl10 and ICl12 with τ2 = 99 ns and 100 ns, respectively.

Table 4.
The optical bandgap and the parameters used to fit the PL decay curve using the bi-exponential function.
4. Conclusions
The thermal and optical properties of two series of laterally chlororinated Schiff base liquid crystals were studied. To explore their molecular self-assemblies, DSC and POM were used. The length of the terminal alkoxy chain at one end and the lateral chloro substituent at the other end determine the thermal stability of the only type of LC phase that was seen, which includes the nematic phase. Furthermore, all of the derivatives that have been produced have extremely high thermal stability and broad enantiotropic nematic temperature mesomorphic ranges. Moreover, uneven trends and small magnitudes of the entropy changes related to the N-isotropic are found, independent of the length of the terminal alkoxy chain (n). It might be caused by the comparatively high clearing temperature values and the high molecular biaxiality promotion of the lateral Cl substituent, which together reduce N-isotropic entropy changes. The lateral Cl substitution induces the formation of nematic phases, as was demonstrated by comparisons between the examined materials and their associated neat compounds as well as their structurally isomeric derivatives reported in the literature. The synthesized LC samples exhibit broad absorption in the UV-visible region and energy bandgap was found to be decreased with the increase of alkoxy side chain length corresponds to the decrease of Urbach tail. The PL intensity noted to be decreased for the samples with for longer alkoxy side chain owing to an increase of non-radiative recombination and, consequently, delayed lifetime of the excited charge carrier.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst13050835/s1, Figures S1–S4: NMR-spectra for compounds.
Author Contributions
Conceptualization, M.T.K.; Methodology, S.A.A.-Z., N.M., Y.A.J. and H.A.A.; Validation, V.J.; Formal analysis, S.A.A.-Z., M.T.K., Y.A.J. and H.A.A.; Investigation, V.J., N.M. and H.A.A.; Resources, S.A.A.-Z., M.T.K. and V.J.; Data curation, N.M.; Writing—original draft, S.A.A.-Z., V.J. and Y.A.J.; Writing—review & editing, S.A.A.-Z., M.T.K., N.M. and H.A.A.; Visualization, Y.A.J.; Funding acquisition, S.A.A.-Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Scientific Research Deanship at University of Ha’il, Saudi Arabia, through project number RG-21018.
Data Availability Statement
The data used for research described in this manuscript are available upon request from corresponding authors: ahoda@sci.cu.edu.eg (H.A.A.); s.alzahrane@uoh.edu.sa (S.A.A.-Z.).
Acknowledgments
The authors extend their appreciation to the Scientific Research Deanship at the University of Ha’il, Saudi Arabia, through project number RG-21018.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Veerabhadraswamy, B.N.; Rao, D.S.S.; Yelamaggad, C.V. Ferroelectric Liquid Crystals: Synthesis and Thermal Behavior of Optically Active, Three-Ring Schiff Bases and Salicylaldimines. Chem.–Asian J. 2018, 13, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Hsu, C.-C.; Chen, L.-W.; Cheng, Y.-L. The effect of position of (S)-2-octyloxy tail on the formation of frustrated blue phase and antiferroelectric phase in Schiff base liquid crystals. Soft Matter 2014, 10, 9343–9351. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Gowda, A.; Jacob, L.; Joy, N.; Philip, R.; Pratibha, R.; Kumar, S. Thermal and nonlinear optical studies of newly synthesized EDOT based bent-core and hockey-stick like liquid crystals. New J. Chem. 2018, 42, 2047–2057. [Google Scholar] [CrossRef]
- Omar, A.Z.; Alazmi, M.L.; Alsubaie, M.S.; Hamed, E.A.; Ahmed, H.A.; El-Atawy, M.A. Synthesis of New Liquid-Crystalline Compounds Based on Terminal Benzyloxy Group: Characterization, DFT and Mesomorphic Properties. Molecules 2023, 28, 3804. [Google Scholar] [CrossRef]
- Alamro, F.S.; Gomha, S.M.; Shaban, M.; Altowyan, A.S.; Abolibda, T.Z.; Ahmed, H.A. Optical investigations and photoactive solar energy applications of new synthesized Schiff base liquid crystal derivatives. Sci. Rep. 2021, 11, 15046. [Google Scholar] [CrossRef]
