Simple Summary
Surgical removal of tumors in children is often difficult because their tumors can grow into nearby healthy tissues and lie close to vital structures. Surgeons must balance removing as much tumor as possible while preserving healthy anatomy. Fluorescence-guided surgery is an emerging technique that uses (non-)specific imaging agents and imaging systems to visualize tumors, blood flow, and vital structures during an operation, potentially improving surgical precision. Although this approach has been widely studied in adults, its use in pediatric oncology is still limited. This review outlines current evidence on how fluorescence guidance is being applied in children, including its benefits and limitations, and discusses the unique challenges of developing more specific imaging agents for pediatric tumors. It also explores strategies to advance this technology toward routine clinical use. If these efforts are successful, fluorescence-guided surgery may enhance surgical accuracy, reduce recurrence risk, and improve long-term outcomes for children with cancer.
Abstract
Background/Objectives: Achieving complete, yet safe tumor resections are particularly challenging in pediatric oncology due to infiltrative tumor growth patterns, small patient size, and the close proximity to critical structures. Fluorescence-guided surgery (FGS) enhances visualization of anatomy, tissue perfusion, and tumor tissue in real time, potentially improving surgical precision. While widely explored in adults, its application in pediatric oncology remains limited. This review summarizes current evidence on FGS in pediatric oncology, with emphasis on the unique challenges inherent to this field. Finally, strategies to accelerate clinical translation and assess the potential clinical value are proposed. Methods: A narrative review of the literature was conducted using PubMed and Embase to identify English-language publications on FGS in pediatric oncology up to September 2025. Search terms included Fluorescence, Pediatrics, Neoplasms, and Surgery. Results: Studies commonly reported that indocyanine green (ICG) aids in lymph node mapping, hepatoblastoma resection, and visualization of vascular structures and tissue perfusion. However, its non-specific nature and lack of histopathological validation limits diagnostic precision in tumor imaging. Tissue-specific agents are being investigated in first-in-humans trials to improve sensitivity and specificity, and to identify ureters and nerves. Conclusions: In this review, the challenging roadmap for advancing FGS in pediatric oncology is presented. Closing current gaps will require coordinated efforts in target discovery, agent design, and clinical validation. If successful, FGS can evolve from a promising tool into an indispensable clinical technique that enhances surgical precision, reduces recurrence, and ultimately improves long-term outcomes for children with cancer.
1. Introduction
For pediatric oncology surgeons adhering to the fundamental principles of surgical oncology—which involve radical resections, accurate disease staging through detection of lymph nodes and avoiding injury to critical structures—can be extremely challenging [1,2]. The challenges mainly lie in infiltrative tumor growth, the size of the pediatric patient and the proximity of critical anatomical structures.
As a consequence, the success of these surgical resections largely depends on the surgeon’s expertise, guided by visual and tactile feedback during the procedure [3]. Irradical resections may lead to incomplete tumor removal, thereby increasing the risk of local recurrence and the need for adjuvant treatment intensification. Moreover, surgical imprecision can result in unnecessary damage, for example, to vital structures and severe postoperative functional impairment. Apart from increasing local recurrence-free and overall survival rates, complete resections may help reduce total dosages of adjuvant chemo- and or radiotherapy. This is particularly important in pediatric patients, who are especially vulnerable to long-term adverse effects of cancer treatment, such as impaired growth and development, organ dysfunction, and the development of secondary malignancies [4].
The inherent challenge of balancing the extent of resection—avoiding both undertreatment and overtreatment—underscores the critical need for intraoperative technologies that enhance surgical precision and support real-time clinical decision making. In this context, both for the adult and pediatric population, there is a growing interest in intraoperative visualization techniques, such as fluorescence-guided surgery (FGS). FGS enables real-time visualization of vital anatomical structures or specific tissues of interest. This technique is based on the Stokes shift principle [5], whereby an external light source excites a fluorescent molecule to a higher energy state. As the molecule returns to its ground state, it emits a photon of lower energy. In clinical practice, after intravenous or local administration of a fluorescent agent, the emitted light is captured using a near-infrared (NIR) imaging system and displayed on the screen in real time (Figure 1).
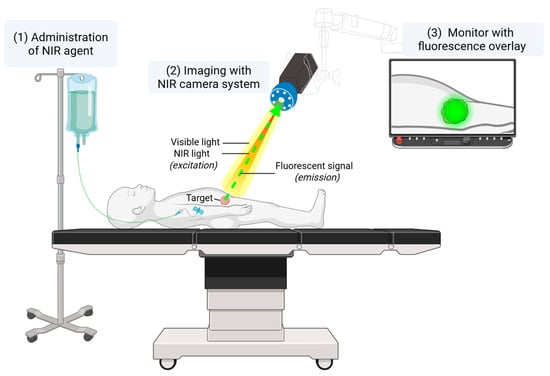
Figure 1.
A schematic overview of a clinical FGS setup. A NIR fluorescent agent is administered intravenously or locally, either preoperatively or intraoperatively. The NIR camera system enables visible light as well as NIR light, which excites the fluorescent agent. The emitted light is then captured by the camera and displayed in real time on a monitor.
Fluorescent agents used in FGS can be divided into two main categories: non-targeted and targeted agents. Commonly used non-targeted agents include the FDA- and EMA-approved indocyanine green (ICG), methylene blue (MB) and sodium fluorescein. These agents have been increasingly implemented in adult and pediatric surgical oncology for various applications, including lymph node identification, perfusion assessment, and solid tumor delineation [6]. To improve the precision of FGS and to explore broader clinical indications, research over the last years has focused on the development of targeted fluorescent agents, including antibodies, antibody fragments, peptides, or small molecules conjugated to a fluorescent dye. These agents are designed to bind selectively to tissue-specific biomarkers, thereby reflecting distinct cellular processes. Adequate target validation is particularly relevant in a pediatric context, as pediatric tumors frequently have an embryonal origin and differ substantially from adult malignancies in their biological characteristics. This target validation requires linking fluorescence to histology to confirm binding specificity and to assess receptor heterogeneity during image processing. Furthermore, applying uniform definitions for quantitative metrics is necessary to account for the unique biological variability encountered intraoperatively. Ultimately, targeted fluorescence imaging aims to enhance the accuracy of tissue delineation and improve surgical outcomes.
While in adult oncologic surgery, numerous clinical trials are investigating the use of targeted fluorescent agents, the application of targeted agents for FGS in pediatric oncology remains in its infancy [7]. Most clinical studies have concentrated on non-targeted agents, primarily evaluating safety, proof-of-concept, and the technical or diagnostic performance of this technique. However, evidence remains limited regarding understanding the relation between pathology and fluorescence of either non-targeted or targeted agents, its impact on intraoperative decision making and the added value for the patient.
In this review, we provide a comprehensive and up-to-date overview of the current literature on FGS in pediatric oncology, organized by clinical indication and highlighting recent technological and translational advancements in the field (Figure 2). Unique in this review, we specifically focus on the unique challenges inherent to pediatric oncology. Finally, we propose concrete strategies to accelerate clinical translation and critically assess the potential clinical value of FGS in pediatric oncologic surgery.
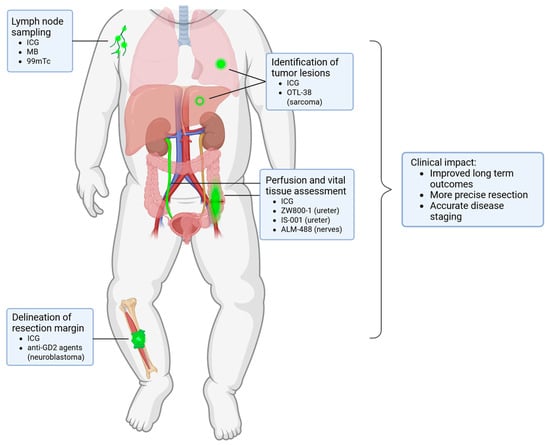
Figure 2.
Overview of the clinical applications of FGS within pediatric oncology with the fluorescent agents per indication.
2. Materials and Methods
A literature search was conducted in Pubmed and Embase for articles in English published up to August 2025 that reported the use of fluorescence imaging in pediatric oncology. The search strategy included the terms ‘Fluorescence’, ‘Pediatrics’, ‘Neoplasms’, and ‘Surgery’, and was supplemented with relevant title and abstract keywords to provide a comprehensive review of relevant articles. The search string was developed with our institutional medical library and is provided in the Supplementary Materials. Our narrative review excluded: studies that were not related to (surgical) oncology, adult population, non-English language, case reports or reviews, brain or eye neoplasms, or publications unrelated to (fluorescence) image-guided surgery. The study selection involved two independent researchers (D.S., L.S.) who identified all relevant articles. Conflicting interpretations were resolved by consulting a third reviewer (W.T.) to reach a consensus. Of the 1046 identified articles in our initial search, 40 full-text publications were included based on the search string. Based on the screened articles, studies were grouped into three intended clinical applications: lymph node identification, vital structure imaging, or tumor tissue visualization. Afterwards, the studies were divided into non-targeted or targeted agents (Table 1). Quality and risk of bias assessment was not performed, as this narrative review aimed to describe current practice and propose strategies for clinical translation.

Table 1.
Overview of FGS in pediatric oncology.
3. Identification of Lymph Nodes
Fluorescent dyes have been used to facilitate lymphatic mapping and sentinel lymph node biopsy in surgical oncology for decades. Until recently, the sentinel lymph node procedure (SNP) in pediatric oncology involved the use of a radiotracer and an optional blue dye injection around the tumor site, commonly MB, for visual guidance. MB is visible without imaging system; however, an important downside of MB is the fact that handling the injection site can lead to diffuse blue staining of the surroundings, obscuring the target area. Moreover, MB is associated with a relatively low efficacy (60%), limited penetration depth, and risk of severe allergic reactions [48,49,50]. Therefore, ICG guidance has gained increasing interest as a safer and more effective alternative to blue dyes, as reported by Campwala et al. and Johnston et al. [8,9]. Thus far, the use of ICG during SNP in children has been described for melanoma, squamous cell carcinoma, and soft tissue sarcomas located in the extremities, head and neck, or trunk.
Jeremiasse et al. reported that ICG was particularly useful for nodes in the inguinal area, head and neck regions, and in transit sites compared to the axilla, likely due to its maximum penetration depth leading to reduced utility for deeper-lying sentinel lymph nodes [10]. To overcome this limited penetration depth, novel optical depth-sensing approaches, such as dual-band systems capable of simultaneously detecting ICG and PpIX, are being developed to enhance deep-tissue fluorescence [51]. Moreover, frequently cited additive values included the detection of additional (sentinel) lymph nodes, and the transcutaneous visualization of sentinel lymph nodes when the agent was administered prior to incision [11], which occurred in about two-thirds of cases. This capability may aid in surgical incision planning (Figure 3) and potentially reduce time under anesthesia. In most studies, ICG was combined with Technetium-99 m nanocolloid to enhance detection of deeper lymph nodes. In adults with breast cancer, ICG alone achieved comparable sentinel lymph node detection [52], which may be relevant for future pediatric applications.
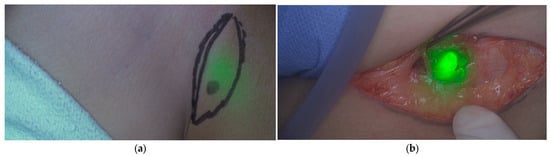
Figure 3.
ICG fluorescence of the sentinel lymph node located in the inguinal region of a pediatric patient with melanoma. Pseudo-colored fluorescence overlay: (a) fluorescent lymph node observed through the skin before surgical incision; (b) fluorescent lymph node localized in vivo. Images were obtained from our Sentinel Lymph Node Procedure Study Using Fluorescence Imaging with ICG (NL71166.041.20).
In pediatric oncology, sampling multiple lymph nodes is a key component of staging certain pediatric tumors, primarily for Wilms tumors and paratesticular rhabdomyosarcomas [53,54,55,56], which may influence intensification of treatment. Thus far, lymph node harvesting for pediatric testicular rhabdomyosarcoma under ICG guidance has only been reported during retroperitoneoscopic surgery, as described by Mansfield et al. and Pio et al. [12,13], where ICG was injected intraoperatively into the spermatic cord under ultrasound guidance to enhance visualization of lymphatic drainage pathways. The first node showing ICG avidity was designated as the sentinel node and was tumor-positive in some cases. Similarly, ICG-guided lymphatic mapping and nodal sampling have been described in Wilms tumor [14,15], achieved through peri-hilar injection or ipsilateral intra-parenchymal injection. These studies showed that the use of ICG in fluorescence-guided surgery increases the number of nodes detected. This is an important consideration given current guidelines recommending sampling of ≥7 nodes in Wilms tumor, despite a median of only 4 typically being reported [54,57]. Although the intra-parenchymal approach was technically more successful than the peri-hilar approach, it can be more challenging in tumors lacking clearly visible healthy renal parenchyma, for example, during upfront resection of larger tumors.
In general, ICG fluorescence in pediatric oncology enables the visualization of lymph nodes for mapping purposes. In this context, FGS is used to identify anatomical structures rather than malignancy, making ICG a safe and valuable technique for surgeons. However, whether it actually improves the precision of nodal sampling or reduces the risk of treatment-related morbidity and complications such as vascular injury is currently being investigated in an ongoing randomized controlled trial (ISRCTN26150156).
4. Imaging of Vital Structures
4.1. Non-Specific Tissue Imaging
Within pediatric oncology, identification of vital structures in proximity of the tumor, such as blood vessels, nerves and ureters is essential for safe tumor excision. Fluorescence can also play a critical role in perfusion imaging (Figure 4). Aung et al. evaluated the use of ICG during rotationplasty to visualize perfusion during pediatric sarcoma resection in three patients [16]. Vascular management is essential for a successful rotationplasty and prevents tissue necrosis and complications. ICG allows surgeons to visualize blood vessels, including the perivascular system of nerves, in real time during tumor resection and rotationplasty, providing insight into tissue perfusion and helping to reduce postoperative complications such as wound healing problems or flap necrosis. During surgery, it helps to identify anatomical variations. Another advantage is the possibility of repeated administration, which can be useful when dissecting neurovascular bundles from the tumor. The authors concluded that ICG fluorescence serves as a safe adjunct that supports surgical decision making by providing essential information on tissue perfusion when planning complex ablative and reconstructive procedures, such as rotationplasties.
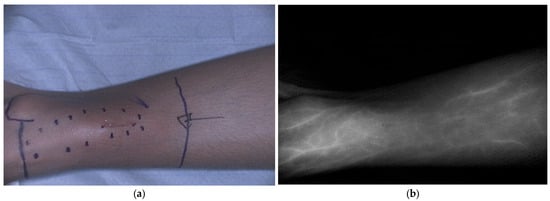
Figure 4.
ICG fluorescence showing vascular perfusion, including the vena saphena magna, before primary bone tumor resection in a pediatric patient. (a) Bright field image; (b) Corresponding black-and-white fluorescence image. ICG was administered intravenously before surgery. Data derived from our clinical study Fluorescence with ICG in Sarcoma Surgery (PMC CRC 2024-003).
To aid intraoperative guidance during pediatric minimally invasive procedures, Esposito and colleagues have consistently highlighted the value of ICG fluorescence imaging. Across their series, ICG was applied intravenously during the resection of abdominal masses and lymphomas. They concluded that ICG was useful for identification of vascular anatomy of the abdominal mass, for determining the plane of resection during mesenteric division, and for perfusion assessment of the bowel [17]. Esposito and colleagues found that ICG helped to assess the vascularity of the ovary, salpinx and uterus after tumor excision [18,19,20]. For paratubal lesions, ICG helped to check the vascular permeability of the fallopian tube after resection [21]. Intraoperative ICG injection, allowed for fast (~60 s) and safe visualization of both critical anatomical structures and pathological entities, thereby potentially preserving organ function and patient’s safety.
Collectively, these studies demonstrate that ICG fluorescence imaging could serve as an adjunct in pediatric oncologic surgery by enabling real-time visualization of vascular anatomy and soft tissue perfusion during tumor resections and reconstructive procedures. Currently, this technique cannot replace image-guided localization techniques; however, ICG could assist surgeons in real-time intraoperative decision making in complex pediatric oncological surgery, allowing them to perform precise resections with the goal of mitigating complications such as ischemia, wound healing problems, or inadvertent injury to adjacent structures. Its rapid onset, possibility for repeated dosing, and ability to distinguish normal from pathological structures support precise resections, reduce complications, and facilitate organ preservation, thereby enhancing both surgical safety and outcomes in complex procedures.
4.2. Advances in Tissue-Specific Imaging Approaches
While no studies in pediatric oncology have yet reported the use of tissue-specific fluorescence to identify vital anatomical structures, several promising advances have emerged in adult surgery [58]. Firstly, multiple approaches for accurate intraoperative identification of nerve tissue to avoid iatrogenic nerve injuries and corresponding morbidities have been made [59]. Lee et al. explored the use of bevonescein (ALM-488), a small peptide labeled with fluorescein binding to the structural extracellular matrix component of the nerves, in head and neck surgery in a phase 1 trial (NCT04420689) [60]. Specific binding to nerve tissue was observed and the injection of bevonescein was considered safe. Consequently, bevonescein is currently being explored in phase 2 clinical trials for nerve visualization in head and neck surgery (NCT06227585, including adults and children aged 16 years and older) and for abdominopelvic surgery in adults (NCT06662097). In addition, nerve-specific oxazine based fluorophores are being explored in pre-clinical studies [61]. Imaging the nerves can be beneficial for different surgical indications, making these studies highly relevant.
Secondly, intraoperative identification of the ureters is gaining interest, for example, to prevent injury during laparoscopic abdominopelvic procedures in adults. Instead of using ureteral stents, which can itself result in complications such as infection, prolonged need for antibiotics or iatrogenic ureteral damage [62,63], NIR fluorescence imaging could provide non-invasive and real-time visualization of the ureters during surgery. De Valk et al. addressed this challenge with a novel zwitterionic NIR fluorophore, nizaracianine triflutate or ZW800-1, specifically engineered for ureter visualization [64]. Unlike conventional highly anionic NIR dyes, ZW800-1 facilitates exclusive renal clearance, thereby enabling clear and consistent ureter delineation for several hours in real time. Currently, a phase 2/3 trial (NCT06101745) is running to investigate the safety and effectiveness of ZW800-1 in ureter visualization. Moreover, Farnam et al. developed IS-001, a cyanine NIR ureter probe for intravenous injection [65]. In this phase 1 study, they concluded that IS-001 was safe and renally excreted, allowing enhanced ureter visualization. A phase 3 study is currently evaluating the safety and efficacy of IS-001 for ureter delineation in robotic-assisted gynecological surgery (NCT05954767). Both ZW800-1 and IS-001 enable clear and consistent real-time ureter delineation using clinically available imaging platforms. However, within pediatric surgical oncology, ZW800-1 is of particular interest given its efficacy at low doses (1–2.5 mg ZW800-1 compared to 10–40 mg IS-001) and its prolonged imaging window (3 h with 2.5 mg ZW800-1 compared to 1 h with 30 mg IS-001). These properties not only minimize exposure risk but also provide greater flexibility during complex and lengthy procedures, making it especially well-suited for pediatric applications.
As discussed above, tissue-specific agents hold significant clinical potential; however, their implementation in pediatric practice has not yet been realized.
5. Visualization of Pediatric Tumors
5.1. Non-Specific Imaging
Another indication of interest for fluorescence imaging is the identification of the tumor location and tumor margins during surgery, both at the primary site and metastatic sites. Clinically approved fluorescent agents, such as ICG and sodium fluorescein, have been employed for this purpose in surgical oncology. Hereafter, the indications are described per tumor type.