- Gomha, S.M.; Ahmed, H.A.; Shaban, M.; Abolibda, T.Z.; Khushaim, M.S.; Alharbi, K.A. Synthesis, optical characterizations and solar energy applications of new Schiff base materials. Materials 2021, 14, 3718. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.A.; Alshabanah, L.A.; Gomha, S.M.; Abolibda, T.Z.; Shaban, M.; Ahmed, H.A. Synthesis and Mesomorphic and Electrical Investigations of New Furan Liquid Crystal Derivatives. Front. Chem. 2021, 9, 711862. [Google Scholar] [CrossRef]
- Al-Zahrani, S.A.; Khan, M.T.; Jevtovic, V.; Masood, N.; Jeilani, Y.A.; Ahmed, H.A.; Alfaidi, F.M. Liquid Crystalline Mixtures with Induced Polymorphic Smectic Phases Targeted for Energy Investigations. Crystals 2023, 13, 645. [Google Scholar] [CrossRef]
- Deng, X.; Wen, X.; Zheng, J.; Young, T.; Lau, C.F.J.; Kim, J.; Green, M.; Huang, S.; Ho-Baillie, A. Dynamic study of the light soaking effect on perovskite solar cells by in-situ photoluminescence microscopy. Nano Energy 2018, 46, 356–364. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; Gomha, S.M.; Shaban, M. Synthesis, Mesomorphic, and Solar Energy Characterizations of New Non-Symmetrical Schiff Base Systems. Front. Chem. 2021, 9, 686788. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, A.; Stoilova, A.; Dimov, D.; Yordanov, D.; Zhivkov, I.; Weiter, M. Synthesis and photochromic properties of some N-phthalimide azo-azomethine dyes. A DFT quantum mechanical calculations on imine-enamine tautomerism and trans-cis photoisomerization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Ovdenko, V.; Kolendo, A. New bent-shaped azomethine monomers for optical applications. Mol. Cryst. Liq. Cryst. 2016, 640, 113–121. [Google Scholar] [CrossRef]
- Komissarova, O.A.; Lukyanov, B.S.; Lukyanova, M.B.; Ozhogin, I.V.; Mukhanov, E.L.; Korobov, M.S.; Minkin, V.I. New indoline spiropyrans containing azomethine fragment. Russ. Chem. Bull. 2017, 66, 2122–2125. [Google Scholar] [CrossRef]
- Stoilova, A.; Georgiev, A.; Nedelchev, L.; Nazarova, D.; Dimov, D. Structure-property relationship and photoinduced birefringence of the azo and azo-azomethine dyes thin films in PMMA matrix. Opt. Mater. 2019, 87, 16–23. [Google Scholar] [CrossRef]
- Georgiev, A.; Kostadinov, A.; Ivanov, D.; Dimov, D.; Stoyanov, S.; Nedelchev, L.; Yancheva, D. Synthesis, spectroscopic and TD-DFT quantum mechanical study of azo-azomethine dyes. A laser induced trans-cis-trans photoisomerization cycle. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 263–274. [Google Scholar] [CrossRef]
- Reddy, D.S.; Reddy, G.S.; Beatriz, A.; Corey, E.J. Contrasting Diastereoselectivity between Cyclic Nitrones and Azomethine Ylides. Stereocontrolled Pathways to cis-anti-anti-cis-Oxazatetraquinanes from a Bicyclic Nitrone. Org. Lett. 2021, 23, 5445–5447. [Google Scholar] [CrossRef]
- Gray, G. Thermotropic Liquid Crystals; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Ha, S.-T.; Ng, M.-Y.; Subramaniam, R.T.; Ito, M.M.; Saito, A.; Watanabe, M.; Lee, S.-L.; Bonde, N.L. Mesogenic azomethiane esters with different end groups: Synthesis and thermotropic properties. Int. J. Phys. Sci. 2010, 5, 1256–1262. [Google Scholar]
- Collings, P.; Goodby, J. Introduction to Liquid Crystals: Chemistry and Physics, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis: Abingdon, UK, 2019. [Google Scholar] [CrossRef]
- Yeap, G.; Osman, F.; Imrie, C. Non-symmetric dimers: Effects of varying the mesogenic linking unit and terminal substituent. Liq. Cryst. 2015, 42, 543–554. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Abaza, A.H.; Saad, G.R. Effect of exchange of terminal substituents on the mesophase behaviour of some azo/ester compounds. Liq. Cryst. 2014, 41, 1559–1568. [Google Scholar] [CrossRef]
- Aldahri, T.H.; Alaasar, M.; Ahmed, H.A. The influence of core fluorination on the phase behaviour of rod-like mesogens. Liquid Cryst. 2023, 1–10. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Omar, A.Z.; Alazmi, M.L.; Alsubaie, M.S.; Hamed, E.A.; Ahmed, H.A. Synthesis and characterization of new imine liquid crystals based on terminal perfluoroalkyl group. Heliyon 2023, 9, e14871. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.Z.; El-Atawy, M.A.; Alsubaie, M.S.; Alazmi, M.L.; Ahmed, H.A.; Hamed, E.A. Synthesis and Computational Investigations of New Thioether/Azomethine Liquid Crystal Derivatives. Crystals 2023, 13, 378. [Google Scholar] [CrossRef]
- Naoum, M.M.; Metwally, N.H.; Abd Eltawab, M.M.; Ahmed, H.A. Polarity and steric effect of the lateral substituent on the mesophase behaviour of some newly prepared liquid crystals. Liq. Cryst. 2015, 42, 1351–1369. [Google Scholar] [CrossRef]