5.1.1. Hepatoblastoma
NIR imaging with ICG is increasingly utilized in pediatric hepatoblastoma surgery, where it serves as a complementary tool for identifying multifocal lesions and the demarcation of tumor tissue. ICG shows prolonged retention in tumor tissue compared to hepatocytes due to leaky vascular capillaries and defective lymphatic clearance (i.e., the enhanced permeability and retention (EPR) effect) [66], which hinders its excretion into bile. In most studies, ICG was intravenously administered at 24–96 h before surgery to minimize background fluorescence, with dosages ranging from 0.1–0.5 mg/kg [22,23,24,25,26,27,28,29]. ICG-guided surgery enabled sensitive detection of primary and residual hepatoblatoma, liver satellite lesions, and metastases including millimeter-sized nodules in the abdominal cavity and lungs [30,31,32,33,67]. In addition, ICG fluorescence helped surgeons in visualizing lesions in the liver that were not identified on preoperative imaging. Although ICG fluorescence is highly sensitive, false positivity may lead to unnecessary resection and increased site-specific morbidity, such as hemorrhage and biliary complications in the liver [68,69,70]. Moreover, pretreated lesions often showed heterogeneous or absent fluorescence but based on limited research no clear association with pathology has been seen thus far.
5.1.2. Renal Tumors
ICG-guided nephron-sparing surgery (NSS) is feasible for pediatric renal cancers, with ICG administered intravenously either the day before surgery (1.5 mg/kg) or a fixed intraoperative intravenous dosage (2.5 or 5 mg) [34,35]. In both case series, Wilms tumor and renal cell carcinoma were hypofluorescent and demonstrated an ‘inverse pattern’ of NIR signal. This was attributed to the downregulation of bilitranslocase, which is highly expressed in normal renal tubules and mediates uptake of organic anions, including ICG [71,72]. In contrast, ICG fluorescence was higher in a malignant rhabdoid tumor of the kidney relative to its healthy parenchyma, likely as a result of the EPR effect [66]. NIR-guided tumor localization during NSS succeeded in most kidneys and could potentially reduce time under anesthesia; however, it has not yet shown to improve the rate of margin positivity, which can lead to an escalation of therapy (e.g., adjuvant radiotherapy) to mitigate the associated risk of local recurrence. Additionally, conditions such as tumor adhesions to perirenal tissues and reduced blood supply due to renal artery embolization may influence fluorescence patterns, making this technique less suitable in some cases. Most Wilms tumor cases received upfront chemotherapy, but histological responses varied widely, making it unclear how practical NIR imaging with ICG is for delineating the tumor–renal boundary after neoadjuvant treatment, which is a challenge for all solid tumors [73]. Furthermore, the ICG avidity of pulmonary Wilms tumor metastases has been inconsistent as described in a few patients [34,36].
5.1.3. Abdominal Tumors and Lymphoma
Esposito et al. recently described the use of ICG during laparoscopic excision of abdominal masses in pediatric patients [17]. In this study, ICG was administered intravenously to visualize the vascular anatomy of the abdominal mass during primary tumor resection. Additionally, they found that ICG helped to identify adnexal tumors, which appeared hypofluorescent relative to healthy tissue [18,19,20]. This technique helped the surgeon define and preserve the resection margins, guiding surgical decision making for potential ovarian-sparing surgery. Intraoperative ICG injection, allowed for fast (~60 s) visualization of both critical anatomical structures and pathological entities, thereby potentially positively impacting oncological radicality while preserving organ function and patient’s safety.
5.1.4. Bone and Soft Tissue Sarcoma
Primary bone and soft tissue sarcomas can be visualized using NIR imaging in adults, with a handful of adolescents included. The application of ICG was regarded useful for achieving complete curettage of locally aggressive benign bone tumors [37], such as giant cell tumor of bone. It was considered particularly important for intralesional curettage, as this has been associated with higher rates of local recurrence compared to wide resection [74], but carries a lower risk of complications. Additionally, the use of fluorescence also frequently helped surgeons identify tumor residue in the wound. ICG uptake was observed in a substantial proportion of malignant bone tumors in a case series [38], which has been suggested to reduce the need for extensive bone resection. ICG fluorescence imaging also guided skull bone tumor resection by visualizing occult tumors, enabling complete removal with appropriate margins, and thus, holding promise for other anatomical sites [39]. In multiple centers, ICG has been used for the identification of metastases of high-grade pediatric sarcomas [36,40,41,67], with intravenous injection (0.5–1.5 mg/kg) frequently administered the day before surgery to reduce background noise. Pulmonary lesions of osteosarcoma, Ewing sarcoma, rhabdomyosarcoma, and non-rhabdomyosarcoma, containing viable disease, fluoresced during surgery (Figure 5). This is promising for procedures with limited or no tactile feedback, such as thoracoscopy, and may help identify additional subpleural nodules missed by preoperative computed tomography (CT) or palpation during open surgery. In some children with osteosarcoma, lesions containing sporadic viable tumor cells either did or did not fluoresce, and lesions without viable tumor did not fluoresce [41]. For now, it is suggested that ICG can be used as an adjunct to other localization methods but cannot replace interventional radiology techniques, such as CT-guided placement of a hook, wire, or injection of EVOH polymer. However, these methods also carry the risk of mispositioning, migration, pneumothorax, and pain [40].
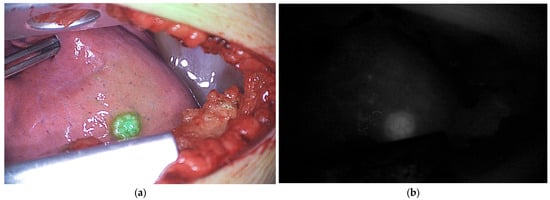
Figure 5.
ICG fluorescence of vital pulmonary metastases in a pediatric patient with osteosarcoma during thoracotomy. (a) Pseudo-colored fluorescence overlay image; (b) Corresponding black-and-white fluorescence image. Data derived from our clinical study Fluorescence with ICG in Sarcoma Surgery (PMC CRC 2024-003).
5.1.5. Peripheral Nerve Sheath Tumors
The feasibility of fluorescence-guided resection of peripheral nerve sheath tumors has been reported by Vetrano et al., with an intravenous injection of 1 mg/kg sodium fluorescein (560 nm) after intubation [42]. This case series included young adults with schwannomas and neurofibromas, primarily located in the extremities or the brachial plexus region. In peripheral nerves, fluorescein may extravasate into the endoneurium, similar to tumors in the central nervous system, where the blood–brain barrier is disrupted. In this series, fluorescein enabled delineation and resection of tumor tissue after opening the tumor pseudocapsule, while functionally intact nerve fibers were identified using direct intraoperative electrophysiological stimulation. Neurofibromas were removed piecemeal, and fluorescein was regarded useful for detecting small tumor remnants that were not visible using ambient light. Notably, plexiform neurofibromas have the potential for malignant transformation. No adverse reactions were reported; however, phototoxic reactions upon sun exposure, including severe pain and allergies, have been described in the literature, which may limit further implementation [75].
5.1.6. Otolaryngologic Malignancies
Richard et al. reported on the feasibility of ICG NIR imaging for pediatric head and neck cancers, using an intravenous dosage of 1.5 mg/kg the day before surgery [43]. ICG NIR imaging revealed tumor extensions that were both undetectable on preoperative imaging and intraoperatively under ambient light and/or by palpation, facilitating more precise and safer resections. ICG NIR was also evaluated for local metastases and altered the surgical strategy in one case, prompting conversion to bilateral neck dissections. Despite ICG’s high sensitivity and specificity, positive tumor margins were observed in two of eight cases (25%), highlighting the need to combine this technique with other intraoperative imaging modalities, such as magnetic resonance imaging or ultrasound, to minimize residual disease.
In conclusion, while fluorescence-guided surgery with non-specific agents such as ICG appears promising for several pediatric solid tumors, its clinical utility remains unproven, with robust evidence currently only available for hepatoblastoma. For other tumor types, results are still exploratory and hampered by non-specific binding and variability in tumor histology and biology. Consequently, non-specific agents are prone to false-positive and false-negative signals, especially in highly vascularized or inflamed tissues. In tumors with infiltrative growth into surrounding normal tissue, FGS may therefore provide limited additional value for complete resection or tumor margins and may mislead surgical decision making. In addition, it may support intraoperative decision making that surgery might not be curative and/or that adjuvant treatment strategies may be more appropriate. To better define its value, a large prospective clinical trial (NCT04084067) has recently been initiated in the United States, including patients with osteosarcoma, Ewing sarcoma, rhabdomyosarcoma, non-rhabdomyosarcoma, neuroblastoma, renal and liver tumors, as well as other rare entities. This study will not only assess the added value of ICG-guided margin delineation and metastasis detection but will also explore factors such as uptake differences between primary and metastatic lesions, the influence of prior treatments, and the detection of residual disease.
5.2. Tissue-Specific Tumor Imaging
To address limitations in sensitivity and specificity associated with non-targeted agents, there is growing interest in targeted fluorescent agents. Several studies have highlighted the potential of targeted FGS for future applications in pediatric oncology [76,77]. However, progress in developing and implementing these agents remains limited, not only due to the scarcity of validated pediatric-specific targets, but also because of the limited availability of targeted agents, such as antibodies, that can be safely administered in children and that demonstrate the required high specificity for tumor cells with minimal binding to healthy tissues. Ideal imaging agents must therefore combine high tumor cell specificity and binding affinity with minimal off-target uptake and low toxicity. In adult cancers, antibodies and peptides directed against various oncological molecular targets such as the endothelial growth factor receptor, vascular endothelial growth factor A, carcinoembryonic antigen have been labeled with NIR dyes to delineate tumors, but such targets are not suitable in the pediatric setting due to the different origin of pediatric tumors [78,79]. Within pediatric oncology, two promising targets for FGS have recently emerged: the disialoganglioside (GD2) antigen, targeted by anti-GD2-based agents, for neuroblastoma and some sarcomas and the folate receptor, targeted by pafolacianine, for pulmonary metastases of (osteo)sarcoma. Until now, these agents have not yet been investigated in children.
5.2.1. Targeting Neuroblastoma with Anti-GD2 Based Imaging Probes
Neuroblastoma surgery poses a high risk of complications as the tumor’s infiltrative growth into surrounding organs and vessels makes the resection challenging. While neoadjuvant therapy often induces tumor volume reduction, it also creates tissue heterogeneity, such as necrosis, fibrosis, matured neuroblastic tissue, and calcifications, which further complicates the distinction between tumor and healthy tissue [80]. Targeted FGS aims to allow for precise resection (≥95% tumor removal) of complex tumors while sparing vital adjacent structures, which both affect the prognosis and quality of life of the patient [81,82,83]. In neuroblastoma, this approach is particularly promising given the tumor-specific overexpression of GD2, which is minimally present in normal tissues and therefore represents an attractive target for both imaging and therapy. Anti-GD2, or Dinutuximab-beta, is currently used for immunotherapy in high-risk patients [84]. Importantly, GD2 is present on the various subtypes and stages of neuroblastoma and is retained on the cell membrane after induction chemotherapy, supporting its potential for targeted FGS [85].
Wellens et al. conjugated anti-GD2 to IRDye800CW, which showed specific binding to human neuroblastoma cells both in vitro and in vivo using xenograft mouse models [44]. Across patient-derived neuroblastoma organoids a universal, yet heterogeneous expression of GD2 was observed. Even tumors with low GD2 expression levels or tumors pretreated with anti-GD2 still provided real-time fluorescence signal. Similarly, Privitera et al. conjugated anti-GD2 to two NIR dyes, IRDye800CW and to IR12, and tested them in multiple cell lines [45]. Both conjugates are promising FGS probes and can be used in the NIR and shortwave infrared imaging (SWIR) window, with NIR providing more signal but SWIR providing sharper imaging at depth which could be beneficial for small lesions or lesions adhering to vital structures. To overcome limited depth penetration, Rosenblum et al. evaluated the potential of dual-labeled 111In-αGD2-IR800, consisting of both a radio-guided surgery (RGS) component and a FGS component in xenograft mouse models [46]. The gamma decay from the RGS component, 111In, can be used for finding the tumor localization at depth while the FGS component, αGD2-IR800, can be used for precisely visualizing the tumor margins for optimal resection. All studies demonstrated highly specific binding of anti-GD2 to neuroblastoma and promising results to use this antibody for FGS. The highest tumor to background (TBR) was achieved between two and six days [44,45,46]. To investigate anti-GD2-800CW in a clinical trial, a first-in-human phase Ib/II clinical trial has recently been opened in the Netherlands (EUCT 2023-507596-22-00).
5.2.2. Targeting Pulmonary Metastases of (Osteo)Sarcoma with Pafolacianine
Surgery for pulmonary metastases is in some pediatric cancers essential, especially for tumors that are less responsive or resistant to adjuvant therapy, such as osteosarcomas and non-rhabdomyosarcomas soft tissue sarcomas [86]. Complete resection of these lesions is necessary to increase the chance of survival [87]. Similarly to ICG, targeted FGS could enhance surgical precision which could potentially influence surgical planning and long-term outcomes.
ICG has been widely applied to localize metastatic nodules. However, low specificity was observed and a need for more accurate techniques is preferred. Pafolacianine, also known as CYTALUX or OTL-38, targets the folate receptor and has successfully been used to improve surgical outcomes for primary and metastatic ovarian and lung tumors in adults [88,89]. In children, the folate receptor is also expressed in osteosarcomas [90]. Lehane et al. investigated the potential of using pafolacianine in four young adults (18–25 years) with pulmonary nodules of patients with osteosarcoma and Ewing sarcoma [47]. One additional lesion was found with the use of fluorescence in a patient with osteosarcoma, which was confirmed to contain malignant tissue. All osteosarcoma lesions that were identified under white light were fluorescent, of which two contained benign intraparenchymal lymph nodes. These false positive results were likely due to folate receptor-beta found on activated macrophages within benign lymph nodes. In the Ewing sarcoma patient, a 4 mm nodule was intraoperatively not distinguishable by fluorescence with faint fluorescence on back-table imaging, which was histologically proven malignant. These results showed that pafolacianine was safe and feasible but highlighted the need for careful target selection and validation in pediatric populations, where tumor biology may differ significantly from adults. Currently, two clinical trials started to investigate the potential of pafolacianine for pediatric pulmonary metastatic disease (NCT06235125) and for pediatric extracranial solid tumors (NCT06915727).
Collectively, these studies suggest that targeted FGS holds considerable promise in pediatric setting, especially for reducing the false positive results; however, the field is still waiting for the results of the first clinical trials.
6. Future Directions
Current evidence demonstrates that FGS in pediatric oncology is feasible and provides added value in selected applications. ICG has a well-established safety profile and has been shown to aid in lymph node mapping, hepatoblastoma surgery, and visualization of vascular structures and tissue perfusion, highlighting its key strengths. In these applications, FGS is most robust as it used to clarify anatomical details and less likely to mislead the surgical decision making. However, its non-specific nature, the predominantly anecdotal nature of the available evidence and the current lack of histopathological validation may limit broader use within pediatric oncology. In particular, ICG can visualize tumor tissue but does not specifically identify tumor lesions or define resection margins, residual viable tumor, or tumor-positive lymph nodes. These potential false-positives or false-negatives for malignancy could mislead surgical decision making and have a negative effect on the clinical outcome. Similar to adult oncology, the EPR effect is variable due to multiple factors including tumor type, volume, location, and the presence of necrosis and/or vascular mediators such as bradykinin and prostaglandins [73,91]. For example, inflammatory tissue express similarities to cancer in vascular mediator activity that can lead to false-positive fluorescence [92]. Increased fluorescence intensity in healthy tissue can also occur due to high vascularity [93]. Such strongly perfused tissues are prone to false-positive fluorescence signals, complicating intraoperative interpretation. Careful reporting of both tumor signal and surrounding tissue characteristics is therefore essential for optimizing the use of ICG, especially when looking at dosing and timing. These weaknesses also underscore the need for more specific fluorescence-guided approaches. Beyond improving intraoperative visualization, advances in FGS may also enable therapeutic extensions, such as photodynamic therapy. By combining tumor-specific fluorescence with light-induced cytotoxicity, these theranostic approaches could potentially enhance local tumor control while preserving surrounding healthy tissue [94,95]. However, in pediatric oncology, such applications remain largely exploratory and will critically depend on the development and validation of highly specific targeting agents tailored to the unique biological characteristics of pediatric tumors [95].
The development and validation of these targeted tissue-specific agents represents an important opportunity that may address these inaccuracies of non-specific agents improving sensitivity and specificity, ultimately enabling safer resections and more tailored treatment strategies. Current strategies for the development of tissue-specific agents often build on established targets from immunotherapy or adapt targets validated in adult oncology to pediatric cancers, as this may accelerate implementation compared to developing entirely novel targets. However, pediatric tumors frequently have an embryonal origin and differ substantially from adult malignancies in biological characteristics. Compared with adult cancers, they generally have a lower tumor mutational burden, which limits the availability of suitable molecular targets for tumor-specific imaging approaches, and they often arise in tissue types that are rarely encountered in adult oncology. Ideal targets should be highly expressed on tumor tissue with minimal off-target binding. Once identified, an appropriate targeting vehicle such as an antibody, antibody fragment, peptide, or small molecule must be selected, each with characteristic trade-offs in specificity, tissue penetration, and pharmacokinetics [96,97]. Antibodies offer high affinity but slower systemic clearance due to their larger size, which can result in prolonged background signal from circulating unbound agent and may require administration several days prior to surgery to allow sufficient clearance [98]. In contrast, smaller fragments and peptides provide faster tumor uptake and background clearance, sometimes at the expense of reduced stability. Small molecules are easier to synthesize and optimize, allow rapid clearance, but are generally less specific. The final step involves pairing the vehicle with a fluorescent dye compatible for intraoperative imaging.
Identified agents should undergo rigorous preclinical validation across in vitro, ex vivo, and in vivo models to confirm specificity, distribution, and safety prior to clinical translation [99]. Translation into humans is critical, as preclinical models cannot fully replicate pediatric tumor biology, and the safety profile of targeted agents must be carefully considered, which may pose a threat. Regulatory approval for first-in-child trials represents a key milestone, as fluorescent agents are generally classified as investigational medicinal products and are subject to drug development regulations. While comprehensive toxicology, pharmacokinetic, and manufacturing data are usually required, many standard tests for therapeutic medicinal products may be less relevant for optical agents, which are administered at much lower doses, that do not require any therapeutic effect. Consequently, early pediatric trials should focus on pharmacokinetics and safety at clinically relevant optical imaging doses, together with optimization of timing of administration. Placed in the broader landscape of emerging targeted optical agents, which promise greater molecular specificity but will demand even stricter methodological rigor, ICG offers a valuable benchmark. Its well-established safety profile and straightforward clinical implementation make it a practical first-line agent to determine which pediatric tumor types can be visualized with adequate sensitivity and specificity, and which will ultimately require the development of more tissue-specific next-generation agents.
To ensure reliable interpretation and broader translation, standardized and transparent reporting is essential, underscoring the need for more systematic and consistent quantitative assessment and reporting. This emphasizes the need for uniform definitions for quantitative metrics (tumor-to-background ratio, mean fluorescence intensity) to be able to compare clinical trials and different agents. These metrics are not only clinical variables, but do also reflects biological variability of tumors. As emphasized by Tummers et al., early-phase studies should incorporate: (1) intraoperative assessment at multiple timepoints and imaging settings to localize tumors, evaluate resection margins, and to identify distant lesions or lymph nodes; (2) specimen mapping with preserved orientation during bread-loafing and paraffin embedding to enable correlation between fluorescence and pathology; and (3) target validation in paraffin-embedded tissue by linking fluorescence to histology, thereby confirming binding specificity and assessing receptor heterogeneity [100]. In addition, quantitative parameters such as mean fluorescence intensity, sensitivity, and specificity must be consistently reported to allow comparison across studies and enable robust clinical translation.