- Ahmed, H.; Saad, G. Mesophase behaviour of laterally di-fluoro-substituted four-ring compounds. Liq. Cryst. 2015, 42, 1765–1772. [Google Scholar] [CrossRef]
- Al-Zahrani, S.A.; Khan, M.T.; Jevtovic’, V.; Masood, N.; Jeilani, Y.A.; Ahmed, H.A. Design of Liquid Crystal Materials Based on Palmitate, Oleate, and Linoleate Derivatives for Optoelectronic Applications. Molecules 2023, 28, 1744. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Ahmed, H. Liquid crystalline behaviour of model compounds di-laterally substituted with different polar groups. Liq. Cryst. 2011, 38, 511–519. [Google Scholar] [CrossRef]
- Naoum, M.; Ahmed, H. Effect of dipole moment and conformation on the mesophase behavior of di-laterally substituted phenylazophenyl benzoate liquid crystals. Thermochim. Acta 2011, 521, 202–210. [Google Scholar] [CrossRef]
- Naoum, M.; Mohammady, S.; Ahmed, H. Lateral protrusion and mesophase behaviour in pure and mixed states of model compounds of the type 4-(4′-substituted phenylazo)-2-(or 3-) methyl phenyl-4′-alkoxy benzoates. Liq. Cryst. 2010, 37, 1245–1257. [Google Scholar] [CrossRef]
- Vora, R.A.; Patel, D.N. Mesogenic Homologous Series Containing Chloro Group as Ortho and Para Substituent. Mol. Cryst. Liq. Cryst. 1983, 103, 127–135. [Google Scholar] [CrossRef]
- Goodby, J.; Collings, P.; Kato, T.; Tschierske, C. Handbook of Liquid Crystals, 2nd ed.; Wiley: Weinheim, Germany, 2014; Volume 8. [Google Scholar]
- Blinov, L. Structure and Properties of Liquid Crystals; Springer: London, UK, 2011. [Google Scholar]
- Patel, J.S.; Meyer, R.B. Flexoelectric electro-optics of a cholesteric liquid crystal. Phys. Rev. Lett. 1987, 58, 1538–1540. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, B.; Lehmann, P.; Coles, H.J. A new series of chiral nematic bimesogens for the flexoelectro-optic effect. Liq. Cryst. 1999, 26, 1235–1249. [Google Scholar] [CrossRef]
- Gray, G.W. Molecular Structure and the Properties of Liquid Crystals; Academic Press: Cambridge, MA, USA, 1962. [Google Scholar]
- Gorkunov, M.V.; Osipov, M.A.; Kocot, A.; Vij, J.K. Molecular model of biaxial ordering in nematic liquid crystals composed of flat molecules with four mesogenic groups. Phys. Rev. E 2010, 81, 061702. [Google Scholar] [CrossRef] [PubMed]
- Klingbiel, R.T.; Genova, D.J.; Criswell, T.R.; Van Meter, J.P. Comparison of the dielectric behavior of several schiffbase and phenyl benzoate liquid crystals. J. Am. Chem. Soc. 1974, 96, 7651–7655. [Google Scholar] [CrossRef]
- Bogojawlenski, A.; Winogradov, N.Z. Über das Verhalten von Schmelz-und Klärungskurvenflüssiger Kristalle und ihrer Mischungen. Z. Phys. Chem. 1908, 64, 229–242. [Google Scholar] [CrossRef]
- Dewar, M.J.; Schroeder, J.P. P-alkoxy-and p-carbalkoxybenzoates of diphenols. A new series of liquid crystalline compounds. J. Org. Chem. 1965, 30, 2296–2300. [Google Scholar] [CrossRef]
- Khan, M.T.; Shkir, M.; Alhouti, B.; Almohammedi, A.; Ismail, Y.A.M. Modulation of optical, photophysical and electrical properties of poly(3-hexylthiophene) via Gd:CdS nanoparticles. Optik 2022, 260, 169092. [Google Scholar] [CrossRef]
- Khan, M.Y.; Almohammedi, A.; Shkir, M.; Aboud, S.W. Effect of Ag2S nanoparticles on optical, photophysical and electrical properties of P3HT thin films. Luminescence 2021, 36, 761–768. [Google Scholar] [CrossRef]
- Almohammedi, A.; Khan, M.T.; Benghanem, M.; Aboud, S.; Shakir, M.; Alfaify, S. Elucidating the impact of PbI2 on photophysical and electrical properties of poly(3-hexythiophene). Mater. Sci. Semicond. Process. 2020, 120, 105272. [Google Scholar] [CrossRef]
- Alhazime, A.A.; Mohamed, S.H.; Khan, M.T.; Awad, M.A. Effect of CuS buffer layer on the structural and optical properties of TeO2 nanowires: A comparative study. Phys. Scr. 2022, 97, 095807. [Google Scholar] [CrossRef]
- Khan, M.T.; Almohammedi, A.; Kazim, S.; Ahmed, S. Charge carrier dynamics in perovskite solar cells. In Perovskite Solar Cells: Materials, Processes, and Devices; Wiley-VCH: Hoboken, NJ, USA, 2021; pp. 389–429. ISBN 9783527347155. [Google Scholar] [CrossRef]
- Khan, F.; Khan, M.T.; Rehman, S.; Al-Sulaiman, F. Analysis of electrical parameters of p-i-n perovskites solar cells during passivation via N-doped graphene quantum dots. Surf. Interfaces 2022, 311, 02066. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).