Despite promising applications, FGS faces several inherent challenges. Interpretation remains highly dependent on the surgeon’s expertise, as differentiating between true and non-specific fluorescence can be subjective. Variability in fluorescence intensity, influenced by vascular inflow dynamics and tissue composition, further confuses intraoperative decision making. Additionally, tumor heterogeneity and complex tumor biology (e.g., coexistence of viable tumor, necrosis, and fibrosis) can affect the consistency and reliability of fluorescence signals. Nevertheless, histopathological confirmation remains indispensable to determine whether the observed fluorescence accurately represents malignant tissue and to validate the diagnostic performance of the technique. While quantitative methods may help standardize interpretation, no universally accepted protocols are currently available, limiting reproducibility and broader adoption.
7. Conclusions
In conclusion, the outlined steps represent a challenging yet clinically realistic and pediatric-specific roadmap for advancing fluorescence-guided surgery in pediatric oncology. Closing current gaps will require coordinated efforts in target discovery, imaging agent design, and clinical validation, as well as collaboration across pediatric oncology centers to standardize methods and reporting. If successful, fluorescence-guided surgery in pediatric oncology can evolve from a promising tool into an indispensable clinical approach that enhances surgical precision, reduces recurrence, and ultimately improves long-term outcomes for children with cancer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers18010149/s1, literature search string.
Author Contributions
Conceptualization, D.C.S., L.H.M.v.S. and W.S.F.J.T.; methodology, D.C.S., L.H.M.v.S., M.A.J.v.d.S., A.L.V., M.H.W.A.W., A.F.W.v.d.S. and W.S.F.J.T.; software, D.C.S., L.H.M.v.S. and W.S.F.J.T.; validation, D.C.S., L.H.M.v.S., M.A.J.v.d.S., A.F.W.v.d.S. and W.S.F.J.T.; formal analysis, D.C.S., L.H.M.v.S. and W.S.F.J.T.; investigation, D.C.S., L.H.M.v.S. and W.S.F.J.T.; resources, D.C.S., L.H.M.v.S. and W.S.F.J.T.; data curation, D.C.S., L.H.M.v.S., M.A.J.v.d.S., A.F.W.v.d.S. and W.S.F.J.T.; writing—original draft preparation, D.C.S., L.H.M.v.S. and W.S.F.J.T.; writing—review and editing, D.C.S., L.H.M.v.S., M.A.J.v.d.S., A.L.V., M.H.W.A.W., A.F.W.v.d.S. and W.S.F.J.T.; visualization, D.C.S., L.H.M.v.S. and W.S.F.J.T.; supervision, M.A.J.v.d.S., A.L.V., M.H.W.A.W., A.F.W.v.d.S. and W.S.F.J.T.; project administration, D.C.S., L.H.M.v.S. and W.S.F.J.T.; funding acquisition, M.A.J.v.d.S., M.H.W.A.W. and A.F.W.v.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding from the KWF Dutch Cancer Society.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Princess Maxima Center for Pediatric Oncology (protocol code NL71166.041.20, date of approval 14 May 2020 and PMC CRC 2024-003 date of approval 18 November 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. Some of the data are not publicly available due to confidentially and in accordance with the Dutch Personal Data Protection Act.
Acknowledgments
The authors thank Felix Weijdema of the Utrecht University Library for his assistance in identifying and accessing key literature that supported the preparation of this review.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FGS | Fluorescence-guided surgery |
| NIR | Near-infrared |
| ICG | Indocyanine green |
| MB | Methylene blue |
| SNP | Sentinel lymph node procedure |
| EPR | Enhanced permeability and retention |
| NSS | Nephron-sparing surgery |
| CT | Computed Tomography |
| SWIR | Shortwave infrared imaging |
| GD2 | Disialoganglioside |
References
- Davidoff, A.M. Advocating for the Surgical Needs of Children with Cancer. J. Pediatr. Surg. 2022, 57, 959–966. [Google Scholar] [CrossRef]
- von Allmen, D. Pediatric Surgical Oncology: A Brief Overview of Where We Have Been and the Challenges We Face. Semin. Pediatr. Surg. 2019, 28, 150864. [Google Scholar] [CrossRef]
- Birkmeyer, J.D.; Stukel, T.A.; Siewers, A.E.; Goodney, P.P.; Wennberg, D.E.; Lucas, F.L. Surgeon Volume and Operative Mortality in the United States. N. Engl. J. Med. 2003, 349, 2117–2127. [Google Scholar] [CrossRef]
- Latoch, E.; Zubowska, M.; Młynarski, W.; Stachowicz-Stencel, T.; Stefanowicz, J.; Sławińska, D.; Kowalczyk, J.; Skalska-Sadowska, J.; Wachowiak, J.; Badowska, W.; et al. Late Effects of Childhood Cancer Treatment in Long-Term Survivors Diagnosed before the Age of 3 Years—A Multicenter, Nationwide Study. Cancer Epidemiol. 2022, 80, 102209. [Google Scholar] [CrossRef] [PubMed]
- Keereweer, S.; Van Driel, P.B.A.A.; Snoeks, T.J.A.; Kerrebijn, J.D.F.; Baatenburg de Jong, R.J.; Vahrmeijer, A.L.; Sterenborg, H.J.C.M.; Löwik, C.W.G.M. Optical Image-Guided Cancer Surgery: Challenges and Limitations. Clin. Cancer Res. 2013, 19, 3745–3754. [Google Scholar] [CrossRef] [PubMed]
- van Manen, L.; Handgraaf, H.J.M.; Diana, M.; Dijkstra, J.; Ishizawa, T.; Vahrmeijer, A.L.; Mieog, J.S.D. A Practical Guide for the Use of Indocyanine Green and Methylene Blue in Fluorescence-Guided Abdominal Surgery. J. Surg. Oncol. 2018, 118, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.C.H.; Lau, K.C.; Wong, K.K.Y. Fluorescence-Guided Pediatric Surgery: The Past, Present, and Future. J. Pediatr. Surg. Open 2024, 5, 100106. [Google Scholar] [CrossRef]
- Campwala, I.; Vignali, P.D.A.; Seynnaeve, B.K.; Davit, A.J.; Weiss, K.; Malek, M.M. Utility of Indocyanine Green for Sentinel Lymph Node Biopsy in Pediatric Sarcoma and Melanoma. J. Pediatr. Surg. 2024, 59, 1326–1333. [Google Scholar] [CrossRef]
- Johnston, M.E.; Farooqui, Z.A.; Nagarajan, R.; Pressey, J.G.; Turpin, B.; Dasgupta, R. Fluorescent-Guided Surgery and the Use of Indocyanine Green Sentinel Lymph Node Mapping in the Pediatric and Young Adult Oncology Population. Cancer 2023, 129, 3962–3970. [Google Scholar] [CrossRef]
- Jeremiasse, B.; van Scheltinga, C.E.J.T.; Smeele, L.E.; Tolboom, N.; Wijnen, M.H.W.A.; van der Steeg, A.F.W. Sentinel Lymph Node Procedure in Pediatric Patients with Melanoma, Squamous Cell Carcinoma, or Sarcoma Using Near-Infrared Fluorescence Imaging with Indocyanine Green: A Feasibility Trial. Ann. Surg. Oncol. 2023, 30, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Pio, L.; Richard, C.; Zaghloul, T.; Murphy, A.J.; Davidoff, A.M.; Abdelhafeez, A.H. Sentinel Lymph Node Mapping with Indocyanine Green Fluorescence (ICG) for Pediatric and Adolescent Tumors: A Prospective Observational Study. Sci. Rep. 2024, 14, 30135. [Google Scholar] [CrossRef]
- Mansfield, S.A.; Murphy, A.J.; Talbot, L.; Prajapati, H.; Maller, V.; Pappo, A.; Singhal, S.; Krasin, M.J.; Davidoff, A.M.; Abdelhafeez, A. Alternative Approaches to Retroperitoneal Lymph Node Dissection for Paratesticular Rhabdomyosarcoma. J. Pediatr. Surg. 2020, 55, 2677–2681. [Google Scholar] [CrossRef]
- Pio, L.; Zaghloul, T.; Abdelhafeez, A.H. Indocyanine Green Fluorescence-Guided Lymphadenectomy with Single Site Retroperitoneoscopy in Children. J. Pediatr. Urol. 2023, 19, 491–492. [Google Scholar] [CrossRef]
- Pachl, M.J. Fluorescent Guided Lymph Node Harvest in Laparoscopic Wilms Nephroureterectomy. Urology 2021, 158, 189–192. [Google Scholar] [CrossRef]
- Abdelhafeez, A.H.; Davidoff, A.M.; Murphy, A.J.; Arul, G.S.; Pachl, M.J. Fluorescence-Guided Lymph Node Sampling Is Feasible during up-Front or Delayed Nephrectomy for Wilms Tumor. J. Pediatr. Surg. 2022, 57, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Heidekrueger, P.I.; Geis, S.; Von Kunow, F.; Taeger, C.; Strauss, C.; Wendl, C.; Brebant, V.; Broer, P.N.; Prantl, L.; et al. A Novel Indication for Indocyanine Green (ICG): Intraoperative Monitoring of Limb and Sciatic Nerve Perfusion during Rotationplasty for Sarcoma Patients. Clin. Hemorheol. Microcirc. 2018, 70, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Del Conte, F.; Cerulo, M.; Gargiulo, F.; Izzo, S.; Esposito, G.; Spagnuolo, M.I.; Escolino, M. Clinical Application and Technical Standardization of Indocyanine Green (ICG) Fluorescence Imaging in Pediatric Minimally Invasive Surgery. Pediatr. Surg. Int. 2019, 35, 1043–1050. [Google Scholar] [CrossRef]
- Esposito, C.; Settimi, A.; Del Conte, F.; Cerulo, M.; Coppola, V.; Farina, A.; Crocetto, F.; Ricciardi, E.; Esposito, G.; Escolino, M. Image-Guided Pediatric Surgery Using Indocyanine Green (ICG) Fluorescence in Laparoscopic and Robotic Surgery. Front. Pediatr. 2020, 8, 314. [Google Scholar] [CrossRef]
- Esposito, C.; Masieri, L.; Cerulo, M.; Castagnetti, M.; Del Conte, F.; Di Mento, C.; Esposito, G.; Tedesco, F.; Carulli, R.; Continisio, L.; et al. Indocyanine Green (ICG) Fluorescence Technology in Pediatric Robotic Surgery. J. Rob. Surg. 2024, 18, 209. [Google Scholar] [CrossRef] [PubMed]
- Ciro, E.; Vincenzo, C.; Mariapina, C.; Fulvia, D.C.; Vincenzo, B.; Giorgia, E.; Roberto, C.; Lepore, B.; Castagnetti, M.; Califano, G.; et al. Review of a 25-Year Experience in the Management of Ovarian Masses in Neonates, Children and Adolescents: From Laparoscopy to Robotics and Indocyanine Green Fluorescence Technology. Children 2022, 9, 1219. [Google Scholar] [CrossRef]
- Esposito, C.; Blanc, T.; Di Mento, C.; Ballouhey, Q.; Fourcade, L.; Mendoza-Sagaon, M.; Chiodi, A.; Cardone, R.; Escolino, M. Robotic-Assisted Surgery for Gynecological Indications in Children and Adolescents: European Multicenter Report. J. Rob. Surg. 2024, 18, 20. [Google Scholar] [CrossRef]
- Cho, Y.J.; Namgoong, J.-M.; Kwon, H.H.; Kwon, Y.J.; Kim, D.Y.; Kim, S.C. The Advantages of Indocyanine Green Fluorescence Imaging in Detecting and Treating Pediatric Hepatoblastoma: A Preliminary Experience. Front. Pediatr. 2021, 9, 635394. [Google Scholar] [CrossRef]
- Feng, J.; Qin, H.; Yang, W.; Cheng, H.; Xu, J.; Han, J.; Mou, J.; Wang, H.; Ni, X. Tumor-Background Ratio Is an Effective Method to Identify Tumors and False-Positive Nodules in Indocyanine-Green Navigation Surgery for Pediatric Liver Cancer. Front. Pediatr. 2022, 10, 875688. [Google Scholar] [CrossRef]
- Kitagawa, N.; Shinkai, M.; Mochizuki, K.; Usui, H.; Miyagi, H.; Nakamura, K.; Tanaka, M.; Tanaka, Y.; Kusano, M.; Ohtsubo, S. Navigation Using Indocyanine Green Fluorescence Imaging for Hepatoblastoma Pulmonary Metastases Surgery. Pediatr. Surg. Int. 2015, 31, 407–411. [Google Scholar] [CrossRef]
- Lake, C.M.; Bondoc, A.J.; Dasgupta, R.; Jenkins, T.M.; Towbin, A.J.; Smith, E.A.; Alonso, M.H.; Geller, J.I.; Tiao, G.M. Indocyanine Green Is a Sensitive Adjunct in the Identification and Surgical Management of Local and Metastatic Hepatoblastoma. Cancer Med. 2021, 10, 4322–4343. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Wu, Y.; Su, J.; Chen, L.; Liao, M.; Zhao, Z.; Lu, Z.; Xiong, X.; Jin, S.; Deng, X. Deploying Indocyanine Green Fluorescence-Guided Navigation System in Precise Laparoscopic Resection of Pediatric Hepatoblastoma. Cancers 2022, 14, 6057. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, M.; Li, J.; Tan, T.; Yang, J.; Pan, J.; Hu, C.; Zou, Y.; Yang, T. Clinical Application of Indocyanine Green Fluorescence Imaging in the Resection of Hepatoblastoma: A Single Institution’s Experiences. Front. Surg. 2022, 9, 932721. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, R.S.; Patel, K.R.; Yang, T.; Nguyen, H.N.; Masand, P.; Vasudevan, S.A. Pathologic Correlation with near Infrared-Indocyanine Green Guided Surgery for Pediatric Liver Cancer. J. Pediatr. Surg. 2022, 57, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Ohno, M.; Fujino, A.; Kanamori, Y.; Irie, R.; Yoshioka, T.; Miyazaki, O.; Uchida, H.; Fukuda, A.; Sakamoto, S.; et al. Fluorescence-Guided Surgery for Hepatoblastoma with Indocyanine Green. Cancers 2019, 11, 1215. [Google Scholar] [CrossRef]
- Liu, S.; Feng, J.; Ren, Q.; Qin, H.; Yang, W.; Cheng, H.; Yao, X.; Xu, J.; Han, J.; Chang, S.; et al. Evaluating the Clinical Efficacy and Limitations of Indocyanine Green Fluorescence-Guided Surgery in Childhood Hepatoblastoma: A Retrospective Study. Photodiagn. Photodyn. Ther. 2023, 44, 103790. [Google Scholar] [CrossRef]
- Shen, Q.; Liu, X.; Pan, S.; Li, T.; Zhou, J. Effectiveness of Indocyanine Green Fluorescence Imaging in Resection of Hepatoblastoma. Pediatr. Surg. Int. 2023, 39, 181. [Google Scholar] [CrossRef]
- Souzaki, R.; Kawakubo, N.; Matsuura, T.; Yoshimaru, K.; Koga, Y.; Takemoto, J.; Shibui, Y.; Kohashi, K.; Hayashida, M.; Oda, Y.; et al. Navigation Surgery Using Indocyanine Green Fluorescent Imaging for Hepatoblastoma Patients. Pediatr. Surg. Int. 2019, 35, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Tanaka, M.; Kitagawa, N.; Nozawa, K.; Shinkai, M.; Goto, H.; Tanaka, Y. Clinicopathological Study of Surgery for Pulmonary Metastases of Hepatoblastoma with Indocyanine Green Fluorescent Imaging. Pediatr. Blood Cancer 2022, 69, e29488. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafeez, A.H.; Murphy, A.J.; Brennan, R.; Santiago, T.C.; Lu, Z.; Krasin, M.J.; Bissler, J.J.; Gleason, J.M.; Davidoff, A.M. Indocyanine Green-Guided Nephron-Sparing Surgery for Pediatric Renal Tumors. J. Pediatr. Surg. 2022, 57, 174–178. [Google Scholar] [CrossRef]
- Feng, J.; Yang, W.; Qin, H.; Xu, J.; Liu, S.; Han, J.; Li, N.; He, L.; Wang, H. Clinical Application of Indocyanine Green Fluorescence Imaging Navigation for Pediatric Renal Cancer. Front. Pediatr. 2023, 11, 1108997. [Google Scholar] [CrossRef]
- Harris, A.C.; Choudhury, S.; Pachl, M. Early Results of Minimally Invasive Fluorescent Guided Pediatric Oncology Surgery with Delivery of Indocyanine Green during Induction of Anesthesia. Photodiagn. Photodyn. Ther. 2023, 42, 103639. [Google Scholar] [CrossRef] [PubMed]
- Brookes, M.J.; Chan, C.D.; Crowley, T.P.; Ragbir, M.; Ghosh, K.M.; Beckingsale, T.; Rankin, K.S. Intraoperative Near-Infrared Fluorescence Guided Surgery Using Indocyanine Green (ICG) May Aid the Surgical Removal of Benign Bone and Soft Tissue Tumours. Surg. Oncol. 2024, 55, 102091. [Google Scholar] [CrossRef]
- Wang, H.; Ji, T.; Qu, H.; Yan, T.; Li, D.; Yang, R.; Tang, X.; Guo, W. Indocyanine Green Fluorescence Imaging May Detect Tumour Residuals during Surgery for Bone and Soft-Tissue Tumours. Bone Jt. J. 2023, 105-B, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Asayama, B.; Sato, K.; Fukui, T.; Okuma, M.; Nakagaki, Y.; Nakagaki, Y.; Osato, T.; Nakamura, H. Skull Bone Tumor Resection with Intraoperative Indocyanine Green Fluorescence Imaging: A Series of Four Surgical Cases. Interdiscip. Neurosurg. 2017, 9, 8–13. [Google Scholar] [CrossRef]
- Abdelhafeez, A.H.; Mothi, S.S.; Pio, L.; Mori, M.; Santiago, T.C.; McCarville, M.B.; Kaste, S.C.; Pappo, A.S.; Talbot, L.J.; Murphy, A.J.; et al. Feasibility of Indocyanine Green-Guided Localization of Pulmonary Nodules in Children with Solid Tumors. Pediatr. Blood Cancer 2023, 70, e30437. [Google Scholar] [CrossRef]
- Jeremiasse, B.; Hulsker, C.C.C.; van den Bosch, C.H.; Buser, M.A.D.; van der Ven, C.P.; Bökkerink, G.M.J.; Wijnen, M.H.W.A.; Van der Steeg, A.F.W. Fluorescence Guided Surgery Using Indocyanine Green for Pulmonary Osteosarcoma Metastasectomy in Pediatric Patients: A Feasibility Study. EJC Paediatr. Oncol. 2023, 2, 100019. [Google Scholar] [CrossRef]
- Vetrano, I.G.; Acerbi, F.; Falco, J.; Devigili, G.; Rinaldo, S.; Messina, G.; Prada, F.; D’Ammando, A.; Nazzi, V. Fluorescein-Guided Removal of Peripheral Nerve Sheath Tumors: A Preliminary Analysis of 20 Cases. J. Neurosurg. 2021, 134, 260–269. [Google Scholar] [CrossRef]
- Richard, C.; White, S.; Williams, R.; Zaghloul, T.; Helmig, S.; Sheyn, A.; Abramson, Z.; Abdelhafeez, H. Indocyanine Green near Infrared-Guided Surgery in Children, Adolescents, and Young Adults with Otolaryngologic Malignancies. Auris Nasus Larynx 2023, 50, 576–585. [Google Scholar] [CrossRef]
- Wellens, L.M.; Deken, M.M.; Sier, C.F.M.; Johnson, H.R.; de la Jara Ortiz, F.; Bhairosingh, S.S.; Houvast, R.D.; Kholosy, W.M.; Baart, V.M.; Pieters, A.M.M.J.; et al. Anti-GD2-IRDye800CW as a Targeted Probe for Fluorescence-Guided Surgery in Neuroblastoma. Sci. Rep. 2020, 10, 17667. [Google Scholar] [CrossRef]
- Privitera, L.; Waterhouse, D.J.; Preziosi, A.; Paraboschi, I.; Ogunlade, O.; Da Pieve, C.; Barisa, M.; Ogunbiyi, O.; Weitsman, G.; Hutchinson, J.C.; et al. Shortwave Infrared Imaging Enables High-Contrast Fluorescence-Guided Surgery in Neuroblastoma. Cancer Res. 2023, 83, 2077–2089. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, L.T.; Sever, R.E.; Gilbert, R.; Guerrero, D.; Vincze, S.R.; Menendez, D.M.; Birikorang, P.A.; Rodgers, M.R.; Jaswal, A.P.; Vanover, A.C.; et al. Dual-Labeled Anti-GD2 Targeted Probe for Intraoperative Molecular Imaging of Neuroblastoma. J. Transl. Med. 2024, 22, 940. [Google Scholar] [CrossRef]
- Lehane, A.; Polites, S.F.; Dodd, A.; Goldstein, S.D.; Lautz, T.B. Let It Glow: Intraoperative Visualization of Pulmonary Metastases Using Pafolacianine, a Next-generation Fluorescent Agent, for Young Adults Undergoing Pulmonary Metastasectomy. Pediatr. Blood Cancer 2024, 71, e31293. [Google Scholar] [CrossRef]
- van den Berg, N.S.; Brouwer, O.R.; Schaafsma, B.E.; Mathéron, H.M.; Klop, W.M.C.; Balm, A.J.M.; van Tinteren, H.; Nieweg, O.E.; van Leeuwen, F.W.B.; Valdés Olmos, R.A. Multimodal Surgical Guidance during Sentinel Node Biopsy for Melanoma: Combined Gamma Tracing and Fluorescence Imaging of the Sentinel Node through Use of the Hybrid Tracer Indocyanine Green-(99m)Tc-Nanocolloid. Radiology 2015, 275, 521–529. [Google Scholar] [CrossRef]
- Mertes, P.M.; Malinovsky, J.M.; Mouton-Faivre, C.; Bonnet-Boyer, M.C.; Benhaijoub, A.; Lavaud, F.; Valfrey, J.; O’Brien, J.; Pirat, P.; Lalourcey, L.; et al. Anaphylaxis to Dyes during the Perioperative Period: Reports of 14 Clinical Cases. J. Allergy Clin. Immunol. 2008, 122, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, V.M.; Brown, A.C.; Szocik, J.F.; Pass, H.A.; Moline, S.; De, S.K.; Domino, E.F. Allergic Reactions to Isosulfan Blue during Sentinel Node Biopsy--a Common Event. Surgery 2001, 130, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.B.; Reed, M.S.; Cao, X.; García, H.A.; Ochoa, M.I.; Jiang, S.; Hasan, T.; Doyley, M.M.; Pogue, B.W. Combined Dual-Channel Fluorescence Depth Sensing of Indocyanine Green and Protoporphyrin IX Kinetics in Subcutaneous Murine Tumors. JBO 2024, 30, S13709. [Google Scholar] [CrossRef] [PubMed]
- Pitsinis, V.; Kanitkar, R.; Vinci, A.; Choong, W.L.; Benson, J. Results of a Prospective Randomized Multicenter Study Comparing Indocyanine Green (ICG) Fluorescence Combined with a Standard Tracer Versus ICG Alone for Sentinel Lymph Node Biopsy in Early Breast Cancer: The INFLUENCE Trial. Ann. Surg. Oncol. 2024, 31, 8848–8855. [Google Scholar] [CrossRef]
- Saltzman, A.F.; Smith, D.E.; Gao, D.; Ghosh, D.; Amini, A.; Aldrink, J.H.; Dasgupta, R.; Gow, K.W.; Glick, R.D.; Ehrlich, P.F.; et al. How Many Lymph Nodes Are Enough? Assessing the Adequacy of Lymph Node Yield for Staging in Favorable Histology Wilms Tumor. J. Pediatr. Surg. 2019, 54, 2331–2335. [Google Scholar] [CrossRef]
- Vujanić, G.M.; Gessler, M.; Ooms, A.H.A.G.; Collini, P.; Coulomb-l’Hermine, A.; D’Hooghe, E.; de Krijger, R.R.; Perotti, D.; Pritchard-Jones, K.; Vokuhl, C.; et al. The UMBRELLA SIOP–RTSG 2016 Wilms Tumour Pathology and Molecular Biology Protocol. Nat. Rev. Urol. 2018, 15, 693–701. [Google Scholar] [CrossRef]
- Dome, J.S.; Fernandez, C.V.; Mullen, E.A.; Kalapurakal, J.A.; Geller, J.I.; Huff, V.; Gratias, E.J.; Dix, D.B.; Ehrlich, P.F.; Khanna, G.; et al. Children’s Oncology Group’s 2013 Blueprint for Research: Renal Tumors. Pediatr. Blood Cancer 2013, 60, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Walterhouse, D.O.; Barkauskas, D.A.; Hall, D.; Ferrari, A.; De Salvo, G.L.; Koscielniak, E.; Stevens, M.C.G.; Martelli, H.; Seitz, G.; Rodeberg, D.A.; et al. Demographic and Treatment Variables Influencing Outcome for Localized Paratesticular Rhabdomyosarcoma: Results from a Pooled Analysis of North American and European Cooperative Groups. J. Clin. Oncol. 2018, 36, 3466–3476. [Google Scholar] [CrossRef]
- Geller, J.I.; Hong, A.L.; Vallance, K.L.; Evageliou, N.; Aldrink, J.H.; Cost, N.G.; Treece, A.L.; Renfro, L.A.; Mullen, E.A. COG Renal Tumor Committee Children’s Oncology Group’s 2023 Blueprint for Research: Renal Tumors. Pediatr. Blood Cancer 2023, 70, e30586. [Google Scholar] [CrossRef]
- Bou-Samra, P.; Muhammad, N.; Chang, A.; Karsalia, R.; Azari, F.; Kennedy, G.; Stummer, W.; Tanyi, J.; Martin, L.; Vahrmeijer, A.; et al. Intraoperative Molecular Imaging: 3rd Biennial Clinical Trials Update. JBO 2023, 28, 050901. [Google Scholar] [CrossRef]
- Wang, L.G.; Gibbs, S.L. Improving Precision Surgery: A Review of Current Intraoperative Nerve Tissue Fluorescence Imaging. Curr. Opin. Chem. Biol. 2023, 76, 102361. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Orosco, R.K.; Bouvet, M.; Richmon, J.D.; Berman, B.J.; Crawford, K.L.; Hom, M.; Nguyen, Q.T.; Rosenthal, E.L. Intraoperative Nerve-Specific Fluorescence Visualization in Head and Neck Surgery: A Phase 1 Trial. Nat. Commun. 2025, 16, 6060. [Google Scholar] [CrossRef]
- Montaño, A.R.; Masillati, A.; Szafran, D.A.; Shams, N.A.; Hubbell, G.E.; Barth, C.W.; Gibbs, S.L.; Wang, L.G. Matrix-Designed Bright near-Infrared Fluorophores for Precision Peripheral Nerve Imaging. Biomaterials 2025, 319, 123190. [Google Scholar] [CrossRef]
- Dyer, R.B.; Chen, M.Y.; Zagoria, R.J.; Regan, J.D.; Hood, C.G.; Kavanagh, P.V. Complications of Ureteral Stent Placement. RadioGraphics 2002, 22, 1005–1022. [Google Scholar] [CrossRef]
- Lange, D.; Bidnur, S.; Hoag, N.; Chew, B.H. Ureteral Stent-Associated Complications—Where We Are and Where We Are Going. Nat. Rev. Urol. 2015, 12, 17–25. [Google Scholar] [CrossRef]
- de Valk, K.S.; Handgraaf, H.J.; Deken, M.M.; Sibinga Mulder, B.G.; Valentijn, A.R.; Terwisscha van Scheltinga, A.G.; Kuil, J.; van Esdonk, M.J.; Vuijk, J.; Bevers, R.F.; et al. A Zwitterionic Near-Infrared Fluorophore for Real-Time Ureter Identification during Laparoscopic Abdominopelvic Surgery. Nat. Commun. 2019, 10, 3118. [Google Scholar] [CrossRef]
- Farnam, R.W.; Richard GArms, I.I.I.; Klaassen, A.H.; Sorger, J.M. Intraoperative Ureter Visualization Using a Near-Infrared Imaging Agent. J. Biomed. Opt. 2019, 24, 066004. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafeez, A.; Talbot, L.; Murphy, A.J.; Davidoff, A.M. Indocyanine Green-Guided Pediatric Tumor Resection: Approach, Utility, and Challenges. Front. Pediatr. 2021, 9, 689612. [Google Scholar] [CrossRef]
- Busweiler, L.A.D.; Wijnen, M.H.W.A.; Wilde, J.C.H.; Sieders, E.; Terwisscha van Scheltinga, S.E.J.; van Heurn, L.W.E.; Ziros, J.; Bakx, R.; Heij, H.A. Surgical Treatment of Childhood Hepatoblastoma in the Netherlands (1990–2013). Pediatr. Surg. Int. 2017, 33, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Erginel, B.; Gun Soysal, F.; Keskin, E.; Kebudi, R.; Celik, A.; Salman, T. Pulmonary Metastasectomy in Pediatric Patients. World J. Surg. Oncol. 2016, 14, 27. [Google Scholar] [CrossRef]
- van Dorp, M.; Wolfhagen, N.; Torensma, B.; Dickhoff, C.; Kazemier, G.; Heineman, D.J.; Schreurs, W.H. Pulmonary Metastasectomy and Repeat Metastasectomy for Colorectal Pulmonary Metastases: Outcomes from the Dutch Lung Cancer Audit for Surgery. BJS Open 2023, 7, zrad009. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-K.; Hsieh, M.-L.; Chen, S.-Y.; Liu, C.-Y.; Lin, P.-H.; Kan, H.-C.; Pang, S.-T.; Yu, K.-J. Clinical Benefits of Indocyanine Green Fluorescence in Robot-Assisted Partial Nephrectomy. Cancers 2022, 14, 3032. [Google Scholar] [CrossRef]
- Montanic, S.; Terdoslavich, M.; Rajcevic, U.; De Leo, L.; Bonin, S.; Serbec, V.C.; Passamonti, S. Development and Characterization of a Novel mAb against Bilitranslocase—A New Biomarker of Renal Carcinoma. Radiol. Oncol. 2013, 47, 128–137. [Google Scholar] [CrossRef]
- Zajac, J.; Liu, A.; Hassan, S.; Gibson, A. Mechanisms of Delayed Indocyanine Green Fluorescence and Applications to Clinical Disease Processes. Surgery 2024, 176, 386–395. [Google Scholar] [CrossRef]
- Klenke, F.M.; Wenger, D.E.; Inwards, C.Y.; Rose, P.S.; Sim, F.H. Giant Cell Tumor of Bone: Risk Factors for Recurrence. Clin. Orthop. Relat. Res. 2011, 469, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Danis, R.P.; Wolverton, S.; Steffens, T. Phototoxicity from Systemic Sodium Fluorescein. Retina 2000, 20, 370–373. [Google Scholar] [CrossRef]
- Goldstein, S.D.; Heaton, T.E.; Bondoc, A.; Dasgupta, R.; Abdelhafeez, A.; Davidoff, A.M.; Lautz, T.B. Evolving Applications of Fluorescence Guided Surgery in Pediatric Surgical Oncology: A Practical Guide for Surgeons. J. Pediatr. Surg. 2021, 56, 215–223. [Google Scholar] [CrossRef]
- Paraboschi, I.; De Coppi, P.; Stoyanov, D.; Anderson, J.; Giuliani, S. Fluorescence Imaging in Pediatric Surgery: State-of-the-Art and Future Perspectives. J. Pediatr. Surg. 2021, 56, 655–662. [Google Scholar] [CrossRef]
- Debie, P.; Devoogdt, N.; Hernot, S. Targeted Nanobody-Based Molecular Tracers for Nuclear Imaging and Image-Guided Surgery. Antibodies 2019, 8, 12. [Google Scholar] [CrossRef]
- Schouw, H.M.; Huisman, L.A.; Janssen, Y.F.; Slart, R.H.J.A.; Borra, R.J.H.; Willemsen, A.T.M.; Brouwers, A.H.; van Dijl, J.M.; Dierckx, R.A.; van Dam, G.M.; et al. Targeted Optical Fluorescence Imaging: A Meta-Narrative Review and Future Perspectives. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4272–4292. [Google Scholar] [CrossRef] [PubMed]
- Dumba, M.; Jawad, N.; McHugh, K. Neuroblastoma and Nephroblastoma: A Radiological Review. Cancer Imaging 2015, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Zwaveling, S.; Tytgat, G.A.M.; van der Zee, D.C.; Wijnen, M.H.W.A.; Heij, H.A. Is Complete Surgical Resection of Stage 4 Neuroblastoma a Prerequisite for Optimal Survival or May >95% Tumour Resection Suffice? Pediatr. Surg. Int. 2012, 28, 953–959. [Google Scholar] [CrossRef]
- Von Allmen, D.; Davidoff, A.M.; London, W.B.; Van Ryn, C.; Haas-Kogan, D.A.; Kreissman, S.G.; Khanna, G.; Rosen, N.; Park, J.R.; La Quaglia, M.P. Impact of Extent of Resection on Local Control and Survival in Patients From the COG A3973 Study With High-Risk Neuroblastoma. J. Clin. Oncol. 2017, 35, 208–216. [Google Scholar] [CrossRef]
- Jain, A.; Peters, N.J.; Samujh, R.; Trehan, A.; Malik, M.A.; Madan, R.; Dogra, S.; Solanki, S.; Singh, J.; Kanojia, R.P.; et al. Outcome Analysis of Surgical Complications in Pediatric Solid Tumors: A Retrospective Clinical Study. Pediatr. Surg. Int. 2025, 41, 201. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 Antibody with GM-CSF, Interleukin-2, and Isotretinoin for Neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef]
- Croteau, N.J.; Heaton, T.E. Pulmonary Metastasectomy in Pediatric Solid Tumors. Children 2019, 6, 6. [Google Scholar] [CrossRef]
- Heaton, T.E.; Davidoff, A.M. Surgical Treatment of Pulmonary Metastases in Pediatric Solid Tumors. Semin. Pediatr. Surg. 2016, 25, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Tanyi, J.L.; Randall, L.M.; Chambers, S.K.; Butler, K.A.; Winer, I.S.; Langstraat, C.L.; Han, E.S.; Vahrmeijer, A.L.; Chon, H.S.; Morgan, M.A.; et al. A Phase III Study of Pafolacianine Injection (OTL38) for Intraoperative Imaging of Folate Receptor-Positive Ovarian Cancer (Study 006). J. Clin. Oncol. 2023, 41, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, I.S.; Martin, L.W.; Rice, D.C.; Blackmon, S.H.; Slade, H.B.; Singhal, S. ELUCIDATE Study Group Pafolacianine for Intraoperative Molecular Imaging of Cancer in the Lung: The ELUCIDATE Trial. J. Thorac. Cardiovasc. Surg. 2023, 166, e468–e478. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Kolb, E.A.; Qin, J.; Chou, A.; Sowers, R.; Hoang, B.; Healey, J.H.; Huvos, A.G.; Meyers, P.A.; Gorlick, R. The Folate Receptor Alpha Is Frequently Overexpressed in Osteosarcoma Samples and Plays a Role in the Uptake of the Physiologic Substrate 5-Methyltetrahydrofolate. Clin. Cancer Res. 2007, 13, 2557–2567. [Google Scholar] [CrossRef]
- Maeda, H.; Fang, J.; Inutsuka, T.; Kitamoto, Y. Vascular Permeability Enhancement in Solid Tumor: Various Factors, Mechanisms Involved and Its Implications. Int. Immunopharmacol. 2003, 3, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Tummers, Q.R.J.G.; Hoogstins, C.E.S.; Peters, A.A.W.; de Kroon, C.D.; Trimbos, J.B.M.Z.; van de Velde, C.J.H.; Frangioni, J.V.; Vahrmeijer, A.L.; Gaarenstroom, K.N. The Value of Intraoperative Near-Infrared Fluorescence Imaging Based on Enhanced Permeability and Retention of Indocyanine Green: Feasibility and False-Positives in Ovarian Cancer. PLoS ONE 2015, 10, e0129766. [Google Scholar] [CrossRef]
- Dirheimer, L.; Cortese, S.; Dolivet, G.; Merlin, J.L.; Marchal, F.; Mastronicola, R.; Bezdetnaya, L. Fluorescence Imaging-Assessed Surgical Margin Detection in Head and Neck Oncology by Passive and Active Targeting. Mol. Diagn. Ther. 2025, 29, 465–481. [Google Scholar] [CrossRef]
- Cai, Y.; Chai, T.; Nguyen, W.; Liu, J.; Xiao, E.; Ran, X.; Ran, Y.; Du, D.; Chen, W.; Chen, X. Phototherapy in Cancer Treatment: Strategies and Challenges. Signal Transduct. Target. Ther. 2025, 10, 115. [Google Scholar] [CrossRef]
- Mazur, A.; Koziorowska, K.; Dynarowicz, K.; Aebisher, D.; Bartusik-Aebisher, D. Photodynamic Therapy for Treatment of Disease in Children—A Review of the Literature. Children 2022, 9, 695. [Google Scholar] [CrossRef]
- Mieog, J.S.D.; Achterberg, F.B.; Zlitni, A.; Hutteman, M.; Burggraaf, J.; Swijnenburg, R.-J.; Gioux, S.; Vahrmeijer, A.L. Fundamentals and Developments in Fluorescence-Guided Cancer Surgery. Nat. Rev. Clin. Oncol. 2022, 19, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Kattner, P.; Strobel, H.; Khoshnevis, N.; Grunert, M.; Bartholomae, S.; Pruss, M.; Fitzel, R.; Halatsch, M.-E.; Schilberg, K.; Siegelin, M.D.; et al. Compare and Contrast: Pediatric Cancer versus Adult Malignancies. Cancer Metastasis Rev. 2019, 38, 673–682. [Google Scholar] [CrossRef]
- Giuliani, S.; Paraboschi, I.; McNair, A.; Smith, M.; Rankin, K.S.; Elson, D.S.; Paleri, V.; Leff, D.; Stasiuk, G.; Anderson, J. Monoclonal Antibodies for Targeted Fluorescence-Guided Surgery: A Review of Applicability across Multiple Solid Tumors. Cancers 2024, 16, 1045. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasse, B.; Rijs, Z.; Angoelal, K.R.; Hiemcke-Jiwa, L.S.; de Boed, E.A.; Kuppen, P.J.K.; Sier, C.F.M.; van Driel, P.B.A.A.; van de Sande, M.A.J.; Wijnen, M.H.W.A.; et al. Evaluation of Potential Targets for Fluorescence-Guided Surgery in Pediatric Ewing Sarcoma: A Preclinical Proof-of-Concept Study. Cancers 2023, 15, 3896. [Google Scholar] [CrossRef]
- Tummers, W.S.; Warram, J.M.; vn den Berg, N.S.; Miller, S.E.; Swijnenburg, R.-J.; Vahrmeijer, A.L.; Rosenthal, E.L. Recommendations for Reporting on Emerging Optical Imaging Agents to Promote Clinical Approval. Theranostics 2018, 8, 5336–5347. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.